Abstract
Prostate cancer is a heterogeneous malignancy, with clinical courses widely differing between indolent and aggressive lethal disease. This heterogeneity calls for a more personalized approach towards diagnosis, prognosis, treatment decision, monitoring and follow-up of patients. In this review, we discuss the possibilities and drawbacks of detecting RNA biomarkers in biological fluids to improve disease-specific survival and quality of life. In particular, we examine literature on long non-coding RNAs in blood and urine of prostate cancer patients. We thereby specifically focus on the need for standard operation procedures on many different levels, analytical validation, clinical validation, and assessment of clinical utility. We argue that thorough multi-step validation of putative biomarkers is necessary for successful translation into clinical prostate cancer care. Our recommendations may also prove useful to biomarker research in other cancers.
1. Introduction
Cancer is not one disease, but a heterogeneous group of malignancies. Even within one cancer type substantial differences exist, and prostate cancer is no exception to that biological observation. Indeed, disease heterogeneity is reflected by the different clinical courses of indolent versus aggressive and lethal prostate cancers. As a matter of fact, the five-year overall survival of prostate cancer patients is 98.9% [1], whereas prostate cancer is still the third-leading cause of death by cancer in men [2]. In other words, the majority of prostate cancers are not life-threatening, but aggressive prostate cancers are still estimated to kill more than 29,000 men in the United States of America in 2018 [3]. This heterogeneity makes prostate cancer rather difficult to treat and far from a well-controlled disease, which is also indicated by the fact that only 30% of metastasized cases survive longer than 5 years [3]. This disease diversity calls for a more personalized approach towards diagnosis, treatment and follow-up of patients.
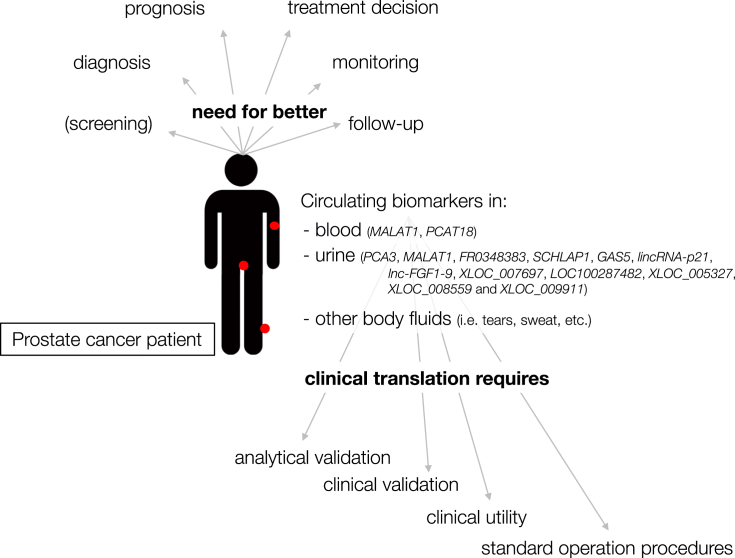
Liquid biopsy, a technique that assesses tumor markers in biological fluids, has the potential to become a low-cost, fast and minimally invasive tool for personalized clinical care. However, the detection of clinically useful biomarkers in blood, urine and other body fluids from cancer patients still has several hurdles to take. Once researchers overcome the obstacles described in this review, new liquid biomarkers have the potential to vastly improve today's clinical care of prostate cancer patients. After summarizing the expectations and limitations of biomarkers and liquid RNA biopsies, we review current literature on long non-coding RNAs (lncRNAs) in body fluids from prostate cancer patients. LncRNAs are RNA molecules that do not code for proteins and are longer than 200 nucleotides [4]. They play an important role in many cancer-related biological processes, from regulation of proliferation to metastasis [5]. Notably, several lncRNAs show a tissue- or cancer (sub)type-specific expression pattern, which makes them excellent candidate biomarkers for personalized medicine [6]. A general overview of the topics discussed in this review article is depicted in Fig. 1.
Fig. 1.
Graphical overview of the topics discussed in this review article.
2. What should we expect from new prostate cancer biomarkers?
In general, good biomarkers can be used for cancer screening, diagnosis, prognosis, treatment decisions and monitoring of cancer patients during/after treatment [7].
Prostate cancer screening. Heated debates have been held over the benefits-to-harms ratio of prostate cancer screening using prostate specific antigen (PSA)-levels in serum. Current recommendations advise against the use of population-based PSA-screening for prostate cancer and stress the importance of shared decision-making [8]. The results of the two large PLCO (Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial) and ERSPC (European Randomized Study of Screening for Prostate Cancer) trials suggest that the benefits of PSA-screening currently seem not to outweigh the harms, although one study showed contamination because patients in the control arm also underwent screening [8]. In particular, up to 75% of the biopsies executed after increased serum PSA-levels reveal no cancer at all [9]. These unnecessary biopsies – which are the result of PSA being prostate-specific rather than prostate-cancer specific – have important emotional and physical side-effects such as infection, hematospermia, hematuria or rectal bleeding [10]. In addition, biopsies very often detect low-grade disease that would rather benefit from active surveillance instead of intense treatment [11]. However, over-diagnosis and overtreatment of clinically insignificant prostate cancer that will never cause any impairment to the patient is a problem and should be avoided. The entire PSA screening controversy is beyond the scope of this article, but new liquid biomarkers for primo-diagnosis able to distinguish between prostate cancer and other (benign) prostate diseases on the one hand, and between indolent and aggressive disease on the other hand could shed an entirely new light on the (dis)advantages of prostate cancer screening. By all means, any cancer screening should reduce cancer-specific mortality and, albeit more challenging, overall mortality [12].
Diagnosis. The gold standard for prostate cancer diagnosis is a needle core biopsy, often performed after increased PSA-levels or suspicious digital rectal exam. However, biopsies can miss up to 28% of all prostate cancers [2]. By definition, a needle core biopsy only investigates small parts of the prostate gland, which augments the likelihood of false-negative results. Systemic, liquid biomarkers have the potential to circumvent this problem.
Prognosis. Currently, one of the strongest prognostic factors for prostate cancer outcome is the Gleason-score that is based on the microscopic architecture and appearance of cells in biopsy tissue [13]. The score is calculated by grading the predominant histological pattern and the highest pattern on a scale from 1 to 5. An (updated) Gleason score of 3 + 3 corresponds to a very low-risk grade 1 cancer, whereas high-risk grade 5 cancers have Gleason scores of 4 + 5, 5 + 4 or 5 + 5. Three other risk groups lie in between these two extremes. However, scoring based on biopsy tissue poses the threat of under-grading in up to 17% of cases because of inherent sampling error [2]. Indeed, due to tumor heterogeneity or multifocal lesions, Gleason scores may sometimes not reflect the true aggressiveness of the tumor. In addition, the development of clinically useful tests to clearly and unambiguously predict the future course of the disease is still a work in progress. Again, new liquid biomarkers could overcome the fact that a tissue biopsy only captures a fraction of the tumor.
Treatment decisions. In contrast to prognostic biomarkers, which inform about outcome independent of the treatment received, predictive biomarkers provide information on the likely benefit from treatment [14,15]. However, biomarkers predictive of treatment toxicity and/or therapeutic effectiveness are largely unexplored [1]. New disease (sub)type-specific liquid biomarkers could help guide the decision of whether to choose for active surveillance, surgery, (adjuvant) radiation therapy, androgen deprivation therapy, chemotherapy, immunotherapy and/or newly developed targeted therapies.
Monitoring and follow-up. Finally, closely monitoring the effect of a certain therapy, especially in aggressive metastatic castration-resistant prostate cancer (mCRPC), is extremely valuable [16]. Early prediction of treatment failure or relapse and indications when to switch gears could – obviously together with the development of new therapies – reduce future prostate cancer-specific mortality. Minimally invasive tests based on liquid biomarkers for therapy monitoring promise to be affordable and fast, and make it possible to evaluate tumor marker levels on a regular, serial basis. In addition, follow-up of patients who received local treatment (such as external radiotherapy, brachytherapy or focal therapy) is currently not straightforward. The PSA bounce phenomenon, for instance, often leads to uncertainty and needless additional clinical tests that could be avoided with better liquid biomarkers.
