Abstract
The emergence of non-coding RNAs (ncRNAs) has challenged the central dogma of molecular biology that dictates that the decryption of genetic information starts from transcription of DNA to RNA, with subsequent translation into a protein. Large numbers of ncRNAs with biological significance have now been identified, suggesting that ncRNAs are important in their own right and their roles extend far beyond what was originally envisaged. ncRNAs do not only regulate gene expression, but are also involved in chromatin architecture and structural conformation. Several studies have pointed out that ncRNAs participate in heart disease; however, the functions of ncRNAs still remain unclear. ncRNAs are involved in cellular fate, differentiation, proliferation and tissue regeneration, hinting at their potential therapeutic applications. Here, we review the current understanding of both the biological functions and molecular mechanisms of ncRNAs in heart disease and describe some of the ncRNAs that have potential heart regeneration effects.
Keywords: Non-coding RNAs, Cardiac regeneration, Cardiac fate, Proliferation, Differentiation, Reprograming
1. Introduction
Cardiovascular disease is a leading cause of death around the world, and is often associated with acute myocardial infarction (MI) resulting in chronic heart failure [1]. The acute shortage of oxygen supply leads to a progressive loss of cardiomyocytes through apoptosis and necrosis. Unlike in lower organisms such as zebrafish [2], in adult mammalian hearts cardiomyocytes show only limited proliferation and regeneration capacity [3]. To understand the potential roles of the genome in regulating cardiac regeneration, genome-wide transcriptome profiling and epigenomics in cardiomyocytes have been studied from various perspectives [4], [5], [6], [7], [8]. Data from these detailed genetic analyses have begun to reveal the complex transcriptional networks and epigenetic states associated with cardiomyocytes. Some of these findings have already provided new insights and allowed the creation of start-up platforms to remodel transcriptional circuits, as well as reshaping the epigenetic landscape of cardiomyocytes as novel strategies to stimulate heart regeneration [8], [9], [10].
The decoding of the human genome has revealed that less than 2% of the total genome comprises protein-encoding genes, with the remainder being non-protein coding [11], [12], [13]. Through the advancement of technologies such as microarrays and next-generation sequencing (NGS), it has been found that 98% of non-coding sequences are actively transcribed to produce non-coding transcripts, termed as ncRNAs. ncRNAs are divided into two groups by an arbitrary length cutoff, small non-coding RNAs (sncRNAs) and long non-coding RNAs (lncRNAs) [14]. The biological functions and molecular mechanisms of ncRNAs have been studied intensively and they have recently been identified as crucial players in the cardiovascular system.
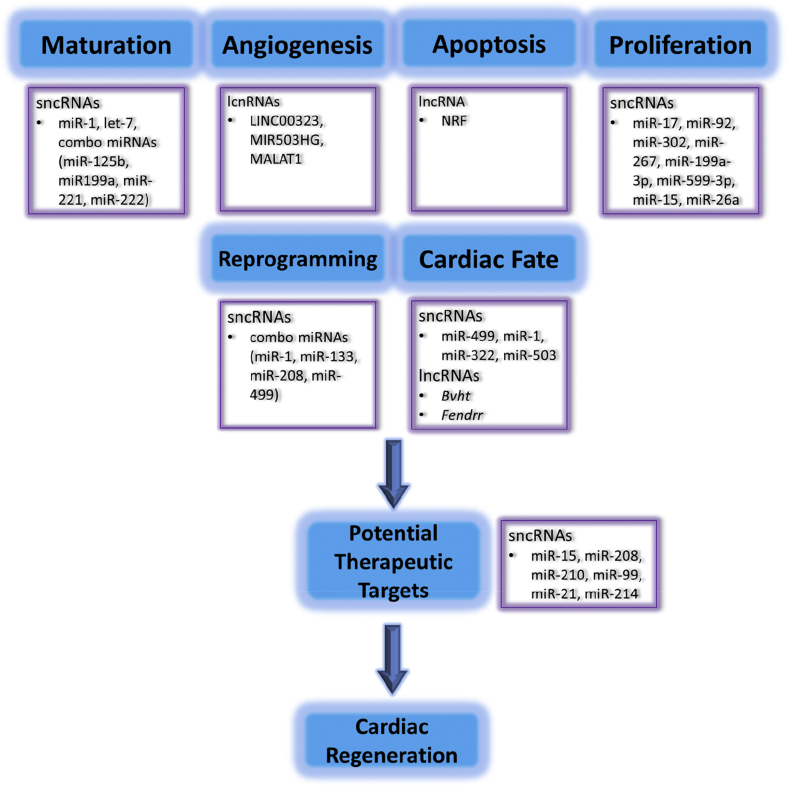
Cardiac regeneration can be achieved through several approaches such as induction of endogenous cardiomyocyte proliferation, manipulation of cardiac fate by differentiation from an induced pluripotent state, and trans-differentiation from endogenous non-myocytes to cardiomyocytes [15], [16], [17], [18], [19], [20]. All of these events are performed through transcriptional modulation of defined genes. Recently, emerging evidence has shown that non-coding RNAs (ncRNAs) play an important role in regulating genetic networks through transcriptional modulation and epigenetics remodelling, and can sequentially govern cardiomyocyte fate and potentially mediate heart regeneration [21], [22]. In this review, we summarize the biological role and functional mechanisms of ncRNAs, as well as potential therapeutic targets in regulating cardiomyocyte regeneration (Fig. 1).
Fig. 1.
The functional roles of ncRNAs in cardiac regeneration.
2. Small NON-CODING RNAs
Small non-coding RNAs (sncRNAs) are RNAs comprising around 22 nucleotides. They are classified as miRNAs, siRNAs or PIWI-interacting RNAs (piRNA) according to their biogenesis pathways [23]. Recently, somatic piRNA-like RNAs have also been discovered [24]. Among these sncRNAs, microRNAs (miRNAs) are the most widely explored. miRNAs are transcribed by RNA polymerase II, and the primary miRNAs (Pri-miRNAs) are cleaved by RNase III Drosha in the nucleus and then exported into the cytoplasm. Cytoplasmic miRNA precursor (pre-miRNA) is further digested by another RNase III protein, Dicer. Argonaute (Ago) protein binds to one strand of mature miRNA and guides the miRNA to the 3′UTR of mRNA by sequence complementarity-based targeting. This targeting leads to mRNA degradation or deadenylation, or inhibits the translation process [25]. Interestingly, studies in microRNA transgenic mice suggest that cardiac microRNAs can indirectly regulate cardiac microRNA expression [26]. In cardiac regenerative medicine, miRNAs play several roles, including cell survival, proliferation, differentiation/reprogramming, angiogenesis and inflammation [27]. One of the most important and difficult issues is to increase the number of functional cardiomyocytes to replace injured cells following injury. Several different approaches have been explored including cardiomyocyte proliferation, stem cell differentiation and cardiac fibroblast proliferation, as discussed below.
2.1. MICRORNAs as inducers of cardiomyocyte proliferation
In rodent models, the rate of cardiomyocyte proliferation is high during development and declines after birth. A second peak of cardiomyocyte cell-cycle entry, measured by DNA labelling, results in binucleation [28]. It is generally accepted that the mammalian heart has limited reparative capacity because cardiomyocyte proliferation is restricted [29]. Indeed, the annual rate of cardiomyocyte turnover in the adult mouse heart under normal conditions is less than 1% [30]. However, a degree of cardiomyocyte proliferation can be stimulated after injury [30]. In humans, cardiomyocyte renewal has also been observed, suggesting that the mammalian heart has the capacity, although limited, to re-enter the cell system [3]. Therefore, induction of cardiomyocyte proliferation may be contemplated as an approach to replenish damaged cardiomyocytes for cardiac repair.
In zebrafish, the heart can be fully replaced with new myocardium post injury [2]. The proliferating cardiomyocytes in the uninjured and injured hearts of zebrafish are around 3% and 20%, respectively [2], whereas in mammalian hearts proliferating cardiomyocytes are around 0.0006% and 0.0083% [31], [32]. RNA sequencing comparing the differentially expressed miRNAs in the injured hearts of zebrafish and mice found that miR-26a is downregulated in the zebrafish heart but does not change in mouse hearts following injury [33]. Inhibition of miR-26a stimulates the proliferation of mouse neonatal cardiomyocytes in vitro and in vivo through regulation of cell cycle negative regulators [33].
Developmental studies using mouse models reveal that several miRNAs are involved in governing the proliferation of cardiomyocytes. Genetic deletion of miR-17-92 cluster (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92a-1 are members of the miR-17-92 cluster) results in postnatal lethality, displaying major defects in the lung and heart [34]. Another study has shown smaller hearts in cardiac specific miR-17-92 knockout mice, owing to the decreased proliferation capacity of cardiomyocytes in both the neonatal and adult hearts [35]. Cardiac deletion of miR-302-367 also leads to thinning of ventricular walls due to reduced cardiomyocyte proliferation in the embryonic heart [36]. Intriguingly, overexpression of both miR-17-92 and miR-302-267 was able to induce cardiomyocyte proliferation in embryonic and postnatal hearts [35], [36]. The ectopic expression of both miRNAs reduces scar formation and improves the function of infarcted hearts [35], [36]. A comparison of gene expression in miR-17-92 knockout mice and transgene mice identified a tumor suppressor gene phosphatase and tensin homolog (PTEN) as a target of miR-19a/b. Overexpression of PTEN significantly reduced miR-19a/b-induced proliferation of mouse neonatal cardiomyocytes [35]. Using HITS-CLIP (throughput RNA sequencing by cross-linking immunoprecipitation), the Hippo pathway was found to be regulated by miR-302 [36]. The Hippo pathway is a negative regulator of cardiomyocyte proliferation and heart size during development [37].
