Abstract
Purpose: To compare treatment outcomes and adverse events between concurrent chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-RT) and conventional concurrent chemoradiotherapy with cisplatin and 5-fluorouracil (CF-RT).
Methods and Materials: We retrospectively investigated treatment outcomes and adverse events in 121 patients with advanced esophageal cancer who underwent concurrent chemoradiotherapy with CF-RT (n = 83) or DCF-RT (n = 38). In the CF-RT group, patients were administered cisplatin (70 mg/m2) and 5-fluorouracil (700 mg/m2) for 5 days; in the DCF-RT group, patients were administered docetaxel (50 mg/m2), cisplatin (50 mg/m2), and 5-fluorouracil (500 mg/m2) for 5 days. The radiotherapy dose was 1.8-2 Gy per session, up to a total of 50-60 Gy.
Results: The complete response (CR) rate was 37.8% in the CF-RT group and 52.6% in the DCF-RT group. Overall survival (OS) rates at 2 and 3 years were 45.0% and 37.5%, respectively, in the CF-RT group and 62.9% and 56.7%, respectively, in the DCF-RT group, with a significant intergroup difference (p = 0.032). Progression-free survival rates at 2 and 3 years were 44.1% and 36.9%, respectively, in the CF-RT group and 45.0% and 45.0%, respectively, in the DCF-RT group (p = 0.10). Local control rates at 2 and 3 years were 59.1% and 54.6%, respectively, in the CF-RT group and 71.8% and 71.8%, respectively, in the DCF-RT group (p = 0.12). The incidence of Grade 3/4 leukopenia was 55.4% (n = 46) in the CF-RT group and 78.9% (n = 30) in the DCF-RT group, with a significant intergroup difference (p = 0.022). The incidence of Grade 3/4 neutropenia was 47.0% (n = 39) in the CF-RT group and 65.8% (n = 25) in the DCF-RT group, with a notable albeit not statistically significant difference between the groups (p = 0.054). There were no significant intergroup differences in anemia, thrombocytopenia, radiation-induced dermatitis, radiation esophagitis, or late adverse events.
Conclusions: Rates of OS and CR were improved after treatment with DCF-RT compared with CF-RT. Although DCF-RT-treated patients had higher rates of leukopenia, treatment safety was ensured through proper management of myelotoxicity. DCF-RT is a promising treatment regimen for advanced esophageal cancer.
Keywords: esophageal cancer, concurrent chemoradiotherapy, docetaxel, cisplatin, 5-fluorouracil, overall survival
Introduction
Esophageal cancer is one of the most difficult cancers to cure and has a poor prognosis. Despite technological advances in treatment modalities, treatment outcomes for locally advanced esophageal cancer are poor1-12, because most patients present with advanced stage disease, with lymph node metastasis already evident at the time of diagnosis.13
Concurrent chemoradiotherapy (CRT) is currently the first-line treatment for inoperable or unresectable esophageal cancer.14 The standard regimen for concurrent CRT combines cisplatin plus 5-fluorouracil (5-FU) (CF) with radiation to achieve good clinical outcomes and a radiosensitizing effect.2,3 However, treatment outcomes of CF are slightly inferior to those of surgery, hovering around 26%-27% for clinical T1-3, N0-1 cases1,15 and 31% for clinical T1-4, N0-1 cases.2
Recent studies have reported favorable outcomes in patients treated with CF plus docetaxel (DCF) instead of CF alone as preoperative chemotherapy or therapy for postoperative recurrence.16-23
However, very few studies have used DCF and radiotherapy concurrently.24-26 In contrast, DCF is frequently combined with radiotherapy as concurrent CRT (DCF-RT) to treat patients with advanced head and neck carcinoma, achieving high response rates.27 In this study, we therefore compared treatment outcomes of DCF-RT and conventional concurrent CRT with CF (CF-RT). To verify the superiority of DCF-RT over CF-RT, we investigated the effectiveness of our treatment strategy for esophageal cancer and the incidence of acute and late adverse events.
Methods and Materials
Patients
The study subjects comprised 121 patients (113 men, 8 women) with stage II, III, or IV esophageal cancer who underwent curative concurrent CRT at Shimane University Hospital and Dokkyo Medical University Hospital between September 2006 and December 2015. Cases were reviewed retrospectively. Esophageal cancer was diagnosed according to the Union for International Cancer Control TNM staging system 2009 (UICC 2009). Esophagoscopy, barium esophagography, helical computed tomography (CT) scans, and endoscopic ultrasonography were performed for clinical staging. Magnetic resonance imaging (MRI), bronchoscopy, and positron emission tomography (PET) were also performed when necessary. Patients with stage IV esophageal cancer were included only when they also had cervical lymph node metastasis or celiac lymph node metastasis, corresponding to the pathological condition previously classified as stage IVa esophageal cancer in UICC 2002. Patients with metastasis to other lymph nodes or distant organs were excluded.
This study was approved by the institutional review board of Shimane University Hospital and Dokkyo Medical University Hospital, and conducted in accordance with the Declaration of Helsinki. Before consenting in writing to participate in the study, all patients were informed in detail of the study purpose and procedures for chemotherapy and radiotherapy, and were told that they were free to reject treatment regimens.
