Abstract
Circulating microRNAs (miRNAs/miRs) have been reported as diagnostic biomarkers for esophageal cancer (EC) diagnosis. However, contrasting results have been achieved in different studies. In the present study, a meta-analysis was performed, based on the systematic search of PubMed and Web of Science, to evaluate the diagnostic value of circulating miRNAs in the peripheral blood in EC. The top 5 most-studied miRNAs were selected for confirmation by reverse transcription quantitative-polymerase chain reaction using the blood plasma of 125 patients with esophageal squamous cell carcinoma (ESCC) and 125 healthy individuals from Henan, China. A total of 45 studies from 22 articles, regarding 33 miRNAs were considered in the meta-analysis. The pooled sensitivity and specificity were both 0.79 (95% confidence interval, 0.76–0.82 for both). Among the 5 miRNAs considered (miR-21, miR-223, miR-375, miR-25 and miR-100), miR-21 and miR-223 were significantly overexpressed whereas miR-375 expression was reduced in patients with ESCC compared with healthy individuals (all P<0.001). The areas under the curves (AUCs) were 0.80, 0.73, and 0.69 for miR-21, miR-223, and miR-375, respectively. The AUCs increased when discriminating between patients with early ESCC in stage 0–I and the non-invasive carcinoma stage Tis-T1 stage from controls. Thus, it was concluded that plasma miR-21, miR-223 and miR-375 may serve as non-invasive diagnostic biomarkers in patients with ESCC, especially early ESCC in stages 0–I and Tis-T1.
Keywords: esophageal squamous cell carcinoma, microRNA, meta-analysis, plasma, diagnosis, biomarker
Introduction
Esophageal cancer (EC) is the 8th-most prevalent and the 6th most lethal type of malignant cancer globally; esophageal squamous cell carcinoma (ESCC) is the predominant pathological type of EC (1). According to the GLOBOCAN estimates, in 2012 the incidence of EC varied by >21-fold internationally (1,2). North-central China is positioned on the ‘esophageal cancer belt’ (3); the Henan province is representative of this region. Due to the lack of symptoms at the onset of EC, the majority of patients with EC are diagnosed and treated at an intermediate or advanced histological stage, when lymph node or distant metastasis, may have occurred. Consequently, the 5-year survival rate of EC remains low, at 15–25% (4,5).
Although treatments for EC, including surgery, radiation, and chemotherapy, have been greatly improved over the past decades, the disease continues to be associated with a relatively poor prognosis. A number of studies have demonstrated that autologous cellular antigens, including established serological tumor biomarkers or their corresponding autoantibodies, could invoke an immune reaction in patients with cancer (6–8). However, such biomarkers are rapidly degraded or cleared from the circulatory system (9). Furthermore, the majority of these biomarkers are insufficient for the early detection of EC (10,11). Therefore, the discovery of novel tumor biomarkers is urgently required to detect carcinogenesis at an early stage as accurately as possible and improve the prognosis of patients with EC.
MicroRNAs (miRNAs/miRs) are small non-coding single-stranded RNAs of 20–25 nucleotides. They have been demonstrated to exist stably in cell-free body fluids, including the saliva, plasma, serum and urine. The first circulating miRNA potential biomarker for cancer diagnosis was identified in 2008 (12). MiRNAs continue to attract research attention for this purpose, and promising diagnostic markers have been identified for breast cancer (13), liver cancer (14), lung cancer (15), colorectal cancer (16), uterine cancer (17), glioma (18) and gastroesophageal cancer (19); a number of such biomarkers function as oncogenes or tumor-suppressor genes (20). Numerous studies have associated various circulating miRNAs with EC (21). However, different research groups have drawn contrasting conclusions regarding the same plasma miRNAs in EC. MiR-375 expression was demonstrated to be upregulated in EC by Li et al (22), and downregulated by Komatsu et al (23). Other studies demonstrated that the expression of miR-375 was significantly downregulated in other types of cancer, particularly in tumors of the digestive tract (24–27). Verification of these previous conclusions is required.
In the present study, a meta-analysis of previously studied circulating miRNAs in plasma or serum was performed to assess their diagnostic efficiency in EC. The 5 most studied miRNAs were selected as candidate markers, and their expression levels in plasma were quantified using reverse transcription quantitative-polymerase chain reaction (RT-qPCR). The results were used to verify and evaluate their diagnostic value in a validation group of ESCC samples.
Subjects and methods
Study population and sample collection
A total of 125 patients were histopathologically diagnosed with primary ESCC at the Henan Cancer Hospital (Henan, China) between April 2013 and December 2014. The patients had undergone no prior surgery, chemotherapy or radiotherapy. The 7th edition of the International Union Against Cancer (UICC) tumor-node-metastasis (TNM) staging system (28) was used for the classification of the patients with ESCC. Peripheral whole blood was collected from each patient in EDTA-K2 anti-coagulant tubes prior to any treatment. Plasma was separated within 2 h of collection by centrifugation at 3,000 × g for 10 min at 4°C. The samples were then stored at −80°C until required. A total of 125 age- and sex-matched individuals were selected from the annual health examinations during the same period, and were enrolled as healthy controls. Patients with symptoms suggestive of cancer, digestive tract diseases or immune diseases were excluded. The present study was approved by the Institute Research Ethics Committee of Henan Cancer Hospital and Zhengzhou University. Written informed consent was obtained from all participants.
Meta-analysis of the diagnostic value of circulating miRNAs to diagnose EC
A systematic literature search in the PubMed and Web of Science databases was performed, considering published studies in English or Chinese until April 2016. The major medical subject heading search terms were as follows: ‘esophageal neoplasms’, ‘microRNAs’, ‘blood’, ‘diagnosis’ and ‘sensitivity and specificity’, ‘biomarkers’ and ‘tumor’. Manual retrieval was also applied by tracing references to identify additional records.
All relevant publications were included based on the following criteria: i) Patients were diagnosed with primary EC; ii) studies concentrated on circulating miRNAs in serum or plasma; and iii) studies provided sufficient data for the construction of 2×2 tables. The exclusion criteria were: i) Duplicate studies; ii) publications not in Chinese or English; iii) reviews, meta-analyses, case reports, editorials and meeting records; iv) studies without blood samples, or with samples extracted subsequent to surgery, chemotherapy or radiotherapy; v) studies that did not provide sufficient data to calculate the sensitivity, specificity and AUC of miRNAs and diagnose EC; vi) studies focusing only on the survival and prognosis of EC; and vii) studies focusing only on the molecular mechanisms of miRNAs in EC.
Data were extracted from the included studies by two independent reviewers, and disagreements were resolved through discussion. The extracted information is provided in Table I. When a training group and a validation group appeared in one article simultaneously, they were treated as independent studies in the meta-analysis. From this meta-analysis, the top 5 most-studied miRNAs were selected as the candidate markers for further verification.
Table I.
The main characteristics of eligible studies in the systematic meta-analysis.