High throughput screening using a synthetic miRNA library has identified miRNAs that can increase cardiomyocyte proliferation in new-born mouse hearts [38]. After refinement and selection using cardiomyocytes of different species, and also fully differentiated cardiomyocytes from adult hearts, miR-199a-3p and miR-590-3p emerged as the candidates with the greatest potential for further investigation. Using adeno-associated virus 9 (AAV9) delivery to overexpress either miRNA resulted in the restoration of cardiac function following injury, with reduction in infarct size and an increase in the number of proliferating cardiomyocytes [38]. Three genes were downregulated by miR-199a-3p and miR-590-3p transfection and two of them were direct targets of these two microRNA: Homer1 and Hopx. Homer1 is a calcium regulator and Hopx is a repressor of embryonic cardiomyocyte proliferation [38]. Knockdown of these genes enhanced neonatal cardiomyocyte proliferation [38].
Exercise is known to be beneficial to the cardiovascular system. Exercise induces physiological cardiac hypertrophy and induces cardiac protective microRNAs [39]. For example, miR-222 is an exercise-induced cardio protective microRNA and is known to play a role in cardiomyocyte proliferation [40]. Overexpression of miR-222 increased cell size and proliferating cells in neonatal cardiomyocytes [40]. Inhibition of miR-222 reduced exercise-induced cardiac hypertrophy and increased the number of proliferating cardiomyocytes [40]. Its target genes p27 and Hipk1 also contributed to the effect of miR-222 in cell proliferation [40]. Interestingly, an elevation of circulating miR-222 could be detected after acute exercise in heart failure patients [40].
In addition to these positive regulators, there are miRNAs that negatively regulate cardiomyocyte proliferation. The miR-15 family has been reported to be involved in regulating the postnatal switch of neonatal mouse cardiomyocytes to terminal differentiation [41]. This miR-15 family is upregulated shortly after birth and contributes to cardiomyocyte mitotic arrest by regulating Chek1, a gene that has been implicated in the regulation of mitosis [41]. Overexpression of miR-195 (a member of the miR-15 family) impairs cardiomyocyte proliferation during the embryonic stage and the regeneration capability of P1 neonatal mice after injury [41], [42]. Importantly, inhibition of the miR-15 family stimulates the proliferation of adult cardiomyocytes and promotes the functional recovery of heart muscle after MI injury [42].
2.2. MICRORNAs control cardiac fate and maturation
In addition to enhancing the proliferation capability of existing cardiomyocytes, injecting cardiac stem cells or cardiomyocytes derived from pluripotent stem cells is another therapeutic approach in the field of heart regeneration. miRNAs have been shown to regulate or enhance the differentiation of stem cell-derived cardiomyocytes. For example, miR-499, which is located in the intron of the Myh7b gene, enhances myocyte differentiation of cardiac stem cells by repressing Sox6 and Rod1 [43]. The injection of cardiac stem cells overexpressing miR-499 further improved the ventricular function and restored myocardial mass in infarcted rat hearts. This effect is attributed to the formation of functionally competent cardiomyocytes from cardiac stem cells [43]. The level of miR-499 has also been shown to be increased during cardiomyocyte differentiation of human embryonic stem cells [44], [45]. Furthermore, ectopic expression of miR-499 in these human embryonic stem cells promotes their ventricular specification [44].
In addition to miR-499, miR-1, which is highly enriched in cardiomyocytes, also promotes the differentiation of cardiomyocytes from human pluripotent stem cells [44], [45], [46], [47]. Forced expression of miR-1 enhances mesoderm lineage genes, while inhibiting the differentiation of human embryonic stem cells into ectodermal or endodermal lineages [46]. An increased number of beating embryoid bodies was further generated from these cells compared to control embryonic stem cells. The same effect was also demonstrated in differentiating human induced pluripotent stem (iPS) cells [47]. Expression of miR-1 enhanced the expression of cardiac transcription factors and sarcomeric genes and by inhibiting the WNT and fibroblast growth factor (FGF) signalling pathways, expression of miR-1 promoted human cardiomyocyte formation and suppressed endothelial cell differentiation of human iPS cells [47].
In a study to identify miRNAs involved in regulating early cardiac fate, high-throughput sequencing was performed to examine the miRNAs which are enriched in Mesp1-lineage cells [48]. This study used a Mesp1 genetic tracing system that allowed isolation of Mesp1-lineage cells during embryonic stem cell differentiation. Among 140 miRNAs that are enriched in Mesp1 YFP + embryonic stem cells, the miR-322/-503 cluster bears MESP1-binding sites and is specifically expressed in the looping heart. Exogenous expression of miR-322/-503 was also found to drive embryonic stem cells toward the cardiac fate [48].
Directing these pluripotent stem cells toward cardiac fate is known to increase the yield of cardiomyocytes, which is necessary to provide a scalable number of cells for future cardiac stem cell therapy [19]. Several studies have observed a ventricular arrhythmia following the transplantation of these pluripotent stem cell-derived cardiomyocytes, which is a significant obstacle in their application to translational medicine [20], [49]. This complication is usually due to the immaturity of these pluripotent stem cell-derived cardiomyocytes, which bear a neonate-like morphology, metabolism, electrophysiology and contractile function. Several approaches are being actively pursued to enhance the maturation of these immature cardiomyocytes towards the adult phenotype. It has been demonstrated that miR-1 can aid the electrophysiological maturation of cardiomyocytes [44]. Another study has identified let-7 as an miRNA that can enhance the metabolic maturation of these fetal-like cardiomyocytes with an increase in fatty acid metabolism, while decreasing PI3/AKT/insulin signalling. This increase in maturation is also accompanied by an increase in cell area and contraction force [50]. In our lab, we have identified a combination of miRNAs (miR-125b, miR-199a, miR-221 and miR-222) that can promote the maturation of these embryonic stem cell-derived cardiomyocytes through their natural interaction with endothelial cells [51]. The exogenous addition of this miR-combo enhanced the maturation-associated phenotype including larger cell area, lower resting membrane potential, increased sarcomeres and improved Connexin-43 organization of both mouse and human cardiomyocytes differentiated from embryonic stem cells.
2.3. MICRORNAs as direct reprogramming factors
Another potential method that can be utilized in repairing the injured heart is direct reprogramming of other non-cardiac cells into cardiomyocytes [16], [52]. In addition to several cardiac transcription factors that have been reported to directly reprogram fibroblasts into cardiomyocyte-like cells (iCM), a combination of miRNAs has also been identified which can successfully generate iCMs from non-myocytes [53]. Based on current knowledge of cardiac development and differentiation, the authors selected 6 candidate miRNAs that potentially determine cardiac cell fate. Following several selections of different combinations, they identified a combination of 4 miRNAs (miRNAs 1, 133, 208, and 499; miR-combo) which were able to directly convert cardiac fibroblasts into cardiomyocytes [45], [53]. Both in vitro and in vivo experiments have demonstrated that the introduction of this miR-combo into cardiac fibroblasts is capable of reprogramming fibroblasts into cells that express cardiomyocyte-specific genes and proteins and exhibit calcium oscillations and electrophysiological characteristics of iCM [53], [54]. In the same study, the authors also used double transgenic mice (Fsp1Cre/tdTOMATO transgenic mice) that can genetically label all cardiac fibroblasts, to isolate and analyse the reprogrammed fibroblasts. Although these tdTOMATO cells showed a range of morphological structure including small non-myocytes and intermediate reprogrammed cells, the tdTOMATO/Troponin T double positive cells exhibit mature cardiac markers with action potential similar to tdTOMATO negative cardiomyocytes [54]. The delivery of lentivirus expressing the same miR-combo into the injured heart significantly increased the ejection fraction and reduced the infarct size [53], [54].
2.4. Circulating MICRORNAs as diagnostic biomarkers in cardiovascular diseases
In 2008, circulating miRNAs were discovered in the bloodstream in the context of cancer detection [55]. In the plasma, pri-miRNAs and mature miRNAs are released from cells and carried by vesicles, such as exosomes and microvesicles, or proteins, such as Ago2 and HDL, to prevent their degradation by ribonucleases [56], [57]. In addition to this normal release, necrotic and apoptotic cells also passively release miRNAs into the plasma with Ago2 protein [58], [59] or apoptotic bodies [60]. It is believed that circulating microRNAs carried by exosomes may mediate intracellular communication [61], [62]. To serve as a biomarker for detecting cardiovascular diseases, tissue-specific and disease-related miRNAs, such as miR-1, miR133a, miR-208a, and miR-499, are important candidates. These microRNAs were found to be elevated in the plasma within one hour after left anterior descending artery ligation [63].
miR-1 is an evolutionarily-conserved, cardiac and muscle specific miRNA accounting for approximately half of the miRNAs in the heart [64]. It negatively regulates ventricular cardiomyocyte proliferation during cardiogenesis [65] and inhibits cell growth during cardiac hypertrophy [66]. miR-1 is upregulated in acute myocardial infarction (MI) in both human and animal hearts [67], [68] and its upregulation induces arrhythmogenesis post-MI by targeting the expression of potassium channels and connexin43 [68]. Overexpression of miR-1 in mice induces cardiac remodelling through targeting calmodulin and cardiac myosin light chain kinase (cMLCK) proteins, as well as elevating ROS stress through interfering with the expression of antioxidant enzymes [69], [70]. Normally, miR-1 is nearly undetectable in the plasma. However, it can be detected in the plasma of MI patients at the onset of disease, and an increased level of miR-1 is correlated with prolonged QRS [71], [72], [73], [74]. Circulating miR-1 can also be detected in animal MI models [71], [73], suggesting that the mechanism regulating miR-1 release from injured hearts may be similar. Although the elevation of plasma miR-1 does not totally reflect the upregulation of miR-1 in ischemic hearts, it may serve as an adequate biomarker candidate for MI.