Patient characteristics are shown in Table 1. Median age was 68 (range 45-86) years. European Clinical Oncology Group performance status (PS) was 0-1 in 111 patients and 2-4 in 10 patients. Histological type was squamous cell carcinoma (SCC) in 120 patients and adenocarcinoma in 1. For chemotherapy, cisplatin and 5-FU were administered to 81 patients (CF-RT group) and docetaxel, cisplatin, and 5-FU to 38 patients (DCF-RT group).
Table 1.
Patient characteristics
| CF-RT group | DCF-RT group | sum | p value | |
|---|---|---|---|---|
| No. of cases | 83 | 38 | 121 | |
| Age, years | 45-86 | 51-79 | p=0.42 | |
| (median) | (68) | (67.5) | ||
| Sex | p=0.24 | |||
| Male | 79 | 34 | 113 | |
| Female | 4 | 4 | 8 | |
| PS(ECOG) | p=0.42 | |||
| 0-1 | 75 | 36 | 111 | |
| 2-4 | 8 | 2 | 10 | |
| Histology | p=0.50 | |||
| Squamous cell carcinoma | 82 | 38 | 120 | |
| Adenocarcinoma | 1 | 0 | 1 | |
| Timing of CRT | p=0.55 | |||
| First CRT with no prior treatment | 69 | 27 | 96 | |
| Postoperative adjuvant CRT | 3 | 3 | 6 | |
| Salvage CRT for postoperative local recurrence or mediastinal lymph node metastasis | 11 | 8 | 19 | |
| Primary site | p=0.75 | |||
| Cervical esophagus | 9 | 7 | 16 | |
| Upper thoracic esophagus | 14 | 5 | 19 | |
| Middle thoracic esophagus | 36 | 11 | 47 | |
| Lower thoracic esophagus | 11 | 7 | 18 | |
| Abdominal esophagus | 2 | 0 | 2 | |
| (Isolated lymph node recurrence in mediastinal lymph node region) | (11) | (8) | (19) | |
| Stage(UICC2009) | p=0.98 | |||
| I | 0 | 0 | 0 | |
| II | 9 | 3 | 12 | |
| III | 47 | 18 | 65 | |
| IV | 17 | 9 | 26 | |
| (Isolated lymph node recurrence in mediastinal lymph node region) | (11) | (8) | (19) | |
| Radiation dose | p=0.075 | |||
| 50 Gy | 8 | 9 | 17 | |
| 60 Gy | 75 | 29 | 104 |
CF; cisplatin and 5-fluorouracil, RT; radiation therapy, DCF; docetaxel, cisplatin and 5-fluorouracil, PS; performance status, ECOG; Europe Clinical Oncology Group, CRT; chemoradiation therapy, UICC; Union for International Cancer Control
Treatment was performed as curative CRT in 96 patients (69 CF-RT, 27 DCF-RT) with no prior treatment history, as adjuvant CRT in 5 patients (2 CF-RT, 3 DCF-RT) with residual tumor after radical surgery for esophageal cancer, or as curative CRT for salvage in 18 patients (10 CF-RT, 8 DCF-RT) with isolated mediastinal lymph node metastasis (recurrence) after radial surgery for esophageal cancer. After excluding cases of salvage CRT for recurrent mediastinal lymph node metastasis, 103 patients were staged according to UICC 2009. Twelve patients were categorized as stage II (9 CF-RT, 3 DCF-RT); 65 as stage III (47 CF-RT, 18 DCF-RT); and 26 as stage IV (17 CF-RT, 9 DCF-RT).
Chemotherapy and Radiation Therapy
All patients underwent chemotherapy and radiotherapy concurrently. In the CF-RT group, cisplatin (70 mg/m2) was administered via intravenous drip infusion on day 1, and 5-FU (700 mg/m2) via continuous intravenous drip infusion on days 1-5. In the DCF-RT group, docetaxel and cisplatin (both 50 mg/m2) were administered via intravenous drip infusion on day 1, and 5-FU (500 mg/m2) via continuous intravenous drip infusion on days 1-5. Patients underwent 2 cycles of chemotherapy during radiotherapy when no deterioration in overall health or occurrence of adverse events was verified. Patients with severe neutropenia were immediately administered granulocyte-colony stimulating factor (G-CSF).
For radiotherapy, 3-dimensional treatment planning was used to plan external beam radiation from a linear accelerator. Heterogeneity correction and a convolution/superposition algorithm were used to calculate radiation dose. The radiation dose per session was 1.8-2 Gy, and the radiation field was based on the area of tumor invasion, lymph node metastasis, PS, and previous medical history. The total radiation dose was 50-60 Gy. Attention was paid to the percentage of lung volume receiving 20 Gy or more, and the radiation field was changed as needed to prevent the maximum radiation dose to the spinal cord from exceeding 50 Gy.
