Abstract
Platinum(II) complexes such as cisplatin, carboplatin and oxaliplatin are clinically approved for the therapy of various solid tumors. Challenging pathogenic properties of cancer cells and the response of cancers towards platinum-based drugs are strongly influenced by non-coding small RNA molecules, the microRNAs (miRNAs). Both increased platinum activity and formation of tumor resistance towards platinum drugs are controlled by miRNAs. This review gives an overview of the interactions between platinum-based drugs and miRNAs, and their influence on platinum activity in various cancer types is discussed.
Keywords: MicroRNA, Cisplatin, Carboplatin, Oxaliplatin, Platinum complexes, Anticancer drugs
Abbreviations: CBDCA, cyclobutane-1,1-dicarboxylate; DACH, 1,2-diaminocyclohexane; DDP, cisplatin; dTTP, deoxythymidine triphosphate; EGCG, (−)-epigallocatechin-3-gallate; EOX, epirubicin/oxaliplatin/xeloda; FOLFOX, folinate/5-FU/oxaliplatin; 5-FU, 5-fluorouracil; GC, gemcitabine/cisplatin, gastric cancer; LNA, locked nucleic acid; MVAC, methotrexate/vinblastine/adriamycin/cisplatin; XELOX, xeloda/oxaliplatin
1. Introduction
The platinum(II) complex cisplatin has become a salient drug in the therapy of solid tumors since Rosenberg and coworkers have discovered the anticancer activity of cisplatin in 1969 (Fig. 1) [1]. Cisplatin binds to DNA, i.e., its main cellular target, via coordination to the N-7 atom of purine bases such as guanine leading to toxic DNA cross-links [2], [3]. Carboplatin and oxaliplatin feature additional anticancer drugs clinically approved in the USA and the EU (Fig. 1) [2], [3], [4]. However, drug resistance and toxic side-effects limit the application of these platinum complexes [3], [4], [5], [6]. Thus, many new platinum complexes were designed such as Pt-complex-conjugates, trans-Pt complexes, Pt(IV) complexes, heteronuclear complexes and N-heterocyclic carbene complexes in order to overcome the eminent drawbacks of cisplatin, carboplatin and oxaliplatin [7], [8], [9], [10], [11].
Fig. 1.
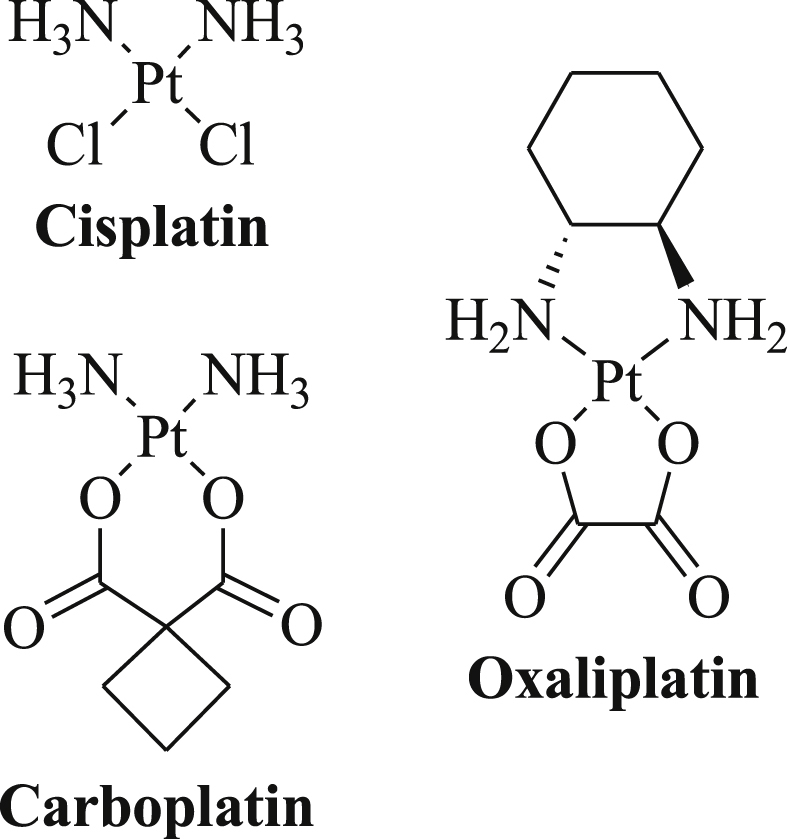
Structures of the platinum(II) complexes cisplatin, carboplatin and oxaliplatin.
MicroRNAs (miRNAs) feature another possibility to investigate the modes of platinum resistance and activity in order to design improved therapy options. About two thousand miRNAs featuring highly conserved non-coding RNAs of 22–23 nucleotides regulate circa one-third of all human genes including genes involved in significant cellular processes such as cell proliferation, cell differentiation and cell death [12], [13]. MiRNAs have become valuable tools for the establishment of prognoses for cancer patients because of the characteristic miRNA expression patterns in tumor tissues [14], [15], [16], [17], [18]. In addition, certain miRNAs regulate the survival of drug-resistant cancer stem-like cells (CSCs) that are responsible for relapse [19], [20]. MiRNAs are also involved in the regulation of metastasis formation, in particular, in the control of the epithelial-to-mesenchymal transition (EMT) of cancer cells [21], [22].
In most cases, mature miRNAs bind to the 3'-untranslated region (3′UTR) of the target messenger RNAs (mRNAs) and, thus, inhibit the translation of these mRNAs [23]. One miRNA species is able to regulate various genes, and both tumor suppressor miRNAs and oncogenic miRNAs (oncomirs) are dysregulated in various human cancer types [24], [25]. The detailed understanding of the modes of action of miRNAs with effects on cellular cancer-related processes is crucial for the development of improved anticancer therapies [26], [27], [28]. A recent review published in this journal before dealt with miRNA-modulating natural phenols and terpenoids and their effects on various tumors [29]. This review provides an overview of widely applied anticancer active platinum complexes (cisplatin, carboplatin, oxaliplatin) and their interactions with miRNAs.
2. Platinum complexes and their interactions with miRNAs
2.1. Cisplatin
Cisplatin, cis-(diammine)dichloridoplatinum(II), was the first platinum complex that was approved for anticancer therapy by the FDA in the USA in 1978 [30]. The chlorido ligands of the square-planar complex cisplatin can be replaced by S- and N-bionucleophiles of proteins and nucleic acids in the target cells and, in particular, the resulting DNA damage (DNA crosslinks, e.g., 1,2-intrastrand crosslinks) by cisplatin leads to apoptosis induction [2], [3], [30]. Cisplatin is widely applied against testicular cancer, non-small and small cell lung cancer, ovarian cancer, cervix carcinoma, prostate carcinoma, endometrial cancer, bladder cancer, melanoma, various sarcomas, and head-and-neck cancer [30]. It is given to the cancer patients as intravenous chloride infusions and the supplemented chloride salt prevents ligand exchange of cisplatin molecules in aqueous solutions [30]. Since the main mode of action of cisplatin is binding to DNA via guanine bases, the interactions of cisplatin with miRNAs, which represent short oligonucleotides, are of particular interest in order to get deeper insights into the modes of cisplatin action in cancer cells.
2.1.1. Cisplatin, miRNAs and ovarian cancer
Ovarian cancer solely affects women and features one of the main applications of cisplatin-based therapies and the microRNA profile of cisplatin-resistant ovarian cancer cells when compared with cisplatin-sensitive ovarian cancer cells was investigated [31]. In fact, five miRNAs (miRPlus-F1064, miR-300, miR-193b, miR-642, miR-1299) were up-regulated while six miRNAs (let-7c, miR-20b, miR-542-3p, miR-625, miRPlus-F1147, miRPlus-F1231) were downregulated in the resistant ovarian cell line A2780/CP70 when compared with the sensitive A2780 cells and was accompanied by significant influences on various signaling pathways (e.g., MAPK, TGF-β, Wnt, mTOR, Notch), apoptosis, actin cytoskeleton and proteasomal mechanisms [31]. Cisplatin-resistance of ovarian cancer cells was also induced by downregulation of the tumor suppressor let-7i [32]. Downregulated let-7i was associated with shorter PFS (progression free survival) in late-stage ovarian cancer patients [32]. In addition, suppression of miR-29 inhibited cisplatin-mediated apoptosis by induction of COL1A1 (collagen type I alpha I) in ovarian cancer cells [33]. MiR-30a sensitized ovarian cancer cells to cisplatin treatment by suppression of ETAR (endothelin-1/ETA receptor) and inhibition both of Akt signaling and of MAPK (mitogen-activated protein kinase) signaling [34]. The increased expression of miR-30c-2 sensitized cisplatin-resistant ovarian cancer cells via inhibition of the oncoprotein Bcl-9 [35]. The ABC-transporter ABCD2 was suppressed by miR-30d in ovarian cancer cells which was associated with enhanced apoptosis induction by cisplatin possibly via increased cisplatin accumulation in the cancer cells [36]. Suppressed miR-130a levels were observed from cisplatin-resistant ovarian cancer cells, and induced miR-130a expression downregulated XIAP (X-linked inhibitor of apoptosis) leading to cisplatin-sensitivity [37]. Another study of the miRNA profile of cisplatin-resistant ovarian cancer cells revealed lower levels of let-7e, miR-30c, miR-130a, and miR-335 in the resistant cancer cells [38]. In epithelial ovarian cancer, upregulated miR-136 augmented cisplatin-sensitivity significantly [39]. Further to this, the tumor suppressor miR-155 enhanced cisplatin-induced apoptosis in SKOV3 and A2780 ovarian cancer cells by inhibition of XIAP [40]. Both miR-152 and miR-185 were suppressed in cisplatin-resistant ovarian cancer cells and expression of miR-152 and miR-185 overcame cisplatin resistance via suppression of DNMT1 (DNA methyltransferase 1) [41]. EMT in EOC patients was correlated with reduced miR-186 expression and upregulated Twist1 transcription factor, while increased miR-186 expression was able to revert EMT and to increase cisplatin activity in ovarian cancer cells via suppression of Twist1 [42]. MiR-199a inhibited mTOR (mammalian target of rapamycin) expression and signaling in ovarian cancer cells leading cisplatin-sensitivity [43]. IGROV-1 ovarian cancer cells exhibited increased cisplatin activity due to induced expression of miR-302b and inhibition of HDAC4 (histone deacetylase 4) expression [44]. The tumor suppressor miR-449a inhibited Notch-1 expression associated with promoted cisplatin sensitivity in ovarian cancer cells [45]. Another tumor suppressor, miR-509-3p, blocked XIAP expression and increased cisplatin-mediated apoptosis induction in epithelial ovarian cancer cells [46]. MiR-519d features another XIAP inhibitor expressed in ovarian cancer cells that respond well to cisplatin treatment [47]. MiR-770-5p was shown to target ERCC2 (excision repair cross-complementation group 2) and, thus, blocked cisplatin resistance formation by ovarian cancer [48]. In addition, the expression of miR-873 blocked MDR1 (multidrug-resistance protein 1, Pgp) expression leading to improved cisplatin response by ovarian cancer cells and inhibition of multidrug resistance formation [49].
In contrast to that, inhibition of miR-9 sensitized ovarian cancer cells to cisplatin treatment [50]. Expression of the oncomir miR-21 led to cisplatin resistance of ovarian cancer cells via suppressed PDCD4 (programmed cell death protein 4) levels [51]. Its passenger strand, miR-21-3p, also increased cisplatin resistance in various ovarian cancer cell lines via suppression of NAV3 (neuron navigator 3) [52]. Interestingly, the natural isoquinoline alkaloid berberine suppressed miR-21 in SKOV3 ovarian cancer cells and augmented cisplatin activity in these cancer cells via induction of PDCD4 [53]. In platinum-resistant ovarian cancer patients, high levels of miR-27a were discovered which was associated with poor prognosis [54]. In addition, cisplatin-resistant ovarian cancer cells exhibited upregulated miR-31 expression leading to the suppression of KCNMA1 (potassium channel calcium activated large conductance subfamily M alpha, member 1) [55]. High expression of miR-93 blocked PTEN (phosphatase and tensin homolog) expression and inhibited cisplatin-induced apoptosis in cisplatin-resistant ovarian cancer cells via induction of Akt-signaling [56]. Further to this, the expression of miR-106a suppressed PDCD4 and induced cisplatin resistance in ovarian cancer [57]. Oncogenic miR-125b mediated cisplatin resistance in ovarian cancer cells via suppression of pro-apoptotic Bak1 [58]. Interestingly, another group has shown that expression of miR-130a and miR-374a reduced the activity of cisplatin in ovarian cancer cells accompanied by increased expression of the drug efflux pumps MDR1 and Pgp [59]. This is in contrast to the previous finding that miR-130a contributed to cisplatin-sensitivity via XIAP inhibition in ovarian cancer cells [37]. Taxol-resistant A2780/Taxol ovarian cancer cells also showed reduced sensitivity to cisplatin via upregulated miR-130b which was correlated with increased expression of MDR1, Pgp (P-glycoprotein) and GST-π (glutathione S-transferase) [60]. In addition, miR-141 expression was responsible for cisplatin resistance in ovarian cancer cells via downregulation of KEAP1 (Kelch-like ECH-associated protein 1) [61]. Omental metastases of epithelial ovarian cancers revealed increased levels of miR-150 and miR-146a leading to survival of ovarian cancer cells and cisplatin resistance [62]. Oncogenic miR-214 suppressed PTEN expression and activated Akt signaling accompanied by cisplatin resistance in ovarian cancer cells [63]. Cisplatin resistance of ovarian papillary serous carcinoma was associated with expression of miR-224-5p leading to inhibition of PRKCD (protein kinase C δ) [64]. The expression of miR-376c inhibited ALK7 (activin receptor-like kinase 7) expression in epithelial ovarian cancer cells leading to cisplatin resistance [65]. Another PTEN inhibitor of ovarian cancer cells is featured by miR-630, and suppression of miR-630 increased cisplatin activity in epithelial ovarian carcinoma cells [66]. Recently, it was shown that oncogenic miR-630 levels were increased after cisplatin treatment via induction of the transcription factor E2F1 and of the miRNA-processing Drosha protein [67]. A list of miRNAs involved in cisplatin-resistance and –sensitivity of ovarian cancers is given in Table 1.