Although miR-133a can be transcribed from the same genome locus as miR-1 [65], it plays a protective role in the heart and is downregulated in MI [75] and cardiac hypertrophy [76], [77]. Overexpression of miR-133a attenuates cardiac hypertrophy through targeting the cytoskeleton dynamic regulators RhoA and Cdc42 and a cardiogenesis related factor, Nelf-A [76]. miR-133a also inhibits fibrosis through targeting connective tissue growth factor (CTGF) in both cardiomyocytes and cardiac fibroblasts [77]. Transplantation of miR-133-overexpressing cardiac progenitor cells into ischemic hearts protects heart function by reducing fibrosis, cardiac hypertrophy and enhancing cardiomyocyte proliferation and vascularization [78]. Interestingly, miR-133a is downregulated in the infarcted myocardium but is elevated in the plasma [71], [79], [80] and the plasma expression level in patients with acute coronary syndrome is significantly associated with risk of death [81].
miR-208a is encoded by an intron of the Myh6 gene [82]. Deletion of miR-208a inhibits cardiac hypertrophy induced by pressure-overload or hypothyroidism and restricts β-MHC expression, a gene associated with heart failure, under these stresses. On the other hand, overexpression of miR-208a upregulates β-MHC expression [83]. Additionally, miR-208a-overexpressing transgenic mice display cardiac hypertrophy and arrhythmic phenotypes [84], indicating that miR-208a mediates pathological cell growth, cardiac electrical conduction and β-MHC gene expression by targeting Thrap1 and myostatin [83], [84]. In human heart tissues, miR-208 is significantly upregulated in both MI and dilated cardiomyopathy (DCM) patients [75], [85] and its expression level is associated with clinical outcomes of DCM [85]. The level of miR-208 in the plasma of healthy people is undetectable, while miR-208a is detected in the plasma within four hours of symptom onset in more than 90% of MI patients. Surprisingly, the detection timing is even earlier than cardiac troponin-T, the a gold standard biomarker used in diagnosis of heart injury [63].
miR-499 is encoded in the Myh7b gene [86], is downregulated in MI [87] and is upregulated in human hypertrophied and failing hearts [88]. It plays both protective and detrimental roles in cardiomyocytes in different heart diseases [87], [88]. Overexpression of miR-499 in mice inhibits cardiomyocyte apoptosis through targeting α- and β-isoforms of calcineurin catalytic subunits that cause mitochondrial fission protein Drp1 accumulation [87]. However, miR-499 transgenic mice develop dilated cardiomyopathy after 20 weeks-old through targeting of Akt and MAPKs and altering phosphorylation of heat shock protein 90 (HSP90β) and protein serine/threonine phosphatase 1-α (PP1α) [88]. Plasma miR-499 can also be considered as a biomarker of MI [86], [89], [90], [91], and elevation of plasma miR-499 can be detected as early as one hour post infarction, with an expression level highly correlated with CKMB and cTnI [90]. In particular, miR-499 can also serve as a biomarker of non-ST-elevation MI in elderly patients [92].
Cardiac troponin I (cTnI) and troponin T (cTnT) are current clinical markers of cardiac injury which can be detected elevated in the blood 4 h after MI [93]. miR-208, on the other hand, can be detected one hour after MI [63] and miR-1 and miR-133 can be detected only 15 min after MI [94]. The detection sensitivity of miR-208 and cTnI in acute MI patients are around 91% and 85%, respectively [63]. Recently, using multiple microRNAs with or without combination of cTnI was found to improve diagnostic accuracy [95], [96]. This suggests that circulating microRNAs may be suitable as alternative, or perhaps superior, biomarkers for early detection of heart injury. However, there are some general limitations of using circulating microRNAs as disease markers. One significant challenge is the lack of appropriate endogenous controls to evaluate the expression level [62], [97]. Another challenge is low amount of plasma/serum microRNA [62]. Methodology of circulating microRNA extraction and detection platform may also cause inconsistency [98], [99].
2.5. MICRORNAs as therapeutic targets
miRNAs and their antisense oligonucleotides are small and can be chemically modified, and so are considered as potential therapeutic targets [99]. For example, there is a current phase II clinical trial evaluating the use of anti-miR-122 a therapeutic to target hepatitis C virus (HCV) infection [99], [100]. In addition, there are several miRNA-based therapies being developed for cardiovascular diseases [101]. So far, anti-miR-15 for the treatment of myocardial infarction is in the preclinical stage [102]. A list of current miRNA-based therapies is presented in Table 1.
Table 1.
miRNAs as potential therapeutic targets.
| miRNA | Disease model | Species | Delivery method | Outcome | Ref. |
|---|---|---|---|---|---|
| anti-miR-15 | MI | Pig | i.v. injection of LNA through the ear vein | Cardiac protection from ischemic injury | [103] |
| anti-miR-208 | Hypertension induced heart failure | Rat | s.c. injection of LNA | Reduce cardiac remodelling | [105] |
| anti-miR-34 | Transverse aortic constriction (TAC) | Mouse | s.c. injection of LNA | attenuate pathological cardiac remodelling | [135] |
| anti-199b | TAC | Mouse | i.p injection of antagomir | prevent and reverse cardiac remodelling | [136] |
| anti-miR-320 | Ischemia/reperfusion (I/R) | Mouse | i.v. injection of antagomir | reduce infarction size | [137] |
| anti-miR-652 | TAC | Mouse | s.c. injection of LNA | Rescue heart function and attenuates cardiac hypertrophy | [138] |
| anti-miR-154 | TAC | Mouse | s.c. injection of LNA | attenuate cardiac remodelling and lung congestion | [139] |
| anti-miR-92 | I/R | Pig | LNA injeciton through i.v. or regionally antegrade into the left anterior descending artery or retrograde into the anterior interventricular vein with a catheter | protecte against cardiomyocyte cell death, enhance angiogenesis and has anti-inflammatory effects | [140] |
| anti-miR-34 | Aging/MI | Mouse | i.v. injection of LNA | inhibits age-induced and ischemia-induced cardiomyocyte cell death and cardiac dysfunction | [141] |
| miR-210 overexpression | MI | Mouse | i.m. injection of minicircle DNA carrying miR-210 precursor | Improve cardiac function through regulation of angiogenesis and antiapoptosis | [106] |
| miR-99a overexpression | MI | Mouse | i.m. injection of lentiviruses carrying miR-99a precursor | Improve cardiac function and survival rate | [107] |
| miR-21 overexpression | MI | Mouse | i.m. injection of lentiviruses carrying miR-99a precursor | Reduce cardiac fibrosis and apoptosis | [108] |
| miR-24 overexpression | MI | Mouse | i.m. injection of lipofectamine 2000 mediated miR-24 mimic | Reduce cariomyocyte apoptosis and attenuated infarct size | [142] |
i.m. - intramuscular, s.c. - subcutaneous, i.v. – intravenous, i.p. – intraperitoneal.
In murine and porcine models of ischemic reperfusion, the miR-15 family (miR-15a, −15b, −16, −195, and −497) are upregulated one day after MI, and found to localize in both the infarcted area and the border zone of heart, and are upregulated for several weeks [103]. In this study, locked nucleic acid (LNA)-modified anti-miR-15 chemistries (8- or 16-mer) were used to treat the ischemic heart. Inhibition of miR-15 was achieved after systemic delivery of the therapeutic, resulting in reduction of infarct size and cardiac remodelling, and improvement in heart function [103]. This is an example of using modified oligonucleotides targeting disease-related microRNAs as a therapeutic strategy. Notably, this study was performed in a pig model of MI, which more closely resembles the anatomy and pathophysiology of human heart injury [104]. In addition, knockdown of miR-15 family increases mitotic cardiomyocytes in neonatal mice [41], which imply a potential role of miR-15 in cardiomyocyte proliferation.
In addition to miR-15, miR-208 is another potential therapeutic target. miR-208 not only serves as a biomarker of cardiomyopathy [75], [85], but is also a potential target for therapy of cardiovascular diseases. As mentioned in the previous section, miR-208 plays a critical role in pathological growth of cardiomyocytes and abnormal cardiac conduction [83], [84]. Inhibition of miR-208a by subcutaneous delivery improved cardiac function and the overall survival rate in Dahl hypertensive rats by reducing cardiac hypertrophy and fibrosis [105]. These beneficial effects of anti-miR-208 therapies indicate their potential for treating cardiovascular diseases.
Not only miRNA antisense, but miRNA precursor can serve as the “drug” for treatment of cardiovascular diseases. For example, overexpression of miR-210, miR-99, miR-21 and miR-214 in vivo reduces cell apoptosis and protects heart function after MI [106], [107], [108], [109]. The protective function of miR-210 is realized through positive regulation of angiogenesis and anti-apoptosis via targeting Efna3, an anti-angiogenic factor, and Ptp1b, which induces apoptosis [106]. miR-99 protects cell apoptosis through enhancing autophagy in ischemic mouse hearts [107] while miR-21 downregulates collagen and fibronectin expression to reduce fibrosis in the infarcted heart [108].
Although chemical modification of oligonucleotides increases the stability of anti-miRNAs or miRNA mimics in the body, their pharmacokinetics and pharmacodynamics are poorly understood [101]. Furthermore, it appears that they do not show immediate therapeutic effects – rather, taking a number of days to show the protective effects [101]. Systemic delivery also causes oligonucleotide accumulation in the liver and kidneys [101]. Improving the targeting of injured tissue and controlling the efficiency and efficacy of anti-miR drugs will be challenges in the development of microRNA therapy [110].
