Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating lung disease characterized by excessive scarring and fibroblast activation. We previously showed that fibroblasts from patients with IPF are hypermethylated at the CDKN2B gene locus, resulting in decreased CDKN2B expression. Here, we examine how diminished CDKN2B expression in normal and IPF fibroblasts affect fibroblast function, and how loss of CDKN2B contributes to IPF pathogenesis. We first confirmed that protein expression of CDKN2B was diminished in IPF lungs in situ. Loss of CDKN2B was especially notable in regions of increased myofibroblasts and fibroblastic foci. The degree of CDKN2B hypermethylation was particularly elevated in patients with radiographic honeycombing, a marker of more advanced fibrosis, and increased DNA methylation correlated with decreased expression. Although CDKN2B is traditionally considered a cell cycle inhibitor, loss of CDKN2B did not result in an increase in fibroblast proliferation, but instead was associated with an increase in myofibroblast differentiation. An increase in myofibroblast differentiation was not observed when CDKN2A was silenced. Loss of CDKN2B was associated with an increase in the transcription factors serum response factor and myocardin-related transcription factor A, and overexpression of CDKN2B in IPF fibroblasts inhibited myofibroblast differentiation. Finally, decreased CDKN2B expression was noted in fibroblasts from a murine model of fibrosis, and Cdkn2b−/− mice developed greater histologic fibrosis after bleomycin injury. These findings identify a novel function for CDKN2B that differs from its conventional designation as a cell cycle inhibitor and demonstrate the importance of this protein in pulmonary fibrosis.
Keywords: pulmonary fibrosis, p15, INK4B, myofibroblast, DNA methylation
Fibrosing disorders represent one of the most common and severe causes of morbidity and mortality worldwide (1). Fibroblasts are the major effector cells of fibrosis responsible for matrix remodeling and the development of pathologic scars. Proliferation and expansion of fibroblast populations allow for the accumulation of these cells at sites of tissue injury (2). Differentiation of fibroblasts into activated myofibroblasts, such as through the actions of transforming growth factor (TGF)-β1, stimulates further extracellular matrix production and encourages wound contraction (3). Idiopathic pulmonary fibrosis (IPF), a particularly severe form of fibrotic lung disease of unknown cause, is characterized by an accumulation of myofibroblasts into fibroblastic foci that form at the leading edge of pathologic lesions (4).
Several studies have shown that fibroblasts within fibrotic disorders exhibit a phenotype that contributes to the excessive scarring and architectural distortion associated with disease. We and others previously showed that fibroblasts from patients with IPF possess epigenetic modifications that contribute to their profibrotic phenotype (5–9). In particular, IPF fibroblasts exhibit global changes in DNA methylation that affect the regulation of many genes, and CDKN2B was one of the top genes identified to be hypermethylated in IPF cells (10).
The CDKN2B gene encodes for CDKN2B (p15, INK4B), which belongs to the INK4 class of cell cycle inhibitors (11). CDKN2B, in addition to other members of the INK4 family CDKN2A (p16, INK4A), CDKN2C (p18, INK4C), and CDKN2D (p19, INK4D), possesses ankyrin repeats that allow it to bind and disrupt the interaction of cyclin-dependent kinase (CDK) 4/6 with cyclin D, thereby inhibiting the activity of CDK4/6 (12, 13). Given the critical role of CDK4/6 and cyclin D in promoting progression through the G1 checkpoint, CDKN2B serves as an important inhibitor of cell proliferation and cell cycle. CDKN2B lies adjacent to CDKN2A on human chromosome 9 and the entire CDKN2A-ARF-CDKN2B locus has shown to be frequently mutated or epigenetically silenced in many cancers (14–16). Whereas the Cip/Kip family of cell cycle inhibitors, such as p21, p27, and p57, has been shown to bind multiple partners and modulate pleiotropic functions, including DNA repair, apoptosis, and senescence (17), the function of CDKN2B has traditionally been limited to cell cycle inhibition because of its specificity toward inhibiting CDK4/6 (12).
Because CDKN2B was identified as hypermethylated and expression decreased in fibroblasts from patients with IPF (10), we sought to identify the functional consequence of diminished CDKN2B expression in fibroblasts. Although it was hypothesized that decreased CDKN2B in fibroblasts would contribute to fibrosis through increased fibroblast proliferation, cell proliferation was surprisingly decreased when CDKN2B was suppressed in normal fibroblasts. Instead, loss of CDKN2B resulted in an increase in myofibroblast differentiation and activation. These data demonstrate a unique and distinct function for CDKN2B in the context of fibroblast biology and the role it has in the pathogenesis of pulmonary fibrosis. Some of the results of these studies have been previously reported in the form of an abstract (18).
Methods
Cell Culture
Normal primary human lung fibroblasts (CCL210) and human bronchial epithelial cells (BEAS-2B) were obtained from American Type Culture Collection. Fibroblasts from patients with IPF and fibroblasts from normal, nonfibrotic control subjects were expanded from lung tissue as detailed in the data supplement and as previously described (19, 20). All cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Invitrogen) supplemented with 10% FBS (Hyclone) and 1% penicillin/streptomycin (Invitrogen) and studied between passages 4 and 7.
siRNA and CDKN2B Overexpression
Fibroblasts were plated at 2.5 × 105 cells per well in six-well plates overnight before transfection with either 25 nM control siRNA (ON-TARGETplus nontargeting pool D-001810) or siRNA against CDKN2B (ON-TARGETplus SMARTpool L-003245) or CDKN2A (ON-TARGETplus SMARTpool L-011007) (all from Dharmacon). All transfections were performed using RNAiMax (Invitrogen) in OptiMEM (Invitrogen). In some experiments, transfected cells were concomitantly treated with CCG-203971 (30 μM, no. 15075; Cayman Chemical), an inhibitor to serum response factor (SRF) and myocardin-related transcription factor (MRTF)-A. After 24 hours, cells were washed, serum starved in DMEM for 24 hours, and then treated for 24 hours in DMEM with or without TGF-β1 (2 ng/ml 100-B; R&D Systems), fibroblast growth factor (FGF) (25 ng/ml; R&D Systems), platelet-derived growth factor (PDGF) (20 ng/ml; EMD Millipore), or 10% serum.
To overexpress CDKN2B, the coding sequence of CDKN2B (NM_004936.3) was cloned into the plasmid pAdTrack-CMV (no. 16405; Addgene). Fibroblasts plated at 2.5 × 105 cells per well in six-well plates were transfected with 1 μg of either the control (pAdTrack-CMV) or CDKN2B-overexpressing plasmid using Lipofectamine LTX with PLUS Reagent (no. 15338100; Invitrogen) in OptiMEM. Cells were washed after 24 hours, and treated with or without TGF-β1 (2 ng/ml) for 24 hours.