3. On the need for biomarker validation and adequate reporting
Biomarkers, both liquid and non-liquid, can be powerful tools in the personalized care of cancer patients. However, the biomarker field has to deal with two main issues. On the one hand, there is a superfluity of putative biomarkers reported in scientific literature that never make it to the clinic. On the other hand, some biomarker tests that were never proven to improve patient care are commercially available [7,17]. Both are the result of a vicious cycle, tremendously well described by Daniel Hayes and colleagues, where poor reporting, inadequate funding and the absence of biomarker validations are all interconnected [18].
Notably, whatever the use of a specific biomarker test will be, new tests should be analytically validated, clinically validated and their clinical utility should be demonstrated before applying them in routine clinical practice. As reviewed last year in the context of RNA sequencing by Byron and colleagues, analytical validation refers to the accuracy and reliability of a certain test to measure a specific biomarker molecule [19]. In other words: how well does the test measure the specific biomarker of interest? Important factors to assess are, amongst others, analytical sensitivity, analytical specificity, repeatability and reproducibility [19]. Subsequently, if a biomarker can be measured accurately in a reliable way, the clinical validity of the test should be evaluated. How well does the biomarker detect or predict a clinical diagnosis or outcome? Key factors here are clinical sensitivity, clinical specificity, and positive and negative predictive value. Finally, and most importantly, a biomarker test should inform clinical decisions and improve disease outcome, which is indicated by the level of clinical utility. However, and in contrast to the more rigorous safety and efficacy regulations before new drugs hit the market, the regulatory environment for commercial use of tumor biomarker tests – especially when it comes to clinical utility – is much less clear [7]. As will be described below, only a minority of lncRNAs reported to be detectable in body fluids of prostate cancer patients has been investigated on these three levels to date. Clearly, to unleash the full potential of biomarkers in personalized cancer care, both investigators, scientific journals, funding agencies and regulatory agencies urgently need to improve research quality by thoroughly thinking through study designs, by stimulating and striving towards reproducible results and by conducting all necessary validation tests.
4. The promises and drawbacks of liquid RNA biopsies
Cancer biomarkers that are detectable in body fluids undeniably have great potential, and liquid biopsies are emerging as an extremely attractive application of this potentiality. As a matter of fact, tumors are known to shed different types of tumor-derived molecules in body fluids: cell-free DNA, RNA (fragments) and proteins, circulating tumor cells, extracellular vesicles, etc. [20]. Assessing the levels of these markers in liquid biopsies has the potential to add a new, minimally invasive layer to the diagnosis, treatment and follow-up of prostate cancer patients. Next to being easy, low-cost and fast, liquid biopsies are in principle able to overcome tumor heterogeneity or the multifocal nature of certain tumors by taking a more systemic view. Finally, a liquid snapshot of the tumor can be obtained repeatedly, as many times as needed [21]. We expect that the implementation of liquid biopsies in clinical prostate cancer care might be even smoother compared to some other cancers. First, prostate cancer has a long history with blood-based PSA tests. Second, the likelihood of detecting tumor markers before radical prostatectomy in urine, a body fluid very easy to collect, is quite high because of the anatomical location of the prostate.
RNA has substantial advantages over DNA when it comes to investigating disease biology in order to develop new biomarkers. First, RNA expression is by definition dynamic and fluctuates according to the internal needs of a (cancer) cell. Second, the expression pattern of several RNA molecules or fragments thereof, in particular lncRNAs, is highly tissue- or disease-state-specific [6]. Finally, focusing on RNA also allows the investigation of non-coding RNAs, fusion transcripts, splice variants and RNA editing events [22]. Identifying which cancer (sub)type a particular patient is confronted with, in which stage the cancer is and which treatment will yield the best results is considered the Holy Grail of cancer biomarker research [23]. Because of their specificity, especially lncRNAs detectable in body fluids have the potential to meet the expectations, but some essential obstacles still need to be tackled.
Indeed, although many putative liquid RNA biomarkers have been published, the RNA liquid biopsy field currently struggles with too many pre-analytical variables that have never been rigorously tested. In particular, it is very well possible that different factors (such as time of blood draw, blood collection tube, needle, diet of the patient, blood fraction (e.g. platelet-rich plasma, platelet-free plasma, platelets, etc.), RNA isolation protocol, and many others) influence RNA abundance levels in body fluids, both inside and outside extracellular vesicles [24]. Therefore, we believe there is an urgent need for standardization of sample collection, RNA isolation and data-analysis. As a matter of fact, we consider standard operating procedures as an extremely important prerequisite before liquid RNA biopsy research can be translated in clinically useful applications.
To standardize the use of lncRNAs in liquid biopsies we suggest first setting up comprehensive RNA sequencing studies to assess the impact of different pre-analytical variables in a controlled and systematic way. Sequencing plasma fractions that were collected and prepared in various ways will provide valuable information on the effect of numerous factors on lncRNA abundance, quality, fragmentation and yield. Factors we expect to have a substantial influence are the blood collection tube, time between collection and plasma preparation, plasma type (number and intensity of centrifugation steps), RNA isolation method, storage conditions, etc. Afterwards, all results should be made available in a public database where researchers can track the influence of different pre-analytical variables on their lncRNA(s) of interest. Based on the collaborative efforts of the liquid biopsy research community, international guidelines to study lncRNA biomarkers can then be implemented. It should be noted that the ideal protocols and procedures will likely depend on the intended application. Our lab is currently coordinating the extracellular RNA quality control study (exRNAQC) in which many of the aforementioned experiments will be conducted.
Finally, technological improvements for RNA quantification are still needed. For instance, total RNA sequencing (to detect both coding and non-coding RNA) and extracellular vesicle isolation methods are still difficult to implement in many research laboratories and would benefit from further technological improvement, especially when these methods will have to be implemented in future routine clinical practice.
5. LncRNAs in prostate cancer body fluids
A literature search for lncRNAs detectable in body fluids of prostate cancer patients, based on the keywords specified in the methods section, returned 28 articles. In these articles, 17 so-called lncRNAs are described to be detectable in blood or urine of prostate cancer patients. Of note, SPINK1 [25] and PTI-1 [26] – at some point described as non-coding RNAs – should be classified as protein-coding genes according to Ensembl hg38 and GeneCards [27,28]. The other lncRNA genes are listed in Table 1.
Table 1.
Overview of circulating lncRNAs in prostate cancer. PCa = prostate cancer, BPH = benign prostatic hyperplasia, ROC = receiver operating characteristic, AUC = area under the curve, DRE = digital rectal exam, PSA = prostate-specific antigen, mCRPC = metastatic castration-recurrent prostate cancer.
