Abstract
Accurate and reproducible assessments of experimental lung injury and inflammation are critical for basic and translational research. In particular, investigators use various methods for BAL and euthanasia; however, the impact of these methods on assessments of injury and inflammation is unknown. To define potential effects, we compared methods of lavage and euthanasia in uninjured mice and after a mild lung injury model (ozone). C57BL/6J male mice (8–10 weeks old) underwent BAL after euthanasia with ketamine/xylazine, carbon dioxide (CO2), or isoflurane. BAL methods included 800 μl of isotonic solution instilled and withdrawn three times, and one or three passive fills and drainage to 20 cm H2O. Parallel experiments were performed 24 hours after 3 hours of ozone (O3) exposure at 2 ppm. BAL total cell counts/differentials and total protein/albumin were determined. Lung histology was evaluated for lung inflammation or injury. BAL cells were cultured and stimulated with PBS, PMA, or LPS for 4 hours and supernatants were evaluated for cytokine content. In uninjured mice, we observed differences due to the lavage and euthanasia methods used. The lavage method increased total cells and total protein/albumin in uninjured and O3-exposed mice, with the 800-μl instillation having the highest values. Isoflurane increased total BAL cells, whereas CO2 euthanasia increased the total protein/albumin levels in uninjured mice. These effects limited our ability to detect differences in BAL injury measures after O3 exposure. In conclusion, the method used for lavage and euthanasia affects measures of lung inflammation/injury and should be considered a variable in model assessments.
Keywords: BAL, euthanasia, lung inflammation, lung injury, ozone
Clinical Relevance
Experimental methods have the potential to alter assessments of acute lung injury models. We demonstrate how the methods used for lung sampling and euthanasia alter measures of acute lung injury in mice before injury.
Assessments of acute lung injury measure the pulmonary response to experimental challenge by irritants, pathogens, or toxicological agents. These challenges are used to model human diseases such as acute respiratory distress syndrome; a syndrome defined by diffuse pulmonary infiltrates, severe hypoxemia, and respiratory failure, which causes substantial morbidity and mortality (1). Beyond acute respiratory distress syndrome, less severe forms of lung injury alter pulmonary and systemic immune functions to limit or prime further responses, with both benefits and detriments to host survival and function (2–4). Because of the importance of lung injury, multiple animal exposure models have been developed to determine mechanisms and define potential treatments (5, 6). These models require accurate and reproducible methods to generate lung injury and, similarly, to assess the severity of injury.
To address the relevance and accuracy of animal injury models and assay methods, the American Thoracic Society convened a consensus group, which generated a workshop report titled “Features and Measurements of Experimental Acute Lung Injury in Animals.” In this document, Matute-Bello and colleagues defined methods of modeling and evaluating lung injury, and discussed the use of various assays to define epithelial permeability, measures of BAL inflammation, and a lung injury scoring method (7). Although the document addressed several important issues, it did not address specific technical concerns, such as the methods used for euthanasia and BAL, and whether they alter injury assessments. Prior work suggested potential differences in inflammatory measures based on lavage characteristics and the type of anesthesia and/or euthanasia used (8–11). However, no study has comprehensively considered these effects together.
In the present study, we performed an evaluation of measures of lung injury using various methods of lavage and euthanasia. We found that values for total cell counts and total protein/albumin were elevated based on the lavage method used, but the relative levels remained consistent among the different lavage methods. In addition, we identified significant differences in measures of cellular inflammation and injury in uninjured mice based on the method of euthanasia. Furthermore, these differences affected our ability to measure differences after a mild injury model. These data suggest that one should consider differences in lavage and euthanasia methods when evaluating experimental lung injury models.
Methods
Mice and Injury Method
C57BL/6J male mice (8–10 weeks old) were purchased from The Jackson Laboratory. Experiments were conducted in accordance with National Institutes of Health guidelines, and the protocols were approved by the Animal Care and Use Committee at Duke University. To generate lung injury, mice were exposed to filtered air (FA) or ozone (O3) for 3 hours, and 24 hours after exposure were assessed for lung injury.
Euthanasia Protocol
Mice were killed before BAL by isoflurane, a ketamine/xylazine mixture, or carbon dioxide (CO2). All methods of euthanasia conform to current 2013 American Veterinary Association Guidelines (12). Isoflurane overdose was administered in a bell glass jar located in a chemical fume hood until complete cessation of respiration was observed, and the mice were monitored for an additional 1 minute to confirm lack of respiration. The animals were elevated above isoflurane liquid during the overdose. Ketamine (100 mg/kg) and xylazine (10 mg/kg) injections were administered intraperitoneally before necropsies were performed. Necropsies of ketamine/xylazine-administered mice were performed after a reduction in respiratory rate and lack of toe-pinch response were observed. CO2 was provided in a plexiglass chamber from an external CO2 tank with a fill rate < 30% of the chamber. The mice were monitored until cessation of respiration was observed, at which point the CO2 was discontinued, and then monitored for an additional minute to confirm apnea. Between animals, the chamber was tipped over to completely empty the CO2 chamber.
BAL Fluid Protocol
After the mice were killed, three BAL methods were used. In the first method, a small nick was made in the trachea and an 18-gauge catheter was placed in the trachea and sutured in place. Then, 800 μl of isotonic saline (sodium chloride, 0.9% [wt/vol]) was slowly instilled and then withdrawn. This fluid was instilled and withdrawn three times in total. The other methods relied on passive fill/drainage. PE-60 tubing (Clay Adams) was used to cannulate the trachea. The tubing was connected to 12-inch tubing from an infusion set (Terumo). Saline was infused via the tubing to 20 cm H2O via connection to syringe on a ring stand until the lung reached total lung capacity. The fluid was then passively drained and the volume was recorded. This fill/drainage procedure was performed once in one group and three times in another group. Cells from the BAL fluid were isolated by centrifugation (1,500 rpm, 10 min) and the supernatant was used for assessment of total protein and albumin. Cells were counted using a Cellometer K2 (Nexcelom Bioscience). After the cell counts were determined, the cells were immobilized by cytospin, stained with Diff-Quik (Fisher Scientific), and counted in a blinded fashion to define cell differentials.
