Abstract
Objectives: To develop a methodology for predicting operative times for robot-assisted radical prostatectomy (RARP) using preoperative patient, disease, procedural, and surgeon variables to facilitate operating room (OR) scheduling.
Methods: The model included preoperative metrics: body mass index (BMI), American Society of Anesthesiologists score, clinical stage, National Comprehensive Cancer Network risk, prostate weight, nerve-sparing status, extent and laterality of lymph node dissection, and operating surgeon (six surgeons were included in the study). A binary decision tree was fit using a conditional inference tree method to predict operative times. The variables most associated with operative time were determined using permutation tests. Data were split at the value of the variable that results in the largest difference in mean for surgical time across the split. This process was repeated recursively on the resultant data.
Results: A total of 1709 RARPs were included. The variable most strongly associated with operative time was the surgeon (surgeons 2 and 4—102 minutes shorter than surgeons 1, 3, 5, and 6, p < 0.001). Among surgeons 2 and 4, BMI had the strongest association with surgical time (p < 0.001). Among patients operated by surgeons 1, 3, 5, and 6, RARP time was again most strongly associated with the surgeon performing RARP. Surgeons 1, 3, and 6 were on average 76 minutes faster than surgeon 5 (p < 0.001). The regression tree output in the form of box plots showed operative time median and ranges according to patient, disease, procedural, and surgeon metrics.
Conclusion: We developed a methodology that can predict operative times for RARP based on patient, disease and surgeon variables. This methodology can be utilized for quality control, facilitate OR scheduling, and maximize OR efficiency.
Keywords: : robot-assisted, prostatectomy, operative time, cost, quality control, scheduling
Introduction
Prostate cancer (PCa) is the most common cancer for men in the United States and is one of the most expensive cancers to treat. Medical expenditures for PCa treatment amount to $1.3 billion annually, with inpatient care accounting for nearly 50% of total spending.1 Radical prostatectomy (RP) is the treatment of choice for the majority of patients with localized PCa. Robot-assisted radical prostatectomy (RARP) increased from 14% in 2004 to 80% in 2014 of RP because RARP has lower intraoperative blood loss, fewer complications, and shorter length of hospital stay than open RP.2,3 RARP has significantly higher costs, which include capital cost (USD 1.5–2 million) and annual maintenance (USD 150,000) that result in direct hospitalization cost ≥USD 2500.4–8 Attempts have been made to reduce costs associated with RARP. Ramirez et al. found that excluding high-cost energy instruments (averaging $250 per instrument per case) reduced RARP costs by 40%.7 Better scheduling and more efficient utilization of operating room (OR) time can increase the profitability of hospitals and account for the higher associated expenditure of RARP.
The OR accounts for almost 30% of total hospital charges.9 Longer operative times increase medical costs, with each OR minute adding roughly $15 to overall hospital expenditures.10 Managing OR scheduling is a challenging task. Confounding factors, such as patient body mass index (BMI), disease stage, procedural complexity, and surgeon experience, have been shown to influence the length of operative times and influence intraoperative costs, and therefore should be considered.11–13 Extended time gaps between operations in addition to late start and stop times can lead to suboptimal OR utilization, which can potentially decrease profitability and can increase costs associated with staffing, and reduce patient satisfaction.9 We aimed to develop a methodology for scheduling RARP that considers the variability in patient and disease characteristics, procedural modifications (such as nerve-sparing or lymph node dissection) and surgeon volume and experience. We hypothesize that by utilizing this methodology, OR times can be managed more efficiently.
Methods
We retrospectively reviewed 2000 RARPs performed at Roswell Park Cancer Institute performed by six different surgeons from 2004 to 2017. Operative time was defined as time elapsed between skin incision and wound closure. Relevant preoperative patient, disease, procedural, and surgeon variables were included in the predictive model. Patient variables included age, BMI, American Society of Anesthesiologists (ASA) score, Charlson Comorbidity Index (CCI), history of prior abdominal surgery, and history of prior irradiation. Disease variables included National Comprehensive Cancer network (NCCN) risk stratification, Gleason grade, prostate weight, and preoperative prostate-specific antigen (PSA) value. Procedural variables included planned extent and laterality of pelvic lymph node dissection (pLND) and nerve-sparing laterality. Patients were excluded who had any missing preoperative variables or operating start and end times.