| gene | reference | fluid | samples | main findings liquid lncRNA | analytical validation | clinical validation | clinical utility | normalization |
|---|---|---|---|---|---|---|---|---|
| PCA3 | Nicholson et al., 2015 | post-DRE urine | meta-analysis | prostate-cancer specific expression | extensively assessed | extensively assessed | assessed | meta-analysis |
| MALAT1 | Ren et al., 2013 | plasma and serum | 8 PCa/25 PCa/87 PCa vs. 82 BPH vs. 23 healthy | stably detectable in plasma, able to discriminate between PCa and non-PCa, and between PCa and BPH; higher in PCa | stability (freeze/thaw, incubation time, storage time, acid-base treatment, RNase degradation). | tumor source, xenograft presence, ROC/AUC, sensitivity, specificity. | not assessed | fixed liquid/RNA volume |
| MALAT1 | Xue et al., 2015 | plasma | 63 PCa, 32 BPH, 50 healthy | able to discriminate PCa from healthy controls and PCa from BPH, higher in PCa; panel of MD-miniRNA and PSA has better performance | not assessed | ROC/AUC | not assessed | β-actin |
| MALAT1 | Wang et al., 2014 | post-DRE urine | 218 (discovery), 216 (multicenter validation) | MALAT1 score significantly higher in men with positive biopsy compared to negative biopsy; could avoid up to 47% avoidable biopsies in PSA grey zone without missing any high-grade cancers | not assessed | detection rate; AUC in univariate regression analysis and predictive accuracy and AUC in multivariable regression, in both overall patient group and PSA grey zone; Everything in discovery and validation cohort | net benefit, net reduction in avoidable biopsies, missed (high-grade) prostate cancers in both overall patient group and PSA grey zone; everything in discovery and validation cohort | normalisation intrinsic to calculation of the score |
| FR0348383 | Zhang et al., 2015 | post-DRE urine | 213 cases with PSA > 4 ng/ml and/or abnormal DRE | high FR0348383 score correlated with probability of positive biopsy; could avoid up to 52% biopsies without missing any high grade cancers in PSA grey zone cohort | three different urine collections? | detection rate; predictive accuracy of variables in univariate and multivariable regression, ROC/AUC, in entire cohort and PSA grey zone cohort | net benefit, net reduction in avoidable biopsies, missed (high-grade) prostate cancer numbers in PSA grey zone cohort | normalisation intrinsic to calculation of the score |
| SCHLAP1 | Prensner et al., 2014 | post-DRE urine | 256 | higher expression in Gleason 7 vs. Gleason 6, and higher levels in high-risk vs. low-risk | not assessed | not assessed | not assessed | GAPDH/KLK3 average |
| GAS5 | Isin et al., 2015 | exosomes from post-DRE urine | 30 PCa, 49 BPH | detected, but no difference between PCa and BPH | not assessed | not assessed | not assessed | GAPDH |
| lincRNA-p21 | Isin et al., 2015 | exosomes from post-DRE urine | 30 PCa, 49 BPH | significant higher levels in PCa compared to BPH | not assessed | sensitivity and specificity. | not assessed | GAPDH |
| PCAT18 | Crea et al., 2014 | plasma | 25 healthy, 25 primary PCa, 25 mCRPC | significant incremental increase from healthy individuals to primary PCa to mCRPC | not assessed | not assessed | not assessed | GAPDH and HPRT |
| AK024556, XLOC_007697, LOC100287482, XLOC_005327, XLOC_008559 and XLOC_009911 | Lee et al., 2014 | urine | 13 PCa, 14 healthy | all six markers detected in urine, up-regulated compared to healthy urine | not assessed | not assessed | not assessed | GAPDH |
Some of these lncRNAs play an important role in prostate cancer progression and metastasis, and have been intensely researched in tissue samples or as potential therapeutic targets. However, in this review we mainly focus on the detection in body fluids and investigate to which level these putative lncRNA biomarkers are analytically and clinically validated in blood or urine.
The specific role and function of these lncRNAs in prostate cancer malignancy is reviewed elsewhere [25,26,29]. As a matter of fact, it is not necessary to know the exact function of a molecule for it to be a good biomarker. To describe the amount of lncRNAs detectable in body fluids, we deliberately choose to talk about ‘levels’ or ‘abundance’ instead of ‘expression’. In our opinion, expression refers to an active endogenous cellular process, while body fluids rather contain lncRNAs than actively expressing them. It should also be noted that lncRNA fragments can be detected in body fluids and extracellular vesicles, adding an additional layer to data-analysis of RNA sequencing experiments.
5.1. An important note on lncRNA nomenclature
Because lncRNA names are not as standardized as is the nomenclature for coding genes, we intended to use LNCipedia 5.0 IDs for all lncRNAs described in this review [4]. However, when identifying lncRNA locations by UCSC in silico PCR using the primers described in the respective articles, we made some interesting observations.
First, the amplicon generated by the MALAT1 primers overlaps with a lncRNA exon on the opposite strand [30]. Because classical RT-qPCR (in contrast to strand-specific RT-qPCR) cannot make a distinction between sense and antisense transcripts, it is possible that the MALAT1 primers also amplify lnc-LTBP3-2. The same is true for PCAT18, whose amplicon overlaps with the antisense lncRNA AQP4-AS1 [31].
What is more, MD-miniRNA (short for MALAT1-derived miniRNA) is described as an independent lncRNA in literature [32,33]. Notably, the same research group published two different articles, one on MALAT1 and one on MD-miniRNA. However, the same primer pair was used to detect both genes. We argue that MD-miniRNA should not be given a separate name and should be identified as MALAT1.
Next, the amplicon generated by both primers for FR0348383 does not overlap with any coding or non-coding exon in LNCipedia or RefSeq, and thus no corresponding LNCipedia ID could be identified [34]. Raw RNA sequencing data from the experiment seem not to be publicly available to check for transcripts or reads ourselves.
In addition, lincRNA-p21 is known as a murine gene and the amplicon also seems not to overlap with any exon in the human hg38 build, because of which no corresponding LNCipedia ID could be identified [35]. However, in 2010 the lab of John Rinn investigated the locus in the human genome and designed primers that amplified human sequences, which were used in the article described in this review [36].
Finally, six lncRNAs are described using identifiers corresponding to specific microarray probes [37]. Because identical identifiers can match different genes in various microarray and RNA sequencing experiments, we do not recommend using them in published articles. Instead accession numbers or sequences should be provided. For one lncRNA, AK024556, the authors mention the alternative gene name SPRY4-IT1, which corresponds to LNCipedia ID lnc-FGF1-9 in the current release. For the other five lncRNAs, we looked up the probe sequence (using online Agilent SurePrint G3 Human GE v2 probe information) and used it as input for LNCipedia searches, returning five gene names. XLOC_007697 corresponds to lnc-SPATA31A6-6, XLOC_005327 to LINC01564, XLOC_008559 to lnc-RPP30-2 and XLOC_009911 to lnc-HNF1A-1. One microarray probe however, LOC100287482, corresponds to the protein coding gene SMK1.
In conclusion, since this is a literature review, we decided to report both the gene names used in the respective articles and the corresponding LNCipedia IDs. Yet, we want to signal our nomenclature observations to the scientific community and recommend to critically investigate the locations, sequences and names of the lncRNAs in question before conducting any further experiments. An overview of the primer and microarray probe sequences can be found in Table 2.
Table 2.
Overview of RT-qPCR primer sequences and microarray probe sequences used to quantify lncRNAs in liquid biopsies.
| gene | reference | forward primer | reverse primer | LNCipedia ID | remark |
|---|---|---|---|---|---|
|
MALAT1 (MD-miniRNA) |
Ren et al., 2013 | CTTCCCTAGGGGATTTCAGG | GCCCACAGGAACAAGTCCTA | MALAT1 | lnc-LTBP3-2 on other strand |
|
MALAT1 (MD-miniRNA) |
Xue et al., 2015 | not mentioned | not mentioned | MALAT1 | lnc-LTBP3-2 on other strand |
| MALAT1 | Wang et al., 2014 | CTTCCCTAGGGGATTTCAGG | GCCCACAGGAACAAGTCCTA | MALAT1 | lnc-LTBP3-2 on other strand |
| FR0348383 | Zhang et al., 2015 | TAAACCTCCTTATCACATGCAGAA | GGACACCGTAGATTCTAGGACACT | not available | |
| SCHLAP1 | Prensner et al., 2014 | TGGACACAATTTCAAGTCCTCA | CATGGTGAAAGTGCCTTATACA | SCHLAP1 | |
| GAS5 | Isin et al., 2015 | CTTCTGGGCTCAAGTGATCCT | TTGTGCCATGAGACTCCATCAG | GAS5 | |
| lincRNA-p21 | Isin et al., 2015 | GGGTGGCTCACTCTTCTGGC | TGGCCTTGCCCGGGCTTGTC | not available | |
| PCAT18 | Crea et al., 2014 | AGGAGACAGGCCCCAGATTT | TGAAGTGCTGGGACAACGTA | PCAT18 | AQP4-AS1 on other strand |
| AK024556, XLOC_007697 | Lee et al., 2014 | ENSG00000274721 TTGGAGCCATTGTACGTGTCGGGAACATATCAGAACACCGAGAATAGCGTCATGTCATAA |
(chr5:141697230–141697290) (chr9:044182789–044182730) |
lnc-FGF1-9 lnc-SPATA31A6-6 |
A_21_P0006269 |
| LOC100287482 | ACTTCCTCTGTTATGCCAGATATGGTTAGCCACTTTGGTTTTTTAGGAGCTATAGGATGG | (chr7:129152443–129152502) | protein coding (SMKR1) | A_21_P0013271 (NM_001195243) | |
| XLOC_005327 | AGAGCTCAAAAGGCAAGAAATCAGCAAGAGAGAGAGATGAAGCATGAGAAATGAGCAAAA | (chr6:53495838–53495897) | LINC01564 | A_19_P00802433 | |
| XLOC_008559 | CATGTGTGTGCATGTGAGAGAGAGAGAGAAATAGGTTTTAATCCTTTGTTCTTTTCTTAT | (chr10:92749981–92750040) | lnc-RPP30-2 | A_21_P0007070 | |
| XLOC_009911 | AAGTGAATCTGGAGCTTAGATGGAGAGAGAAGAGAGAGATTAATTGAGGCCCCAGGTACT | (chr12:121343059–121343118) | lnc-HNF1A-1 | A_21_P0007854 |
5.2. PCA3
PCA3 is without a doubt the most widely studied lncRNA in body fluids of prostate cancer patients. Prostate cancer-specific overexpression of PCA3 (DD3) was first described by Bussemakers and colleagues in 1999 [38]. In 2012, the American Food and Drug administration (FDA) approved the Progensa PCA3 urine test for helping clinicians whether or not to recommend a repeat biopsy after a negative biopsy result [39]. In addition, the test received a CE-mark for use in the European Union [40]. An independent meta-analysis of the analytical validity, clinical validity, clinical utility and cost-effectiveness of the urine PCA3 test is way beyond the scope of this review article. What is more, an extensive 192-page long report that investigated all four topics above was published in 2015 [40]. In short, analytical validity was reviewed using six studies, indicating that there were issues with the precision of the PCA3 measurements. Fifteen studies assessing clinical validity indicated insufficient evidence to identify appropriate threshold values for clinical use. In addition, the test did not improve discrimination compared to clinical assessment and magnetic resonance imaging. No studies were found analyzing clinical utility and cost-effectiveness. Therefore, the authors of the report built a de novo model indicating that the PCA3 test had no value for money for UK's National Health Service. In conclusion, the report could not confirm the clinical benefit of the PCA3 assay. Consequently, the National Institute for Health and Care Excellence does not recommend the test in men who already had a negative (or unclear) prostate biopsy.