Animals
Wild-type, Cdkn2b+/−, and Cdkn2b−/− mice on a C57Bl/6 background were bred and studied at 6–10 weeks of age. Mice were treated intratracheally with either saline (50 μl PBS) or bleomycin (0.9 U/kg body weight reconstituted in 50 μl of PBS; B5507; Sigma-Aldrich). Weights were measured at baseline and every 7 days. Mice were killed at Day 21 after bleomycin and the entire lung was collected for hydroxyproline measurements. For some mice, lungs were washed with saline and perfused with formalin at a pressure of 25 cm H2O before being fixed in formalin, sectioned, and stained with hematoxylin and eosin and trichrome. The degree of fibrosis was assessed in a blinded, independent fashion using the Ashcroft scoring scale (21).
Study Approval
All animal studies were approved by the University of Michigan Institutional Animal Care and Use Committee. All studies involving clinical data and patient-derived cells were performed with written informed consent and approved by the University of Michigan Institutional Review Board. In some samples, cells and tissues were obtained from deidentified deceased individuals during warm autopsy, and were deemed Institutional Review Board exempt.
Detailed methods of immunohistochemistry, immunofluorescence, DNA methylation analysis, cell proliferation assay, PCR, immunoblot, hydroxyproline measurements, and ELISA for CXCL1 are described in further detail in the data supplement.
Results
Decreased Expression of CDKN2B Was Associated with More Severe Fibrosis
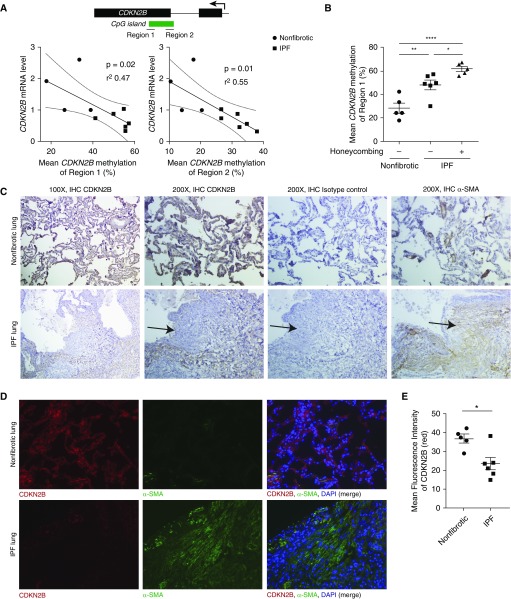
We previously showed that CDKN2B was hypermethylated and its expression decreased in fibroblasts from patients with IPF (10). To determine whether the level of DNA methylation correlated with decreased expression, we compared the expression of CDKN2B among different IPF cell lines with mean levels of DNA methylation, assayed at two different regions, of the CDKN2B gene (Figure 1A). An inverse correlation was observed between the degree of DNA methylation and level of CDKN2B mRNA. Because levels of CDKN2B methylation were variable among different IPF cell lines, we next compared the level of DNA methylation with patient characteristics from which these cells were derived. Fibroblasts from patients with IPF not only exhibited increased DNA methylation compared with normal control subjects (Figure 1B), but the level of methylation was notably higher in fibroblasts from patients with radiographic evidence of honeycombing on computed tomography scans, which is often associated with more advanced fibrosis.
Figure 1.
Increased CDKN2B methylation was observed in idiopathic pulmonary fibrosis (IPF) patients with radiographic honeycombing and was associated with decreased gene expression. Fibroblasts from normal (nonfibrotic) lungs and the lungs of patients with IPF were assayed for CDKN2B DNA methylation by bisulfite pyrosequencing. (A) Mean CDKN2B DNA methylation (averaged over six CpG sites within region 1 and 19 CpG sites within region 2) were compared with levels of CDKN2B mRNA expression using linear regression. (B) Mean levels of CDKN2B methylation were compared between fibroblasts derived from normal lung and IPF lung with and without radiographic evidence of honeycombing. *P < 0.05, **P < 0.01, ****P < 0.0001, one-way ANOVA with Tukey’s multiple comparisons test. (C and D) Lung sections from histologically normal, nonfibrotic lungs and fibrotic areas of IPF were immunostained for CDKN2B, α-smooth muscle actin (α-SMA), or isotype control. (C) Representative images at ×100 and ×200 magnification from three different patients are shown. Arrows indicate the presence of fibroblastic foci. (D) Representative immunofluorescent images of CDKN2B (red), α-SMA (green), DAPI (blue), and merged images at ×200 magnification from four normal and six IPF tissue sections are shown. (E) Mean fluorescence intensity of CDKN2B (red) was measured from lung sections of four different nonfibrotic and six different patients with IPF. The fluorescence intensity for each tissue section was calculated from the mean intensity of 4–10 images taken from each section. *P < 0.05, Student’s t test. IHC = immunohistochemistry.
To examine the expression of CDKN2B in situ, we stained sections of normal and IPF lung for CDKN2B. CDKN2B expression was decreased overall in IPF lung by immunohistochemistry (Figure 1C) and further confirmed by immunofluorescent imaging (Figure 1D). The decreased level of CDKN2B was quantified by fluorescence intensity (Figure 1E).
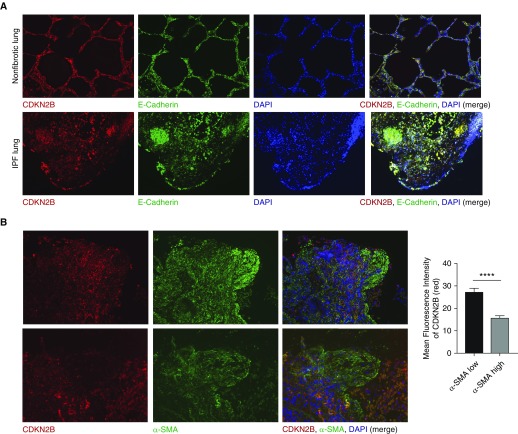
Staining by either immunohistochemistry or immunofluorescence revealed that CDKN2B expression was high in alveolar epithelium and low particularly in cells expressing α-smooth muscle actin (SMA) (Figures 1C and 1D). To better characterize which cells express CDKN2B, we costained sections of normal and IPF lung with antibody to CDKN2B, E-cadherin (a marker of epithelial cells), and α-SMA (a marker of myofibroblasts) (Figure 2). As seen in costains with E-cadherin, CDKN2B colocalizes with both normal and hyperplastic alveolar epithelium, which is often seen in IPF lung (Figure 2A). Conversely, CDKN2B is notably absent in regions of high α-SMA staining, especially within fibroblastic foci (Figure 2B). Together, these data suggest that increased CDKN2B methylation and decreased expression are features of IPF, particularly pronounced in patients with radiographic honeycombing and in microscopic regions of fibroblastic foci consisting of myofibroblasts.
Figure 2.
Expression of CDKN2B was localized to epithelial cells and decreased in regions of increased α-SMA. (A) Normal, nonfibrotic lung and IPF lung were immunostained for CDKN2B (red), E-cadherin (green), and DAPI (blue). Representative images (×100 magnification) from three different patient lines are shown. (B) IPF lung sections were immunostained for CDKN2B (red), α-SMA (green), and DAPI. Shown are representative images from two sections (top, ×100 magnification; bottom, ×200 magnification). Mean fluorescence intensity of CDKN2B was compared between regions of high α-SMA–expressing and low α-SMA–expressing cells (n = 3 different patients, one to six images taken from each patient). ****P < 0.0001, Student’s t test.