3. Long NON-CODING RNAs
Long non-coding RNAs (lncRNAs) are defined as a diverse class of RNA transcripts with a size larger than 200 base-pairs that contain a cryptic open reading frame, without translational ability [12]. lncRNAs are produced through general transcriptional machinery, RNA polymerase II. The expression of lncRNAs is associated with histone marks such as H3K4me2, H3K4me3, H3K9ac, H3K27ac at the transcription start site (TSS) of lncRNAs which share similar histone patterns with protein-coding genes [13]. However, lncRNAs harbor a higher DNA methylation density which is remarkably different from protein-coding genes [111]. This difference may be used to distinguish coding genes from non-coding genes. Several transcriptional factors such as p53, NFkB, Sox2, Oct4 (also known as Pou5f1), Nanog and MYC have all been identified to bind to the TSS of lncRNAs resulting in regulating or transactivating lncRNA expression [112], [113], [114]. lncRNA transcripts subsequently undergo post-transcriptional processing such as 5′ terminal methylguanosine capping, polyadenylation, alternative splicing and RNA editing [112], [115].
3.1. LNCRNAs in the heart
The limited renewal capacity of cardiomyocytes is unable to adequately cope with the damage brought about by heart disease. Understanding the cellular and molecular mechanisms of cardiac development and the response to injury may provide us the knowledge to manipulate regenerative pathways in the heart. Here, we describe several lncRNAs associated with heart disease, as well as lncRNAs which are potentially involved in cardiac regeneration (Table 2).
Table 2.
lncRNAs associated with heart disease and potential therapeutic targets.
| lncRNAs | Species | Model | Outcome | Ref |
|---|---|---|---|---|
| Bvht | mouse | mouse embryonic stem cells | cardiac fate | [21] |
| CDR1AS | human | acute myocardiac infarction | circulating lncRNA | [132] |
| Chaer | mouse | transverse aortic constriction (TAC) | regulates cardiac hypertrophy | [120] |
| Chast | mouse | transverse aortic constriction (TAC) | regulates cardiac hypertrophy | [121] |
| Fendrr | mouse | mouse embryo | cardiac lineage commitment | [143] |
| LINC00323 | human | human umbilical vein Ecs, hypoxia condition | angiogenesis and capilary formation | [127] |
| LIPCAR | human | cardiac remodelling | circulating lncRNA | [131] |
| MIR503HG | human | human umbilical vein Ecs, hypoxia condition | angiogenesis and capilary formation | [127] |
| MALAT1 | human | human umbilical vein Ecs, hypoxia condition | induces angiogenic sprouting and migration | [126] |
| NRF | mouse | ischemia/reperfusion | cardiomyocytes necrosis | [124] |
| SMILR | human | human saphenous vein vascular smooth muscle cells | smooth muscle cell proliferation | [128] |
| SENCR | human | human coronary artery smooth muscle cells | smooth muscle cell migration | [129] |
| ZFAS1 | human | acute myocardiac infarction | circulating lncRNA | [132] |
3.2. LNCRNAs in cardiac development and diseases
A precise spatio-temporal control of gene networks is crucial for both normal cardiac development, and for disease pathogenesis. The discovery of lncRNAs has shown that regulation of complex gene networks is not solely operated by protein-coding genes. Through RNA-sequencing of fetal mouse hearts at different stages during development, a subset of lncRNAs are discovered, which can be assigned into distinct profiles such as cardiac-specific lncRNAs or those which are dynamically expressed during differentiation and maturation [116], [117]. There are approximately 1237 differentially expressed lncRNAs identified throughout the heart development stages, suggesting that these lncRNAs may play a role in regulating gene networks in normal heart development [116]. Similar methods have also been utilized to identify differentially expressed lncRNAs in heart disease models. Via RNA sequencing of samples representing human heart failure, an lncRNA transcriptional profile is generated. In this profile, 3.7% of lncRNAs and 2.5% of pseudogenes were found to be differentially expressed in human heart failure compared to normal donor hearts [118]. Another group also performed RNA deep sequencing on failing human hearts before and after left ventricular assist device (LVAD) implantation. They generated a myocardial transcriptome profile from LVAD-supported conditions and found that lncRNA transcriptome showed a distinct signature that was able to distinguish ischemic hearts of different pathologies and the response to LVAD support [119]. This also provided better biological sample segregation compared to mRNA and miRNA profiles, suggesting that lncRNAs play an important role in cardiac pathology, far beyond simply regulating gene transcription [119]. Recently, a cardiac-enriched lncRNA Chaer (cardiac-hypertrophy-associated epigenetic regulator) has been identified as a key factor for cardiac hypertrophy induced by pressure overload [120]. It is located on chromosome 5 with two exons in the mouse genome, and is highly expressed in the heart [120]. Mechanistically, Chaer directly interacts with enhancer of zeste homolog 2 (EZH2) subunit of PRC2, hence inhibiting methylation of H3 Lys27 (H3K27) at the promoter region of cardiac hypertrophy genes [120]. Another lncRNA Chast (cardiac hypertrophy–associated transcript) was identified using lncRNA microarray analysis on pressure overload-induced cardiac hypertrophy [121]. Ectopic expression of Chast induces hypertrophy both in vitro and in vivo, while inhibiting Chast expression attenuates the hypertrophy condition. Plekhm1 (Pleckstrin homology domain–containing protein family M member 1) is involved in inhibition of autophagy [122]. Chast negatively regulates Plekhm1, which in turn inhibits autophagy in cardiomyocytes and drives cardiac hypertrophy [121]. Interestingly, in vivo GapmeR-mediated silencing of Chast in TAC-induced pathological cardiac remodelling model significantly attenuates cardiac hypertrophy [121]. Furthermore, lncRNA NRF (necrosis-related factor) is an lncRNA that directly binds to miR-873, titrating it out from targeting to RIPK1 (receptor-interacting serine/threonine-protein kinase 1)/RIPK3 (receptor-interacting serine/threonine-protein kinase 3). RIPK1 and 3 are mainly involved in necrotic signalling pathway [123]. Knockdown of NRF is able to reduce necrosis in hearts with MI [124].
3.3. LNCRNAs regulate cardiomyocyte fate
Braveheart (Bvht) is an lncRNA located in the mouse chromosome 18 and is highly expressed in the heart. It has been identified as an essential activator for a core cardiovascular gene network (e.g., MesP1, Gata4, Hand1, Hand2, Nkx2.5, and Tbx5) that drives cardiovascular lineage commitment [21], [125]. Interestingly, Bvht functions upstream of Mesp1 and regulates temporal activation of cardiovascular network genes through modulation of Mesp1. Moreover, Bvht is shown to interact with SUZ12, a component of PRC2, and in turn mediate the epigenetics of the core network genes [21]. Depletion of Bvht in mouse ES cells disturbs and reduces cardiomyocyte differentiation, showing that Bvht has a role in cardiac lineage commitment. However, this discovery was found to be only conserved in mice, as no Bvht homolog has been identified in other species [21]. Another lineage commitment-related lncRNA named Fendrr (FOXF1 adjacent non-coding developmental regulatory RNA) is expressed in the nascent lateral plate mesoderm, which is essential for heart and body wall development in mice [22]. Knockout of Fendrr is embryonically lethal due to developmental defects on the abdominal wall and the heart septum. Fendrr has been demonstrated to interact with PRC2 and TrxG/MLL complexes to modulate chromatin signatures of Foxf1 and Pitx2 [22]. Loss of Fendrr affects the expression of cardiac lineage-determining transcription factors Gata6 and Nkx2.5 [22]. This special ability of lncRNAs to influence cardiac lineage specification implies that they may have potential to be used to modulate cardiac regeneration.
3.4. LNCRNAs regulate NON-MYOCYTE proliferation
In addition to cardiomyocytes, non-myocytes are essential to maintain normal heart function. Endothelial cells play an important role in the heart, particularly during the formation of vasculature in the heart. Following MI, a shortage of oxygen to the heart muscle is the main cause of cardiomyocyte death, and new blood vessel formation is a crucial aspect of heart regeneration. Recently, MALAT1 has been identified with relatively high expression in endothelial cells, especially under hypoxic conditions. Knockdown of MALAT1 induces angiogenic sprouting and migration but not cell proliferation, indicating that MALAT1 controls a phenotypic switch in endothelial cells [126]. Furthermore, there are two other lncRNAs– LINC00323 and MIR503HG, which have been identified in endothelial cells under hypoxic conditions through RNA sequencing and microarray [127]. Knockdown of both of these lncRNAs repressed endothelial transcription factor GATA2, subsequently leading to angiogenic defects and inhibition of capillary formation [127]. In addition to endothelial cells, smooth muscle cells are also involved in coronary artery formation. Smooth muscle induced lncRNA enhances replication (SMILR) is a novel lncRNA that was identified by RNA-sequencing and highly expressed in the nucleus and cytosol after IL1α and PDGF treatment [128]. Knockdown of SMILR significantly reduces cell proliferation, suggesting that SMILR is a regulator of smooth muscle cell proliferation [128]. Through RNA-sequencing of human coronary artery smooth muscle cells, an lncRNA termed smooth muscle and endothelial cell–enriched migration/differentiation-associated long Non-Coding RNA (SENCR) has been identified. SENCR is mainly localized in the cytoplasm, and loss of function studies showed that smooth muscle cell migration is inhibited as well as the expression of smooth muscle contractile genes and Myocardin [129].
3.5. Circulating LNCRNAs
A genome-wide transcriptome of lncRNAs in the whole blood, plasma and heart tissue of mice with heart failure has been reported by Li et al. [130]. This study revealed significant changes in the expression level of lncRNAs in the plasma upon heart failure. For example, the lncRNA LIPCAR was up-regulated in the plasma of patients with cardiac remodelling [131]. lncRNA CDR1AS was also significantly increased in patients with acute MI, while another lncRNA ZFAS1 showed the opposite change [132]. These studies provide evidence suggesting that lncRNAs may serve as new biomarkers for heart diseases, as well as serving as therapeutic guidance for overall cardiovascular status.
