Abstract
Programmed death-ligand 1 (PD-L1) has been reported to be expressed in many types of tumor cells, and bind to PD-1 on T lymphocytes to inhibit immune response. Immunologic checkpoint blockade with antibodies that target the PD1/PD-L1 pathway has demonstrated to have impressive antitumor effects on many malignancies. However, the significance of PD1/PD-L1 pathway in cervical cancer remains unclear. Here we studied PD-L1, PD-1, CD8 and HPV expression in cervical cancer and normal cervix by immunohistochemical staining. Our results showed that there was more frequently positive for PD-L1, PD-1 and CD8 in cervical cancer tissues compared to normal tissues, especially those strongly stained HPV. Additionally, PD-L1, PD-1 and CD8 were more frequently stained in tissues from advanced tumor and tumor with lymphoid nodes or vascular invasion respectively. Tissues from patients with chemotherapy history had over expression of PD-L1 in tumor cells and more PD-1 and CD8 in stromal mononuclear cells, which were identified as tumor infiltrated lymphocytes (TILs). These findings point to a key role of PD-L1 in immune escape of cervical cancer, and provide a rationale for therapeutic targeting of the PD-1/PD-L1 pathway.
Keywords: PD-L1, HPV, cervical cancer, chemotherapy, CD8.
Introduction
Cervical cancer is the fourth most common women malignancy cancer, with an estimated global incidence of over 528,000 new cases per year, and is the second leading death cause of women world widely, with an estimated 266,000 deaths per year 1. The etiology of cervical cancer has been largely attributed to infection of human papillomavirus (HPV), most frequently HPV16 and/or HPV18 2. At present, patients with cervical cancer are treated with radical hysterectomy and pelvic lymphadenectomy or chemoradiation, depending on tumor stage and tumor size 3, 4. However, the number of patients with cervical cancer is still rising and these patients seem to have a poorer survival rate although accepted traditional radical hysterectomy or chemoradiation 5, 6. In addition, these traditional therapies have obvious side-effects. Therefore, there is an urgent need to developing better therapeutic strategy against cervical cancer.
Programmed death ligand 1 (PD-L1) is a protein modulating the adaptive arm of immune system, which is often upregulated on tumor cells and tumor-infiltrating lymphocytes 7, 8. The expression of PD-L1 and PD-1 on tumor cells impairs T-cell mediated immune responses, and also promotes T-cell tolerance during chronic viral infections 9. Increased PD-L1 expression may allow tumors and/or viruses to avoid immune surveillance 10. Various biological inhibitors targeted on PD-1 or PD-L1 have been tested in many clinical trials. Several monoclonal antibodies (mAbs) for blockading the PD-1 or PD-L1, such as nivolumab (BMS-936558) and pembrolizumab (MK-3475), have been studied for cancer immunotherapy and shown to have impressive response in patients with different malignant tumors 11, 12.
Previous studies have shown there were direct correlations between the expression levels of PD-L1 and PD-1, and CIN grade and HPV positive rate, suggesting the PD-1/PD-L1 pathway could impair cervical immunity in high-risk human papillomavirus (HR-HPV)-related CIN 13. Less PD-L1 expression was observed in the cervical squamous cell cancers (51%) than the CIN1-2 lesions (95%) 14. In addition, the expression of PD-L1 in the CD8+ cells that was strongly associated with the area of viral infection 15. Another study reported that the majority of PD-L1 positive cervical cancer was more often to be HPV18-positive than HPV16-positive cervical cancer (83% vs 42%) 14. The expression of PD-L1 is therefore likely to be associated with more advanced stage of cervical cancer 16. Neoadjuvant chemotherapy is one of the main treatments for advanced cervical cancer patients. However, the relationship between PD-L1 and neoadjuvant chemotherapy is still unclear.
In this study, the expressions of PD-1 and PD-L1 in cervical cancer were investigated and their correlations with pathological and clinical characteristics in cervical cancer samples were also studied. Our data showed that there were more frequently positive for PD-L1, PD-1 and CD8 in cervical cancer, especially those strongly stained HPV. Additionally, PD-L1, PD-1 and CD8 were more frequently stained in tissues from advanced tumor and tumor with lymphoid nodes or vascular invasion respectively. Higher positive rates of PD-L1, PD-1 and CD8 expression were also found in tumor tissues from patients with neoadjuvant chemotherapy. These findings point to a significant role of PD-L1 in immune escape of cervical cancer, and provide a rationale for therapeutic targeting of the PD-1/PD-L1 pathway.
