Abstract
Alveolar type II (AT2) epithelial cells are uniquely specialized to produce surfactant in the lung and act as progenitor cells in the process of repair after lung injury. AT2 cell injury has been implicated in several lung diseases, including idiopathic pulmonary fibrosis and bronchopulmonary dysplasia. The inability to maintain primary AT2 cells in culture has been a significant barrier in the investigation of pulmonary biology. We have addressed this knowledge gap by developing a three-dimensional (3D) organotypic coculture using primary human fetal AT2 cells and pulmonary fibroblasts. Grown on top of matrix-embedded fibroblasts, the primary human AT2 cells establish a monolayer and have direct contact with the underlying pulmonary fibroblasts. Unlike conventional two-dimensional (2D) culture, the structural and functional phenotype of the AT2 cells in our 3D organotypic culture was preserved over 7 days of culture, as evidenced by the presence of lamellar bodies and by production of surfactant proteins B and C. Importantly, the AT2 cells in 3D cocultures maintained the ability to replicate, with approximately 60% of AT2 cells staining positive for the proliferation marker Ki67, whereas no such proliferation is evident in 2D cultures of the same primary AT2 cells. This organotypic culture system enables interrogation of AT2 epithelial biology by providing a reductionist in vitro model in which to investigate the response of AT2 epithelial cells and AT2 cell–fibroblast interactions during lung injury and repair.
Keywords: alveolar type II cell, epithelial–mesenchymal interactions, organotypic coculture, lung model
The lung is comprised of more than 40 resident cell types, each with specific physiologic functions (1). Within the alveolus, the alveolar type II (AT2) epithelial cell is particularly important because it has the specialized function of surfactant production and also serves as a resident stem cell that proliferates in response to injury and subsequently differentiates into alveolar type I (AT1) epithelial cells (2–4). Conventional two-dimensional (2D) culture methods have forged major advances in the understanding of the biosynthetic and regenerative capacity of AT2 cells, but knowledge of AT2 cell biology, including the response to injury, has been hindered by the difficulty in culturing AT2 cells in vitro (5). With conventional 2D culture methods, the distinct pulmonary epithelial phenotype of AT2 cells is lost within 3–5 days of culture, as evidenced by the loss of their characteristic cuboidal shape and diminished surfactant production, including the loss of lamellar bodies (6). To date, the barrier to culturing primary AT2 cells has been circumvented by using stable cell lines that approximate AT2 function, but these immortalized cells do not fully recapitulate the biology of primary AT2 cells (5, 6).
Major advances in bioengineering have led to attempts to replicate the microenvironment of the lung, modeling lung development and respiratory disease using human primary or induced pluripotent stem cells (7, 8). These new technologies, which include organoid culture, alveolospheres, and lung-on-a-chip, have advanced the field of lung biology and have demonstrated the importance of culturing cells in three dimensions (3D) (4, 9, 10). Prior work with rat AT2 cells cocultured with fibroblasts and collagen (11, 12), as well as more recent alveolosphere experiments with cocultured human epithelial cells and mesenchyme (13–15), illustrates the important contribution that mesenchymal cells make to the maintenance of AT2 cell survival and function. Other groups have generated complex lung bud organoids with human induced pluripotent stem cells and have demonstrated the spatial organization of many cell types in the developing lung (16). Although these methods are improvements over conventional 2D culture, they have proven difficult to standardize, are not readily scaled for higher throughput, and allow for only limited access to the different cellular compartments, thereby restricting the experimental manipulation and interrogation of the culture system.
Building on prior work in other organ systems (17), we have developed a system of 3D organotypic coculture that can be used with primary human AT2 cells and pulmonary fibroblasts. In contrast to 2D cultures of primary AT2 cells, which lose many of their defining characteristics, we demonstrate that primary lung epithelial cells cultured in this 3D organotypic coculture retain their AT2 physiology and molecular characteristics. This novel method enables routine and standardizable long-term culture of AT2 cells and has the potential for use in modeling their contribution to the emergence and resolution of diseases of the human lung.
Methods
Primary Epithelial Cell Isolation
Second-trimester human lung tissues were isolated as previously published (18, 19), in accordance with protocols approved by the Vanderbilt University Institutional Review Board (details provided in the data supplement).
Isolation of Primary Lung Fibroblasts
Using the same second-trimester fetal lung tissues described above, fetal lung fibroblasts were isolated, and cells between passages 5 and 15 were used for experiments. (Details of cell culture are provided in the data supplement.)
Isolation of Primary Mouse Lung Epithelium
All mouse experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. Lungs were isolated from C57BL/6 mice aged 8–12 weeks and from both males and females, and AT2 cells were isolated using a protocol detailed in the data supplement.
Mouse Lung Epithelium Cell Line
Mouse Lung Epithelium (MLE-15) cells were obtained from Dr. Jeffrey Whitsett (Cincinnati Children’s Hospital, Cincinnati, OH) (20). (Additional culture details are provided in the data supplement.)