3.6. lncRNAs as potential therapeutic targets
The above-mentioned studies demonstrate the biological and mechanistic function of lncRNAs in cardiac development and disease. Therefore, we hypothesize that modulation of lncRNA expression might not only attenuate cardiac disease conditions, but also potentially achieve cardiac regeneration. However, there are several limitations of lncRNAs which must be addressed before they can be therapeutically useful. First, the relationship between sequence conservation and molecular function of lncRNAs in different species is a major concern [133], [134]. Second, most lncRNAs regulate a genetic network and not a single pathway especially for lncRNAs which are involved in epigenetics and chromatin remodelling. Therefore, inhibition of lncRNAs might lead to unexpected complications [133]. Third, lncRNAs may play different roles if they contain different variants or undergo RNA modification such as RNA editing and methylation [133]. Fourth, the subcellular localization of lncRNAs gives rise to their diverse functions. It is known that the same lncRNA present in different cellular compartments such as nucleus, cytosol, or inside mitochondria plays different roles [134].
4. Conclusion
As research into ncRNAs continues, it has become clear that their roles in cardiac differentiation, specification, and regeneration are more critical and more widespread than previously thought. Moreover, high throughput screenings have identified novel ncRNAs that are critically involved in the pathophysiology of different heart diseases. Since ncRNAs can regulate transcriptome and epigenomic circuits at the cellular level, it is expected that improving our understanding of their structural and biochemical properties will further enhance our ability to carry out effective tissue regeneration. Work on ncRNAs is likely to continue for many years, as there are still many ncRNAs waiting to be identified. Some of these unknown ncRNAs are likely the missing pieces of the puzzle that are critical to the future of cardiac regenerative medicine.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank Dr. Bill Cheng and Dr. David Lundy (both at the Institute of Biomedical Sciences, Academia Sinica) for critical analysis, suggestions, and proofreading. This study was supported by the Ministry of Science and Technology (MOST 102-2321-B-001-069, 105-2325-B-001-009 and 105-2314-B-039-051), Taiwan, and the Academia Sinica Translational Medicine Program.
References
- 1.Writing Group M., Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Soliman E.Z., Sorlie P.D., Sotoodehnia N., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B. Heart disease and stroke statistics—2012 update: a report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poss K.D., Wilson L.G., Keating M.T. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabé-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B.A., Druid H., Jovinge S., Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Zhong J.F., Qiu H., Robb MacLellan W., Marbán E., Wang C. Epigenomic reprogramming of adult cardiomyocyte-derived cardiac progenitor cells. Sci. Rep. 2015;5:17686. doi: 10.1038/srep17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Chakravarty T., Zhang Y., Li X., Zhong J.F., Wang C. Single-cell transcriptome and epigenomic reprogramming of cardiomyocyte-derived cardiac progenitor cells. Sci. Data. 2016;3:160079. doi: 10.1038/sdata.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berg C.W., Okawa S., Chuva de Sousa Lopes S.M., van Iperen L., Passier R., Braam S.R., Tertoolen L.G., del Sol A., Davis R.P., Mummery C.L. Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development. 2015;142:3231–3238. doi: 10.1242/dev.123810. [DOI] [PubMed] [Google Scholar]
- 7.Beqqali A., Kloots J., Ward-van Oostwaard D., Mummery C., Passier R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. stem Cells. 2006;24:1956–1967. doi: 10.1634/stemcells.2006-0054. [DOI] [PubMed] [Google Scholar]
- 8.O'Meara C.C., Wamstad J.A., Gladstone R.A., Fomovsky G.M., Butty V.L., Shrikumar A., Gannon J.B., Boyer L.A., Lee R.T. Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ. Res. 2015;116:804–815. doi: 10.1161/CIRCRESAHA.116.304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song K., Nam Y.-J., Luo X., Qi X., Tan W., Huang G.N., Acharya A., Smith C.L., Tallquist M.D., Neilson E.G., Hill J.A., Bassel-Duby R., Olson E.N. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguirre A., Montserrat N., Zacchigna S., Nivet E., Hishida T., Krause Marie N., Kurian L., Ocampo A., Vazquez-Ferrer E., Rodriguez-Esteban C., Kumar S., Moresco James J., Yates Iii John R., Campistol Josep M., Sancho-Martinez I., Giacca M., Izpisua Belmonte J.C. In vivo activation of a conserved microrna program induces mammalian heart regeneration. Cell Stem Cell. 2014;15:589–604. doi: 10.1016/j.stem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., Kodzius R., Shimokawa K., Bajic V.B., Brenner S.E., Batalov S., Forrest A.R.R., Zavolan M., Davis M.J., Wilming L.G., Aidinis V., Allen J.E., Ambesi-Impiombato A., Apweiler R., Aturaliya R.N., Bailey T.L., Bansal M., Baxter L., Beisel K.W., Bersano T., Bono H., Chalk A.M., Chiu K.P., Choudhary V., Christoffels A., Clutterbuck D.R., Crowe M.L., Dalla E., Dalrymple B.P., de Bono B., Gatta G.D., di Bernardo D., Down T., Engstrom P., Fagiolini M., Faulkner G., Fletcher C.F., Fukushima T., Furuno M., Futaki S., Gariboldi M., Georgii-Hemming P., Gingeras T.R., Gojobori T., Green R.E., Gustincich S., Harbers M., Hayashi Y., Hensch T.K., Hirokawa N., Hill D., Huminiecki L., Iacono M., Ikeo K., Iwama A., Ishikawa T., Jakt M., Kanapin A., Katoh M., Kawasawa Y., Kelso J., Kitamura H., Kitano H., Kollias G., Krishnan S.P.T., Kruger A., Kummerfeld S.K., Kurochkin I.V., Lareau L.F., Lazarevic D., Lipovich L., Liu J., Liuni S., McWilliam S., Babu M.M., Madera M., Marchionni L., Matsuda H., Matsuzawa S., Miki H., Mignone F., Miyake S., Morris K., Mottagui-Tabar S., Mulder N., Nakano N., Nakauchi H., Ng P., Nilsson R., Nishiguchi S., Nishikawa S., Nori F., Ohara O., Okazaki Y., Orlando V., Pang K.C., Pavan W.J., Pavesi G., Pesole G., Petrovsky N., Piazza S., Reed J., Reid J.F., Ring B.Z., Ringwald M., Rost B., Ruan Y., Salzberg S.L., Sandelin A., Schneider C., Schönbach C., Sekiguchi K., Semple C.A.M., Seno S., Sessa L., Sheng Y., Shibata Y., Shimada H., Shimada K., Silva D., Sinclair B., Sperling S., Stupka E., Sugiura K., Sultana R., Takenaka Y., Taki K., Tammoja K., Tan S.L., Tang S., Taylor M.S., Tegner J., Teichmann S.A., Ueda H.R., van Nimwegen E., Verardo R., Wei C.L., Yagi K., Yamanishi H., Zabarovsky E., Zhu S., Zimmer A., Hide W., Bult C., Grimmond S.M., Teasdale R.D., Liu E.T., Brusic V., Quackenbush J., Wahlestedt C., Mattick J.S., Hume D.A., Kai C., Sasaki D., Tomaru Y., Fukuda S., Kanamori-Katayama M., Suzuki M., Aoki J., Arakawa T., Iida J., Imamura K., Itoh M., Kato T., Kawaji H., Kawagashira N., Kawashima T., Kojima M., Kondo S., Konno H., Nakano K., Ninomiya N., Nishio T., Okada M., Plessy C., Shibata K., Shiraki T., Suzuki S., Tagami M., Waki K., Watahiki A., Okamura-Oho Y., Suzuki H., Kawai J., Hayashizaki Y. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 12.Kapranov P., Cheng J., Dike S., Nix D.A., Duttagupta R., Willingham A.T., Stadler P.F., Hertel J., Hackermüller J., Hofacker I.L., Bell I., Cheung E., Drenkow J., Dumais E., Patel S., Helt G., Ganesh M., Ghosh S., Piccolboni A., Sementchenko V., Tammana H., Gingeras T.R. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 13.Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D.G., Lagarde J., Veeravalli L., Ruan X., Ruan Y., Lassmann T., Carninci P., Brown J.B., Lipovich L., Gonzalez J.M., Thomas M., Davis C.A., Shiekhattar R., Gingeras T.R., Hubbard T.J., Notredame C., Harrow J., Guigó R. The gencode v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagano T., Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Efe J.A., Hilcove S., Kim J., Zhou H., Ouyang K., Wang G., Chen J., Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 16.Ieda M., Fu J.-D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.J.-D. Fu, Nicole R. Stone, L. Liu, C.I. Spencer, L. Qian, Y. Hayashi, P. Delgado-Olguin, S. Ding, Benoit G. Bruneau, D. Srivastava, Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep.. 1 235–247. [DOI] [PMC free article] [PubMed]
