Abstract
Heart failure is a complex syndrome involving various pathophysiological processes. An increasing body of evidence shows that the myocardial microvasculature is essential for the homeostasis state and that a decompensated heart is associated with microvascular dysfunction as a result of impaired endothelial angiogenic capacity. The intercellular communication between endothelial cells and cardiomyocytes through various signaling molecules, such as vascular endothelial growth factor, nitric oxide, and non-coding RNAs is an important determinant of cardiac microvascular function. Non-coding RNAs are transported from endothelial cells to cardiomyocytes, and vice versa, regulating microvascular properties and angiogenic processes in the heart. Small-exocytosed vesicles, called exosomes, which are secreted by both cell types, can mediate this intercellular communication. The purpose of this review is to highlight the contribution of the microvasculature to proper heart function maintenance by focusing on the interaction between cardiac endothelial cells and myocytes with a specific emphasis on non-coding RNAs (ncRNAs) in this form of cell-to-cell communication. Finally, the potential of ncRNAs as targets for angiogenesis therapy will also be discussed.
1. Introduction
Heart failure (HF) is the final state of various cardiovascular conditions where the heart is no longer capable of supplying sufficient blood to support the physiological demand of the body. Despite currently available therapies, HF remains a chronic condition with high morbidity and mortality rates [1], [2]. Since the pathogenesis of the disease is complex, there is a dire need for a comprehensive and thorough understanding of the pathophysiological processes that lead to the onset and progression of HF in order to improve or develop novel therapeutic options.
Pathological cardiac remodeling is marked by hypertrophic growth of the heart, formation of fibrotic tissue, infiltration of inflammatory cells, and reduced myocardial capillary number. HF is associated with vascular structural remodeling, which leads to disturbed blood flow and heart muscle tissue perfusion [3], [4]. Reduction in capillary density, or capillary rarefaction, occurs in the heart of patients with HF [5], [6], and is determined by the rate of angiogenesis in the heart where endothelial cells (ECs) play a significant role [7], [8]. Despite the conflicting results from past and ongoing clinical trials, pre-clinical in vivo studies show clear improved cardiac function after neovascularization by enhancing capillary density with pro-angiogenic compounds [9], [10], [11]. One of the possible reasons for this discrepancy is the common use of single pro-angiogenic agents, mostly considered insufficient to boost angiogenesis in the heart [12], [13].
MicroRNAs (miRNAs) are short non-coding RNAs (ncRNAs), 21–23 nucleotide-long that function by repressing the expression of their target genes [13], [14]. miRNAs are able to bind complementarily to the 3′ untranslated region (UTR) of more than one hundred target messenger RNAs (mRNAs) [15], [16] involved in regulating common or diverse networks or pathways, allowing the occurrence of a synergistic effect. In contrast, long ncRNAs (lncRNAs) are more than 200 nucleotides in size and exert multiple functions, including functioning as scaffold for transcription factors, or acting as molecular sponges [17]. ncRNAs are established regulators of cardiac capillary formation [18], [19], [20] and their endothelial expression is influenced by various factors released by the adjacent cell types, forming an intercellular communication network. Exosomes have emerged as an essential intercellular communication tool among different cell types in the heart as they are able to carry various biological information, including proteins, lipids, and ncRNAs, from donor cells and affect the behavior of the recipient cells [21], [22]. In this review, we discuss the contribution of microvascular remodeling in the development of HF. We outline the role of ECs and the significance of ncRNAs in the regulation of heart microvasculature. Moreover, we discuss the role of exosomes as an intercellular communication tool affecting endothelial angiogenic capacity. Finally, we discuss angiogenic ncRNAs as potential targets for neovascularization therapy to ameliorate HF.
2. Development of heart vascularization
ECs, among other cell types, are a major determinant of the homeostasis of the heart. They line the interior of myocardial capillaries, forming an endothelial contour, which serves as an anatomical and functional boundary between blood and the surrounding tissues. The formation of blood vessels starts with the formation of a linear heart tube consisting of several layers of cardiomyocytes (CMs) adhering to the EC layer, which develops at embryonic day (E.) 8 in the mouse and E.17-.19 in human. At this point, the heart is avascular and receives nutrients and oxygen supply from its surroundings through diffusion [8]. As the CM layer grows, the diffusion process can no longer maintain nutrient demand, and soon after the heart starts to contract, the primitive vascular plexus forms [23]. The plexus originates from angiogenic precursor cells coming from the pro-epicardial organ and sinus venosus, which will differentiate into ECs and form a primeval capillary network [8], [23]. This early phase of blood vessel formation where blood vessels develop from non-existing vessels is termed vasculogenesis. The vascular plexus expands through EC sprouting from the pre-existing capillaries in a process known as angiogenesis, ultimately developing into an organized network of smaller and larger vessels. At E.13 in the mouse (E.42 in human) this network connects with the aorta, followed by colonization of smooth muscle cells and fibroblasts to form the media and adventitial layer in a process called arteriogenesis [8], [24]. Myocardial thickness increases approximately fourfold during postnatal life due to CM proliferation up to day 7 [25], and hypertrophic growth. The increased metabolic demand of proliferating and hypertrophying CMs is met by the expansion of myocardial capillary density three-to fourfold during the first 3 weeks of postnatal life [8]. Cardiac capillaries and CM growth are proportional to an increase in cardiac mass [26], suggesting that any alterations in these processes may result in myocardial hypoxia and/or ischemia leading to pathological remodeling and eventually HF.
3. Capillary rarefaction in the failing heart
Myocardial growth and angiogenesis are adaptive responses of the heart to an increase in hemodynamic demand. An upregulation of myocardial capillary density has been long observed in response to the presence of physiological stimuli, such as pregnancy and exercise, while an opposite effect occurs in HF [27], [28], [29]. Vascular endothelial growth factor (VEGF) is an angiogenic molecule with a pivotal role in vessel formation of several organs, including the heart [28], [30]. CM growth induced by physiological stimuli through the Akt1 pathway [31], [32], [33] can promote CM growth with simultaneous upregulation of VEGF and promotion of angiogenesis, leading to an increase in capillary number [33]. Activation of Akt in heart muscle-restricted inducible Akt1 transgenic mice increases production of VEGF, with subsequent preservation of cardiac capillary density, indicating the paracrine effect of VEGF on the surrounding myocardial ECs [34]. Hypoxia inducible factor-1α (HIF-1α) and GATA4 are two major positive regulators of VEGF, that are involved in the regulation of VEGF in the heart by acting on distinct regulatory elements of VEGF [30], [35]. An increase in capillary mass, in turn, can maintain cardiac muscle growth by enhancing the secretion of growth factors from the endothelium, such as nitric oxide (NO), which is transferred to CMs and results in the activation of PI3Kγ/Akt pathway [36], [37], [38].
Deregulation of PI3Kγ/Akt/VEGF pathway leads to cardiac dysfunction. VEGF deficiency has been observed during pressure overload, leading to a capillary rarefaction and transition to HF [30], [39]. The myocardium is highly dependent on oxygen and nutrients, and therefore, displays a high capillary number to guarantee ample amount of supplies [36], [40]. Vascularization of the myocardium is essential for cardiac homeostasis, and any abnormalities in this process will impair cardiac function. Capillary rarefaction has been observed in HF induced by various etiologies, including myocardial infarction, hypertension, or cardiomyopathy [6], [41], [42]. Recent studies have also demonstrated capillary rarefaction in HF patients with preserved ejection fraction (HFpEF) and indicate that cardiac endothelial cell remodeling has a causal role in the onset and progression of the disease [30], [43], [44]. An increasing body of evidences shows that stimulation of blood vessel formation in the heart could exert a therapeutic benefit for a failing heart. Neovascularization therapies to induce canonical angiogenesis pathways through the PI3K/Akt1/VEGF axis has been advocated to improve capillary density and subsequent heart function [45], [46]. However, and although animal studies showed promising results, clinical trials have shown none or only very modest effects [46], [47], [48]. This is due to several factors, including the choice of one single pro-angiogenic growth factor, which is considered insufficient to boost angiogenesis in the heart [12], [49]. From this perspective, ncRNAs, given their pleiotropic effects, serve as promising targets for neovascularization therapies in HF.
4. Non-coding RNAs in cardiac angiogenesis
ncRNA species are artificially divided into two groups depending on the length of their nucleotide sequence: small ncRNAs that are less than 200 nucleotide-long, and lncRNAs, which are more than 200 nucleotides in length [50], [51]. Small ncRNAs are functionally subdivided into various categories, including small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), piwi interacting RNAs (piRNAs), and miRNAs. lncRNAs comprise the most heterogeneous and most poorly characterized group of non-coding transcripts to date. In this review, we focus on the contribution of miRNAs and lncRNAs in the regulation of cardiac microvasculature.