Data were summarized using descriptive statistics. A binary decision tree was fit using a conditional inference tree method to predict the distribution of RARP operative times. The conditional inference tree method was applied using the “ctree” algorithm, which is implemented in the “party” package for the R statistical software language. The variable having the strongest association with operative times was determined using permutation tests. Data of this variable were split at the value resulting in the greatest difference in mean for operative times across the split. This process was repeated recursively on the resultant data until no significant association between the remaining variables and RARP operative times was found by the permutation tests. The resulting data sets were called terminal nodes. Each terminal node represents operative time outputs for specific preoperative (patient, disease, procedural, surgeon) inputs.
Comparisons for continuous variables were performed using Kruskal–Wallis tests for equality of distribution by surgeons. For categorical variables Freeman–Halton tests were used to determine if there was independence between surgeon and the variable in question. Due to the sample size Monte Carlo methods were used to estimate p-values with 100,000 iterations. The software package output multiple box plots depict the median, interquartile ranges, and the minimum and the maximum duration of operative times within each terminal node. Operative times were viewed as lognormally distributed.14 A lognormal model was fit within each terminal node to the operative times of patients included in the node. This lognormal model fit made it possible to estimate any quantity associated with the distribution of operative times. All tests were two-sided, with statistical significance defined as p ≤ 0.05. R software was used to perform all statistical analyses (version 3.2, R Core Team [2016]. R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Results
The final analysis comprised 1709 RARPs (Table 1). Mean age was 60 years (standard deviation [SD] 7). Fifteen percent had Gleason sum >7, 16% had Gleason grade 4 + 3 = 7, and 35% had Gleason grade 3 + 4 = 7. The mean prostate weight was 49 g (SD 21), and the mean preoperative PSA value was 7.3 ng/mL (SD 7.5). Forty-nine percent had bilateral, and 6% had unilateral pLND. The median total lymph node yield was 2 (interquartile range [IQR] 0–7). Sixty-eight percent had bilateral and 23% had unilateral nerve-sparing procedures. Median operative time was 221 minutes (IQR 176–283). There was a statistically significant difference between the six surgeons in preoperative patient factors (BMI, ASA score, CCI, prostate weight, prior abdominal surgery, and history of other cancers), and disease characteristics (Gleason grade and NCCN risk). Operative variables and pathologic outcomes were also different among surgeons (Table 1).
Table 1.
Perioperative Outcomes of 1709 Patients Who Underwent Robot-Assisted Radical Prostatectomy
| Variable | Surgeon 1 | Surgeon 2 | Surgeon 3 | Surgeon 4 | Surgeon 5 | Surgeon 6 | Total | p-Value |
|---|---|---|---|---|---|---|---|---|
| No. of patients, n | 544 | 884 | 118 | 19 | 89 | 55 | 1709 | — |
| Preoperative characteristics | ||||||||