In addition, a recommendation from the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group rated the analytical validity of the Progensa PCA3 urine test adequate, although very few studies investigated pre-analytical effects, analytical performance, and diagnostic accuracy of other quantitative assays for PCA3. In contrast to the analytical validity, the clinical validity was rated inadequate to inform decisions about initial or repeat biopsy [41]. No clinical utility studies could be identified. Although the evidence seems to indicate insufficient advantage of the PCA3 kit, the test is still sold and used by many clinicians worldwide. These results exemplify the extreme importance of validating biomarkers on different levels, and in multiple independent studies. In addition, convincing clinicians to assess results of validation studies critically instead of blindly following recommendations from pharmaceutical or diagnostic companies is not always straightforward.
5.3. MALAT1 (or MD-miniRNA)
PolyA + RNA sequencing of 14 prostate cancer tissues and their adjacent normal tissues revealed on average 406 differentially expressed lncRNAs in the 14 different comparisons, including PCA3 and MALAT1 [42]. MALAT1 was found to be overexpressed in 82.5% of prostate cancer compared to normal tissues. In a follow-up study, the same research group tried to validate the presence of MALAT1 and PCA3 in additional tissue samples, and in plasma from eight prostate cancer patients. For the latter part, RT-qPCR primer pairs for nine different amplicons were developed.
The authors identified high plasma levels of one amplicon in particular and named it MD-miniRNA (short for MALAT1 derived miniRNA). A similar result was obtained for a PCA3 amplicon, in the article called PCA3 derived miniRNA, or PD-miniRNA. Assuming that higher abundant transcripts play a more important role, further validation experiments in plasma were carried out with MD-miniRNA and PD-miniRNA only. As described above, the authors of this review propose to use MALAT1 and PCA3, respectively, instead.
Interestingly, the authors investigated the plasma abundance stability in 13 patients by varying freeze/thaw cycles, incubation times at room temperature, storage times in −80 °C freezers, acid-base treatments, and RNase degradation assays. Strikingly, none of these five variables seemed to have a significant influence on plasma RNA levels. By proving that both of the plasma lncRNA fragments are stable, detectable and RNase degradation-resistant, the authors assess many of the pre-analytical variables that are largely ignored in liquid biopsy studies today. What is more, the levels of the lncRNA fragments were compared in parallel samples of EDTA plasma, heparin plasma and serum of 12 additional patients. The results revealed that fragments are largely undetectable in heparin tubes but can be measured in EDTA plasma and serum, although the detected levels are not correlated in both blood fractions.
Because of low diagnostic power of PD-miniRNA, the authors further only validate MD-miniRNA as possible blood-based biomarker for prostate cancer. After evaluating the presence of MD-miniRNA in blood of xenografted mice, MD-miniRNA levels were assessed in 10 additional prostate cancer patients before and 7 days after surgery to verify that the lncRNA fragment is derived from prostate tumors. Indeed, in all patients but one, the MD-miniRNA levels declined after surgery with approximately 10-fold magnitude. Finally, the clinical validity of MD-miniRNA to discriminate 1) between prostate cancer (PCa) and non-PCa, 2) between prostate cancer and benign prostate hyperplasia (BPH) in patients with PSA > 4 ng/ml and 3) between prostate cancer and BPH in patients with PSA levels between 4 and 10 ng/ml was evaluated. Therefore, RT-qPCR levels of plasma MD-miniRNA were measured in 87 prostate cancer patients with positive biopsy, 82 BPH patients with negative biopsy and 23 healthy controls, followed by receiver operating characteristics (ROC) curve analysis comparing serum PSA to plasma MD-miniRNA. The results indicate a slightly higher area under the curve (AUC) for MD-miniRNA in all three settings. Indeed, when comparing plasma MD-miniRNA with serum PSA, AUCs were respectively 0.836 vs. 0.770, 0.841 vs. 0.708 and 0.767 vs. 0.446 in the three settings described above. In addition, sensitivities of 58.6%, 58.6% and 43.5%, and specificities of 84.8%, 84.1% and 81.6% were calculated at a detection cut-off of 867.8 copies of MD-miniRNA per microliter, altogether illustrating that plasma MD-miniRNA might be a promising indicator for discriminating between PCa and non-PCa, and between prostate cancer and BPH, especially in the PSA grey zone (PSA between 4 and 10 ng/ml).
In an independent study, plasma MD-miniRNA was measured using RT-qPCR in a cohort of 63 prostate cancer patients, 32 BPH patients and 50 healthy controls [33]. ROC curve analysis with AUC showed that MD-miniRNA was better able to discriminate prostate cancer from healthy controls on the one hand, and prostate cancer from BPH on the other hand. MD-miniRNA performed better than serum PSA, but the combined panel of the two had the best diagnostic performance. AUC values for prostate cancer vs. control were 0.73 for serum PSA, 0.86 for MD-miniRNA and 0.89 for the panel. AUC values for prostate cancer vs. BPH were 0.66 for serum PSA, 0.79 for MD-miniRNA and 0.82 for the combination of both. Although ROC curves are interesting tools to mutually compare tests, p-values should also be reported. What is more, clinical implementation of biomarker tests also requires the determination of a specific cut-off value, with associated sensitivity and specificity values (and other relevant parameters) – which were not determined by the authors.
In conclusion, while the clinical utility of plasma MD-miniRNA remains to be proven, at least several analytical and clinical validations are in place. Larger, non-Asian, multicenter study cohorts are needed to further validate the results. On a more molecular level, novel RNA sequencing technologies are also likely to shed additional light on the fragmented abundance of lncRNAs in body fluids.
As mentioned above, Ren and colleagues performed polyA + RNA sequencing on 14 pairs of prostate cancer tissue and adjacent normal tissue, amongst others revealing several differentially expressed lncRNAs [42]. In another retrospective and prospective follow-up study, the authors sought to evaluate lncRNA MALAT1 levels in urine samples as a diagnostic biomarker for prostate cancer [30]. Therefore, urinary RT-qPCR levels of MALAT1 and PSA were analyzed in a discovery cohort of 218 patients and a multicenter validation cohort of 216 patients, all scheduled for prostate biopsy because of elevated PSA levels (PSA > 4 ng/ml) and/or suspicious digital rectal exam (DRE). Subsequently, the MALAT1 score was calculated as (MALAT1 RNA/PSA mRNA) x 1000. This is similar to 2Cq(PSA)-Cq(MALAT1) x 1000 (with Cq being the RT-qPCR quantification cycle). Of note, our comparison of the primer sequences revealed that the authors amplify the same MD-miniRNA amplicon as in the article above, although they consistently use the gene name MALAT1 throughout the article.
