Abstract
Cigarette smoke (CS) affects DNA damage and cellular senescence signaling pathways in the pathogenesis of chronic obstructive pulmonary disease (COPD). p16INK4a (p16: a cyclin-dependent kinase inhibitor) is a key marker of cellular senescence, which is induced by CS in lung cells. It is thought that removal of p16 attenuates premature aging by removing senesced cells. However, the role of p16 in CS-induced stress-induced premature senescence (SIPS) and senescence-associated secretory phenotype (SASP) during the development of COPD/emphysema is not known. We hypothesize that p16 regulates cellular senescence and DNA damage/repair molecular signaling targets during chronic CS–induced inflammation and airspace enlargement in mouse models of COPD. We used p16 global knockout (KO) and p16 lung epithelial cell–specific KO (p16CreCC10) mice to determine whether p16 removal in lung epithelium augments or protects against cellular senescence (SIPS and SASP) in chronic CS– and elastase-induced development of COPD/emphysema in mice. p16 KO mice exposed to chronic CS and p16 lung epithelial cell–specific KO mice exposed to elastase did not show attenuation of lung inflammation, altered lung function, or airspace enlargement. p16 KO and p16CreCC10 exposed to CS and elastase showed increases in lung senescence-associated β-galactosidase activity. Thus, removal of p16-positive cells did not protect against airspace enlargement and decline in lung function induced in COPD mouse models. Our findings suggest that p16 is not the only key player associated with CS-induced cellular senescence phenotypes (SIPS and SASP), decline in lung function, and airspace enlargement in COPD/emphysema.
Keywords: cigarette smoke, p16, DNA damage, cellular senescence, COPD
Chronic obstructive pulmonary disease (COPD) is the third leading cause of chronic morbidity and mortality, both in the United States (affecting an estimated 23 million people) and globally. Cigarette smoke (CS), the most important etiological risk factor for the development of COPD, has been shown to cause DNA damage and cellular senescence, leading to premature and accelerated lung aging, predominantly due to lung parenchymal destruction and decline in pulmonary function (1–3). Cellular senescence is a state of irreversible growth arrest, which impairs tissue repair and contributes to the aging process. A link between cellular senescence and premature lung aging has been proposed in the pathogenesis of COPD. Previous reports have shown a critical role of cellular senescence during the development of COPD/emphysema (4–6), but the precise molecular mechanism is not yet known.
The senescent cells are prone to secrete proinflammatory mediators, a phenomenon termed “senescence-associated secretory phenotype” (SASP), which may form a positive loop that reinforces stress-induced cellular senescence (7–11). Lung cellular senescence and inflammatory response are the key events in the pathogenesis of COPD. p16INK4a (a cyclin-dependent kinase inhibitor) is a novel target shown to be associated with cellular senescence in vitro and in vivo. Removal of p16 is shown to delay cellular senescence in progeroid aging mice (12, 13). However, it remains unclear whether p16 induction is responsible for CS-induced lung cellular senescence, and its role in the development of COPD/emphysema is not known. In addition, it remains unclear whether p16 global deletion or targeted deletion in the lung epithelium could augment and or/attenuate against CS-mediated stress–induced premature senescence (SIPS) and SASP during the pathogenesis of COPD/emphysema.
Human studies have shown that telomere attrition (in leukocytes) is associated with decline in lung function and accelerated aging in smokers and patients with COPD (14, 15). We have recently shown that CS exposure increases the levels of p16, p21, p53 (prosenescence markers), and γH2AX phosphorylation (DNA damage marker) in human lung epithelial cells and fibroblasts in vitro, acute/chronic CS–exposed mouse lungs in vivo, as well as in lungs of patients with COPD (16–21). However, it is not known whether p16 induction or p16 removal from lung cells has an explicit role in CS-induced DNA damage and cellular senescence during the development of COPD/emphysema. We hypothesized that induction of prosenescence targets, such as p16, in mouse models of COPD/emphysema augments cellular senescence mechanisms contributing to premature lung aging. Furthermore, we hypothesize that p16 plays an important role in the regulation of CS-induced cellular senescence (SIPS and SASP) during the pathogenesis of COPD. We show here that p16 and p21 levels were induced in chronic CS–exposed mouse lungs with COPD/emphysema. CS caused impaired DNA damage response, which augments chronic CS–induced cellular senescence. In this study, we used mouse models of COPD/emphysema (CS, N-formyl-methionyl-leucyl-phenylalanine [fMLP], and elastase) and novel transgenic mouse strains (p16 global deletion [p16 knockout (KO)] and p16 lung epithelial cell–specific [p16 Cre-CC10] KO mice). Our data clearly demonstrates that deletion of p16, either globally or specifically in the lung epithelium, was not sufficient to mediate protection against SIPS and SASP, suggesting that the involvement of other cellular senescence mechanisms together play an essential role in the pathogenesis of COPD/emphysema.
Methods
See details on the methods in the data supplement.
All the experimental details for mouse strains used in this study are presented in detail in the data supplement, and include the following: mouse models of COPD/emphysema (CS, fMLP, and elastase exposures); measurement of lung mechanics; BAL; labeling of BAL cells for flow cytometry; protein extraction from lung tissues and quantification; Western blot analysis; immunohistochemistry; lung morphometry; proinflammatory cytokine analysis in BAL fluid; measurement of senescence-associated β-galactosidase (SA-β-gal) activity; cellular senescence gene expression panel by nanoString nCounter; and statistical analysis.
p16 Global KO and p16 Lung Epithelial Cell–Specific Conditional KO Mice
Ink4a/Arf-null (B6) mice (B6.129-Cdkn2atm1Rdp/Nci) were obtained from the National Cancer Institute (stock no. 01XB1) (22). Both p16 heterozygous and homozygous KO mice were used. p16CreCC10 mice (club cell–specific p16 deletion) were generated by crossing p16fl/fl mice (p16 conditional KO) (23), recovered from cryopreserved embryos from the Jackson Laboratory provided by Dr. Norman E. Sharpless (stock no. 905917; The University of North Carolina, Chapel Hill) with mice expressing the Cre recombinase transgene under the control of the CC10 promoter (C57BL/6J; obtained from T. J. Mariani, Ph.D., University of Rochester) (see details described in the data supplement).