| Age at prostatectomy, mean (SD) (years) | 61 (6) | 60 (7) | 59 (7) | 61 (7) | 61 (6) | 59 (8) | 60 (7) | 0.18 |
| BMI, mean (SD) (kg/m2) | 29 (5) | 30 (5) | 30 (5) | 31 (5) | 29 (5) | 28 (5) | 29 (5) | 0.04 |
| ASA score, mean (SD) | 2.0 (0.4) | 2.1 (0.4) | 2.1 (0.3) | 2.3 (0.5) | 2.2 (0.4) | 2.1 (0.4) | 2.1 (0.4) | <0.001 |
| PSA value, mean (SD) (ng/mL) | 7.1 (7.0) | 7.3 (76.9) | 6.9 (6.5) | 8.6 (12.0) | 9.4 (14.1) | 7.0 (7.2) | 7.3 (7.5) | 0.27 |
| Prostate weight, mean (SD) (g) | 47 (17) | 51 (20) | 44 (16) | 53 (18) | 47 (19) | 52 (57) | 49 (21) | <0.001 |
| Prior abdominal/pelvic surgery, n (%) | 159 (29) | 239 (27) | 30 (25) | 10 (53) | 33 (38) | 11 (20) | 482 (28) | 0.04 |
| Gleason grade, n (%) | ||||||||
| 6 | 217 (40) | 302 (34) | 37 (31) | 3 (16) | 5 (6) | 26 (47) | 590 (35) | |
| 3 + 4 | 174 (32) | 299 (34) | 47 (40) | 8 (42) | 47 (53) | 18 (33) | 593 (35) | |
| 4 + 3 | 81 (15) | 140 (16) | 25 (21) | 4 (21) | 19 (21) | 8 (15) | 277 (16) | |
| >7 | 72 (13) | 143 (17) | 9 (8) | 4 (21) | 18 (20) | 3 (5) | 249 (15) | <0.001 |
| High NCCN risk | 91 (17) | 194 (22) | 12 (10) | 4 (21) | 22 (25) | 7 (13) | 330 (19) | <0.001 |
| Charlson Comorbidity Index | ||||||||
| 0–3, n (%) | 425 (78) | 668 (76) | 94 (80) | 10 (53) | 58 (65) | 39 (71) | 1294 (76) | |
| 4–7, n (%) | 118 (22) | 214 (24) | 24 (20) | 9 (47) | 29 (33) | 15 (27) | 409 (24) | 0.008 |
| 8+, n (%) | 1 (0.2) | 2 (0.2) | 0 (0) | 0 (0) | 2 (2) | 1 (2) | 6 (0.4) | |
| Nonadenocarcinoma, n (%) | 1 (0.1) | 2 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.2) | 1.00 |
| History of cancer, n (%) | 46 (8) | 86 (10) | 9 (8) | 5 (26) | 13 (15) | 11 (20) | 170 (10) | 0.009 |
| History of smoking, n (%) | 261 (48) | 400 (46) | 54 (46) | 10 (53) | 52 (58) | 31 (56) | 808 (48) | 0.18 |
| Operative outcomes | ||||||||
| Operative time, median (IQR) (minutes) | 277 (240–311) | 182 (151–210) | 274 (220–327) | 216 (190–256) | 354 (320–389) | 286 (250–342) | 221 (176–283) | <.001 |
| EBL, median (IQR) (mL) | 200 (100–350) | 150 (100–300) | 225 (100–488) | 150 (100–225) | 250 (150–400) | 200 (100–300) | 200 (100–300) | <.001 |
| Lymph node dissection, n (%) | ||||||||
| None | 256 (47) | 420 (48) | 49 (42) | 6 (32) | 1 (1) | 33 (60) | 765 (45) | |
| Unilateral | 94 (17) | 6 (1) | 0 (0) | 1 (5) | 0 (0) | 5 (9) | 106 (6) | |
| Bilateral | 194 (36) | 458 (52) | 69 (58) | 12 (63) | 88 (99) | 17 (31) | 838 (49) | <0.001 |
| Nerve sparing, n (%) | ||||||||
| None | 53 (10) | 69 (8) | 12 (10) | 4 (21) | 15 (17) | 8 (15) | 163 (9) | |
| Unilateral | 163 (30) | 133 (15) | 36 (31) | 10 (53) | 41 (46) | 10 (18) | 393 (23) | |
| Bilateral | 328 (60) | 680 (77) | 69 (59) | 5 (26) | 33 (37) | 37 (67) | 1152 (68) | <0.001 |
| Pathologic outcomes | ||||||||
| pT3/T4, n (%) | 209 (38) | 340 (38) | 47 (40) | 10 (53) | 54 (61) | 19 (35) | 709 (42) | 0.01 |
| LNY, mean (SD) | 3 (5) | 4 (5) | 4 (4) | 4 (5) | 8 (5) | 2 (4) | 4 (5) | <0.001 |
| LNY, median (IQR) | 1 (0–6) | 2 (0–7) | 2 (0–7) | 2 (0–10) | 8 (4–11) | 0 (0–3) | 2 (0–7) | <0.001 |
| N1, n (%) | 18 (3) | 21 (2) | 1 (1) | 0 (0) | 7 (8) | 1 (2) | 48 (3) | <0.001 |
| Positive soft tissue surgical margins, n (%) | 168 (31) | 192 (22) | 38 (32) | 6 (32) | 24 (27) | 11 (20) | 439 (26) | 0.002 |
Boldface indicates statistically significant values.