Loss of CDKN2B Was Associated with a Paradoxical Decrease in Fibroblast Proliferation
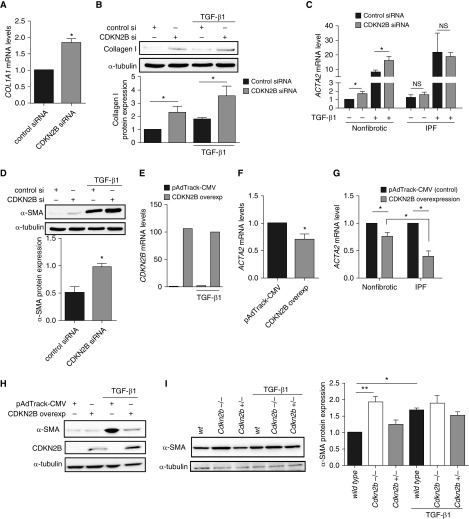
CDKN2B is traditionally considered a cell cycle inhibitor. To examine the functional consequence of a loss in CDKN2B expression in fibroblasts, we silenced CDKN2B expression in normal fibroblasts and examined cell proliferation. Use of CDKN2B-specific siRNA selectively inhibited expression of CDKN2B mRNA (Figure 3A) and protein (Figure 3B) without appreciably affecting expression of other cell cycle inhibitor genes. This level of CDKN2B suppression by siRNA was confirmed in all subsequent siRNA experiments. Because CDKN2B is recognized to inhibit CDK4/6 and has been shown to suppress proliferation in a number of cell types, we hypothesized that fibroblasts treated with CDKN2B siRNA would exhibit increased cell proliferation, which we first observed in unstimulated cells at baseline (10). Multiple subsequent experiments, however, in the presence of growth factors, such as PDGF, FGF, and serum, demonstrated that silencing CDKN2B suppressed fibroblast proliferation (Figure 3C). To ensure that this unexpected result was not merely an artifact of the proliferation assay, we examined the expression of cyclin B1 and cyclin D1, proteins that increase with cell cycle and are often used as markers of increased proliferation. Consistent with that observed with the proliferation assay, silencing CDKN2B in normal fibroblasts resulted in a decrease in both cyclin B1 and cyclin D1 mRNA and protein expression (Figures 3D–3F).
Figure 3.
Loss of CDKN2B in fibroblasts resulted in a decrease in cell proliferation. (A) Normal lung fibroblasts (CCL210) were treated with either control siRNA or siRNA against CDKN2B for 48 hours, and expression of various CDK inhibitors was assayed by RT-PCR (n = 4). (B–F) CCL210 fibroblasts were treated with either control or CDKN2B siRNA for 48 hours before treatment with either medium alone, platelet-derived growth factor (PDGF) (20 ng/ml), fibroblast growth factor (FGF) (25 ng/ml), transforming growth factor (TGF)-β1 (2 ng/ml), or Dulbecco’s modified Eagle medium (DMEM) with 10% serum for 24 hours. (B) Expression of CDKN2B was evaluated by immunoblot. Representative blot of four independent experiments is shown. (C) Cell proliferation was assayed by the CyQuant assay, as described in Methods, with values expressed as arbitrary fluorescent units. Data from a single experiment, which is representative of four independent experiments, are shown. Each experiment was performed in six replicate wells, with mean and SE measurements shown. (D) Levels of CCNB1 (cyclin B1) and CCND1 (cyclin D1) mRNA were assayed by RT-PCR (n = 8). (E) Cyclin B1 (n = 2) and (F) cyclin D1 (n = 3) protein were assayed by immunoblot with representative immunoblots shown. (G) Fibroblasts from wild-type and Cdkn2b−/− mice were assayed for cyclin D1 protein expression at baseline and in the presence of FGF (25 ng/ml). The mean of the relative densitometric values from three independent experiments are indicated below. (H) CDKN2B mRNA levels were assayed from fibroblasts of wild-type, Cdkn2b+/−, and Cdkn2b−/− mice in the presence or absence of TGF-β1 (2 ng/ml) or FGF (25 ng/ml). (I) Fibroblasts from wild-type, Cdkn2b+/−, and Cdkn2b−/− mice were assayed for proliferation by CyQuant assay in the presence of medium alone, FGF (25 ng/ml), PDGF (20 ng/ml), and 10% serum (representative experiment from four independent experiments). (J and K) BEAS-2B, a bronchial epithelial cell line, was treated with control or CDKN2B siRNA. Proliferation of cells in (J) bronchial epithelial growth medium (BEGM) was assayed by CyQuant assay (n = 3) and by expression of (K) cyclin D1 (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test (A, D, J, and K), one-way ANOVA with Tukey’s multiple comparisons test (C and F). arb = arbitrary; cont si = control siRNA; Rel exp = relative expression.
To ensure that the decrease in cell proliferation when CDKN2B was silenced was not due to an unintended artifact of siRNA, we compared fibroblast proliferation in cells derived from wild-type and Cdkn2b knockout (Cdkn2b−/−) mice. In keeping with the siRNA experiments, fibroblasts from Cdkn2b−/− mice exhibited decreased levels of cyclin D1 protein (Figure 3G) and cell proliferation (Figure 3I) at baseline and in the presence of growth factors. Previous studies have shown that, in certain cell types, TGF-β1 increases the expression of CDKN2B, which negatively feeds back to limit proliferation. Fibroblasts from wild-type mice, however, did not exhibit changes in CDKN2B expression by either TGF-β1 or FGF (Figure 3H). Finally, fibroblasts from Cdkn2b+/− exhibited half the level of CDKN2B expression compared with fibroblasts from wild-type mice that resulted in a level of proliferation at baseline and in response to growth factors that were intermediate between wild-type and Cdkn2b−/− cells (Figure 3I).
CDKN2B has been characterized as a cell cycle inhibitor based on many studies, often in cells of epithelial origin. We thus compared the effect of CDKN2B siRNA in fibroblasts with that in BEAS-2B cells, which are derived from bronchial epithelium. As opposed to that observed in fibroblasts, and in keeping with the recognized function of CDKN2B as a cell cycle inhibitor, cell proliferation (Figure 3J) and cyclin D1 levels (Figure 3K) both increased in BEAS-2B cells when CDKN2B was silenced. These findings thus indicate that the actions of CDKN2B in fibroblasts remain distinct from those of epithelial cells and contrast with its previously described role as a cell cycle inhibitor.