Assembly of 3D Organotypic Cocultures
3D organotypic cocultures were assembled on 24-mm Transwells with 3-μm pore polycarbonate membrane inserts (CLS3414; Corning) and placed into deep-well culture plates (catalog no. 355467; Corning) (Figures 1A and 1B). Cocultures were assembled in two layers, comprising a 3D matrix with human fetal lung fibroblasts and a layer of AT2 cells (primary human fetal AT2 cells, mouse AT2 cells, or MLE-15); for complete details of organotypic coculture assembly and processing for analysis, see the data supplement. Full details of control experiments with matrix-free coculture and cell-free AT2 matrix culture are also provided in the data supplement.
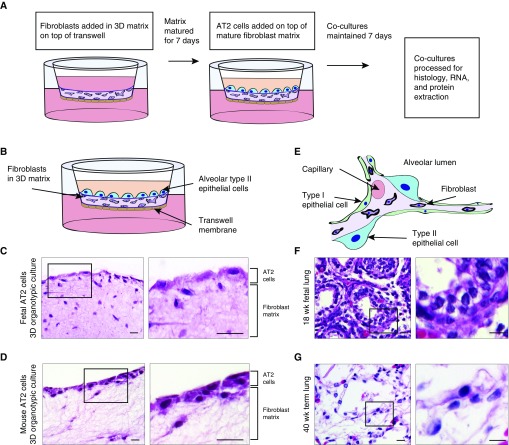
Figure 1.
Three-dimensional (3D) organotypic cocultures mimic the relationship between epithelial and mesenchymal cells. (A) Flowchart depicting the assembly of 3D organotypic cocultures. Fibroblasts were cultured in a 3D matrix, plated on a Transwell membrane, and matured for 7 days. Alveolar type II (AT2) cells were then added and cocultured for 7 days. (B) Schematic overview of 3D organotypic cocultures. (C) Hematoxylin and eosin (H&E) stains of 3D organotypic coculture with fetal lung fibroblasts in matrix and primary human fetal AT2 cells on top layer. 3D culture has been embedded in paraffin on edge and sectioned. (D) H&E stains of 3D organotypic coculture with human fetal lung fibroblasts in matrix and primary mouse AT2 cells in top layer. (E) Schematic of human lung alveolus demonstrating the relationship between AT2 cells and fibroblasts. (F) H&E stains of 18-week human fetal lung. (G) H&E stains of 40-week term infant lung. Scale bars: 25 μm.
2D Culture
AT2 cells were isolated from second-trimester fetal lung as described above and cultured on Matrigel-coated glass coverslips in serum-free Waymouth’s medium with dexamethasone, 8-bromo-cAMP, isobutyl-methylxanthine (DCI) added, as previously described (19). Cells were cultured for 7 days, with medium changed every 24 hours. For the comparison with 3D organotypic cocultures, AT2 cells from the same lung were used. For experiments with added growth factors, keratinocyte growth factor (KGF) at 10 ng/ml and hepatocyte growth factor (HGF) at 20 ng/ml were used, in accordance with previously published experiments using these growth factors and AT2 cells (21).
RNA Isolation and qRT-PCR
Total RNA was isolated and reverse transcribed by standard methods using the Qiagen RNEasy Kit and the Invitrogen SuperScript VILO cDNA Synthesis Kit (Life Technologies). Additional details are provided in the data supplement.
Immunofluorescence
Immunofluorescence was performed as described previously (10, 22, 23). Additional details about specific antibodies, cyclic staining, and microscopy are provided in the data supplement.
Western Blot Analysis
AT2 cells were isolated from fetal lungs that had been cultured as explants in DCI medium for 96 hours and were grown in 3D organotypic cocultures as described above. Human fetal lung explants grown in DCI medium for 6 days were used as positive controls. Full details of protein isolation and IB preparation can be found in the data supplement.
Transmission Electron Microscopy and Scanning Electron Microscopy
Specimens were processed for transmission electron microscopy (TEM) and scanning electron microscopy (SEM) and imaged in the Vanderbilt Cell Imaging Shared Resource Electron Microscopy facility. Details of the fixation and microscopy protocols used for TEM and SEM can be found in the data supplement.
Statistics
Experiments were performed a minimum of three times, with triplicate samples used in qPCR experiments. Quantitative microscopy was performed with a minimum of 25 high-power fields counted per sample. All values are reported as mean ± SD. Statistical analysis was performed using Prism version 7 software (GraphPad Software). A two-tailed Student’s t test was used for two-group comparisons, together with the Shapiro-Wilk test for normality and correction for multiple comparisons using the Holm-Sidak method. One-way ANOVA was used for comparisons between multiple groups. For ANOVA, the Tukey method was used to correct for multiple comparisons. Adjusted P values less than 0.05 were considered to be statistically significant.