Mouse Models of COPD/Emphysema
1) CS exposure: acute/chronic CS exposure
p16 KO, wild-type littermates, and p16fl/fl and p16CreCC10 mice were bred and maintained with a 12-hour light/dark cycle in the vivarium facility of the University of Rochester. For studies involving 3 days or 10 days (acute) and 6 months (chronic) exposures, research-grade cigarettes (3R4F; University of Kentucky, Lexington, KY) were used to generate CS. Mice were exposed to CS according to the Federal Trade Commission protocol (1 puff/min of 2-s duration and 35-ml volume) with a Baumgartner-Jaeger CSM2072i automatic CS generating machine (CH Technologies) (24, 25) (see details described in the data supplement).
2) fMLP exposure
It is also known that p16 deletions in mice develop spontaneous tumorigenesis (22, 26). Therefore, we considered using the fMLP-induced airspace enlargement model (in 21 d) to investigate the role of p16 deletion, which mimics chronic CS–induced emphysema (27, 28) (see details described in the data supplement).
3) Elastase exposure
Mice were suspended at 50–60° by securing the upper incisor teeth to the board after being anesthetized with ketamine (100 mg/kg intraperitoneally). The MicroSpray tip (Penn-Century Inc.) was endotracheally inserted, and 50–100 μl of saline alone or saline containing 1 U of porcine pancreatic elastase (Sigma-Aldrich) was sprayed into the trachea, as described previously (21, 29) (see details described in the data supplement).
Measurement of Lung Mechanics
Lung mechanical properties, including lung compliance, resistance, and elastance, were determined as described previously (21, 29) (see detailed descriptions of all other methods in the data supplement).
Statistical Analysis
Statistical analysis of significance was calculated using one-way ANOVA for multigroup comparisons (Tukey’s multiple comparison test) using GraphPad Prism 7 (GraphPad Software Inc.). The results are shown as the mean (±SEM). P less than 0.05 was considered as statistically significant.
Results
Chronic CS Exposure Augments Cellular Senescence Markers and Reduces DNA Repair Protein in the Lungs
Chronic CS–exposed wild-type mice showed a significant increase in the abundance of p16 and p21 in lung homogenates compared with air-exposed control mice (Figures 1A and 1B). The induction of cellular senescence markers in lung homogenates correlates with increased DNA damage and reduced DNA repair protein. Immunoblot analysis confirmed significant reduction in the levels of nonhomologous end joining DNA repair protein, Ku70, and increase in γH2AX phosphorylation in chronic CS–exposed mice compared with air-exposed control mice (Figures 1A and 1B). This was associated with increased cellular senescence, as measured by SA-β-gal in chronic CS–exposed wild-type (WT) mice compared with air-exposed control mice (data not shown), consistent with our previous report (21). In addition, we also measured p16 expression in the lung epithelium by immunohistochemical staining. Chronic CS exposure significantly induced expression of p16 in the mouse bronchial epithelium, as confirmed by an increase in the staining score performed semiquantitatively in a blinded manner compared with air-exposed controls, which corroborates the immunoblot analysis for p16 in lung homogenates (Figures 1C and 1D). p16INK4a abundance was further confirmed using other targeted antibodies (data not shown). This finding allows us to generate p16 lung epithelial cell–specific conditional KO mice (p16CreCC10) to test our hypothesis.
Figure 1.
Chronic cigarette smoke (CS) exposure induces cellular senescence markers and alters expression of DNA damage response targets in mouse lung. Cellular senescence (p16 and p21) and DNA damage/repair markers (γH2AX and Ku70) in mouse lung were measured in C57BL/6J mice exposed to chronic CS. (A) Abundance of p16, p21, Ku70, and γH2AX (S139) (DNA damage) were assessed in the whole-lung homogenate by immunoblot analysis. GAPDH/β-actin was used as a loading control. (B) The band intensity was measured by densitometry, and data are shown as fold change relative to GAPDH/β-actin control. (C) Representative images of p16 expression in airway epithelium from chronic air– and CS-exposed mouse lung. Arrows indicate immunostaining for p16 dark brown color in airway epithelial cells. Insets show magnified areas. (D) Histogram for immunostaining shows the average staining score for p16 expression in the bronchial region of chronic air– and CS-exposed mouse lung determined semiquantitatively in a blinded manner. Data are shown as mean (±SEM) (n = 3–6/group). **P < 0.01 significant compared with air group control.
Acute 10-Day CS Exposure to p16 KO Mice Shows Attenuation of Inflammatory Cellular Influx, but Not Proinflammatory Cytokines in the Lungs
p16 KO mice exposed to acute/short-term (10-d) CS showed significant reduction in inflammatory cellular influx (neutrophil counts and total cell counts) in the BAL fluid (see Figure E1A in the data supplement). We next determined whether the reduction in inflammatory cellular influx in BAL fluid was associated with reduced proinflammatory mediators in acute 10-day exposed p16 KO and WT mice. We found that reduction in inflammatory cells in p16 KO mice was not associated with decreased proinflammatory cytokine levels in BAL fluid in p16 KO CS-exposed compared with p16 KO air-exposed control (Figure E1B). p16 KO mice exposed to acute CS showed significant increase in monocyte chemoattractant protein-1 (MCP-1) and IL-6 measured in BAL fluid. WT mice exposed to CS showed a modest increase in MCP-1, IL-6, macrophage inflammatory protein-2 (MIP-2), and keratinocyte chemoattractant (KC) cytokines/chemokines compared with p16 KO CS-exposed mice (Figure E1B). Acute CS exposure for 10 days did not affect the SA-β-gal activity measured in lung homogenates from either WT or p16 KO mice (Figure E2A).