Loss of CDKN2B Was Associated with an Increase in Myofibroblast Differentiation
Immunofluorescent and immunohistochemical images demonstrated that CDKN2B expression was particularly diminished in cells expressing α-SMA. Because CDKN2B silencing was associated with an unexpected decrease in fibroblast proliferation, we next examined whether silencing or loss of CDKN2B expression was associated with an increase in myofibroblast differentiation. Fibroblasts, often in the presence of TGF-β1, differentiate into activated myofibroblasts, which are characterized by the increased production of extracellular matrix proteins, such as collagen I, and expression of α-SMA. Silencing of CDKN2B resulted in an increase in collagen I mRNA (Figure 4A) and protein (Figure 4B). The increase in collagen expression was also accompanied by an increase in α-SMA (Figures 4C and 4D). Notably, the increase in α-SMA was observed at the protein and mRNA level both in the presence and absence of exogenous TGF-β1. In contrast to normal lung fibroblasts, IPF lung fibroblasts, which exhibit lower levels of endogenous CDKN2B expression, did not demonstrate an increase in α-SMA when CDKN2B was silenced (Figure 4C). Fibroblasts treated with a plasmid to overexpress CDKN2B (Figure 4E) exhibited decreased expression of α-SMA mRNA and protein (Figures 4F and 4H), and the decrease in α-SMA was more pronounced when the CDKN2B overexpression vector was transfected in IPF fibroblasts (Figure 4G). Finally, fibroblasts from Cdk2nb−/− mice also exhibited increased levels of α-SMA compared with cells from wild-type mice (Figure 4I). The increased level of α-SMA in fibroblasts from Cdkn2b−/− mice was comparable to that observed in fibroblasts from wild-type mice treated with TGF-β1.
Figure 4.
A decrease in CDKN2B expression in fibroblasts was associated with an increase in myofibroblast differentiation. (A) Levels of COL1A1 mRNA were assayed in normal fibroblasts (CCL210) treated with control siRNA or CDKN2B siRNA (n = 8). (B and D) CCL210 fibroblasts were treated with control or CDKN2B siRNA for 48 hours before treatment with TGF-β1 (2 ng/ml) for 24 hours. Levels of (B) collagen I protein (n = 4) and (D) α-SMA (n = 3) were assayed by immunoblot. Representative blots from each experiment are shown. (C) Fibroblasts from normal (nonfibrotic) and IPF lungs were treated with control or CDKN2B siRNA before treatment with TGF-β1 (2 ng/ml) and levels of ACTA2 (α-SMA) mRNA were assayed by RT-PCR (n = 4). (E and F) CCL210 fibroblasts were transfected with a plasmid that overexpressed CDKN2B or control plasmid (pAdTrack-CMV). Levels of (E) CDKN2B mRNA (n = 1) and (F) ACTA2 (n = 3) were assayed by RT-PCR. (G) Fibroblasts from IPF and nonfibrotic control lungs were transfected with pAdTrack-CMV or CDKN2B overexpression plasmid and levels of ACTA2 mRNA was assayed by RT-PCR (n = 4 for nonfibrotic and n = 3 for IPF). (H) CCL210 fibroblasts were transfected with pAdTrack-CMV or CDKN2B overexpression plasmid and α-SMA protein expression was assayed by immunoblot (representative blot from two independent experiments). (I) Fibroblasts were cultured from the lungs of wild-type (wt), Cdkn2b−/−, and Cdkn2b+/− mice and treated with or without TGF-β1 (2 ng/ml) for 24 hours. Levels of α-SMA protein were assayed by immunoblot. Mean densitometry from three independent experiments and a representative blot are shown. *P < 0.05, **P < 0.01, Student’s t test (A, D, and F), one-way ANOVA with Tukey’s multiple comparisons test (B, C, G, and I). NS = not significant.
Loss of CDKN2B Was Associated with an Increase in Expression of Myofibroblast-Related Transcription Factors
To determine how loss of CDKN2B contributes to increased myofibroblast differentiation, we examined the expression of transcription factors, SRF and MRTF-A (also known as megakaryoblastic leukemia 1), recognized to be critical to myofibroblast differentiation (22, 23). Silencing of CDKN2B in normal fibroblasts resulted in an increase in SRF mRNA levels and protein expression (Figures 5A and 5B). Likewise, levels of MRTF-A mRNA (MLK1) and protein (Figures 5C and 5D) were also increased when CDKN2B expression was lost. Fibroblasts from Cdkn2b−/− mice also demonstrated increased protein expression of SRF (Figure 5E) and MRTF-A (Figure 5F). Finally, pretreatment of fibroblasts with the SRF/MRTF-A inhibitor CCG-203971 resulted in decreased expression of α-SMA in both the control and CDKN2B-silenced cells (Figure 4G), indicating that the increase in SRF and MRTF-A when CDKN2B is silenced is necessary for the increase in myofibroblast differentiation.
Figure 5.
Loss of CDKN2B resulted in increased expression of serum response factor (SRF) and myocardin-related transcription factor (MRTF)-A. (A–D) Normal lung fibroblasts treated with either control or CDKN2B siRNA for 48 hours were then treated with TGF-β1 (2 ng/ml) for 24 hours before being assayed for levels of SRF (A, n = 6) and MKL1 (MRTF-A) (C, n = 3) mRNA by RT-PCR and SRF (B) and MRTF-A (D) protein by immunoblot. Representative blots of three independent experiments are shown, with relative densitometry (mean ± SE) indicated. (E and F) Lung fibroblasts from wild-type (wt) and Cdkn2b−/− mice were treated in the presence or absence of TGF-β1 (2 ng/ml) for 24 hours, and expression of SRF (E) and MRTF-A (F) were assayed by immunoblot. Representative blots from three independent experiments are shown. (G) CCL210 fibroblasts were treated with either control or CDKN2B siRNA in the presence or absence of an SRF/MRTF-A inhibitor CCG-203971 (30 μM) for 48 hours. Cells were then treated with or without TGF-β1 (2 ng/ml) for 24 hours and assayed for α-SMA by immunoblot. A representative blot from four independent experiments is shown. *P < 0.05, ***P < 0.001; Student’s t test (B, C, D, and F), one-way ANOVA with Tukey’s multiple comparisons test (A, E, and G). Inh = inhibitor.
Effects of CDKN2B on Fibroblast Proliferation and Myofibroblast Differentiation Was Not Observed with Silencing CDKN2A
CDKN2A and CDKN2B are members of the INK4 family of cell cycle inhibitors, and their actions are often considered redundant, based on similar structural homology and binding affinity to CDK4/6. Although there was no difference in CDKN1A and CDKN1B expression between IPF and nonfibrotic control fibroblasts, levels of CDKN2A were decreased in IPF cells compared with normal controls, just as with CDKN2B (see Figures E1A–E1D in the data supplement). To examine if similar observations of CDKN2B in fibroblasts could be seen with CDKN2A, normal fibroblasts were treated with siRNA against CDKN2A, which effectively suppressed CDKN2A mRNA levels (Figure E1E). In contrast to data observed with CDKN2B, levels of α-SMA were unchanged when CDKN2A was silenced (Figure E1F). Furthermore, fibroblasts treated with siRNA against CDKN2A demonstrated an increase in cyclin B1 (CCNB1) mRNA (Figure E1G), in keeping with the function of CDKN2A as a cell cycle inhibitor. These data demonstrate that the effects of silencing CDKN2A on cell proliferation and markers of myofibroblast differentiation are distinct from that observed with CDKN2B.