Results
3D Organotypic Cocultures Support a Monolayer of Epithelial Cells with Direct Fibroblast Cell Contact
Assembly of the lung epithelium atop matrix-embedded fibroblasts suspended on a Transwell insert generates a 3D organotypic coculture that maintains AT2 cells in close proximity to lung fibroblasts (Figures 1B–1D). This models the interaction that exists between these two cell types in lung tissue (Figures 1E–1G). With hematoxylin and eosin staining, the primary fetal AT2 cells were observed to establish a continuous monolayer on the 3D fibroblast matrix (Figure 1C), as did primary AT2 cells isolated from adult mice (Figure 1D). The coculture of these two cell types in close proximity facilitates paracrine signaling as is found in vivo. Furthermore, the primary AT2 cells appear to have direct contact with the fibroblast cells in a manner that resembles the direct interaction between epithelial cells and fibroblasts in developing fetal lung (Figure 1F) and term infant lung (Figure 1G). To enable direct comparison between our new organotypic model and existing research using established lines, we implemented the model with MLE-15 cells, a mouse cell line that closely resembles human AT2 cells and exhibits surfactant protein production (20). When seeded onto the 3D fibroblast matrix, the MLE-15 cells established a monolayer (see Figure E1A in the data supplement) that emulated the morphology of the human fetal AT2 cells.
Type II Alveolar Epithelial Cells Grown in This 3D Organotypic Coculture System Exhibit Ultrastructural Features Characteristic of AT2 Cells
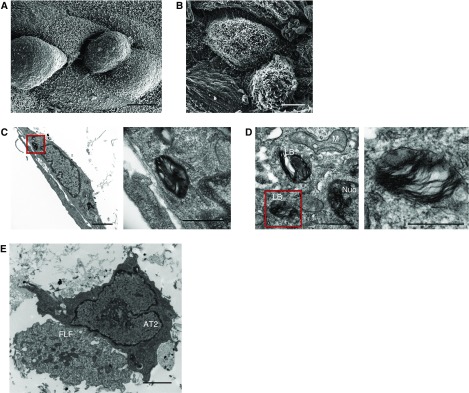
We next examined whether the structural features that define AT2 cells (2) were preserved in our 3D organotypic cultures. Using SEM, we confirmed that AT2 cells in this system exhibited the characteristic cuboidal shape with apical microvilli (Figures 2A and 2B). This is in contrast to 2D culture, where the AT2 cells flatten after 24–48 hours (3). TEM further revealed the presence of lamellar bodies in the AT2 cells (Figures 2C and 2D), a cardinal feature of fully differentiated AT2 cells (5). In addition, TEM demonstrated the direct interaction between AT2 cells and fibroblasts (Figure 2E), a novel feature of our system compared with the separation by an artificial membrane that occurs in traditional Transwell cultures.
Figure 2.
Primary AT2 cells grown in 3D organotypic coculture have preserved ultrastructural features. (A) Scanning electron microscopy of the surface of 3D organotypic coculture demonstrating the cuboidal shape of the primary AT2 cells. Scale bar: 5 μm. (B) Scanning electron microscopy of the 3D organotypic coculture showing the presence of microvilli of the AT2 cells. Scale bar: 5 μm. (C and D) Transmission electron microscopy showing the presence of lamellar bodies in AT2 cells. Panels on the right are enlargements of areas indicated by red boxes. Scale bars in C: 2 μm, with inset scale bar: 660 nm: scale bars in D: 500 nm, with inset scale bar: 1.4 μm. (E) Transmission electron microscopy shows direct contact between AT2 cells and fetal lung fibroblasts (FLF). Scale bar: 2 μm. LB = lamellar body; M = mitochondria; Nuc = nucleus.