| Study population characteristics | Experimental method | Data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year | miR | Trend | Country | Casesa | Controlsa | Sample type | RNA extraction reagent/kit | Normalization control | RT-qPCR type | Cutoff | TP | FP | FN | TN | (Refs.) |
| Ye et al, 2014 | miR-21 | Up | China | 100 (80/20), 59 (43–88) | 50 | Plasma | mirVANA PARIS | miR-16 | SYBR | YI | 97 | 22 | 3 | 28 | (52) |
| Li et al, 2015 | miR-21 | Up | China | 24b (18/6), 68.5 (47–87) | 19 | Plasma | mirVANA PARIS | miR-1228 | TaqMan | 3.89 | 22 | 6 | 2 | 13 | (53) |
| Li et al, 2015 | miR-21 | Up | China | 38 (30/8) | 19 | Plasma | mirVANA PARIS | miR-1228 | TaqMan | YI | 35 | 8 | 3 | 11 | (22) |
| Wang and Zhang, 2012 | miR-21 | Up | China | 31c (23/8), 61 (46–82) | 39 (30/9), 46 (−) | Plasma | Trizol LS | miR-16 | SYBR | 3.37 | 22 | 12 | 9 | 27 | (40) |
| Komatsu et al, 2011 | miR-21 | Up | Japan | 50 (44/6) | 20 | Plasma | mirVANA PARIS | Standard curve | TaqMan | 0.22 | 24 | 3 | 26 | 17 | (23) |
| Wu et al, 2014 | miR-223 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | YI | 40 | 12 | 23 | 51 | (46) |
| Zhang et al, 2012 | miR-223 | Up | China | 113 (88/25) | 67 | Serum | Phenol/ chloroform | Standard curve | TaqMan | 0.71 | 79 | 13 | 34 | 54 | (36) |
| Zhang et al, 2010 | miR-223 | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | YI | 124 | 17 | 25 | 83 | (39) |
| Komatsu et al, 2011 | miR-375 | Down | Japan | 50 (44/6) | 20 | Plasma | mirVANA PARIS | Standard curve | TaqMan | <0.01 | 39 | 5 | 11 | 15 | (23) |
| Li et al, 2015 | miR-375 | Up | China | 38 (30/8) | 19 | Plasma | mirVANA PARIS | miR-1228 | TaqMan | YI | 31 | 3 | 7 | 16 | (22) |
| Wu et al, 2014 | miR-25 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | YI | 47 | 16 | 16 | 47 | (46) |
| Komatsu et al, 2014 | miR-25 | Up | Japan | 20 | 50 | Plasma | mirVANA PARIS | Standard curve | TaqMan | 0.32 | 17 | 7 | 3 | 43 | (45) |
| Wu et al, 2014 | miR-100 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | YI | 48 | 22 | 15 | 41 | (46) |
| Zhang et al, 2010 | miR-100 | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | 0.01 | 95 | 19 | 54 | 81 | (39) |
| Xie et al, 2013 | miR-10b | Down | China | 29b (24/5), (61.3±9.5) | 16 (13/3), (57.5±7.1) | Plasma | mirVANA PARIS | miR-16 | SYBR | 0.56 | 24 | 0 | 5 | 16 | (37) |
| Xu et al, 2015 | miR-10b | Down | China | 50 (27/23) | 50 (30/20) | Serum | mirVANA PARIS | RUN6B | TaqMan | YI | 38 | 8 | 12 | 42 | (49) |
| Zhang et al, 2011 | miR-31 | Up | China | 120 (79/41) 81 (49/32) | 121 (76/45) 81 (43/38) | Serum | miRNeasy Mini | miR-16 | SYBR | 0.01 0.01 | 104 70 | 19 17 | 16 11 | 102 64 | (34) |
| Zhang et al, 2013 | miR-1322 | Up | China | 120 (79/41) 81 (49/32) | 120 (75/45) 81 (43/38) | Serum | miRNeasy Mini | miR-16 | SYBR | 0.01 0.01 | 98 68 | 21 16 | 22 13 | 99 65 | (44) |
| Takeshita et al, 2013 | miR-1246 | Up | Japan | 101 (89/12) | 46 | Serum | mirVANA PARIS | miR-16 | TaqMan | 1.32 | 72 | 12 | 29 | 34 | (43) |
| Zhang et al, 2010 | miR-10a | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | YI | 121 | 20 | 28 | 80 | (39) |
| Zhang et al, 2010 | miR-127 | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | YI | 117 | 13 | 32 | 87 | (39) |
| Zhang et al, 2010 | miR-133a | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | YI | 97 | 17 | 52 | 83 | (39) |
| Zhang et al, 2010 | miR-148b | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | YI | 99 | 13 | 50 | 87 | (39) |
| Zhang et al, 2010 | miR-22 | Up | China | 149 (116/33) | 100 (74/26) | Serum | Trizol | Serum volume | TaqMan | YI | 132 | 14 | 17 | 86 | (39) |
| Li et al, 2015 | miR-16 | Up | China | 38 (30/8) | 19 | Plasma | mirVANA PARIS | miR-1228 | TaqMan | 3.45 | 36 | 8 | 2 | 11 | (22) |
| Li et al, 2015 | miR-185 | Up | China | 38 (30/8) | 19 | Plasma | mirVANA PARIS | miR-1228 | TaqMan | 3.97 | 38 | 8 | 0 | 11 | (22) |
| Liu et al, 2012 | miR-155 | Down | China | 60, (61.9±7.4) | 60, (63.6±8.6) | Plasma | Trizol | RUN6B | SYBR | YI | 38 | 21 | 22 | 39 | (35) |
| Jiang et al, 2015 | miR-218 | Down | China | 106b (69/37) | 60 | Serum | mirVANA PARIS | miR-16 | Molecular beacon | 0.77 | 76 | 14 | 30 | 46 | (48) |
| He et al, 2015 | miR-20a | Up | China | 70 (46/24), | 40, (61.7±6.9) (60.5±8.4) | Plasma | Trizol reagent BD TB-126 | SV40 | SYBR | 4.77 | 45 | 10 | 25 | 30 | (47) |
| He et al, 2015 | let-7a | Down | China | 70 (46/24), | 40, (61.7±6.9) (60.5±8.4) | Plasma | Trizol reagent BD TB-126 | SV40 | SYBR | 6.22 | 52 | 6 | 18 | 34 | (47) |
| Hirajim et al, 2013 | miR-18a | Up | Japan | 106 (87/19) | 54 | Plasma | mirVANA PARIS | Standard curve | TaqMan | 1.99 | 92 | 0 | 14 | 54 | (41) |
| Sharma et al, 2013 | miR-107 | Down | India | 14 | 17 | Serum | QIAamp Viral RNA mini | 5s rRNA | SYBR | YI | 9 | 3 | 5 | 14 | (42) |
| Sun et al, 2015 | miR-718 | Down | China | 120 (79/41) | 51 | Plasma | Trizol reagent BD TB-126 | miR-16 | SYBR | 0.09 | 83 | 17 | 37 | 34 | (51) |
| Guan et al, 2015 | miR-613 | Down | China | 75 (43/32) | 75 | Serum | miRNeasy serum/plasma | miR-16 | SYBR | 0.88 | 61 | 28 | 14 | 47 | (50) |
| Hui et al, 2015 | miR-129 | Up | China | 69 | 14 | Serum | mirVANA PARIS | miR-1228 | TaqMan | 0.39 | 54 | 4 | 15 | 10 | (38) |
| Hui et al, 2015 | miR-365 | Up | China | 69 | 14 | Serum | mirVANA PARIS | miR-1228 | TaqMan | 5.06 | 56 | 2 | 13 | 12 | (38) |
| Hui et al, 2015 | miR-451 | Up | China | 69 | 14 | Serum | mirVANA PARIS | miR-1228 | TaqMan | 0.57 | 57 | 3 | 12 | 11 | (38) |
| Xu et al, 2015 | miR-29c | Down | China | 50 (27/23) | 50 (30/20) | Serum | mirVANA PARIS | RUN6B | TaqMan | YI | 39 | 7 | 11 | 43 | (49) |
| Xu et al, 2015 | miR-205 | Down | China | 50 (27/23) | 50 (30/20) | Serum | mirVANA PARIS | RUN6B | TaqMan | YI | 38 | 7 | 12 | 43 | (49) |
| Wu et al, 2014 | miR-193 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | YI | 47 | 8 | 16 | 55 | (46) |
| Wu et al, 2014 | miR-194 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | YI | 54 | 4 | 15 | 10 | (46) |
| Wu et al, 2014 | miR-483 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | YI | 57 | 3 | 12 | 11 | (46) |
| Wu et al, 2014 | miR-337 | Up | China | 63 (55/8), (62.0±8.3) | 63 (55/8), (59.7±6.8) | Serum | Trizol | let-7d, 7g and 7i | TaqMan | 0.37 | 54 | 4 | 15 | 10 | (46) |
Number (males/females) and the mean (± standard deviation) or median (range) age.
These studies included esophageal squamous cell carcinoma and esophageal adenocarcinoma histological types.
The histological type of the esophageal carcinoma patients was not identified in this study. Ref., reference; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; YI, Youden index; TP, true positive; FP, false positive; FN, false negative, TN, true negative.
RNA purification
RNA was purified from 200 µl plasma using a miRNeasy Serum/Plasma kit (cat. no. 217184; Qiagen GmbH, Hilden, Germany) and eluted with 14 µl of RNase-free water, according to the manufacturer's instructions. The yield of RNA was determined by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA USA). The total extracted RNA was stored at −80°C until required.
RT-qPCR
Quantification of the 5 selected miRNAs was performed using RT-qPCR, with 5S rRNA as a reference for normalization. The reverse transcription of total RNA was performed using a miScript II Reverse Transcription kit (cat. no. 218161; Qiagen GmbH) in the GeneAmp® PCR System 9700 (Applied Biosystems, Foster City, CA, USA). Thermocycling conditions were as follows: 37°C for 60 min followed by 95°C for 5 min. Each reaction consisted of 0.5 µg RNA, 1 µl 10× Nucleics mix, 2 µl 5× HiSpec buffer and 0.5 µl miScript Reverse Transcriptase Mix, with RNase-free water added for a total volume of 10 µl. The reaction mix was diluted to 1:10 using RNase-free water and stored at −20°C until use. The PCR amplification was performed using SYBR Green I Master on the LightCycler® 480 II Real-time PCR Instrument (both from Roche Diagnostics GmbH, Basel, Switzerland), according to the manufacturer's instructions. The thermocycling conditions of the PCR amplification reactions were as following: First an initial denaturation for 10 min at 95°C, then 40 cycles of denaturation at 95°C for 10 sec and annealing at 60°C for 30 sec. The primer sequences are listed in Table II. Each reaction was performed in triplicate. The cycle number at which the fluorescence reached the fixed threshold was referred to as the cycle threshold (Cq). The expression levels of miRNAs were calculated using the 2−ΔΔCq method (ΔΔCq = ΔCqcase - ΔCqcontrol, ΔCq = CqmiRNA - Cqreference) (29).