SD = standard deviation; BMI = body mass index; ASA score = American Society of Anesthesiologists score; PSA = prostate-specific antigen; NCCN = National Comprehensive Cancer Network; EBL = estimated blood loss; LNY = lymph node yield.
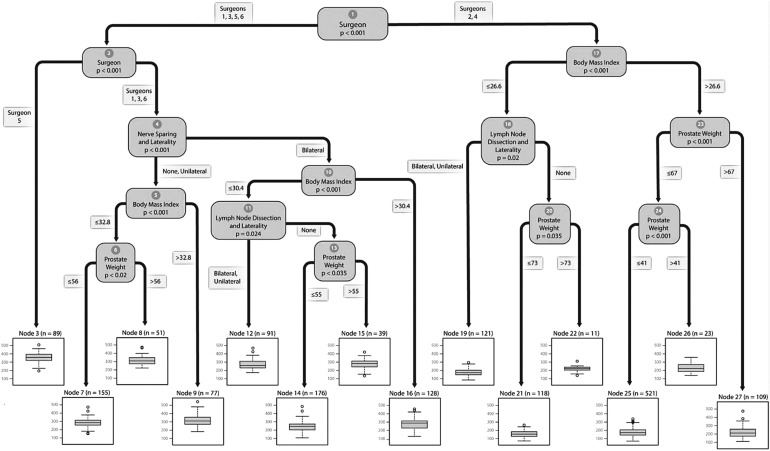
The variable most strongly associated with surgical time was the surgeon and it resulted in the largest mean difference (surgeons 2 and 4—102 minutes shorter than surgeons 1, 3, 5, and 6, p < 0.001) (Fig. 1). For surgeons 2 and 4, RARP time was most strongly associated with BMI; patients with BMI ≤27 kg/m2 had shorter operative times (20 minutes, p < 0.001). The shortest operative time (node 21; 160 minutes) was observed in patients with BMI ≤27 kg/m2, who did not receive pLND, and who had prostate weights ≤73 g. The longest operative time (node 26; 231 minutes) was observed in patients with BMI >41 kg/m2 who had prostate weights ≤67 g.
FIG. 1.
Regression tree showing significant variables associated with operative times for robot-assisted radical prostatectomy.
Among patients operated by surgeons 1, 3, 5, and 6, RARP time was again most strongly associated with the surgeon performing RARP. Surgeons 1, 3, and 6 were on average 76 minutes faster than surgeon 5 (p < 0.001). The shortest operative time (node 14; 244 minutes) was observed in bilateral nerve-sparing procedures by surgeons 1, 3, and 6, performed on patients with BMI ≤30 kg/m2, who did not receive pLND, who had prostate weights ≤55 g. The longest operative time (node 3; 355 minutes) was observed in patients operated on by surgeon 5.
Applying the binary decision tree to scheduling OR times is best illustrated with an example. Assume surgeon 1 had a patient with BMI 25 kg/m2 and prostate weight 50 g. The patient has low-risk PCa and is scheduled for a RARP with bilateral nerve sparing and no pLND. Starting at the top of the regression tree (Fig. 1), we proceed to the left (urologist is surgeon 1). Then, at node 2, we proceed to the right because the urologist is surgeon 1. At node 4, we proceed to the right because the patient is scheduled for bilateral nerve sparing. At node 10, we proceed to the left because patient's BMI is <30 kg/m2. At node 11, we proceed to the right because the patient is not scheduled for pLND. Finally, at node 13, we proceed left because prostate weight is <55 g. From Table 2, we can see that similar operations (n = 176) had mean operative time 244 minutes (SD 57 minutes). Similarly, the median, minimum, maximum, and IQR for similar RARPs are available in Table 2.