Evaluation Methods and Criteria
Tumor response was evaluated 1 month after completion of chemoradiotherapy and evaluated by esophagoscopy and CT scans. The response of primary tumors was evaluated according to the criteria of the Japanese Society for Esophageal Diseases.28 For primary tumors, complete response (CR) was defined as the disappearance of all visible tumors, including ulceration, as confirmed by endoscopy and negative biopsy results. Partial response (PR) was defined as a 50% decrease in the area of the primary tumor; marked morphological improvements were confirmed by endoscopy on the basis of findings such as regression of the tumor, flattening of a raised ulcer margin, and shallowing and clearing of an ulcer base. No change (NC) was defined as a less than 50% reduction or less than 25% increase in the area of the primary tumor; a slight decrease or no change in tumor size was confirmed by endoscopy. Progressive disease (PD) was defined as a 25% increase in the area of the primary tumor; enlargement of the tumor and/or the appearance of new lesion(s) was confirmed by endoscopy. The response of metastatic lesions such as lymph node metastases, was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1).29 CR of lymph node metastases was defined as “a reduction in the short axes of target pathological lymph nodes to < 10 mm. Adverse reactions were evaluated according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE; version 4.0).
Statistical Analysis
The Mann-Whitney U-test and the chi-squared test were used to determine differences between the patient groups in continuous numeric and categorical variables, respectively. Rates of overall survival (OS), progression-free survival (PFS), and local control (LC) were estimated by the Kaplan-Meier method and compared using the log-rank test, with a P-value of less than 0.05 considered statically significant.
Results
All patients completed CRT. The median observation period was 20.1 (range 1.5-86.2) months, and was 19.3 months and 22.1 months in the CF-RT and DCF-RT groups, respectively; the difference between the two groups was not significant (p = 0.76). The median overall treatment time was 44 days in all subjects, 44 days in the CF-RT group, and 42 days in the DCT-RT group; the difference between the two groups was not significant (p = 0.10). The number of cases of delayed treatment by 10 days or longer was 14 (16.9%) in the CF-RT group and 5 (13.2%) in the DCF-RT group; the difference between the two groups was also not significant (p = 0.44).
In the CF-RT group, CR was observed in 31 patients, PR in 39, NC-PD in 12, and unknown response in 1. In the DCF-RT group, CR was observed in 20 patients, PR in 14, and NC-PD in 4. The mean CR rate was higher in the DCF-RT group (52.6%) compared with the CF-RT group (37.8%), although this difference was not statistically significant (p = 0.13; Table 2).
Table 2.
Evaluation of tumor responses
| CF-RT group | DCF-RT group | p value | |
|---|---|---|---|
| p=0.13 | |||
| CR (CR rate) | 31 (38.3%) | 20 (52.6%) | |
| non CR (non CR rate) | 51 (61.4%) | 18 (47.4%) | |
| PR | 39 | 14 | |
| NC-PD | 12 | 4 | |
| not description | 1 (1.2%) | 0 (0%) |