Statistics
Data are expressed as mean ± SE. The statistical difference between groups was assessed by one-way ANOVA followed by Tukey’s test for multiple comparisons. P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 7.02.
Additional details regarding the methods used in this work are provided in the data supplement.
Results
Methods of Euthanasia and BAL Are Variable or Inconsistently Reported in Experimental Lung Injury Models
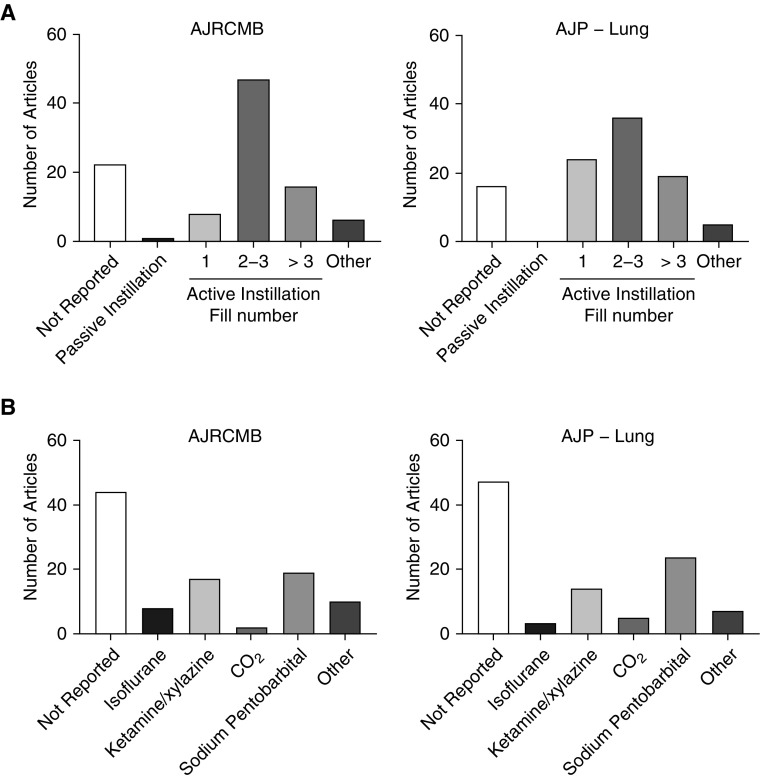
To define the effects of different methods of lavage and euthanasia, we first determined the frequency of reporting and variety of these measures in the published literature. Using published studies of experimental acute lung injury in two highly cited pulmonary journals (American Journal of Respiratory Cell and Molecular Biology and American Journal of Physiology—Lung Cellular and Molecular Physiology), we quantified the studies based on the type of method used. Interestingly, we observed that a large portion of publications in both journals did not report the method of lavage or euthanasia (Figure 1 and Table E1 in the data supplement). In the studies in which the methods were reported, we observed a wide variety of euthanasia and lavage methods. These observations were highly similar between the two journals. Because measures of experimental lung injury and inflammation are critical for determining underlying mechanisms, we hypothesized that the methods of lavage and euthanasia could represent an experimental variable that should be considered when designing experiments or interpreting results. Furthermore, we were interested in assessing the utility of passive fill methods, as such approaches had been infrequently compared with other methods.
Figure 1.
Frequency of euthanasia and BAL methods in experimental models of acute lung injury. (A and B) The 100 most recent articles in the American Journal of Respiratory Cell and Molecular Biology (AJRCMB) and the American Journal of Physiology—Lung Cellular and Molecular Physiology (AJP-Lung) were assessed for methods of (A) euthanasia and (B) BAL. Values are presented as the number of articles in each category. Active instillation values refer to the number of fills.
Methods of Euthanasia and Lavage Alter BAL Cell Counts in Uninjured Murine BAL Fluid
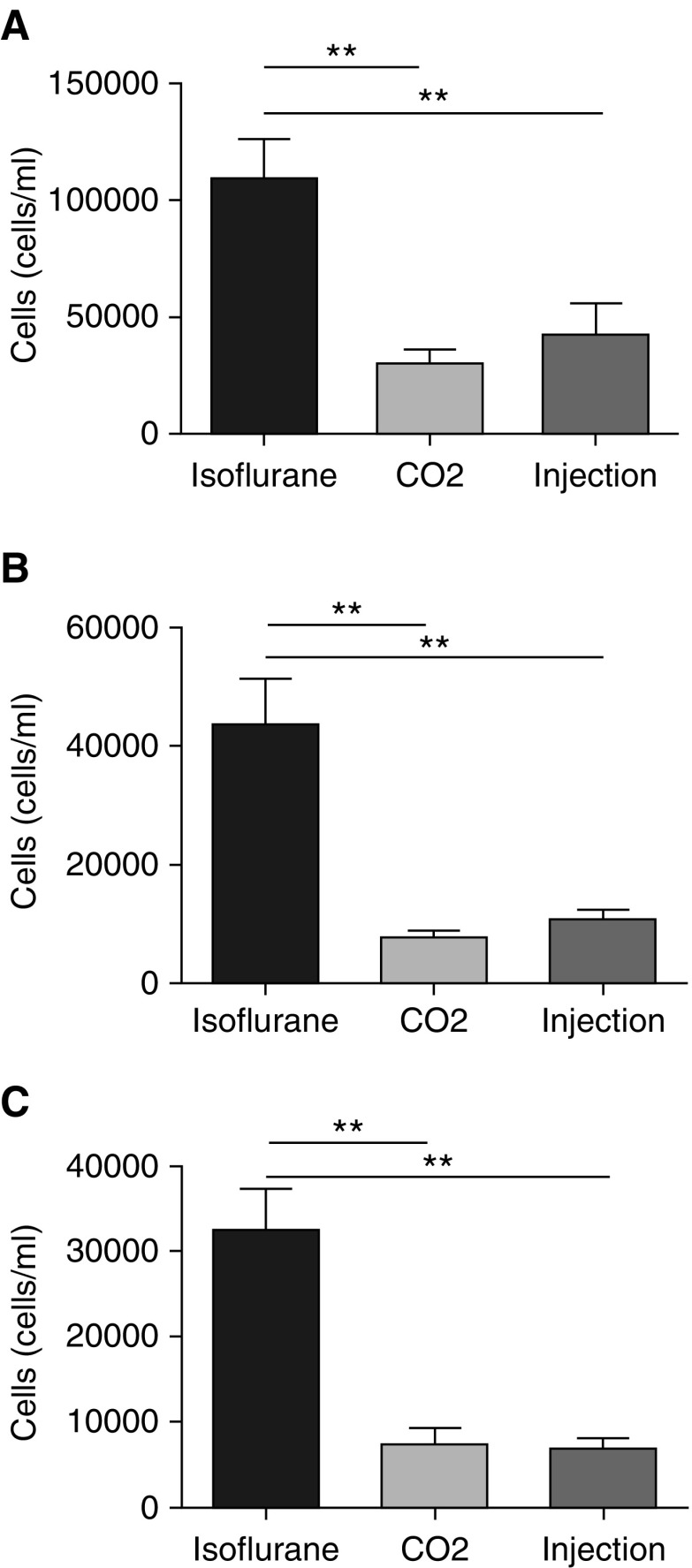
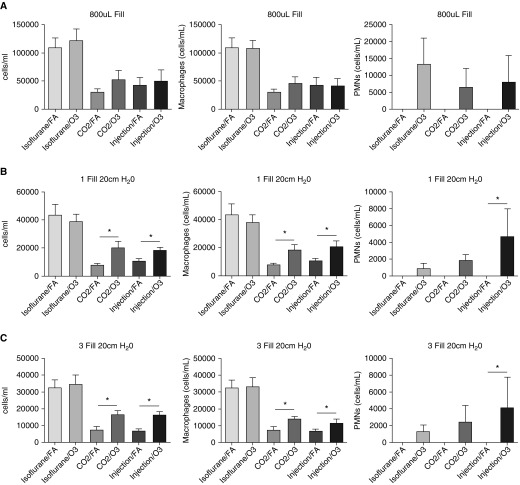