Epithelial Cells and Fibroblasts Exist in the Coculture System as Distinct, Geographically Defined Populations, Retaining Expression of Cell Type–Specific Genes
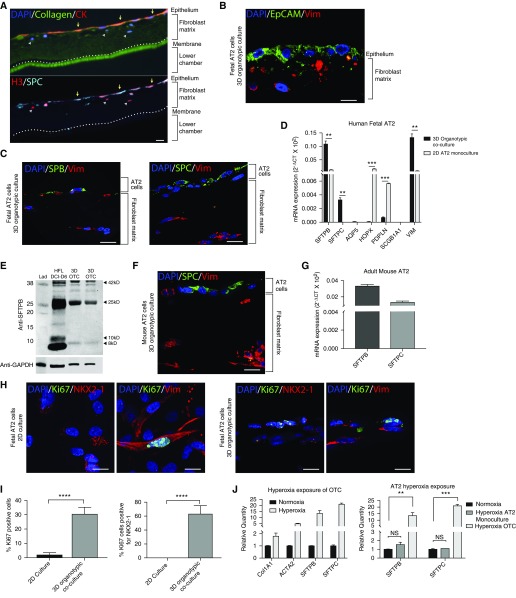
Immunofluorescence demonstrated the presence of distinct populations of epithelial cells (that stained positive for the epithelial markers pan-cytokeratin and Ep-CAM [epithelial cell adhesion molecule]) and fibroblasts (expressing the mesenchymal marker vimentin) that spatially exist in separate regions of the organotypic coculture (Figures 3A and 3B). In other culture models using fibroblasts as a feeder layer for epithelial cells, induction of cell-cycle arrest is often required to prevent fibroblasts from taking over the culture (24). In this organotypic system, however, the 3D matrix allows for physiologic amounts of proliferation in the fibroblast population and direct contact with epithelial cells, but it prevents their overgrowth in the coculture. Cells expressing both pro–surfactant protein C (SP-C) and pan-cytokeratin, a generic marker of epithelial cells, resided only in the top epithelial layer (Figures 3A and 3C), and this region was negative for the mesenchymal marker vimentin (Figures 3A and 3C). The top layer of cells was also positive for surfactant protein B (SP-B) in addition to pro-SP-C, reflecting expression of both surfactant genes SFTPB and SFTPC by qPCR (Figure 3D). Expression of vimentin, a fibroblast marker, was seen only in the lower layer of the organotypic cultures (Figure 3D). As expected, we also found evidence of expression of VIM (vimentin) by qPCR (Figure 3D). qRT-PCR of 3D cultures showed nearly no expression of AT1 markers AQP5 (aquaporin 5), HOPX, and PDPN (podoplanin), with cycle threshold values greater than 30 (Figure 3D). Because club cells have also been shown to express SP-B, we tested for expression of the club cell–specific marker SCGB1A1 and found very little mRNA (cycle threshold, >35) (Figure 3D). When we examined human fetal AT2 cells cultured in 2D conditions for 7 days, we found significant differences in the pattern of expression of AT2 and AT1 cell markers. After 7 days in culture, expression of SFTPB was approximately 10-fold greater in the 3D organotypic cocultures than in the 2D culture, with significantly increased SFTPC expression as well (Figure 3D). Interestingly, the 2D AT2 monocultures exhibited significantly increased expression of AT1 markers PDPN and HOPX compared with 3D organotypic cocultures. Increased expression of the fibroblast marker vimentin in the 3D organotypic cultures was expected, because the organotypic culture contained both AT2 cells and fibroblasts, whereas the 2D cultures contained AT2 depleted of fibroblasts.
Figure 3.
AT2 cells in organotypic culture express surfactant and retain the ability to replicate. (A) Cyclic immunofluorescence of a large section of organotypic coculture (OTC) with staining for epithelial marker pan-cytokeratin (red; CK), type I collagen (green), nuclei (DAPI), pro-surfactant protein C (SPC) (aqua), and nuclear marker histone H3 (red). Scale bar: 100 μm. (B) Immunofluorescence of three-dimensional (3D) OTC with human fetal AT2 cells for epithelial cell adhesion molecule (EpCAM; green) and vimentin (Vim; red). Scale bar: 25 μm. (C) Immunofluorescence of 3D OTC with primary human fetal AT2 cells for surfactant protein B (SPB), pro-SPC (green), and vimentin (red). Scale bars: 25 μm. (D) qRT-PCR expression of SFTPC, SFTPB, AQP5, HOPX, PDPN, SCGB1A1, and VIM in 3D cocultures with primary fetal AT2 cells. (E) Western blot with an antibody that binds immature and mature forms of SPB, with the mature form measuring 8 kD. (F) Immunofluorescence of 3D OTC with primary mouse AT2 cells and human fetal lung fibroblasts for SPC (green) and vimentin (red). Scale bar: 25 μm. (G) qRT-PCR expression of Sftpb and Sftpc in 3D cocultures with primary mouse AT2 cells. (H) Immunofluorescence of human fetal AT2 cells cultured in 2D and 3D for proliferation marker Ki-67 (green), AT2 markers NKX2-1 (red) and Ki-67 (green), and fibroblast marker vimentin (red). Scale bars: 25 μm. (I) Quantification of 1,000 cells per sample showed a significant increase in the percentage of Ki-67–positive cells in 3D culture compared with the same cells cultured in 2D Matrigel (P < 0.0001). There was a significant increase in the percentage of NKX2-1 cells that were also positive for Ki-67 in 3D culture compared with 2D culture (P < 0.0001). (J) Exposure of 3D OTC to hyperoxia replicated many of the transcriptomic features described in bronchopulmonary dysplasia (OTC), with significant increases in COL1A1 and ACTA2 expression. When comparing AT2 cells grown in 2D monoculture or 3D OTC, we observed a greater response to hyperoxia, as demonstrated by the increase in expression of SPB and SPC in OTC. **P < 0.01, ***P < 0.001, ****P < 0.0001. Col1A1 = collagen 1; DCI = dexamethasone, 8-bromo-cAMP, isobutylmethylxanthine; HFL = human fetal lung explants; Lad = ladder; NS = not significant (P > 0.05); SFTPB = surfactant protein B.