Increase in Myofibroblast Differentiation with Loss of CDKN2B Was Not Associated with Changes in Smad Signaling
As silencing of CDKN2B did not result in an increase in cell proliferation, we sought to determine whether loss of CDKN2B influenced that activity of CDK4/6. CDK4/6 is recognized to phosphorylate the protein retinoblastoma (pRb), which typically promotes cell cycling and increases cell proliferation. Silencing CDKN2B in normal fibroblasts resulted in an increase of pRb phosphorylation (Figure E2A), indicating that CDK4/6 activity is indeed increased when CDKN2B expression is suppressed. These data, however, also indicate that loss of CDKN2B can suppress fibroblast proliferation even when pRb phosphorylation is increased.
Several studies have demonstrated that CDK4/6 can further promote cell proliferation and inhibit differentiation by phosphorylating Smad 3 at the threonine 8 (T8) position (24), which results in an inhibition of Smad 3. Although silencing CDKN2B in fibroblasts was associated with an increase in pRb phosphorylation and CDK4/6 activity, we did not observe an increase in Smad 3 (T8) phosphorylation (Figure E2B). Instead, a decrease in phosphorylated Smad 3 (T8) was observed, which is consistent with the observation that loss of CDKN2B promoted myofibroblast differentiation. Loss of CDKN2B has recently been shown to promote TGF-β1 signaling in smooth muscle cells by increasing Smad 3 phosphorylation and decreasing Smad 7 expression (25). Our studies of fibroblasts, however, did not reveal a significant loss of Smad 7 (Figure E2C) or increase in phosphorylation of Smad 2 or Smad 3 (Figure E2D) in cells for which CDKN2B was silenced. These studies thus demonstrate that the increase in myofibroblast differentiation after loss of CDKN2B was not dependent on alterations in Smad signaling.
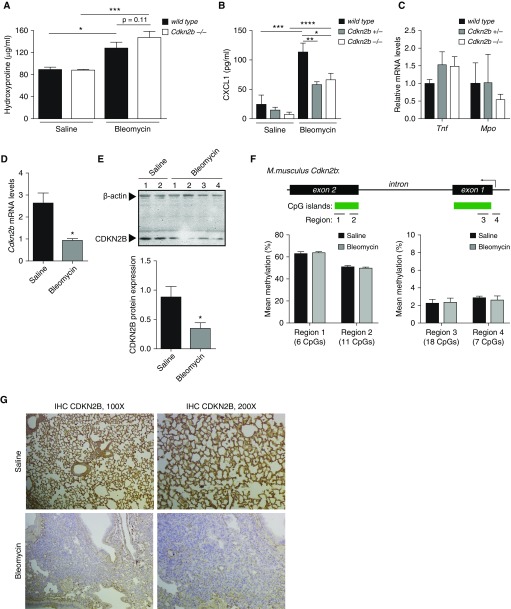
Histologic Levels of Fibrosis Were Worse in Cdkn2b−/− Mice after Intratracheal Bleomycin Injury
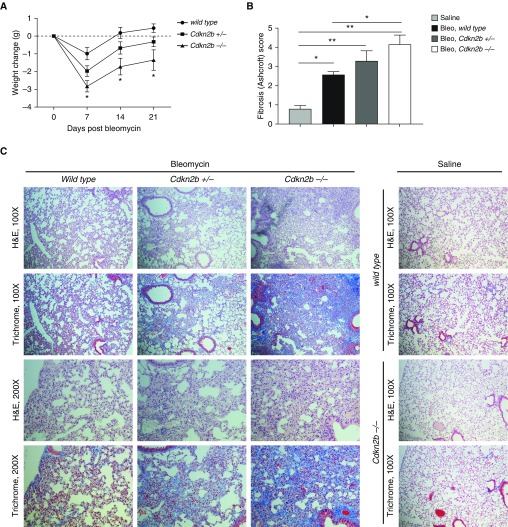
Intratracheal administration of bleomycin in mice is a commonly used model to cause lung injury and subsequent fibrosis in animals. Wild-type, Cdkn2b+/−, and Cdkn2b−/− mice were given a single low dose of bleomycin to approximate mild injury and subsequent fibrosis. Lower doses of bleomycin were used to determine if Cdkn2b−/− mice would develop a worse response to bleomycin than wild type. Cdkn2b−/− mice lost, on average, a greater amount of weight compared with Cdkn2b+/− and wild-type mice (Figure 6A). At Day 21, increased fibrosis was observed from the lungs of Cdkn2b−/− compared with wild-type mice, noted by both hematoxylin and eosin and trichrome staining (Figures 6B and 6C). Cdkn2b+/− mice appeared to develop fibrosis that was intermediate between wild-type and Cdkn2b−/− mice.
Figure 6.
Cdkn2b−/− mice develop worse histologic fibrosis after bleomycin injury. (A) Wild-type (n = 17), Cdkn2b+/− (n = 24), and Cdkn2b−/− (n = 22) mice were weighed at baseline and at 7, 14, and 21 days after bleomycin administration, and the average change in weight compared with baseline is shown. *P < 0.05 in Cdkn2b−/− compared with wild type, one-way ANOVA with Tukey’s multiple comparisons test. (B and C) Wild-type, Cdkn2b+/−, and Cdkn2b−/− mice were treated for 21 days with either saline or bleomycin (Bleo), and lungs were examined by hematoxylin and eosin (H&E) and trichrome staining. (B) Level of fibrosis, as scored by the Ashcroft scale, was determined from each slide, and the mean of each group is shown. (C) Representative images, at ×100 and ×200 magnification, from three mice in each group are shown. *P < 0.05, **P < 0.01, one-way ANOVA with Tukey’s multiple comparisons test.
Hydroxyproline is a commonly used biochemical assay to estimate levels of collagen. Cdkn2b−/− mice exhibited a trend toward increased levels of hydroxyproline in the lungs at Day 21 after bleomycin injury compared with wild-type mice, although the difference was not statistically significant (Figure 7A). To examine why a greater statistical difference in hydroxyproline levels was not observed between wild-type and Cdkn2b−/− mice, we examined the inflammatory response in mice at Day 2 after bleomycin administration. Levels of CXCL1 were higher overall in bleomycin-treated mice, but lower in Cdkn2b+/− and Cdkn2b−/− mice compared with wild-type (Figure 7B). Levels of whole-lung mRNA for Tnf and myeloperoxidase (Mpo), a marker of neutrophil recruitment, were unchanged among mice (Figure 7C). We next examined the expression of CDKN2B in fibroblasts from wild-type mice after bleomycin injury. Notably, levels of CDKN2B mRNA (Figure 7D) and protein (Figure 7E) were decreased in fibroblasts of wild-type mice at Day 21 after bleomycin. Because DNA hypermethylation was noted to account for the difference in CDKN2B expression between normal and IPF fibroblasts, we examined the DNA methylation of Cdkn2b in fibroblasts of mice treated with saline or bleomycin. In contrast to IPF, there was no change in DNA methylation of Cdkn2b in murine fibroblasts after bleomycin-induced fibrosis (Figure 7F). Finally, immunohistochemistry staining for CDKN2B demonstrated decreased expression of CDKN2B in the fibrotic mouse model (Figure 7G). These data indicate that loss of CDKN2B may be an important feature of other models of fibrosis, though the mechanisms by which CDKN2B is lost may be different from that of IPF.