Table II.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction.
| Name | Primer sequence (5′-3′) |
|---|---|
| Universal primer | Unknown (provided by Qiagen) |
| 5S rRNA | GGAGACCGCCTGGGAATA |
| hsa-miR-21 | TAGCTTATCAGACTGATGTTGA |
| hsa-miR-223 | TGTCAGTTTGTCAAATACCCCA |
| hsa-miR-375 | TTTGTTCGTTCGGCTCGCGTGA |
| hsa-miR-25 | CATTGCACTTGTCTCGGTCTGA |
| hsa-miR-100 | AACCCGTAGATCCGAACTTGTG |
hsa-miR, Homo sapiens microRNA.
Statistical analysis
Stata 12.0 software (StataCorp LP, College Station, TX, USA) was used to perform the diagnostic meta-analysis using the Midas module produced by Dwamena (30). The bivariate mixed effects model (31) was used to calculate the main parameters and their corresponding 95% confidence intervals (CIs). The heterogeneity among studies was assessed by I2 tests, and significant heterogeneity was considered to exist when I2 >50% (32). Publication bias was evaluated by Deeks' funnel plots. P<0.1 was considered to indicate a statistically significantly difference.
Non-parametric tests (the Mann-Whitney U test and Kruskal-Wallis test) were used to compare the plasma miRNAs expression levels. The associations between the concentrations of plasma miRNAs and clinicopathological features were evaluated by Fisher's exact test or χ2 test. The analysis of receiver operating characteristic (ROC) curves was performed to evaluate diagnostic power, allowing estimation of the area under the ROC curves (AUCs) with 95% CIs. The Youden index was used to determine the cutoff value of miRNAs (33). GraphPad Prism 6 (GraphPad software Inc., La Jolla, CA, USA) was used to produce figures. A two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Subject characteristics
A total of 125 patients with ESCC and 125 age- and sex-matched healthy controls were included in the study. All subjects were >40 years old, with ESCC patients ranging from 41–80 and healthy individuals from 40–79; the median age was 63 for these two groups. The main clinicopathological features of patients with ESCC are listed in Table III. No data was available for 4 (3.2%) patients regarding the TNM stage, 7 (5.6%) regarding the tumor location and 14 (11.2%) regarding the differentiation grade. There was only data regarding M-status for a further 3 patients. Therefore, in total, there were 7 patients for which data regarding the T and N stage were not available.
Table III.
The association between plasma miRNA expression levels and clinicopathological factors of patients with esophageal squamous cell carcinoma.
| miR-21 | miR-223 | miR-375 | miR-25 | miR-100 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | High | Low | P-value | High | Low | P-value | High | Low | P-value | High | Low | P-value | High | Low | P-value |
| Total | 125 | 39 | 86 | 42 | 83 | 32 | 93 | 38 | 87 | 36 | 89 | |||||
| Sex | 0.213 | 0.134 | 0.998 | 0.802 | 0.753 | |||||||||||
| Male | 76 | 21 | 55 | 23 | 53 | 19 | 57 | 21 | 55 | 19 | 57 | |||||
| Female | 49 | 18 | 31 | 19 | 30 | 13 | 36 | 17 | 32 | 17 | 32 | |||||
| Age | 0.959 | 0.358 | 0.464 | 0.716 | 0.423 | |||||||||||
| <63 | 59 | 20 | 39 | 20 | 39 | 16 | 43 | 19 | 40 | 16 | 43 | |||||
| ≥63 | 66 | 19 | 47 | 22 | 44 | 16 | 50 | 19 | 47 | 20 | 46 | |||||
| Tumor location | 0.097 | 0.123 | 0.668 | 0.003 | 0.928 | |||||||||||
| Upper | 22 | 7 | 15 | 8 | 14 | 5 | 17 | 6 | 16 | 4 | 18 | |||||
| Middle | 65 | 17 | 48 | 16 | 49 | 20 | 45 | 16 | 49 | 24 | 41 | |||||
| Lower | 31 | 15 | 16 | 18 | 13 | 4 | 27 | 15 | 16 | 6 | 25 | |||||
| T stage | 0.006 | 0.002 | <0.001 | 0.001 | <0.001 | |||||||||||
| Tis-T1 | 50 | 25 | 25 | 27 | 32 | 3 | 47 | 25 | 25 | 5 | 45 | |||||
| T2 | 20 | 6 | 14 | 4 | 16 | 10 | 10 | 3 | 17 | 8 | 12 | |||||
| T3-4 | 48 | 8 | 40 | 11 | 37 | 14 | 34 | 9 | 39 | 18 | 30 | |||||
| N stage | 0.122 | 0.001 | <0.001 | <0.001 | <0.001 | |||||||||||
| Negative | 86 | 33 | 53 | 38 | 48 | 16 | 70 | 34 | 52 | 20 | 66 | |||||
| Positive | 32 | 6 | 26 | 4 | 28 | 11 | 21 | 3 | 29 | 11 | 21 | |||||
| M stage | 0.906 | 0.158 | 0.075 | 0.513 | 0.126 | |||||||||||
| Negative | 116 | 38 | 78 | 41 | 75 | 27 | 89 | 36 | 80 | 31 | 85 | |||||
| Positive | 5 | 1 | 4 | 1 | 4 | 2 | 3 | 1 | 4 | 1 | 4 | |||||
| TNM stage | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||||||
| 0/I | 54 | 29 | 25 | 32 | 22 | 3 | 51 | 28 | 26 | 6 | 48 | |||||
| II | 38 | 4 | 34 | 6 | 32 | 16 | 22 | 7 | 31 | 16 | 22 | |||||
| III/IV | 29 | 6 | 23 | 4 | 25 | 10 | 19 | 2 | 27 | 10 | 19 | |||||
| Differentiation grade | 0.453 | 0.654 | 0.016 | 0.772 | 0.012 | |||||||||||
| Well | 15 | 7 | 8 | 6 | 9 | 1 | 14 | 3 | 12 | 1 | 14 | |||||
| Moderate | 46 | 14 | 32 | 14 | 32 | 9 | 37 | 17 | 29 | 11 | 35 | |||||
| Poor | 50 | 16 | 34 | 20 | 30 | 16 | 34 | 13 | 37 | 18 | 32 | |||||
TNM, tumor-node-metastasis; miR/miRNA, microRNA.
Characteristics of the studies included in the meta-analysis
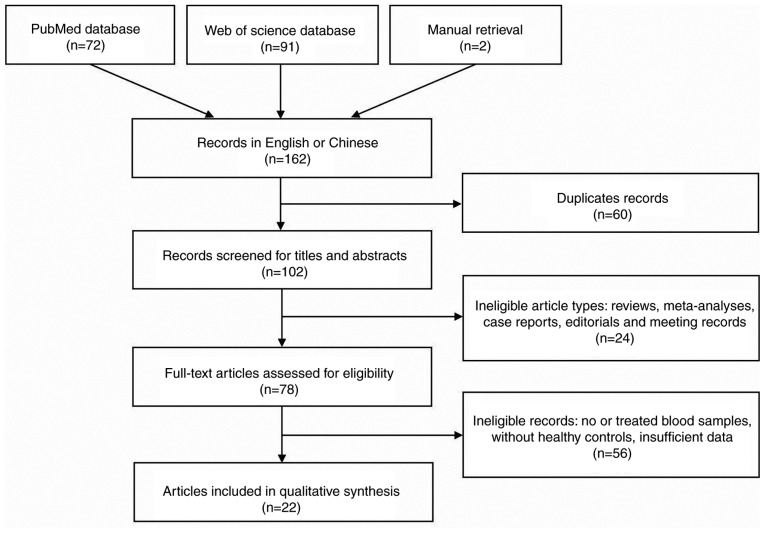
With non-English/Chinese studies excluded, a total of 162 articles were retrieved from the 2 databases and manual retrieval (Fig. 1). Among them, 140 were subsequently excluded according to the exclusion criteria. As a consequence, 22 articles (22,23,34–53) were eventually included in the meta-analysis. Among them, 7 articles (22,38,39,22) studied more than one miRNA. A total of 2 articles (34,44) focused on different miRNAs in the same subjects and contained a validation group. Thus, this meta-analysis contained 45 studies and 33 miRNAs, 1,589 patients with EC and 1,112 healthy individuals.
Figure 1.
Flowchart illustrating the literature identification and selection process.
The main characteristics of each study are listed in Table I. The vast majority of studies considered only ESCC, with just 21 patients from 3 studies (37,48,53) presenting with EAC (esophageal adenocarcinoma). In addition, one study (40) did not provide patient histological types. All studies were published between 2010 and 2016 in Asia, of which 39 were published in China, 5 in Japan and 1 in India. MiR-21, miR-375, miR-223, miR-25, miR-100 and miR-10b were reported twice or more in different articles.