Expression and full proteolytic processing of proSP-B from a 42 kD proprotein to an 8 kD mature protein is a cardinal feature of AT2 cells that is not demonstrated by club cells (25). Western IB of 3D cocultures demonstrated the presence of mature and immature forms of SP-B, as did explants from second-trimester human fetal lung grown for 6 days in DCI (Figure 3E). 3D cocultures using primary mouse AT2 cells and human fetal lung fibroblasts demonstrated results similar to those of human fetal AT2 cells in coculture. Specifically, primary mouse cells demonstrated spatial segregation of SP-C–positive cells in the top layer of cells and vimentin-positive fibroblasts in the bottom layer of cells within the matrix (Figure 3F), as well as maintenance of expression of Sftpb and Sftpc, by qRT-PCR after 7 days in coculture (Figure 3G). Similar results were found in the cocultures in which we combined MLE-15 cells and human fibroblasts (Figures E1B and E1C).
AT2 Cells in 3D Coculture Have Higher Rates of Proliferation than AT2 Cells Grown with Traditional 2D Culture Methods
As mentioned previously, 2D cultures of AT2 cells often require serum-free conditions to minimize fibroblast overgrowth, which also results in suppression of AT2 proliferation. Despite using serum-free culture media for both 3D cocultures and 2D cultures, we observed that 30.4% (± 2.4%) of the total cells in the 3D organotypic coculture culture immunostained positive for Ki-67, a marker of cellular proliferation, whereas only 1.8% (± 0.8%) of cells were Ki-67–positive under 2D conditions (P < 0.0001) (Figures 3H and 3I). Costaining with NKX2-1 (NK2 homeobox 1), a marker specific to AT2 cells, revealed that 62.9% (± 6.0%) of Ki-67–positive cells were positive for NKX2-1 in the 3D coculture. This was in striking contrast to AT2 cells grown in 2D culture for 7 days in DCI medium, where none of the Ki-67 cells were positive for NKX2-1 (Figures 3H and 3I). Instead, all of the Ki-67–positive cells in the 2D cultures were also vimentin positive, whereas less than 30% of the Ki-67–positive cells in 3D coculture were vimentin positive (Figure 3H). The 1.8% of Ki67-positive vimentin-positive cells in the AT2 2D culture likely represent contaminating fibroblasts. Thus, 3D organotypic cocultures preserve the capacity of AT2 cells to proliferate, a critical characteristic of AT2 cells in vivo (26).
To understand the importance of the complex structure of the 3D coculture to the preservation of at AT2 phenotype, we assessed proliferation and apoptosis in AT2 cultures missing key features of the 3D cocultures and in 2D cultures supplemented with growth factors. Human fetal AT2 cells cultured on the complex Matrigel-collagen matrix without any fibroblasts for 7 days demonstrated few surviving AT2 cells, and very little RNA remained. TUNEL staining showed a very high rate of AT2 apoptosis in the absence of matrix-embedded fibroblasts (Figures E2A–E2E). In contrast, less than 5% of TUNEL-positive staining was evident in organotypic cultures constructed using human fetal lung AT2, mouse primary AT2, or MLE-15 cells (P < 0.0001). AT2 cells cultured on the upper surface of Transwell membranes with fibroblasts growing on the bottom surface of the well exhibited increased rates of proliferation, as indicated by Ki-67 immunostaining, but all of the Ki-67–positive cells were vimentin-positive fibroblasts, and none were NKX2-1–positive AT2 cells (Figure E3A). Epithelial cell proliferation in 2D culture did not achieve the amounts seen in 3D organotypic cultures even when KGF, HGF, or both were added to 2D cultures for 7 days, demonstrating no cells immunopositive for both Ki-67 and the AT2 marker NKX2-1 (Figures E4A, E4C, and E4E). Under matrix-free coculture or added growth factor conditions, we observed no detectable SFPTC expression, decreased SFTPB expression, or increased expression of AT1 markers relative to AT2 cells grown in 3D organotypic coculture by qPCR (Figures E3C, E4B, E4D, and E4F).
Organotypic Cocultures Can Be Used to Model Injury and to Examine Cell–Cell Interactions
Hyperoxia has been used in in vivo and in vitro systems to model lung injury, particularly injury occurring after preterm birth (27–29). We exposed our 3D organotypic cocultures to 48 hours of 70% oxygen and found that many of the previously reported transcriptomic features were replicated, including increased expression of COL1A1 (collagen type I, alpha 1 chain) and α-smooth muscle actin (ACTA2) relative to the organotypic cocultures grown in normoxia (Figure 3J). Prior work demonstrated that in AT2 cells, surfactant production is increased in response to hyperoxia (30). We found a greater increase in the expression of SFTPB and SFTPC in the hyperoxia-exposed 3D organotypic cultures than in the hyperoxia-exposed AT2 cells growing in traditional 2D conditions (Figure 3J).