- 18.Cheng Y.Y., Yan Y.T., Lundy D.J., Lo A.H., Wang Y.P., Ruan S.C., Lin P.J., Hsieh P.C. Reprogramming derived gene cocktail increases cardiomyocyte proliferation for heart regeneration. EMBO Mol. Med. 2017;9(2):251–264. doi: 10.15252/emmm.201606558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funakoshi S., Miki K., Takaki T., Okubo C., Hatani T., Chonabayashi K., Nishikawa M., Takei I., Oishi A., Narita M., Hoshijima M., Kimura T., Yamanaka S., Yoshida Y. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016;6:19111. doi: 10.1038/srep19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong J.J.H., Yang X., Don C.W., Minami E., Liu Y.-W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J., Gantz J.A., Fugate J.A., Muskheli V., Gough G.M., Vogel K.W., Astley C.A., Hotchkiss C.E., Baldessari A., Pabon L., Reinecke H., Gill E.A., Nelson V., Kiem H.-P., Laflamme M.A., Murry C.E. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klattenhoff Carla A., Scheuermann Johanna C., Surface Lauren E., Bradley Robert K., Fields Paul A., Steinhauser Matthew L., Ding H., Butty Vincent L., Torrey L., Haas S., Abo R., Tabebordbar M., Lee Richard T., Burge Christopher B., Boyer Laurie A. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grote P., Wittler L., Hendrix D., Koch F., Währisch S., Beisaw A., Macura K., Bläss G., Kellis M., Werber M., Herrmann Bernhard G. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim V.N., Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 24.Ortogero N., Schuster A.S., Oliver D.K., Riordan C.R., Hong A.S., Hennig G.W., Luong D., Bao J.Q., Bhetwal B.P., Ro S., McCarrey J.R., Yan W. A novel class of somatic small RNAs similar to germ cell pachytene piwi-interacting small RNAs. J. Biol. Chem. 2014;289 doi: 10.1074/jbc.M114.613232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 26.Matkovich S.J., Hu Y., Dorn G.W. Regulation of cardiac microRNAs by cardiac microRNAs. Circ. Res. 2013;113:62–71. doi: 10.1161/CIRCRESAHA.113.300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romaine S.P.R., Tomaszewski M., Condorelli G., Samani N.J. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101:921–928. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soonpaa M.H., Kim K.K., Pajak L., Franklin M., Field L.J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. Heart Circ. Physiol. 1996;271:H2183–H2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 29.Laflamme M.A., Murry C.E. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senyo S.E., Steinhauser M.L., Pizzimenti C.L., Yang V.K., Cai L., Wang M., Wu T.-D., Guerquin-Kern J.-L., Lechene C.P., Lee R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soonpaa M.H., Field L.J. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ. Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Soonpaa M.H., Field L.J. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 33.Crippa S., Nemir M., Ounzain S., Ibberson M., Berthonneche C., Sarre A., Boisset G., Maison D., Harshman K., Xenarios I., Diviani D., Schorderet D., Pedrazzini T. Comparative transcriptome profiling of the injured zebrafish and mouse hearts identifies miRNA-dependent repair pathways. Cardiovasc Res. 2016;110:73–84. doi: 10.1093/cvr/cvw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ventura A., Young A.G., Winslow M.M., Lintault L., Meissner A., Erkeland S.J., Newman J., Bronson R.T., Crowley D., Stone J.R., Jaenisch R., Sharp P.A., Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17∼92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Huang Z.-P., Seok H.Y., Ding J., Kataoka M., Zhang Z., Hu X., Wang G., Lin Z., Wang S., Pu W.T., Liao R., Wang D.-Z. MiR-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ. Res. 2013;112:1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian Y., Liu Y., Wang T., Zhou N., Kong J., Chen L., Snitow M., Morley M., Li D., Petrenko N., Zhou S., Lu M., Gao E., Koch W.J., Stewart K.M., Morrisey E.E. A microRNA-hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.3010841. 279ra238–279ra238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R.L., Martin J.F. Hippo pathway inhibits WNT signaling to restrain cardiomyocyte proliferation and heart size. Sci. (New York, NY) 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eulalio A., Mano M., Ferro M.D., Zentilin L., Sinagra G., Zacchigna S., Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 39.Fernandes T., Baraúna V.G., Negrão C.E., Phillips M.I., Oliveira E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H543–H552. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X., Xiao J., Zhu H., Wei X., Platt C., Damilano F., Xiao C., Bezzerides V., Boström P., Che L., Zhang C., Spiegelman B.M., Rosenzweig A. MiR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porrello E.R., Johnson B.A., Aurora A.B., Simpson E., Nam Y.-J., Matkovich S.J., Dorn G.W., van Rooij E., Olson E.N. The miR-15 family regulates post-natal mitotic arrest of cardiomyocytes. Circ. Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porrello E.R., Mahmoud A.I., Simpson E., Johnson B.A., Grinsfelder D., Canseco D., Mammen P.P., Rothermel B.A., Olson E.N., Sadek H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. U. S. A. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosoda T., Zheng H., Cabral-de-Silva M., Sanada F., Ide-Iwata N., Ogórek B., Ferreira-Martins J., Arranto C., D'Amario D., del Monte F., Urbanek K., D'Alessandro D.A., Michler R.E., Anversa P., Rota M., Kajstura J., Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Fu J.-D., Rushing S.N., Lieu D.K., Chan C.W., Kong C.-W., Geng L., Wilson K.D., Chiamvimonvat N., Boheler K.R., Wu J.C., Keller G., Hajjar R.J., Li R.A. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2011;6:e27417. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson K.D., Hu S., Venkatasubrahmanyam S., Fu J.-D., Sun N., Abilez O.J., Baugh J.J.A., Jia F., Ghosh Z., Li R.A., Butte A.J., Wu J.C. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circ. Cardiovasc Genet. 2010;3:426–435. doi: 10.1161/CIRCGENETICS.109.934281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivey K.N., Muth A., Arnold J., King F.W., Yeh R.-F., Fish J.E., Hsiao E.C., Schwartz R.J., Conklin B.R., Bernstein H.S., Srivastava D. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell stem cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu T.-Y., Lin B., Li Y., Arora A., Han L., Cui C., Coronnello C., Sheng Y., Benos P.V., Yang L. Overexpression of microRNA-1 promotes cardiomyocyte commitment from human cardiovascular progenitors via suppressing WNT and FGF signaling pathways. J. Mol. Cell Cardiol. 2013;63:146–154. doi: 10.1016/j.yjmcc.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen X., Soibam B., Benham A., Xu X., Chopra M., Peng X., Yu W., Bao W., Liang R., Azares A., Liu P., Gunaratne P.H., Mercola M., Cooney A.J., Schwartz R.J., Liu Y. MiR-322/-503 cluster is expressed in the earliest cardiac progenitor cells and drives cardiomyocyte specification. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9551–9556. doi: 10.1073/pnas.1608256113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., Ogasawara T., Okada K., Shiba N., Sakamoto K., Ido D., Shiina T., Ohkura M., Nakai J., Uno N., Kazuki Y., Oshimura M., Minami I., Ikeda U. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 50.Kuppusamy K.T., Jones D.C., Sperber H., Madan A., Fischer K.A., Rodriguez M.L., Pabon L., Zhu W.-Z., Tulloch N.L., Yang X., Sniadecki N.J., Laflamme M.A., Ruzzo W.L., Murry C.E., Ruohola-Baker H. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E2785–E2794. doi: 10.1073/pnas.1424042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Desy S., Chen J.-H., Lundy David J., Liu C.-H., Hwang S.-M., Pabon L., Shieh R.-C., Chen C.-C., Wu S.-N., Yan Y.-T., Lee S.-T., Chiang P.-M., Chien S., Murry Charles E., Hsieh Patrick C.H. Defined microRNAs induce aspects of maturation in mouse and human embryonic-stem-cell-derived cardiomyocytes. Cell Rep. 2015;12:1960–1967. doi: 10.1016/j.celrep.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 52.Cao N., Huang Y., Zheng J., Spencer C.I., Zhang Y., Fu J.-D., Nie B., Xie M., Zhang M., Wang H., Ma T., Xu T., Shi G., Srivastava D., Ding S. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352:1216–1220. doi: 10.1126/science.aaf1502. [DOI] [PubMed] [Google Scholar]
- 53.Jayawardena T.M., Egemnazarov B., Finch E.A., Zhang L., Payne J.A., Pandya K., Zhang Z., Rosenberg P., Mirotsou M., Dzau V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayawardena T.M., Finch E.A., Zhang L., Zhang H., Hodgkinson C.P., Pratt R.E., Rosenberg P.B., Mirotsou M., Dzau V.J. MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function. Circ. Res. 2015;116:418–424. doi: 10.1161/CIRCRESAHA.116.304510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O'Briant K.C., Allen A., Lin D.W., Urban N., Drescher C.W., Knudsen B.S., Stirewalt D.L., Gentleman R., Vessella R.L., Nelson P.S., Martin D.B., Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guay C., Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- 57.Cortez M.A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A.K., Calin G.A. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arroyo J.D., Chevillet J.R., Kroh E.M., Ruf I.K., Pritchard C.C., Gibson D.F., Mitchell P.S., Bennett C.F., Pogosova-Agadjanyan E.L., Stirewalt D.L., Tait J.F., Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turchinovich A., Weiz L., Langheinz A., Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B., Hristov M., Köppel T., Jahantigh M.N., Lutgens E., Wang S., Olson E.N., Schober A., Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000610. ra81-ra81. [DOI] [PubMed] [Google Scholar]
- 61.Morel O., Toti F., Hugel B., Freyssinet J.-M. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr. Opin. Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 62.Gupta S.K., Bang C., Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ-cardiovasc Gene. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 63.Wang G.-K., Zhu J.-Q., Zhang J.-T., Li Q., Li Y., He J., Qin Y.-W., Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 64.Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Samal E., Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 66.Ikeda S., He A., Kong S.W., Lu J., Bejar R., Bodyak N., Lee K.-H., Ma Q., Kang P.M., Golub T.R., Pu W.T. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol. 2009;29:2193–2204. doi: 10.1128/MCB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bostjancic E., Zidar N., Stajer D., Glavac D. MicroRNA miR-1 is up-regulated in remote myocardium in patients with myocardial infarction. Folia Biol-Prague. 2010;56:27–31. [PubMed] [Google Scholar]
- 68.Yang B., Lin H., Xiao J., Lu Y., Luo X., Li B., Zhang Y., Xu C., Bai Y., Wang H., Chen G., Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 69.Ai J., Zhang R., Gao X., Niu H.-F., Wang N., Xu Y., Li Y., Ma N., Sun L.-H., Pan Z.-W., Li W.-M., Yang B.-F. Overexpression of microRNA-1 impairs cardiac contractile function by damaging sarcomere assembly. Cardiovasc Res. 2012;95:385–393. doi: 10.1093/cvr/cvs196. [DOI] [PubMed] [Google Scholar]