Figure 7.
Fibroblasts from fibrotic lung exhibit decreased expression of CDKN2B. (A) Wild-type and Cdkn2b−/− mice were given saline (n = 14 wild type, n = 8 Cdkn2b−/−) or bleomycin (n = 11 wild type, n = 14 Cdkn2b−/−) at Day 0, and whole lungs were assessed at Day 21 for levels of hydroxyproline. (B and C) Wild-type, Cdkn2b+/−, and Cdkn2b−/− mice (three to six mice each) were treated with either saline or bleomycin. On Day 2, BAL was assayed for CXCL1 (B) and whole lung was assayed for levels of Tnf and myeloperoxidase (Mpo) (C) mRNA. Shown are levels of Tnf and Mpo in bleomycin-treated mice (Tnf and Mpo levels in saline-treated mice were undetectable). (D and E) Fibroblasts were cultured from the lungs of wild-type mice 21 days after mice were treated with either saline or bleomycin, and examined for levels of Cdkn2b mRNA (D, n = 3) and CDKN2B protein expression (E). (F) Lung fibroblasts were cultured from mice at Day 21 after saline (n = 5) or bleomycin (n = 12), and different regions of the Cdkn2b gene were assessed for DNA methylation by bisulfite pyrosequencing. (G) Lung sections were obtained from wild-type mice 21 days after treatment with saline or bleomycin, and immunostained for CDKN2B expression. Representative images, at ×100 and ×200 magnification, from three mice in each group are shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test (B and C), one-way ANOVA with Tukey’s multiple comparisons test (A).
Discussion
CDKN2B is a cell cycle inhibitor where the loss of expression has been shown to be important in tumorigenesis (14–16) and cardiovascular diseases (26, 27). Here, we demonstrate that loss of CDKN2B in lung fibroblasts may also be critical to the development of pulmonary fibrosis. Not only was the expression of CDKN2B diminished in IPF fibroblasts as we had previously observed (10), but we show, for the first time, that: 1) CDKN2B is diminished in IPF tissue, particularly in fibroblastic foci and α-SMA–expressing cells; 2) the degree of methylation and loss of expression correlates with radiographic honeycombing; 3) loss of CDKN2B resulted in a paradoxical decrease in proliferation unique to fibroblasts; 4) loss of CDKN2B promotes myofibroblast differentiation through increases in SRF and MRTF-A; and 5) Cdkn2b−/− mice develop worse fibrosis compared with wild-type mice after bleomycin injury. CDKN2B is classically considered a cell proliferation checkpoint inhibitor (11, 12) and is often considered a tumor suppressor (28). In fibroblasts, however, loss of CDKN2B actually resulted in a decrease in cell proliferation. These data were at first surprising, as they conflicted with our previous study (10), but our previous study differed in that the effect of silencing CDKN2B in fibroblasts was initially observed without the presence of growth factors. In this study, we found that loss of CDKN2B, whether through siRNA or through the use of fibroblasts from Cdkn2b−/− mice, was associated with a decrease in fibroblast proliferation, as measured by multiple assays of proliferation. This was observed in the presence of multiple growth factors, including FGF, PDGF, and serum. The role of CDKN2B as a cell cycle inhibitor is best understood from studies in epithelial cells (11) and cancer (28, 29), many of which are also epithelial cells in origin. Indeed, silencing CDKN2B in bronchial epithelial cells in our experiments resulted in increased epithelial cell proliferation. That proliferation was decreased when CDKN2B was silenced in fibroblasts provides another example of how epithelial and mesenchymal cells often respond in opposing fashion to a given signal and how CDKN2B function varies by cell type. This “paradox” between fibroblast and epithelial cell responses has often been described in other contexts (30, 31), and may play an important role in their differential response to tissue injury and repair. Prostaglandin E2, for example, has been shown to both promote epithelial cell proliferation and inhibit fibroblast proliferation, even despite the generation of cAMP as a common early signaling mediator (30, 32).
In the context of diminished proliferation, loss of CDKN2B in fibroblasts was associated with an increase in myofibroblast differentiation. Experimentally, loss of CDKN2B was associated with an increase in expression of both SRF and MRTF-A, two transcription factors that are critical to the activation and differentiation of myofibroblasts (22, 23). An inhibitor of SRF/MRTF-A was able to block the increase in α-SMA when CDKN2B was silenced. Interestingly, silencing CDKN2B promoted myofibroblast differentiation, even in the absence of exogenous TGF-β1 or change in matrix environment, which are stimuli traditionally considered necessary for myofibroblast differentiation (33, 34). The increase in myofibroblasts was not associated with an increase in Smad 2 phosphorylation, indicating that there was no increase in endogenous TGF-β1 production. These data suggest that loss of CDKN2B expression in the mesenchymal cells may be sufficient to promote fibrogenesis. This loss of CDKN2B was particularly noted in IPF lung sections with fibroblastic foci and in myofibroblasts expressing α-SMA. That decreased CDKN2B expression was associated with an increase in myofibroblast differentiation and not proliferation also supports studies in IPF that demonstrate an accumulation of myofibroblasts despite a lack of fibroblast proliferation within fibroblastic foci (35, 36). Regional or cell-to-cell variation in CDKN2B expression within IPF lung could also signify transitions where fibroblasts switch signals from a more proliferative state to that of a myofibroblast.
Although SRF and MRTF-A were responsible for the downstream effects of diminished CDKN2B, the upstream signals that mediate the effects when CDKN2B is lost are less clear. p21 (CDKN1A) and p57 (CDKN1C), which belong to the Cip/Kip family of cell cycle inhibitors, bind to multiple partners that allow p21 and p57 to exert pleiotropic actions, including inhibition of apoptosis and DNA repair (17). The expression of p21 and p57, however, was not altered when CDKN2B was silenced, and levels of CDKN1A were not different in IPF versus normal fibroblasts. CDKN2B is recognized to only bind and inhibit CDK4/6–cyclin D complexes (12), and whether CDKN2B targets proteins other than CDK4/6 and cyclin D is unknown. Interestingly, the effects of silencing CDKN2B in fibroblasts was not observed when CDKN2A was silenced, indicating that the function of CDKN2B in fibroblasts is not generalizable to all INK4 family members. Our data show that silencing of CDKN2B in fibroblasts resulted in an increase in CDK4/6 activity, as phosphorylation of pRb, a downstream target of CDK4/6, is increased. CDK4/6 has been shown to inhibit Smad 3 and inhibit differentiation in other cell types by increasing phosphorylation of Smad 3 at the inhibitory T8 position (24, 37). However, silencing CDKN2B in fibroblasts resulted in a decrease in Smad 3 (T8) phosphorylation. Loss of CDKN2B has recently been shown to cause an increase in TGF-β1 signaling in vascular smooth muscle cells due to a decrease in inhibitory Smad 7 expression and an increase in active Smad 3 phosphorylation (25). Our data in fibroblasts, however, did not demonstrate a decrease in Smad 7 expression or increase in TGF-β1 signaling (as assayed by Smad 2 and Smad 3 phosphorylation), indicating that these signaling pathways are not responsible for the increase in myofibroblast differentiation in fibroblasts. The E2F family of transcription factors is often activated as a consequence of CDK4/6 activation and pRb phosphorylation (38). Although E2F proteins are generally felt to bind and activate cell cycle genes, the various E2F isoforms (E2F1–8) allow for a variety of genes to be activated or suppressed (39, 40). E2F4 has been demonstrated to modulate differentiation of hematopoietic progenitor cells (41) and E2F1 has been shown to deregulate apoptosis-related genes in fibroblasts (42). Specific E2F isoforms may thus be responsible for the triggering myofibroblast differentiation in fibroblasts.