Materials and Methods
Patients
The patients who were pathologically diagnosed with cervical cancer in Second Affiliated Hospitals of Chongqing Medical University, First Affiliated Hospitals of Chongqing Medical University and Suining Center Hospital between April 2014 and August 2016 were recruited for this study. A total of 97 patients with cervical cancer, 30 patients with normal cervix uteri and normal tonsillar from 5 patients were analyzed for PD-L1 expression. The following pathological characteristics were collected for all 97 patients: age, gender, primary site, tumor grade according to the 2014 FIGO classification, uterus metastasis, lymphatic metastasis, vascular invasion, previous history of infection including HPV infection and CIN history, information on chemotherapy, operation history and information on radiotherapy.
Immunohistochemistry (IHC) of PD-1, PD-L1, CD8 and HPV
Tumor sections were freshly cut to 4 mm and dipped in 4% formalin for 24 hours. Tumor sections were then embedded and cut into slices. With antigen repaired, PD-L1 immunohistochemistry (rabbit anti-human PD-L1 monoclonal, 1:200,Cell Signaling 13684; rabbit anti-human CD8 monoclonal 1:100, Abcam ab93278; mouse anti-human HPV16/18 monoclonal stoste, Abcam ab51931; rabbit anti-human PD-1 monoclonal 1:50, Abcam ab137132.rabbit anti-human IgG monoclonal 1:500, Abcam ab172730) was performed and the antibody was incubated for one night at 4℃.
PD-L1, CD8 and HPV expression was evaluated on tumor tissues. Tumor infiltrating immune cells were not identified in all cases. The proportion of PD-L1-positive cells was estimated as the percentage of total tumor cells; tumor cells typically showed membranous staining with a variably component of cytoplasmic staining.
The standard for evaluation scale of marks of the situation of dye is below: The staining intensity of immunohistochemistry was divided into 4 levels: negative, weakly positive, moderate positive, strong positive. We scored negative as 0; weakly positive as 1; moderate positive as 2; strong positive as 3.
The evaluation of the specimens was scored as the following principle:
1. CD8, PD-1 score
(1) For the different intensity of the tumor stroma percentage of the total score = the percentage of negative area *0 + the percentage of weakly positive area *1 + the percentage of moderate positive area *2 + the percentage of strong positive area *3. (2) The substantial part of the PD-1, CD8 expression levels were assessed at 4 levels: the tumor cells around, at the edge or in which have a large number of coloration (TIL stain score * area ≥30 points) was scored as 3. The tumor cells around, at the edge or in which have medium amount of coloration (TIL stain score * area ≥10 points) was scored as 2. The tumor cells around, at the edge or in which have a small amount of coloration (TIL stain score * area ≤10 points) was scored as 1. The tumor cells around, at the edge or in which have no coloration or just tumor cells themselves were stained was scored as 0.
2. PD-L1 and HPV score
Evaluation of HPV expression levels for stromal cells. A little positive cells scattered in the stromal cells was scored as 1, clustering positive cells distributed in the part of stromal cells was scored as 2, clustering positive cells distributed in the most of the stromal cells was score as 3.
For PD-1 and CD8, when the tumor stroma was scored over10 or the tumor cells were scored over 2, the specimen was positive. For PD-L1 and HPV, when the tumor cells were scored over 10 or the tumor stroma was scored over 2, the specimen was positive.
Statistical analyses
Correlation between the status of PD-1, PD-L1, CD8 and HPV expression and clinicopathological variables was analyzed using the t-test or the Fisher's exact test as appropriate or one-way analysis of variance. Treatment outcomes were estimated as response rate (RR) and disease control rate (DCR). Descriptive statistics were reported as proportions and medians. Kaplan-Meier estimates were used in the analysis of all time to event variables, and the 95% confidence interval (CI) for the median time to event was computed.