Because cell influx into the airspace is an important measure of pulmonary inflammation, we wanted to determine the effect of methods of lavage and euthanasia on this response. Cell influx is defined as the difference between experimental and control conditions. This difference can be small in models where the injury or insult is mild. In mild injury models where the biologic response is small, it is particularly critical to limit biologic variability in control animals that could reduce the ability to detect differences between the experimental group. We therefore performed experiments designed to determine how various lavage methods and euthanasia affect BAL cell counts from uninjured mice. As described in the Methods section, uninjured mice were killed after administration of isoflurane, ketamine/xylazine, or CO2. Afterward, BAL was performed by active instillation/drainage of 800 μl of PBS × 3 or by passive fill/drainage to 20 cm H2O one or three times. BAL cells were used to determine total cell counts and differentials using an automated cell counter. We observed significant differences based on the method of euthanasia. Uninjured isoflurane-killed mice had significantly higher total cell counts (Figures 2A–2C). The cell differentials and morphologies of the cells revealed these to be 100% macrophages in all of the euthanasia groups (data not shown). These data suggest that isoflurane euthanasia increased total cell numbers in uninjured BAL over other forms of euthanasia.
Figure 2.
BAL total cell counts and differentials in uninjured mice. BAL cell counts and differentials were measured in uninjured C57BL/6J male mice after euthanasia with ketamine/xylazine (injection), isoflurane, or carbon dioxide (CO2). (A–C) Three forms of BAL were performed: (A) instillation and withdrawal of 800 μl of isotonic saline performed three times, (B) passive fill and drainage to 20 cm H2O performed once, and (C) passive fill and drainage to 20 cm H2O performed three times. Cell counts were determined by an automated cell counter and differentials were determined by cytospin. Data are from n = 5 mice per group per experiment, each of which was repeated twice. **P < 0.005 between the different forms of euthanasia.
Using the same data, we also compared effects related to different methods of lavage. The results indicate that the overall number of cells recovered from lavage was increased with the 800-μl fill method compared with the one fill to 20 cm H2O and three fills to 20 cm H2O methods, respectively (Figure E1). This increase in cell numbers was identified in all groups independently of the euthanasia method used, and there was no statistical decrease in cell viability (Figure E2). Overall, these data support the conclusion that the methods of lavage altered BAL total cell numbers in uninjured mice.