CF; cisplatin and 5-fluorouracil, DCF; docetaxel, cisplatin and 5-fluorouracil, CR; complete response, PR; partial response, NC; no change, PD; progressive disease
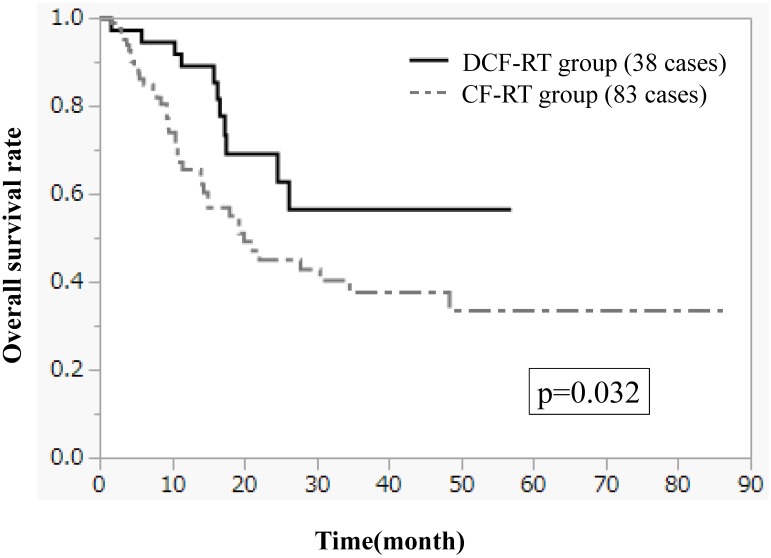
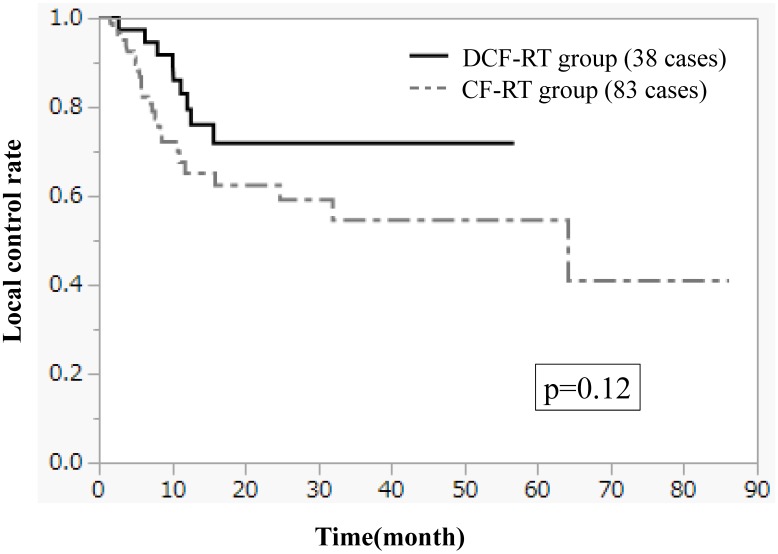
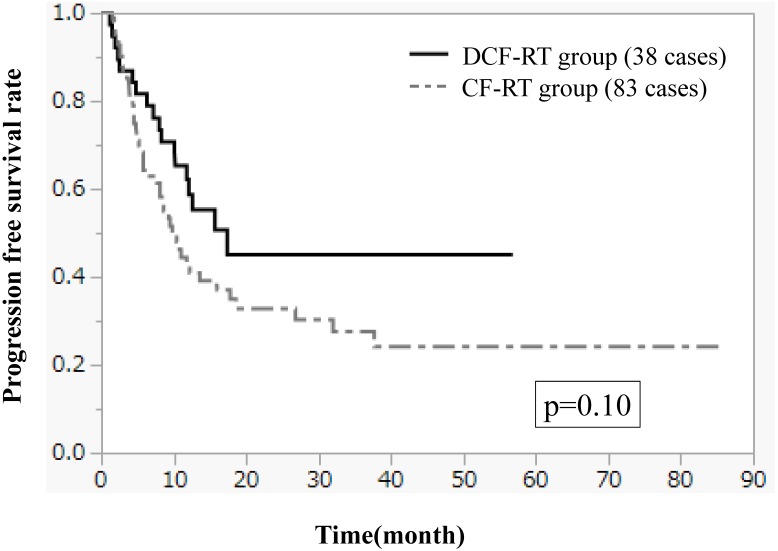
Figures 1-3 show OS, PFS, and LC curves in the CF-RT and DCF-RT groups. OS rates at 2 and 3 years were 45.0% and 37.5%, respectively, in the CF-RT group and 62.9% and 56.7%, respectively, in the DCF-RT group, with a significant intergroup difference (p = 0.032). PFS rates at 2 and 3 years were 44.1% and 36.9%, respectively, in the CF-RT group and 45.0% and 45.0%, respectively in the DCF-RT group; LC rates at 2 and 3 years were 59.1% and 54.6%, respectively, in the CF-RT group and 71.8% and 71.8%, respectively, in the DCF-RT group. There were no significant intergroup differences for PFS or LC rates (p = 0.10 and p = 0.12, respectively).
Figure 1.
Overall survival (OS) curves for the CF-RT (cisplatin, 5-fluorouracil and radiotherapy) and DCF-RT (docetaxel, cisplatin, 5-fluorouracil and radiotherapy) groups. OS rates at 2 and 3 years are 45.0% and 37.5%, respectively, in the CF-RT group and 62.9% and 56.7%, respectively, in the DCF-RT group, with a significant intergroup difference (p = 0.032).
Figure 3.
Local control (LC) curves for the CF-RT and DCF-RT groups. LC rates at 2 and 3 years are 59.1% and 54.6%, respectively, in the CF-RT group and 71.8% and 71.8%, respectively, in the DCF-RT group, with no significant intergroup difference (p = 0.12).
For univariate analysis, patients were divided into subgroups based on patient characteristics as follows: age (< 70 or ≥ 70 years), PS (0-1 or 2-4), treatment background (new or residual/recurrent tumor), and radiation dose (50 or 60 Gy). No significant intergroup difference was observed between the two subgroups for any of these factors (Table 3).
Table 3.
Univariate analysis with overall survival time
| 2-year OS (%) | 3-year OS (%) | p value | |
|---|---|---|---|
| Chemotherapy regimen | p=0.032 | ||
| CF | 45.0 | 37.5 | |
| DCF | 62.9 | 52.7 | |
| Age | p=0.29 | ||
| <70 | 42.4 | 42.4 | |
| ≥70 | 57.7 | 45.5 | |
| PS (ECOG) | p=0.17 | ||
| 0-1 | 52.7 | 43.9 | |
| 2-4 | 32.4 | 32.4 | |
| First treatment or Treatment for residual/recurrent tumor | p=0.29 | ||
| First treatment | 48.9 | 44.6 | |
| Treatment for residual/recurrent tumor | |||
| 63.1 | 21.1 | ||
| Radiation dose | p=0.69 | ||
| 50Gy | 51.7 | 35.2 | |
| 60Gy | 50.2 | 45.2 | |
OS; overall survival rates, CF; cisplatin and 5-fluorouracil, DCF; docetaxel, cisplatin and 5-fluorouracil, PS; performance status, ECOG; Europe Clinical Oncology Group
Recurrence was observed in 62 patients (44 [53.0%] in the CF-RT group and 18 [47.4%] in the DCF-RT group; Table 4), with no significant intergroup difference. Because multiple recurrences were concurrently observed in 62 patients, we analyzed 77 recurrence patterns in these patients. The site of recurrence was locoregional and mediastinal lymph nodes in 32 patients (24 CF-RT group, 8 DCF-RT group) and cervical, supraclavicular, and upper abdominal lymph nodes in 12 patients (7 CF-RT group, 5 DCF-RT group). Recurrence in distant organs was observed in 33 patients (20 CF-RT group, 13 DCF-RT group). No significant intergroup differences in recurrence sites were observed.
Table 4.