Results
Histologic and viral characterization of the samples
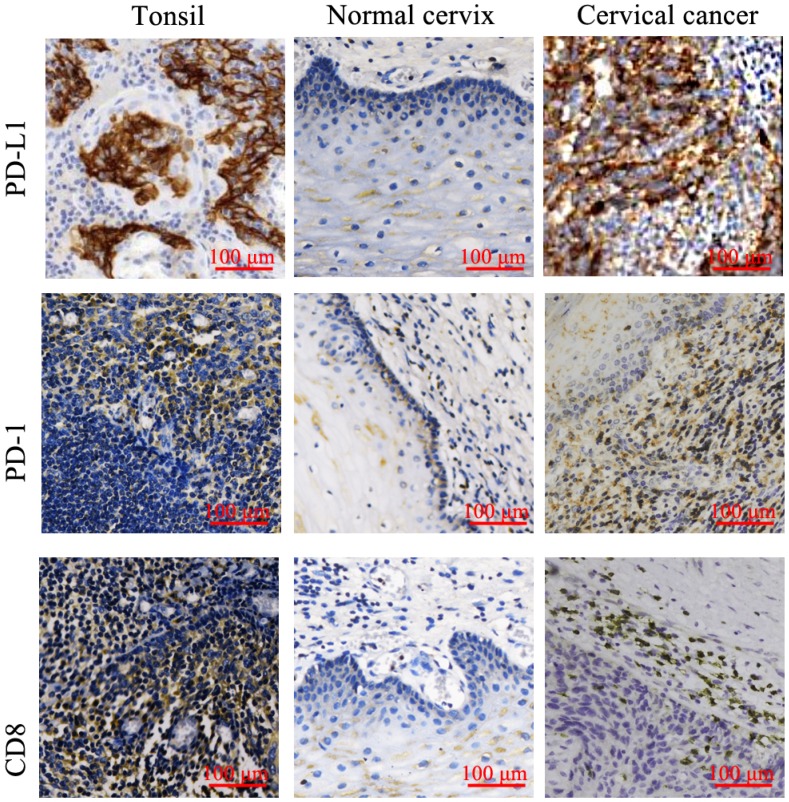
A total of 97 patients who were histopathologically diagnosed with cervical cancer were evaluated in this study. Meanwhile, 30 patients with benign uterine diseases were enrolled as the control group. The baseline demographics and clinical characteristics of 97 cervical cancer patients were shown in Table 1. The examples of the positive staining for PD-L1, PD-1 and CD8 T cells in tonsillitic tissues are showed in Figure 1. To further confirm the detection of T lymphocytes, hematoxylin-eosin (HE) staining was applied (Supplementary 1). In cervical cancer tissues, over-expression of PD-L1 was mostly found in tumor cells and sporadically in stromal cells (Figure 1 and Table 2). However, the PD-1+ cells and CD8+ cells were often found at the periphery of the tumor, with only a few of PD-1+ cells dispersed in the nest of the tumor, suggesting a defect of T lymphocytes infiltration.
Table 1.
Baseline demographics and clinical characteristics of cervical cancer patients.
| Characteristic | Number | Proportion (%) |
|---|---|---|
| Total | 97 | |
| Age (y) | ||
| <50 | 55 | 56.70 |
| ≥50 | 42 | 43.30 |
| Histologic diagnosis | ||
| Adenocarcinoma | 7 | 7.22 |
| Squamous carcinoma | 82 | 84.54 |
| Other | 8 | 8.24 |
| Neoadjuvant chemotherapy | ||
| Yes | 40 | 41.24 |
| No | 32 | 32.99 |
| unknown | 25 | 25.77 |
| Differentiation | ||
| Low differentiation | 12 | 12.37 |
| High differentiation | 40 | 41.24 |
| Unknown | 45 | 46.39 |
| Pathological stage | ||
| I-IIa | 61 | 62.89 |
| IIb-IV | 20 | 20.62 |
| Unknown | 16 | 16.49 |
| Lymph node metastasis | ||
| Yes | 18 | 18.56 |
| No | 60 | 61.85 |
| Unknown | 19 | 19.59 |
| Vascular invasion | ||
| Yes | 13 | 13.40 |
| No | 55 | 56.70 |
| Unknown | 29 | 29.90 |
Figure 1.
Expression of PD-L1, PD-1 and CD-8 in tonsillitic tissues, normal cervix and cervical cancer were examined by IHC. The strongly stained mononuclear cells in tonsillitic tissues were identified as T lymphocytes by clinical pathologists and used as a standard to identify the lymphocytes in the cervical tissues. Expression of PD-L1, PD-1 and CD-8 in 97 samples of cervical cancer tissues and 30 normal cervix tissues were examined by IHC. The typical pictures were shown. PD-L1, PD-1 and CD-8 all had higher expression in cervical cancer. Moreover, PD-1 and CD-8 had similar expression pattern. They were strongly stained in stromal mononuclear cells around the tumor cells. The mononuclear cells were identified as tumor infiltration lymphocytes (TILs). PD-L1 mainly expressed in tumor cells.