Discussion
There is significant evidence that epithelial–mesenchymal interactions are key drivers of normal lung development (31–33) and of the pathology of many forms of lung injury and fibrotic lung disease (34–36). The ability to understand the complex biology and behavior of AT2 cells has been limited by the difficulty of culturing AT2 cells and maintaining the relevant features of their phenotype (5). The development of a 3D organotypic coculture system that preserves direct contact between AT2 cells and mesenchymal cells is therefore a critical step toward developing in vitro models of these diseases. The presented model allows for the interrogation of essential drivers of epithelial–mesenchymal cell interactions by allowing access to each cell type in the coculture for up to 7 days and enabling the manipulation of the growth medium of each compartment. In addition, this system allows for epithelial–mesenchymal interactions to be investigated over at least a 7-day time course, a significant improvement from traditional 2D methods, in which primary AT2 cells begin to lose their characteristic structure and surfactant production within 3–5 days (24, 37).
The lung epithelial cells maintained in the 3D organotypic culture retain many of the specialized functions of AT2 cells. These include lamellar body production and full processing of surfactant proteins, which are considered metrics of fully differentiated AT2 cells in vivo (38). Considering the role of AT2 cells in lung repair, proliferation is essential for AT2 cells to replenish the AT2 cell population and provide progenitor cells that can differentiate into AT1 cells (26). Although significant evidence of differentiation from type II to type I cells occurs in traditional culture systems after 3–5 days, we did not observe such type I cell differentiation in our 3D system. This is not entirely unexpected, because the secreted factors from the fibroblasts that maintain the AT2 phenotype most likely prevent this AT2-to-AT1 differentiation. We found that the maintenance of SP-B and SP-C expression and cellular proliferation were critically dependent on the direct interaction with fibroblasts growing in the 3D matrix. AT2 cells grown in 2D culture (with and without KGF and HGF), cell-free matrix, and matrix-free Transwell cocultures all had significantly less surfactant protein expression and AT2 proliferation than cells growing in 3D organotypic coculture. Although the AT2 cells cultured in matrix-free Transwells and 2D culture with growth factors showed some evidence of proliferation, all of the dividing cells were negative for the AT2 marker NKX2-1. We have not elucidated the precise mechanism by which the organotypic coculture system maintains the AT2 phenotype and promotes AT2 cell proliferation, but we speculate that the fibroblasts growing in the 3D matrix secrete growth factors and cytokines that support the physiology of AT2 cells in a manner that reproduces the in vivo condition. Indeed, one advantage of this system is that it allows for the maintenance of primary AT2 cells without the addition of exogenous growth factors, which might have unintended effects on the lung biology and pathology being modeled. On the basis of work of other groups that have cultured AT2 cells (39), it is possible that transcriptomic changes and epigenetic shifts occur during the cell isolation and culture process, and we did not investigate these changes in our system in this study. Future work to examine the features of each cell type in our organotypic culture system and how these features evolve over time will be important to gain an understanding of the features and limitations of this system and how this system might be used to study lung development and lung diseases that result from AT2 cell injury.
As with all reductionist models, this system has several limitations. It does not measure or replicate all of the functions of AT2 cells; specifically, it does not study fluid transport, vectorial ion transport, or the properties of tight junctions, which have previously been modeled in Transwell cultures (40–42). We have demonstrated lamellar body production by electron microscopy, expression of SFTPB and SFTPC by qPCR, and processing of SP-B by Western IB, but we have not isolated mature surfactant proteins or demonstrated surfactant arranged in a tubular myelin structure from the secreted media, as other models using alveolospheres in Matrigel have done (43). In addition, this system includes only two cell types and does not include endothelial cells or circulating immune cells, both of which have also been implicated as key drivers of lung disease. Future work to expand this model and incorporate these cell types may produce a more complex model that has even greater fidelity to the human lung. Indeed, recent studies of complex alveolospheres and lung buds incorporating multiple cell types represent major advances in in vitro modeling of lung development and disease (4, 16). Benefits of our more simplified system are that the media of each cell type can be accessed and manipulated separately and that the ratio of AT2 cells to fibroblasts can be carefully controlled, in contrast to the more stochastic assembly of alveolosphere-based cocultures.
Currently, the longevity of our coculture is limited by contraction of the matrix layer, which prevents culture of the system beyond 17–21 total days (10–14 d after adding AT2 cells). As the matrix pulls away from the sides of the Transwell insert, separation of the two media compartments is lost, and the surface of the epithelial side of the matrix deforms. Additional studies that change the stiffness and composition of the matrix may provide a solution to this barrier to long-term culture.