4.1. MicroRNAs
miRNAs are most likely the best studied functional, small ncRNAs. They are evolutionarily conserved ∼22-nucleotide single-stranded RNA molecules that function by inhibiting the expression of mRNA targets through Watson–Crick base pairing with their binding site on the 3′UTR of the target transcript. Depending on the binding specificity, the target mRNA may be degraded (mRNA cleavage) or, more commonly, its translation is inhibited (mRNA decay). The varying degree of specificity in the complementarity between the miRNA and the target mRNA allows the same miRNA species to regulate several different mRNAs, simultaneously [52]. The human genome has been estimated to encode more than 1000 miRNA genes [53], which regulate over 60% of protein coding genes [49], [54]. miRNAs are transcribed in the nucleus by RNA polymerase II as primary miRNA (pri-miRNA) transcripts [55]. The pri-miRNAs are then cleaved by the RNase III enzyme Drosha to generate precursor miRNAs (pre-miRNAs), which are subsequently exported to the cytoplasm by exportin-5 [55], [56]. Once in the cytoplasm, pre-miRNAs are further processed by RNase III enzyme Dicer into mature ∼22 nucleotide single stranded miRNAs which are then incorporated into the RNA-induced silencing complex (RISC) [57]. Eventually, the mature miRNAs will guide this complex, through its complementary base pairing to degrade or to inhibit the translation of target genes [58].
miRNAs are involved in the regulation of various biological processes, such as cellular proliferation, differentiation, and migration [20], [59]. Aberrant expression of miRNAs has been observed in different cardiac pathologies, including HF [60], [61], [62] and post-myocardial infarction remodeling [63], [64]. In addition, miRNAs have been reported to regulate various aspects of the cardiact angiogenic response through their direct effect on ECs [18], [65], [66]. The first clue of the involvement of miRNAs in the regulation of angiogenesis was observed in Dicer knockout mice which displayed early mortality during embryonic development caused by impaired angiogenesis [67].
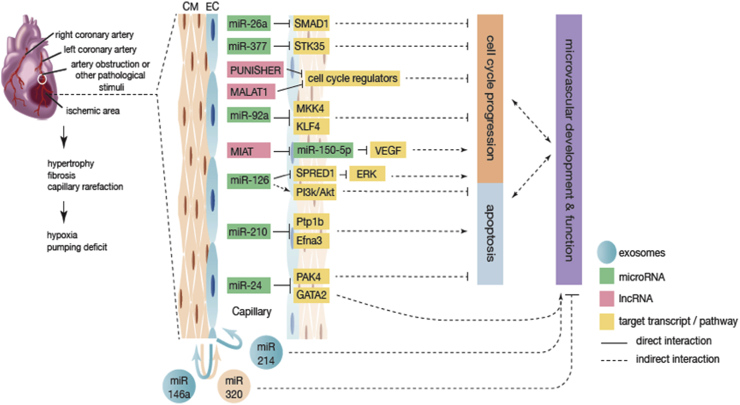
Functional endothelial miRNAs can be categorized into those that impair (anti-angiogenic) and those that induce (pro-angiogenic) endothelial angiogenic properties. miR-92a and miR-24 are two abundant endothelial miRNAs with anti-angiogenic properties. Inhibition of miR-92a improved vascular function and proliferation, with subsequent amelioration of heart function, both in a pig model of ischemia/reperfusion injury [68] and a mouse model of myocardial infarction [18]. miR-92a interacts with MAP kinase kinase 4 (MKK4) and Kruppel-like factors-4 (KLF4), thus interfering with cell cycle progression in ECs [69]. miR-24 is enriched in cardiac ECs and upregulated after an ischemic insult. Inhibition of endothelial miR-24 leads to reduced myocardial infarct size and improved heart function in mice, an effect that is mediated through prevention of EC apoptosis and enhanced vascularity [19]. miR-24 is also able to directly inhibit p21 protein (Cdc42/Rac)-activated kinase 4 (PAK4) and GATA binding protein 2 (GATA2) to improve EC survival and fitness [19] (Fig. 1).
Fig. 1.
The role of ncRNAs in cardiac EC function in HF. In the injured heart, cardiac remodeling, including capillary rarefaction, takes place, leading to relative hypoxia in the heart. In response to these environmental changes, ECs are able to alter their gene expression profiles in a number of ways, including through ncRNA-mediated gene silencing. Interplay with other cell types, such as CMs also occur, which can be mediated by exosomal transfer. Several EC miRNAs and lncRNAs, and their target genes are shown to regulate EC cell cycle progression or apoptosis process, which determine the function and development of cardiac microvascularization.
Similarly, miR-26a [70] and miR-377 [71] are two recently described miRNAs whose inhibition leads to recovery of microvascularization of the heart tissue. miR-26a expression is upregulated in a mouse model of acute myocardial infarction and in human subjects suffering from acute coronary syndromes [70]. SMAD family member 1 (SMAD1) was demonstrated to be the target gene of miR-26a responsible for these effects. Administration of a miR-26a inhibitor to mice subjected to myocardial infarction, led to an increase in SMAD1 expression with subsequent enhancement of angiogenesis, reduced infarct size and improved cardiac function [70]. Heart tissue from patients with HF revealed a significant upregulation of miR-377 expression, a miRNA that inhibits migration and the capacity of ECs and endothelial progenitor cells to form tubes in vitro. Implantation of miR-377-null endothelial progenitor cells into ischemic myocardium resulted in an attenuation of pathological cardiac remodeling [71], an effect that is mediated by the direct pro-angiogenic target of miR-377, serine/threonine kinase 35 (STK35) (Fig. 1). A similar role is exerted by miR-34 whose expression is upregulated in response to stress. Inhibition of all its family members (miR-34a, 34b, and 34c) results in increased capillary density in a mouse model of either myocardial infarction or pressure overload, accompanied by reduced fibrosis and improved heart function [72]. Downregulation of this miRNA leads to upregulation of several target genes, including VEGF, vinculin, protein O-fucosyltranferase 1, Notch1, and semaphorin 4B [72]. Even though the direct role of this miRNA and its target genes in the endothelium remains to be further investigated, it is clear that cardiac endothelial function is affected by the modulation of this miRNA [73].
Another anti-angiogenic miRNA is miR-503 whose expression is upregulated in myocardial microvascular ECs from type 2 diabetic Goto–Kakizaki rats, leading to impaired angiogenesis [74] as observed by reduced proliferation, migration, and network formation capacity of these cells in vitro. These effects were mediated by the suppression of target genes Cyclin E1 (CCNE1) and cell division cycle 25A (cdc25A). Inhibition of miR-503 in the ischemic muscle tissue of diabetic mice leads to improved post-ischemic angiogenesis and blood flow recovery [75]. Given the fact that miR-503 is involved in the regulation of EC angiogenic capacity and that there is dysregulation of the expression of this miRNA in the hearts of diabetic mice, it is plausible that miR-503 also plays a role in regulating myocardial angiogenesis in the development towards HF.
miR-210, a strongly expressed miRNAs under cardiac hypoxic conditions, is considered to be a valid biomarker for chronic HF [76], [77]. The function of miR-210 is, however, debatable. It has been advocated that this miRNA is pro-angiogenic and anti-apoptotic, through direct interaction with protein tyrosine phosphatase 1b (Ptp1b) and ephrin A3 (Efna3) (Fig. 1). Intra-myocardial injections of a minicircle vector carrying miR-210 precursor into a mouse model of myocardial infarction resulted in improved cardiac function in comparison to the untreated, control group. Histological analysis showed a decrease in cellular apoptosis and increased neovascularization [78]. In contrast, another study shows that miR-210 targets HIF-1α and induce a detrimental angiogenic response under hypoxic conditions while its inhibition improves heart function and survival of mice after myocardial infarction [79]. These diverging results could be a product of different transfection methods and efficiencies and/or technical bias, however this still requires clarification.
Another well-known endothelial-enriched pro-angiogenic miR is miR-126, an established fundamental player in the maintenance of vascular integrity and function [65]. This miRNA is encoded within the host gene epidermal growth factor like domain 7 (EGFL-7), known to regulate tubulogenesis [80]. miR-126 is able to potentiate endothelial pro-angiogenic pathways in multiple axis. It directly inhibits sprout-related, EVH1 domain, containing 1 (SPRED1), an inhibitor of the extracellular regulated MAP kinase (ERK), to prevent its anti-angiogenic action. miR-126 is also a potent anti-apoptotic factor through regulation of the PI3K/Akt [81], [82], [83]. While recent findings report a decrease in circulating levels of miR-126 after myocardial infarction, miR-126 expression profiles can also be correlated to cardiac function indexes, strongly supporting the contribution of miR-126 to regulation of proper cardiac function (Fig. 1) [81], [84].
Several other miRNAs were reported to display differential expression and have biological relevance when exposing ECs to different types of stress, such as alterations in the blood flow, inflammatory response, hyperglycemia, and hypoxia (Table 1). While the significance of such findings to the regulation of cardiac microvasculature has not yet been established, we speculate that many of these miRNAs may also play a role in the remodelling of the injured heart by impacting EC function.
Table 1.