p16 Deletion Does Not Protect against Chronic CS–induced Lung Proinflammatory Cytokine Release and Airspace Enlargement
Despite the limitation that p16 KO mice develop early tumorigenesis in other organs, such as the spleen and liver (22, 26), we exposed p16 KO mice to chronic air and CS exposures so as to understand the role of p16INK4a in mediating CS stress–induced cellular senescence (SIPS/SASP) and emphysema in vivo. We initiated chronic CS exposure with p16 Het/KO mice (n = 11). We observed significantly high mortality in p16 KO mice (eight mice died) due to tumor development in the spleen and lymphoid organs, as reported previously (22, 26), during chronic CS exposure (data not shown). We only analyzed limited parameters (proinflammatory cytokines in lung homogenates, airspace enlargement, and gene expression by nanoString) in chronic CS–exposed p16 Het/KO mice (n = 2-3) and unable to perform lung inflammatory cell influx and lung mechanical properties in these mice.
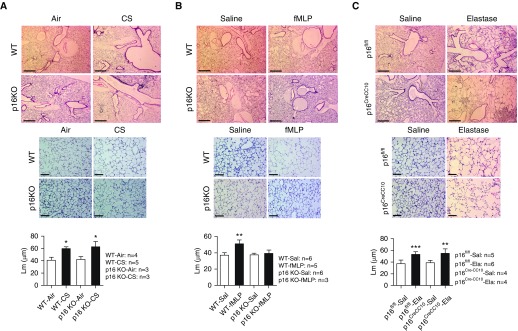
Chronic CS exposure significantly increased the proinflammatory cytokine (MCP-1 [macrophage driven] and KC [neutrophil secreted]) levels in the lung homogenates of WT and p16 KO mice as compared with their respective air-exposed controls, as measured by ELISA (Figures 3A and 3D). We did not observe any protective effect against proinflammatory cytokine release in CS-exposed p16 KO mice compared with WT CS mice. Hematoxylin and eosin–stained lung histology analysis revealed normal lung phenotype in three out of four mice in the p16 KO air group. One of the p16 KO air-exposed mice showed mild lung inflammation, whereas, in chronic CS–exposed p16 Het (2)/KO (1) mice (n = 3), two out of three showed very mild lung inflammation and one mouse showed severe lung inflammation (data not shown). Mean linear intercept (Lm) data show increased airspace enlargement in p16 KO and WT mice exposed to chronic CS compared with their respective air-exposed controls (Figure 5A). We did not observe any protection against chronic CS–induced airspace enlargement/emphysema in p16 KO mice.
Figure 3.
p16 global and lung epithelial cell–specific deletion shows increased proinflammatory response to chronic CS, fMLP, and elastase in BAL fluid. Mice were exposed to chronic CS (6 mo) or aerosolized fMLP or elastase intratracheal injection, as described in Methods. Mice were killed and proinflammatory mediators monocyte chemoattractant protein-1 (MCP-1) and keratinocyte chemoattractant (KC) in the BAL fluid were measured by ELISA after (A and D) chronic CS exposure, (B and E) fMLP aerosolization, and (C and F) elastase injection. Data are shown as mean (±SEM) (n = 3–8/group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-/Sal-exposed controls; ###P < 0.001, significant compared with WT fMLP.
Figure 5.
CS and elastase induced airspace enlargement in p16 global and lung epithelial cell–specific deletion mice, but p16 deletion protected against fMLP–induced airspace enlargement. (A) WT and p16 KO mice exposed to chronic CS and (B) WT mice exposed to aerosolized fMLP after 21 days show increased airspace enlargement measured as mean linear intercept (Lm), but not in p16 KO mice. (C) p16fl/fl and p16CreCC10 mice treated with intratracheal elastase injection after 21 days show increased airspace enlargement compared with respective controls. Representative images of hematoxylin and eosin–stained lung sections were provided. Top panel: original magnification, ×40. Scale bars: 500 μm. Middle panel: original magnification, ×200. Scale bars: 100 μm. Data are shown as mean (±SEM) (n = 3–6/group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-/Sal-exposed controls.
Cellular Senescence–associated Genes Analyzed by nanoString in WT and p16 KO Air or CS Groups
Based on the literature and current knowledge from animal models of cellular senescence, we designed a panel of 41 different mouse cellular senescence genes for differential expression analysis by nanoString nCounter (Table E1). Boxplots were generated using the boxplot function in R, which shows the distribution of normalized transcript levels from different experimental groups (Figure E3A). A heatmap was generated using the heatmap.2 function in the gplot package in R. We removed two genes (RGN and KL) from the heatmap, as their counts were too low for most of the experimental groups. The heatmap shows normalized transcript levels from 39 different cellular senescence genes analyzed from mouse lungs by nanoString nCounter (Figure E3B). WT CS-exposed mice showed significant increases in mRNA transcript levels of key cellular senescence genes, such as MMP12, CCL2, CDKN2A, TERT, and BUB1B, compared with mice in the WT air group (Figure E4). Similarly, when we analyzed p16 KO CS-exposed mice, MMP12, CDKN2A, and PRKAA2 were significantly increased and PCNA and TERC were significantly decreased compared with p16 KO air-exposed mice (Figure E4). In addition, we compared p16 KO air-exposed mice with WT air-exposed mice, which showed significant increases in several target genes, such as BUB1B, CAMP, PCNA, TERC, TERT, CCL2, H2AFX, and EZH2 (Figure E5). There were other target genes, such as HSPA4, HDAC2, TP53BP1, HMGB1, TERF2, CXCL8, PIK3CA, PRKAA2, and PARGC1A, that were significantly downregulated in p16 KO air-exposed mice compared with WT air-exposed mice (Figure E5). Finally, when we analyzed p16 KO CS-exposed mice compared with WT CS mice, only HDAC2 was significantly downregulated (Figure E5). In addition, we included scatter plots for selected cellular senescence genes identified by nanoString that could possibly play an important role in chronic CS–induced cellular senescence (Figure 6).