- 70.Wang L., Yuan Y., Li J., Ren H., Cai Q., Chen X., Liang H., Shan H., Fu Z.D., Gao X., Lv Y., Yang B., Zhang Y. MicroRNA-1 aggravates cardiac oxidative stress by post-transcriptional modification of the antioxidant network. Cell Stress & Chaperones. 2015;20:411–420. doi: 10.1007/s12192-014-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Alessandra Y., Devanna P., Limana F., Straino S., Di Carlo A., Brambilla P.G., Rubino M., Carena M.C., Spazzafumo L., De Simone M., Micheli B., Biglioli P., Achilli F., Martelli F., Maggiolini S., Marenzi G., Pompilio G., Capogrossi M.C. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ai J., Zhang R., Li Y., Pu J., Lu Y., Jiao J., Li K., Yu B., Li Z., Wang R., Wang L., Li Q., Wang N., Shan H., Li Z., Yang B. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010;391:73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Cheng Y., Tan N., Yang J., Liu X., Cao X., He P., Dong X., Qin S., Zhang C. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin. Sci. (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Long G., Wang F., Duan Q., Chen F., Yang S., Gong W., Wang Y., Chen C., Wang D.W. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int J. Biol. Sci. 2012;8:811–818. doi: 10.7150/ijbs.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bostjancic E., Zidar N., Stajer D., Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 76.Care A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P., Bang M.-L., Segnalini P., Gu Y., Dalton N.D., Elia L., Latronico M.V.G., Hoydal M., Autore C., Russo M.A., Dorn G.W., Ellingsen O., Ruiz-Lozano P., Peterson K.L., Croce C.M., Peschle C., Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 77.Duisters R.F., Tijsen A.J., Schroen B., Leenders J.J., Lentink V., van der Made I., Herias V., van Leeuwen R.E., Schellings M.W., Barenbrug P., Maessen J.G., Heymans S., Pinto Y.M., Creemers E.E. MiR-133 and miR-30 regulate connective tissue growth factor : implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- 78.A. Izarra, I. Moscoso, E. Levent, S. Cañón, I. Cerrada, A. Díez-Juan, V. Blanca, I.-J. Núñez-Gil, I. Valiente, A. Ruíz-Sauri, P. Sepúlveda, M. Tiburcy, W.-H. Zimmermann, A. Bernad, MiR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep.. 3 1029-1042. [DOI] [PMC free article] [PubMed]
- 79.Eitel I., Adams V., Dieterich P., Fuernau G., de Waha S., Desch S., Schuler G., Thiele H. Relation of circulating microRNA-133a concentrations with myocardial damage and clinical prognosis in st-elevation myocardial infarction. Am. Heart J. 2012;164:706–714. doi: 10.1016/j.ahj.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Wang F., Long G., Zhao C., Li H., Chaugai S., Wang Y., Chen C., Wang D.W. Plasma microRNA-133a is a new marker for both acute myocardial infarction and underlying coronary artery stenosis. J. Transl. Med. 2013;11:222. doi: 10.1186/1479-5876-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Widera C., Gupta S.K., Lorenzen J.M., Bang C., Bauersachs J., Bethmann K., Kempf T., Wollert K.C., Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J. Mol. Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 82.van Rooij E., Liu N., Olson E.N. MicroRNAs flex their muscles. Trends Genet. 2008;24:159–166. doi: 10.1016/j.tig.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 83.van Rooij E., Sutherland L.B., Qi X., Richardson J.A., Hill J., Olson E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 84.Callis T.E., Pandya K., Seok H.Y., Tang R.-H., Tatsuguchi M., Huang Z.-P., Chen J.-F., Deng Z., Gunn B., Shumate J., Willis M.S., Selzman C.H., Wang D.-Z. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Invest. 2009;119:2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satoh M., Minami Y., Takahashi Y., Tabuchi T., Nakamura M. Expression of microRNA-208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J. Card. Fail. 2010;16:404–410. doi: 10.1016/j.cardfail.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Bhuiyan S.S., Kinoshita S., Wongwarangkana C., Asaduzzaman M., Asakawa S., Watabe S. Evolution of the myosin heavy chain gene MYH14 and its intronic microRNA miR-499: muscle-specific miR-499 expression persists in the absence of the ancestral host gene. BMC Evol. Biol. 2013;13:142. doi: 10.1186/1471-2148-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J.-X., Jiao J.-Q., Li Q., Long B., Wang K., Liu J.-P., Li Y.-R., Li P.-F. MiR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 88.Matkovich S.J., Hu Y., Eschenbacher W.H., Dorn L.E., Dorn G.W. Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ. Res. 2012;111:521–531. doi: 10.1161/CIRCRESAHA.112.265736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corsten M.F., Dennert R., Jochems S., Kuznetsova T., Devaux Y., Hofstra L., Wagner D.R., Staessen J.A., Heymans S., Schroen B. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ-cardiovasc Gene. 2010;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L., Chen X., Su T., Li H., Huang Q., Wu D., Yang C., Han Z. Circulating mir-499 are novel and sensitive biomarker of acute myocardial infarction. J. Thorac. Dis. 2015;7:303–308. doi: 10.3978/j.issn.2072-1439.2015.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adachi T., Nakanishi M., Otsuka Y., Nishimura K., Hirokawa G., Goto Y., Nonogi H., Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin. Chem. 2010;56:1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 92.Olivieri F., Antonicelli R., Lorenzi M., D'Alessandra Y., Lazzarini R., Santini G., Spazzafumo L., Lisa R., La Sala L., Galeazzi R., Recchioni R., Testa R., Pompilio G., Capogrossi M.C., Procopio A.D. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non st-elevation myocardial infarction. Int. J. Cardiol. 2013;167:531–536. doi: 10.1016/j.ijcard.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 93.O'Brien P.J., Smith D.E.C., Knechtel T.J., Marchak M.A., Pruimboom-Brees I., Brees D.J., Spratt D.P., Archer F.J., Butler P., Potter A.N., Provost J.P., Richard J., Snyder P.A., Reagan W.J. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab. Anim. 2006;40:153–171. doi: 10.1258/002367706776319042. [DOI] [PubMed] [Google Scholar]
- 94.Liebetrau C., Möllmann H., Dörr O., Szardien S., Troidl C., Willmer M., Voss S., Gaede L., Rixe J., Rolf A., Hamm C., Nef H. Release kinetics of circulating muscle-enriched microRNAs in patients undergoing transcoronary ablation of septal hypertrophy. J Am. Coll. Cardiol. 2013;62:992–998. doi: 10.1016/j.jacc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 95.Oerlemans M.I.F.J., Mosterd A., Dekker M.S., de Vrey E.A., van Mil A., Pasterkamp G., Doevendans P.A., Hoes A.W., Sluijter J.P.G. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol. Med. 2012;4:1176–1185. doi: 10.1002/emmm.201201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeller T., Keller T., Ojeda F., Reichlin T., Twerenbold R., Tzikas S., Wild P.S., Reiter M., Czyz E., Lackner K.J., Munzel T., Mueller C., Blankenberg S. Assessment of microRNAs in patients with unstable angina pectoris. Eur. Heart J. 2014;35:2106–2114. doi: 10.1093/eurheartj/ehu151. [DOI] [PubMed] [Google Scholar]
- 97.Tang G., Shen X., Lv K., Wu Y., Bi J., Shen Q. Different normalization strategies might cause inconsistent variation in circulating microRNAs in patients with hepatocellular carcinoma. Med. Sci. Monit. 2015;21:617–624. doi: 10.12659/MSM.891028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moldovan L., Batte K.E., Trgovcich J., Wisler J., Marsh C.B., Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol. Med. 2014;18:371–390. doi: 10.1111/jcmm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koshiol J., Wang E., Zhao Y., Marincola F., Landi M.T. vol. 19. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology; 2010. pp. 907–911. (Strengths and Limitations of Laboratory Procedures for MicroRNA Detection). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Rooij E., Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olson E.N. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009008. 239ps233–239ps233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Christopher A.F., Kaur R.P., Kaur G., Kaur A., Gupta V., Bansal P. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016;7:68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hullinger T.G., Montgomery R.L., Seto A.G., Dickinson B.A., Semus H.M., Lynch J.M., Dalby C.M., Robinson K., Stack C., Latimer P.A., Hare J.M., Olson E.N., van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ. Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munz M.R., Faria M.A., Monteiro J.R., Águas A.P., Amorim M.J. Surgical porcine myocardial infarction model through permanent coronary occlusion. Comp. Med. 2011;61:445–452. [PMC free article] [PubMed] [Google Scholar]
- 105.Montgomery R.L., Hullinger T.G., Semus H.M., Dickinson B.A., Seto A.G., Lynch J.M., Stack C., Latimer P.A., Olson E.N., van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A., Fasanaro P., Sun N., Wang X., Martelli F., Robbins R.C., Wu J.C. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Q., Xie J., Li R., Shi J., Sun J., Gu R., Ding L., Wang L., Xu B. Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myocardial infarction. J Cell Mol. Med. 2014;18:919–928. doi: 10.1111/jcmm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gu G.-L., Xu X.-L., Sun X.-T., Zhang J., Guo C.-F., Wang C.-S., Sun B., Guo G.-L., Ma K., Huang Y.-Y., Sun L.-Q., Wang Y.-Q. Cardioprotective effect of microRNA-21 in murine myocardial infarction. Cardiovasc Ther. 2015;33:109–117. doi: 10.1111/1755-5922.12118. [DOI] [PubMed] [Google Scholar]