Table 2.
The positive ration of PD-L1, PD-1, CD8 and HPV in tumor cell or tumor stroma respectively
| PD-L1 | PD-1 | CD-8 | HPV | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Tumor cells | 64 (65.98%) | 33 (34.02%) | 18 (18.56%) | 79 (81.44%) | 20 (20.62%) | 77 (79.38%) | 61 (81.33%) | 14 (18.67%) |
| Stroma | 22 (22.68%) | 75 (77.32%) | 59 (60.82%) | 38 (39.18%) | 55 (56.70%) | 42 (43.30%) | 13 (17.33%) | 62 (82.67%) |
Among 97 cervical cancer samples, 68 cases (70.10%) were positive for PD-L1 staining and 66 cases (68.04%) were positive for PD-1 staining. In contrast, only 7 cases (23.33%) were positive for PD-L1 staining and 9 cases (30.00%) were positive for PD-1 staining in 30 cases of normal cervical samples (Table 3). The positive rates for PD-L1 and PD-1 in cervical cancer samples were significantly higher than that in normal cervical tissues (Table 3). In addition, the positive rate for CD8 in cervical cancer samples was also significantly higher than that in normal cervical tissues (65.98% vs 33.33%, P < 0.01).
Table 3.
The expression of PD-L1, PD-1, CD-8 and HPV in normal cervix samples and cervical cancer samples
| Number of sample | Positive (%) | Negative (%) | P value | |
|---|---|---|---|---|
| PD-L1 | ||||
| Normal cervix | 30 | 7 (23.33) | 23 (76.67) | <0.01* |
| Cervical caner | 97 | 68 (70.10) | 29 (29.90) | |
| PD-1 | ||||
| Normal cervix | 30 | 9(30.00) | 21(70.00) | <0.01* |
| Cervical caner | 97 | 66(68.04) | 31(31.96) | |
| CD8 | ||||
| Normal cervix | 30 | 10(33.33) | 20(66.67) | <0.01* |
| Cervical caner | 97 | 64(65.98) | 33(34.02) | |
| HPV | ||||
| Normal cervix | 30 | 7(23.33) | 13(76.67) | <0.01* |
| Cervical caner | 78 | 65(83.58) | 13(16.42) |
* represents P<0.05.
Expression of PD-1 and PD-L1 in relation to HPV-E6 oncoprotein
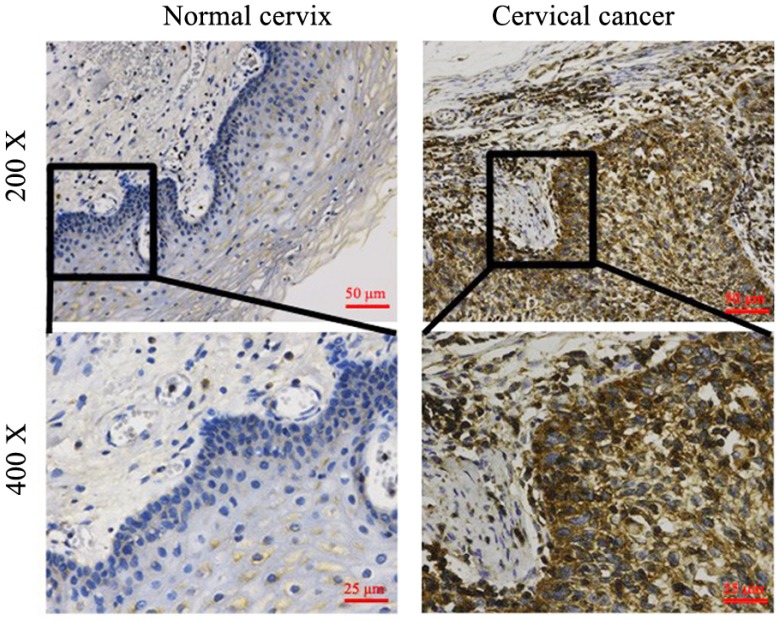
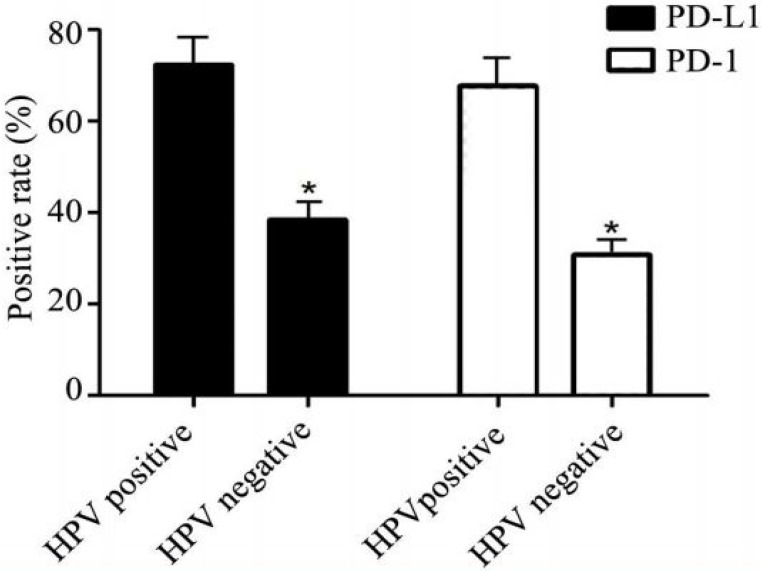
Among 97 cervical cancer patients, 78 patients who had HPV infection history were further performed the HPV-E6 IHC staining in their cancer tissues (Figure 2). As shown in Table 2, 65 out of 78 patients (83.58%) were positive for HPV-E6 staining while only 7 out of 30 (23.33%) normal cervical samples were positive for HPV-E6. Importantly, the positive rates for PD-L1 and PD-1 were significantly higher in HPV-E6 positive cancer tissues than that in HPV-E6 negative cancer tissues (Figure 3 and Table 4), suggesting the over-expression of PD-L1 and PD-1 was related to HPV-E6 oncoprotein.