Table 2.
Mean, Standard Deviation, Ranges, Median, and Interquartile Range (Gray) for Operative Times for Each Node
| Node | Patients in node (n) | Mean operative time (minutes) | SD (minutes) | Minimum operative time (minutes) | Maximum operative time (minutes) | 25th percentile (minutes) | Median operative time (minutes) | 75th percentile (minutes) |
|---|---|---|---|---|---|---|---|---|
| 3 | 89 | 355 | 57 | 193 | 509 | 320 | 354 | 389 |
| 7 | 155 | 283 | 50 | 159 | 475 | 256 | 282 | 316 |
| 8 | 51 | 316 | 58 | 226 | 475 | 275 | 311 | 344 |
| 9 | 77 | 324 | 67 | 186 | 542 | 279 | 322 | 359 |
| 12 | 91 | 274 | 57 | 178 | 466 | 230 | 260 | 306 |
| 14 | 176 | 244 | 57 | 112 | 483 | 210 | 242 | 277 |
| 15 | 39 | 277 | 64 | 134 | 423 | 246 | 280 | 314 |
| 16 | 128 | 284 | 70 | 132 | 460 | 235 | 285 | 316 |
| 19 | 121 | 178 | 43 | 84 | 297 | 152 | 177 | 209 |
| 21 | 118 | 160 | 36 | 76 | 263 | 133 | 156 | 182 |
| 22 | 11 | 217 | 47 | 141 | 314 | 200 | 226 | 238 |
| 25 | 521 | 184 | 44 | 73 | 340 | 152 | 184 | 209 |
| 26 | 23 | 231 | 58 | 141 | 356 | 188 | 227 | 272 |
| 27 | 109 | 213 | 57 | 113 | 475 | 176 | 205 | 251 |
Discussion
PCa is responsible for about 25% of male cancers in the United States and results in ∼70,000 RARPs performed annually.15,16 The objective of this study was to develop a patient-based model for estimating RARP operative times. An accurate estimate of operative times facilitates scheduling, decreases unexpected idle time between cases, and minimizes staffing costs associated with overtime use.9,17
Different strategies exist for OR scheduling that commonly include open, block, and modified block strategies. Open scheduling is done by assigning an OR at the convenience of the surgeon on a “first-come, first-served” basis. Block scheduling assigns each surgeon a block of time into which they arrange their procedures. Modified block combines open and block strategies; scheduling is done by blocking a portion of time, marking the rest as open, and releasing any remaining time that is unused.17 The key to maximizing OR utilization is to block off the appropriate amount of time for each operation using different variables that may affect its duration.
Historical data averaging the operative times for a surgeon's last 10 cases and using a surgeon's estimation of how long an operation will take have been explored as mean for scheduling operative times, but these techniques did not show reliable predictive validity.18 OR utilization has been reported as low as 80% of desired targets, which significantly increases medical expenditures.19 Accurate prediction of OR times helps manage the OR by anticipating fluid load challenges and planning for intensive vs high dependency bed requirements.12 Surgery planning and scheduling is complex due to so much uncertainty. Multiple patient, disease, procedural, and surgeon variables can influence operative time. Our methodology considers these factors, and generates an individualized scheduling time that is unique for each patient. A main advantage of using the algorithm is that each institution can develop its own “decision-tree” based on their surgeons and patients.
Different statistical models have been proposed for estimating operative times. Selecting the appropriate method is based on examining the data distribution, where linear regression can be used in cases of normal distribution. Intelligent-based models and data mining techniques are used to predict operative times, although the initial results have been unsatisfactory.20 Our study used a multilevel conditional inference tree model that handles complex interactions among variables, and determines the contribution of each variable at each level to predict operative times. Tree-based models are advantageous because they can be scaled to large numbers of explanatory variables, can fit data that are not normally distributed, and can be easily interpreted and utilized by nonstatisticians.12 It is noteworthy that the tree will change from one practice to another and will be tailored based on the institution's own historical data, therefore accounting for each surgeon's experience and also for the learning curve.