Functional miRNAs in ECs exposed to pathological stimuli prevalent in cardiovascular diseases.
| miRNAs | Targets | Pathological Stimuli | Model | Effect | References |
|---|---|---|---|---|---|
| miR-15a | FGF2 VEGF |
Ischemia | Murine Hindlimb | Anti-angiogenic | [85] |
| miR-19a | CCND1 | Dysregulated Flow | HUVEC | Anti-proliferation | [86] |
| miR-19/221/222 | PGC-1α | Inflammation | HAEC | Pro-apoptotic | [87] |
| miR-21 | PTEN | Dysregulated Flow | HUVEC | Anti-inflammation Anti-apoptotic |
[88] |
| miR-92 | KLF2 | Dysregulated Flow | HUVEC | Anti-angiogenic Pro-inflammation |
[89] |
| miR-100 | mTOR | Ischemia | Murine Hindlimb | Anti-angiogenic | [90] |
| miR-101 | Cul3 | Ischemia | HUVEC | Pro-angiogenic | [91] |
| miR-101 | mTOR | Dysregulated Flow | HUVEC | Anti-proliferation | [92] |
| miR-106b∼25 | PTEN | Ischemia | Murine Hindlimb HUVEC |
Pro-angiogenic | [93] |
| miR-107 | Dicer1 | Ischemia | MCAO mice HUVEC |
Pro-angiogenic | [94] |
| miR-132/212 | Rasa1 Spred1 Spry1 |
Ischemia | Murine Hindlimb | Pro-angiogenic | [95] |
| miR-155 | AT1R VEGFR2 |
Ischemia | MCAO mice | Anti-angiogenic | [96], [97] |
| miR-155 | AT1R Ets1 |
Inflammation | HUVEC | Anti-inflammation Anti-angiogenic |
[98] |
| miR-200c | ZEB1 | Ischemia | Murine Hindlimb HUVEC |
Anti-angiogenic | [99] |
| miR-221/222 | Ets1 | Inflammation | HUVEC | Anti-inflammation | [98] |
| miR-223 | RPS6KB1 | Ischemia | CMEC | Anti-angiogenic | [100] |
| miR-365 | Bcl2 | Inflammation | HUVEC | Pro-angiogenic Anti-inflammation |
[101] |
| miR-424 | Cul2 | Ischemia | HUVEC | Pro-angiogenic | [102] |
| miR-663 | KLF4 CEBPB ATf3 |
Dysregulated Flow | HUVEC | Pro-inflammation | [103] |
4.2. LncRNAs
lncRNAs differentially regulate gene expression. Prior to transcription, lncRNAs can act as a scaffold to recruit and coordinate the assembly of epigenetic complexes. They are able to interfere with transcription by serving as a decoy or competing with transcription factors, while potentially also being able to inhibit RNA polymerase II activity. Post-transcriptionally, lncRNAs can affect gene expression by interacting with mRNAs and causing their destabilization [104], [105], [106]. Moreover, lncRNAs can serve as sponges for miRNAs [107].
lncRNAs identified to be involved in cardiac microvascular dysfunction are myocardial infarction-associated transcript (MIAT) and PUNISHER (Fig. 1). Expression of MIAT was first reported as a predictor of myocardial infarction [108] and later related to pathological angiogenesis [109]. MIAT is able to sponge miR-150-5p, which is responsible for abnormal upregulation of VEGF, and thus promoting pathological reduced angiogenesis and microvascular dysfunction [109]. PUNISHER is a novel endothelial-specific lncRNA conserved in zebrafish, mice and human, named retrospectively according to the phenotype it induces in zebrafish. It appears to be an essential regulator of vessel formation as its inhibition results in severe vascular defects, which negatively correlates with cell cycle- and endothelial fitness-related gene expression, and positively correlates with cell adhesion-related gene expression [110].
While comprehensive studies on the role of lncRNAs in the regulation of cardiac microvasculature are scarce, these few reports provide some insights into the topic. Several lncRNAs are known to regulate EC function and behaviour, and despite not having been proven to exert their role in the heart, it is plausible that they play a role in fine tuning of cardiac function [17], [111]. The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a pro-angiogenic lncRNA that is upregulated under hypoxic conditions and fundamentally controls the switch between endothelial proliferative and migratory phenotypes [112], [113]. Another noncoding transcript that may impact on cardiac microvasculature is an antisense RNA transcript named ANRIL (antisense noncoding RNA in the INK4 locus) [114]. ANRIL is expressed in both vascular endothelial and coronary smooth muscle cells. Single nucleotide polymorphism variants of ANRIL have been linked to angiogenesis and atherosclerosis [115], [116]. In addition to ANRIL, lncRNA maternally expressed gene 3 (MEG3) has been described to play a role in diabetes-related microvascular dysfunction. MEG3 expression levels are significantly low in the retina of streptozotocin-induced diabetic mice and MEG3 knockdown exaggerates retinal microvascular dysfunction, shown by increased microvascular leakage, inflammation, and capillary degeneration [117].
Other angiogenesis-related lncRNAs are LINC00323 and MIR503HG. Silencing of LINC00323 in human umbilical vein endothelial cells (HUVECs) inhibited cell proliferation, migration, and tube formation capacity. A direct interaction between LINC00323 and eIF4A3/GATA2 suggests that LINC00323 acts as a scaffold controlling the expression of these two endothelial transcription factors. MIR503HG represses miR-424 expression in an hypoxia-dependent fashion and its inhibition leads to upregulation of miR-424 with subsequent impairment of EC proliferation and migration. MIR503HG seems to regulate endothelial angiogenic capacity by altering the expression of GATA2 via modulation of miR-424 expression levels [118].
SENCR is a human vascular-enriched lncRNA whose expression is abundant in ECs and positively correlates with Friend Leukemia Integration virus 1 (FLI1) expression, a regulator of endothelial development. The levels of this lncRNA are altered in vascular tissue and cells derived from patients with limb ischemia and with premature coronary artery disease. Overexpression of SENCR in HUVECs induces cell proliferation, migration, and tube formation capacity [119]. Two other lncRNAs which promote EC function are platelet-activating factor acetyl hydrolase 1B1 (PAFAH1B1, also known as Lis1) and NONHSAT073641. Downregulation of these lncRNAs leads to impaired endothelial tube formation and decreased sprouting. PAFAH1B1 promotes the expression of Matrix Gla Protein (MGP), a positive regulator of endothelial angiogenic capacity, as it is required for active histone marks and binding of RNA Polymerase II to the transcriptional start site of MGP. Although NONHSAT073641 positively regulates angiogenesis to a similar extent as PAFAH1B1, its molecular mechanism is still unknown [120].
5. Exosomes as an intercellular communication tool between cardiomyocytes and endothelial cells
ncRNAs can be transferred and influence the behaviour of other cells adjacent to their cell of origin. The mechanism of this transport can be either through direct cell-to-cell contact or through a paracrine-like action. Even though ncRNAs can travel through gap junctions from one cell to another [121], [122], [123], extracellular transportation has been the focus of most recent studies. The majority of secreted RNAs are chaperoned by another biological entity, including exosomes, which protects them from RNAse-mediated degradation [124], [125]. Exosomes are small (ranging from 40 to 100 nm) cup-shaped, double-membraned extracellular vesicles secreted by the vast majority of human cell types [126]. They begin as intracellular vesicles, known as endosomes, which result from the inward budding of the cell membrane. Once the endosome is formed, there is an invagination of its membrane, leading to accumulation of intraluminal vesicles within the larger multivesicular bodies (MVBs). The outer membrane of MVBs retains much of the plasma membrane composition, while its internal vesicles can incorporate cytosolic components [127]. MVBs can fuse with lysosomes for degradation of their cargos, or with the plasma membrane in order to release their internal vesicles, referred to as exosomes [128]. The process of exosome biogenesis differentiate these vesicles from other extracellular vesicles that arise from the outward budding of the cell membrane, apoptotic bodies, or necrotic blebs of the plasma membrane [129]. Exosomes can enter target cells through a variety of mechanisms such as ligand-receptor binding, membrane fusion, or endocytosis [130]. Exosomes mostly maintain the membrane characteristics of their parent cell which is enriched in cholesterol, sphingomyelin, glycolipids and ceramide [131]. Specific proteins, residing on the surface of the exosomes including tetraspanins (CD9, CD63, and CD81), can be used as exosomal markers. Exosome secretion depends on Rab27a and Rab27b which mediate the anchoring to the plasma membrane [132], [133]. Several mechanisms are involved in the specific sorting of exosomal cargos, including endosomal-sorting complexes required for transport (ESCRT), tetraspanins and lipid-dependent mechanism. The ESCRT complex, which is composed of several sub-complexes, produce vesicles through inward budding of MVBs and sort mono-ubiquitinated proteins into them. Tetraspanins function to sort different cargos and interact with other transmembrane proteins, cytosolic proteins and lipids, and to organize the exosome membrane into tetraspanin-enriched domains [131], [134]. Lipid-dependent mechanisms involve the synthesis of ceramide by a rate limiting enzyme neutral sphingomyelinase 2 (nSMase2), which can be inhibited by nSMase2 inhibitor compounds such as GW4869. Ceramide triggers the invagination of exosomes into MVBs and this pathway is considered responsible for the uptake of miRNAs into exosomes [135], [136].
miRNAs have been reported to transfer within exosomes [137]. Following RNA-induced silencing complex (RISC) disassembly, some miRNAs are integrated into the intraluminal vesicles within MVBs [124]. Previous studies showed that miRNAs are selectively incorporated into exosomal vesicles [138] and can be enriched differently than the parent-cell type [139]. The different theories as to how the selection for exosomal transport occurs are still debatable [140]. Exosomes were initially described as a mechanism used by reticulocytes to discard redundant receptors and proteins complexes as they develop into erythrocytes [141]. In cancer, exosomes have been shown to pass malignancy from cancer cells to the surrounding areas which are less or non-malignant [142], [143]. In addition, exosomes are now emerging as important tools of intercellular communication among different cell types in the heart [21], [131], [133].