Table 1.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in ovarian cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| let-7c, let-7e, let-7i, miR-20b, miR-29, miR-30a, miR-30c, miR-30c-2, miR-30d, miR-130a(!), miR-136, miR-152, miR-155, miR-185, miR-186, miR-199a, miR-302b, miR-335, miR-449a, miR-509-3p, miR-519d, miR-542-3p, miR-625, miR-770-5p, miR-873, miRPlus-F1147, miRPlus-F1231 | miR-9, miR-21, miR-21-3p, miR-27a, miR-27b, miR-31, miR-93, miR-106a, miR-125b, miR-130a(!), miR-130b, miR-141, miR-146a, miR-150, miR-193b, miR-214, miR-224-5p, miR-300, miR-374a, miR-376c, miR-630, miR-642, miR-1299, miRPlus-F1064 |
The processing of miRNAs into their mature form strongly depends on the enzyme Dicer, and suppression of Dicer induced chemoresistance and invasive cancer forms [68]. In particular, knockdown of Dicer reduced the activity of cisplatin in A2780 ovarian cancer cells [68]. More recently, inhibition of the RNA-binding protein DGCR8 (digeorge critical region 8), which processes primary miRNA (pri-miRNA) into precursor miRNA (pre-miRNA) together with the RNAse III Drosha in the nucleus, enhanced the activity of cisplatin via downregulation of miR-27b [69].
2.1.2. Cisplatin, miRNAs and lung cancer
Non-smokers are almost exclusively affected by non-small cell lung cancer (NSCLC), while smoking patients usually suffer from small-cell lung cancer (SCLC), and over one million people (both men and women) die of lung cancer worldwide every year [70], [71]. Platinum complexes such as cisplatin and carboplatin have become crucial parts of chemotherapeutic treatments both of small-cell and of non-small-cell lung cancers, and have improved the survival of lung cancer patients significantly when administered in combination with other anticancer drugs such as gemcitabine, etoposide, vinorelbine or paclitaxel [70], [71]. The role of miRNAs for the anti-lung cancer activity of cisplatin was thoroughly investigated, and the tumor suppressor miR-15a-3p enhanced the activity of cisplatin in NSCLC cells via suppression of the anti-apoptotic Bcl-2 protein [72]. Expression of let-7c sensitized cisplatin-resistant A549/DDP NSCLC cells to cisplatin treatment via suppression of the ABC-transporter ABCC2 and Bcl-xl [73]. Let-7i was suppressed in cisplatin-resistant A549/DDP NSCLC cells as well as the long non-coding RNA (lncRNA) AK126698 which lead to activation of the Wnt/β-catenin signaling pathway [74]. The expression of miR-17 family miRNAs (miR-17, miR-20a, miR-20b) in A549 NSCLC cells blocked cisplatin resistance by inhibition of the TGF-β (transforming growth factor) signaling pathway and repression of TGF-β receptor 2 (TGF-βR2) [75]. Suppression of miR-17 and miR-92 families in NSCLC was correlated with cisplatin resistance via upregulation of CDKN1A (cyclin-dependent kinase inhibitor 1A) and of the double-strand break repair protein RAD21 leading to efficient DNA repair [76]. Downregulation of miR-26a was associated with resistance to cisplatin treatment in NSCLC cells, and restored miR-26a expression increased cisplatin activity via inhibition of HMGA2/E2F1/Akt signaling pathway and suppression of Bcl-2 [77]. Reduced levels of miR-29b were found in NSCLC tissues and restored miR-29b expression sensitized NSCLC cells to cisplatin via downregulation of CDK6 (cyclin-dependent kinase 6), DNMT3B and Mcl-1 (myeloid cell leukemia sequence 1) expression [78]. Restoration of miR-34a expression in lung tumors led to improved survival of cisplatin-treated KP mice bearing lung tumors [79]. MiR-34a-mediated suppression of PEBP4 increased cisplatin activity in resistant lung cancer cells [80]. In addition, miR-34a sensitized cisplatin-resistant lung cancer cells independent of their p53 activity state and the combination of miR-34a expression with cisplatin treatment seemed feasible [81]. Drug-resistant A549/DDP NSCLC cells showed reduced levels of miR-135a/b associated with cisplatin resistance via upregulation of Mcl-1 [82]. Increased levels of miR-138 were accompanied by reduced ERCC1 expression leading to enhanced cisplatin activity in NSCLC cells [83]. The tumor suppressor miR-148b sensitized drug-resistant NSCLC cells to cisplatin via inhibition of DNMT1 [84]. Further to this, miR-181b expression inhibited TGF-βR1/Smad signaling leading to cisplatin sensitivity of NSCLC cells [85]. MiR-181b also downregulated anti-apoptotic Bcl-2 associated with increased cisplatin activity in drug-resistant A549 NSCLC cells [86]. Expression of let-7c and miR-200b reverted EMT in lung cancer cells and re-sensitized cancer cells to treatment with erlotinib and cisplatin [87]. The miR-200bc/429 cluster was downregulated in cisplatin-resistant A549/DDP NSCLC cells associated with upregulated anti-apoptotic Bcl-2 and XIAP, and expression of miR200bc/429 led to increased apoptosis induction by cisplatin [88]. In SCLC cells, suppressed miR-200b expression was observed leading to cisplatin resistance via ZEB2 (zinc-finger E-box-binding homeobox 1) [89]. In addition, expression of miR-200c restored cisplatin activity in H1266 NSCLC cells via upregulation of E-cadherin and suppression of N-cadherin [90]. Cisplatin-mediated tumor cell growth inhibition and apoptosis induction was promoted by expression of miR-216a in NSCLC cells leading to suppression of eIF4B (eukaryotic initiation factor 4B) and ZEB1 [91]. The tumor suppressor miR-217 sensitized lung cancer cells to cisplatin treatment via KRAS (Kirsten-rat sarcoma) downregulation [92]. The expression of miR-375 was reduced in ERCC1-positive NSCLC tumors associated with reduced cisplatin activity [93]. In addition, upregulation of miR-378 sensitized cisplatin-resistant A549/DDP and Anip973/DDP lung adenocarcinoma cells to cisplatin treatment via suppression of secreted clusterin (sCLU) [94]. Suppression of Akt signaling and Bcl-2 was observed from A549 lung cancer cells expressing miR-451 which led to increased sensitivity to cisplatin treatment [95]. NSCLC cells expressing miR-495 exhibited higher cisplatin activity than cells with suppressed miR-495, and miR-495 blocked the expression of the copper transporter ATPase ATP7A, which is involved in multidrug resistance [96]. Downregulated miR-497 was observed from cisplatin-resistant A549/DDP NSCLC cells associated with induction of anti-apoptotic Bcl-2 protein expression [97]. Reduced expression of miR-503 was also detected in A549/DDP NSCLC cells, and induced miR-503 expression enhanced cisplatin activity via Bcl-2 suppression [98]. The tumor suppressor miR-509-3-5p sensitized A549 NSCLC cells to cisplatin treatment via PLK1 (polo-like kinase 1) suppression and cell cycle arrest [99]. MiR-630 suppressed CDC7 (cell division cycle 7) kinase and augmented cisplatin activity in A549 cells [100]. NSCLC patients expressing significant levels of miR-638 revealed longer survival and better response to cisplatin chemotherapy [101].
MiR-15b induced cisplatin resistance of lung cancer cells by downregulation of PEBP4 (phosphatidylethanolamine-binding protein 4) [102]. The oncogenic miR-21 enhanced cisplatin resistance in A549 lung cancer cells by downregulation of PTEN and induction of anti-apoptotic Bcl-2, and miR-21 expression can be applied as a marker for cisplatin response and survival prognoses in NSCLC patients [103]. Suppression of oncogenic miR-25 leading to CDC24 downregulation augmented cisplatin activity in A549 NSCLC cells [104]. MiR-25 was also overexpressed in SCLC cells associated with cisplatin resistance and expression of CDK2 (cyclin-dependent kinase 2) and cyclin E2 [105]. Single nucleotide polymorphism (SNP) in the pre-miR-27a led to increased levels of the oncogene miR-27a associated with poorer response to cisplatin in Chinese NSCLC patients harbouring this genetic polymorphism [106]. Expression of miR-31 blocked cisplatin-mediated apoptosis in NSCLC cells via inhibition of the ABCB9 transporter [107]. Inhibition of the PTEN repressor miR-92b sensitized resistant A549/DPP lung cancer cells to cisplatin [108]. Cisplatin treatment of A549 NSCLC cells increased p53 levels and reduced Bcl-2 and miR-98 expression, a negative regulator of p53, and upregulated miR-98 suppressed p53, which is a crucial protein for cisplatin-induced apoptosis [109]. However, there's another report about miR-98 which described cisplatin sensitivity in miR-98 expressing A549 cells associated with increased HMGA2 levels and lowered Bcl-2 levels [110]. (−)-Epigallocatechin-3-gallate (EGCG), the major natural catechin of the tea plant Camellia sinensis, suppressed oncogenic miR-98-5p leading to induction of p53 expression and increased cisplatin activity in NSCLC cells [111]. MiR-100 was upregulated in cisplatin-resistant SCLC cells leading to suppression of HOXA1 (homeobox A1) [112]. Both miR-106 and miR-150 functioned as oncogenes in A549 lung cancer cells, and inhibition of miR-106 and miR-150 by antisense oligonucleotides enhanced the activity of cisplatin in these cells [113]. Increased miR-106a expression led to cisplatin resistance in A549 NSCLC cells via suppression of the ABC transporter ABCA1, which is a transporter involved in cisplatin uptake [114]. Another oncomir, miR-128-2, suppressed the p21-repressor E2F5 in NSCLC cells leading to inhibition of apoptosis induction by cisplatin [115]. Expression of miR-145 decreased cisplatin activity in NSCLC cells by suppression of CDK6 [116]. In addition, suppressed miR-155 led to decreased Bax levels and increased apoptosis induction by cisplatin in A549 NSCLC cells [117]. Oncogenic miR-186* was suppressed by treatment with curcumin, a natural phenolic derivative from the roots of Curcuma longa (turmeric), and reduced miR-186* levels were associated with augmented cisplatin activity in resistant A549/DDP NSCLC cells [118]. Cisplatin-resistant A549/DDP cells also showed increased levels of miR-192, and inhibition of miR-192 sensitized these lung cancer cells to cisplatin via suppression of Bcl-2 and upregulation of Bax [119]. MiR-205 features another PTEN repressor and miR-205 expression was associated with cisplatin resistance in NSCLC cells [120]. Upregulated miR-224 contributed to cisplatin resistance of lung adenocarcinoma cells via p21(WAF1/CIP1) suppression [121]. The miR-499 rs3746444T > C polymorphism was identified as a marker for bad prognosis and cisplatin resistance in lung cancer patients [122]. A list of miRNAs involved in cisplatin-resistance and –sensitivity of lung cancers is given in Table 2.
Table 2.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in lung cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| let-7c, let-7i, miR-15a-3p, miR-17, miR-20a, miR-20b, miR-26a, miR-29b, miR-34a, miR-98(!), miR-135a/b, miR-138, miR-148b, miR-181b, miR-200b, miR-200c, miR-216a, miR-375, miR-378, miR-429, miR-451, miR-495, miR-497, miR-503, miR-509-3-5p, miR-630, miR-638 | miR-15b, miR-21, miR-25, miR-27a, miR-31, miR-92b, miR-98(!), miR-98-5p, miR-100, miR-106, miR-106a, miR-128-2, miR-145, miR-150, miR-155, miR-186*, miR-192, miR-205, miR-224, miR-499 rs3746444T > C |
2.1.3. Cisplatin, miRNAs and breast cancer
Among female cancer patients, breast cancer diseases represent the major cause of death though mortality rates were reduced by 36% since 1989 [123]. The efficacy of cisplatin treatment in breast cancer patients was correlated with certain miRNA expression profiles [123]. Cisplatin-resistance of breast cancer cells was induced by downregulation of the tumor suppressor let-7i [32]. MiR-7 and miR-345 suppressed the multidrug resistance associated transporter MRP1 in cisplatin-resistant MCF-7/DDP breast cancer cells [124]. In addition, miRNAs of the miR-200 family were downregulated in cisplatin-resistant MCF-7/DDP cells [124]. MiR-128 induced apoptosis in a p53-independent way and increased cisplatin activity in MCF-7 breast cancer cells [125]. BRCA1 (breast cancer 1) protein expression was induced in cisplatin-resistant MCF-7/DDP breast cancer cells because of suppression of miR-218 and restoration of miR-218 expression sensitized these cells to cisplatin again [126]. The expression of miR-638 was downregulated in breast cancer cells and induced miR-638 expression sensitized triple-negative breast cancer cells to cisplatin treatment [127].