Methods of Euthanasia and Lavage Alter BAL Albumin and Total Protein Measures in Uninjured Mice
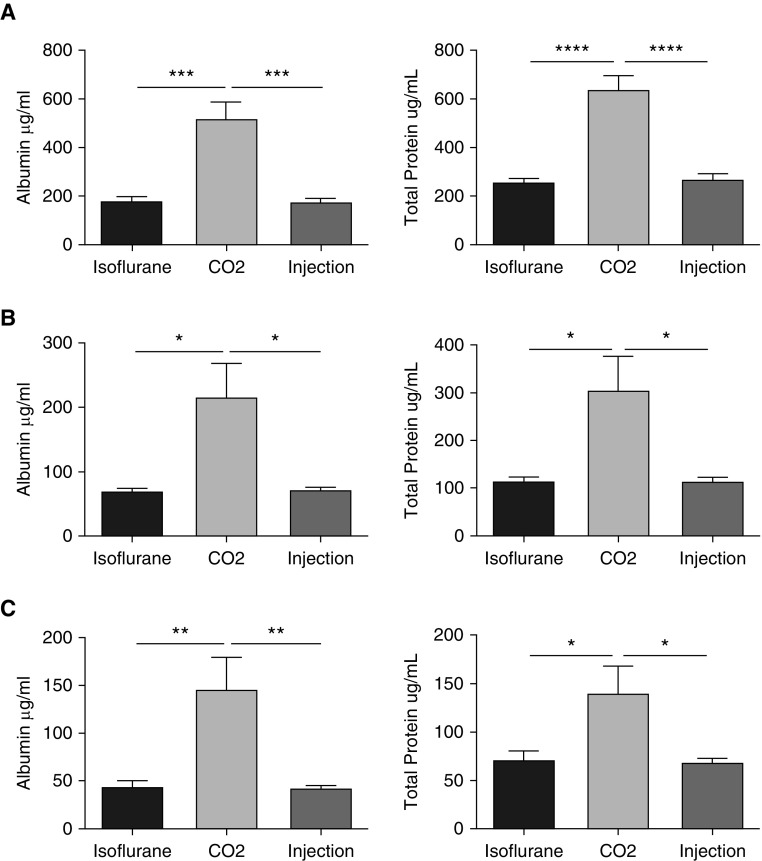
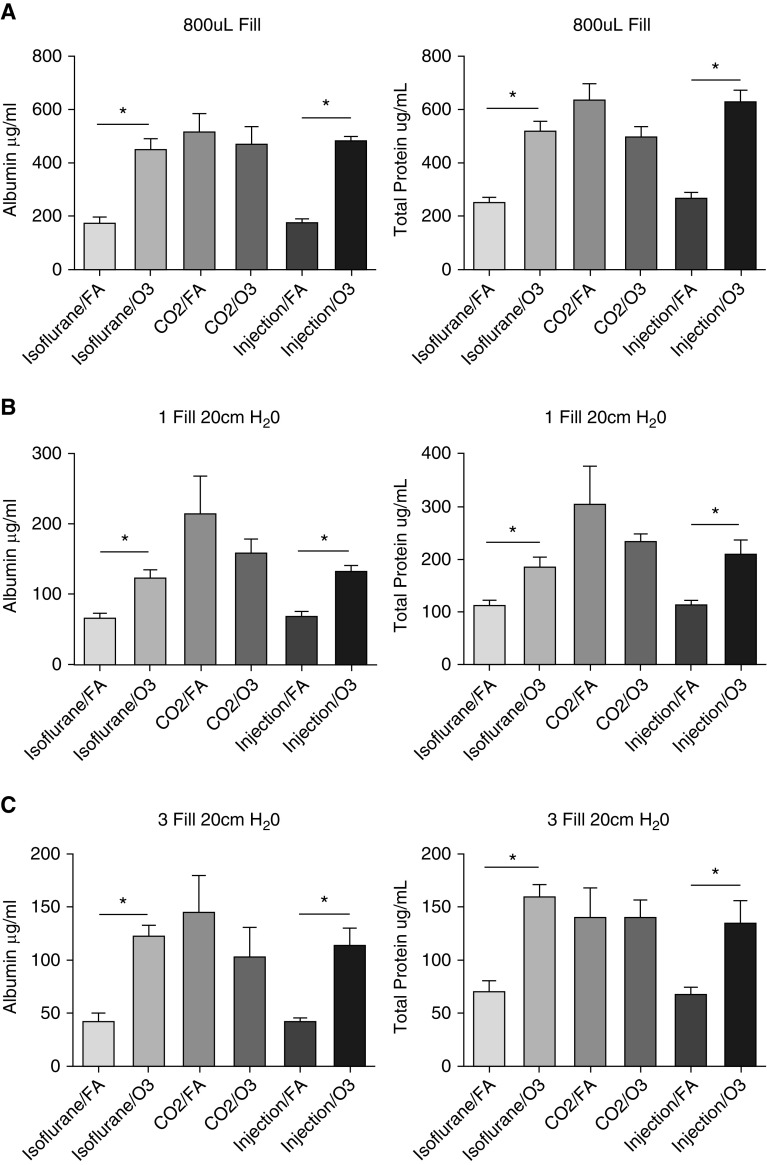
Because the total cell counts were altered based on the methods of lavage and euthanasia used, we were interested in determining the effects on other measures of lung injury, such as BAL total protein and albumin. We noted significant effects based on the method of euthanasia and/or lavage. As was the case with total cell counts, there were significant differences in the BAL total protein and albumin levels from uninjured mice based on the method of euthanasia. CO2-killed mice showed significantly increased BAL albumin compared with the ketamine/xylazine- and isoflurane-killed mice (Figure 3). Furthermore, there was greater variability in the measured values in the CO2 group, as reflected by increases in the SEM and SD that were independent of the lavage method used (Table E2). This suggests that the method of euthanasia used in uninjured mice also affects multiple measures of experimental lung injury.
Figure 3.
BAL albumin and total protein levels in uninjured mice. BAL was done and cell-free lavage fluid was used to determine albumin and total protein levels by colorimetric assays. (A–C) This was performed in uninjured C57BL/6J male mice after euthanasia with ketamine/xylazine (injection), isoflurane, or CO2, and using three forms of BAL: (A) instillation and withdrawal of 800 μL of isotonic saline performed three times, (B) passive fill and drainage to 20 cm H2O performed once, and (C) passive fill and drainage to 20 cm H2O performed three times. Data are from n = 5 mice per group per experiment, each of which was repeated twice. *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.00005 between the different forms of euthanasia.
To determine the effect of the lavage method on BAL albumin and total protein measures, we also analyzed the effects of various lavage methods (Figure E3). In the isoflurane (Figure E3A), CO2 (Figure E3B), and injection (Figure E3C) groups, the 800-μl fill method resulted in the highest levels of BAL albumin and total protein when compared with the other two lavage methods. There was a clear trend, though not significantly different in all comparisons, for the BAL albumin and total protein measures to be higher in the one-fill group than in the three fills to 20 cm H2O group. The effect of lavage appeared to be independent of the method of euthanasia in this analysis, as the same effect was observed in all of the euthanasia groups. These data suggest that the method of lavage affects the measured level of BAL albumin and total protein in uninjured mice.