In vitro experiments established that the communication between CMs and cardiac ECs through exosomal miRNA transfer is an effective way of modulating gene expression and affectting the biology of these cells. Exosomes from diabetic rats-derived CMs are enriched in anti-angiogenic miR-320 but deficient of pro-angiogenic miR-126. Mouse cardiac ECs are able to incorporate these exosomes, which eventually lead to impairment of their proliferative, migration, and tube formation capacities (Fig. 1). This is associated with downregulation of the exosomal miR-320 target genes insulin-like growth factor 1 (IGF1) and E26 avian leukemia oncogene 2 (Ets2), in the recipient ECs [144].
Independent studies have postulated that vesicular miR-126-deficiency under pathological conditions can result in a decrease of endothelial neovascularization potential [145], and that this phenotypical change can be rescued by extracellular transfer of functional miR-126 [146]. In line with these results, miR-126 along with miR-210, were found to be upregulated in the exosomes derived from cardiac ECs under hypoxic conditions. Overexpression of HIF on ECs also increases the expression of these miRNAs in the exosomes, which can be incorporated into cardiac progenitor cells to drive a pro-survival phenotype, proving their functionality across multiple cell types. Accordingly, delivery of these progenitor cells to mouse hearts after myocardial infarction leads to improved ejection fraction [147]. In a similar process, miR-146a is able to induce detrimental phenotypes in ECs, which results in the inhibition of angiogenesis in a mouse model for peripartum cardiomyopathy. Transfer of exosomal miR-146a to CMs changes their metabolic activity and contractility, leading to impairment of heart function [148]. A recent study determined the pro-angiogenic nature of miR-214 [149], adding to the controversy in literature. While miR-214 seems to play a role in exosome-mediated signaling to promote EC angiogenic capacity [149], it can also function as an anti-angiogenic agent, once its direct transfection on ECs leads to reduced sprouting and tube formation [150]. Nevertheless, this miRNA was found to be secreted within EC-derived exosomes and be able to modulate endothelial angiogenic properties (Fig. 1).
lncRNAs have not yet been as much the focus of research as their smaller counterparts in the context of extracellular transport and intercellular communication, particularly in the field of cardiovascular diseases. However, interesting observations have been made, indicating that lncRNAs are selectively loaded and enriched in exosomes from cancer cell lines, including the previously described MALAT1 [151]. Another lncRNA, LINC00152, is present in plasma-derived exosomes of gastric cancer patients. The majority of this lncRNA in plasma is derived from exosomes, establishing it as a diagnostic marker for gastric cancer [152]. Moreover, the lncRNA HOTAIR was found in exosomes isolated from serum of laryngeal squamous cell carcinoma patients and its expression is elevated in patients with lymph node metastasis in comparison to those without metastasis [153]. Furthermore, it has been demonstrated that hepatocellular cancer cells secrete exosomes containing various lncRNAs, including HOTAIR, HULC, linc-ROR and H19 which, in vitro, can be uptaken by ECs and induce angiogenesis by promoting EC re-organization into tubular-like structures and an increase in the expression of VEGF and its receptor [154].
Taken together, these studies emphasize the importance of crosstalk and subsequent exchange of gene expression regulators between multiple cell types in the heart. We expect that more comprehensive studies of exosomal ncRNA function in cardiovascular regulation in health and disease will keep yielding interesting and therapy-oriented results.
6. Angiogenic ncRNAs as potential therapeutic targets for heart failure
An increasing number of evidence supports the relevance of capillary rarefaction in the development of HF. Patients with end-stage HF due to idiopathic dilated cardiomyopathy [5], [6], ischemic cardiomyopathy, and inflammatory cardiomyopathy [5] have demonstrated a reduction in cardiac capillary density. As capillary rarefaction leads to a decrease in coronary flow reserve [155], which is correlated with poor tissue perfusion and an abnormal oxygen consumption pattern [156], promotion of angiogenesis serves as a potential therapeutic tool for HF. In fact, studies have shown the efficacy of several pro-angiogenic factors in improving heart function in animal models of HF. Administration of VEGF and Ang-1 to a porcine model of myocardial infarction results in an increase in vascular density, myocardial perfusion and function [45]. Treatment of a rat model of chronic HF induced by coronary artery ligation with combination of fibroblast and hepatocyte growth factor stimulates cardiac angiogenesis and arteriogenesis, leading to improved myocardial function and perfusion, as measured by magnetic resonance imaging [46]. In addition, several approved drugs, including pitavastatin and benidipine, categorized as statins and calcium-channel blockers, respectively, have been reported to induce myocardial angiogenesis and improve contractility in a mouse model of pressure overload [157] and in Dahl-salt sensitive rats [158]. Despite the efficacy of angiogenesis therapy observed in animal studies, conflicting results have emerged from clinical trials. Several randomized clinical trials, including AGENT [47], VIVA [46], and KAT [48], have shown only modest cardiac function improvement after fibroblast growth factor (FGF) or VEGF gene therapy. This may be due to several factors, including patients selection, delivery strategy, and the chosen growth factors [49]. A single pro-angiogenic gene that is commonly used to promote angiogenesis [12] might be considered insufficient to boost angiogenesis in the heart. From this perspective, modulation of ncRNAs offers a promising new potential therapeutic strategy. miRNAs are able to regulate multiple genes often coordinating one single signaling pathway, or several pathways, leading to a stronger synergistic effect than the effect of a single therapeutic target. miRNAs are also an interesting therapeutic option since they are comprised of only ≈22 nucleotides that can easily be inhibited or overexpressed. In addition, these nucleotide sequences are highly conserved across multiple species, favoring the translation from preclinical animal studies to clinical trials in humans [14].
The delivery of miRNAs to ECs as a mean of angiogenesis therapy has been reported. miR-126 delivery to ECs through circulating microparticles [146] or apoptotic bodies [82] induces vascular repair in vivo. Therapeutic delivery of miRNAs to ECs was also performed by intravenous injection of liposome-encapsulated miRNAs but this method did not only specifically target ECs, as miRNAs were also detected in leukocytes and other organs, including liver, spleen, and kidney [159]. Another delivery method so-called ultrasound-targeted microbubble destruction (UTMD) has been described to solve this cell specificity issue. Delivery of miR-126 to the ECs of vessels in ischemic rat hind limb with this method resulted in downregulation of known miR-126 target genes only in ECs and not in liver and spleen as common off-target organs, and increased vessel length, vascular density, and tissue perfusion [160]. UTMD is shown to be an efficient cell-specific method of miRNA delivery and less invasive in comparison to other delivery methods [13]. Although the study was performed in the model of hind limb ischemia, it is likely that it can also be applied to study the effect of miRNA delivery to cardiac ECs in the context of pathological remodeling. An increasing number of evidence supports the notion of pro- and anti-angiogenic miRNAs exerting their effect during the development of cardiac pathologies. Even though there are less known regarding their therapeutic applications, lncRNAs are associated with EC function, and their pleotropic function, including being a sponge for miRNAs, renders their potential to modulate a specific process, including angiogenesis. In addition, therapeutically targeting lncRNAs could result in less off-target effects, due to their tissue specificity and their ability to regulate miRNA/mRNA networks [161]. Modulation of angiogenesis-related-ncRNAs is associated with improvement or worsening of heart function. Therefore, both miRNAs and lncRNAs have the potential to serve as therapeutic targets to restore cardiac microvascularization in the progression of HF.
7. Conclusion and future perspectives
Myocardial growth and angiogenesis response are reciprocal adaptive reactions of the heart. Physiological CM growth can induce an increase in capillary density in the heart, which in turn, enhances growth factors secretion from endothelium, maintaining heart muscle growth. This process indicates interplay between CM and cardiac ECs to maintain cardiac homeostasis. The canonical molecular mechanism involves the activation of PI3K/Akt1 pathway in CMs, leading to the secretion of VEGF and subsequent activation of ECs and stimulation of angiogenesis. Capillary rarefaction is a hallmark of a failing heart, which occur in the heart of patients with HF of different etiologies. Neovascularization therapies targeting PI3K/Akt1/VEGF axis are efficacious in animal models but unsuccessful in clinical trials, which could be due to the inefficiency of the single pro-angiogenic factors used in the studies.
ncRNAs emerge as new players involved in cardiac cellular crosstalk, affecting cardiac microvasculature homeostasis. The role of miRNAs is prominent in the myocardial vascularization with several miRNAs, including miR-92, -24, −26, −377, and 34, shown to be anti-angiogenic [18], [68], [70], [71], and several others, such as miR-126 and -210, to be pro-angiogenic [82], [83]. lncRNAs, being a larger counterpart of miRNAs, have emerged as new players involved in the regulation of heart tissue vascularization. MIAT and PUNISHER are two well-known lncRNAs involved in vessel formation in the heart [110]. Extracellular transportation through exosomes has been shown to mediate miRNAs transfer between ECs and CMs and to affect critical functions on both cell types. lncRNAs, similar to miRNAs, can also be transported within exosomes and although their relevance in cardiac disease remains to be further elucidated, available data endorse their use as a biomarker or therapeutic target to treat pathologies of the heart.