Initial recurrence pattern
| CF-RT group | DCF-RT group | p value | |
|---|---|---|---|
| No. of patients | 44 patients (53.0%) | 18 patients (47.4%) | p=0.56 |
| No. of recurrent sites | 51 sites | 26 sites | |
| No. of local relapses and recurrences of the mediastinum lymph node region | 24 patients (24 sites) | 8 patients (8 sites) | p=0.49 |
| No. of recurrences of the neck, supraclavicular and abdominal lymph node | 7 patients (7 sites) | 5 patients (5 sites) | p=0.63 |
| No. of distant metastases | 20 patients (20 sites) | 13 patients (13 sites) | p=0.35 |
CF; cisplatin and 5-fluorouracil, DCF; docetaxel, cisplatin and 5-fluorouracil
A total of 49 patients died. In the CF-RT group (38 deaths), the cause of death was primary cancer in 31 patients; other in 3 (empyema, pneumonia due to methicillin-resistant Staphylococcus aureus, and aspiration pneumonia); and treatment-related in 4 (a combination of radiation pneumonitis and aspiration pneumonia in 1, interstitial pneumonia in 1, sudden death from massive bleeding due to aortoesophageal fistula in 1, and recurrent septic shock due to esophagobronchial fistula in 1. In the DCF-RT group (11 deaths), the cause of death was primary cancer in 9 patients, cancer in multiple organs in 1 (hypopharyngeal cancer), and other in 1 (aspiration pneumonia).
Acute adverse events were classified according to the CTCAE (Table 5). Regarding hematological toxicities, the incidence of Grade 3/4 leukopenia was 55.4% (n = 46) in the CF-RT group and 78.9% (n = 30) in the DCF-RT group, with a significant intergroup difference (p = 0.022). The incidence of Grade 3/4 neutropenia was 47.0% (n = 39) in the CF-RT group and 65.8% (n = 25) in the DCF-RT group; the between-group difference was notable but not statistically significant (p = 0.054). No significant intergroup difference was observed in anemia or thrombocytopenia. Additionally, Grade 3/4 elevation in serum aspartate transaminase, alanine transaminase, bilirubin, or creatinine was seldom observed in both groups.
Table 5.
Acute adverse events (CTCAE ver4.0)
| Grade | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | Grade 0-2 | Grade 3-5 | p value | ||
| (1) Hematotoxicity | ||||||||||
| Anemia | p=0.68 | |||||||||
| CF-RT group | 2 | 23 | 43 | 15 | 0 | 0 | 68 | 15 (18.1%) | ||
| DCF-RT group | 1 | 9 | 23 | 5 | 0 | 0 | 33 | 5 (13.2%) | ||
| Leukopenia | p=0.022 | |||||||||
| CF-RT group | 2 | 8 | 27 | 35 | 9 | 0 | 37 | 46 (55.4%) | ||
| DCF-RT group | 3 | 0 | 5 | 22 | 8 | 0 | 8 | 30 (78.9%) | ||
| Neutropenia | p=0.054 | |||||||||
| CF-RT group | 3 | 24 | 17 | 32 | 7 | 0 | 44 | 39 (47.0%) | ||
| DCF-RT group | 3 | 2 | 8 | 17 | 8 | 0 | 13 | 25 (65.8%) | ||
| Thrombopenia | p=0.80 | |||||||||
| CF-RT group | 17 | 48 | 14 | 4 | 0 | 0 | 79 | 4 (4.8%) | ||
| DCF-RT group | 8 | 24 | 3 | 2 | 1 | 0 | 35 | 3 (7.9%) | ||
| AST increasing | p=0.69 | |||||||||
| CF-RT group | 60 | 20 | 3 | 0 | 0 | 0 | 83 | 0 (0%) | ||
| DCF-RT group | 30 | 6 | 1 | 1 | 0 | 0 | 37 | 1 (2.6%) | ||
| ALT increasing | p=0.94 | |||||||||
| CF-RT group | 49 | 25 | 6 | 3 | 0 | 0 | 80 | 3 (3.6%) | ||
| DCF-RT group | 27 | 8 | 1 | 2 | 0 | 0 | 36 | 2 (5.3%) | ||
| Bilirubin increasing | p=1.0 | |||||||||
| CF-RT group | 76 | 5 | 2 | 0 | 0 | 0 | 83 | 0 (0%) | ||
| DCF-RT group | 32 | 5 | 1 | 0 | 0 | 0 | 38 | 0 (0%) | ||
| Creatinin increasing | p=1.0 | |||||||||
| CF-RT group | 76 | 7 | 0 | 0 | 0 | 0 | 83 | 0 (0%) | ||
| DCF-RT group | 28 | 10 | 0 | 0 | 0 | 0 | 38 | 0 (0%) | ||
| (2) Non-hematotoxicity | ||||||||||
| Radiation induced dermatitis | p=0.94 | |||||||||
| CF-RT group | 22 | 35 | 25 | 1 | 0 | 0 | 82 | 1 (1.2%) | ||
| DCF-RT group | 24 | 12 | 2 | 0 | 0 | 0 | 36 | 2 (5.3%) | ||
| Radiation esophagitis | p=0.58 | |||||||||
| CF-RT group | 12 | 34 | 26 | 11 | 0 | 0 | 72 | 11 (13.3%) | ||
| DCF-RT group | 17 | 9 | 9 | 3 | 0 | 0 | 35 | 3 (7.9%) | ||
CTCAE; Common Terminology Criteria for Adverse Events, CF; cisplatin and 5-fluorouracil, DCF; docetaxel, cisplatin and 5-fluorouracil, AST; Aspartate transaminase, ALT; Alanine transaminase
Regarding non-hematological adverse events, Grade 3/4 radiation-induced dermatitis was observed in 1 patient in the CF-RT group and 0 in the DCF-RT group, and Grade 3/4 radiation esophagitis was observed in 11 (13.3%) patients in the CF-RT group and 3 (7.9%) patients in the DCF-RT group, with no significant intergroup difference (p = 0.58). Late adverse events included Grade 1 radiation pneumonitis in 9 patients, Grade 2 in 2, Grade 3 in 1, and Grade 5 in 1 in the CF-RT group, and Grade 1 and 3 radiation pneumonitis in 2 and 1 patient, respectively, in the DCF-RT group. Other adverse events in the CF-RT group included Grade 5 aortoesophageal fistula in 1 patient, esophagobronchial fistula in 1 patient, Grade 4 gastrointestinal bleeding in 1, Grade3 esophageal stricture in 1, Grade3 esophageal perforation in 1, and Grade2 esophagocutaneous fistula in 1, Grade 2 pleural effusion in 3, and Grade 2 pericardial effusion in 3. In the DCF-RT group, Grade 2 pleural effusion was observed in 2 patients and Grade 2 and 3 pericardial effusion in 1 each. No significant intergroup difference in adverse events was observed.