Lavage and Euthanasia Methods Do Not Alter Uninjured Lung Histology
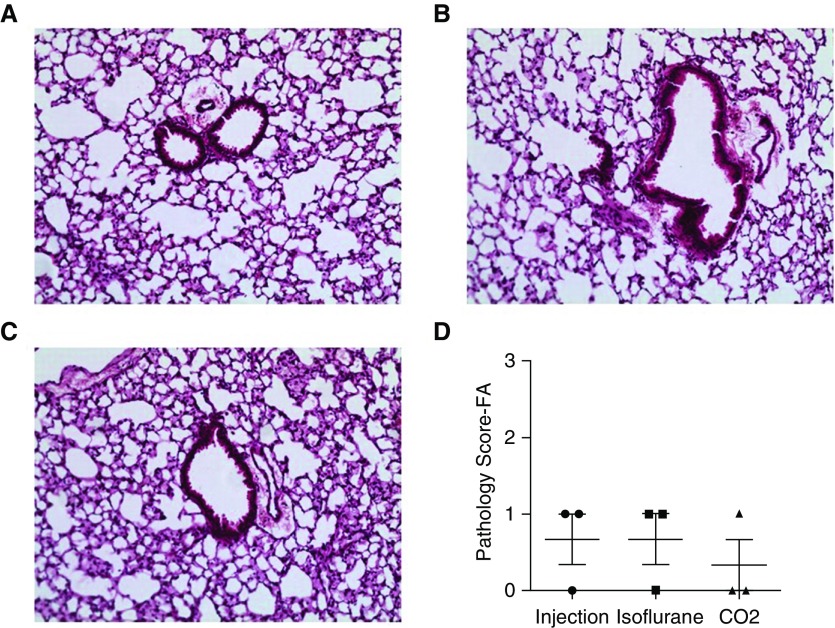
Histology is frequently used as a standard assessment in lung injury models. To determine whether alterations due to the euthanasia method were present in histology, we performed lung injury scoring on lung tissue sections from uninjured mice. Because the total cell count and albumin/total protein data suggested greater variability with the euthanasia method, we focused on the method of euthanasia as a potential histologic modifier. Tissue sections were obtained from the left lobe after different forms of euthanasia were conducted. We performed hematoxylin-and-eosin staining, and visualized tissue sections for evidence of inflammation and injury. We did not observe significant changes in the representative sections (Figures 4A–4C). To quantify this observation, we scored tissue sections in a blinded fashion based on a previously published protocol (13). There was minimal evidence of lung pathology based on scoring, and no significant difference between the groups (Figure 4D). This scoring supports the observed histology and suggests that the euthanasia or lavage-dependent effects on total cells and total protein/albumin in uninjured mice were not associated with changes in lung histology.
Figure 4.
Lung histology and scoring in uninjured mice. (A–C) Representative images of uninjured C57BL/6J mice after the different forms of euthanasia ([A] injection, [B] isoflurane, and [C] CO2). (D) Histology scoring was performed on lung tissue samples. Scoring was performed on n = 3 biologic replicates. FA = filtered air.
Alveolar Macrophages Obtained after Different Forms of Euthanasia Have Similar Responses to In Vitro PMA and LPS Stimulation
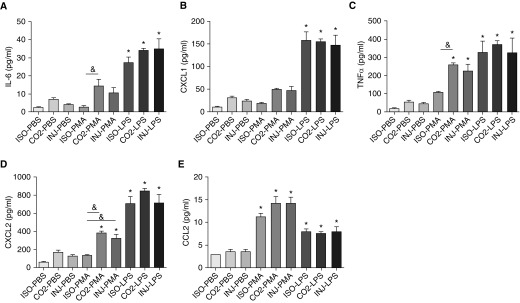
The differences in cellular inflammation and injury due to euthanasia raised the question as to whether immune cells are functionally altered by the method of euthanasia. To examine this issue, we stimulated alveolar macrophages obtained from uninjured mice after different forms of euthanasia and quantified their cytokine production. BAL was performed after isoflurane, CO2, or ketamine/xylazine euthanasia to obtain alveolar macrophages. The cells were cultured overnight and then stimulated for 4 hours with PBS, PMA (1 μM), or LPS (20 ng/ml). LPS and PMA were selected because they are widely used for in vitro stimulation of macrophages. LPS is a prototypical Toll-like receptor 4 (TLR4) agonist, whereas PMA is a specific activator of protein kinase C and therefore activates TLR4 signaling via different pathways. After stimulation, the supernatants and cells were collected for analysis. We focused on cytokines that are known to be increased after in vitro macrophage stimulation. These included IL-6, C-X-C Motif Chemokine Ligand 1 (CXCL1, also known as KC), TNF-α, CXCL2 (also known as MIP-2), and C-C Motif Chemokine Ligand 2 (CCL2, also known as MCP-1). To determine potential control-stimulated differences, we compared the PBS groups based on the euthanasia methods used (Figure E4). Although the cytokine levels were low and of uncertain biologic significance, we observed differences. Compared with isoflurane, the ketamine/xylazine and CO2 euthanasia–obtained alveolar macrophages had higher cytokine responses (Figure E4). This observation was strongest for the CO2 euthanasia–obtained alveolar macrophages. Based on these responses, we sought to determine whether there were differences in the in vitro responses to stimulation. After LPS administration, we observed no difference in the cytokine responses based on the method of euthanasia (Figure 5), which we confirmed using RT-PCR on the cultured alveolar macrophages (Figure E5). This supports the notion that the method of euthanasia does not modify the in vitro alveolar macrophage response to LPS. After PMA stimulation, there was no difference in the cytokine responses between the ketamine/xylazine and CO2 euthanasia groups (Figure 5). Trends were evident in the PMA responses between isoflurane-killed alveolar macrophages and ketamine/xylazine- or CO2-killed alveolar macrophages (Figure 5). These responses were statistically different with IL-6, TNF-α, and CXCL2 (Figure 5; bars with the & symbol denote significance). Overall, this suggests that the alveolar macrophage responses to LPS are independent of the euthanasia method, but PMA responses may be altered.
Figure 5.
Cytokine production after in vitro stimulation of alveolar macrophages obtained using different forms of euthanasia. Alveolar macrophages were obtained by BAL after administration of isoflurane (ISO), CO2, or ketamine/xylazine (INJ), cultured overnight, and then stimulated for 4 hours in PBS, 1 μM PMA, or 20 ng/ml LPS. (A–E) After stimulation, the cell culture supernatants were collected for cytokine analysis. (A) IL-6, (B) CXCL1, (C) TNF-α, (D) CXCL2, and (E) CCL2 were assessed by multiplex ELISA. BAL macrophages were pooled from n = 10 mice per method of euthanasia for in vitro stimulations. Each stimulation was performed in triplicate. *P < 0.05 between PBS and euthanasia method–matched stimulation groups; &P < 0.05 between cells with the same stimulation but different forms of euthanasia.