The power of ncRNAs lies on their pleiotropicity, as they are capable to simultaneously regulate the expression of more than one gene, often regulating the same network or pathway, thereby synergistically enhancing the outcome of the regulation. Since angiogenesis therapy has been shown promising to treat HF, at least at the pre-clinical level, the potential of angiogenesis-related ncRNAs as strong modulators of angiogenesis in the heart should be further explored. The cell specificity and the applicability of the current delivery methods are also essential to be elucidated in order to extract the most beneficial effect of these angiogenesis-related ncRNAs not only in animal models, but also in clinical trials. Exosomes can be an alternative delivery method for ncRNAs. Both natural and modified exosomes, have been used as a tool to carry biological entities to target cells. It has been demonstrated that modified exosomes carrying a neuron-specific protein on their surface can transfer their siRNA cargo to mouse brains [162]. Exosomes carrying recombinant proteins and tumor antigens expressed by cancer vaccines were reported to have a therapeutic effect in phase I clinical trials on patients with melanoma [163]. Furthermore, several stem cell-derived exosomes were shown to induce tissue regeneration after ischemia [164], [165]. Despite these interesting possibilities for the use of exosomal delivery to treat diseases, a better understanding of their complex structure and cargoes, and their potential off-target effects is necessary before translation into the clinic.
FGF2 = Fibroblast Growth Factor 2; VEGF=Vascular Endothelial Growth Factor; CCDN1 = Cyclin D1; PGC-1α = Peroxisome proliferative activated receptor, Gamma, Coactivator 1 alpha; PTEN=Phosphatase and Tensin homolog; KLF2 = Krüppel-like factor 2; mTOR = mechanistic Target Of Rapamycin; Cul3 = Cullin 3; Dicer1 = Dicer ribonuclease 1; Rasa1 = RAS p21 protein activator 1; Spred1 = Sprouty-related, EVH1 domain containing 1; Spry1 = Sprouty RTK signalling antagonist 1; AT1R = Angiotensin Receptor 1; VEGFR2 = Vascular Endothelial Growth Factor Receptor 2; Ets1 = E26 avian leukemia oncogene 1, transcription factor; ZEB1 = Zinc finger E-box Binding homeobox 1; RPS6KB1 = Ribosomal Protein S6 Kinase, Polypeptide 1; Bcl2 = B-cell CLL/lymphoma 2; Cul2 = Cullin 2; KLF4 = Krüppel-Like Factor 4; CEBPB=CCAAT/enhancer binding protein beta; ATf3 = activating transcription factor 3; HAEC=Human Aortic Endothelial Cells; REC = Retinal microvascular Endothelial Cells; HUVEC=Human Umbilical Vein Endothelial Cells; MCAO = Middle Cerebral Artery Occlusion.
References
- 1.Nichols M., Townsend N., Scarborough P., Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 2014;35:2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S. Executive summary: heart disease and stroke statistics-2013 update: a report from the american heart association. Circulation. 2013;127(1):143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Sansone R., Stanske B., Keymel S., Schuler D., Horn P., Saeed D., Boeken U., Westenfeld R., Lichtenberg A., Kelm M. Macrovascular and microvascular function after implantation of left ventricular assist devices in end-stage heart failure: role of microparticles. J. heart lung Transplant. 2015;34(7):1–12. doi: 10.1016/j.healun.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Heiss C., Rodriguez-Mateos A., Kelm M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxidants redox Signal. 2015;22(14):1230–1242. doi: 10.1089/ars.2014.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karch R., Neumann F., Ullrich R., Neumüller J., Podesser B.K., Neumann M., Schreiner W. The spatial pattern of coronary capillaries in patients with dilated, ischemic, or inflammatory cardiomyopathy. Cardiovasc. Pathol. 2005;14(3):135–144. doi: 10.1016/j.carpath.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Abraham D., Hofbauer R., Schäfer R., Blumer R., Paulus P., Miksovsky a, Traxler H., Kocher a, Aharinejad S. Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circulation Res. 2000;87(8):644–647. doi: 10.1161/01.res.87.8.644. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J., Shing Y. Angiogenesis. J. Biol. Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 8.Luttun A., Carmeliet P. De novo vasculogenesis in the heart. Cardiovasc. Res. 2003;58(2):378–389. doi: 10.1016/s0008-6363(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 9.Harada K., Grossman W., Friedman M., Edelman E.R., Prasad P.V., Keighley C.S., Manning W.J., Sellke F.W., Simons M. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J. Clin. Invest. 1994;94(2):623–630. doi: 10.1172/JCI117378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano F.J., Ping P., McKirnan M.D., Nozaki S., DeMaria A.N., Dillmann W.H., Mathieu-Costello O.H.H. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat. Med. 1996;2(5):534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 11.Unger E.F., Banai S., Shou M., Jaklitsch M., Hodge E., Correa R., Jaye M.E.S. No TitleA model to assess interventions to improve collateral blood flow: continuous administration of agents into the left coronary artery in dogs. Cardiovasc Res. 1993;27(5):785–791. doi: 10.1093/cvr/27.5.785. [DOI] [PubMed] [Google Scholar]
- 12.Huang M., Chan D.A., Jia F., Xie X., Li Z., Hoyt G., Robbins R.C., Chen X., Giaccia A.J., Wu J.C. Short hairpin RNA interference therapy for ischemic heart disease. Circulation. 2008;118(14 Suppl):S226–S233. doi: 10.1161/CIRCULATIONAHA.107.760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H.S., Fish J.E. Neovascularization driven by MicroRNA delivery to the endothelium. Arteriosclerosis, Thrombosis, Vasc. Biol. 2015;35(11):2263–2265. doi: 10.1161/ATVBAHA.115.306558. [DOI] [PubMed] [Google Scholar]
- 14.van Rooij E., Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014;6(7):851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis B., Burge C., Bartel D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are MicroRNA. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Lu J., Clark A.G. 2012. Impact of microRNA Regulation on Variation in Human Gene Expression; pp. 1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida S., Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circulation Res. 2015;116(4):737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 18.Bonauer A., Carmona G., Iwasaki M., Mione M., Koyanagi M., Fischer A., Burchfield J., Fox H., Doebele C., Ohtani K. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 19.Fiedler J., Jazbutyte V., Kirchmaier B.C., Gupta S.K., Lorenzen J., Hartmann D., Galuppo P., Kneitz S., Pena J.T.G., Sohn-Lee C. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124(6):720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann D., Thum T. MicroRNAs and vascular (dys)function. Vasc. Pharmacol. 2011;55(4):92–105. doi: 10.1016/j.vph.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Cervio E., Barile L., Moccetti T., Vassalli G. Exosomes for intramyocardial intercellular communication. Stem Cells Int. 2015:2015. doi: 10.1155/2015/482171. (482171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson C., Vicencio J.M., Yellon D.M., Davidson S.M. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J. Endocrinol. 2016;228(2):R57–R71. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- 23.Riley P.R., Smart N. Vascularizing the heart. Cardiovasc. Res. 2011;91(2):260–268. doi: 10.1093/cvr/cvr035. [DOI] [PubMed] [Google Scholar]
- 24.Oka T., Akazawa H., Naito A.T., Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circulation Res. 2014;114(3):565–571. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoud A.I., Kocabas F., Muralidhar S a, Kimura W., Koura A.S., Thet S., Porrello E.R., Sadek H. a. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497(7448):249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anversa P., Levicky V., Beghi C., McDonald S.L., Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circulation Res. 1983;52(1):57–64. doi: 10.1161/01.res.52.1.57. [DOI] [PubMed] [Google Scholar]
- 27.Nabeebaccus A., Zheng S., Shah A.M. Heart failure - potential new targets for therapy. Br. Med. Bull. 2016:1–12. doi: 10.1093/bmb/ldw025. June 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomanek R.J. Response of the coronary vasculature to myocardial hypertrophy. J. Am. Coll. Cardiol. 1990;15(3):528–533. doi: 10.1016/0735-1097(90)90620-5. [DOI] [PubMed] [Google Scholar]
- 29.Brown M., Egginton S., Kingdom U. Angiogenesis in skeletal and cardiac muscle. Physiol. Rev. 1992;72(2) doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu I., Minamino T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Debosch B., Treskov I., Lupu T.S., Weinheimer C., Kovacs A., Courtois M., Muslin A.J. 2006. Akt1 Is Required for Physiological Cardiac Growth. [DOI] [PubMed] [Google Scholar]
- 32.Buss S.J., Riffel J.H., Malekar P., Hagenmueller M., Asel C., Zhang M., Weiss C., Katus H.A., Hardt S.E., Weiss C. 2012. Chronic Akt Blockade Aggravates Pathological Hypertrophy and Inhibits Physiological Hypertrophy; pp. 420–430. [DOI] [PubMed] [Google Scholar]