Figure 2.
HPV expression in normal cervix and cervical cancer. 100 samples of cervical cancer tissues and 30 normal cervix tissues were examined by IHC. The typical pictures were shown. HPV over-expression in tissues from cancer patients.
Figure 3.
PD-L1 expression correlated with HPV expression. In the HPV positive group, the rate of PD-L1 was higher than that in HPV negative group. Similarly, HPV positive patients had higher probability to be PD-1 positive simultaneously. * P<0.05.
Table 4.
The expression of HPV in cervical cancer patients
| Pathology characteristics | Number of samples | Positive (%) | Negative (%) | P value | |
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | Yes | 40 | 35(87.50%) | 6 (12.50%) | 0.912 |
| No | 25 | 21(84.00%) | 4(16.00%) | ||
| Differentiation | Low differentiation | 12 | 11(91.67%) | 1(8.33%) | 0.206 |
| High differentiation | 31 | 23(74.19%) | 8(25.81%) | ||
| Pathological stage | I-IIa | 46 | 34(73.91%) | 12(26.09%) | 0.047* |
| IIb-IV | 20 | 19(95.00%) | 1(5.00%) | ||
| Lymph node metastasis | Yes | 14 | 13(92.86%) | 1(7.14%) | 0.227 |
| No | 47 | 37(78.72%) | 10(21.28%) | ||
| vascular invasion | Yes | 11 | 10(90.91%) | 1(9.09%) | 0.450 |
| No | 43 | 35(81.40%) | 8(18.60%) | ||
* represents P<0.05.
Correlation between PD-L1, PD-1 and CD8 expression on tumor cells and tumor malignance and metastasis
The correlation between PD-L1 expression and clinic pathological characteristics was further investigated. Although no significant correlation between the positive rate of PD-L1 staining and the differentiation status of tumor cells was identified (P = 0.344, Table 5), a significantly more frequent PD-L1 staining were found in tumor tissues from patients with higher FIGO stage (90.00% stage IIb-IV vs 62.30% stage I-IIa; P = 0.0199), lymph node metastasis (P = 0.039) or vessel invasion (P = 0.046), suggesting upregulation of PD-L1 played an important role in tumor progression in cervical cancer. A similar pattern of PD-1 and CD8 expressions were identified as well (Table 6 and 7), indicating more T cell-medicated immune responses were activated to against tumor malignance.
Table 5.