The majority of the miRNAs were reported as overexpressed in ESCC. However, different studies arrived at opposing conclusions regarding the expression of miR-375. All eligible studies in this meta-analysis used RT-qPCR to determine the expression levels of miRNAs in plasma (n=16) or serum (n=29), of which 13 studies performed RT-qPCR with SYBR-Green while 32 studies used probes. The expression levels of miRNAs were relatively or absolutely quantified using the 2−ΔCq or 2−ΔΔCq methods. The methods for normalization varied from total serum volume to reference controls, including SV40, RUN6B, 5S rRNA, the combination of let-7d, 7g and 7i, miR-16, miR-1228 or other synthetic miRNAs for the construction of standard curves. Cutoff values were set according to the Youden index and varied from <0.01 to 6.22. The overall quality of the included studies in the meta-analysis was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (54), and the result was relatively moderate.
Evaluation of the meta-analysis
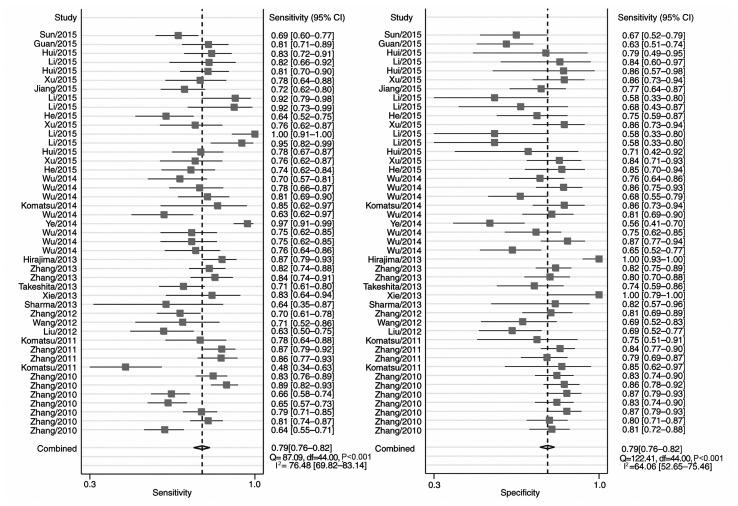
Fig. 2 includes the forest plots for 45 studies of the sensitivity and specificity of miRNA detection for EC diagnosis. Heterogeneity between studies in sensitivity and specificity were recorded; the I2 values were 76 and 64%, respectively. Overall pooled sensitivity and specificity were 0.79 (95% CI, 0.76–0.82 for both), and the AUC was 0.86 (95% CI, 0.83–0.89), indicating a relatively high accuracy. As the majority of patients presented with ESCC, the pooled ESCC estimates were close to the overall estimates (Table IV).
Figure 2.
Forest plot of the pooled sensitivity and specificity of microRNAs in the detection of esophageal cancer. The squares and horizontal lines reflect the study-specific sensitivity and specificity estimates and their corresponding 95% CIs. The diamonds represent the combined estimate. CI, confidence interval; Q, Choran's Q for heterogeneity; df, degrees of freedom.
Table IV.
Summary of the estimates of diagnostic value, with 95% CIs.
| Category | Sensitivity | Specificity | LRP | LRN | DOR | AUC |
|---|---|---|---|---|---|---|
| Country | ||||||
| China | 0.79 (0.76–0.82) | 0.79 (0.76–0.81) | 3.7 (3.3–4.2) | 0.26 (0.23–0.30) | 14 (11–18) | 0.86 (0.82–0.89) |
| Non-China | 0.72 (0.58–0.82) | 0.88 (0.68–0.96) | 5.8 (1.9–17.7) | 0.32 (0.19–0.54) | 18 (4–87) | 0.85 (0.81–0.88) |
| Sample type | ||||||
| Serum | 0.76 (0.72–0.79) | 0.81 (0.75–0.85) | 3.9 (3.0–5.1) | 0.30 (0.25–0.35) | 13 (9–19) | 0.84 (0.81–0.87) |
| Plasma | 0.82 (0.76–0.86) | 0.78 (0.75–0.82) | 3.8 (3.2–4.4) | 0.24 (0.19–0.30) | 16 (12–22) | 0.86 (0.83–0.89) |
| ESCC only | 0.79 (0.75–0.82) | 0.79 (0.76–0.82) | 3.8 (3.3–4.4) | 0.27 (0.23–0.31) | 14 (11–18) | 0.86 (0.83–0.89) |
| Overall | 0.79 (0.76–0.82) | 0.79 (0.76–0.82) | 3.8 (3.3–4.3) | 0.27 (0.23–0.31) | 14 (11–18) | 0.86 (0.83–0.89) |
CIs, confidence intervals; LRP, likelihood ratio positive; LRN, likelihood ratio negative; DOR, diagnostic odds ratio; AUC, area under the ROC curve; ESCC, esophageal squamous cell carcinoma.
Since only one study was performed in India, the studies were divided into 2 subgroups: China and non-China. Subgroup analyses based on countries and sample types were performed (Table IV). The China subgroup had a higher pooled sensitivity compared with the non-China subgroup (0.79 vs. 0.72), but a lower pooled specificity (0.79 vs. 0.88). Regarding sample types, the pooled sensitivity was 0.82 vs. 0.76, and the specificity 0.78 vs. 0.81, when comparing plasma to serum. Heterogeneity was observed within each subgroup, suggesting that country and sample types were not the major sources of heterogeneity in the meta-analysis. In the meta-regression analyses, differences in the pooled sensitivity and specificity were statistically significant for the following covariates: The number of cases and controls, the method used to perform RT-qPCR and sample type. Hence, the sources of inter-study heterogeneity remain unclear.
The Deeks' funnel plot was used to explore the potential publication bias. The P-value of the slope coefficient was 0.881, indicating no distinct asymmetry and a relatively low probability of publication bias in the meta-analysis.
Comparison of the 5 candidate miRNAs in patients with ESCC compared with healthy controls
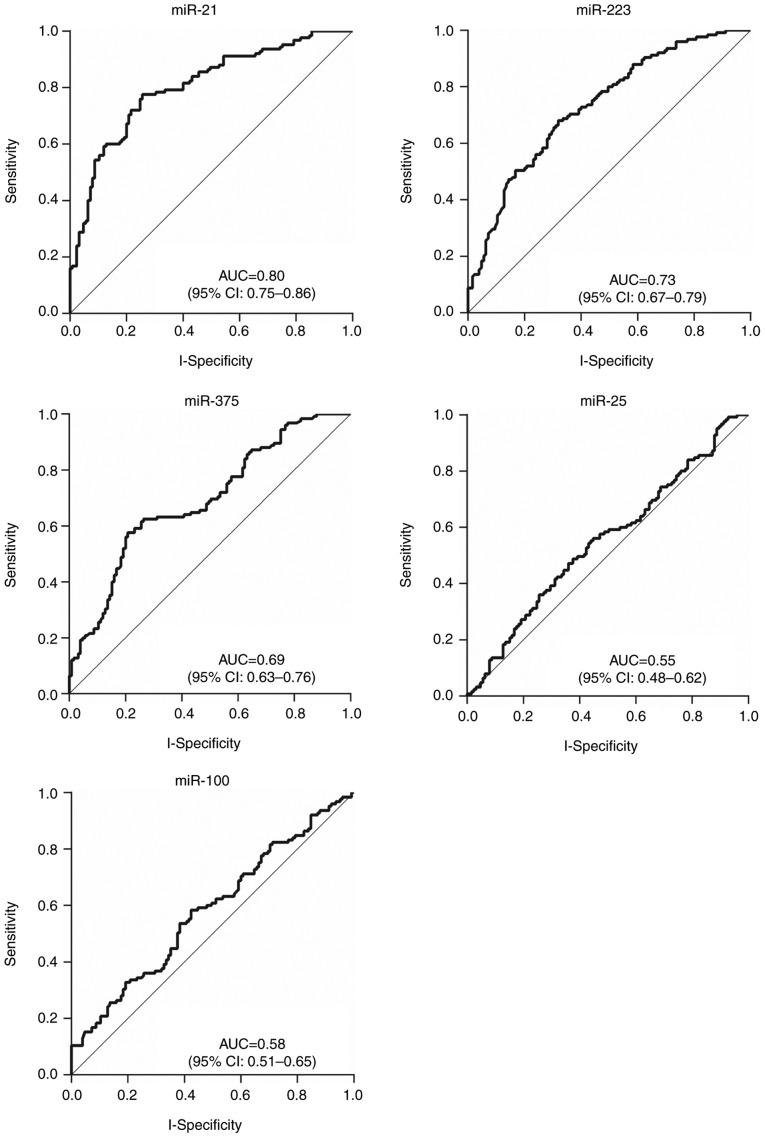
The plasma expression levels of the 5 candidate miRNAs, miR-21, miR-223, miR-100, miR-25, and miR-375, were determined using RT-qPCR. Analyses of ROC curves were conducted to evaluate their diagnostic values. Although the results revealed statistically significant differences in the expression of the 4 miRNAs (P=0.025 for miR-100, and P<0.001 for miR-21, miR-223, and miR-375), only those ≥2-fold upregulated or ≤0.5-fold downregulated between the blood of patients with ESCC and controls were considered to be meaningful. Consequently, miR-21 and miR-223 expression was determined to be upregulated whereas miR-375 was downregulated in patients with ESCC compared with healthy individuals (Table V). The corresponding AUCs were 0.80 (95% CI, 0.75–0.86) for miR-21, 0.73 (95% CI, 0.67–0.79) for miR-223 and 0.69 (95% CI, 0.63–0.76) for miR-375, respectively (Table V; Fig. 3).