- 33.Shiojima I., Yefremashvili M., Luo Z., Kureishi Y., Takahashi A., Tao J., Rosenzweig A., Kahn C.R., Abel E.D., Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J. Biol. Chem. 2002;277(40):37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 34.Shiojima I., Sato K., Izumiya Y., Schiekofer S., Ito M., Liao R., Colucci W.S., Kenneth W. Disruption of coordinated tissue growth and angiogenesis in the heart contributes to the progression from adaptive hypertrophy to heart failure. J. Cardiac Fail. 2005;11(8):S278. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heineke J., Auger-messier M., Xu J., Oka T., Sargent M.A., York A., Klevitsky R., Vaikunth S., Duncan S.A., Aronow B.J. Cardiomyocyte GATA4 functions as a stress- responsive regulator of angiogenesis in the murine heart. J. Clin. Invest. 2007;117(11):3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirziu D., Chorianopoulos E., Moodie K.L., Palac R.T., Zhuang Z.W., Tjwa M., Roncal C., Eriksson U., Fu Q., Elfenbein A. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J. Clin. Investigation. 2007;117(11):3188–3197. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaba I.M., Zhuang Z.W., Li N., Jiang Y., Martin K.A., Sinusas A.J., Papademetris X., Simons M., Sessa W.C., Young L.H. No triggers rgs4 degradation to coordinate angiogenesis and cardiomyocyte growth. J. Clin. Investigation. 2013;123(4):1718–1731. doi: 10.1172/JCI65112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert N.A., Johnston C.A., Cappell S.D., Kuravi S., Kimple A.J., Willard F.S., Siderovski D.P. Regulators of G-protein Signaling accelerate GPCR signaling kinetics and govern sensitivity solely by accelerating GTPase activity. Proc. Natl. Acad. Sci. 2012;109(6):2175. doi: 10.1073/pnas.0912934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izumiya Y., Shiojima I., Sato K., Sawyer D.B., Colucci W.S., Walsh K. 2006. Vascular Endothelial Growth Factor Blockade Promotes the Transition from Compensatory Cardiac Hypertrophy to Failure in Response to Pressure Overload; pp. 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heineke J. Wag the dog: how endothelial cells regulate cardiomyocyte growth. Arteriosclerosis, Thrombosis, Vasc. Biol. 2012;32(3):545–547. doi: 10.1161/ATVBAHA.111.242784. [DOI] [PubMed] [Google Scholar]
- 41.Naito A.T., Okada S., Minamino T., Iwanaga K., Liu M.L., Sumida T., Nomura S., Sahara N., Mizoroki T., Takashima A. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circulation Res. 2010;106(11):1692–1702. doi: 10.1161/CIRCRESAHA.109.214346. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida M., Shiojima I., Ikeda H., Komuro I. Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J. Mol. Cell. Cardiol. 2009;47(5):698–705. doi: 10.1016/j.yjmcc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Paulus W.J., Tschöpe C., PH D. 2013. State-of-the-art Paper and Commentary a Novel Paradigm for Heart Failure with Preserved Ejection Fraction Comorbidities Drive Myocardial Dysfunction and Remodeling. 62(4) [DOI] [PubMed] [Google Scholar]
- 44.Mohammed S.F., Hussain S., Mirzoyev S.A., Edwards W.D., Maleszewski J.J., Redfield M.M. 2014. Fibrosis in Heart Failure with Preserved Ejection Fraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao Z., Chen B., Tan X., Zhao Y., Wang L., Zhu T., Cao K., Yang Z., Kan Y.W., Su H. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc. Natl. Acad. Sci. U. S. A. 2011;108(5):2064–2069. doi: 10.1073/pnas.1018925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banquet S., Gomez E., Nicol L., Edwards-Lévy F., Henry J.P., Cao R., Schapman D., Dautreaux B., Lallemand F., Bauer F. Arteriogenic therapy by intramyocardial sustained delivery of a novel growth factor combination prevents chronic heart failure. Circulation. 2011;124(9):1059–1069. doi: 10.1161/CIRCULATIONAHA.110.010264. [DOI] [PubMed] [Google Scholar]
- 47.Grines C.L., Watkins M.W., Helmer G., Penny W., Brinker J., Marmur J.D., West A., Rade J.J., Marrott P., Hammond H.K. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105(11):1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 48.Hedman M. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the kuopio. Circulation. 2003;107(>21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 49.Meloni M., Marchetti M., Garner K., Littlejohns B., Sala-Newby G., Xenophontos N., Floris I., Suleiman M.-S., Madeddu P., Caporali A. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol. Ther. 2013;21(7):1390–1402. doi: 10.1038/mt.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carninci P. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 51.Birney E., Stamatoyannopoulos J a, Dutta A., Guigó R., Gingeras T.R., Margulies E.H., Weng Z., Snyder M., Dermitzakis E.T., Thurman R.E. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gori M., Trombetta M., Santini D., Rainer A. Tissue engineering and microRNAs: future perspectives in regenerative medicine. Expert Opin. Biol. Ther. 2015:1–22. doi: 10.1517/14712598.2015.1071349. [DOI] [PubMed] [Google Scholar]
- 53.Bentwich I., Avniel A., Karov Y., Aharonov R., Gilad S., Barad O., Barzilai A., Einat P., Einav U., Meiri E. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005;37(7):766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 54.Filipowicz W., Bhattacharyya S.N., Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 55.Kim V.N. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cel. Biol. 2005;6(May):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 56.Lee Y., Kim M., Han J., Yeom K.-H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. Eur. Mol. Biol. Organ. J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartel D.P., Lee R., Feinbaum R. MicroRNAs;: genomics, biogenesis, mechanism, and function genomics. miRNA Genes. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 58.Khvorova A., Reynolds A., Jayasena S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115(2):209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 59.Karp X., Ambros V. Developmental biology. Encountering microRNAs in cell fate signaling. Science. 2005;310(5752):1288–1289. doi: 10.1126/science.1121566. [DOI] [PubMed] [Google Scholar]
- 60.da Costa Martins P.A., Salic K., Gladka M.M., Armand A.-S., Leptidis S., El Azzouzi H., Hansen A., Coenen-de Roo C.J., Bierhuizen M.F., van der Nagel R. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010;12(12):1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 61.El Azzouzi H., Leptidis S., Dirkx E., Hoeks J., Van Bree B., Brand K., McClellan E.A., Poels E., Sluimer J.C., Van Den Hoogenhof M.M.G. The Hypoxia-Inducible MicroRNA Cluster miR-199a∼214 targets myocardial PPAR and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013;18(3):341–354. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Dirkx E., Gladka M.M., Philippen L.E., Armand A.-S., Kinet V., Leptidis S., el Azzouzi H., Salic K., Bourajjaj M., da Silva G.J.J. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nat. Cell Biol. 2013;15(11):1282–1293. doi: 10.1038/ncb2866. [DOI] [PubMed] [Google Scholar]
- 63.Roy S., Khanna S., Hussain S.A., Biswas S., Azad A., Rink C., Gnyawali S., Shilo S., Nuovo G.J., Sen C.K. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van E.Rooij, Sutherland L.B., Thatcher J.E., Dimaio J.M., Naseem R.H., Marshall W.S., Hill J.A., Olson E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. 2008;105(35) doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S., Aurora A.B., Johnson B.A., Qi X., Mcanally J., Hill J.A., Richardson J.A., Bassel-duby R., Olson E.N., Hill A. The endothelial-specific MicroRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fasanaro P., Alessandra Y.D., Stefano V Di, Melchionna R., Romani S., Pompilio G., Capogrossi M.C., Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase. J. Biol. Chem. 2008;283(23):15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang W.J., Yang D.D., Na S., Sandusky G.E., Zhang Q., Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 2005;280(10):9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 68.Hinkel R., Penzkofer D., Z??hlke S., Fischer A., Husada W., Xu Q.F., Baloch E., Van Rooij E., Zeiher A.M., Kupatt C. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128(10):1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 69.Iaconetti C., Polimeni A., Sorrentino S., Sabatino J., Pironti G., Esposito G., Curcio A., Indolfi C. Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res. Cardiol. 2012;(5):107. doi: 10.1007/s00395-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 70.Icli B., Wara A.K.M., Moslehi J., Sun X., Plovie E., Cahill M., Marchini J.F., Schissler A., Padera R.F., Shi J. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ. Res. 2013;113:1231–1241. doi: 10.1161/CIRCRESAHA.113.301780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joladarashi D., Srikanth Garikipati V.N., Thandavarayan R.A., Verma S.K., Mackie A.R., Khan M., Gumpert A.M., Bhimaraj A., Youker K.A., Uribe C. Enhanced cardiac regenerative ability of stem cells after ischemia-reperfusion injury: role of human CD34+ cells deficient in microRNA-377. J. Am. Coll. Cardiol. 2015;66(20):2214–2226. doi: 10.1016/j.jacc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernardo B.C., Gao X.-M., Winbanks C.E., Boey E.J.H., Tham Y.K., Kiriazis H., Gregorevic P., Obad S., Kauppinen S., Du X.-J. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. U. S. A. 2012;109(43):17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao T., Li J., Chen A.F. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. American journal of physiology. Endocrinol. metabolism. April 2010;2010(299):E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X.H., Qian R., Zhang W., Chen S., Jin H., Hu R. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin. Exp. Pharmacol. Physiology. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 75.Caporali A., Meloni M., Vo C., Bonci D., Sala-newby G.B., Addis R., Spinetti G., Losa S., Masson R., Baker A.H. Deregulation of microRNA-503 contributes to diabetes mellitus – induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Mol. Cardiol. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 76.Endo K., Naito Y., Ji X., Nakanishi M., Noguchi T., Goto Y., Nonogi H., Ma X., Weng H., Hirokawa G. MicroRNA 210 as a biomarker for congestive heart failure. Biol. Pharm. Bull. 2013;36(1):48–54. doi: 10.1248/bpb.b12-00578. [DOI] [PubMed] [Google Scholar]