The expression of PD-L1 was correlated to neoadjuvant chemotherapy, tumor stage and metastasis in cervical cancer patients
| Pathology characteristics | Number of samples | Positive (%) | Negative (%) | P value | |
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | Yes | 40 | 35(87.50%) | 5(12.50%) | <0.010* |
| No | 32 | 14(43.75%) | 18(56.25%) | ||
| Differentiation | Low differentiation | 12 | 9(75.00%) | 3(25.00%) | 0.344 |
| High differentiation | 40 | 24(60.00%) | 16(40.00%) | ||
| Pathological stage | I-IIa | 61 | 38(62.30%) | 23(37.70%) | 0.0199* |
| IIb-IV | 20 | 18(90.00%) | 2(10.00%) | ||
| Lymph node metastasis | Yes | 18 | 15(83.33%) | 3(16.67%) | 0.039* |
| No | 60 | 37(61.67%) | 23(38.33%) | ||
| Vascular invasion | Yes | 13 | 11(84.62%) | 2(15.38%) | 0.046* |
| No | 55 | 30(54.55%) | 25(45.45%) | ||
* represents P<0.05.
Table 6.
The expression of PD-1 was correlated to neoadjuvant chemotherapy, tumor stage and metastasis in cervical cancer patients
| Pathology characteristics | Number of samples | Positive (%) | Negative (%) | P value | |
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | Yes | 40 | 35(87.50%) | 5(12.50%) | 0.001* |
| No | 32 | 17(53.12%) | 15(46.88%) | ||
| Differentiation | Low differentiation | 12 | 10(83.33%) | 2(16.67%) | 0.136 |
| High differentiation | 40 | 24(60.00%) | 16(40.00%) | ||
| Pathological stage | I-IIa | 61 | 37(60.67%) | 24(39.33%) | 0.015* |
| IIb-IV | 20 | 18(90.00%) | 2(10.00%) | ||
| Lymph node metastasis | Yes | 18 | 16(88.89%) | 2(11.11%) | 0.017* |
| No | 60 | 35(58.33%) | 25(41.67%) | ||
| vascular invasion | Yes | 13 | 12(92.31%) | 1(7.69%) | 0.016* |
| No | 55 | 31(56.36%) | 24(43.67%) | ||
* represents P<0.05.
Table 7.
The expression of CD8 was correlated to neoadjuvant chemotherapy, tumor stage and metastasis in cervical cancer patients
| Pathology characteristics | Number of samples | Positive (%) | Negative (%) | P value | |
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | Yes | 40 | 35(87.50%) | 5(12.50%) | <0.010* |
| No | 32 | 16(50.00%) | 16(50.00%) | ||
| Differentiation | Low differentiation | 12 | 10(83.33%) | 2(16.67%) | 0.103 |
| High differentiation | 40 | 23(57.50%) | 17(42.5%) | ||
| Pathological stage | I-IIa | 61 | 36(59.02%) | 25(40.98%) | 0.034* |
| IIb-IV | 20 | 17(85.00%) | 3(15.00%) | ||
| Lymph node metastasis | Yes | 18 | 16(88.89%) | 2(11.11%) | 0.017* |
| No | 60 | 35(58.33%) | 25(41.67%) | ||
| Vascular invasion | Yes | 13 | 12(92.31%) | 1(7.69%) | <0.010* |
| No | 55 | 29(52.73%) | 26(47.27%) | ||
* represents P<0.05.
PD-L1, PD-1 and CD8 expression in relation to neoadjuvant chemotherapy in cervical cancer
The expression of PD-L1, PD-1, and CD8 of specimens from patients with or without neoadjuvant chemotherapy were directly compared (Table 5-7). The positive rates of PD-L1, PD-1 and CD8 in patients with neoadjuvant chemotherapy were significantly higher than those in patients without neoadjuvant chemotherapy (PD-L1: 87.50% vs 43.75%, P < 0.01; PD-1: 87.5% vs 53.12%, P=0.001; CD8: 87.50% vs 50.00%, P < 0.01).
The differentiation status, FIGO stage, lymph node metastasis and vessel invasion between two cohorts of patients were further compared. However, no statistic difference of characteristics was found between these two cohorts (Table 8), suggesting up-regulation of PD-L1 and increased number of T cells in patients previously treated with neoadjuvant chemotherapy was not due to difference in tumor malignance.
Table 8.