Figure 6.
p16 global and lung epithelial cell–specific deletion mice exposed to fMLP and elastase show no effect on senescence-associated β-galactosidase (SA-β-gal) activity in the lung. SA-β-gal activity was measured in lung homogenates (after 1 and 3 h of incubations) from (A) WT and p16 KO mice exposed to aerosolized fMLP after 21 days, and (B) both p16fl/fl and p16CreCC10 mice treated with Ela after 21 days. Data are shown as mean (±SEM) (n = 6–10/group).
p16 Deletion Shows Protection against fMLP-induced Lung Proinflammatory Response, Lung Function, and Airspace Enlargement
Due to the limitations mentioned previously here, using p16 global KO mice, we employed another model of emphysema (i.e., the fMLP-mediated emphysema model). This is based on the previous findings that fMLP can induce airspace enlargement in mice (17, 27, 28). We found that fMLP exposure caused a significant increase in neutrophil percentage and total cell counts in p16 KO mice compared with p16 KO saline-exposed controls (Figures 2A and 2E). Total cell counts, with the exception of the percentage of neutrophils, were significantly increased in WT fMLP-exposed mice compared with WT saline-exposed control mice (Figures 2A and 2E). The percentage of lymphocytes remained significantly unaltered in both the WT and p16 KO fMLP groups compared with saline-exposed controls (Figure 2G). In addition, the percentage of macrophages was significantly reduced in the p16 KO fMLP group compared with the WT fMLP and p16 saline-exposed controls, which correlates with increased neutrophil percentages observed in the p16 KO-fMLP group (Figures 2C and 2E). p16 KO mice exposed to fMLP showed a significant increase in MCP-1 levels compared with both p16 KO saline- and WT fMLP-exposed mice (Figure 3B). When we analyzed KC levels, WT fMLP-exposed mice showed significant increases in KC release compared with WT saline- and p16 fMLP-exposed mice (Figure 3E).
Figure 2.
p16 global and lung epithelial cell–specific deletion shows increased inflammatory cellular influx in response to N-formylmethionyl-leucyl-phenylalanine (fMLP) and elastase in BAL fluid. Mice were exposed to aerosolized fMLP or elastase intratracheal injection, as described in Methods. After 21 days from first exposure, mice were killed and BAL fluid was used for differential cell counts. (A and B) Total cell counts per milliliter in BAL fluid were determined by acridine orange and propidium iodide staining using cellometer. Percentages of (C and D) F4/80+ macrophages, (E and F) LY6B.2+ neutrophils, and (G and H) CD8a+ lymphocytes were determined by flow cytometry. Data are shown as mean (±SEM) (n = 4–7/group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective saline (Sal)-exposed controls; ##P < 0.01, significant compared with wild-type (WT) fMLP. Ela = elastase; KO = knockout.
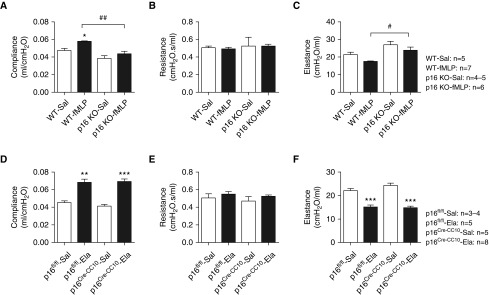
p16 KO mice exposed to fMLP showed protection against altered lung mechanical properties compared with WT mice. fMLP exposure in WT mice significantly increased lung compliance and decreased lung elastance compared with WT saline-exposed mice (Figures 4A and 4C), whereas p16 KO saline- and fMLP-exposed mice did not show alterations in lung compliance and elastance (Figures 4A and 4C). When we compared lung mechanical properties between the WT fMLP- and the p16 KO saline/fMLP group, lung compliance was significantly reduced in both p16 KO saline/fMLP (Figure 4A). Similarly, the elastance measured in WT fMLP compared with the p16 KO saline/fMLP group were significantly increased (Figure 4C). The lung resistance was not affected in both saline- and fMLP-exposed WT and p16 KO mice (Figure 4B).
Figure 4.
p16 global and lung epithelial cell–specific deletion shows differential response to lung mechanics after fMLP aerosolization and elastase injection. (A) Lung compliance, (B) resistance, and (C) elastance were measured in WT and p16 KO mice exposed to aerosolized Sal/fMLP after 21 days. Similarly, (D) lung compliance, (E) resistance, and (F) elastance were measured in p16fl/fl and p16CreCC10 mice treated with Sal/Ela injection after 21 days. Data are shown as mean (±SEM) (n = 3–8 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-/Sal-exposed controls; #P < 0.05, ##P < 0.01, significant compared with WT fMLP.
WT fMLP-exposed mice showed a significant increase in airspace enlargement/emphysema measured as Lm, suggesting more alveolar destruction in the lungs compared with WT saline-exposed controls (Figure 5B), whereas p16 KO mice exposed to fMLP showed attenuation in airspace enlargement compared with p16 KO saline-exposed controls (Figure 5B). There is no evidence that fMLP induces cellular senescence in the lungs. We, for the first time, determined if fMLP exposure in p16 deletion can augment/attenuate cellular senescence in the lungs. We did not observe any significant change in the SA-β-gal activity measured in lung homogenates from saline- and fMLP-exposed WT and p16 KO mice (Figure 6A).