Concerning oncomirs, TGF-β-induced miR-21 expression contributed to cisplatin resistance in breast cancer cells [128]. In line with this finding, transfection of MCF-7 breast cancer cells with an anti-miR-21 oligonucleotide increased cisplatin activity in these cells [129]. In addition, cisplatin-resistant MCF-7/DDP cells showed increased levels of oncogenic miR-10a, miR-146a, miR-221 and miR-222, which control the expression of BRCA1 (breast cancer 1), p27 and ERα (estrogen receptor α) [124]. MiR-221 knockdown also induced pro-apoptotic Bim expression leading to enhanced cisplatin activity in breast cancer cells [130]. Suppression of miR-203 expression by anti-miR-203 increased cisplatin activity in MCF-7 breast cancer cells by induction of SOCS3 (suppressor of cytokine signaling 3) [131]. Overexpression of miR-569 contributed to aggressiveness of breast tumors including increased cancer cell survival and proliferation by downregulation of TP53INP1, and suppression of miR-569 augmented cisplatin activity in breast cancers [132]. A list of miRNAs involved in cisplatin-resistance and –sensitivity of breast cancers is given in Table 3.
Table 3.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in breast cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| let-7i, miR-7, miR-128, miR-200, miR-218, miR-345, miR-638 | miR-10a, miR-21, miR-146a, miR-203, miR-221, miR-222, miR-569 |
2.1.4. Cisplatin, miRNAs, head-and-neck cancers and esophageal cancers
Head-and-neck cancers are mostly squamous cell carcinomas that can affect oral (tongue), nasopharyngeal, and laryngeal parts of the cancer patient, and head-and-neck squamous cell carcinoma (HNSCC) is the 6th most common cancer worldwide [133]. HNSCC is particularly prevalent in India where 30% of all cancers are HNSCC [134], [135]. Esophageal cancers represent the 8th most common cancer worldwide and squamous cell carcinomas (ESCC) are predominant among esophageal cancer cases as well [136]. Cisplatin-based therapies are applied for head-and-neck and esophageal cancers though the response rate in the latter case is only moderate (19–40%) [136], [137], [138]. Certain miRNAs can play a role concerning the response of head-and-neck and esophageal cancers to cisplatin. In HNSCC cells, cisplatin treatment led to suppression of miR-181a, miR-374a, and miR-519a, while miR-630 expression was induced [139]. Inhibition of miR-630 led to increased tumor cell survival, while inhibition of miR-181a, miR-374a and miR-519a reduced cell survival [139]. Nasopharyngeal carcinoma (NPC), which is prevalent in South China and Southeast Asia, is induced by the Epstein-Barr virus (EBV) and EBV-encoded BART Cluster 1 miRNAs reduced cisplatin activity via LMP1 suppression [140]. Induction of the tumor suppressor miR-16 after CDK4 knockdown in NPC cells was associated with enhanced cisplatin activity [141]. NPC cells with EBV-encoded LMP1 (latent membrane protein 1) induced miR-21 expression leading to inhibition of cisplatin-induced apoptosis via downregulation of PDCD4 and Fas-L [142]. MiR-21 also reduced cisplatin activity in tongue squamous cell carcinomas via suppression of PDCD4 [143]. Downregulation of miR-30a in HNSCC enhanced cisplatin activity and blocked cell motility via inhibition of Bcl-2 [144]. In addition, expression of miR-98 in HNSCC cells enhanced cisplatin resistance by suppression of HMGA2 (high mobility group A2) [145]. Upregulation of miR-125a and miR-125b in cisplatin-treated NPC cells silenced cisplatin-mediated apoptosis induction by suppression of p53 [146]. Induction of miR-101-3p in laryngeal cancer (LC) cells augmented cisplatin resistance because miR-101-3p inhibited RIPK1 (receptor-interacting serine/threonine kinase 1) expression [147]. Another oncomir, miR-222, was inhibited by antisense-miR-222 in oral squamous cell carcinoma (OSCC) cells leading to PUMA (p53 upregulated modulator of apoptosis) expression and increased cisplatin activity [148]. In addition, expression of miR-134 in OSCC cells increased cisplatin activity [149]. Tongue squamous cell carcinoma (TSCC) revealed high miR-24 expression associated with cisplatin resistance and PTEN suppression [150]. Further to this, TSCC tumors with suppressed miR-200c and miR-15b expression exhibited resistance to cisplatin-based chemotherapy and poorer prognosis of TSCC patients [151]. In contrast to that, TSCC cells with increased miR-23a and miR-214 expression levels were cisplatin-resistant because of suppression of TOP2B (DNA topoisomerase II beta) [152]. Cisplatin-resistant LC cells (Hep-2/v cells) exhibited upregulated miR-210 and miR-923 expression while miR-93 was suppressed in the multidrug-resistant cancer cells, this miRNA profile of resistant LC cells was accompanied by suppression of serine protease HTRA1 and NUPR1 (nuclear protein 1), and induction of RGS10 (regulator of G-protein signaling 10) expression [153].
Esophageal squamous cell carcinoma (ESCC) cells with reduced expression of let-7b and let-7c showed cisplatin resistance and restored let-7b/c expression in ESCC cells sensitized these cells to cisplatin treatment via downregulation of IL-6/STAT-3 (interleukin 6/signal transducer and activator of transcription 2) signaling [154]. Induced expression of let-7g and let-7i in esophageal cancer cells suppressed ABCC10 expression leading to increased cisplatin activity [155]. Patients suffering from ESCC or EAC (esophagogastric adenocarcinoma), most of them received cisplatin-based chemotherapies, with increased miR-21 levels exhibited shorter survival times [156]. In addition, ESCC cells with suppressed miR-27a (via antagomir miR-27a transfection) showed increased cisplatin activity because of Bcl-2 inhibition and Bax induction [157]. Pathologic complete response (pCR) in patients suffering from esophageal adenocarcinoma who received chemoradiation including 5-FU (5-fluorouracil) and platinum (cisplatin or oxaliplatin) therapy was correlated with reduced expression of miR-99b, miR-145*, miR-451, and miR-505* [158]. The proton pump inhibitor esomeprazole sensitized esophageal cancer cells (both ESCC and EAC) to cisplatin treatment and revealed increased expression of miR-141 and miR-200b as well as downregulated miR-376a levels [159]. However, miR-141 was also described as an oncomir highly expressed in cisplatin-resistant ESCC cells leading to YAP1 (yes-associated protein 1) suppression [160]. EAC cells (SK-GT-4) expressing miR-145 exhibited distinct cisplatin resistance and promoted cancer cell invasion [161]. MiRNA polymorphisms also play a role for cisplatin resistance and the TT genotypes of miR-196a2 rs11614913 and miR-125a rs12976445 were associated with reduced survival of ESCC patients receiving platinum-based therapy (cisplatin or oxaliplatin) [162]. Cisplatin treatment of ESCC and EAC cells exhibited changes in miRNA expression associated with cell survival, three miRNAs (miR-342-3p, miR-425, miR-320a) were upregulated by cisplatin in ESCC cells, while two miRNAs (miR-548d-5p, miR-455-3p) were suppressed in EAC cells [163]. In addition, increased expression of miR-200c was correlated with cisplatin resistance in esophageal cancer patients via induction of Akt signaling and inhibition of PP2A (protein phosphatase 2A) [164]. MiR-330-3p features another oncomir that suppressed PDCD4 in ESCC cells accompanied by inhibition of cisplatin-mediated apoptosis [165]. Expression of the tumor suppressor miR-507 augmented cisplatin activity in ESCC tumors via suppression of NRF2 (NF-E2-related factor 2) [166]. Another study of the miRNA profile of cisplatin-resistant ESCC and EAC cells revealed eleven dysregulated miRNAs in EAC cells (miR-191-5p and miR-638 were upregulated, and let-7e-5p, miR-31-5p, miR-125-5p, miR-181a-5p, miR-181b-5p, miR-200a-3p, miR-200b-3p, miR-200b-5p, and miR-455-3p were suppressed) and one upregulated miRNA (miR-130-3p) in ESCC cells [167]. A list of miRNAs involved in cisplatin-resistance and –sensitivity of head-and-neck cancers and esophageal cancers is given in Table 4.
Table 4.
Micro-RNAs involved in cisplatin-resistance and –sensitivity in head-and-neck cancers and esophageal cancers.
| Sensitivity | Resistance | |
|---|---|---|
| Head-and-neck cancer | miR-15b, miR-16, miR-93, miR-134, miR-200c, miR-630 | BART Cluster 1 miRNAs (EBV), miR-21, miR-23a, miR-24, miR-30a, miR-98, miR-101-3p, miR-125a, miR-125b, miR-181a, miR-210, miR-214, miR-222, miR-374a, miR-519a, miR-923 |
| Esophageal cancer | let-7b, let-7c, let-7e-5p, let-7g, let-7i, miR-31-5p, miR-125-5p, miR-141(!), miR-181a-5p, miR-181b-5p, miR-200a-3p, miR-200b, miR-200b-3p, miR-200b-5p, miR-455-3p, miR-507, miR-548d-5p | miR-21, miR-27a, miR-99b, miR-125a rs12976445, miR-130-3p, miR-141(!), miR-145, miR-145*, miR-191-5p, miR-196a2 rs11614913, miR-200c, miR-320a, miR-330-3p, miR-342-3p, miR-376a, miR-425, miR-451, miR-505*, miR-638 |
2.1.5. Cisplatin, miRNAs and gastric cancers
Gastric cancer (GC) belongs to the most common cancers worldwide (in particular, in Asia) and features the 2nd most cause of cancer deaths [168]. Dysregulation of tumor suppressors and oncogenes was observed from gastric cancers, including the modulation of miRNA expression in the course of carcinogenesis and chemotherapy [169], [170]. Advanced gastric cancer diseases are commonly treated with cisplatin, however, increased toxicity and emergence of resistance represent significant drawbacks of this drug [171]. A strong influence of miRNAs on cisplatin activity in gastric cancers was assumed. Expression of oncogenic miR-20a in gastric cancers activated NF-κB (nuclear factor κB) signaling via repression of NFKBIB (= IκBβ) leading to cisplatin resistance of GC cells [172]. In addition, miR-20a induced cisplatin resistance in GC cells by downregulation of EGR2 (early growth response 2) [173]. Oncogenic miR-21 suppressed PTEN and induced Akt signaling leading to cisplatin resistance of GC cells [174]. Cisplatin treatment induced the expression of miR-29c and suppressed the oncogenic targets of miR-29c (catenin-δ and Rho signaling), which was associated with reduced metastasis formation and lower relapse rates in gastric cancer patients [175]. Expression of miR-30a increased cisplatin activity in GC cells via inhibition of Snail and vimentin and reversal of EMT [176]. In addition, expression of miR-101 enhanced cisplatin activity in cisplatin-resistant SGC7901/DDP cells by inhibition of VEGF-C (vascular endothelial growth factor C) expression [177]. Sensitivity of GC cells to cisplatin was promoted by downregulation of oncogenic miR-141 and induction of the miR-141 target KEAP1, and Helicobacter pylori bacteria were able to suppress miR-141 in GC cells and to augment cisplatin activity [178]. However, another study on miR-141 and the long-coding RNA (lncRNA) H19 showed that miR-141 expression downregulated the oncogenic lncRNA H19 leading to proliferation inhibition and increased cisplatin activity [179]. Expression of the tumor suppressor miR-200c promoted cisplatin activity in resistant SGC7901/DDP GC cells via induced expression of E-cadherin and pro-apoptotic Bax as well as downregulation of anti-apoptotic Bcl-2 [180]. In addition, RhoE was identified as a target of miR-200c and suppression of RhoE sensitized cisplatin-resistant GC cells to cisplatin [181]. Expression of miR-218 in GC cells (SGC7901) enhanced the sensitivity of these cancer cells to cisplatin treatment, and miR-218 was induced in advanced GC patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy [182]. In contrast to that, cisplatin-resistant GC cells exhibited increased miR-223 expression, which contributed to drug resistance by downregulation of FBXW7 (F-box and WD repeat domain containing 7) [183]. GC tissues and cells infected by H. pylori showed upregulated miR-223 expression, which may play a role for the emergence of cisplatin resistance in gastric cancers [183]. This is in contrast to the observation that H. pylori suppressed miR-141 expression in GC cells associated with increased cisplatin activity [178]. In addition, overexpression of miR-362 in GC cells (BGC-823, SGC-7901) inhibited cisplatin-mediated apoptosis induction via activation of NF-κB [184]. Downregulation of miR-375 contributed to cisplatin resistance of GC cells (SGC7901/DDP) because of induction of the receptor tyrosine kinase ERBB2 and activation of Akt signaling [185]. Activation of NF-κB signaling induced the expression of miR-425 leading to PTEN suppression and cisplatin resistance [186]. The tumor suppressor miR-449a inhibited Bcl-2 and cyclin D1 expression in GC cells and, thus, enhanced cisplatin-induced apoptosis [187]. In addition, the tumor suppressor miR-503 inhibited Bcl-2 and IGF1R (insulin-like growth factor receptor 1) expression associated with increased apoptosis induction by cisplatin [188]. Upregulation of miR-765 sensitized BGC-823/DDP cells to cisplatin via inhibition of CIAPIN1 (cytokine-induced apoptosis inhibitor 1) expression [189]. MiR-1271 expression also sensitized GC cells to cisplatin treatment via inhibition of IGFR1, IRS1, mTOR and Bcl-2 expression [190]. Samples of gastric cancer patients exhibited upregulated expression of six miRNAs (let-7g, miR-1, miR-16, miR-34, miR-181, miR-342) which were associated with chemosensitivity to cisplatin treatment [191]. Further to this, miR-181a blocked autophagy in GC cells and increased cisplatin activity in SGC7901/DDP cells [192]. A list of miRNAs involved in cisplatin activity in gastric cancers is given in Table 5.