Characteristics of patients with neoadjuvant chemotherapy and patients without neoadjuvant chemotherapy
| Patients with neoadjuvant chemotherapy | Patients without neoadjuvant chemotherapy | P value | |
|---|---|---|---|
| Differentiation | 0.558 | ||
| Low differentiation | 16 | 15 | |
| High differentiation | 5 | 7 | |
| Pathological stage | 0.495 | ||
| I-IIa | 32 | 29 | |
| IIb-IV | 2 | 0 | |
| Lymph node metastasis | 0.073 | ||
| Yes | 23 | 24 | |
| No | 10 | 3 | |
| Vascular invasion | 0.075 | ||
| Yes | 18 | 23 | |
| No | 8 | 2 |
Discussion
In this study, we investigated the expressions of PD-1 and PD-L1 in cervical cancer cells and their correlations with pathological and clinical characteristics in patients with cervical cancer. Our results have demonstrated the over-expression of PD-L1, PD-1, CD8 and HPV-E6 in most of the cervical cancer tissues, which were related to the tumor stage and grade, lymphatic metastasis and vascular invasion. The expressions of PD-L1, PD-1 and CD8 in patients with or without neoadjuvant chemotherapy were also compared.
Our results showed that PD-L1 expressed on the surface of tumor cells overwhelmingly presented in cervical cancer tissues, while most of the CD8+ and PD1+ cells were in stroma around the nest of tumor tissues (Figure 1 and Table 2). CD8+ T cells among tumor infiltrating cells (TILs) were demonstrated to have in situ function and act as direct cancer cell killers 17, 18. The presence of CD8+ T cells was only found at the tumor invasive margin in cervical cancers, which suggesting a defect of T lymphocytes infiltration. Considering the PD-L1/PD-1 axis is one of the most well-known immune-checkpoint pathways as a mechanism for tumor cells to evade and inhibit immune response, the defect of T-lymphocytes infiltration was possibly due to the overexpression of PD-L1 19. Further studies are needed to investigate whether the blockade the PD-L1/PD-1 pathway could unlock the potency of T cell-mediated immunity in patients with cervical cancer.
In this study, the expression of HPV-E6 oncoprotein was found in most of the squamous cancer, which was consistent with previous reports 20. In addition, the PD-L1, PD-1 and CD8 were also strongly stained in HPV positive cancer tissues. Similar results could be found in HPV-associated head and neck squamous cell carcinomas which is another tumor caused by HPV infection 21. It suggested that PD-L1/PD-1 interaction might create an immune-privileged site for HPV infection, and support further development of tumor by resisting immune response. However, the underlying mechanisms for the activation of PD-L1/PD-1 pathway in HPV-associated cancers are yet to know, and its role played in other HPV-associated cancers including genital and lung cancers need further studies.
We have also demonstrated the over-expression of PD-L1, PD1 and CD8 was associated with aggressive features such as clinical lymph nodes metastatic or vascular invasion. It indicates that the detection of over-expression of PD-L1, PD-1 or CD8 in cervical cancer tissue from patients could be used as biomarkers of cervical cancer to evaluate the malignancy of cervical cancer. However, we failed to find the link between the expression of PD-L1, PD1 and CD8 and prognosis of patients due to limitation of completing follow-up information. Further studies to investigate whether the expression of PD-L1 and CD8+ TILs could be served as prognostic biomarkers in cervical cancer are needed.
The efficacy of neoadjuvant chemotherapy for patients at stage Ib2 or IIa2 with tumor over 4 cm had been proved, and were widely used to shrank tumor to offer patients opportunities for the surgery. However, previous studies have shown that the chemotherapy increased PD-L1 expression in epithelial ovarian cancer, thymoma, melanoma and glioblastoma 22-24. In vitro study has also demonstrated that the cisplatin could also induce expression of PD-L1 in PD-L1-negative epithelial ovarian cancer cell lines 21. In this study, an increase ratio of PD-L1 positivity was found in tissues from patients after neoadjuvant chemotherapy compared with patients without neoadjuvant chemotherapy, even the clinical features of tumor stage, tumor differentiation, node metastasis and vascular invasion between these two cohorts have no statistic difference. Although it could not conclude the treatment activate PD-L1/PD-1 pathway, it suggested that neoadjuvant chemotherapy might associated with the increase of PD-L1 expression. Therefore, the over expression of PD-1 and PD-L1 suggested potential chemotherapy resistance, which is of guiding significance for medicine application in future. However, more studies on the PD-L1/PD-1 expression in pre/post chemotherapy specimens are needed to understand the underlying mechanisms and correlation with the chemotherapy treatment efficacy.
Taken together, the present results confirm the relative high proportion of PD-L1 expression in tissues from patients with cervical cancer, and the positive rate increases with tumor progression. These observations suggested that targeting the PD-1/PD-L1 axis may be a promising immunotherapy of cervical cancer, especially for patients in advanced stage. The elevated level of PD-L1 expression in patients with neoadjuvant chemotherapy also suggested the potential combination strategies, such as anti-PD-1/PD-L1 drugs with/after neoadjuvant chemotherapy.