Table V.
Diagnostic values of the 5 candidate miRNAs in differentiating patients with esophageal squamous cell carcinoma from controls.
| Biomarker | Fold change | P-value | Sensitivity | Specificity | AUC (95% CI) |
|---|---|---|---|---|---|
| miR-21 | 3.13 | 0.000 | 0.74 | 0.78 | 0.80 (0.75–0.86) |
| miR-223 | 3.88 | 0.000 | 0.68 | 0.68 | 0.73 (0.67–0.79) |
| miR-100 | 0.72 | 0.164 | 0.58 | 0.58 | 0.58 (0.51–0.65) |
| miR-25 | 1.44 | 0.025 | 0.54 | 0.57 | 0.55 (0.48–0.62) |
| miR-375 | 0.37 | 0.000 | 0.78 | 0.59 | 0.69 (0.63–0.76) |
miR/miRNA, microRNA; AUC, area under the curve; CI, confidence interval.
Figure 3.
Receiver operating characteristic curves for the discrimination of esophageal squamous cell carcinoma patients from healthy individuals by 5 plasma microRNAs. miR, microRNA; AUC, area under the curve; CI, confidence interval.
Associations of the expression levels of plasma miRNAs with ESCC clinicopathological factors
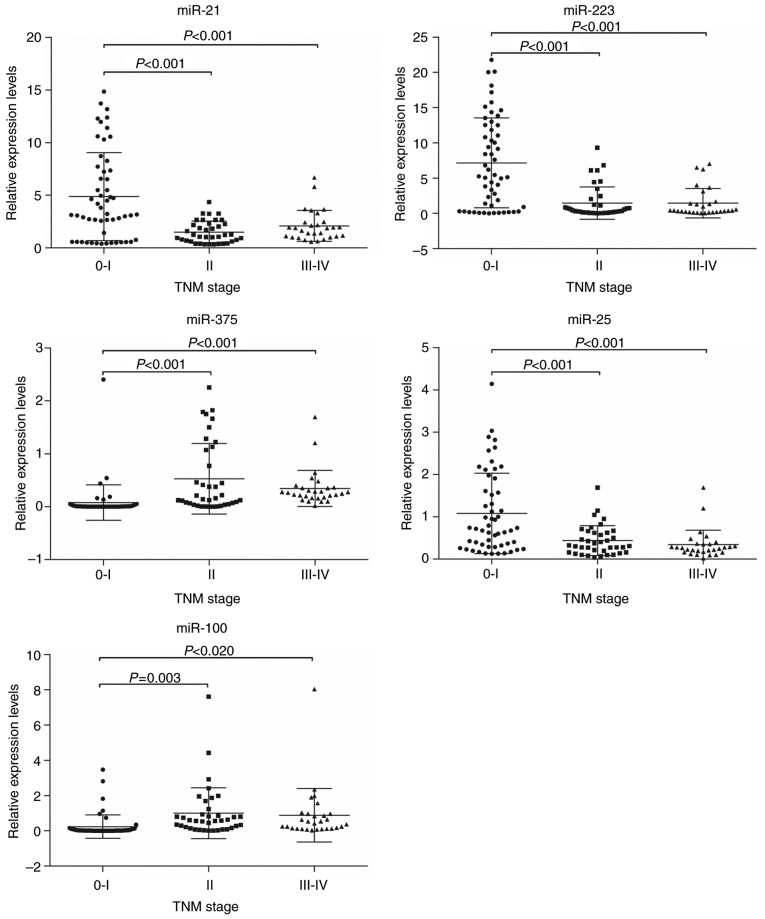
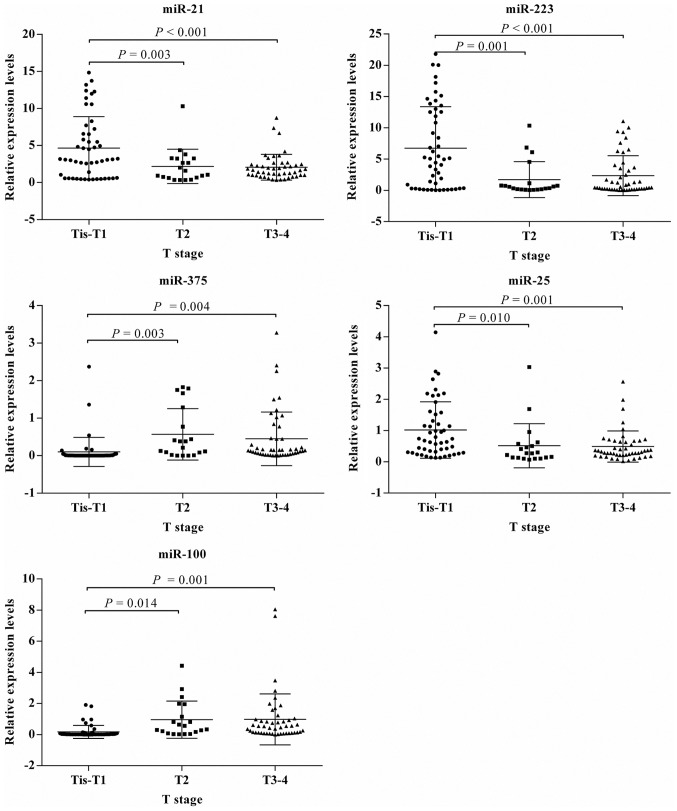
Based on the mean expression level, the patients were divided into a high-expression group and a low-expression group for each miRNA. Age and sex were not associated with the expression of any miRNA (P>0.05). The expression level of miR-25 was significantly associated with tumor location (P<0.001), and was higher for tumors of the lower-esophagus than those in the upper- and middle-esophagus. MiR-375 and miR-100 were associated with differentiation grade (P=0.019 and P=0.010, respectively), but there was no significant association between the expression of the other miRNAs and the differentiation grade. The expression levels of all 5 plasma miRNAs were also significantly associated with the TNM stage, particularly the T stage. However, the M stage was not associated with the expression of any miRNA (Table IV). The statistical association of miRNA expression level with TNM stage (Fig. 4) and T stage (Fig. 5) were consistent for all the miRNAs considered. The expression levels of miR-21, miR-223, and miR-25 were higher, whereas miR-100 and miR-375 were lower in patients with stage 0–I or Tis-T1 tumors than those in other stages.
Figure 4.
Association of plasma microRNA expression levels with the TNM stage of esophageal squamous cell carcinoma. The longer line demonstrates the mean value of the relative expression levels of miRNA, while the shorter lines demonstrate standard deviations. miR, microRNA; TNM, tumor-node-metastasis.
Figure 5.
Association of plasma microRNA expression levels with the T stage of esophageal squamous cell carcinoma. The longer line demonstrates the mean value of the relative expression levels of miRNA, while the shorter ones refer to the corresponding standard deviations. miR, microRNA.