Table 5.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in gastric cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| let-7g, miR-1, miR-16, miR-29c, miR-30a, miR-34, miR-101, miR-141(!), miR-181, miR-181a, miR-200c, miR-218, miR-342, miR-375, miR-449a, miR-503, miR-1271 | miR-20a, miR-21, miR-141(!), miR-223, miR-362, miR-425 |
2.1.6. Cisplatin, miRNAs, hepatomas and renal cancers
Hepatocellular carcinoma (HCC) represents the 5th most common cancer worldwide and the 3rd most fatal cancer disease (more than 600.000 deaths per year) [193]. Renal cell carcinoma (RCC) is the most common kidney cancer leading to more than 13.000 deaths per year in the USA [194]. The activity of cisplatin against both cancer types was investigated and certain miRNAs played crucial roles for the performance of cisplatin. HCC patients who did not respond to cisplatin-based chemotherapy exhibited twelve upregulated miRNAs (miR-10a-1, miR-23a-1, miR-24, miR-26a, miR-27a, miR-30c, miR-30e, miR-106b, miR-133b, miR-199a, miR-199-3p, miR-200b) [195]. In addition, the regulation of ten miRNAs was correlated with patient survival and cisplatin activity, i.e., suppression of miR-602-2 and upregulation of nine miRNAs (let-7g-2, miR-21-2, miR-31-3, miR-98-1, miR-107-1, miR-181a-2, miR-210-1, miR-491-1, miR-664-1) were associated with better survival rates in HCC patients who received cisplatin treatment [195]. Expression of miR-27b enhanced cisplatin activity in HCC cells (HepG2) by p53 activation and inhibition of CYP1B1 (cytochrome P450 1B1) expression [196]. In contrast to that, the expression of oncogenic miR-1180 activated NF-κB signaling in HCC cells leading to cisplatin resistance [197]. Cisplatin-treated HCC cells showed altered miRNA expressions (upregulated miR-34a, miR-99a and miR-338-3p expression) which may contribute to growth inhibition and apoptosis induction by cisplatin [198]. In addition, expression of miR-133a and miR-326 downregulated the anti-apoptotic Bcl-xl protein which was associated with increased cisplatin activity in HCC cells [199]. MiR-182 levels are increased in HCC patients receiving cisplatin-based chemotherapy and it was shown that miR-182 increased cisplatin resistance in HCC cells by suppression of TP53INP1 (tumor protein 53-induced nuclear protein 1) expression [200].
In renal cell carcinoma cells (RCC), levels of PTENP1 (a pseudogene of PTEN) were low and suppressed by oncogenic miR-21. Increased expression of PTENP1 was accompanied by increased cisplatin activity in RCC cells [201]. Like in HCC cells, p53 activation and CYP1B1 suppression were observed from RCC cells expressing miR-27b, which was associated with augmented cisplatin activity [196]. A list of miRNAs involved in cisplatin activity in hepatomas and renal cancers is given in Table 6.
Table 6.
Micro-RNAs involved in cisplatin-resistance and –sensitivity in hepatomas and renal cancers.
| Sensitivity | Resistance | |
|---|---|---|
| Hepatoma | let-7g-2, miR-21-2, miR-27b, miR-31-3, miR-34a, miR-98-1, miR-99a, miR-107-1, miR-133a, miR-181a-2, miR-210-1, miR-326, miR-338-3p, miR-491-1, miR-664-1 | miR-10a-1, miR-23a-1, miR-24, miR-26a, miR-27a, miR-30c, miR-30e, miR-106b, miR-133b, miR-182, miR-199a, miR-199-3p, miR-200b, miR-602-2, miR-1180 |
| Renal cancer | miR-27b | miR-21 |
2.1.7. Cisplatin, miRNAs, bladder cancer and colon cancer
Bladder cancer is the fourth most common cancer among men of the West, and standard chemotherapy includes cisplatin in combination with methotrexate, vinblastine, and doxorubicin (MVAC) or in combination with gemcitabine (GC) [202]. However, toxicity and drug resistance feature severe drawbacks of cisplatin therapy, which is in parts regulated by miRNAs. Bladder cancer cells that responded well to cisplatin treatment showed reduced levels of miR-138 and increased expression of miR-27a and miR-642 [202]. Restored miR-27a expression in cisplatin-resistance bladder cancer cells led to increased cisplatin activity by suppression of the cystine/glutamate exchanger SLC7A11 [203]. Cisplatin-induced expression of miR-34a in bladder cancer cells inhibited CD44 expression and potentiated cisplatin activity [204]. In addition, expression of miR-101 sensitized bladder cancer cells to cisplatin treatment by suppression of COX-2 (cyclooxygenase-2) [205]. MiR-1182 also sensitized bladder cancer cells to cisplatin via hTERT (human telomerase reverse transcriptase) inhibition [206]. In contrast to that, miR-150 expression downregulated PDCD4 and contributed to cisplatin resistance of muscle-invasive bladder cancer cells [207].
Colon cancer is the 3rd most cancer worldwide and cisplatin plays only a negligible role for the treatment of colorectal cancers (CRC) because of inefficacy and drug resistance [208]. Nevertheless, the influence of certain miRNAs on the activity of cisplatin in colon cancer was investigated. Expression of miR-128 in colon cancer cells (HCT-116) increased the apoptosis induction by cisplatin independent of the p53 state via upregulation of PUMA and suppression of Akt signaling [125]. In addition, expression of miR-203 sensitized colon cancer cells to cisplatin treatment via inhibition of SIK2 (salt-inducible kinase 2) [209]. Induced expression of miR-497 by non-toxic doses of the DNA-methylation inhibitor decitabine in HCT-116 colon cancer cells increased cisplatin activity in these cancer cells and sensitized colon cancer stem cells to cisplatin treatment [210]. A list of miRNAs involved in cisplatin activity in bladder cancers and colon cancers is given in Table 7.
Table 7.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in bladder cancers and colon cancers.
| Tumor suppressors | Oncogenes | |
|---|---|---|
| Bladder cancer | miR-27a, miR-34a, miR-101, miR-642, miR-1182 | miR-138, miR-150 |
| Colon cancer | miR-128, miR-203, miR-497 | – |
2.1.8. Cisplatin, miRNAs, cervical cancer and prostate cancer
Cervical cancer (CC) is one of the most common female cancers with high mortality rates (ranked 2nd among female cancers) [211]. Globally, there are ca. half a million new cases and ca. 230.000 women die of this cancer per year. Most cervical cancer patients suffer from squamous cell carcinomas (ca. 80%) while a minor group is affected by adenocarcinomas (5–20%) [212]. Cisplatin is applied in neoadjuvant chemotherapy of cervical cancer and this platinum complex increased miR-34a and miR-605 expression levels as well as p53 expression in cervical cancer samples from cancer patients [213]. HeLa CC cells, which expressed miR-30a, increased cisplatin-mediated apoptosis via suppression of Beclin-1 and inhibition of autophagy [214]. In addition, expression of miR-125a sensitized resistant CC cells to cisplatin by suppression of STAT-3 [215]. Induction of miR-124 and miR-520b suppressed Eps8 and potentiated cisplatin activity in HeLa CC cells [216]. MiR-506 also sensitized CC cells to cisplatin via inhibition of hedgehog signaling and suppression of Gli3 [217]. In contrast to that, expression of miR-181a led to cisplatin resistance in CC cells by inhibition of pro-apoptotic PRKCD (protein kinase C delta) [218]. Further to this, inhibition of miR-199a by anti-miR-199a oligonucleotides augmented cisplatin activity in CC cells [219]. MiR-93 and miR-106b contributed to cisplatin resistance in CC cells by targeting the cell-cycle checkpoint protein RAD1 [220].
Prostate cancer (PCa) is the second most cause of death of male cancer patients in the West [133]. PCa cells expressing miR-29b increased apoptosis induction by cisplatin by suppression of Akt3 and DNMT3b [221]. In addition, suppression of miR-31 and miR-205 led to cisplatin resistance by upregulation of anti-apoptotic Bcl-w and E2F6 [222]. Inhibition of autophagy via suppression of lysosome-associated proteins RAB27A (a Ras-related protein) and LAMP3 (lysosomal associated membrane protein 3) by miR-205 in castration-resistant PCa cells was accompanied by enhanced cisplatin sensitivity in these cancer cells [223]. In contrast to that, expression of oncogenic miR-125b mediated cisplatin resistance by downregulation of p53, Bak1 and PUMA [224]. A list of miRNAs involved in cisplatin activity in cervical and prostate cancers is given in Table 8.
Table 8.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in cervical cancers and prostate cancers.
| Tumor suppressors | Oncogenes | |
|---|---|---|
| Cervical cancer | miR-30a, miR-34a, miR-124, miR-125a, miR-506, miR-520b, miR-605 | miR-93, miR-106b, miR-181a, miR-199a |
| Prostate cancer | miR-29b, miR-31, miR-205 | miR-125b |
2.1.9. Cisplatin, miRNAs and miscellaneous cancer diseases
Testicular germ cell tumors (TGCT) are the most common solid tumors among young men and the survival rate of TGCT patients has improved much since the approval of cisplatin in the late 1970's [30]. Nevertheless, the influence of a few miRNAs on cisplatin activity in TGCT was investigated and the expression of miR-302a increased cisplatin activity in TGCT cells (NT2) via suppression of p21 [225]. In addition, NT2 cells expressing miR-383 exhibited suppressed PNUTS (a subunit of protein phosphatase 1) and histone H2AX, which was associated with augmented cisplatin activity [226].
Endometrial cancer (EC) represents a female malignancy with poor prognosis. Expression of miR-134 in human EC stem cells was downregulated leading to reduced cisplatin activity, while miR-134 expression suppressed POGLUT1 (protein O-glucosyltransferase 1) expression and Notch signaling which was accompanied by distinct in vivo tumor growth inhibition [227]. The inhibition of miR-200b* in endometrioid EC cells increased the anticancer activity of cisplatin [228]. Increased expression of miR-200b, miR-200c and miR-429 in EC was associated with cisplatin resistance, however, the binding site SNP rs1045385 A > C in the miRNA response element (MRE) of the 3′-UTR (3′ untranslated region) of their target gene AP-2α blocked the binding of miR-200b/200c/429 to the MRE of AP-2α leading to upregulated expression of the tumor suppressor AP-2α and increased cisplatin activity [229], [230].
Medulloblastomas (MB) belong to the most common pediatric neoplasms of the central nervous system [231]. Expression of miR-34a in MB cells suppressed MAGE-A (melanoma associated antigen) expression and induced p53 activity associated with enhanced cisplatin efficacy [231]. Neuroblastomas (NB) originating from the sympathetic nervous system causes 15% of all pediatric cancer deaths [232]. MYCN amplified high-risk NB cells expressing miR-497 revealed downregulated cell cycle regulator WEE1, which was accompanied by enhanced apoptosis induction by cisplatin [232]. In addition, suppression of miR-520f in cisplatin-resistant NB cells (SK-N-AsCis24) led to increased NAIP (neural apoptosis inhibitory protein) expression and inhibition of cisplatin-mediated apoptosis induction [233]. In adults, gliomas represent the most lethal brain malignancies, and it was shown that miR-136 expression in glioma cells suppressed the E2F1 oncogene leading to cisplatin sensitivity in glioma cells [234].
In addition, miRNAs function as key regulators in pancreatic cancers (PaCa), and suppression of miR-374b was associated with cisplatin resistance in pancreatic cancers [235]. In addition, expression of miR-34 sensitized pancreatic cancer cells to cisplatin treatment via suppression of Bcl-2 and Notch1/2 [236]. Gallbladder cancer (GBC) is the most common cancer of the biliary tract with poor survival rates, and it was shown that expression of miR-145 increased cisplatin activity in GBC cells via downregulation of the multidrug resistance associated protein 1 (MRP1) [237].