Previous studies have demonstrated the utility of 3D organotypic coculture between fibroblasts and epithelial cells in other organ systems, as well as in transformed cancer cell lines from the lung (17, 44). Our experimental method differs from these methods in the composition of the mesenchymal matrix and in the use of primary lung fibroblasts to create a tissue culture matrix that is hospitable to fragile primary AT2 cells, creating an opportunity to study their intrinsic vulnerability to injury and characterize their basic biologic features. Understanding the specialized AT2 cell and its critical contribution to lung development, injury, and repair is of paramount importance in understanding the pathobiology of multiple human lung diseases and developing new therapeutic strategies. This system of 3D organotypic coculture not only enables the study of the epithelial cells themselves but also allows for the investigation of interaction between the epithelium and the underlying stroma, a critical communication that is central to repair after lung injury. We have demonstrated that at least a portion of the transcriptomic response of AT2 cells and fibroblasts to hyperoxia can be replicated in this model, and in fact the AT2 response to hyperoxia appears to be related to the epithelial–mesenchymal interactions afforded by the 3D model. Future development of this model will allow modeling of disease using human primary cells as well as mouse primary cells from genetically modified animals. By replicating the epithelial–mesenchymal proximity found natively in the alveolus, we have created a system that allows for the organotypic cultivation and manipulation of AT2 cells and fibroblasts, thus creating a path for broadening the understanding of the biology of the distal lung in development and disease. Importantly, the ease of use and scalability of this model system will enable high-throughput discovery and preclinical screening of therapeutic agents that may lead to novel interventional strategies.
Acknowledgments
Acknowledgment
Experiments were performed in part with the use of the Vanderbilt University Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, and DK5963). The authors are grateful to Austin McKissack for technical support and Brian Hackett for thoughtful discussions.
Footnotes
Supported by National Institutes of Health (NIH) grants K12 HD087023 (J.M.S.S.), R01CA218526 (A.Z.), GM108807 (S.H.G.), U01-HL101794 (J.T.B.), HL119503 (L.R.Y.), K08HL130595 (J.A.K.), P01HL92870 (T.S.B.), R01HL085317 (T.S.B.), and F31-DE025477-01A1 (H.L.); the Department of Veterans Affairs (T.S.B.); the Francis Family Foundation (J.A.K.); the Pulmonary Fibrosis Foundation (J.A.K.); and the Julia Carrell Stadler Chair in Pediatrics (S.H.G.).
Author Contributions: Conception and design: J.M.S.S., C.S.J., H.L., J.W., E.J.P., J.T.B., L.R.Y., J.A.K., S.K., L. Gleaves, A.E., L. Goetzl, T.S.B., S.H.G., and A.Z.; performed experiments: J.M.S.S., C.S.J., J.W., C.L.C., S.K., P.W., A.E., and A.Z.; analysis and interpretation: J.M.S.S., E.J.P., J.T.B., L.R.Y., J.A.K., T.S.B., S.H.G., and A.Z.; drafting of the manuscript: J.M.S.S. and A.Z.; and editing of the manuscript: J.M.S.S., C.S.J., J.W., E.J.P., J.T.B., L.R.Y., J.A.K., S.K., T.S.B., S.H.G., and A.Z.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0442MA on April 6, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc. 2008;5:763–766. doi: 10.1513/pats.200803-025HR. [DOI] [PubMed] [Google Scholar]
- 2.Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO. Morphometric characteristics of cells in the alveolar region of mammalian lungs. Am Rev Respir Dis. 1983;128:S42–S46. doi: 10.1164/arrd.1983.128.2P2.S42. [DOI] [PubMed] [Google Scholar]
- 3.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beers MF, Moodley Y. When is an alveolar type 2 cell an alveolar type 2 cell? A conundrum for lung stem cell biology and regenerative medicine. Am J Respir Cell Mol Biol. 2017;57:18–27. doi: 10.1165/rcmb.2016-0426PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 7.Aurora M, Spence JR. hPSC-derived lung and intestinal organoids as models of human fetal tissue. Dev Biol. 2016;420:230–238. doi: 10.1016/j.ydbio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, et al. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife. 2016;5:e19732. doi: 10.7554/eLife.19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson DC, Alva-Ornelas JA, Sucre JM, Vijayaraj P, Durra A, Richardson W, et al. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med. 2017;6:622–633. doi: 10.5966/sctm.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shannon JM, Jennings SD, Nielsen LD. Modulation of alveolar type II cell differentiated function in vitro. Am J Physiol. 1992;262:L427–L436. doi: 10.1152/ajplung.1992.262.4.L427. [DOI] [PubMed] [Google Scholar]