Discussion
Mean CR rate, the primary endpoint of this study, was 37.8% in the CF-RT group compared with 52.6% in the DCF-RT group, although the difference was not statistically significant. These rates are very similar to those found in previous studies; Higuchi et al. treated patients with DCF-RT and achieved a CR rate of 52.4%26), while Ohtsu et al. attained a CR rate of 33% using CF-RT.30 In our study, OS rates were significantly improved and PFS and LC rates were favorable in the DCF-RT group. Taken together, our findings suggest that the anticancer effect of CRT is more potent with DCF-RT than with CF-RT.
In a study of preoperative CRT with DCF for locally advanced esophageal cancer, Zanoni et al. observed a pathological CR rate of 43% in patients with SCC, and 5-year OS and disease-related survival rates were 43% and 49%, respectively.24 This led the authors to conclude that the addition of a taxane to CF-CRT was beneficial. Pasini et al. also observed pathological CR in 35 (47%) of 74 patients who underwent surgery immediately after DCF therapy.16 Docetaxel exerts anticancer effects by suppressing the depolymerization of microtubules and blocking cell division. The agent also increases the radiosensitization effect by arresting cells in G2/M, a cell cycle phase that is highly vulnerable to radiation, thereby increasing the radiosensitization effect.31 A previous study also reported that cisplatin and taxane-containing regimens suppress the expression of genes associated with dihydropyrimidine dehydrogenase, an enzyme that degrades 5-FU.32 The strong anticancer effects of DCF-RT may be attributable to these molecular mechanisms.
While a radiation dose of 50 Gy became standard in the United States and Europe after the outcome of the RTOG9405/INT0123 trial was reported2, 60 Gy is often used in Asian countries, including Japan. Adenocarcinoma is the major type of esophageal cancer in Western countries, but SCC is more common in Japan and other Asian countries. Compared with adenocarcinoma, SCC is more sensitive to radiation compared with adenocarcinoma; accordingly, 60 Gy is used rather frequently in Japan and other Asian countries for higher curability.
In the present study, OS and PFS rates did not differ significantly between patients treated with 50 Gy versus 60 Gy, but LC was slightly higher in the latter. Suh et al. compared treatment outcomes between patients treated with 50 Gy versus 60 Gy for advanced esophageal cancer (mostly SCC) and found that the latter group had significantly better locoregional control (2-year locoregional control rate, 69% versus 32%, p < 0.01) and prolonged PFS (2-year PFS rate, 47% versus 20%, p = 0.01).33 Median OS was 18 months in the 50-Gy group and 28 months in the 60-Gy group, with no significant intergroup difference (p = 0.26). However, Suh et al. reported that multivariate analysis revealed that 60 Gy or higher radiotherapy was a significant prognostic factor for improved locoregional control, PFS, and OS, suggesting that it is more beneficial to use 60 Gy.33
Hematological toxicities are the most frequent and important adverse events in therapy with DCF.
Few patients had Grade 3/4 anemia or thrombocytopenia in the present study, but 78.9% and 65.8% of patients had Grade 3/4 leukopenia and neutropenia, respectively, in the DCF-RT group. These findings are similar to those of previous work: the incidence of Grade 3/4 leukopenia and neutropenia was 91.9% and 75.7%, respectively, in a study conducted by Miyazaki et al.25 and 71.4% and 57.1%, respectively, in another study conducted by Higuchi et al.26
In general, the number of neutrophils decreases rapidly in patients treated with DCF-RT.34 In the present study, we also observed cases in which patients who originally had no leukopenia or neutropenia developed Grade 4 leukopenia or neutropenia in a matter of 4-5 days. We performed complete blood counts 3 times a week during and after chemotherapy and administered G-CSF as soon as cell counts began to decrease. Despite these efforts, some patients developed Grade 4 leukopenia/neutropenia, suggesting that it may be necessary to consider using long-acting G-CSF agents such as pegfilgrastim in the future.