In anaplastic thyroid carcinoma (ATC, a rare but aggressive thyroid cancer), levels of the tumor suppressor miR-30d were downregulated while induced miR-30d expression sensitized ATC cells to cisplatin treatment by suppression of Beclin-1 and inhibition of autophagic tumor cell survival [238].
The most prevalent bone cancer is osteosarcoma (OS) and high expression of miR-133a was associated with poor survival [239]. OS cells treated with cisplatin showed higher levels of miR-133a, and inhibition of miR-133a expression sensitized OS cells to cisplatin treatment [239]. The expression of miR-103 and miR-107 sensitized OS cells (U2OS) to cisplatin treatment via inhibition of homologous recombination (HR) and inhibition of the formation of RAD51 foci [240]. In addition, U2OS cells, which expressed miR-138, showed repression of histone H2AX expression, inhibition of HR repair and enhanced sensitivity to cisplatin treatment [241].
In order to investigate the role of Marek's Disease Virus Type 1 miRNA miR-M3 (MDV1-miR-M3) from a chicken herpes virus for cisplatin sensitivity, chicken fibroblast cells (DF-1) were transfected with MDV1-miR-M3 [242]. The expression of MDV1-miR-M3 in DF-1 cells inhibited cisplatin-mediated apoptosis induction via suppression of Smad2 (mothers against decapentaplegic 2) [242]. A list of miRNAs involved in cisplatin activity in miscellaneous cancers is given in Table 9.
Table 9.
MicroRNA tumor suppressors and oncogenes correlated with cisplatin activity in miscellaneous cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| miR-30d (ATC), miR-34 (PaCa), miR-34a (MB), miR-103 (OS), miR-107 (OS), miR-134 (EC), miR-136 (glioma), miR-138 (OS), miR-145 (GBC), miR-302a (TGCT), miR-374b (PaCa), miR-383 (TGCT), miR-497 (NB), miR-520f (NB) | miR-133a (OS), miR-200b (EC), miR-200b* (EC), miR-200c (EC), miR-429 (EC), MDV1-miR-M3 (chicken fibroblasts) |
2.2. Carboplatin
Carboplatin, cis-diammine(cyclobutane-1,1-dicarboxylate-O,O′)platinum(II), was the second platinum complex that was approved for anticancer therapy and it has shown reduced side-effects and lower toxicity than cisplatin [30]. Because of its improved toxicity profile, carboplatin has begun to replace cisplatin in various solid tumors such as ovarian cancer, lung cancer, head-and-neck cancer, and cervix carcinomas and it is given to the patients as intravenous infusions [30]. In the carboplatin molecule, the chlorido ligands of cisplatin were replaced by the O,O’-chelating ligand cyclobutane-1,1-dicarboxylate (CBDCA) which reduced the reactivity of carboplatin when compared with cisplatin [30]. Nevertheless, carboplatin formed the same DNA adducts as cisplatin and exhibited cross-resistance to cisplatin [30].
2.2.1. Carboplatin, miRNAs and ovarian cancer
Patients who suffered from EOC (epithelial ovarian cancer) and received carboplatin treatment were investigated for their miRNA expression profile, and high expression of miR-181-5p as well as high phospho-Smad2 levels were associated with poor prognosis and shorter survival [243]. The ABC-transporter ABCD2 was suppressed by miR-30d in ovarian cancer cells associated with enhanced carboplatin-mediated apoptosis induction possibly via increased platinum accumulation in the cancer cells [36]. Reduced miR-148b-5p expression in EOC patients who received decitabine plus carboplatin chemotherapy exhibited shorter progression free survival [244]. In addition, carboplatin-resistance was observed from ovarian cancer cells that express miR-193b* leading to suppression of CRIM1 (cysteine-rich transmembrane BMP regulator 1) [245]. The expression of the miR-200 family miRNAs miR-200c and miR-141 mimics in ovarian cancer cells led to carboplatin resistance [246]. In contrast to that, increased expression of miR-200 family miRNAs in ovarian cancer patients who received paclitaxel-carboplatin therapy was associated with improved response to chemotherapy and prolonged progression free survival because of reduction of β-tubulin III protein levels [247]. Levels of small nuclear RNA (snRNA) RNU2-1f (also described as miR-1246 and miR-1290, both miRNAs are fragmented forms of RNU2-1f) was increased in sera of EOC patients, and high RNU2-1f concentrations were associated with carboplatin-resistance in EOC patients [248]. EOC ascites tumors exhibited upregulated miR-21 and miR-214 expression as well as carboplatin resistance [249]. High levels of miR-27a were observed from platinum-resistant (cisplatin or carboplatin) ovarian cancer patients, which was associated with poor prognosis [54]. Further to this, high levels of let-7a in ovarian cancer patients were accompanied by weaker response to paclitaxel-carboplatin chemotherapy and shorter survival [250]. A list of miRNAs involved in carboplatin activity in ovarian cancers is given in Table 10.
Table 10.
MicroRNA tumor suppressors and oncogenes correlated with carboplatin activity in ovarian cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| miR-30d, miR-148b-5p, miR-200 family(!) | let-7a, miR-21, miR-27a, miR-141, miR-181-5p, miR-193b*, miR-200c(!), miR-214, RNU2-1f (miR-1246, miR-1290) |
2.2.2. Carboplatin, miRNAs and lung cancer
NSCLC (non-small cell lung cancer) cells with high miR-19b and low miR-146a expression were correlated with shorter survival and lymph node metastasis promotion in NSCLC patients receiving platinum-based chemotherapy (e.g., carboplatin) [251]. In addition, NSCLC patients receiving platinum therapy (either carboplatin or cisplatin) who showed partial response to platinum treatment had low levels of oncogenic miR-21 [252]. Increased levels of the oncogene miR-27a associated with poorer response to carboplatin were observed from Chinese NSCLC patients who harboured a single nucleotide polymorphism (SNP) in the pre-miR-27a [106]. NSCLC patients with increased levels of miR-135a*, miR-196b, and miR-1290 showed improved response to platinum-based chemotherapy (either carboplatin or cisplatin) [253]. In A549 and H1975 lung cancer cells, expression of the tumor suppressor miR-218 inhibited tumor cell growth and invasion, while expression of oncogenic miR-205 led to carboplatin resistance via upregulation of anti-apoptotic Mcl-1 and survivin as well as suppression of pro-apoptotic PARP (poly ADP ribose polymerase), caspase-3, and Bax proteins [254]. A list of miRNAs involved in carboplatin activity in lung cancers is given in Table 11.
Table 11.
MicroRNA tumor suppressors and oncogenes correlated with carboplatin activity in lung cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| miR-135a*, miR-146a, miR-196b, miR-218, miR-1290 | miR-19b, miR-21, miR-27a, miR-205 |
2.2.3. Carboplatin, miRNAs and miscellaneous cancers
Carboplatin-resistant Huh-7 hepatocellular carcinoma (HCC) cells showed increased expression of miR-27b, miR-146a, miR-146b-5p, miR-181a, and miR-181d [255]. In contrast to that, the expression of miR-621 in breast cancer cells sensitized these cancer cells to carboplatin treatment via inhibition of FBXO11 and increased p53 activity [256]. In addition, increased expression of miR-218 in cervical cancer (CC) cells augmented carboplatin activity via suppression of cyclin D1 followed by cell cycle arrest [257]. In retinoblastoma (RB) cells, miR-34a inhibited HMGB1 expression leading to enhanced activity of chemotherapeutic agents including carboplatin [258]. A list of miRNAs involved in carboplatin activity in miscellaneous cancers is given in Table 12.
Table 12.
MicroRNA tumor suppressors and oncogenes correlated with carboplatin activity in miscellaneous cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| miR-34a (RB), miR-218 (CC), miR-621 (breast cancer) | miR-27b (HCC), miR-146a (HCC), miR-146b-5p (HCC), miR-181a (HCC), miR-181d (HCC) |
2.3. Oxaliplatin
Oxaliplatin differs structurally from cisplatin and carboplatin, and this Pt(II) complex consists of an N,N’-chelating ligand (trans-1R,2R-diaminocyclohexane, DACH) and the O,O’-chelating ligand oxalate [30]. Due to the bulky organic DACH ligand of oxaliplatin, the DNA adducts built by oxaliplatin were more toxic when compared with cisplatin adducts and the oxaliplatin-DNA-adducts were not recognized by the DNA mismatch repair system [30]. Oxaliplatin showed no cross-resistance to cisplatin and carboplatin in colorectal cancers (CRC) which are resistant to cisplatin and carboplatin treatment [30]. Consequently, oxaliplatin was approved for the treatment of colorectal cancers and is mostly given as intravenous infusions in combination with 5-FU and folinate (= FOLFOX regimen) [30].
2.3.1. Oxaliplatin, miRNAs and colorectal cancers
Treatment of CRC (colorectal cancer) cells (HCT-8) with oxaliplatin led to suppression of six oncogenic miRNAs (miR-92a, miR-93, miR-191, miR-197, miR-222, miR-1826) which likely contributed to the potent oxaliplatin activity against CRC [259]. In contrast to that, oxaliplatin-resistant CRC cells showed upregulated miR-203 expression via inhibition of ATM kinase expression [260]. MiR-153 expression also led to oxaliplatin resistance by downregulation of FOXO3a (forkhead box O3a) [261]. High levels of miR-20a were found in oxaliplatin-resistant SW620 CRC cells leading to BNIP2 (Bcl-2/adenovirus E1B 19 kDa interacting protein 2) targeting, and inhibition of miR-20a sensitized SW620 cells to oxaliplatin treatment [262]. It was found that deregulated stromal cells produced oncogenic miR-21 leading to inhibition of oxaliplatin-mediated apoptosis induction in CRC cells and to enhanced invasiveness of CRC cells via suppression of the MMP2 (matrix metalloproteinase 2) inhibitor RECK (reversion-inducing cysteine-rich protein motif with Kazal motifs) [263]. The biguanide metformin suppressed miR-21 expression in CRC cells via inhibition of Wnt signaling leading to increased oxaliplatin activity [264]. Further to this, miR-34a expression was downregulated by oxaliplatin in CRC cells, which was associated with oxaliplatin resistance [265]. Interestingly, the new and orally applicable Pt(IV) drug candidate satraplatin induced expression of the tumor suppressor miR-34a in CRC cells and, thus, may overcome platinum resistance [265]. However, another study reported of oxaliplatin-induced p53 activation and miR-34a upregulation in CRC cells, which was accompanied by downregulation of E2F1, DUT-N, and genes of the thymidylate biosynthesis leading to reduced dTTP levels [266]. Repression of the tumor suppressor let-7 family by LIN28B reduced oxaliplatin activity in CRC cells (HCT-116, SW480) as well [267]. Expression of miR-133a increased oxaliplatin-induced apoptosis in CRC cells by suppression of RFFL (ring finger and FYVE-like domain containing E3-ubiquitin protein ligase) [268]. Oxaliplatin activity was also increased by miR-139-5p via suppression of anti-apoptotic Bcl-2 protein expression [269]. Oxaliplatin-resistant CRC cells were sensitized to oxaliplatin treatment by increased miR-297 levels, which reduced the levels of the platinum-resistance associated MRP2 (MDR-associated protein 2) protein in the CRC cells [270]. Expression of miR-1915 sensitized oxaliplatin-resistant CRC cells (HCT-116/L-OHP) to oxaliplatin treatment by downregulation of anti-apoptotic Bcl-2 protein expression [271]. In addition, expression of miR-1915 and miR-1914* increased the activity of oxaliplatin in resistance CRC cells by suppression of NFIX (nuclear factor I/X) [272].
Rectal cancer patients who responded well to oxaliplatin-capecitabine radiochemotherapy (i.e., who showed complete response) exhibited upregulated miR-622 and miR-630 expression [273]. In addition, patients suffering from metastatic CRC showed better responses to XELOX therapy (combination of capecitabine/xeloda and oxaliplatin) and longer progression-free survival in case of high miR-126 levels [274]. Overexpression of miR-107 and miR-99a-3p was associated with improved outcome in patients with metastatic CRC treated with 5-FU and oxaliplatin [275]. Advanced CRC patients with low miR-148a expression showed poorer survival and worse response to oxaliplatin treatment [276]. In addition, patients suffering from KRAS-mutated CRC with increased miR-200b and reduced miR-146 levels exhibited longer survival times after treatment with oxaliplatin in combination with capecitabine and the antibody cetuximab [277]. Upregulation of miR-196b-5p and miR-592 also increased the potency of the XELOX regimen in metastatic CRC patients [278]. In contrast to that, upregulated expression of five serum miRNAs (miR-20a, miR-130, miR-145, miR-216, miR-372) was observed from oxaliplatin-resistant CRC patients [279]. However, upregulated miR-143 and miR-145 enhanced activity of oxaliplatin in CRC cells by suppression of CD44 (cluster of differentiation 44), KRAS, KLF5 (Krüppel-like factor 5) and BRAF (v-Raf murine sarcoma viral oncogene homolog B1) [280]. In addition, higher expression of miR-106a, miR-130b and miR-484 was determined in patients with oxaliplatin-resistant CRC when compared with responders [281]. Increased expression of miR-320e also led to worse outcome in advanced CRC patients who received oxaliplatin treatment [282]. Further to this, overexpression of miR-27b, miR-181b, and miR-625-3p was discovered in non-responding metastatic CRC patients after treatment with oxaliplatin (XELOX or FOLFOX) and these three oncogenic miRNAs were also upregulated in oxaliplatin-resistant CRC cells (HCT-116/oxPt) [283]. A list of miRNAs involved in oxaliplatin activity in colon cancers is given in Table 13.