- 12.Becker PM, Tran TS, Delannoy MJ, He C, Shannon JM, McGrath-Morrow S. Semaphorin 3A contributes to distal pulmonary epithelial cell differentiation and lung morphogenesis. PLoS One. 2011;6:e27449. doi: 10.1371/journal.pone.0027449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44:794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170:1134–1148.e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YW, Huang SX, de Carvalho ALRT, Ho SH, Islam MN, Volpi S, et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol. 2017;19:542–549. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomans HA, Arnold SA, Quast LL, Andl CD. Esophageal squamous cell carcinoma invasion is inhibited by activin A in ACVRIB-positive cells. BMC Cancer. 2016;16:873. doi: 10.1186/s12885-016-2920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales LW, Angampalli S, Guttentag SH, Beers MF, Feinstein SI, Matlapudi A, et al. Maintenance of differentiated function of the surfactant system in human fetal lung type II epithelial cells cultured on plastic. Pediatr Pathol Mol Med. 2001;20:387–412. doi: 10.1080/15513810109168622. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283:L940–L951. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J Biol Chem. 1995;270:6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- 21.Mason RJ, Lewis MC, Edeen KE, McCormick-Shannon K, Nielsen LD, Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;282:L249–L258. doi: 10.1152/ajplung.00027.2001. [DOI] [PubMed] [Google Scholar]
- 22.Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell. 2014;15:199–214. doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sucre JMS, Deutsch GH, Jetter CS, Ambalavanan N, Benjamin JT, Gleaves LA, et al. A shared pattern of β-catenin activation in bronchopulmonary dysplasia and idiopathic pulmonary fibrosis. Am J Pathol. 2018;188:853–862. doi: 10.1016/j.ajpath.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin M, Bhandari R, Hamilton G, Chan YC, Powell JT. Alveolar type II cell-fibroblast interactions, synthesis and secretion of surfactant and type I collagen. J Cell Sci. 1993;105:423–432. doi: 10.1242/jcs.105.2.423. [DOI] [PubMed] [Google Scholar]
- 25.Guttentag SH, Beers MF, Bieler BM, Ballard PL. Surfactant protein B processing in human fetal lung. Am J Physiol. 1998;275:L559–L566. doi: 10.1152/ajplung.1998.275.3.L559. [DOI] [PubMed] [Google Scholar]
- 26.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sucre JM, Vijayaraj P, Aros CJ, Wilkinson D, Paul M, Dunn B, et al. Posttranslational modification of β-catenin is associated with pathogenic fibroblastic changes in bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2017;312:L186–L195. doi: 10.1152/ajplung.00477.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sucre JM, Wilkinson D, Vijayaraj P, Paul M, Dunn B, Alva-Ornelas JA, et al. A three-dimensional human model of the fibroblast activation that accompanies bronchopulmonary dysplasia identifies Notch-mediated pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2016;310:L889–L898. doi: 10.1152/ajplung.00446.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2015;2:49. doi: 10.3389/fmed.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wikenheiser KA, Wert SE, Wispé JR, Stahlman M, D’Amore-Bruno M, Singh G, et al. Distinct effects of oxygen on surfactant protein B expression in bronchiolar and alveolar epithelium. Am J Physiol. 1992;262:L32–L39. doi: 10.1152/ajplung.1992.262.1.L32. [DOI] [PubMed] [Google Scholar]
- 31.Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ, et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev. 2015;32:98–105. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis KJR, Hall JK, Kiyotake EA, Christensen T, Balasubramaniam V, Anseth KS. Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system. Biomaterials. 2018;155:124–134. doi: 10.1016/j.biomaterials.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy R, Navarro S, et al. Targeted type 2 alveolar cell depletion: a dynamic functional model for lung injury repair. Am J Respir Cell Mol Biol. 2016;54:319–330. doi: 10.1165/rcmb.2014-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster CD, Varghese LS, Skalina RB, Gonzales LW, Guttentag SH. In vitro transdifferentiation of human fetal type II cells toward a type I-like cell. Pediatr Res. 2007;61:404–409. doi: 10.1203/pdr.0b013e3180332c6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell. 2017;21:472–488.e10. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, et al. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 40.Bove PF, Dang H, Cheluvaraju C, Jones LC, Liu X, O’Neal WK, et al. Breaking the in vitro alveolar type II cell proliferation barrier while retaining ion transport properties. Am J Respir Cell Mol Biol. 2014;50:767–776. doi: 10.1165/rcmb.2013-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang X, Song Y, Zemans R, Hirsch J, Matthay MA. Fluid transport across cultured rat alveolar epithelial cells: a novel in vitro system. Am J Physiol Lung Cell Mol Physiol. 2004;287:L104–L110. doi: 10.1152/ajplung.00176.2003. [DOI] [PubMed] [Google Scholar]
- 42.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, et al. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L242–L249. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 43.Yu W, Fang X, Ewald A, Wong K, Hunt CA, Werb Z, et al. Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol Biol Cell. 2007;18:1693–1700. doi: 10.1091/mbc.E06-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correia LL, Johnson JA, McErlean P, Bauer J, Farah H, Rassl DM, et al. SOX2 drives bronchial dysplasia in a novel organotypic model of early human squamous lung cancer. Am J Respir Crit Care Med. 2017;195:1494–1508. doi: 10.1164/rccm.201510-2084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]