The MALAT1 score was significantly higher in patients with a positive biopsy compared to negative biopsies, in both the overall discovery patient group and in a subgroup of patients with PSA levels in the grey zone. In addition, prostate cancer detection rates in subjects with low, intermediate, high and very high MALAT1 scores were reported. Comparable results were obtained in the validation cohort.
Next, univariate analyses were included for different variables (MALAT1 score, age, total PSA, percentage free PSA, etc.) and the area under the curve (AUC) for each variable was reported in both the overall discovery patient group and the PSA grey zone subgroup. Subsequently, two different multivariable models (one with and one without MALAT1 score) were evaluated for predictive accuracy and AUC values, revealing that the model including the MALAT1 score performed slightly better in both the entire patient group and the PSA grey zone subgroup. AUCs were 0.815 for the model without MALAT1 score vs. 0.840 for the model with MALAT1 score in the entire patient group and 0.821 vs. 0.853 in the PSA grey zone subgroup. The results could all be validated, with AUCs of 0.817 vs. 0.833 in the entire validation cohort and 0.772 vs. 0.779 in the PSA grey zone subgroup of the validation cohort.
A hint towards clinical utility is given by decision curve analysis where the net benefit and net reduction in avoidable biopsies are calculated for 7 different levels of threshold probabilities, ranging from 10% to 40%. Threshold probabilities reflect the theoretical point at which a patient would decide to undergo a certain procedure, because from that level on the benefits outweigh the harms. For instance, a cut-off value of 25% implies that a patient would decide not to be scheduled for prostate biopsy when he was told he had an estimated probability of less than 25% of having prostate cancer. Conversely, the patient would take the biopsy if his estimated probability of prostate cancer was equal to or higher than 25% [43]. The authors focus on patients in the PSA grey zone, while results for the entire group are described in supplementary files. In addition, the numbers of missed prostate cancers and high-grade prostate cancers are reported for threshold probabilities between 15% and 40%. At a cut-off value of 25%, the model including MALAT1 score could avoid slightly more unnecessary biopsies, while missing the same number or fewer cancer diagnoses, depending on whether looking at the discovery or validation cohort. Notably, the differences in avoidable biopsy percentages strongly differ between discovery and validation cohort (47% vs. 30%), pointing towards the importance of including an independent cohort in biomarker validation studies. Finally, sensitivity and specificity were compared between MALAT1 score and percentage free PSA at specific cut-off values. In the discussion, the authors mention four shortcomings in their study design: relatively small sample size, no comparison with PCA3 score (due to the PCA3 kit not being available in China), no comparison with existing nomograms and not taking into account whether patients undergo a first or repeat biopsy. Further, large-scale studies including additional (analytical and clinical) validation will indeed be needed to confirm the findings. As a side note, the authors mention that MALAT1 is also reported to be involved in other cancer types (hepatocellular carcinoma, lung adenocarcinoma, colorectal cancer, etc.). One should be careful with clinical implementation of so-called ‘specific’ lncRNA biomarker tests, as patients with increased serum PSA levels and a positive (hypothetical) MALAT1 test could, in theory, suffer from another cancer in combination with BPH, for instance. Therefore, pan-cancer-wide screening of putative lncRNA biomarkers should be an additional validation step.
5.4. FR0348383
As already described, Ren and colleagues previously revealed several differentially expressed lncRNAs after RNA sequencing 14 pairs of prostate cancer tissue and matched adjacent normal tissue from patients who underwent radical prostatectomy [42]. In a third follow-up study, the same group investigated whether one of the top differentially expressed lncRNAs, FR0348383, could have diagnostic value in urine samples [34]. Again, first-catch urine samples after DRE were collected from patients scheduled for prostate biopsy because of elevated PSA (PSA > 4.0 ng/ml) and/or abnormal DRE results. The FR0348383 score, being the ratio of PSA and FR0348383 mRNA measured by RT-qPCR multiplied by 1000, was calculated in 213 patients whose urine samples contained sufficient RNA.
An increasing FR0348383 score was significantly correlated with an increasing probability of a positive biopsy. In addition, prostate cancer detection rates and univariate and multivariable regression analysis with predictive accuracy of each variable were described, the latter both for the entire cohort and for a sub-cohort of patients with grey zone PSA. The authors applied similar clinical validation experiments as in their previous article to assess whether FR0348383 score, PSA, PSA density (the ratio of PSA level and prostate volume) and percentage free PSA could accurately discriminate between patients with and without prostate cancer. ROC curve analyses and AUC calculations for the entire cohort revealed that FR0348383 score and PSA were comparable (AUC of 0.760 vs. 0778, p = 0.740). In the PSA grey zone cohort, FR0348383 (AUC: 0.815) significantly outperformed PSA (AUC: 0.562, p = 0.020) and percentage free PSA (AUC: 0.599, p = 0.039), while FR0348383 score AUC was higher but not significantly different compared to PSA density (AUC: 0.645, p = 0.124). The combination of FR0348383 score and PSA density had the highest AUC (0.876), but was not statistically different from FR0348383 score alone.
Subsequently, net benefit, net reduction in avoidable biopsies and number of missed (high-grade) prostate cancers were reported for six different threshold probabilities, ranging from 15% to 40%. These decision curves revealed that the FR0348383 score could save 52% of avoidable biopsies in patients with grey zone PSA at a threshold probability of 30%, without missing any high-grade prostate cancer. What is more, the authors state in their methods section that three different urine samples of all patients were collected to demonstrate reliability and concordance of the biomarker. However, it is not entirely clear how these triplicates are handled in the article. In conclusion, the authors clearly value the importance of biomarker validation, but severe analytical validation of the test itself was not performed and some additional levels of clinical validation and clinical utility (such as another (blinded) sample set, prospective trial design, multicenter design, measuring impact on patient care, etc.) remain to be conducted.
5.5. SCHLAP1
Prensner and colleagues performed microarray experiments in 960 tissue samples from prostate cancer patients treated with radical prostatectomy. They identified lncRNA SCHLAP1 as the highest-ranked overexpressed gene in cancers with metastatic progression compared to non-metastatic cases [44]. Several experiments and analyses were performed to investigate the prognostic value of SCHLAP1 expression in tissues, in order to identify patients at high risk for metastatic disease progression. Also urinary levels of SCHLAP1 were assessed in 230 post-DRE urine samples. Apart from correlating SCHLAP1 levels with Gleason score and risk group, no biomarker validations were performed. It is clear that, although the prognostic value of SCHLAP1 in tissue samples yielded promising results, further optimization for a possible use as biomarker in urine is still needed. This limitation is also comprehensively acknowledged by the authors.
5.6. GAS5 and lincRNA-p21
Isin and colleagues collected urine samples after digital rectal exam of 30 prostate cancer patients and 49 patients with BPH [35]. Subsequently, ‘exosomal’ RNA was extracted using the Norgen Biotek “urine exosome RNA isolation kit”. Of note, the authors do not describe any quality control of the isolated extracellular vesicles by western blot, electron microscopy or nanoparticle characterization. Yet, evaluation and reporting of exosome quality control is vital for research in the field of extracellular vesicles [45]. Although we cannot be entirely sure that the reported lncRNAs reside inside exosomes, lincRNA-p21 and GAS5 were evaluated by RT-qPCR in all urine samples.
GAS5 levels were similar in urine of prostate cancer and BPH patients, whereas the authors report a statistically significant higher lincRNA-p21 abundance in prostate cancer compared to BPH. Perhaps a limitation for a robust biomarker, the RNA levels of lincRNA-p21 are very low. AUC was calculated for lincRNA-p21 (0.663) and the (clinical) sensitivity and specificity to discriminate BPH from prostate cancer were calculated for lincRNA-p21 alone (sensitivity: 67% and specificity: 63%) and in combination with PSA (52% and 94%), the latter achieving higher specificity. Of note, the lincRNA-p21 cut-off value was set at 0.181 and the median ‘exosomal’ levels were reported as 0.071 in BPH and 0.163 in prostate cancer.