Table 13.
MicroRNA tumor suppressors and oncogenes correlated with oxaliplatin activity in colorectal cancers.
| Tumor suppressors | Oncogenes |
|---|---|
| let-7 family, miR-34a, miR-99a-3p, miR-107, miR-126, miR-133a, miR-139-5p, miR-143, miR-145(!), miR-148a, miR-196b-5p, miR-200b, miR-297, miR-592, miR-622, miR-630, miR-1914*, miR-1915 | miR-20a, miR-21, miR-27b, miR-92a, miR-93, miR-106a, miR-130, miR-130b, miR-145(!), miR-146, miR-153, miR-181b, miR-191, miR-197, miR-203, miR-216, miR-222, miR-320e, miR-372, miR-484, miR-625-3p, miR-1826 |
2.3.2. Oxaliplatin, miRNAs, esophageal and gastric cancers
In addition to CRC, oxaliplatin has also found entrance to the treatment of other solid cancers that are hard to tackle such as esophageal cancer or gastric cancer. Pathologic complete response (pCR) to chemoradiation including 5-FU (5-fluorouracil) and platinum (cisplatin or oxaliplatin) therapy was associated with reduced expression of miR-99b, miR-145*, miR-451, and miR-505* in patients suffering from esophageal adenocarcinoma (EAC) [158]. MiRNA polymorphisms also play a role for oxaliplatin resistance and reduced survival of esophageal squamous cell carcinoma (ESCC) patients receiving platinum-based therapy (cisplatin or oxaliplatin) were correlated to the TT genotypes of miR-196a2 rs11614913 and miR-125a rs12976445 [162].
The oncogenes miR-21 and miR-181b were strongly expressed in advanced gastric cancer tissues (GC), and advanced GC patients with suppressed expression of miR-21 and miR-181b exhibited better response to oxaliplatin and prolonged survival [284]. In addition, metastatic or recurrent GC patients with high levels of miR-1 and miR-27a showed lower response rates and shorter overall survival after 5-FU/oxaliplatin chemotherapy [285]. Another study reported of suppression of miR-20b, miR-27a, and miR-181a accompanied by increased expression of MDR1, HIF1A (hypoxia-inducible factor 1-alpha) and HIPK2 (homeodomain-interacting protein kinase 2) in GC patients with progressing disease when compared with responders to EOX chemotherapy (epirubicin, oxaliplatin, capecitabine) [286]. In addition, patients suffering from advanced GC revealed better responses to oxaliplatin treatment when the miR-129 expression level was low [287]. GC patients who received palliative chemotherapy (5-FU in combination with platinum complexes, cisplatin or oxaliplatin) showed shorter time to progression in association with overexpression of five miRNAs (miR-150, miR-342-3p, miR-181b, miR-221, miR-224) and suppression of miR-520 h, while shorter overall survival was associated with increased expression of miR-150, miR-192, miR-224, miR-375, and miR-342-3p [288]. A list of miRNAs involved in oxaliplatin activity in esophageal and gastric cancers is given in Table 14.
Table 14.
MicroRNA tumor suppressors and oncogenes correlated with oxaliplatin activity in esophageal and gastric cancers.
| Tumor suppressors | Oncogenes | |
|---|---|---|
| Esophageal cancer | – | miR-99b, miR-125a rs12976445, miR-145*, miR-196a2 rs11614913, miR-451, miR-505* |
| Gastric cancer | miR-20b, miR-27a(!), miR-181a, miR-520 h | miR-1, miR-21, miR-27a(!), miR-129, miR-150, miR-181b, miR-192, miR-221, miR-224, miR-342-3p, miR-375 |
2.3.3. Oxaliplatin, miRNAs and miscellaneous cancers
The combination of gemcitabine and oxaliplatin showed only slight benefits in hepatocellular carcinoma (HCC) patients. UCP2 (mitochondrial uncoupling protein 2) expression in HCC patients was identified as resistance factor for chemotherapy in HCC patients, while induced expression of miR-214 reduced the UCP2 levels in HCC and inhibited HCC growth [289]. In contrast to that, expression of miR-93 increased oxaliplatin resistance in hepatoma cells by suppression of PTEN [290]. In oxaliplatin-resistant pancreatic cancer cells (AsPc-10R), the expression of miR-21 and miR-221 was twice as high as in the AsPc-1 parent cells leading to suppression of PTEN, PDCD4, Maspin and TPM1 (tropomyosin 1) [291].
2.4. MiRNAs modulated in various platinum-sensitive and resistant cancers as drugs or drug targets
The expression of certain miRNAs was modulated in various platinum-resistant or platinum-treated cancers and, thus, these miRNAs feature suitable drug targets (Table 15). Concerning tumor suppressor miRNAs, tumors expressing let-7 family miRNAs (let-7c, let-7g, let-7i), miR-16, miR-20b, miR-29b, miR-30d, miR-34a, miR-107, miR-196b, miR-218, miR-497, and miR-503 were often associated with enhanced platinum sensitivity. Hence, treatment of resistant tumors with these miRNAs or suitable mimics likely increase platinum anticancer activity. In addition, certain oncogenic miRNAs are involved in various platinum-resistant cancers, such as miR-10a, miR-21, miR-24, miR-99b, miR-106a, miR-125b, miR-150, miR-191, miR-192, miR-214, miR-221, miR-222, miR-224, miR-374a, and miR-425. The inhibition of these oncomirs by potent miRNA antagonists (antagomirs) represents another target for improved platinum-based chemotherapy. Numerous miRNAs such as miR-27a, miR-31, miR-93, miR-98, miR-101, miR-128, miR-130, miR-133a, miR-138, miR-141, miR-145, miR-146a, miR-155, miR-181a, miR-199a, miR-203, miR-205, miR-375, miR-429, miR-451, miR-630, miR-638, miR-642, miR-1290 and members of the miR-200 family are either tumor suppressors or oncomirs depending on the cancer type.
Table 15.
MicroRNAs (tumor suppressors and oncogenes) correlated with platinum activity in several cancers.
| Tumor suppressors | Oncogenes | Tumor suppressors/Oncogenes (depending on cancer type) |
|---|---|---|
| let-7c, let-7g, let-7i, miR-16, miR-20b, miR-29b, miR-30d, miR-34a, miR-107, miR-196b, miR-218, miR-497, miR-503 | miR-10a, miR-21, miR-24, miR-99b, miR-106a, miR-125b, miR-150, miR-191, miR-192, miR-214, miR-221, miR-222, miR-224, miR-374a, miR-425 | miR-27a, miR-31, miR-93, miR-98, miR-101, miR-128, miR-130, miR-133a, miR-138, miR-141, miR-145, miR-146a, miR-155, miR-181a, miR-199a, miR-200, miR-203, miR-205, miR-375, miR-429, miR-451, miR-630, miR-638, miR-642, miR-1290 |
The quick and facile degradation of RNA molecules as well as their low cell permeability limited the application of accurate miRNAs (either mimics or antagomirs) for anticancer therapy. The liposomal formulation MRX34 of a tumor suppressor miRNA-34a mimic (Mirnarx Therapeutics) showed adequate stability and cell delivery of the applied miRNA-34a mimic and led to the initiation of phase I clinical trials with MRX34 [292]. Since miR-34a augmented the anticancer activity of cisplatin, carboplatin and oxaliplatin, the combination of MRX34 with platinum complexes appears to be promising for future clinical trials of various solid tumor diseases. Further to this, an increased lipophilicity of RNA molecules was generated by modification with locked nucleic acids (LNA). LNAs possess a methylene bridge between the 2’-oxygen and the 4’-carbon of the ribose moiety and a prominent example of an LNA-modified antagomir is Miravirsen (developed by Santaris Pharma), which targets miR-122 expressed in the human liver [292]. In addition, several dietary factors and natural products that are easily obtained from natural sources provide a rich supply of low-cost, safe and easily available miRNA-modulators for the improvement of platinum-based chemotherapy [29], [293].
3. Conclusions
The approved anticancer Pt(II) complexes cisplatin, carboplatin and oxaliplatin modulated the miRNA profile of various solid tumors. Vice versa, several miRNAs were identified that influenced platinum activity either positively or negatively. Since intrinsic or acquired platinum resistance as well as toxic side-effects of the cytotoxic platinum complexes are regulated by miRNAs, a profound knowledge of the interactions between anticancer active platinum complexes and miRNAs is of high importance. A better understanding of the roles of miRNAs for the activity of platinum complexes will certainly lead to improved anticancer therapy regimens based on platinum compounds, and to better survival rates and prognoses due to the circumvention of platinum resistance and the improvement of patient life quality.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Rosenberg B., VanCamp L., Trosko J.E., Mansour V.H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson E.R., Lippard S.J. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Lippard S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 4.Kartalou M., Essigmann J.M. Mechanisms of resistance to cisplatin. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 5.Oh G.S., Kim H.J., Shen A., Lee S.B., Khadka D., Pandit A., So H.S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014;12:55–65. doi: 10.5049/EBP.2014.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schobert R., Biersack B., Dietrich A., Grotemeier A., Müller T., Kalinowski B., Knauer S., Voigt W., Paschke R. Monoterpenes as drug shuttles: cytotoxic (6-aminomethylnicotinate) dichloridoplatinum(II) complexes with potential to overcome cisplatin resistance. J. Med. Chem. 2007;50:1288–1293. doi: 10.1021/jm061379o. [DOI] [PubMed] [Google Scholar]
- 8.Zoldakova M., Biersack B., Kostrhunova K., Ahmad A., Padhye S., Sarkar F.H., Schobert R., Brabec V. (Carboxydiamine)Pt(II) complexes of a combretastatin A-4 analogous chalcone: the influence of the diamine ligand on DNA binding and anticancer effects. Med. Chem. Commun. 2011;2:493–499. [Google Scholar]
- 9.Najajreh Y., Perez J.M., Navarro-Ranninger C., Gibson D. Novel soluble cationic trans-diaminedichloroplatinum(II) complexes that are active against cisplatin resistant ovarian cancer cell lines. J. Med. Chem. 2002;45:5189–5195. doi: 10.1021/jm0201969. [DOI] [PubMed] [Google Scholar]
- 10.Liu W., Gust R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013;42:755–773. doi: 10.1039/c2cs35314h. [DOI] [PubMed] [Google Scholar]
- 11.Muenzner J.K., Rehm T., Biersack B., Casini A., de Graaf I.A.M., Worwutputtapong P., Noor A., Kempe R., Brabec V., Kasparkova J., Schobert R. Adjusting the DNA interaction and anticancer activity of Pt(II) N-heterocyclic carbene complexes by steric shielding of the trans leaving group. J. Med. Chem. 2015;58:6283–6292. doi: 10.1021/acs.jmedchem.5b00896. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S.K., Arora S., Averett C., Singh S., Singh A. Modulation of microRNAs by phytochemicals in cancer: underlying mechanisms and translational significance. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/848710. Article ID 848710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarnieri D.J., DiLeone R.J. MicroRNAs: a new class of gene regulators. Ann. Med. 2008;40:197–208. doi: 10.1080/07853890701771823. [DOI] [PubMed] [Google Scholar]
- 14.Jeong H.C., Kim E.K., Lee J.H., Yoo H.N., Kim J.K. Aberrant expression of let-7a miRNA in the blood of non-small cell lung cancer patients. Mol. Med. Rep. 2011;4:383–387. doi: 10.3892/mmr.2011.430. [DOI] [PubMed] [Google Scholar]