Acknowledgments
This work was supported by the Zhaoke Pharmaceutical (Hefei) Company Limited and Lee's Pharmaceutical (Hong Kong) Limited.
Author contributions
HL and LH contributed to the design of the study and drafting the manuscript. YM and XH contributed to the immunohistochemistrical staining processing. SL, XH, MSKW, and XL contributed to the data analysis, data collection. YM, HL and JH contributed to the drafting of the manuscript. YM, HL and LH reviewed the manuscript. All authors read and approved the final report.
References
- 1.Burk RD, Chen Z, Saller C. et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insinga RP, Perez G, Wheeler CM, Koutsky LA, Garland SM, Leodolter S. et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev. 2011;20(2):287–296. doi: 10.1158/1055-9965.EPI-10-0791. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H, Li L, Su H, Lin B, Zhang X, Xue S. et al. Concurrent paclitaxel/cisplatin chemoradiotherapy with or without consolidation chemotherapy in high-risk early-stage cervical cancer patients following radical hysterectomy: preliminary results of a phase III randomized study. Oncotarget. 2016;7(43):70969. doi: 10.18632/oncotarget.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luvero D, Plotti F, Aloisi A, Capriglione S, Ricciardi R, Miranda A. et al. Patients treated with neoadjuvant chemotherapy+ radical surgery+ adjuvant chemotherapy in locally advanced cervical cancer: long-term outcomes, survival and prognostic factors in a single-center 10-year follow-up. Medical Oncology. 2016;33(10):110. doi: 10.1007/s12032-016-0830-0. [DOI] [PubMed] [Google Scholar]
- 5.Park JY, Kim DY, Kim JH, Kim YT, Nam JH. et al. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. British journal of cancer. 2010;102(12):1692–1698. doi: 10.1038/sj.bjc.6605705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh GK, RE Azuine, M Siahpush. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. International Journal of MCH and AIDS. 2015;1(1):17–30. doi: 10.21106/ijma.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, JP Allison. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 8.Pennock GK, LQ Chow. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist. 2015;20(7):812. doi: 10.1634/theoncologist.2014-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penalozamacmaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE. et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. The Journal of experimental medicine. 2014;211(9):1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Z, Chen Y, Zhao S, Yang Z, Yao X, Guo S. et al. Intrahepatic PD-1/PD-L1 up-regulation closely correlates with inflammation and virus replication in patients with chronic HBV infection. Immunological investigations. 2009;38(7):624–63. doi: 10.1080/08820130903062210. [DOI] [PubMed] [Google Scholar]
- 11.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends in molecular medicine. 2015;21(1):24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Medicine. 2013;2(5):662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, Song Y, Lu YL, Sun LZ, Wang HW. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139(4):513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD-L1 in cervical intraepithelial neoplasia and cervical cancers. Modern Pathology. 2015;28(12):1594. doi: 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Park JS, Jeong YH, Son J, Ban YH, Lee BH. et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. Journal of Immunology. 2015;194(12):5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 16.Heeren AM, Simone P, Bleeker Maaike CG, Gaarenstroom KN, Jacobus VDV, Kenter GC. et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Modern Pathology. 2016;29(7):753–763. doi: 10.1038/modpathol.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda Y, Kiyotani K, Yew PY, Sato S, Imai Y, Yamaguchi R. et al. Clinical significance of T cell clonality and expression levels of immune-related genes in endometrial cancer. Oncology Reports. 2017;37(5):2603–2610. doi: 10.3892/or.2017.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sine Hadrup, Marco Donia, Per thor Straten. Effector CD4 and CD8 T Cells and Their Role in the Tumor Microenvironment. Cancer Microenvironment. 2013;6(2):123–133. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunology Immunotherapy. 2007;56(5):739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexi A, Wright MPH, Brooke E, Howitt MD, Andrea P, Myers MD. et al. Oncogenic mutations in cervical cancer. Cancer. 2013;119(21):3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B. et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Research. 2013;73(6):1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F. et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Annals of Oncology. 2016;28(3):651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 23.Derer A, Spiljar M, Baumler M, Hecht M, Fietkau R, Frey B. et al. Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Frontiers in Immunology. 2016;7(2):610. doi: 10.3389/fimmu.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsuya Y, Horinouchi H, Asao T, Kitahara S, Goto Y, Kanda S. et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4–10. doi: 10.1016/j.lungcan.2016.05.007. [DOI] [PubMed] [Google Scholar]