Acute 3-Day CS Exposure to p16 Lung Epithelial Cell–Specific Conditional KO Shows Attenuation of Inflammatory Cellular Influx and Proinflammatory Cytokines in the Lungs
p16CreCC10 mice exposed to acute CS showed significant reduction in inflammatory cellular influx (neutrophil and total cell counts) in the lungs compared with p16fl/fl mice (Figure E7A). This reduction in inflammatory cellular influx observed in BAL fluid was associated with suppression of selective proinflammatory cytokines (SASP: MCP-1 and KC) in BAL fluid of CS-exposed p16CreCC10 compared with p16fl/fl mice (Figure E7B). We further determined whether cell-specific deletion of p16 can protect against acute CS–induced cellular senescence. Acute 3-day CS exposure did not show an increase in SA-β-gal activity measured in lung homogenates from p16fl/fl and p16CreCC10 compared with their respective air-exposed controls (Figure E2B). It is possible that lung epithelial cell–specific removal of p16 does not have any bearing on acute CS–induced cellular senescence response.
p16 Lung Epithelial Cell–Specific Conditional KO Did Not Show Protection against Elastase-induced Lung Proinflammatory Response, Lung Function, and Airspace Enlargement
Elastase exposure significantly increased the percentage of T lymphocytes and modestly increased percentage of neutrophils recruitment into the lungs in p16CreCC10 mice compared with p16fl/fl saline controls (Figures 2F and 2H). Similarly, elastase exposure increased the total cell counts in both p16fl/fl and p16CreCC10 mice compared with their respective saline controls (Figure 2B). Although the neutrophils (%) were not significantly increased, this contributes to the reduction in the percentage of macrophages observed in BAL fluid after elastase exposure in p16fl/fl and p16CreCC10 mice compared with their respective saline-exposed controls (Figures 2D and 2F). p16CreCC10 mice exposed to elastase did not show augmented proinflammatory response compared with elastase-exposed p16fl/fl mice. Both p16fl/fl and p16CreCC10 exposed to elastase showed significant increases in MCP-1 and KC levels, as measured by ELISA, compared with their respective saline-exposed controls (Figures 3C and 3F).
p16CreCC10 and p16fl/fl mice exposed to elastase showed significant increases in lung compliance compared with their respective saline-exposed controls (Figure 4D). As expected, we found that elastase exposure in p16CreCC10 and p16fl/fl mice significantly reduced lung elastance in comparison with their respective saline-exposed controls (Figure 4F). However, we did not observe any significant alteration in lung resistance among p16CreCC10 and p16fl/fl exposed elastase and saline-exposed controls (Figure 4E). Elastase exposure caused significant increases in airspace enlargement/emphysema in p16CreCC10 and p16fl/fl mice, as measured by lung morphometry analysis (Figure 5C). Our data suggest that p16 deletion in lung epithelial cells did not protect against elastase-induced airspace enlargement. We found a modest increase in SA-β-gal activity from elastase-exposed p16CreCC10 and p16fl/fl mice compared with their respective saline-exposed controls (Figure 6B). Overall, these data suggest that p16 deletion in lung epithelium did not protect against elastase-induced inflammatory cellular influx, proinflammatory cytokines (SIPS and SASP), lung mechanical properties, airspace enlargement, or premature lung aging.
Discussion
CS has been shown to cause DNA damage and cellular senescence, leading to premature aging of the lung (5, 6, 30). We and others have shown that abundance of p16, p21, and p53 were increased by CS exposure in lung epithelial cells and fibroblasts in vitro, mouse lungs, and lungs from smokers and patients with COPD (5, 6, 16, 17, 20, 21, 30–33). The role and mechanism underlying SIPS and SASP in development of COPD/emphysema are unknown. Cyclin-dependent kinase inhibitor (p16INK4a) is induced during cellular senescence, and is considered as a marker of cells undergoing cellular senescence. The role of p16 in CS-induced cellular senescence in vivo in mouse models is nebulous. Furthermore, it is thought that deletion of p16 will rescue cells undergoing senescence, and may provide protection against lung-injurious/emphysematous response. It has been shown that upregulation of p16INK4a and p19ARF is associated with cellular senescence, which is linked to SASP and inflammatory response (2, 7, 34), as well as decline in lung function (35). In this study, we examined the role of p16 in CS-induced inflammatory responses, whether p16 removal/deletion would rescue lung cells against CS-induced SIPS (senescence-associated β-gal activity) and SASP (e.g., proinflammatory mediators’ release) in lung cells.
p16INK4a is involved in irreversible and permanent cell cycle arrest during DNA damage response observed during cellular senescence. p16 upregulation limits cell proliferation induced by various stressful stimuli: oxidative damage, telomere dysfunction, and DNA damage. We hypothesized that p16 plays an important role in the regulation of CS-induced cellular senescence (SIPS and SASP) in lung cells in mouse models of COPD/emphysema. CS caused stress-induced cellular senescence which was associated with increased SA-β-gal activity, p21 and p16 expression in mouse lungs. We then used the strategy of p16 deletion/elimination of these cells in p16 KO mice exposed to CS (acute and chronic), and other valid models (fMLP and elastase) of COPD/emphysema.