Diagnostic value of the 5 candidate miRNAs for early ESCC
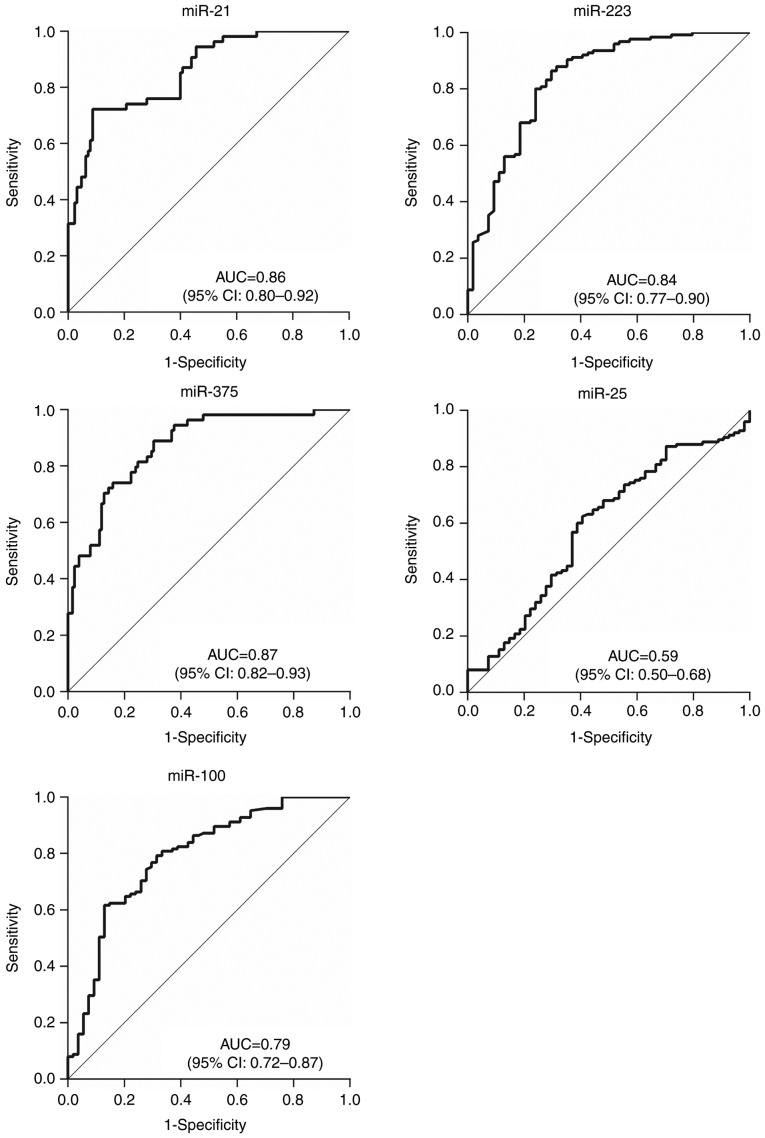
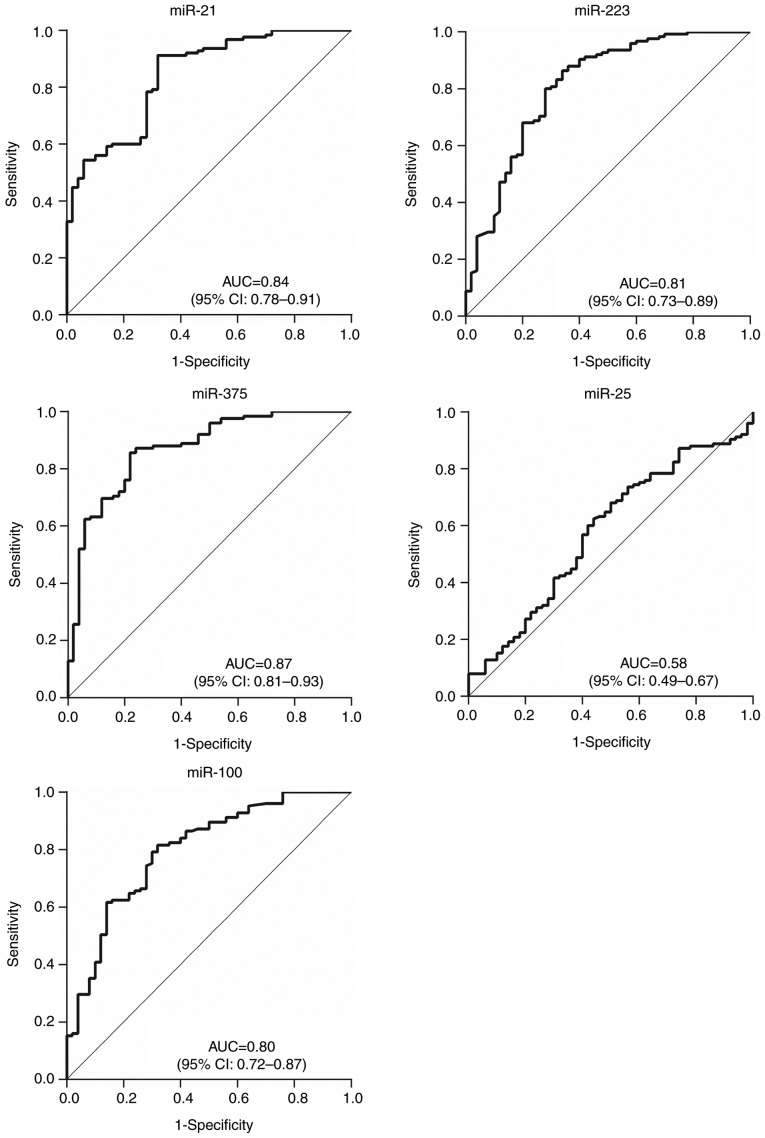
ROC curves were constructed and analyzed to evaluate the diagnostic values of the 5 candidate miRNAs for differentiating patients with ESCC in stages 0–I (Fig. 6) and Tis-T1 (Fig. 7) from healthy individuals. The results indicated that the 3 abnormally expressed miRNAs had relatively high AUCs of 0.86 (95% CI, 0.80–0.92) for miR-21, 0.83 (95% CI, 0.77–0.90) for miR-223 and 0.87 (95% CI, 0.82–0.93) for miR-375 when comparing patients in stage 0–I to healthy patients; the AUCs when comparing patients with Tis-T1 tumors to all healthy patients were 0.84 (95% CI, 0.78–0.91), 0.81 (95% CI, 0.73–0.89) and 0.87 (95% CI, 0.81–0.93) respectively. This indicates that these miRNAs could be effective in providing early diagnosis. The diagnostic value of plasma miR-100 was also improved, from AUC 0.58 (95% CI, 0.51–0.65) for all patients with ESCC, to 0.79 (95% CI, 0.72–0.87) for stage 0–I and 0.80 (95% CI, 0.72–0.87) for stage Tis-T1.
Figure 6.
Receiver operating characteristic curve analyses of 5 plasma microRNAs for differentiating early esophageal squamous cell carcinoma patients in stage 0–I from healthy individuals. miR, microRNA; AUC, area under curve; CI, confidence interval.
Figure 7.
Receiver operating characteristic curve analyses of 5 plasma microRNAs for differentiating early esophageal squamous cell carcinoma patients with stage T0-1 tumors from healthy individuals. miR, microRNA; AUC, area under curve; CI, confidence interval.
Discussion
It has been demonstrated that miRNAs are remarkably stable, reproducible and consistent in clinical studies (55). Furthermore, they can be easily detected by microarray or RT-qPCR for EC diagnosis, which is advantageous over conventional detection methods for EC, including endoscopic and pathologic examination. Numerous studies have explored the associations between miRNAs and various cancers. However, a large proportion of this research was performed in tissues or cell lines, and studies in the context of bodily fluids are rare. Compared with other bodily fluids, blood is generally more applicable as it is easily attained in a relatively noninvasive manner and can be stored for long periods. Circulating miRNAs are highly resistant to RNase A-digestion and other adverse environments, including repeated freeze-thaw cycles, prolonged storage at room temperature and extreme pH conditions (56). Thus, circulating miRNAs have provided a promising scope for the discovery of diagnostic biomarkers since 2008 (12).
Considering that a number of circulating miRNAs have been identified for the detection of EC, a meta-analysis was conducted in the present study to evaluate the diagnostic values of circulating miRNAs in plasma or serum in EC. The results revealed a relatively high accuracy, with an AUC of 0.86. In the subgroup and meta-regression analyses, it was demonstrated that the covariates of country, sample type, size of cases and controls, and the detection method used to perform RT-qPCR varied in the pooled sensitivity and specificity. However, heterogeneity remained evident in all subgroups based on these covariates, indicating that the major sources of heterogeneity were not identified through these analyses.
Although the expression levels of the circulating miRNAs were all quantified using RT-qPCR, the heterogeneity identified in the meta-analysis may be due to the varying RT-qPCR methods and quantification methods of the studies analyzed. The reagent used for RNA extraction from serum or plasma also varied, as did the standardized control (57). Furthermore, only miRNAs significantly overexpressed or reduced in expression were selected from the eligible studies in the present meta-analysis. For example, Li et al (22) selected 9 miRNAs to evaluate their diagnostic values, but only 4 differentially expressed miRNAs were identified in the study. Furthermore, all studies were designed as case-controls, which may also contribute to the heterogeneity. Ethnicity would be more likely to be the source of heterogeneity instead of country, as all study subjects were confined to Asia.
The present meta-analysis concentrated only on patients who had not undergone surgery, chemotherapy or radiotherapy in order to achieve a better reflection of the diagnostic efficiency of circulating miRNAs. Wang et al (58) concluded that the sample type was a source for heterogeneity, potentially due to the negligence of the value of I2 in subgroup and meta-regression analyses.
Since the majority of miRNAs considered in the studies included in the meta-analysis were reported only once, the top 5 most-studied miRNAs (miR-21, miR-25, miR-100, miR-223 and miR-375) were selected as candidate markers. According to the meta-analysis, all 5 miRNAs have been identified as overexpressed in EC, other than miR-375, for which there are opposing results regarding its expression (22,23). The present study used specifically selected patient samples and RT-qPCR to validate the previous results. Only the expression levels of plasma miR-21, miR-223 and miR-375 were significantly different in patients with ESCC compared with controls. Among them, miR-223 was the most significantly upregulated (fold change, 3.88) and miR-21 had the highest diagnostic value (AUC, 0.80). None of the miRNAs were associated with sex or age, which was in accordance with previous reports (21,58,59). All 5 miRNAs were associated with the T and N TNM stages, with the exception of miR-21, which was associated only with T stage. M stage was not associated with any of the 5 miRNAs. Only miR-25 was found to be associated with tumor location, with tumors from the lower esophagus exhibiting a higher expression of miR-25.