Compared with normal lung fibroblasts, fibroblasts from the lungs of patients with IPF demonstrate features that are felt to contribute to a more aggressive, fibrotic phenotype. We were able to inhibit myofibroblast differentiation in IPF fibroblasts by overexpressing CDKN2B in IPF cells, suggesting that drugs that inhibit CDK4/6, such as palbociclib, which is being considered for breast cancer (43), may have therapeutic benefit in IPF. Some of the phenotypic differences between IPF and normal fibroblasts can be attributed to epigenetic changes, and CDKN2B was the top gene that we identified to be hypermethylated in an unbiased methylation array of IPF fibroblasts (10). We observed that CDKN2B expression was not only decreased in IPF fibroblasts, but the degree of DNA hypermethylation, which was inversely correlated with its expression, was associated with the presence of honeycombing in patients with IPF. The presence of honeycombing on computed tomography scan is a feature often observed in patients with more advanced disease, and the degree of CDKN2B hypermethylation may serve as a biomarker and/or potential mechanism for increased fibrosis and disease progression. We previously showed that the DNA methylation inhibitor 5-aza-2′-deoxycytidine (decitabine) can restore CDKN2B expression in vitro (10); whether decitabine, which is in clinical use to treat leukemia, has therapeutic benefit in IPF remains to be determined. The entire CDKN2A-p14ARF-CDKN2B genetic locus is frequently silenced by DNA methylation in many cancers (44, 45); along with our studies of CDKN2B, p14ARF is likewise hypermethylated in IPF cells (5). These data thus suggest that this locus is a potential “hotspot” of epigenetic regulation for a variety of diseases from cancer to fibrosis.
We investigated the role of CDKN2B in the fibroblasts of IPF lung, but whether its expression and function are altered in other cell types within the fibrotic lung is unknown. Immunohistochemistry and immunofluorescent staining suggest that CDKN2B may be globally suppressed in fibrotic lung, although it was found to be expressed within alveolar epithelium. Whereas loss of CDKN2B in fibroblastic foci may contribute to the increase in α-SMA expression and myofibroblast differentiation, a loss of CDKN2B in alveolar epithelial cells may be necessary for epithelial cells to proliferate and re-epithelialize injured tissue. Thus, loss of CDKN2B in epithelial cells may be protective in early lung injury, and may explain why the increase in hydroxyproline was not as great as one might expect in global Cdkn2b−/− mice compared with wild type. Lower levels of CXCL1 in Cdkn2b−/− mice after bleomycin injury may also explain why differences in hydroxyproline were not more marked, and indicate that Cdkn2b−/− mice have an even greater fibrotic response to a lower level of injury. Finally, because CDKN2B expression was lost in fibroblasts from wild-type mice after bleomycin exposure, this may also explain why a greater difference in hydroxyproline was not noted between wild-type and Cdkn2b−/− mice. Interestingly, the loss of CDKN2B expression in bleomycin-treated mice could not be attributed to an increase in DNA methylation, suggesting that other mechanisms may be responsible for the decrease in CDKN2B expression. This could be due to changes in transcription factors that regulate CDKN2B expression or other epigenetic mechanisms, such as histone modifications, which have been shown to modulate CDKN2A/CDKN2B expression in cancers and other cell types (46). These findings thus provide another example of mechanistic differences between bleomycin mouse models and IPF.
CDKN2B lies within chromosome 9p21, which is a genetic locus that has been shown by genome-wide association studies to be one of the most highly associated with cardiovascular disease (26, 47). The candidate gene(s) within the 9p21 locus that contributes to the increased risk of cardiovascular disease is unknown. Recent studies suggest that loss of CDKN2B may promote atherosclerosis through changes in endothelial and smooth muscle cell biology (27, 48); our findings provide a novel role for CDKN2B in modulating fibroblast biology and fibroblast differentiation. It would be intriguing to determine whether CDKN2B also inhibits myofibroblasts within blood vessel walls, which may provide another mechanism by which polymorphisms in the 9p21 genetic locus contribute to vascular remodeling. Other studies have demonstrated that CDKN2B and/or CDK4 actions may have other pleiotropic roles, including regulating hematopoiesis (49), glucose metabolism (50), and Wnt signaling (51), the latter of which has also been attributed to fibrogenesis (52).
In conclusion, CDKN2B expression is decreased in IPF fibroblasts and in animal models of acquired fibrosis. Decreased CDKN2B expression, however, was not associated with an increase in fibroblast proliferation, and instead, was associated with a stimulation of myofibroblast differentiation even in the absence of traditional myofibroblast differentiating signals. The increase in differentiation was associated with an increase in SRF and MRTF-A expression. CDKN2B expression was particularly diminished in areas of fibroblastic foci, and cells from patients with more advanced fibrosis exhibited a greater degree of hypermethylation. These findings thus identify a novel role for CDKN2B in fibroblast biology and demonstrate the importance of CDKN2B in the pathogenesis of IPF.
Acknowledgments
Acknowledgment
The authors thank Thomas Sisson and Natalya Subbotina for their assistance with the hydroxyproline assay, Amir Lagstein for his assistance with scoring fibrosis on histopathology, Natalie Walker for her assistance with immunofluorescence, and L. R. Penke for his thoughtful discussions and insight.
Footnotes
This work was supported by a National Institutes of Health grant R01 HL127203 (S.K.H.).