As expected, chronic CS exposure resulted in an increased lung inflammatory cell influx associated with the release of proinflammatory mediators; however, p16 depletion had little or no protective effect on these responses. Similarly, airspace enlargement was not affected by p16 deletion in chronic CS exposed mice. There are some caveats in this model (e.g., p16 global KO mice develop tumors in several organs [e.g., liver, spleen, etc.]); as such, results from p16 KO must be interpreted cautiously. Due to the limiting factors stated previously here, we used another model of COPD/emphysema in p16 global KO mice. As described previously, it is known that fMLP instillation/exposure induces emphysematous responses in mouse lungs (17, 27, 28). fMLP treatment increases lung elastase burden, decreases lung elastin, and induces emphysematous responses. fMLP induced inflammatory and injurious responses in WT mice, whereas it was attenuated in p16 KO mice. Airspace enlargement/emphysema measured as Lm was markedly increased as a result of alveolar destruction by fMLP exposure in WT, which was attenuated in p16 global KO mice. These findings were surprising, which may be due to the neutrophil-derived inflammatory response, where p16 may play a role in the attenuation of lung destruction rather than other factors (CS or elastase), which are macrophage or epithelial driven. Furthermore, our observation of reduction in injurious responses may be due to the lung destruction, independent of DNA damage–initiated cellular senescence caused by fMLP.
Elastase exposure was aggressive in producing airspace enlargement in p16 lung epithelial cell–specific KO mice. Lung function, airspace enlargement (Lm), differential cell counts, and proinflammatory mediators (cytokines) in BAL fluid of p16fl/fl and p16CreCC10 mice exposed to elastase were augmented compared to saline control. These observations parallel what we observed in the chronic CS exposure model (SIPS and SASP). The involvement of p16 may be limited to the epithelial/alveolar cell cycle, and deletion of cell cycle inhibitor in the lung epithelium may lead to further augmentation of damaging/injurious responses in COPD/emphysema models. Furthermore, it may be surmised that p16 deletion in lung epithelium may be indirectly related to governing cellular senescence (as p16 may be just a marker of cellular senescence or upregulated when the cells are undergoing senescence) and/or that p16 is not the sole factor for inducing senescence in lung cells.
Our observations of chronic CS exposures and other models of emphysema are in contrast to what we observed in response to acute 10-day CS exposure in p16 KO mice. Acute CS exposure in p16 KO (10 d) and p16 CreCC10 mice (3 d) showed a significant reduction in inflammatory cellular influx (neutrophils) in the lung compared with WT or p16fl/fl mice. This was associated with suppression of specific proinflammatory cytokines in BAL fluid, but not cellular senescence, as measured by SA-β-gal activity. This raises the possibility that cell cycle inhibitors, such as p16, are involved in regulation of inflammatory response as we noticed in p21-deficient mice (17). However, chronic destruction of airspace is independent of p16 (because this occurred in lung structural cells) where cellular senescence occurred. It remains unclear whether p16 deficiency has any role during chronic CS exposure–mediated immunosenescence (senescence in immune-inflammatory cells). A previous report shows an ectopic expression of p16INK4a-mediated suppression of LPS-induced IL-6 expression in macrophages (36). In addition, it was found that senescent macrophages with physiological expression of p16 upregulated IL-6 production when p16 was targeted by siRNA (36). Our data from acute CS–exposed p16 KO mice show reduced neutrophil influx in BAL fluid, but increased release of macrophage-specific cytokines, MCP-1 and IL-6, suggesting cyclin-dependent kinase (CDK)–inhibitory proteins differentially regulate inflammatory response during acute CS–induced oxidative stress. Recent studies have provided strong evidence that nuclear CDKs support the expression of inflammatory genes (37). Strategies to therapeutically target CDK involvement in proinflammatory gene expression will allow us to devise a novel therapy against SASP mediators in chronic inflammatory lung diseases.
Prior studies have demonstrated a role for p16 in the differentiation of monocytes into inflammatory M1 macrophages, which, in turn, participates in T lymphocyte polarization toward senescence-inducing T-helper type 1 cells (38, 39). B cell senescence occurs during aging, and the expression levels of both aging markers, p16INK4a and p14/p19ARF, increase in all B cell lineages (pro-B, pre-B, and IgM+ mature B cells) (40, 41). Ectopic expression of p16INK4a and p14/p19ARF in young pro-/pre-B cells mimics the effects of aging by reducing cell growth and survival. In contrast, downregulation/knockdown of CDKN2A locus or gene promotes proliferation and predisposition of B cells to leukemogenesis (41). It has been shown in murine models that hematopoietic stem cells (HSCs) accumulate DNA damage and senescence markers as they age. It is possible that chronic CS exposure could affect the HSC replicative capacities and thus increase the overall HSC pool, with impaired differentiation potential over time and, ultimately, an effect on immune system homeostasis (42, 43).
Recently, we have shown that telomere protection protein 1 (TPP1) plays an important role in protecting CS-induced telomeric DNA damage and cellular senescence in lungs of chronic CS–exposed mice (20). Furthermore, p16INK4a exerts a protective effect against dysfunctional telomere–induced ataxia-telangiectasia mutated and Rad3-related (ATR)-dependent DNA damage response in proliferating cells (44). This suggests that p16 induction and telomeric DNA damage response are intertwined. However, our data from chronic CS–exposed p16 KO mice do not show protection against CS-induced DNA damage and cellular senescence, suggesting that p16INK4a may not be required to protect against dysfunctional telomeric DNA damage response and cellular senescence in the mouse model of COPD/emphysema. Thus, removal of p16-positive cells did not protect against airspace enlargement or decline in lung function induced in COPD mouse models. Similarly, removal of p16 in the lung epithelium did not protect mice against decline in lung function or airspace enlargement induced by elastase.
We believe that p16 KO/lung epithelial cell–specific p16 deletion in acute CS exposure models show early repair responses, such as reduced inflammatory cellular influx and proinflammatory cytokines release. During chronic CS exposure, the contribution of p16INK4a-regulated SIPS- and SASP-mediated cellular senescence, along with cell-intrinsic and cell-extrinsic factors, drives the lung aging process (45). Our findings suggest that p16INK4a plays a critical role in the regulation of lung inflammatory response, but is not associated with CS-induced cellular senescence phenotypes (SIPS and SASP) against decline in lung function and airspace enlargement in COPD/emphysema. The role of p16 in different lung cell types (airway smooth muscle cells, endothelial cells, and fibroblasts) needs to be investigated before drawing any conclusion on whether p16 is associated with CS-induced cellular senescence phenotypes (SIPS and SASP), as well as decline in lung function and airspace enlargement in COPD/emphysema.