Considering that a patient's prognosis is improved by an early diagnosis, ROC curves were drawn to evaluate the diagnostic values of plasma miRNAs in differentiating patients with early ESCC (in stages 0–I and Tis-T1) from healthy controls. Potentially due to the grouping method, except for miR-25, the expression levels of all the miRNAs in patients with 0–I or Tis-T1 tumors were significantly and consistently altered compared to patients with medium (stage II) and advanced (stage III–IV), or T2 and T3-4 tumors. The AUC for miR-100 increased from 0.58 in all patients with ESCC to 0.79 in stage 0–I and 0.80 in stage Tis-T1, and the AUC for miR-375 increased from 0.69 in all patients with ESCC to 0.87 in stage 0–I and 0.87 in stage Tis-T1. Thus, it is concluded that plasma miR-21, miR-223, miR-100 and miR-375 have the potential to be used as biomarkers for the diagnosis of patients with ESCC, particularly in the early stages of disease.
MiR-21 has been studied in various types of cancer; it is hypothesized that it functions as an oncogene and is upregulated in various types of solid tumor. It is widely involved in cell growth, proliferation, invasion, intravasation and metastasis by targeting tumor suppressor genes, including phosphate and tensin homolog (60,61), programmed cell death 4 (62,63), tropomyosin 1 (64), Sprouty2 (65), acidic nuclear phosphoprotein 32 and SWI/SNF-related, matrix associated, actin dependent regulator of chromatin A4 (66). Previous studies have demonstrated that miR-21 was associated with chemoresistance (61,67) as well as prognosis (23,68) in ESCC.
MiR-375 is predominantly expressed in the pancreatic islets (24). Its expression has been reported as downregulated in multiple types of malignancy, including pancreatic adenocarcinomas (69), gastric cancer (24), head and neck squamous cell carcinoma (70) and liver cancer (71). Janus kinase 2, MAX interactor 1, and aryl hydrocarbon receptor have been identified as targets of miR-375 that are involved in the regulation of carcinogenesis (24,72). There is considerable evidence that miR-375 is a tumor suppressor gene (26). The present study corroborates this hypothesis, and not the findings of Li et al (22). One possible reason for these differing conclusions may be error in the original experiment or the subsequent analysis. The expression level of miR-375 may have been interfered with by other factors, for example, blood sample pollution. Alternatively, miR-375 may serve distinct roles (oncogene or tumor suppressor gene) in different types of cancer, as is the case for miR-223 and miR-25. Further research is required to explore the mechanism of miR-375 in ESCC.
Previous studies have indicated that miR-223 and miR-25 are overexpressed in many types of cancer, and may promote cell proliferation, migration and invasion. However, miR-223 expression has been reported as downregulated in primary small cell lung cancer (73), and miR-25 may inhibit the proliferation of colon cancer and anaplastic thyroid carcinoma cells, thus acting as a tumor suppressor gene (74,75).
The present study focused only on the diagnostic value of circulating miRNAs in EC. A range of miRNAs have been identified to exhibit altered expression in EC. These potential biomarkers may be superior in sensitivity to the established serologic biomarkers, including squamous cell carcinoma antigen (SCCA) and carcinoembryonic antigen (CEA) (10,11). However, issues remain regarding the applicability of circulating miRNAs as diagnostic biomarkers. No clear consensus exists for the normalization of miRNA detection in serum or plasma, nor does a consistent method for analyzing RT-qPCR data, which leads to the poor repeatability of results. This is a limitation when determining the value of miRNAs for diagnosis. Therefore, it remains unclear which single miRNA, or panel of miRNAs, could be used effectively for the early detection of ESCC. Thus, further efforts are required to set the relevant standards to avoid futile and repetitive studies in the future. The survival rate for ESCC remains poor even following surgery, radiotherapy and chemotherapy. Although it has not yet been satisfactorily achieved, alterations to miRNA expression have the potential to be developed into novel therapies in the future. Considerable efforts are required before miRNA-based methods can be clinically applied.
In conclusion, the present study verified that miR-21 and miR-223 were over-expressed, whereas miR-375 expression was reduced, in patients with ESCC compared with healthy individuals. The identified miRNAs may serve as non-invasive biomarkers for the diagnosis of ESCC, particularly early ESCC. MiR-100 is also a promising biomarker for the early diagnosis of ESCC. Further large-scale prospective studies are required to confirm the clinical diagnostic value of circulating miRNAs in ESCC.
Acknowledgements
The present study was supported by grants from the General Program of National Natural Science Foundation of China (grant no. 81372371), the Science and Technology Programs for Tackling Key Problems in Henan province (grant nos. 162102310037 and 201503200), the Zhongyuan Scholar Program (grant no. 162101510006) and the Major Project of Science and Technology in Henan province (grant no. 161100311400).
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 4.Law S, Wong J. The current management of esophageal cancer. Adv Surg. 2007;41:93–119. doi: 10.1016/j.yasu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JY, Looi KS, Tan EM. Identification of tumor-associated antigens as diagnostic and predictive biomarkers in cancer. Methods Mol Biol. 2009;520:1–10. doi: 10.1007/978-1-60327-811-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu BC, Wang LD, Pei H. Tumor-associated autoantibody measurement for screening esophageal cancer. J Clin Exp Med. 2007 [Google Scholar]
- 8.Dai L, Ren P, Liu M, Imai H, Tan EM, Zhang J. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin Immunol. 2014;152:127–139. doi: 10.1016/j.clim.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KS, LaBaer J. The sentinel within: Exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mroczko B, Kozłowski M, Groblewska M, Łukaszewicz M, Nikliński J, Jelski W, Laudański J, Chyczewski L, Szmitkowski M. The diagnostic value of the measurement of matrix metalloproteinase 9 (MMP-9), squamous cell cancer antigen (SCC) and carcinoembryonic antigen (CEA) in the sera of esophageal cancer patients. Clin Chim Acta. 2008;389:61–66. doi: 10.1016/j.cca.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Łukaszewicz-Zając M, Mroczko B, Kozłowski M, Nikliński J, Laudański J, Szmitkowski M. Higher importance of interleukin 6 than classic tumor markers (carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis Esophagus. 2012;25:242–249. doi: 10.1111/j.1442-2050.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57:84–91. doi: 10.1373/clinchem.2010.151845. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Cheng Q, Zhang BH, Zhang MZ. Circulating microRNAs as biomarkers in hepatocellular carcinoma screening: A validation set from China. Medicine (Baltimore) 2015;94:e603. doi: 10.1097/MD.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z, Zhao C, Wang Z. MicroRNAs as ideal biomarkers for the diagnosis of lung cancer. Tumour Biol. 2014;35:10395–10407. doi: 10.1007/s13277-014-2330-1. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Zhao W, Yu H, Wang Y, Liu Y, Xie C. A comprehensive meta-analysis of MicroRNAs for predicting colorectal cancer. Medicine (Baltimore) 2016;95:e2738. doi: 10.1097/MD.0000000000002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou C, Tan G, Feng S. Clinical significance of microRNA expressions in diagnosing uterine cancer and predicting lymph node metastasis. Tumour Biol. 2014;35:10789–10798. doi: 10.1007/s13277-014-2382-2. [DOI] [PubMed] [Google Scholar]
- 18.Qu S, Guan J, Liu Y. Identification of microRNAs as novel biomarkers for glioma detection: A meta-analysis based on 11 articles. J Neurol Sci. 2015;348:181–187. doi: 10.1016/j.jns.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143:35–47. doi: 10.1053/j.gastro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Tian T, Xia LL, Ding Y, Cormier RT, He Y. Circulating miRNAs as novel potential biomarkers for esophageal squamous cell carcinoma diagnosis: A meta-analysis update. Dis Esophagus. 2017;30:1–9. doi: 10.1093/dote/dox070. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Yu Q, Shi Z, Li P, Fu S. Circulating microRNAs in esophageal squamous cell carcinoma: Association with locoregional staging and survival. Int J Clin Exp Med. 2015;8:7241–7250. [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H, Kosuga T, Iitaka D, Konishi H, Shiozaki A, et al. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2011;105:104–111. doi: 10.1038/bjc.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J, Zhou T. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Li Y, Zhou J, Xu J, Peng C, Ye F, Shen Y, Lu W, Wan X, Xie X. miR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am J Pathol. 2011;179:2580–2588. doi: 10.1016/j.ajpath.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, Qin YR, Guan XY. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut. 2012;61:33–42. doi: 10.1136/gutjnl-2011-300178. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, Moriyama M. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339–2349. doi: 10.1158/0008-5472.CAN-09-2777. [DOI] [PubMed] [Google Scholar]