Late adverse events, which are important factors in CRT with esophageal cancer, included radiation pneumonitis, pleural effusion, cardiac dysfunction, pericardial effusion, esophageal stricture, and perforation in the present study. Our findings did not differ substantially from those of previous studies using CF-RT.35,36 Radiotherapy has been reported to significantly increase cardiac death rates, suggesting that long-term follow-up may be necessary.37
This study has certain limitations. First, the effects of selection bias are not negligible, as this is a retrospective study. As shown in Table 1, there were no significant differences in any of the characteristics of patients between the two groups. Nevertheless, we plan to continue accumulating more cases so that we can perform matched-pair comparison in the future. Also, favorable outcomes of this study may prompt a prospective study using a new modified protocol. We are in the process of designing a prospective study according to the present protocol, for which it will be particularly important that patients are well matched with regard to clinical stages and radiation fields. Also, the proportion of elderly patients was slightly higher in the CF-RT group than in the DCF-RT group because the expected toxicity of DCF-RT was slightly greater than that of CF-RT. Although the median ages of the two groups were roughly the same and did not differ significantly (Table 1), a possible selection bias remained. Second, the DCF-RT group had fewer patients than the CF-RT group. To improve statistical precision, the number of patients undergoing DCF-RT will be increased in our future prospective study.
In conclusion, DCF-RT has an extremely potent anticancer effect and extends overall patient survival compared with CF-RT albeit with some limitations. It may be necessary to prevent or manage leukopenia and neutropenia—for example, with G-CSF—but it is very likely that DCF-RT will be a promising treatment regimen for esophageal cancer in the future.
Figure 2.
Progression free survival (PFS) curves for the CF-RT and DCF-RT groups. PFS rates at 2 and 3 years are 44.1% and 36.9%, respectively, in the CF-RT group and 45.0% and 45.0%, respectively, in the DCF-RT group, with no significant intergroup difference (p = 0.10).
Acknowledgments
The authors acknowledge the support of the Department of Radiotherapy, Gastrointestinal Surgery and Gastroenterology at Shimane University Hospital and Dokkyo Medical University Hospital.
Abbreviations
- CRT
chemoradiotherapy
- 5-FU
5-fluorouracil
- CF
cisplatin and 5-fluorouracil
- DCF
docetaxel, cisplatin, and 5-fluorouracil
- DCF-RT
concurrent CRT with DCF
- CF-RT
concurrent CRT with CF
- UICC
the Union for International Cancer Control TNM staging system
- CT
computed tomography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- PS
performance status
- SCC
squamous cell carcinoma
- GCF
granulocyte-colony stimulating factor
- CR
complete response
- PR
partial response
- NC
no change
- PD
progressive disease
- CTCAE
Common Terminology Criteria for Adverse Events
- OS
overall survival
- PFS
progression-free survival
- LC
local control.
References
- 1.Cooper JS, Guo MD, Herskovic A. et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Pajak TF, Ginsberg RJ. et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 3.Bedenne L, Michel P, Bouche O. et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 4.Stahl M, Stuschke M, Lehmann N. et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–7. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 5.Shimada Y, Imamura M, Sato F. et al. Indications for abdominal para-aortic lymph node dissection in patients with esophageal squamous cell carcinoma. Surgery. 2002;132:93–9. doi: 10.1067/msy.2002.125486. [DOI] [PubMed] [Google Scholar]
- 6.Herskovic A, Leichman L, Lattin P. et al. Chemo/radiation with and without surgery in the thoracic esophagus: the Wayne State experience. Int J Radiat Oncol Biol Phys. 1988;15:655–62. doi: 10.1016/0360-3016(88)90308-2. [DOI] [PubMed] [Google Scholar]
- 7.Li QW, Zhu YJ, Zhang WW. et al. Chemoradiotherapy for Synchronous Multiple Primary Cancers with Esophageal Squamous Cell Carcinoma: a Case-control Study. J Cancer. 2017;8:563–9. doi: 10.7150/jca.17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida K, Ando N, Yamamoto S. et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516) Jpn J Clin Oncol. 2004;34:615–9. doi: 10.1093/jjco/hyh107. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita H, Seto Y, Takenaka R. et al. Survival comparison between radical surgery and definitive chemoradiation in 267 esophageal squamous cell carcinomas in a single institution: A propensity-matched study. PLoS One. 2017;12:e0177133. doi: 10.1371/journal.pone.0177133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamaki Y, Sasaki R, Ejima Y. et al. Efficacy of intraoperative radiotherapy targeted to the abdominal lymph node area in patients with esophageal carcinoma. J Radiat Res. 2012;53:882–91. doi: 10.1093/jrr/rrs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Qiu B, Luo G. et al. TNM staging matched-pair comparison of surgery after neoadjuvant chemoradiotherapy, surgery alone and definitive chemoradiotherapy for thoracic esophageal squamous cell carcinoma. J Cancer. 2017;8:683–90. doi: 10.7150/jca.17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forastiere AA, Orringer MB, Perez-Tamayo C. et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118–23. doi: 10.1200/JCO.1993.11.6.1118. [DOI] [PubMed] [Google Scholar]