Our nanoString mRNA transcript analysis clearly demonstrates a role of matrix metalloproteinase 12 (MMP12) in CS-exposed WT and p16 Het/KO mice during the development of CS-induced COPD/emphysema. Previously, it has been shown that MMP12 KO mice are protected against chronic CS–induced emphysema, demonstrating the role of MMP12 in the pathogenesis of COPD/emphysema (46). We found that chronic CS exposure in WT mice significantly induced cellular senescence marker, p16 (CDNK2A), confirmed by nanoString. One of the major SASP cytokine CCL2 genes (MCP-1) is significantly induced by chronic CS exposure, as confirmed in BAL fluid, and lung homogenates by ELISA was elevated at the mRNA level in lung tissues identified by nanoString. We found that chronic CS exposure increased the mRNA transcript levels of mouse mitotic checkpoint gene, BUB1B, a novel Bub1 family member, that is expressed in a cell cycle–dependent manner (47). In addition, we measured expression levels of telomere length–related genes, such as TERT, TERC, and TERF2, in chronic CS–exposed WT and p16 Het/KO mouse lungs. WT CS-exposed mice showed increased expression of TERT and decreased expression of TERF2, whereas the p16 Het/KO CS-exposed mice showed reduced expression of TERC, suggesting that differential expression of telomere length–related genes play an important role in the regulation of CS-induced cellular senescence. Together, these data suggest that the cellular senescence program induced by CS in WT mice leads to upregulation of genes involved in cellular senescence, which is further induced by CS in p16-deficient mice. However, the induction of these genes is independent of alveolar/epithelial destruction in p16-ablated mice.
Further work using imaging on p16INK4a induction (by total body luciferase imaging) in p16 luc mice will address and differentiate the involvement of the cell-dependent role of p16 in cellular senescence programing and emphysema (34). Future work using the senolytic drugs in animal models of COPD/emphysema may also be important. Senolytic drugs are currently emerging, and is based on removal of senesced cells or inhibition of pathways involved in cellular senescence (e.g., prosurvival signaling molecules, such as Bcl-xl, JAK/STAT, SIRT1, and mTOR pathways) (48–50). These drugs are known to have antiinflammatory properties (anti-SASP); prior studies have been conducted in cell lines/in vitro to assess the effects of senolytic compounds, but in vivo studies in COPD/emphysema are challenging to perform.
In conclusion, p16 deletion attenuates acute CS–induced inflammatory responses, but is not associated with clearance of senesced cells or stopping the progression of lung phenotype (airspace enlargement and lung mechanical properties), at least in the animal models of COPD/emphysema (CS/fMLP/elastase) described in this study. Hence, p16 global deletion may not be a promising approach in attenuation of COPD/emphysema as a result of several caveats observed in p16 KO mice exposed to chronic CS. Furthermore, p16-deficient mice have a higher mortality rate due to tumor development in other lymphoid organs as they age. p16 is required to maintain normal tissue homeostasis and limit tumor progression. Lack of p16 possibly increases CS-induced SIPS/SASP and systemic damage–associated molecular patterns, leading to lung inflammation, injury, and COPD/emphysema. Similarly, lung epithelial cell–specific deletion of p16 also did not show protection against elastase-induced inflammatory response or cellular senescence in mouse models of COPD/emphysema. However, the use of p16INK4a-based senescence ablation systems, such as p16-3MR (p16INK4a-trimodality reporter: 50 kb of the p16INK4a promoter driving monomeric red fluorescent protein) or INK-ATTAC (p16INK4a apoptosis through targeted activation of caspase: a small, senescence-responsive fragment of the p16INK4a [CDKN2A] promoter to drive green fluorescent protein [GFP] expression in the INK-ATTAC transgene) and/or INK-NTR mice (generated by replacing the FK506-binding protein-Casp8-IRES-EGFP segment of the INK-ATTAC transgene cassette with an EGFP-NTR fusion gene) in COPD/emphysema models would be promising (13, 51, 52). Future uses of novel senolytic drugs (e.g., tyrosine kinase inhibitor, dasatinib; flavonoid, quercetin; navitoclax [ABT-263], etc.) and possible combinations in the in vivo model of COPD/emphysema appear promising based upon data currently available from a mouse model of pulmonary fibrosis (53, 54). Another approach is to target SASP factors using a SASP modulator (rapamycin, bromodomain containing 4 [BRD4], NF-κB, or p38 inhibitors) (55–58). This may have ramifications in the development of senolytics and SASP inhibitors in COPD.
Acknowledgments
Acknowledgment
The authors thank Drs. Hongwei Yao, Meimei Yin, Tanveer Ahmad, and Chad Lerner for their technical assistance.