Several variables were significantly different among the six surgeons. However, not all of them were clinically relevant, such as ASA score (mean = 2 for all), BMI, and prostate weight (minor difference). The main differences were related to the disease stage (Gleason grade and NCCN risk), and patient characteristics (CCI, prior abdominal surgery, and history of other cancers). Disease and patient-related factors are the main determinants of performing LND and nerve-sparing procedures, in addition to the surgeon experience and skill. Therefore, although disease stage and risk were not significant in the ctree, their implication on the procedure (i.e., the decision to perform a nerve-sparing procedure or LND) significantly contributed to OR time. Technically, Gleason 8 disease itself may not pose additional difficulty to RARP if it is organ confined, but a Gleason 6 disease in a large prostate with bilateral nerve sparing may take a longer time. For example, although surgeon 2 had the second highest proportion of high-risk patients (22%) and the highest nerve-sparing rates (77%), he had the shortest median operative time among all surgeons. This reflects that OR time is most likely determined by a combination of surgeon skill and experience, in addition to the patient and disease-related factors, or procedural modifications (such as LND and nerve sparing). The strength of our model is that it considers interactions between all the variables and also sorts them according to the contribution of each. Postoperative variables (pT, lymph node yield [LNY], N1, and positive margins) were not included in the ctree as they will not be useful in a tool intended for preoperative use.
Several studies have examined the different factors that may contribute to operative times in RARP. In agreement with our findings, prior studies have linked obesity with prolonged operative times for RARP.21 Age has been associated with operative times for radical cystectomy, where older patients were found to have shorter operative times, which may reflect surgeons' concern about the possible hazardous effects of prolonged anesthesia and operative times.11 However, this study did not find age as a significant predictor. In agreement with our findings, Martin et al. reported no significant effect of prior surgery or radiation on surgical times for RARP.22 Prior abdominal surgeries or pelvic radiation may cause adhesions and altered fascial planes. Such history increases surgical complexity, heightens the risk of intraoperative complications, and may increase operative times.12,23,24 However, this study did not find abdominal surgeries or pelvic radiation as significant predictors.
Several disease-related variables can affect RARP operative times. Prior studies have shown a clear association between biopsy Gleason score and preoperative PSA values and longer operative times.25,26 We did not find an association with Gleason score, PSA value, or NCCN risk group. Although advanced disease may render RARP more challenging, early detection of high-risk patients, while the disease is still prostate confined, may negate some of the adverse factors that increase the risk of intraoperative complications and time.
Multiple studies have reported a relationship between prostate size (volume or weight) and operative times. Larger prostates were associated with longer surgical times, where each 10 cc increase in prostate gland size increased operative time by 2.7 minutes.25,27,28 Our study demonstrated that larger prostate weights were associated with longer operative times. Adjunct procedures (such as pLND) have been linked with longer operative times.26 Our study demonstrated that any form of pLND was associated with increased RARP times. These procedures require additional time for dissecting lymphatic tissue from the major vessels and for controlling lymphatic vessels to avoid lymphocele. Procedural modifications (bladder neck or urethral sparing, nerve-sparing techniques) may affect operative times.29,30 Yong et al. reported nerve sparing as an independent predictor of prolonged operative time.28 In contrast, our results demonstrated that bilateral nerve sparing was associated with shorter operative times compared with unilateral or non-nerve-sparing procedures. This may be explained by the fact that surgeons who perform nerve-sparing procedures are usually more experienced and would take shorter time (surgeon is most predictive variable of operative time in this study). Several studies have shown that surgeon experience influences RARP operative times.21,26,28 Simon et al. in a study of six hospitals showed that each additional RARP per year per institution is associated with a 0.80- to 0.89-minute decrease in operative time.26 We found that surgeons 2 and 4, who performed 52% of the total RARPs, had shorter operative times compared with the remaining surgeons. Patients who underwent bilateral nerve-sparing procedures had lower average BMI than those who underwent unilateral or no nerve-sparing (the second most predictive variable in our study) procedure.