- 13.Igaki H, Kato H, Tachimori Y. et al. Prognostic evaluation for squamous cell carcinomas of the lower thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg. 2001;19:887–93. doi: 10.1016/s1010-7940(01)00701-1. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Han S, Gu F. et al. A retrospective comparison of taxane and fluorouracil-based chemoradiotherapy in patients with inoperable esophageal squamous cell carcinoma. J Cancer. 2016;7:1066–73. doi: 10.7150/jca.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.al-Sarraf M, Martz K, Herskovic A. et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277–84. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 16.Pasini F, de Manzoni G, Zanoni A. et al. Neoadjuvant therapy with weekly docetaxel and cisplatin, 5-fluorouracil continuous infusion, and concurrent radiotherapy in patients with locally advanced esophageal cancer produced a high percentage of long-lasting pathological complete response: a phase 2 study. Cancer. 2013;119:939–45. doi: 10.1002/cncr.27822. [DOI] [PubMed] [Google Scholar]
- 17.Muro K, Hamaguchi T, Ohtsu A. et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15:955–9. doi: 10.1093/annonc/mdh231. [DOI] [PubMed] [Google Scholar]
- 18.Rigas JR, Dragnev KH, Bubis JA. Docetaxel in the treatment of esophageal cancer. Semin Oncol. 2005;32:S39–51. doi: 10.1053/j.seminoncol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Ilson DH, Ajani J, Bhalla K. et al. Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol. 1998;16:1826–34. doi: 10.1200/JCO.1998.16.5.1826. [DOI] [PubMed] [Google Scholar]
- 20.Einzig AI, Neuberg D, Remick SC. et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrontestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996;13:87–93. doi: 10.1007/BF02993858. [DOI] [PubMed] [Google Scholar]
- 21.Chiarion-Sileni V, Corti L, Ruol A. et al. Phase II trial of docetaxel, cisplatin and fluorouracil followed by carboplatin and radiotherapy in locally advanced oesophageal cancer. Br J Cancer. 2007;96:432–8. doi: 10.1038/sj.bjc.6603585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanoni A, Verlato G, Giacopuzzi S. et al. Neoadjuvant concurrent chemoradiotherapy for locally advanced esophageal cancer in a single high-volume center. Ann Surg Oncol. 2013;20:1993–9. doi: 10.1245/s10434-012-2822-4. [DOI] [PubMed] [Google Scholar]
- 23.Satake H, Tahara M, Mochizuki S. et al. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol. 2016;78:91–9. doi: 10.1007/s00280-016-3062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higuchi K, Koizumi W, Tanabe S. et al. A phase I trial of definitive chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) for advanced esophageal carcinoma: Kitasato digestive disease and oncology group trial (KDOG 0501) Radiother Oncol. 2008;87:398–404. doi: 10.1016/j.radonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki T, Sohda M, Tanaka N. et al. Phase I/II study of docetaxel, cisplatin, and 5-fluorouracil combination chemoradiotherapy in patients with advanced esophageal cancer. Cancer Chemother Pharmacol. 2015;75:449–55. doi: 10.1007/s00280-014-2659-6. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi K, Komori S, Tanabe S. et al. Definitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: a phase 2 trial (KDOG 0501-P2) Int J Radiat Oncol Biol Phys. 2014;89:872–9. doi: 10.1016/j.ijrobp.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Katori H, Tsukuda M. Comparison of induction chemotherapy with docetaxel, cisplatin, and 5-fl uorouracil (TPF) followed by radiation vs. concurrent chemoradiotherapy with TPF in patients with locally advanced squamous cell carcinoma of the head and neck. Clin Oncol (R Coll Radiol) 2005;17:148–52. doi: 10.1016/j.clon.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Kuwano H, Nishimura Y, Ohtsu A. et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2007 edition. Esophagus. 2008;5:61–73. [Google Scholar]
- 29.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Ohtsu A, Boku N, Muro K. et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21. doi: 10.1200/JCO.1999.17.9.2915. [DOI] [PubMed] [Google Scholar]
- 31.Mason KA, Hunter NR, Milas M. et al. Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res. 1997;3(12Pt 1):2431–8. [PubMed] [Google Scholar]
- 32.Schneider S, Uchida K, Brabender J. et al. Down-regulation of TS, DPD, ERCC 1, GST-Pi, EGFR, and HER2 gene expression after neoadjuvant three-modality treatment in patients with esophageal cancer. J Am Coll Surg. 2005;200:336–44. doi: 10.1016/j.jamcollsurg.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 33.Suh YG, Lee IJ, Koom WS. et al. High-dose versus standard-dose radiotherapy with concurrent chemotherapy in stages II-III esophageal cancer. Jpn J Clin Oncol. 2014;44:534–40. doi: 10.1093/jjco/hyu047. [DOI] [PubMed] [Google Scholar]
- 34.Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Sal. et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217–23. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- 35.Ishikura S, Nihei K, Ohtsu A. et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 36.Kato K, Muro K, Minashi K. et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–90. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Gharzai L, Verma V, Denniston KA. et al. Radiation therapy and cardiac death in long-term survivors of esophageal cancer: an analysis of the surveillance, epidemiology, and end result database. PLoS One. 2016;11:e0158916. doi: 10.1371/journal.pone.0158916. [DOI] [PMC free article] [PubMed] [Google Scholar]