Footnotes
This work was supported by National Institute of Health (NIH) grants 2R01HL085613 (I.R.) and 3R01HL085613-07S1, and in part by the University of Rochester Clinical and Translational Science Award UL1 TR002001 (D.L.) from the National Center for Advancing Translational Sciences of the NIH.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: I.K.S., K.R., J.G., and I.R. conceived and designed the experiments; I.K.S., K.R., J.G., and D.L. performed the experiments and analyzed the data; I.K.S. and I.R. wrote and edited the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0390OC on February 15, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2012;9:62–63. doi: 10.1513/pats.201201-012MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karrasch S, Holz O, Jörres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102:1215–1230. doi: 10.1016/j.rmed.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Sandford A, Man P, Sin DD. Is the aging process accelerated in chronic obstructive pulmonary disease? Curr Opin Pulm Med. 2011;17:90–97. doi: 10.1097/mcp.0b013e328341cead. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F, Onizawa S, Nagai A, Aoshiba K. Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res. 2011;12:78. doi: 10.1186/1465-9921-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 6.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35:681–688. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion Nat Cell Biol 200911973–979.[Published erratum appears in Nat Cell Biol 11:1272.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:566–571. doi: 10.1164/rccm.200809-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rode L, Bojesen SE, Weischer M, Vestbo J, Nordestgaard BG. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax. 2013;68:429–435. doi: 10.1136/thoraxjnl-2012-202544. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, et al. Impaired mitophagy leads to cigarette smoke stress–induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, et al. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Respir Cell Mol Biol. 2008;39:7–18. doi: 10.1165/rcmb.2007-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao H, Sundar IK, Gorbunova V, Rahman I. P21–PARP-1 pathway is involved in cigarette smoke–induced lung DNA damage and cellular senescence. PLoS One. 2013;8:e80007. doi: 10.1371/journal.pone.0080007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundar IK, Rashid K, Sellix MT, Rahman I. The nuclear receptor and clock gene REV-ERBα regulates cigarette smoke–induced lung inflammation. Biochem Biophys Res Commun. 2017;493:1390–1395. doi: 10.1016/j.bbrc.2017.09.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad T, Sundar IK, Tormos AM, Lerner CA, Gerloff J, Yao H, et al. Shelterin telomere protection protein 1 reduction causes telomere attrition and cellular senescence via sirtuin 1 deacetylase in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2017;56:38–49. doi: 10.1165/rcmb.2016-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122:2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 23.Monahan KB, Rozenberg GI, Krishnamurthy J, Johnson SM, Liu W, Bradford MK, et al. Somatic p16(INK4a) loss accelerates melanomagenesis. Oncogene. 2010;29:5809–5817. doi: 10.1038/onc.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, et al. Cigarette smoke–mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–L1186. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- 25.Rajendrasozhan S, Chung S, Sundar IK, Yao H, Rahman I. Targeted disruption of NF-kappaB1 (p50) augments cigarette smoke–induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling. Am J Physiol Lung Cell Mol Physiol. 2010;298:L197–L209. doi: 10.1152/ajplung.00265.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 27.Cavarra E, Martorana PA, de Santi M, Bartalesi B, Cortese S, Gambelli F, et al. Neutrophil influx into the lungs of beige mice is followed by elastolytic damage and emphysema. Am J Respir Cell Mol Biol. 1999;20:264–269. doi: 10.1165/ajrcmb.20.2.3235. [DOI] [PubMed] [Google Scholar]
- 28.Cavarra E, Martorana PA, Gambelli F, de Santi M, van Even P, Lungarella G. Neutrophil recruitment into the lungs is associated with increased lung elastase burden, decreased lung elastin, and emphysema in alpha 1 proteinase inhibitor–deficient mice. Lab Invest. 1996;75:273–280. [PubMed] [Google Scholar]
- 29.Yao H, Arunachalam G, Hwang JW, Chung S, Sundar IK, Kinnula VL, et al. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc Natl Acad Sci USA. 2010;107:15571–15576. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80:59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- 32.Müller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, et al. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7:32. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyunoya T, Monick MM, Klingelhutz AL, Glaser H, Cagley JR, Brown CO, et al. Cigarette smoke induces cellular senescence via Werner’s syndrome protein down-regulation. Am J Respir Crit Care Med. 2009;179:279–287. doi: 10.1164/rccm.200802-320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorrentino JA, Krishnamurthy J, Tilley S, Alb JG, Jr, Burd CE, Sharpless NE. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J Clin Invest. 2014;124:169–173. doi: 10.1172/JCI70960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, et al. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight. 2016;1:e87732. doi: 10.1172/jci.insight.87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami Y, Mizoguchi F, Saito T, Miyasaka N, Kohsaka H. p16(INK4a) exerts an anti-inflammatory effect through accelerated IRAK1 degradation in macrophages. J Immunol. 2012;189:5066–5072. doi: 10.4049/jimmunol.1103156. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz ML, Kracht M. Cyclin-dependent kinases as coregulators of inflammatory gene expression. Trends Pharmacol Sci. 2016;37:101–113. doi: 10.1016/j.tips.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Cudejko C, Wouters K, Fuentes L, Hannou SA, Paquet C, Bantubungi K, et al. p16INK4a deficiency promotes IL-4–induced polarization and inhibits proinflammatory signaling in macrophages. Blood. 2011;118:2556–2566. doi: 10.1182/blood-2010-10-313106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signer RA, Montecino-Rodriguez E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22:3115–3120. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 43.Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Sharpless N, Chang S. p16(INK4a) protects against dysfunctional telomere–induced ATR-dependent DNA damage responses. J Clin Invest. 2013;123:4489–4501. doi: 10.1172/JCI69574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke–induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 47.Davenport JW, Fernandes ER, Harris LD, Neale GA, Goorha R. The mouse mitotic checkpoint gene bub1b, a novel bub1 family member, is expressed in a cell cycle–dependent manner. Genomics. 1999;55:113–117. doi: 10.1006/geno.1998.5629. [DOI] [PubMed] [Google Scholar]
- 48.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demaria M. Senescent cells: new target for an old treatment? Mol Cell Oncol. 2017;4:e1299666. doi: 10.1080/23723556.2017.1299666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:pii:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasdemir N, Banito A, Roe JS, Alonso-Curbelo D, Camiolo M, Tschaharganeh DF, et al. Brd4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 2016;6:612–629. doi: 10.1158/2159-8290.CD-16-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, et al. Erratum: mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1370. doi: 10.1038/ncb3243. [DOI] [PubMed] [Google Scholar]
- 57.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response–independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]