5.7. PCAT18
In 2014, Crea and colleagues performed RNA sequencing of two xenografted cell lines derived from a needle biopsy of the same prostate cancer patient [46]. Remarkably, the cells display very different metastatic characteristics when injected in NOD-SCID mice. Differential expression analysis between the metastatic and non-metastatic xenograft-derived cell lines revealed several differentially expressed lncRNAs. The transcript with highest expression in the metastatic xenograft was named PCAT18 by the authors. In the article, the levels of PCAT18 are also assessed by RT-qPCR in plasma of 25 healthy controls, 25 primary prostate cancer patients and 25 patients with mCRPC. The PCAT18 RNA levels significantly increase from healthy individuals to patients with primary and subsequently metastatic prostate cancer. While PCAT18 is proposed as a novel biomarker for metastatic prostate cancer, no validation experiments are performed to date.
lnc-FGF1-9 (SPRY4-IT1/AK024556), lnc-SPATA31A6-6 (XLOC_007697), SMKR1 (LOC100287482), LINC01564 (XLOC_005327), lnc-RPP30-2 (XLOC_008559) and lnc-HNF1A-1 (XLOC_009911)
Based on differential expression analysis in prostate cancer cell lines and patient tissue samples, Lee and colleagues described six so-called lncRNAs for further investigation (lnc-FGF1-9 (AK024556/SPRY4-IT1), lnc-SPATA31A6-6 (XLOC_007697), SMKR1 (LOC100287482), LINC01564 (XLOC_005327), lnc-RPP30-2 (XLOC_008559) and lnc-HNF1A-1 (XLOC_00991)) [37]. However, one microarray probe corresponds to the protein coding gene SMKR1. All markers could be detected by RT-qPCR in urine samples from 13 prostate cancer patients and were significantly higher compared to urine of 14 heathy controls. In addition, there was no significant difference between urine of healthy controls and 9 BPH patients. Unfortunately, no direct comparison between prostate cancer patients and BPH patients was shown. Further, no validation tests for the biomarker(s) were performed.
6. Normalization of biomarker levels
All body fluid lncRNAs in this review were detected using the reverse transcription quantitative PCR (RT-qPCR) method. When evaluating RNA expression levels in cellular RNA from tissues or cells, normalization of the raw Cq values is extremely important to account for possible variation introduced at various levels, from differences in extraction yield and input concentration to efficiency of enzymatic reactions [47]. Normalizing to stably expressed endogenous reference genes is able to eliminate potential bias in a sample-specific way. Although many researchers still normalize to only one reference gene, the MIQE guidelines clearly state that normalization against a single reference gene is unacceptable unless authors prove that the gene is invariantly expressed under the experimental conditions described. What is more, the optimal number and the choice of reference genes should be experimentally determined [48]. Unfortunately, the MIQE guidelines from 2009 are not yet used by the majority of investigators when designing RT-qPCR experiments.
In contrast to evaluating expression levels in cellular RNA, normalization of biomarker data in liquid biopsies is somewhat different. As a matter of fact, RT-qPCR for biomarkers is not aimed at obtaining the most accurate estimate of ‘real’ expression but rather at optimizing the discriminatory power of a quantitative test. The normalization method to be used (if any) is entirely dependent on whether normalizing raw values improves the performance of a specific biomarker test. Alternative to reference genes, it should be considered to use fixed input volumes of fluid and/or RNA eluate (in combination with spike-in molecules, see further).
The last column of Table 1 indicates the normalization strategy for the liquid lncRNAs detected in prostate cancer patients. The majority of studies use GADPH (either alone or in combination) as reference gene. One study opted for fixed liquid/RNA input volumes, whereas two other articles from the same research group calculated (intrinsically normalized) scores based on Cq values of the lncRNA of interest and PSA.
In addition to evaluating the normalization strategy, we also plea for the use of exogenous spike-in molecules as processing controls. Spiking external controls should be part of any biomarker analytical validation experiment, to get a better grip on variation introduced during RNA extraction, reverse transcription and qPCR or any other quantification method. In the end, the biomarker(s) could be normalized using either endogenous reference genes or exogenous spikes; the normalization strategy that results in best biomarker performance is clearly most appropriate in that given experimental setup.
7. Are other lncRNAs detectable in body fluids?
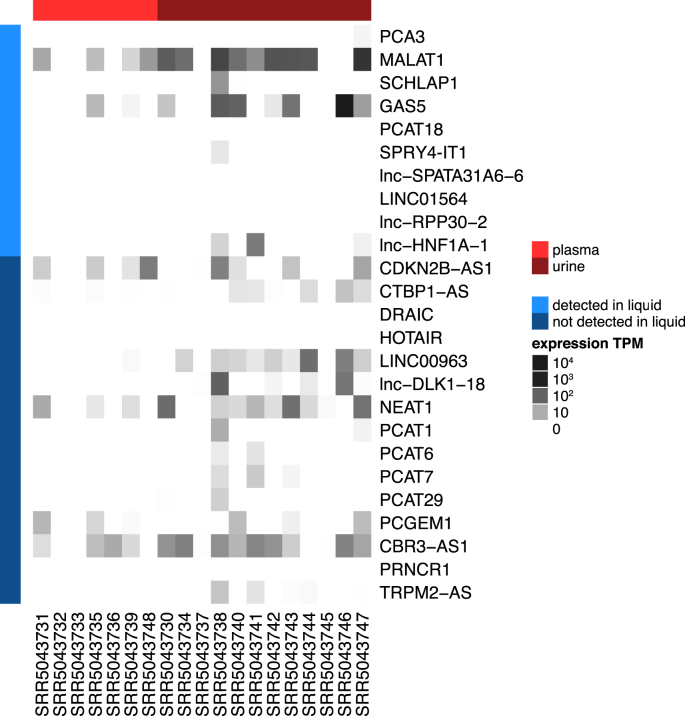
During our search for lncRNAs detectable in body fluids, we came across several other lncRNAs implicated in prostate cancer tissue that are not yet reported to be present in blood, urine or other body fluids. Examples are lncRNAs with LNCipedia IDs CDKN2B-AS1 (also known as ANRIL), CTBP1-AS, DRAIC, HOTAIR, Linc00963, Lnc-MX1-1, lncRNA-ATB (no LNCipedia ID), lnc-DLK1-18 (MEG3), NEAT1, PCAT1, PCAT6, PCAT7, PCAT29, PCGEM1, CBR3-AS1, PRNCR1, SOCS2-AS1 (no LNCipedia ID), and TRPM2-AS [26,29,[49], [50], [51], [52], [53]]. To make this review complete, we investigated whether any of these lncRNAs are present in publicly available total RNA sequencing data from prostate cancer patient liquid biopsies. Total RNA sequencing is, in contrast to small RNA sequencing and polyA + RNA sequencing, also able to detect (non-polyadenylated, polyA-) lncRNAs in an unbiased way. Because all lncRNAs in Table 1 were detected in urine, plasma, serum or exosomes, we focused our literature search on these four liquid biopsy types. To our surprise, we found only one study matching our interests (see Fig. 1) .
In 2016, Nikitina and colleagues performed whole transcriptome profiling in urine and plasma samples from 14 prostate cancer patients and 3 BPH patients [54,55]. Some samples were collected before and others after treatment. For one patient, all four sample types are available, i.e. both urine and plasma, before and after radical prostatectomy. We extracted FASTQ files from the publicly available IonTorrent data and quantified counts and transcripts per million (TPM) using Kallisto [56]. Next, we checked the levels of the lncRNA genes reported to be involved in prostate cancer. Only some of the liquid lncRNAs described in this review were detected in blood and/or urine. What is more, certain lncRNAs that have not been identified in body fluids of prostate cancer patients yet, seem to circulate (Fig. 2). As mentioned above, all lncRNAs in Table 1 were detected in the respective papers using the RT-qPCR methodology. We expect that optimization of total RNA sequencing procedures in body fluids will hugely impact the identification of novel biomarkers for prostate cancer.
Fig. 2.
Heat map constructed from publicly available total RNA sequencing data of lncRNAs known to be involved in prostate cancer. The lncRNAs in light blue were already described in literature to be detectable in liquids. The lncRNAs in dark blue are known to be involved in prostate carcinogenesis, but never before described in liquids.