Methods of Euthanasia and Lavage Alter the Ability to Assess BAL Measures of Mild Lung Injury after Acute O3 Exposure
Given our observations that methods of euthanasia and lavage affected measures of inflammation and injury in uninjured mice, we were interested in determining whether this would limit our ability to define pulmonary inflammation and injury in experimental lung injury models. Because we hypothesized that this variability would be most problematic in mild injury models, we focused on a noninfectious and environmentally relevant injury model using O3. O3 is a gas that forms via a chemical reaction between oxides of nitrogen and volatile organic compounds in the presence of sunlight. As a criterion pollutant, it is an important cause of air pollution, with known effects on both pulmonary inflammation and injury (14–17). We performed whole-body exposures in C57BL/6J mice to FA or O3 at 2 ppm for 3 hours. At 24 hours after exposure, we performed BAL to obtain measures of lung inflammation and injury. Consistent with our evaluations in uninjured mice, we found that the method of lavage affected the FA total cell counts, with the 800-μl method having the highest cell numbers, followed by the one- and three-fill gravity methods, respectively (Figure 6). This was not associated with any change in cell viability between the lavage groups (Figures E2A–E2C). We determined that compared with the other lavage methods, it was more difficult to detect differences between FA and O3 exposures in the 800-μl group (Figures 6A–6C). This suggests that after O3 exposure, passive fill methods may be more appropriate for determining total cell counts than the active method tested in this study. Differences were also appreciated based on the method of euthanasia. We observed that the higher basal total cell counts in the isoflurane euthanasia group did not allow for definition of an O3 BAL cell response (Figures 6A–6C). This is despite evidence of a PMN influx in all of the euthanasia groups. In the ketamine/xylazine and CO2 euthanasia groups, we were able to detect reproducible cellular differences between the FA and O3 exposures, and this was most prominent with BAL macrophages.
Figure 6.
Ozone (O3) total cell counts and differentials based on the method of lavage or euthanasia. Male C57BL/6J mice underwent FA or O3 exposure at 2 ppm for 3 hours. At 24 hours after exposure, the mice were killed by isoflurane, CO2, or ketamine/xylazine injection, and underwent BAL (800 μL fill, one fill to 20 cm H2O, and three fills to 20 cm H2O). (A–C) BAL cells were analyzed for total cell counts and differentials based on the method of euthanasia or lavage. Data from n = 5 mice per lavage group per method of euthanasia, each of which was repeated once. *P < 0.05 between the FA and O3 groups. PMNs = polymorphonuclear cells.
We performed a similar analysis for albumin and total protein (Figure 7). As opposed to the total cells and differentials, we did not observe a difference in our ability to detect O3-induced BAL albumin and total protein based on the method of lavage. Albumin/total protein levels were highest after O3 exposure using the 800-μl lavage method, followed by the one and three passive fills, respectively. However, the delta between FA and O3 based on these lavage methods remained similar. Significant differences were observed based on the method of euthanasia. Due to the increased FA BAL albumin/total protein, we were unable to define O3 exposure responses in the CO2 euthanasia group. In contrast, differences were appreciated between FA and O3 in the isoflurane and ketamine/xylazine groups. Therefore, after O3 exposure, CO2 euthanasia limits one’s ability to detect an O3-induced enhancement of BAL albumin and total protein.
Figure 7.
O3 BAL albumin and total protein based on the method of lavage or euthanasia. Male C57BL/6J mice were exposed to FA or O3 exposure at 2 ppm for 3 hours. At 24 hours after exposure, the mice were killed by isoflurane, CO2, or ketamine/xylazine injection, and underwent BAL (800 μL fill, one fill to 20 cm H2O, and three fills to 20 cm H2O). (A–C) The BAL fluid was assessed for albumin and total protein based on the method of euthanasia or lavage. Data from n = 5 mice per group per lavage group per method of euthanasia, each of which was repeated once. *P < 0.05 between the FA and O3 groups.
Discussion
Accurate and reproducible measures of lung injury and inflammation in experimental animal models are critical to define causative mechanisms and identify novel therapeutics. Because assessments of injury and inflammation are typically defined as a change from a control exposure, it is critical to limit the variability in these measurements. Several potential sources of variability exist and require consideration, including biologic conditions, assay methods, and sample-collection/processing methods. High variability causes a host of experimental issues, such as a reduced ability to detect differences between control and experimental groups that either leads to erroneous conclusions or requires substantially increased numbers of animals to power experiments. In the present study, we carefully considered the effect of methods of BAL and euthanasia on baseline measures of lung injury and inflammation. We identified a significant effect of the method of euthanasia on the assessment of BAL cellular inflammation, and measures of BAL total protein and albumin. To clarify the influence of euthanasia on the assessment of lung injury in a mild injury model, we performed whole-animal O3 exposures and then assessed the effects of the lavage or euthanasia methods. The results confirmed that the method of euthanasia alters the ability to detect differences in this model system.
Although other measures of lung injury exist, the popularity and ease of BAL make it the most common initial method to screen lung injury models. Despite this popularity, however, we found that BAL methods vary substantially among investigators (Figure 1). This limits the generalizability and reproducibility of data sets between laboratories. Researchers have attempted to standardize lavage methods. Song and colleagues performed BAL using various numbers of attempts at manual administration and aspiration of 30 ml/kg of chilled saline to define the optimal number of lavages in rats (10). They concluded that three lavage attempts were optimal, and validated their observations after bleomycin injury. Bleomycin generates a robust injury response (18, 19). Given the scale of injury, subtle differences in control groups may not affect one’s ability to discriminate between experimental and control groups. Alternatively, in mild injury models, the effect of variations in control measures can be augmented due to the lower injury levels. To address this concern, we performed experiments to compare methods of lavage. These included manual administration and aspiration, and either one or three passive fills (to 20 cm H2O) and drainage. We used passive gravity-fill methods because they have been used by other investigators (16, 20, 21) but have not been compared with active methods. The major difference between the two types of BAL methods is that the 800-μl manual administration and aspiration method resulted in increased total cell counts and total protein/albumin levels in both the control and experimental groups compared with the passive-fill methods. Interestingly, in the O3 exposure model, the increase in FA total cell counts with the 800-μl method limited our ability to measure O3-induced differences. This was despite evidence of BAL neutrophilia and increased total protein/albumin levels consistent with known in vivo O3 responses. These data suggest that passive fills may offer improved fidelity in mild injury models such as O3 exposure compared with the active method performed in this study.