- 77.Zaccagnini G., Maimone B., Di Stefano V., Fasanaro P., Greco S., Perfetti A., Capogrossi M.C., Gaetano C., Martelli F. Hypoxia-induced miR-210 modulates tissue response to acute peripheral ischemia. Antioxidants redox Signal. 2013;21(8):1–12. doi: 10.1089/ars.2013.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A., Fasanaro P., Sun N., Wang X., Martelli F. MicroRNA-210 as a novel therapy for treatment of. Circulation. 2010;122:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y., Pan X., Fan Y., Hu X., Liu X., Xiang M., Wang J. Dysregulated expression of microRNAs and mRNAs in myocardial infarction. Am. J. Transl. Res. 2015;7(11):2291–2304. [PMC free article] [PubMed] [Google Scholar]
- 80.Francisco S.S., Francisco S.S. The endothelial-cell-derived secreted factor Eg 7 regulates vascular tube formation. Nature. 2004;428(April):1–5. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 81.Wei X.J., Han M., Yang F.Y., Wei G.C., Liang Z.G., Yao H., Ji C.W., Xie R.S., Gong C.L., Tian Y. Biological significance of miR-126 expression in atrial fibrillation and heart failure. Braz. J. Med. Biol. Res. 2015;48(11):1–7. doi: 10.1590/1414-431X20154590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B., Hristov M., Köppel T., Jahantigh M.N., Lutgens E. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 83.Chen L., Wang J., Wang B., Yang J., Gong Z., Zhao X., Zhang C., Du K. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann. Hematol. 2016;95(3):365–374. doi: 10.1007/s00277-015-2567-9. [DOI] [PubMed] [Google Scholar]
- 84.Long G., Wang F., Duan Q., Chen F., Yang S., Gong W., Wang Y., Chen C., Wang D.W. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int. J. Biol. Sci. 2012;8(6):811–818. doi: 10.7150/ijbs.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin K.J., Olsen K., Hamblin M., Zhang J., Schwendeman S.P., Chen Y.E. Vascular endothelial cell-specific MicroRNA-15a inhibits angiogenesis in hindlimb ischemia. J. Biol. Chem. 2012;287(32):27055–27064. doi: 10.1074/jbc.M112.364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin X., Wang X., Wang Y., Tang Z., Cui Q., Xi J., Li Y.-S.J., Chien S., Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107(7):3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue Y., Wei Z., Ding H., Wang Q., Zhou Z., Zheng S., Zhang Y., Hou D., Liu Y., Zen K. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis. 2015;241(2):671–681. doi: 10.1016/j.atherosclerosis.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 88.Weber M., Baker M.B., Moore J.P., Searles C.D. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophysical Res. Commun. 2010;393(4):643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu W., Xiao H., Laguna-Fernandez A., Villarreal G., Jr., Wang K.C., Geary G.G., Zhang Y., Wang W.C., Huang H.D., Zhou J., Li Y.S., Chien S., Garcia-Cardena G.S.J. Flow-dependent regulation of krüppel-like factor 2 is mediated by MicroRNA-92a. Circulation. 2011;124(5):181–204. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grundmann S., Hans F.P., Kinniry S., Heinke J., Helbing T., Bluhm F., Sluijter J.P.G., Hoefer I., Pasterkamp G., Bode C. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123(9):999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 91.Kim J.-H., Lee K.-S., Lee D.-K., Kim J., Kwak S.-N., Ha K.-S., Choe J., Won M.-H., Cho B.-R., Jeoung D. Hypoxia-Responsive MicroRNA-101 promotes angiogenesis via heme oxygenase-1/vascular endothelial growth factor Axis by targeting cullin 3. Antioxidants redox Signal. 2014;0(0):1–14. doi: 10.1089/ars.2014.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen K., Fan W., Wang X., Ke X., Wu G., Hu C. MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem. Biophysical Res. Commun. 2012;427(1):138–142. doi: 10.1016/j.bbrc.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 93.Semo J., Sharir R., Afek A., Avivi C., Barshack I., Maysel-Auslender S., Krelin Y., Kain D., Entin-Meer M., Keren G. The 106b???25 microRNA cluster is essential for neovascularization after hindlimb ischaemia in mice. Eur. Heart J. 2014;35(45):3212–3223. doi: 10.1093/eurheartj/eht041. [DOI] [PubMed] [Google Scholar]
- 94.Li Y., Mao L., Gao Y., Baral S., Zhou Y., Hu B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci. Rep. 2015;5:13316. doi: 10.1038/srep13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumarswamy R., Volkmann I., Beermann J., Napp L.C., Jabs O., Bhayadia R., Melk A., Ucar A., Chowdhury K., Lorenzen J.M. Vascular importance of the miR-212/132 cluster. Eur. Heart J. 2014;5325272:3224–3231. doi: 10.1093/eurheartj/ehu344. [DOI] [PubMed] [Google Scholar]
- 96.Meng Y.-C., Ding Z.-Y., Wang H.-Q., Ning L.-P., Wang C. Effect of microRNA-155 on angiogenesis after cerebral infarction of rats through AT1R/VEGFR2 pathway. Asian Pac. J. Trop. Med. 2015;8(10):829–835. doi: 10.1016/j.apjtm.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 97.Caballero-Garrido E., Pena-Philippides J.C., Lordkipanidze T., Bragin D., Yang Y., Erhardt E.B., Roitbak T. Vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J. Neurosci. 2015;35(36):12446–12464. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu N., Zhang D., Chen S., Liu X., Lin L., Huang X., Guo Z., Liu J., Wang Y., Yuan W. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 99.Magenta a, Cencioni C., Fasanaro P., Zaccagnini G., Greco S., Sarra-Ferraris G., Antonini a, Martelli F., Capogrossi M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell death Differ. 2011;18(10):1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dai G.H., Ma P.Z., Song X.B., Liu N., Zhang T., Wu B. MicroRNA-223-3p inhibits the angiogenesis of ischemic cardiac microvascular endothelial cells via affecting RPS6KB1/hif-1a signal pathway. PLoS ONE. 2014;9(10):1–14. doi: 10.1371/journal.pone.0108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qin B., Xiao B., Liang D., Xia J., Li Y., Yang H. MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem. Biophysical Res. Commun. 2011;410(1):127–133. doi: 10.1016/j.bbrc.2011.05.118. [DOI] [PubMed] [Google Scholar]
- 102.Ghosh G., Subramanian I.V., Adhikari N., Zhang X., Joshi H.P., Basi D., Chandrashekhar Y.S., Hall J.L., Roy S., Zeng Y. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J. Clin. Invest. 2010;120(11):4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ni C., Qiu H., Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. American journal of physiology. Heart circulatory physiology. 2011;30322:1762–1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sparmann A., van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nature reviews. Cancer. 2006;6(11):846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 105.Guil S., Soler M., Portela A., Carrère J., Fonalleras E., Gómez A., Villanueva A., Esteller M. Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nat. Struct. Mol. Biol. 2012;19(7):664–670. doi: 10.1038/nsmb.2315. [DOI] [PubMed] [Google Scholar]
- 106.Khalil A.M., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D., Thomas K., Presser A., Bernstein B.E., van Oudenaarden A. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U. S. A. 2009;106(28):11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ling H., Fabbri M., Calin G. a. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nature reviews. Drug Discov. 2013;12(11):847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ishii N., Ozaki K., Sato H., Mizuno H., Saito Susumu, Takahashi A., Miyamoto Y., Ikegawa S., Kamatani N., Hori M. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J. Hum. Genet. 2006;51(12):1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 109.Yan B., Yao J., Liu J.Y., Li X.M., Wang X.Q., Li Y.J., Tao Z.F., Song Y.C., Chen Q., Jiang Q. LncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circulation Res. 2015;116(7):1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 110.Kurian L., Aguirre A., Sancho-Martinez I., Benner C., Hishida T., Nguyen T.B., Reddy P., Nivet E., Krause M.N., Nelles D.A. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation. 2015;131(14):1278–1290. doi: 10.1161/CIRCULATIONAHA.114.013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ounzain S., Crippa S., Pedrazzini T. Small and long non-coding RNAs in cardiac homeostasis and regeneration. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 2013;1833(4):923–933. doi: 10.1016/j.bbamcr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 112.Ji P., Diederichs S., Wang W., Boing S., Metzger R., Schneider P.M., Tidow N., Brandt B., Buerger H., Bulk E. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):6087–6097. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 113.Michalik K.M., You X., Manavski Y., Doddaballapur A., Zörnig M., Braun T., John D., Ponomareva Y., Chen W., Uchida S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circulation Res. 2014;114(9):1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 114.Helgadottir A., Thorleifsson G., Manolescu A. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1494. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 115.Holdt L.M., Beutner F., Scholz M., Gielen S., G??bel G., Bergert H., Schuler G., Thiery J., Teupser D. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arteriosclerosis, Thrombosis, Vasc. Biol. 2010;30(3):620–627. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- 116.Burd C.E., Jeck W.R., Liu Y., Sanoff H.K., Wang Z., Sharpless N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6(12):1–15. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qiu G.-Z., Tian W., Fu H.-T., Li C.-P., Liu B. Long non-coding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem. Biophysical Res. Commun. 2016;471(1):135–141. doi: 10.1016/j.bbrc.2016.01.164. [DOI] [PubMed] [Google Scholar]
- 118.Fiedler J., Breckwoldt K., Remmele C.W., Hartmann D., Dittrich M., Pfanne A., Just A., Xiao K., Kunz M., M??ller T. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J. Am. Coll. Cardiol. 2015;66(18):2005–2015. doi: 10.1016/j.jacc.2015.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boulberdaa M., Scott E., Ballantyne M., Garcia R., Descamps B., Angelini G.D., Brittan M., Hunter A., Mcbride M., Mcclure J. A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Mol. Ther. 2016;24(5):978–990. doi: 10.1038/mt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Josipovic I., Fork C., Preussner J., Prior K., Iloska D., Vasconez A.E., Labocha S., Looso M., Pullamsetti S.S., Geisslinger G. PAFAH1B1 and the lncRNA NONHSAT073641 maintain an angiogenic phenotype in human endothelial cells. Acta Physiol. 2016;218:13–27. doi: 10.1111/apha.12700. [DOI] [PubMed] [Google Scholar]