- 28.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors. 7th edition. Wiley-Blackwell; Oxford, UK: 2009. [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Dwamena B. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Stat Softw Components. 2007;14 [Google Scholar]
- 31.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang T, Wang Q, Zhao D, Cui Y, Cao B, Guo L, Lu SH. The oncogenetic role of microRNA-31 as a potential biomarker in oesophageal squamous cell carcinoma. Clin Sci (Lond) 2011;121:437–447. doi: 10.1042/CS20110207. [DOI] [PubMed] [Google Scholar]
- 35.Liu R, Liao J, Yang M, Shi Y, Peng Y, Wang Y, Pan E, Guo W, Pu Y, Yin L. Circulating miR-155 expression in plasma: A potential biomarker for early diagnosis of esophageal cancer in humans. J Toxicol Environ Health A. 2012;75:1154–1162. doi: 10.1080/15287394.2012.699856. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Li Y, Wang C, Guan X, Chen X, Zeng K, Zhang C, Zhang C. Sequencing and identification of mircORNAs from a genome-wide expression profile in serum of esophageal squamous cell carcinoma. J Clin Lab Sci. 2012 [Google Scholar]
- 37.Xie Z, Chen G, Huang J, Li Z. The diagnostic significance of plasma miR-10 for esophageal cancer. Guangdong Med J. 2013;34:2465–2468. [Google Scholar]
- 38.Hui B, Chen X, Hui L, Xi R, Zhang X. Serum miRNA expression in patients with esophageal squamous cell carcinoma. Oncol Lett. 2015;10:3008–3012. doi: 10.3892/ol.2015.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, Dai J, Hu Z, Zhou X, Chen L, et al. Expression profile of microRNAs in serum: A fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 40.Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol. 2012;138:1659–1666. doi: 10.1007/s00432-012-1244-9. [DOI] [PubMed] [Google Scholar]
- 41.Hirajima S, Komatsu S, Ichikawa D, Takeshita H, Konishi H, Shiozaki A, Morimura R, Tsujiura M, Nagata H, Kawaguchi T, et al. Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2013;108:1822–1829. doi: 10.1038/bjc.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma P, Saraya A, Gupta P, Sharma R. Decreased levels of circulating and tissue miR-107 in human esophageal cancer. Biomarkers. 2013;18:322–330. doi: 10.3109/1354750X.2013.781677. [DOI] [PubMed] [Google Scholar]
- 43.Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y, Ikeda N, Isozaki Y, Maruyama T, Akanuma N, et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer. 2013;108:644–652. doi: 10.1038/bjc.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang T, Zhao D, Wang Q, Yu X, Cui Y, Guo L, Lu SH. MicroRNA-1322 regulates ECRG2 allele specifically and acts as a potential biomarker in patients with esophageal squamous cell carcinoma. Mol Carcinog. 2013;52:581–590. doi: 10.1002/mc.21880. [DOI] [PubMed] [Google Scholar]
- 45.Komatsu S, Ichikawa D, Hirajima S, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Arita T, Konishi H, Shiozaki A, et al. Plasma microRNA profiles: Identification of miR-25 as a novel diagnostic and monitoring biomarker in oesophageal squamous cell carcinoma. Br J Cancer. 2014;111:1614–1624. doi: 10.1038/bjc.2014.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C, Wang C, Guan X, Liu Y, Li D, Zhou X, Zhang Y, Chen X, Wang J, Zen K, et al. Diagnostic and prognostic implications of a serum miRNA panel in oesophageal squamous cell carcinoma. PLoS One. 2014;9:e92292. doi: 10.1371/journal.pone.0092292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He FC, Meng WW, Qu YH, Zhou MX, He J, Lv P, Ming L. Expression of circulating microRNA-20a and let-7a in esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:4660–4665. doi: 10.3748/wjg.v21.i15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Z, Song Q, Yang S, Zeng R, Li X, Jiang C, Ding W, Zhang J, Zheng Y. Serum microRNA-218 is a potential biomarker for esophageal cancer. Cancer Biomark. 2015;15:381–389. doi: 10.3233/CBM-150480. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Yao Y, Meng F, Qian X, Jiang X, Li X, Gao Z, Gao L. Predictive value of serum miR-10b, miR-29c, and miR-205 as promising biomarkers in esophageal squamous cell carcinoma screening. Medicine (Baltimore) 2015;94:e1558. doi: 10.1097/MD.0000000000001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan S, Wang C, Chen X, Liu B, Tan B, Liu F, Wang D, Han L, Wang L, Huang X, et al. miR-613: A novel diagnostic and prognostic biomarker for patients with esophageal squamous cell carcinoma. Tumour Biol. 2016;37:4383–4391. doi: 10.1007/s13277-015-4271-8. [DOI] [PubMed] [Google Scholar]
- 51.Sun L, Dong S, Dong C, Sun K, Meng W, Lv P, Yin H, Ming L, He F. Predictive value of plasma miRNA-718 for esophageal squamous cell carcinoma. Cancer Biomark. 2016;16:265–273. doi: 10.3233/CBM-150564. [DOI] [PubMed] [Google Scholar]
- 52.Ye M, Ye P, Zhang W, Rao J, Xie Z. Diagnostic values of salivary versus and plasma microRNA-21 for early esophageal cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:885–889. (In Chinese) [PubMed] [Google Scholar]
- 53.Li BX, Shi ZL, Yu Q, Fu S. Dynamic monitoring of miR-21 in peripheral blood before and after radiotherapy in patients with esophageal carcinoma and its clinical implication. Tumor. 2015;35:550–555. [Google Scholar]
- 54.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2 Group: QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 55.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 57.Redshaw N, Wilkes T, Whale A, Cowen S, Huggett J, Foy CA. A comparison of miRNA isolation and RT-qPCR technologies and their effects on quantification accuracy and repeatability. Biotechniques. 2013;54:155–164. doi: 10.2144/000114002. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Wang Q, Zhang N, Ma H, Gu Y, Tang H, Xu Z, Gao Y. Identification of microRNAs as novel biomarkers for detecting esophageal squamous cell carcinoma in Asians: A meta-analysis. Tumor Biol. 2014;35:11595–11604. doi: 10.1007/s13277-014-2350-x. [DOI] [PubMed] [Google Scholar]
- 59.Wan J, Wu W, Che Y, Kang N, Zhang R. Insights into the potential use of microRNAs as a novel class of biomarkers in esophageal cancer. Dis Esophagus. 2016;29:412–420. doi: 10.1111/dote.12338. [DOI] [PubMed] [Google Scholar]
- 60.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma WJ, Lv GD, Tuersun A, Liu Q, Liu H, Zheng ST, Huang CG, Feng JG, Wang X, Lin RY, et al. Role of microRNA-21 and effect on PTEN in Kazakh's esophageal squamous cell carcinoma. Mol Biol Rep. 2011;38:3253–3260. doi: 10.1007/s11033-010-0480-9. [DOI] [PubMed] [Google Scholar]
- 62.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 63.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- 64.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 65.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell. 2008;19:3272–3282. doi: 10.1091/mbc.e08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schramedei K, Mörbt N, Pfeifer G, Läuter J, Rosolowski M, Tomm JM, von Bergen M, Horn F, Brocke-Heidrich K. MicroRNA-21 targets tumor suppressor genes ANP32A and SMARCA4. Oncogene. 2011;30:2975–2985. doi: 10.1038/onc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurashige J, Kamohara H, Watanabe M, Tanaka Y, Kinoshita K, Saito S, Hiyoshi Y, Iwatsuki M, Baba Y, Baba H. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2012;106:188–192. doi: 10.1002/jso.23064. [DOI] [PubMed] [Google Scholar]
- 68.Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S, Kawaguchi T, Arita T, Shiozaki A, Fujiwara H, et al. Prognostic impact of circulating miR-21 and miR-375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin Biol Ther. 2012;12(Suppl 1):S53–S59. doi: 10.1517/14712598.2012.681373. [DOI] [PubMed] [Google Scholar]
- 69.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 70.Avissar M, Christensen BC, Kelsey KT, Marsit CJ. MicroRNA expression ratio is predictive of head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15:2850–2855. doi: 10.1158/1078-0432.CCR-08-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 72.Mathé EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, Braun R, Reimers M, Kumamoto K, Hughes D, et al. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: Associations with survival. Clin Cancer Res. 2009;15:6192–6200. doi: 10.1158/1078-0432.CCR-09-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miko E, Czimmerer Z, Csánky E, Boros G, Buslig J, Dezso B, Scholtz B. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res. 2009;35:646–664. doi: 10.3109/01902140902822312. [DOI] [PubMed] [Google Scholar]
- 74.Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H, Wang W, Zhang L, Zhang X, Tang Q, et al. MicroRNA-25 functions as a potential tumor suppressor in colon cancer by targeting Smad7. Cancer Lett. 2013;335:168–174. doi: 10.1016/j.canlet.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 75.Esposito F, Tornincasa M, Pallante P, Federico A, Borbone E, Pierantoni GM, Fusco A. Down-regulation of the miR-25 and miR-30d contributes to the development of anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J Clin Endocrinol Metab. 2012;97:E710–E718. doi: 10.1210/jc.2011-3068. [DOI] [PubMed] [Google Scholar]