8. Discussion
In this review, we first discussed how (novel) biomarkers could help improve diagnosis, prognosis, treatment decisions, monitoring and follow-up of prostate cancer patients. We next touched upon the importance of analytical and clinical validation, adequate reporting and a well-thought-out study design when investigating biomarkers. Subsequently, we considered some advantages and problems concerning liquid RNA biopsies, to find out which lncRNAs are reported to be present in body fluids of prostate cancer patients in the next paragraph. PCA3 is available as a commercial test, and MALAT1, FR0348383, SCHLAP1, GAS5, lincRNA-p21, PCAT18, lnc-FGF1-9 (AK024556 or SPRY4-IT1), lnc-SPATA31A6-6 (XLOC_007697), LINC01564 (XLOC_005327), lnc-RPP30-2 (XLOC_008559) and lnc-HNF1A-1 (XLOC_009911) were all detectable in either plasma, serum, urine or urinary ‘exosomes’. While all of them are proposed as putative biomarkers at some point, the levels of validation differ widely between studies. Yet, decently validated biomarkers are much more likely to be included in further translational studies. There is thus both room for further validation of known liquid lncRNA biomarkers in prostate cancer, and for discovery and subsequent validation of novel ones. As a matter of fact, the future of big data is likely to produce an overwhelming amount of candidate biomarkers. Utilizing such biomarkers to reduce cancer mortality and to improve patient quality of life will largely depend on excellent study designs, analytical and clinical validation of the tests, demonstration of clinical utility and detailed reporting to ensure reproducibility.
To illustrate this, we want to highlight the interesting approach the group of Yinghao Sun employed: after RNA sequencing of 14 matched tumor/normal pairs, they reported validation experiments for four different biomarkers in body fluids (MALAT1, PCA3 and FR0348383), in three follow-up papers. As is obvious from Table 1, they conduct more clinical and analytical validation experiments than any other published article reporting a putative liquid biomarker.
As a side note, it might be possible that Sun's group also tried to validate other biomarkers, without satisfying results. It would be a big improvement for biomarker research, and for science in general, if it was common practice to also report these ‘negative’ findings in scientific journals or curated online databases. This could avoid wasting valuable resources of other researchers who are trying to investigate exactly the same question, and who will likely also not report their results if the conclusions are ‘negative’.
At the same time, reproducible research will also rely upon the use of standard operation procedures on many different levels. For instance, standardized protocols to prepare blood plasma will be needed. As a matter of fact, plasma is now prepared with different blood collection tubes and according to varying centrifugation steps in labs and clinics. However, lncRNA signals are likely to differ between platelet-rich plasma, platelet-poor plasma and platelet-free plasma, all of which can be isolated separately by varying centrifugation speeds and durations.
On a more biological level, it remains to be elucidated what the exact source of a specific cancer-related lncRNA in body fluids is: does it come from the tumor or is it the result of a more systemic (immune) response? In addition, current analyses do not indicate if the lncRNA (fragment) is present in circulating tumor cells or extracellular vesicles, whether it is complexed with certain proteins or if it travels entirely free, either fragmented or not. An interesting approach could be to compare RNA from total plasma with RNA isolated from extracellular vesicles of the same patient. Extracellular vesicle RNA research in cancer seems promising because exosomes by definition carry a cargo originating from their host cell, but standardization in isolation methods is urgently needed [45]. In addition, tumor-educated platelets are now emerging as new, interesting blood components to search for biomarkers [57,58]. It goes without saying that also the detection of coding mRNA can be extremely valuable in the clinical management of prostate cancer patients. One example is the SelectMDx test to identify patients at increased risk for aggressive disease, which measures mRNA levels of DLX1 and HOXC6 in post-DRE urine.
Finally, apart from actions on the researcher's side, the biomarker field would also benefit from the involvement of ‘biomarker experts’ as peer-reviewers. Rather than assigning manuscripts to reviewers familiar with the biology of the gene or with the disease under study, at least one expert acquainted with specialized biomarker statistics and guidelines should be included in the peer-review process. Whether the research community already comprises a sufficient number of experts, or whether these should be educated in specialized teaching programs remains an open question. In addition, efforts to explain biomarker experiment design and statistics in a clear, comprehensible way to life science researchers is without a doubt extremely valuable, since most of the statistical courses are too theoretical for many biomedical researchers and clinicians.
In conclusion, we are hopeful that detecting carcinogenesis-related biomarkers in body fluids will improve diagnosis, prognosis, treatment decision, monitoring and follow-up of (prostate) cancer patients in the near future. However, we are running the risk of overhyping the promise if putative biomarkers are not appropriately validated at different levels, and standardized protocols are not implemented soon. This will hamper translation into clinical practice and might ultimately not lead to any reduction in cancer-related mortality or progress in quality of life.
9. Methods
To identify lncRNAs in body fluids of prostate cancer patients, we performed a PubMed search using keywords “lncRNA” or “long non-coding RNA” AND “prostate cancer” AND “serum” or “plasma” or “circulation” or “circulating” or “urine” or “exosomes” or “CTC” or “body fluid” or “cell-free” or “extracellular”. The search was last performed on Sept 25, 2017, and returned 28 articles. Detailed reading of these articles led us to more articles on biomarkers, lncRNAs and prostate cancer, of which the most relevant ones to the reader are included in the review.
To seek for publicly available total RNA sequencing datasets, we performed a PubMed search using keywords “total RNA sequencing” or “RNA sequencing” or “RNA-seq” or “RNAseq” AND “prostate cancer” AND “plasma” or “urine” or “serum” or “exosomes”. Given that RNA sequencing of liquid biopsies is a rapidly evolving research field, we last performed this search immediately before submitting the review, on December 14, 2017.
Acknowledgements
Hetty Helsmoortel is a postdoctoral fellow and Celine Everaert a doctoral fellow with the Research Foundation Flanders. The team is supported by a research grant from Stand up to Cancer.
References
- 1.Gaudreau P.-O., Stagg J., Soulières D., Saad F. The present and future of biomarkers in prostate cancer: proteomics, genomics, and immunology advancements. Biomarkers Canc. 2017;8s2 doi: 10.4137/BIC.S31802. BIC.S31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwin M.S., Tan H.-J. The diagnosis and treatment of prostate cancer: a review. Jama. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 3.2017. https://seer.cancer.gov/statfacts/html/prost.html
- 4.Volders P.-J., Helsens K., Wang X., Menten B., Martens L., Gevaert K. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:D246–D251. doi: 10.1093/nar/gks915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Canc. Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer M.K., Niknafs Y.S., Malik R., Singhal U., Sahu A., Hosono Y. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes D.F. Biomarker validation and testing. Molecular Oncology. 2014;9:960–966. doi: 10.1016/j.molonc.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinsky P.F., Prorok P.C., Kramer B.S. Prostate cancer screening - a perspective on the current state of the evidence. N. Engl. J. Med. 2017;376:1285–1289. doi: 10.1056/NEJMsb1616281. [DOI] [PubMed] [Google Scholar]
- 9.Prensner J.R., Rubin M.A., Wei J.T., Chinnaiyan A.M. Beyond PSA: the next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003180. 127rv3–127rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. Eau-estro-siog guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Klotz L. Prostate cancer overdiagnosis and overtreatment. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:204–209. doi: 10.1097/MED.0b013e328360332a. [DOI] [PubMed] [Google Scholar]
- 12.Prasad V., Lenzer J., Newman D.H. Why cancer screening has never been shown to “save lives–”and what we can do about it. Br. Med. J. 2016;352:h6080–h6084. doi: 10.1136/bmj.h6080. [DOI] [PubMed] [Google Scholar]