- 121.Wolvetang E.J., Pera M.F., Zuckerman K.S. Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem. Biophysical Res. Commun. 2007;363(3):610–615. doi: 10.1016/j.bbrc.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 122.Brink P.R., Valiunas V., Gordon C., Rosen M.R., Cohen I.S. Can gap junctions deliver? Biochimica Biophysica Acta - Biomembr. 2012;1818(8):2076–2081. doi: 10.1016/j.bbamem.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 123.Hong X., Sin W.C., Harris A.L., Naus C.C. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget. 2015;6(17) doi: 10.18632/oncotarget.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu L., Yang B.F., Ai J. MicroRNA transport: a new way in cell communication. J. Cell. Physiology. 2013;228(8):1713–1719. doi: 10.1002/jcp.24344. [DOI] [PubMed] [Google Scholar]
- 125.Mo M.H., Chen L., Fu Y., Wang W., Fu S.W. Cell-free circulating miRNA biomarkers in cancer. J. Cancer. 2012;3(1):432–448. doi: 10.7150/jca.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Niel G., Porto-Carreiro I., Simoes S., Raposo G. Exosomes: a common pathway for a specialized function. J. Biochem. 2006;140(1):13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 127.Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Simons M., Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 129.Kalluri R. The biology and function of exosomes in cancer. J. Clin. Investigation. 2016;126(4):1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Turturici G., Tinnirello R., Sconzo G., Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. American journal of physiology. Cell physiol. 2014;306(7):C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 131.Emanueli C., Shearn A.I.U., Angelini G.D., Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vasc. Pharmacol. 2015;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okoye I.S., Coomes S.M., Pelly V.S., Czieso S., Papayannopoulos V., Tolmachova T., Seabra M.C., Wilson M.S. Exosomes suppress pathogenic t helper 1 cells. Immunity. 2014;41(1):89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Das S., Halushka M.K. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc. Pathol. 2015;24(4):199–206. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 134.Palma J., Yaddanapudi S.C., Pigati L., Havens M.A., Jeong S., Weiner G.A., Mary K., Weimer E., Stern B., Hastings M.L. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40(18):9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic MicroRNAs regulate cancer cell. J. Biol. Chem. 2013;288(15):10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory mechanisms and intercellular transfer of MicroRNAs in living cells. J. Biol. Chem. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.L., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 138.Guduric-Fuchs J., O'Connor A., Camp B., O'Neill C.L., Medina R.J. Simpson D a. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13(1):357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goldie B.J., Dun M.D., Lin M., Smith N.D., Verrills N.M., Dayas C.V., Cairns M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42(14):9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Exosome Mi S., MicroRNA Exosomal. Trafficking, sorting, and function. Genomics. Proteomics Bioinforma. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan B.-T., Teng K., Wu C., Adam M., Johnstone R.M. Electron Microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985;101(September):942–945. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zomer A., Maynard C., Pegtel D.M., Rheenen J Van, Schiffelers R.M., Wit E De, Berenguer J., Inge S., Ellenbroek J., Wurdinger T. In vivo imaging reveals extracellular vesicle-mediated phenocopying of Metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clayton A., Clayton A. Immunity and the microenvironment Cancer cells use exosomes as tools to manipulate immunity and the microenvironment. Oncoimmunology. 2016;1:78–80. doi: 10.4161/onci.1.1.17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang X., Huang W., Liu G., Cai W., Millard R.W., Wang Y., Chang J., Peng T., Fan G.C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zampetaki A., Kiechl S., Drozdov I., Willeit P., Mayr U., Prokopi M., Mayr A., Weger S., Oberhollenzer F., Bonora E. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in type 2 diabetes. Circulation Res. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 146.Jansen F., Yang X., Hoelscher M., Cattelan A., Schmitz T., Proebsting S., Wenzel D., Vosen S., Franklin B.S., Fleischmann B.K. Endothelial microparticle-mediated transfer of microRNA-126 promotes vascular endothelial cell repair via spred1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128(18):2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 147.Ong S.-G.S.-G., Lee W.H., Huang M., Dey D., Kodo K., Sanchez-Freire V., Gold J.D., Wu J.C. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal MicroRNA transfer. Circulation. 2014;130(11_suppl_1):S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Halkein J., Tabruyn S.P., Ricke-Hoch M., Haghikia A., Nguyen N.Q.N., Scherr M., Castermans K., Malvaux L., Lambert V., Thiry M. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investigation. 2013;123(5):2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.BWM Van Balkom, OG De Jong, Smits M., Brummelman J., K Den Ouden, PM De Bree, MAJ Van Eijndhoven, Pegtel D.M., Stoorvogel W., Thomas W. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2016;121(19):3997–4007. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 150.Van Mil A., Grundmann S., Goumans M.J., Lei Z., Oerlemans M.I., Jaksani S., Doevendans P.A., Sluijter J.P.G. MicroRNA-214 inhibits angiogenesis by targeting Quaking and reducing angiogenic growth factor release. Cardiovasc. Res. 2012;93(4):655–665. doi: 10.1093/cvr/cvs003. [DOI] [PubMed] [Google Scholar]
- 151.Gezer U., Özgür E., Cetinkaya M., Isin M., Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol. Int. 2014;38(9):1076–1079. doi: 10.1002/cbin.10301. [DOI] [PubMed] [Google Scholar]
- 152.Li Q., Shao Y., Zhang X., Zheng T. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumor Biol. 2015;3:2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 153.Wang J., Zhou Y., Lu J. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med. Oncol. 2014;31:148. doi: 10.1007/s12032-014-0148-8. [DOI] [PubMed] [Google Scholar]
- 154.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M. CD90 + liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:1–11. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsagalou E.P., Anastasiou-Nana M., Agapitos E., Gika A., Drakos S.G., Terrovitis J.V., Ntalianis A., Nanas J.N. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2008;52(17):1391–1398. doi: 10.1016/j.jacc.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 156.van den Heuvel A.F., van Veldhuisen D.J., van der Wall E.E., Blanksma P.K., Siebelink H.-M.J., Vaalburg W.M., van Gilst W.H., Crijns H.J.G. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2000;35(1):19–28. doi: 10.1016/s0735-1097(99)00499-4. [DOI] [PubMed] [Google Scholar]
- 157.Kameda Y., Hasegawa H., Kubota A., Tadokoro H., Kobayashi Y., Komuro I., Takano H. Effects of pitavastatin on pressure overload-induced heart failure in mice. Circulation J. 2012;76(5):1159–1168. doi: 10.1253/circj.cj-11-1114. [DOI] [PubMed] [Google Scholar]
- 158.Nishizawa T., Cheng X.W., Jin Z., Obata K., Nagata K., Hirashiki A., Sasaki T., Noda A., Takeshita K., Izawa H. Ca(2+) channel blocker benidipine promotes coronary angiogenesis and reduces both left-ventricular diastolic stiffness and mortality in hypertensive rats. J. Hypertens. 2010;28(7):1515–1526. doi: 10.1097/HJH.0b013e328339fd3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun X., Icli B., Wara A.K., Belkin N., He S., Kobzik L., Hunninghake G.M., Vera M.P., Registry M., Blackwell T.S. MicroRNA-181b regulates NF-κB – mediated vascular inflammation. J. Clin. Invest.. 2012;122(6):1–18. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cao W.J., Rosenblat J.D., Roth N.C., Kuliszewski M.A., Matkar P.N., Rudenko D., Liao C., Lee P.J.H., Leong-Poi H. Therapeutic angiogenesis by ultrasound-mediated MicroRNA-126-3p delivery. Arteriosclerosis, Thrombosis, Vasc. Biol. 2015;35(11):2401–2411. doi: 10.1161/ATVBAHA.115.306506. [DOI] [PubMed] [Google Scholar]
- 161.Ballantyne M., Mcdonald R., Baker A. lncRNA/MicroRNA interactions in the vasculature. Clin. Pharmacol. Ther. 2016;99(5):494–501. doi: 10.1002/cpt.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.El-andaloussi S., Lee Y., Lakhal-littleton S., Li J., Seow Y., Gardiner C., Alvarez-erviti L., Sargent I.L., Wood M.J.A. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012;7(12):2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 163.Rountree R.B., Mandl S.J., Nachtwey J.M., Dalpozzo K., Do L., Lombardo J.R., Schoonmaker P.L., Brinkmann K., Dirmeier U., Laus R. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011;71(15):5235–5244. doi: 10.1158/0008-5472.CAN-10-4076. [DOI] [PubMed] [Google Scholar]
- 164.Mackie A.R., Klyachko E., Thorne T., Schultz K.M., Millay M., Ito A., Kamide C.E., Liu T., Gupta R., Sahoo S. Sonic hedgehog – modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circulation Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ong S.-G., Lee W.H., Huang M., Dey D., Kodo K., Sanchez-Freire V., Gold J.D., Wu J.C. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal MicroRNA transfer. Circulation. 2014;130(11_suppl_1):S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]