- 15.Mallick R., Patnaik S.K., Yendamuri S. Micro RNAs and lung cancer: biology and applications in diagnosis and prognosis. J. Carcinog. 2010;9:8. doi: 10.4103/1477-3163.67074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patnaik S.K., Mallick R., Yendamuri S. Detection of microRNAs in dried serum blots. Anal. Biochem. 2010;407:147–149. doi: 10.1016/j.ab.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothe F., Ignatiadis M., Chaboteaux C., Haibe-Kains B., Kheddoumi N., Majjaj S., Badran B., Fayyad-Kazan H., Desmedt C., Harris A.L., Piccart M., Sotiriou C. Global microRNA expression profiling identifies miR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enerly E., Steinfeld I., Kleivi K., Leivonen S.-K., Aure M.R., Russnes H.G., Ronneberg J.A., Johnsen H., Navon R., Rodland E., Mäkelä R., Naume B., Perälä M., Kallioniemi O., Kristensen V.N., Yakhini Z., Borresen-Dale A.-L. MiRNA-mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS One. 2011;6:e16915. doi: 10.1371/journal.pone.0016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang-Verslues W.W., Chang P.-H., Wei P.-C., Yang C.-Y., Huang C.-K., Kuo W.-H., Shew J.-Y., Chang K.-J., Lee E.Y.-H.P., Lee W.-H. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via down-regulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 20.Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., Dirbas F.M., Somlo G., Pera R.A., Lao K., Clarke M.F. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory P.A., Bracken C.P., Bert A.G., Godall G.J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 22.Yu F., Jiao Y., Zhu Y., Wang Y., Zhu J., Cui X., Liu Y., He Y., Park E.-Y., Zhang H., Lv X., Ma K., Su F., Park J.H., Song E. MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J. Biol. Chem. 2012;287:465–473. doi: 10.1074/jbc.M111.280768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartels D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 24.Wu S., Huang S., Ding J., Zhao Y., Liang L., Liu T., Zhan R., He X. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene. 2010;29:2302–2308. doi: 10.1038/onc.2010.34. [DOI] [PubMed] [Google Scholar]
- 25.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 26.di Leva G., Garofolo M., Croce C.M. MicroRNAs in cancer. Ann. Rev. Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouyssou J.M., Manier S., Huynh D., Issa S., Roccaro A.M., Ghobrial I.M. Regulation of microRNAs in cancer metastasis. Biochim. Biophys. Acta. 2014;22:255–265. doi: 10.1016/j.bbcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biersack B. Current state of phenolic and terpenoidal dietary factors and natural products as non-coding RNA/microRNA modulators for improved cancer therapy and prevention. Non-coding RNA Res. 2016 doi: 10.1016/j.ncrna.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voigt W., Dietrich A., Schmoll H.-J. Cisplatin und seine Analoga. Pharm. Unserer Zeit. 2006;35:134–143. doi: 10.1002/pauz.200500162. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S., Kumar A., Shah P.P., Rai S.N., Panguluri S.K., Kakar S.S. MicroRNA signature of cis-platin resistant vs. cis-platin sensitive ovarian cancer cell lines. J. Ovarian Res. 2011;4:17. doi: 10.1186/1757-2215-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang N., Kaur S., Volinia S., Greshock J., Lassus H., Hasegawa K., Liang S., Leminen A., Deng S., Smith L., Johnstone C.N., Chen X.-M., Liu C.-G., Huang Q., Katsaros D., Calin G.A., Weber B.L., Bützow R., Croce C.M., Coukos G., Zhang L. MicroRNA microarray identifies let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68:10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu P.-N., Yan M.-D., Lai H.-C., Huang R.-L., Chou Y.-C., Lin W.-C., Yeh L.-T., Lin Y.-W. Downregulation of miR-29 contributes to cisplatin resistance of ovarian cancer cells. Int. J. Cancer. 2014;134:542–551. doi: 10.1002/ijc.28399. [DOI] [PubMed] [Google Scholar]
- 34.Sestito R., Cianfrocca R., Rosano L., Tocci P., Semprucci E., di Castro V., Caprara V., Bagnato A., Ferrandina G., Sacconi A., Blandino G. MiR30a inhibits endothelin A receptor and chemoresistance in ovarian carcinoma. OncoTarget. 2016;7:4009–4023. doi: 10.18632/oncotarget.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia W., Eneh J.O., Ratnaparkhe S., Altman M.K., Murph M.M. MicroRNA-30c-2 expressed in ovarian cancer cells suppresses growth factor-induced cellular proliferation and downregulates the oncogene BCL9. Mol. Cancer Res. 2011;9:1732–1745. doi: 10.1158/1541-7786.MCR-11-0245. [DOI] [PubMed] [Google Scholar]
- 36.LaCroix B., Gamazon E.R., Lenkala D., Im H.K., Geeleher P., Ziliak D., Cox N.J., Huang R.S. Integrative analyses of genetic variation, epigenetic regulation, and the transcriptome to elucidate the biology of platinum sensitivity. BMC Genomics. 2014;15:292. doi: 10.1186/1471-2164-15-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X., Huang L., Zhao Y., Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim. Biophys. Sin. 2013;45:995–1001. doi: 10.1093/abbs/gmt113. [DOI] [PubMed] [Google Scholar]
- 38.Sorrentino A., Liu C.-G., Addario A., Peschle C., Scambia G., Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol. Oncol. 2008;111:478–486. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H., Liu S., Wang G., Wu X., Ding Y., Guo G., Jiang J., Cui S. Expression of miR-136 is associated with the primary cisplatin resistance of human epithelial ovarian cancer. Oncol. Rep. 2015;33:591–598. doi: 10.3892/or.2014.3640. [DOI] [PubMed] [Google Scholar]
- 40.Chen W., Huang L., Hao C., Zeng W., Luo X., Li X., Zhou L., Jian S., Chen Z., He Y. MicroRNA-155 promotes apoptosis in SKOV3, A2780, and primary cultured ovarian cancer cells. Tumor Biol. 2016 doi: 10.1007/s13277-016-4804-9. [DOI] [PubMed] [Google Scholar]
- 41.Xiang Y., Ma N., Wang D., Zhang Y., Zhou J., Wu G., Zhao R., Huang H., Wang X., Qiao Y., Li F., Han D., Wang L., Zhang G., Gao X. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabine. Oncogene. 2014;33:378–386. doi: 10.1038/onc.2012.575. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X., Shen H., Yin X., Long L., Xie C., Liu Y., Hui L., Lin X., Fang Y., Cao Y., Xu Y., Li M., Xu W., Li Y. MiR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin. Oncogene. 2016;35:323–332. doi: 10.1038/onc.2015.84. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z., Ting Z., Li Y., Chen G., Lu Y., Hao X. MicroRNA-199a is able to reverse cisplatin resistance in human ovarian cancer cells though the inhibition of mammalian target of rapamycin. Oncol. Lett. 2013;6:789–794. doi: 10.3892/ol.2013.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Cecco L., Berardi M., Sommariva M., Cataldo A., Canevari S., Mezzanzanica D., Iorio M.V., Tagliabue E., Balsari A. Increased sensitivity to chemotherapy induced by CpG-ODN treatment is mediated by microRNA modulation. PLoS One. 2013;8:e58849. doi: 10.1371/journal.pone.0058849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y., Chen Q., Qin R., Zhang K., Li H. MicroRNA-449a reduces cell survival and enhances cisplatin-induced cytotoxicity via downregulation of NOTCH1 in ovarian cancer cells. Tumor Biol. 2014;35:12369–12378. doi: 10.1007/s13277-014-2551-3. [DOI] [PubMed] [Google Scholar]
- 46.Chen W., Zeng W., Li X., Xiong W., Zhang M., Huang Y., Zhou L., Jiang S. MicroRNA-509-3p increases the sensitivity of epithelial ovarian cancer cells to cisplatin-induced apoptosis. Pharmacogen. 2016;17:187–197. doi: 10.2217/pgs.15.166. [DOI] [PubMed] [Google Scholar]
- 47.Pang Y., Mao H., Shen L., Zhao Z., Liu R., Liu P. MiR-519d represses ovarian cancer cell proliferation and enhances cisplatin-mediated cytotoxicity in vitro by targeting XIAP. OncoTargets Ther. 2014;7:587–597. doi: 10.2147/OTT.S60289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H., Yu X., Ding Y., Zhao J., Wang G., Wu X., Cui S., Jiang J., Peng C., Guo G.Z. MiR-770-5p inhibits cisplatin chemoresistance in human ovarian cancer by targeting ERCC2. Oncotarget. 2016 doi: 10.18632/oncotarget.10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu D., Li X., Meng X., Yan J., Zong Z. MicroRNA-873 mediates multidrug resistance in ovarian cancer cells by targeting ABCB1. Tumor Biol. 2016 doi: 10.1007/s13277-016-4944-y. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H.-M., Wei W., Sun Y.-H., Gao J.-H., Wang Q., Zheng J.-H. MicroRNA-9 promotes tumorigenesis and mediates sensitivity to cisplatin in primary epithelial ovarian cancer cells. Tumor Biol. 2015;36:6867–6873. doi: 10.1007/s13277-015-3399-x. [DOI] [PubMed] [Google Scholar]
- 51.Echevarría-Vargas I.M., Valiyeva F., Vivas-Mejía P.E. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 2014;9:e97094. doi: 10.1371/journal.pone.0097094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pink R.C., Samuel P., Massa D., Caley D.P., Brooks S.A., Carter D.R.F. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol. Oncol. 2015;137:143–151. doi: 10.1016/j.ygyno.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 53.Liu S., Fang Y., Shen H., Xu W., Li H. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim. Biophys. Sin. 2013;45:756–762. doi: 10.1093/abbs/gmt075. [DOI] [PubMed] [Google Scholar]
- 54.Eitan R., Kushnir M., Lithwick-Yanai G., Ben David M., Hoshen M., Glezerman M., Hod M., Sabah G., Rosenwald S., Levavi H. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol. Oncol. 2009;114:253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Samuel P., Pink R.C., Caley D.P., Currie J.M.S., Brooks S.A., Carter D.R.F. Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumor Biol. 2016;37:2565–2573. doi: 10.1007/s13277-015-4081-z. [DOI] [PubMed] [Google Scholar]
- 56.Fu X., Tian J., Zhang L., Chen Y., Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586:1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Li H., Xu H., Shen H., Li H. MicroRNA-106a modulates cisplatin sensitivity by targteting PDCD4 in human ovarian cancer cells. Oncol. Lett. 2014;7:183–188. doi: 10.3892/ol.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong F., Sun C., Wang Z., Han L., Weng D., Lu Y., Chen G. Journal of Huazhong University of Science and Technology, Medical Sciences. Medicine. 2011;31:543–549. doi: 10.1007/s11596-011-0487-z. [DOI] [PubMed] [Google Scholar]
- 59.Li N., Yang L., Wang H., Yi T., Jia X., Chen C., Xu P. MiR-130a and miR-374a function as novel regulators of cisplatin resistance in human ovarian cancer A2780 cells. PLoS One. 2015;10:e0128886. doi: 10.1371/journal.pone.0128886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zong C., Wang J., Shi T.-M. MicroRNA 130b enhances drug resistance in human ovarian cancer cells. Tumor Biol. 2014;35:12151–12156. doi: 10.1007/s13277-014-2520-x. [DOI] [PubMed] [Google Scholar]
- 61.van Jaarsveld M.T.M., Helleman J., Boersma A.W.M., van Kuijk P.F., van Ijcken W.F., Despierre E., Vergote I., Mathijsen R.H.J., Berns E.M.J.J., Verweij J., Prothof J., Wiemer E.A.C. MiR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32:4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 62.Vang S., Wu H.-T., Fischer A., Miller D.H., MacLaughlan S., Douglass E., Steinhoff M., Collins C., Smith P.J.S., Brard L., Brodsky A.S. Identification of ovarian cancer metastatic miRNAs. PLoS One. 2013;8:e58226. doi: 10.1371/journal.pone.0058226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang H., Kong W., He L., Zhao J.-J., O'Donnell J.D., Wang J., Wenham R.M., Coppola D., Kruk P.A., Nicosia S.V., Cheng J.Q. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 64.Zhao H., Bi T., Qu Z., Jiang J., Cui S., Wang Y. Expression of miR-224-5p is associated with the original cisplatin resistance of ovarian papillary serous carcinoma. Oncol. Rep. 2014;32:1003–1012. doi: 10.3892/or.2014.3311. [DOI] [PubMed] [Google Scholar]
- 65.Ye G., Fu G., Cui S., Zhao S., Bernaudo S., Bai Y., Ding Y., Zhang Y., Yang B.B., Peng C. MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: implications for chemoresistance. J. Cell Sci. 2011;124:359–368. doi: 10.1242/jcs.072223. [DOI] [PubMed] [Google Scholar]
- 66.Zou Y.T., Gao J.Y., Wang H.L., Wang Y., Wang H., Li P.L. Downregulation of microRNA-630 inhibits cell proliferation and invasion and enhances chemosensitivity in human ovarian carcinoma. Gen. Mol. Res. 2015;14:8766–8777. doi: 10.4238/2015.July.31.25. [DOI] [PubMed] [Google Scholar]
- 67.Cao J.-X., Li S.-Y., An G.-S., Mao Z.-B., Jia H.-T., Ni J.-H. E2F1-regulated DROSHA promotes miR-630 biosynthesis in cisplatin-exposed cancer cells. Biochem. Biophys. Res. Commun. 2014;450:470–475. doi: 10.1016/j.bbrc.2014.05.138. [DOI] [PubMed] [Google Scholar]
- 68.Kuang Y., Cai J., Li D., Han Q., Cao J., Wang Z. Repression of Dicer is associated with invasive phenotype and chemoresistance in ovarian cancer. Oncol. Lett. 2013;5:1149–1154. doi: 10.3892/ol.2013.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Y., Tian P., Yang C., Liang Z., Li M., Sims M., Lu L., Zhang Z., Li H., Pfeffer L.M., Yue J. Silencing the double-stranded RNA binding protein DGCR8 inhibits ovarian cancer cell proliferation, migration, and invasion. Pharm. Res. 2015;32:769–778. doi: 10.1007/s11095-013-1219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper S., Spiro S.G. Small cell lung cancer: treatment review. Respirology. 2006;11:241–248. doi: 10.1111/j.1440-1843.2006.00850.x. [DOI] [PubMed] [Google Scholar]