- 13.Jennifer Gordetsky J.E. Grading of prostatic adenocarcinoma: current state and prognostic implications. Diagn. Pathol. 2016;11:58. doi: 10.1186/s13000-016-0478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballman K.V. Biomarker: predictive or prognostic? J. Clin. Oncol. 2015;33:3968–3971. doi: 10.1200/JCO.2015.63.3651. [DOI] [PubMed] [Google Scholar]
- 15.Italiano A. Prognostic or Predictive? It's time to get back to definitions! J. Clin. Oncol. 2011;29 doi: 10.1200/JCO.2011.38.3729. 4718–4718. [DOI] [PubMed] [Google Scholar]
- 16.Wallace T.J., Torre T., Grob M., Yu J., Avital I., Brücher B. Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J. Canc. 2014;5:3–24. doi: 10.7150/jca.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. Reporting recommendations for tumor marker prognostic studies (REMARK) J. Natl. Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 18.Hayes D.F., Allen J., Compton C., Gustavsen G., Leonard D.G.B., McCormack R. Breaking a vicious cycle. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005950. 196cm6–196cm6. [DOI] [PubMed] [Google Scholar]
- 19.Byron S.A., Van Keuren-Jensen K.R., Engelthaler D.M., Carpten J.D., Craig D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and Challenges. Nat. Rev. Genet. 2016 doi: 10.1038/nrg.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Meo A., Bartlett J., Cheng Y., Pasic M.D., Yousef G.M. Liquid biopsy: a step forward towards precision medicine in urologic malignancies. Mol. Canc. 2017;16:80. doi: 10.1186/s12943-017-0644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini K.L., Sanda M.G., Moreno C.S. RNA biomarkers to facilitate the identification of aggressive prostate cancer. Mol. Aspect. Med. 2015;45:37–46. doi: 10.1016/j.mam.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchinson L., DeVita V.T., Jr. The holy Grail of biomarkers. Nat. Rev. Clin. Oncol. 2009;6 doi: 10.1038/nrclinonc.2009.145. 553–553. [DOI] [PubMed] [Google Scholar]
- 24.Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martignano F., Rossi L., Maugeri A., Gallà V., Conteduca V., De Giorgi U. Urinary RNA-based biomarkers for prostate cancer detection. Clin. Chim. Acta. 2017;473:96–105. doi: 10.1016/j.cca.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Su Y.-J., Yu J., Huang Y.-Q., Yang J. Circulating long noncoding RNA as a potential target for prostate cancer. Ijms. 2015;16:13322–13338. doi: 10.3390/ijms160613322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubbard T., Barker D., Birney E., Cameron G., Chen Y., Clark L. The Ensembl genome database project. Nucleic Acids Res. 2002;30:38–41. doi: 10.1093/nar/30.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebhan M., ChalifaCaspi V., Prilusky J., Lancet D. GeneCards: integrating information about genes, proteins and diseases. Trends Genet. 1997;13 doi: 10.1016/s0168-9525(97)01103-7. 163–163. [DOI] [PubMed] [Google Scholar]
- 29.Misawa A., Takayama K.I., Inoue S. Long non-coding RNAs and prostate cancer. Canc. Sci. 2017;431:350. doi: 10.1111/cas.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F., Ren S., Chen R., Lu J., Shi X., Zhu Y. Development and prospective multicenter evaluation of the long noncoding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancer. Oncotarget. 2014;5:11091–11102. doi: 10.18632/oncotarget.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crea F., Watahiki A., Quagliata L., Xue H., Pikor L., Parolia A. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget. 2014;5:764–774. doi: 10.18632/oncotarget.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren S., Wang F., Shen J., Sun Y., Xu W., Lu J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. EJC (Eur. J. Cancer) 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Xue D., Zhou C.-X., Shi Y.-B., Lu H., He X.-Z. MD-miniRNA could be a more accurate biomarker for prostate cancer screening compared with serum prostate-specific antigen level. Tumor Biol. 2015;36:3541–3547. doi: 10.1007/s13277-014-2990-x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Ren S.C., Shi X.L., Liu Y.W., Zhu Y.S., Jing T.L. A novel urinary long non-coding RNA transcript improves diagnostic accuracy in patients undergoing prostate biopsy. Prostate. 2015;75:653–661. doi: 10.1002/pros.22949. [DOI] [PubMed] [Google Scholar]
- 35.Işın M., Uysaler E., Özgür E., Köseoğlu H., Şanlı Ö., Yücel Ö.B. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front. Genet. 2015;6:168. doi: 10.3389/fgene.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huarte M., Guttman M., Feldser D., Garber M., Koziol M.J., Kenzelmann-Broz D. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee B., Mazar J., Aftab M.N., Qi F., Shelley J., Li J.-L. Long noncoding RNAs as putative biomarkers for prostate cancer detection. J. Mol. Diagn. 2014;16:615–626. doi: 10.1016/j.jmoldx.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bussemakers M.J.G., van Bokhoven A., Verhaegh G.W., Smit F.P., Karthaus H.F.M., Schalken J.A. DD3:A new prostate-specific gene, highly overexpressed in prostate cancer. Canc. Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 39.Food D. Administration, PMA approval. PROGENSA PCA3 assay. 2013. www.accessdata.fda.gov/cdrh_docs/pdf10/p100033a.pdf 1-34.
- 40.Nicholson A., Mahon J., Boland A., Beale S., Dwan K., Fleeman N. The clinical effectiveness and cost-effectiveness of the PROGENSA® prostate cancer antigen 3 assay and the Prostate Health Index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol. Assess. 2015;19:1–192. doi: 10.3310/hta19870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group Recommendations from the EGAPP Working Group: does PCA3 testing for the diagnosis and management of prostate cancer improve patient health outcomes? Genet. Med. 2014;16:338–346. doi: 10.1038/gim.2013.141. [DOI] [PubMed] [Google Scholar]
- 42.Ren S., Peng Z., Mao J.-H., Yu Y., Yin C., Gao X. RNA-seq analysis of prostate cancer in the Chinese population identifies recurrent gene fusions, cancer-associated long noncoding RNAs and aberrant alternative splicings. Cell Res. 2012;22:806–821. doi: 10.1038/cr.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kattan M.W. 2009. Encyclopedia of Medical Decision Making. [Google Scholar]
- 44.Prensner J.R., Zhao S., Erho N., Schipper M., Iyer M.K., Dhanasekaran S.M. RNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1. Lancet Oncol. 2014;15:1469–1480. doi: 10.1016/S1470-2045(14)71113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Deun J., Mestdagh P., Agostinis P., Akay Ö., Anand S., Anckaert J. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Br. J. Pharmacol. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 46.Crea F., Watahiki A., Quagliata L., Xue H., Pikor L., Parolia A. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget. 2014;5:764–774. doi: 10.18632/oncotarget.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 49.Willard S.S., Koochekpour S. Regulators of gene expression as biomarkers for prostate cancer. Am J Cancer Res. 2012;2:620–657. [PMC free article] [PubMed] [Google Scholar]
- 50.Ramalho-Carvalho J., Fromm B., Henrique R., Jerónimo C. Deciphering the function of non-coding RNAs in prostate cancer. Canc. Metastasis Rev. 2016;35:235–262. doi: 10.1007/s10555-016-9628-y. [DOI] [PubMed] [Google Scholar]
- 51.Chandra Gupta S., Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: from biomarkers to therapeutic targets. Int. J. Canc. 2017;140:1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]
- 52.Xu S., Xu S., Yi X.-M., Yi X.-M., Tang C.-P., Tang C.-P. Long non-coding RNA ATB promotes growth and epithelial-mesenchymal transition and predicts poor prognosis in human prostate carcinoma. Oncol. Rep. 2016;36:10–22. doi: 10.3892/or.2016.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma W., Chen X., Ding L., Ma J., Jing W., Lan T. The prognostic value of long noncoding RNAs in prostate cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:57755–57765. doi: 10.18632/oncotarget.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikitina A.S., Babenko V.V., Babalyan K.A., Vasiliev A.O., Govorov A.V., Prilepskaya E.A. Primary candidate rna biomarker screening by RNA-seq for prostate cancer diagnostics. Biomed Khim. 2015;61:781–784. doi: 10.18097/PBMC20156106781. [DOI] [PubMed] [Google Scholar]
- 55.Nikitina A.S., Sharova E.I., Danilenko S.A., Selezneva O.V., Butusova T.B., Vasiliev A.O. Datasets for next-generation sequencing of DNA and RNA from urine and plasma of patients with prostate cancer. Data in Brief. 2017;10:369–372. doi: 10.1016/j.dib.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 57.Sol N., Wurdinger T. Platelet RNA signatures for the detection of cancer. Canc. Metastasis Rev. 2017;36:263–272. doi: 10.1007/s10555-017-9674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Best M.G., Vancura A., Wurdinger T. Platelet RNA as a circulating biomarker trove for cancer diagnostics. J. Thromb. Haemostasis. 2017;15:1295–1306. doi: 10.1111/jth.13720. [DOI] [PubMed] [Google Scholar]