Another important technical component of BAL in animals is the procedural use of anesthesia/euthanasia. Several different methods of euthanasia have been used by a variety of investigators, including cervical dislocation, intraperitoneal injections of ketamine/xylazine or barbiturates (such as phenobarbital), and inhaled agents such as isoflurane and CO2. To define the effect of various euthanasia methods on markers of lung inflammation and injury, we compared ketamine/xylazine, isoflurane, and CO2. We observed differences in airspace cellular influx of cells and injury in uninjured mice that depended on the the method of euthanasia utilized. Isoflurane consistently generated higher total cell counts, whereas CO2 increased total protein/albumin. These effects limited our ability to define lung inflammation and injury after O3 exposure. Overall, intraperitoneal administration of ketamine/xylazine had the least effect on uninjured/FA total cells or total protein/albumin, and provided the best ability to discriminate differences after O3 exposure. This suggests that the method of euthanasia in mild experimental lung injury models can substantially alter one’s ability to define differences between control and experimental groups.
Interestingly, several groups have studied the effects of anesthetic agents on pulmonary immune responses. The majority of these studies focused on anesthetics as a therapeutic modality, with some demonstrating a beneficial effect of large doses of ketamine (22) and either pre- or post-treatment with isoflurane (23, 24) for LPS-induced lung injury. The mechanisms of these effects are not completely clear, but they have been suggested to involve limiting reactive oxygen species–mediated inflammasome activation or preserving epithelial integrity (24, 25). Few studies have focused on how these agents alter lung injury measures with euthanasia. In the present study, we did not detect euthanasia-mediated differences in O3-induced inflammation or injury, as O3-exposed animals exhibited similar levels of neutrophil influx, cytokines, and total protein/albumin. Instead, we observed that an overdose of isoflurane substantially increased uninjured BAL total cell numbers without altering other measures of lung injury (BAL total protein/albumin, histology, and BAL cytokines). The mechanism of this effect is unclear. Given the short time interval between euthanasia and BAL, the increased number of macrophages is unlikely to be a consequence of recruitment from the circulation, proliferation of resident lung macrophages, or maturation of macrophages from monocytes. Alternatively, isoflurane could improve the release of airspace macrophages that are resistant to BAL, consistent with research suggesting that alveolar macrophages can be attached to the epithelium (26) and are poorly lavaged under homeostatic conditions (27). Despite the observation of increased BAL macrophages from uninjured mice, there was no difference in the functional response of the macrophages to in vitro LPS, a prototypical TLR4 ligand. Differences were observed in the response to PMA, an activator of protein kinase C. Further studies are required to confirm the mechanism of this observation and to clarify whether there is a difference in the cytokine responses based on the type of stimuli.
CO2 is the most commonly used method of euthanasia in rodents. This is due to its ease of use, low cost, and the fact that it can be used on large numbers of animals. It also has been used in the context of terminal experimental procedures for organ harvest. This is despite evidence that inhalation of CO2 can cause pulmonary injury (28). Danneman and colleagues demonstrated that terminal CO2 inhalation led to intra-alveolar hemorrhage and serosanguinous nasal discharge (29), and this was supported by BAL studies (9, 28). The present study supports concerns about CO2 as a method of euthanasia before lung harvest, and extends prior observations by defining this method’s potential impact on mild lung injury assessments. These data suggest that CO2 should be used with care in studies where lung injury is a primary assessment.
We should note some limitations of the current study. Although our goal was to determine whether different lavage methods affect measures of lung injury, it was not possible to test every method. Additionally, we did not test every potential combination of injectable or inhalational anesthetics. For example, as presented in Figure 1, several different active methods of lavage have been used in the literature and were not tested in the present study. Therefore, it is possible that different lavage methods or anesthetics could differentially alter measures of lung inflammation and injury. Furthermore, we only performed these studies on C57BL/6J mice. Rodent O3 responses exhibit well-known strain-specific response effects (14, 30). Similar strain-specific differences in the lavage or euthanasia method–related effects were not explored, although interstrain differences have been demonstrated (31). Therefore, strain-related variations in the response to euthanasia may require consideration by investigators during initial experimental model development. Despite these limitations, the present study supports the consideration of methods of euthanasia as an experimental variable and a potential modifier of outcomes.
In summary, we determined that lavage and euthanasia methods alter baseline measures of lung injury and inflammation. The alterations were more pronounced with certain euthanasia methods, particularly the use of isoflurane (increased total cells) and CO2 (increased total protein/albumin). Additionally, these effects limited our ability to detect differences between control and experimental groups in a mild injury model (O3). Overall, these data highlight lavage and euthanasia methods as potential experimental variables, and have important implications for power calculations in experimental lung injury models. They also emphasize the importance of accurately reporting these methods in publications to allow for greater reproducibility of results by different research groups.
Footnotes
Supported by National Institutes of Health grants R01 ES013611, K08 HL105537, and R01 ES027574 (R.M.T.), and a Health Effects Institute Walter A. Rosenblith Award (K.M.G.). The research described in this article was conducted under a contract to the Health Effects Institute, an organization jointly funded by the United States Environmental Protection Agency (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. Ozone exposures were performed using chambers in the Duke University Rodent Inhalation and Physiology Shared Resource Facility.