Author Contributions: A.M.S. designed the experimental studies, conducted the experiments, and analyzed the data; H.B.K. and P.T. conducted the experiments and analyzed the data; N.J.L. provided Cdkn2b−/− mice; E.S.W. provided normal and idiopathic pulmonary fibrosis tissues and cell lines; and S.K.H. designed the experimental studies, conducted the experiments, analyzed the data, and wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0298OC on February 8, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis: ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 5.Cisneros J, Hagood J, Checa M, Ortiz-Quintero B, Negreros M, Herrera I, et al. Hypermethylation-mediated silencing of p14(ARF) in fibroblasts from idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L295–L303. doi: 10.1152/ajplung.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol. 2009;29:4325–4339. doi: 10.1128/MCB.01776-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SK, Fisher AS, Scruggs AM, White ES, Hogaboam CM, Richardson BC, et al. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol. 2010;177:2245–2255. doi: 10.2353/ajpath.2010.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SK, Scruggs AM, Donaghy J, Horowitz JC, Zaslona Z, Przybranowski S, et al. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis. 2013;4:e621. doi: 10.1038/cddis.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:610–618. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SK, Scruggs AM, McEachin RC, White ES, Peters-Golden M. Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS One. 2014;9:e107055. doi: 10.1371/journal.pone.0107055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-β–induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 12.Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol. 1999;287:821–828. doi: 10.1006/jmbi.1999.2640. [DOI] [PubMed] [Google Scholar]
- 13.Russo AA, Tong L, Lee JO, Jeffrey PD, Pavletich NP. Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumour suppressor p16INK4a. Nature. 1998;395:237–243. doi: 10.1038/26155. [DOI] [PubMed] [Google Scholar]
- 14.Drexler HG. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells. Leukemia. 1998;12:845–859. doi: 10.1038/sj.leu.2401043. [DOI] [PubMed] [Google Scholar]
- 15.Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378:F115–F177. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 16.Sharpless NE. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat Res. 2005;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scruggs AM, Koh HB, Leeper NJ, Penke LRK, Huang SK. Decreased expression of the cell cycle inhibitor CDKN2B promotes pulmonary fibrosis through increased myofibroblast differentiation rather than increased proliferation [abstract] Am J Respir Crit Care Med. 2016;193:A4018. [Google Scholar]
- 19.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogaboam CM, Carpenter KJ, Evanoff H, Kunkel SL. Approaches to evaluation of fibrogenic pathways in surgical lung biopsy specimens. Methods Mol Med. 2005;117:209–221. doi: 10.1385/1-59259-940-0:209. [DOI] [PubMed] [Google Scholar]
- 21.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du KL, Chen M, Li J, Lepore JJ, Mericko P, Parmacek MS. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. J Biol Chem. 2004;279:17578–17586. doi: 10.1074/jbc.M400961200. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Ip HS, Lu MM, Clendenin C, Parmacek MS. A serum response factor–dependent transcriptional regulatory program identifies distinct smooth muscle cell sublineages. Mol Cell Biol. 1997;17:2266–2278. doi: 10.1128/mcb.17.4.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 25.Nanda V, Downing KP, Ye J, Xiao S, Kojima Y, Spin JM, et al. CDKN2B regulates TGFβ signaling and smooth muscle cell investment of hypoxic neovessels. Circ Res. 2016;118:230–240. doi: 10.1161/CIRCRESAHA.115.307906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helgadottir A, Thorleifsson G, Magnusson KP, Grétarsdottir S, Steinthorsdottir V, Manolescu A, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 27.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–1097. doi: 10.1172/JCI70391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krimpenfort P, Ijpenberg A, Song JY, van der Valk M, Nawijn M, Zevenhoven J, et al. p15Ink4b is a critical tumour suppressor in the absence of p16Ink4a. Nature. 2007;448:943–946. doi: 10.1038/nature06084. [DOI] [PubMed] [Google Scholar]
- 29.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 30.Maher TM, Evans IC, Bottoms SE, Mercer PF, Thorley AJ, Nicholson AG, et al. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:73–82. doi: 10.1164/rccm.200905-0674OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang SK, White ES, Wettlaufer SH, Grifka H, Hogaboam CM, Thannickal VJ, et al. Prostaglandin E(2) induces fibroblast apoptosis by modulating multiple survival pathways. FASEB J. 2009;23:4317–4326. doi: 10.1096/fj.08-128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cha SI, Groshong SD, Frankel SK, Edelman BL, Cosgrove GP, Terry-Powers JL, et al. Compartmentalized expression of c-FLIP in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42:140–148. doi: 10.1165/rcmb.2008-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leppäranta O, Pulkkinen V, Koli K, Vähätalo R, Salmenkivi K, Kinnula VL, et al. Transcription factor GATA-6 is expressed in quiescent myofibroblasts in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42:626–632. doi: 10.1165/rcmb.2009-0021OC. [DOI] [PubMed] [Google Scholar]
- 37.Baughn LB, Di Liberto M, Niesvizky R, Cho HJ, Jayabalan D, Lane J, et al. CDK2 phosphorylation of Smad2 disrupts TGF-β transcriptional regulation in resistant primary bone marrow myeloma cells. J Immunol. 2009;182:1810–1817. doi: 10.4049/jimmunol.0713726. [DOI] [PubMed] [Google Scholar]
- 38.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 39.Ertosun MG, Hapil FZ, Osman Nidai O. E2F1 transcription factor and its impact on growth factor and cytokine signaling. Cytokine Growth Factor Rev. 2016;31:17–25. doi: 10.1016/j.cytogfr.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Hsu J, Sage J. Novel functions for the transcription factor E2F4 in development and disease. Cell Cycle. 2016;15:3183–3190. doi: 10.1080/15384101.2016.1234551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enos ME, Bancos SA, Bushnell T, Crispe IN. E2F4 modulates differentiation and gene expression in hematopoietic progenitor cells during commitment to the lymphoid lineage. J Immunol. 2008;180:3699–3707. doi: 10.4049/jimmunol.180.6.3699. [DOI] [PubMed] [Google Scholar]
- 42.Kitamura H, Ozono E, Iwanaga R, Bradford AP, Okuno J, Shimizu E, et al. Identification of novel target genes specifically activated by deregulated E2F in human normal fibroblasts. Genes Cells. 2015;20:739–757. doi: 10.1111/gtc.12268. [DOI] [PubMed] [Google Scholar]
- 43.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor–positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 44.Herman JG, Civin CI, Issa JP, Collector MI, Sharkis SJ, Baylin SB. Distinct patterns of inactivation of p15INK4B and p16INK4A characterize the major types of hematological malignancies. Cancer Res. 1997;57:837–841. [PubMed] [Google Scholar]
- 45.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 46.Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cluett C, McDermott MM, Guralnik J, Ferrucci L, Bandinelli S, Miljkovic I, et al. The 9p21 myocardial infarction risk allele increases risk of peripheral artery disease in older people. Circ Cardiovasc Genet. 2009;2:347–353. doi: 10.1161/CIRCGENETICS.108.825935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leeper NJ, Raiesdana A, Kojima Y, Kundu RK, Cheng H, Maegdefessel L, et al. Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:e1–e10. doi: 10.1161/ATVBAHA.112.300399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolff L, Bies J. p15Ink4b functions in determining hematopoietic cell fates: implications for its role as a tumor suppressor. Blood Cells Mol Dis. 2013;50:227–231. doi: 10.1016/j.bcmd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y, Dominy JE, Choi YJ, Jurczak M, Tolliday N, Camporez JP, et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510:547–551. doi: 10.1038/nature13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol. 2010;20:453–460. doi: 10.1016/j.tcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]