This study provides a unique methodology for predicting OR times at the individual patient level, but it has several limitations. Limitations inherent to retrospective study design are well recognized. The Current Procedure Terminology (CPT) code defines the operative time as the time elapsed from incision to wound closure. Nonoperative times that include delays in patient arrival, frozen sections, anesthesia induction, patient discharge, and turnover are needed to calculate the overall OR time. Unfortunately, these times were not recorded often. Still, developing a reliable OR schedule relies primarily on accurately estimating the time needed to perform the operation rather than turnover or cleaning times.12 Operations that take significantly more or less time to perform can lead to OR underutilization. Events such as unexpected intraoperative findings induce variability between actual and scheduled times that cannot be avoided. Another limitation is that our study only included RARPs performed by six surgeons at a single high-volume referral institution, which may include variation in the technique among these surgeons, and may limit generalizability of the results. However, the methodology can be replicated with institution-specific data to allow institutions to produce their own decision trees.
Conclusion
A large database of RARP patients was used retrospectively to develop a methodology for estimating operative times based on preoperative patient, disease, procedural, and surgeon metrics that can be used to assist the OR for scheduling RARP more efficiently.
Abbreviations Used
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- IQR
interquartile range
- NCCN
National Comprehensive Cancer Network
- OR
operating room
- PCa
prostate cancer
- pLND
pelvic lymph node dissection
- PSA
prostate-specific antigen
- RARP
robot-assisted radical prostatectomy
- RP
radical prostatectomy
- SD
standard deviation
Acknowledgments
This research was supported in part by funding from the National Cancer Institute of the National Institutes of Health under award number R25CA181003 and Roswell Park Alliance Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Penson DF, Chan JM; Urologic Diseases in America Project. Prostate cancer. J Urol 2007;177:2020–2029 [DOI] [PubMed] [Google Scholar]
- 2.Hu JC, O'Malley P, Chughtai B, et al. . Comparative effectiveness of cancer control and survival after robot-assisted versus open radical prostatectomy. J Urol 2017;197:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs EF, Boris R, Masterson TA. Advances in robotic-assisted radical prostatectomy over time. Prostate Cancer 2013;2013:902686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Close A, Robertson C, Rushton S, et al. . Comparative cost-effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of men with localised prostate cancer: A health technology assessment from the perspective of the UK National Health Service. Eur Urol 2013;64:361–369 [DOI] [PubMed] [Google Scholar]
- 5.Kim SP, Shah ND, Karnes RJ, et al. . Hospitalization costs for radical prostatectomy attributable to robotic surgery. Eur Urol 2013;64:11–16 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PL, Gu X, Lipsitz SR, et al. . Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol 2011;29:1517–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez D, Ganesan V, Nelson RJ, Haber GP. Reducing costs for robotic radical prostatectomy: Three-instrument technique. Urology 2016;95:213–215 [DOI] [PubMed] [Google Scholar]
- 8.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Hu JC. Hospital volume, utilization, costs and outcomes of robot-assisted laparoscopic radical prostatectomy. J Urol 2012;187:1632–1637 [DOI] [PubMed] [Google Scholar]
- 9.Vargas LG, May JH, Spangler W, Stanciu A, Strum DP. (2008) Operating Room Scheduling and Capacity Planning. In: Anesthesia Informatics. Health Informatics. Springer, New York, NY: 978-0-387-76417-7. 10.1007/978-0-387-76418-4_19 [DOI] [Google Scholar]