Author Contributions: A.B. assisted with the experimental design, performed experiments, and assisted with drafting the manuscript. M.J.Y., S.W.R., and K.M.G. performed pathology scoring of the lung tissue samples, performed the literature review, and assisted with drafting the manuscript. R.M.T. conceived of the area of investigation, led all studies, performed experiments, collected and analyzed data, and drafted the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0357OC on February 26, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JW, Free ME, Li Z, Andrews LN, Nakano H, Cook DN. Ozone activates pulmonary dendritic cells and promotes allergic sensitization through a Toll-like receptor 4-dependent mechanism. J Allergy Clin Immunol. 2010;125:1167–1170. doi: 10.1016/j.jaci.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frush BW, Li Z, Stiles JV, Cotter SF, Shofer SL, Foster WM, et al. Ozone primes alveolar macrophage-derived innate immunity in healthy human subjects. J Allergy Clin Immunol. 2016;138:1213–1215. doi: 10.1016/j.jaci.2016.03.052. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, Gregory D, Smith A, Kobzik L. MARCO regulates early inflammatory responses against influenza: a useful macrophage function with adverse outcome. Am J Respir Cell Mol Biol. 2011;45:1036–1044. doi: 10.1165/rcmb.2010-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthay MA, Howard JP. Progress in modelling acute lung injury in a pre-clinical mouse model. Eur Respir J. 2012;39:1062–1063. doi: 10.1183/09031936.00204211. [DOI] [PubMed] [Google Scholar]
- 7.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson RF. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp Toxicol Pathol. 2005;57:155–159. doi: 10.1016/j.etp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Henderson RF, Lowrey JS. Effect of anesthetic agents on lavage fluid parameters used as indicators of pulmonary injury. Lab Anim Sci. 1983;33:60–62. [PubMed] [Google Scholar]
- 10.Song JA, Yang HS, Lee J, Kwon S, Jung KJ, Heo JD, et al. Standardization of bronchoalveolar lavage method based on suction frequency number and lavage fraction number using rats. Toxicol Res. 2010;26:203–208. doi: 10.5487/TR.2010.26.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacher SE, Johnson C, Jessop F, Holian A, Migliaccio CT. Murine pulmonary inflammation model: a comparative study of anesthesia and instillation methods. Inhal Toxicol. 2010;22:77–83. doi: 10.3109/08958370902929969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AVMA. AVMA guidelines for the euthanasia of animals. 2013 edition; 2013 [accessed 2018 Jan 17]. Available from: https://www.avma.org/kb/policies/documents/euthanasia.pdf.
- 13.Gowdy KM, Madenspacher JH, Azzam KM, Gabor KA, Janardhan KS, Aloor JJ, et al. Key role for scavenger receptor B-I in the integrative physiology of host defense during bacterial pneumonia. Mucosal Immunol. 2015;8:559–571. doi: 10.1038/mi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, et al. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet. 1997;17:475–478. doi: 10.1038/ng1297-475. [DOI] [PubMed] [Google Scholar]
- 15.Kleeberger SR, Reddy S, Zhang LY, Jedlicka AE. Genetic susceptibility to ozone-induced lung hyperpermeability: role of Toll-like receptor 4. Am J Respir Cell Mol Biol. 2000;22:620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- 16.Tighe RM, Li Z, Potts EN, Frush S, Liu N, Gunn MD, et al. Ozone inhalation promotes CX3CR1-dependent maturation of resident lung macrophages that limit oxidative stress and inflammation. J Immunol. 2011;187:4800–4808. doi: 10.4049/jimmunol.1101312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 17.Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, et al. Ambient ozone primes pulmonary innate immunity in mice. J Immunol. 2007;179:4367–4375. doi: 10.4049/jimmunol.179.7.4367. [DOI] [PubMed] [Google Scholar]
- 18.Tighe RM, Liang J, Liu N, Jung Y, Jiang D, Gunn MD, et al. Recruited exudative macrophages selectively produce CXCL10 after noninfectious lung injury. Am J Respir Cell Mol Biol. 2011;45:781–788. doi: 10.1165/rcmb.2010-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY, Choeng HC, Ahn C, Cho SH. Early and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis. Yonsei Med J. 2009;50:68–77. doi: 10.3349/ymj.2009.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, et al. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem. 2009;284:11309–11317. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 22.Yang J, Li W, Duan M, Zhou Z, Lin N, Wang Z, et al. Large dose ketamine inhibits lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2005;54:133–137. doi: 10.1007/s00011-004-1334-5. [DOI] [PubMed] [Google Scholar]
- 23.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–517. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Yin N, Peng Z, Li B, Xia J, Wang Z, Yuan J, et al. Isoflurane attenuates lipopolysaccharide-induced acute lung injury by inhibiting ROS-mediated NLRP3 inflammasome activation. Am J Transl Res. 2016;8:2033–2046. [PMC free article] [PubMed] [Google Scholar]
- 25.Englert JA, Macias AA, Amador-Munoz D, Pinilla Vera M, Isabelle C, Guan J, et al. Isoflurane ameliorates acute lung injury by preserving epithelial tight junction integrity. Anesthesiology. 2015;123:377–388. doi: 10.1097/ALN.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehn B, Bruch J, Zou T, Hobusch G. Recovery of rat alveolar macrophages by bronchoalveolar lavage under normal and activated conditions. Environ Health Perspect. 1992;97:11–16. doi: 10.1289/ehp.929711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conlee KM, Stephens ML, Rowan AN, King LA. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim. 2005;39:137–161. doi: 10.1258/0023677053739747. [DOI] [PubMed] [Google Scholar]
- 29.Danneman PJ, Stein S, Walshaw SO. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci. 1997;47:376–385. [PubMed] [Google Scholar]
- 30.Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, et al. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol. 2004;31:69–77. doi: 10.1165/rcmb.2003-0001OC. [DOI] [PubMed] [Google Scholar]
- 31.Fisher S, Burgess WL, Hines KD, Mason GL, Owiny JR. Interstrain differences in CO2-induced pulmonary hemorrhage in mice. J Am Assoc Lab Anim Sci. 2016;55:811–815. [PMC free article] [PubMed] [Google Scholar]