- 109.Yang X., Qin Y., Shao S., Yu Y., Zhang C., Dong H., Lv G., Dong S. MicroRNA-214 inhibits left ventricular remodeling in an acute myocardial infarction rat model by suppressing cellular apoptosis via the phosphatase and tensin homolog (PTEN) Int. Heart J. 2016;57:247–250. doi: 10.1536/ihj.15-293. [DOI] [PubMed] [Google Scholar]
- 110.Li Z., Rana T.M. Therapeutic targeting of microRNAs: current status and future challenges. Nat. Rev. Drug Discov. 2014;13:622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 111.Sati S., Ghosh S., Jain V., Scaria V., Sengupta S. Genome-wide analysis reveals distinct patterns of epigenetic features in long non-coding RNA loci. Nucleic Acids Res. 2012;40:10018–10031. doi: 10.1093/nar/gks776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., Cabili M.N., Jaenisch R., Mikkelsen T.S., Jacks T., Hacohen N., Bernstein B.E., Kellis M., Regev A., Rinn J.L., Lander E.S. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D., Khalil A.M., Zuk O., Amit I., Rabani M., Attardi L.D., Regev A., Lander E.S., Jacks T., Rinn J.L. A large intergenic non-coding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hart J.R., Roberts T.C., Weinberg M.S., Morris K.V., Vogt P.K. MYC regulates the non-coding transcriptome. Oncotarget. 2014;5:12543–12554. doi: 10.18632/oncotarget.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rose D., Hiller M., Schutt K., Hackermüller J., Backofen R., Stadler P.F. Computational discovery of human coding and non-coding transcripts with conserved splice sites. Bioinformatics. 2011;27:1894–1900. doi: 10.1093/bioinformatics/btr314. [DOI] [PubMed] [Google Scholar]
- 116.Zhu J.G., Shen Y.H., Liu H.L., Liu M., Shen Y.Q., Kong X.Q., Song G.X., Qian L.M. Long noncoding RNAs expression profile of the developing mouse heart. J Cell Biochem. 2014;115:910–918. doi: 10.1002/jcb.24733. [DOI] [PubMed] [Google Scholar]
- 117.Matkovich S.J., Edwards J.R., Grossenheider T.C., de Guzman Strong C., Dorn G.W. Epigenetic coordination of embryonic heart transcription by dynamically regulated long noncoding RNAs. Proc. Natl. Acad. Sci. 2014;111:12264–12269. doi: 10.1073/pnas.1410622111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Salvo T.G., Guo Y., Su Y.R., Clark T., Brittain E., Absi T., Maltais S., Hemnes A. Right ventricular long noncoding RNA expression in human heart failure. Pulm. Circ. 2015;5:135–161. doi: 10.1086/679721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang K.-C., Yamada K.A., Patel A.Y., Topkara V.K., George I., Cheema F.H., Ewald G.A., Mann D.L., Nerbonne J.M. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014;129:1009–1021. doi: 10.1161/CIRCULATIONAHA.113.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Z., Zhang X.-J., Ji Y.-X., Zhang P., Deng K.-Q., Gong J., Ren S., Wang X., Chen I., Wang H., Gao C., Yokota T., Ang Y.S., Li S., Cass A., Vondriska T.M., Li G., Deb A., Srivastava D., Yang H.-T., Xiao X., Li H., Wang Y. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat. Med. 2016;22:1131–1139. doi: 10.1038/nm.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Viereck J., Kumarswamy R., Foinquinos A., Xiao K., Avramopoulos P., Kunz M., Dittrich M., Maetzig T., Zimmer K., Remke J., Just A., Fendrich J., Scherf K., Bolesani E., Schambach A., Weidemann F., Zweigerdt R., de Windt L.J., Engelhardt S., Dandekar T., Batkai S., Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf1475. 326ra322–326ra322. [DOI] [PubMed] [Google Scholar]
- 122.McEwan David G., Popovic D., Gubas A., Terawaki S., Suzuki H., Stadel D., Coxon Fraser P., de Stegmann D. Miranda, Bhogaraju S., Maddi K., Kirchof A., Gatti E., Helfrich Miep H., Wakatsuki S., Behrends C., Pierre P., Dikic I. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol Cell. 2015;57:39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 123.Vandenabeele P., Declercq W., Van Herreweghe F., Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci. Signal. 2010;3 doi: 10.1126/scisignal.3115re4. re4-re4. [DOI] [PubMed] [Google Scholar]
- 124.Wang K., Liu F., Liu C.Y., An T., Zhang J., Zhou L.Y., Wang M., Dong Y.H., Li N., Gao J.N., Zhao Y.F., Li P.F. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23(8):1394–1405. doi: 10.1038/cdd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xue Z., Hennelly S., Doyle B., Gulati Arune A., Novikova Irina V., Sanbonmatsu Karissa Y., Boyer Laurie A. A g-rich motif in the lncRNA Braveheart interacts with a zinc-finger transcription factor to specify the cardiovascular lineage. Mol Cell. 2016;64:37–50. doi: 10.1016/j.molcel.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Michalik K.M., You X., Manavski Y., Doddaballapur A., Zörnig M., Braun T., John D., Ponomareva Y., Chen W., Uchida S., Boon R.A., Dimmeler S. The long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014;114(9):1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 127.Fiedler J., Breckwoldt K., Remmele C.W., Hartmann D., Dittrich M., Pfanne A., Just A., Xiao K., Kunz M., Müller T., Hansen A., Geffers R., Dandekar T., Eschenhagen T., Thum T. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J Am. Coll. Cardiol. 2015;66:2005–2015. doi: 10.1016/j.jacc.2015.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ballantyne M.D., Pinel K., Dakin R., Vesey A.T., Diver L., Mackenzie R., Garcia R., Welsh P., Sattar N., Hamilton G., Joshi N., Dweck M.R., Miano J.M., McBride M.W., Newby D.E., McDonald R.A., Baker A.H. Smooth muscle enriched long noncoding RNA SMILR regulates cell proliferation. Circulation. 2016;133:2050–2065. doi: 10.1161/CIRCULATIONAHA.115.021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bell R.D., Long X., Lin M., Bergmann J.H., Nanda V., Cowan S.L., Zhou Q., Han Y., Spector D.L., Zheng D., Miano J.M. Identification and initial functional characterization of a human vascular cell–enriched long noncoding RNA. Arterioscler. Thromb. Vasc. Biol. 2014;34:1249–1259. doi: 10.1161/ATVBAHA.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li D., Chen G., Yang J., Fan X., Gong Y., Xu G., Cui Q., Geng B. Transcriptome analysis reveals distinct patterns of long noncoding RNAs in heart and plasma of mice with heart failure. PLoS One. 2013;8:e77938. doi: 10.1371/journal.pone.0077938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kumarswamy R., Bauters C., Volkmann I., Maury F., Fetisch J., Holzmann A., Lemesle G., de Groote P., Pinet F., Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014;114:1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y., Sun L., Xuan L., Pan Z., Li K., Liu S., Huang Y., Zhao X., Huang L., Wang Z., Hou Y., Li J., Tian Y., Yu J., Han H., Liu Y., Gao F., Zhang Y., Wang S., Du Z., Lu Y., Yang B. Reciprocal changes of circulating long non-coding RNAs ZFAS1 and CDR1AS predict acute myocardial infarction. Sci. Rep. 2016;6:22384. doi: 10.1038/srep22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boon R.A., Jaé N., Holdt L., Dimmeler S. Long noncoding RNAs: from clinical genetics to therapeutic targets? J Am. Coll. Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 134.McMullen Julie R., Drew Brian G. Long non-coding RNAs (lncRNAs) in skeletal and cardiac muscle: potential therapeutic and diagnostic targets? Clin. Sci. 2016;130:2245–2256. doi: 10.1042/CS20160244. [DOI] [PubMed] [Google Scholar]
- 135.Bernardo B.C., Gao X.-M., Winbanks C.E., Boey E.J.H., Tham Y.K., Kiriazis H., Gregorevic P., Obad S., Kauppinen S., Du X.-J., Lin R.C.Y., McMullen J.R. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.da Costa Martins P.A., Salic K., Gladka M.M., Armand A.-S., Leptidis S., el Azzouzi H., Hansen A., Coenen-de Roo C.J., Bierhuizen M.F., van der Nagel R., van Kuik J., de Weger R., de Bruin A., Condorelli G., Arbones M.L., Eschenhagen T., De Windt L.J. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. cell Biol. 2010;12:1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 137.Ren X.-P., Wu J., Wang X., Sartor M.A., Jones K., Qian J., Nicolaou P., Pritchard T.J., Fan G.-C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting Hsp20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bernardo B.C., Nguyen S.S., Winbanks C.E., Gao X.-M., Boey E.J.H., Tham Y.K., Kiriazis H., Ooi J.Y.Y., Porrello E.R., Igoor S., Thomas C.J., Gregorevic P., Lin R.C.Y., Du X.-J., McMullen J.R. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J. 2014;28:5097–5110. doi: 10.1096/fj.14-253856. [DOI] [PubMed] [Google Scholar]
- 139.Bernardo B.C., Nguyen S.S., Gao X.-M., Tham Y.K., Ooi J.Y.Y., Patterson N.L., Kiriazis H., Su Y., Thomas C.J., Lin R.C.Y., Du X.-J., McMullen J.R. Inhibition of miR-154 protects against cardiac dysfunction and fibrosis in a mouse model of pressure overload. Sci. Rep. 2016;6:22442. doi: 10.1038/srep22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hinkel R., Penzkofer D., Zühlke S., Fischer A., Husada W., Xu Q.-F., Baloch E., van Rooij E., Zeiher A.M., Kupatt C., Dimmeler S. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128:1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 141.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S., Kaluza D., Treguer K., Carmona G., Bonauer A., Horrevoets A.J.G., Didier N., Girmatsion Z., Biliczki P., Ehrlich J.R., Katus H.A., Muller O.J., Potente M., Zeiher A.M., Hermeking H., Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 142.Qian L., Van Laake L.W., Huang Y., Liu S., Wendland M.F., Srivastava D. MiR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp. Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grote P., Wittler L., Hendrix D., Koch F., Währisch S., Beisaw A., Macura K., Bläss G., Kellis M., Werber M., Herrmann Bernhard G. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]