- 10.Macario A. What does one minute of operating room time cost? J Clin Anesth 2010;22:233–236 [DOI] [PubMed] [Google Scholar]
- 11.Filson CP, Tan HJ, Chamie K, Laviana AA, Hu JC. Determinants of radical cystectomy operative time. Urol Oncol 2016;34:431.e417–e424 [DOI] [PubMed] [Google Scholar]
- 12.Hussein AA, May PR, Ahmed YE, et al. . Development of a patient and institutional-based model for estimation of operative times for robot-assisted radical cystectomy: Results from the International Robotic Cystectomy Consortium. BJU Int 2017;120:695–701 [DOI] [PubMed] [Google Scholar]
- 13.Schuster M, Standl T, Wagner JA, Berger J, Reimann H, am Esch JS. Effect of different cost drivers on cost per anesthesia minute in different anesthesia subspecialties. Anesthesiology 2004;101:1435–1443 [DOI] [PubMed] [Google Scholar]
- 14.Davila MP. A Methodology for Scheduling Operating Rooms Under Uncertainty. University of South Florida, 2013. http://scholarcommons.usf.edu/etd/4463/ [Google Scholar]
- 15.Mohler JL, Armstrong AJ, Bahnson RR, et al. . Prostate cancer, version 1.2016. J Natl Compr Canc Netw 2016;14:19–30 [DOI] [PubMed] [Google Scholar]
- 16.Singh I, Hemal AK. Robotic-assisted radical prostatectomy in 2010. Expert Rev Anticancer Ther 2010;10:671–682 [DOI] [PubMed] [Google Scholar]
- 17.Fei H, Meskens N, Chu C. A planning and scheduling problem for an operating theatre using an open scheduling strategy. Comput Ind Eng 2010;58:221–230 [Google Scholar]
- 18.Strum DP, Sampson AR, May JH, Vargas LG. Surgeon and type of anesthesia predict variability in surgical procedure times. Anesthesiology 2000;92:1454–1466 [DOI] [PubMed] [Google Scholar]
- 19.Association HFM. Achieving operating room efficiency through process integration. Healthc Financ Manag 2003;57:suppl 1–7 following 112 [PubMed] [Google Scholar]
- 20.Combes C, Meskens N, Rivat C, Vandamme J-P. Using a KDD process to forecast the duration of surgery. Int J Prod Econ 2008;112:279–293 [Google Scholar]
- 21.Carter SC, Lipsitz S, Shih YC, Nguyen PL, Trinh QD, Hu JC. Population-based determinants of radical prostatectomy operative time. BJU Int 2014;113:E112–E118 [DOI] [PubMed] [Google Scholar]
- 22.Martin AD, Desai PJ, Nunez RN, et al. . Does a history of previous surgery or radiation to the prostate affect outcomes of robot‐assisted radical prostatectomy? BJU Int 2009;103:1696–1698 [DOI] [PubMed] [Google Scholar]
- 23.Castle EP, Pruthi RS. Robotic Surgery of the Bladder. Berlin: Springer, 2014 [Google Scholar]
- 24.Yuh BE, Ciccone J, Chandrasekhar R, et al. . Impact of previous abdominal surgery on robot-assisted radical cystectomy. JSLS 2009;13:398–405 [PMC free article] [PubMed] [Google Scholar]
- 25.Violette PD, Mikhail D, Pond GR, Pautler SE. Independent predictors of prolonged operative time during robotic-assisted radical prostatectomy. J Robot Surg 2015;9:117–123 [DOI] [PubMed] [Google Scholar]
- 26.Simon RM, Howard LE, Moreira DM, et al. . Predictors of operative time during radical retropubic prostatectomy and robot-assisted laparoscopic prostatectomy. Int J Urol 2017;24:618–623 [DOI] [PubMed] [Google Scholar]
- 27.Link BA, Nelson R, Josephson DY, et al. . The impact of prostate gland weight in robot assisted laparoscopic radical prostatectomy. J Urol 2008;180:928–932 [DOI] [PubMed] [Google Scholar]
- 28.Yong DZ, Tsivian M, Zilberman DE, Ferrandino MN, Mouraviev V, Albala DM. Predictors of prolonged operative time during robot-assisted laparoscopic radical prostatectomy. BJU Int 2011;107:280–282 [DOI] [PubMed] [Google Scholar]
- 29.Khoder WY, Waidelich R, Seitz M, et al. . Do we need the nerve sparing radical prostatectomy techniques (intrafascial vs. interfascial) in men with erectile dysfunction? Results of a single-centre study. World J Urol 2015;33:301–307 [DOI] [PubMed] [Google Scholar]
- 30.Schlomm T, Heinzer H, Steuber T, et al. . Full functional-length urethral sphincter preservation during radical prostatectomy. Eur Urol 2011;60:320–329 [DOI] [PubMed] [Google Scholar]