- 71.Haura E.B. Treatment of advances non-small-cell lung cancer: a review of current randomized clinical trials and an examination of emerging therapies. Cancer Control. 2001;8:326–336. doi: 10.1177/107327480100800404. [DOI] [PubMed] [Google Scholar]
- 72.Cetintas V.B., Vardarh A.T., Düzgün Z., Kaymaz B.T., Acikgöz E., Aktug H., Can B.K., Gündüz C., Eroglu Z. MiR-15a enhances the anticancer effects of cisplatin in the resistant non-small cell lung cancer cells. Tumor Biol. 2016;37:1739–1751. doi: 10.1007/s13277-015-3950-9. [DOI] [PubMed] [Google Scholar]
- 73.Zhan M., Qu Q., Wang G., Zhou H. Let-7c sensitizes acquired cisplatin-resistant A549 cells by targeting ABCC2 and Bcl-XL. Pharmazie. 2013;68:955–961. [PubMed] [Google Scholar]
- 74.Yang Y., Li H., Hou S., Hu B., Liu J., Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8:e65309. doi: 10.1371/journal.pone.0065309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang Z., Yin J., Fu W., Mo Y., Pan Y., Dai L., Huang H., Li S., Zhao J. MiRNA 17 family regulates cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC. PLoS One. 2014;9:e94639. doi: 10.1371/journal.pone.0094639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J., Fu W., Liao H., Dai L., Jiang Z., Pan Y., Huang H., Mo Y., Li S., Yang G., Yin J. The regulatory and predictive functions of miR-17 and miR-92 families on cisplatin resistance of non-small cell lung cancer. BMC Cancer. 2015;15:731. doi: 10.1186/s12885-015-1713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y., Heng P., Zhang Y., Yang J., Jiang G., Fan J. Decreased microRNA-26a expression causes cisplatin resistance in human non-small cell lung cancer. Cancer Biol. Ther. 2016;17:515–525. doi: 10.1080/15384047.2015.1095405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Y., Crawford M., Mao Y., Lee R.J., Davis I.C., Elton T.S., Lee L.J., Nana-Sinkam S.P. Therapeutic delivery of microRNA-29b by cationic lipoplexes for lung cancer. Mol. Ther. Nucl. Acids. 2013;2:e84. doi: 10.1038/mtna.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue W., Dahlman J.E., Tammela T., Khan O.F., Sood S., Dave A., Cai W., Chirino L.M., Yang G.R., Bronson R., Crowley D.G., Sahay G., Schroeder A., Langer R., Anderson D.G., Jacks T. Small RNA combination therapy for lung cancer. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3553–E3561. doi: 10.1073/pnas.1412686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu G., Chen G., Huang B., Wu S. Downregulation of PEBP4, a target of miR-34a, sensitizes drug-resistant lung cancer cells. Tumor Biol. 2014;35:10341–10349. doi: 10.1007/s13277-014-2284-3. [DOI] [PubMed] [Google Scholar]
- 81.Wang X., Dong K., Gao P., Long M., Lin F., Weng Y., Ouyang Y., Ren J., Zhang H. MicroRNA-34a sensitizes lung cancer cell lines to DDP treatment independent of p53 status. Cancer Biother. Radiopharm. 2013;28:45–50. doi: 10.1089/cbr.2012.1218. [DOI] [PubMed] [Google Scholar]
- 82.Zhou L., Qiu T., Xu J., Wang T., Wang J., Zhou X., Huang Z., Zhu W., Shu Y., Liu P. MiR-135a/b modulate cisplatin resistance of human lung cancer cell line by targeting MCL1. Pathol. Oncol. Res. 2013;19:677–683. doi: 10.1007/s12253-013-9630-4. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q., Zhong M., Liu W., Li J., Huang J., Zheng L. Alterations of microRNAs in cisplatin-resistant human non-small cell lung cancer cells (A549/DDP) Exp. Lung Res. 2011;37:427–434. doi: 10.3109/01902148.2011.584263. [DOI] [PubMed] [Google Scholar]
- 84.Sui C., Meng F., Li Y., Jiang Y. MiR-148b reverses cisplatin-resistance in non-small cell cancer cells via negatively regulating DNA (cytosine-5)-methyltransferase 1 (DNMT1) expression. J. Transl. Med. 2015;13:132. doi: 10.1186/s12967-015-0488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Chen X., Meng Q., Jing H., Lu H., Yang Y., Cai L., Zhao Y. MiR-181b regulates cisplatin chemosensitivity and metastasis by targeting TGFβR1/Smad signaling pathway in NSCLC. Sci. Rep. 2015;5:17618. doi: 10.1038/srep17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu W., Shan X., Wang T., Shu Y., Liu P. MiR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int. J. Cancer. 2010;127:2520–2529. doi: 10.1002/ijc.25260. [DOI] [PubMed] [Google Scholar]
- 87.Ahmad A., Maitah M.Y., Ginnebaugh K.R., Li Y., Bao B., Gadgeel S.M., Sarkar F.H. Inhibition of hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs. J. Hematol. Oncol. 2013;6:77. doi: 10.1186/1756-8722-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu W., Xu H., Zhu D., Zhi H., Wang T., Wang J., Jiang B., Shu Y., Liu P. MiR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother. Pharmacol. 2012;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 89.Fang S., Zeng X., Zhu W., Tang R., Chao Y., Guo L. Zinc finger E-box-binding homeobox 2 (ZEB2) regulated by miR-200b contributes to multi-drug resistance of small cell lung cancer. Exp. Mol. Pathol. 2014;96:438–444. doi: 10.1016/j.yexmp.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 90.Ceppi P., Mudduluru G., Kumarswamy R., Rapa I., Scagliotti G.V., Papotti M., Allgayer H. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol. Cancer Res. 2010;8:1207–1216. doi: 10.1158/1541-7786.MCR-10-0052. [DOI] [PubMed] [Google Scholar]
- 91.Wang R.-T., Xu M., Xu C.-X., Song Z.-G., Jin H. Decreased expression of miR216a contributes to non-small-cell lung cancer progression. Clin. Cancer Res. 2014;20:4705–4716. doi: 10.1158/1078-0432.CCR-14-0517. [DOI] [PubMed] [Google Scholar]
- 92.Guo J., Feng Z., Huang Z., Wang H., Lu W. MicroRNA-217 functions as a tumour suppressor gene and correlates with cell resistance to cisplatin in lung cancer. Mol. Cells. 2014;37:664–671. doi: 10.14348/molcells.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friboulet L., Barrios-Gonzales D., Commo F., Olaussen K.A., Vagner S., Adam J., Goubar A., Dorvault N., Lazar V., Job B., Besse B., Validire P., Girard P., Lacroix L., Hasmats J., Dufour F., André F., Soria J.-C. Molecular characteristics of ERCC1-negative versus ERCC1-positive tumors in resected NSCLC. Clin. Cancer Res. 2011;17:5562–5572. doi: 10.1158/1078-0432.CCR-11-0790. [DOI] [PubMed] [Google Scholar]
- 94.Chen X., Jiang Y., Huang Z., Li D., Chen X., Cao M., Meng Q., Pang H., Sun L., Zhao Y., Cai L. MiRNA-378 reverses chemoresistance to cisplatin in lung adenocarcinoma cells by targeting secreted clusterin. Sci. Rep. 2016;6:19455. doi: 10.1038/srep19455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H., Bian H., De W., Wang C. miRNA-451 resensitizes non-small cell lung cancer A549/DDP cells to cisplatin resistance and mechanisms involved. Xiandai Zhongliu Yixue. 2012;20:1767–1771. [Google Scholar]
- 96.Song L., Li Y., Li W., Wu S., Li Z. MiR-495 enhances the sensitivity of non-small cell lung cancer cells to platinum by modulation of copper-transporting P-type adenosine triphosphatase (ATP7A) J. Cell. Biochem. 2014;115:1234–1242. doi: 10.1002/jcb.24665. [DOI] [PubMed] [Google Scholar]
- 97.Zhu W., Zhu D., Lu S., Wang T., Wang J., Jiang B., Shu Y., Liu P. MiR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med. Oncol. 2012;29:384–391. doi: 10.1007/s12032-010-9797-4. [DOI] [PubMed] [Google Scholar]
- 98.Qiu T., Zhou L., Wang T., Xu J., Wang J., Chen W., Zhou X., Huang Z., Zhu W., Shu Y., Liu P. MiR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2. Int. J. Mol. Med. 2013;32:593–598. doi: 10.3892/ijmm.2013.1439. [DOI] [PubMed] [Google Scholar]
- 99.Wang X.-H., Lu Y., Liang J.-J., Cao J.-X., Jin Y.-Q., An G.-S., Ni J.-H., Jia H.-T., Li S.-Y. MiR-509-3-5p causes aberrant mitosis and anti-proliferative effect by suppression of PLK1 in human lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2016;478:676–682. doi: 10.1016/j.bbrc.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 100.Cao J.-X., Lu Y., Qi J.-J., An G.-S., Mao Z.-B., Jia H.-T., Li S.-Y., Ni J.-H. MiR-630 inhibits proliferation by targeting CDC7 kinase, but maintains the apoptotic balance by targeting multiple modulators in human lung cancer A549 cells. Cell Death Dis. 2014;5:e1426. doi: 10.1038/cddis.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang F., Lou J., Cao Y., Shi X., Wang P., Xu J., Xie E., Xu T., Sun R., Rao J., Huang P., Pan S., Wang H. MiR-638 is a new biomarker for outcome prediction of non-small cell lung cancer patients receiving chemotherapy. Exp. Mol. Med. 2015;47:e162. doi: 10.1038/emm.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Z., Zhang L., Yao Q., Tao Z. MiR-15b regulates cisplatin resistance and metastasis by targeting PEBP4 in human lung carcinoma cells. Cancer Gene Ther. 2015;22:108–114. doi: 10.1038/cgt.2014.73. [DOI] [PubMed] [Google Scholar]
- 103.Gao W., Lu X., Liu L., Xu J., Feng D., Shu Y. MiRNA-21, a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol. Ther. 2012;13:330–340. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 104.Yang T., Chen T., Li Y., Gao L., Zhang S., Wang T., Chen M. Downregulation of miR-25 modulates non-small cell lung cancer cells by targeting CDC42. Tumor Biol. 2015;36:1903–1911. doi: 10.1007/s13277-014-2793-0. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Z., Liu J., Wang C., Wang Y., Jiang Y., Guo M. MicroRNA-25 regulates small cell lung cancer cell development and cell cycle through cyclin E2. Int. J. Clin. Exp. Pathol. 2014;7:7726–7734. [PMC free article] [PubMed] [Google Scholar]
- 106.Xu J., Yin Z., Shen H., Gao W., Qian Y., Pei D., Liu L., Shu Y. A genetic polymorphism in pre-miR-27a confers clinical outcome of non-small cell lung cancer in a Chinese population. PLoS One. 2013;8:e79135. doi: 10.1371/journal.pone.0079135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dong Z., Zhong Z., Yang L., Wang S., Gong Z. MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small cell lung cancer cells by regulating the drug transporter ABCB9. Cancer Lett. 2014;343:249–257. doi: 10.1016/j.canlet.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 108.Li Y., Li L., Guan Y., Liu X., Meng Q., Guo Q. MiR-92b regulates the cell growth, cisplatin chemosensitivity of A549 non small cell lung cancer cell line and target PTEN. Biochem. Biophys. Res. Commun. 2013;440:604–610. doi: 10.1016/j.bbrc.2013.09.111. [DOI] [PubMed] [Google Scholar]
- 109.Zhang S., Zhang C., Li Y., Wang P., Yue Z., Xie S. MiR-98 regulates cisplatin-induced A549 cell death by inhibiting TP53 pathway. Biomed. Pharmacother. 2011;65:436–442. doi: 10.1016/j.biopha.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Xiang Q., Tang H., Yu J., Yin J., Yang X., Lei X. MicroRNA-98 sensitizes cisplatin-resistant human lung adenocarcinoma cells by up-regulation of HMGA2. Pharmazie. 2013;68:274–281. [PubMed] [Google Scholar]
- 111.Zhou D.H., Wang X., Feng Q. EGCG enhances the efficacy of cisplatin by downregulating has-miR-98-5p in NSCLC A549 cells. Nutr. Cancer. 2014;66:636–644. doi: 10.1080/01635581.2014.894101. [DOI] [PubMed] [Google Scholar]
- 112.Xiao F., Bai Y., Chen Z., Li Y., Luo L., Huang J., Yang J., Guo L. Downregulation of HOXA1 gene affects small cell lung cancer cell survival and chemoresistance under the regulation of miR-100. Eur. J. Cancer. 2014;50:1541–1554. doi: 10.1016/j.ejca.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 113.Wang P.-Y., Li Y.-J., Zhang S., Li Z.-L., Yue Z., Xie N., Xie S.-Y. Regulating A549 cells growth by ASO inhibiting miRNA expression. Mol. Cell. Biochem. 2010;339:163–171. doi: 10.1007/s11010-009-0380-2. [DOI] [PubMed] [Google Scholar]
- 114.Ma Y., Li X., Cheng S., Wie W., Li Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphate-binding cassette A1. Mol. Med. Rep. 2015;11:625–632. doi: 10.3892/mmr.2014.2688. [DOI] [PubMed] [Google Scholar]
- 115.Donzelli S., Fontemaggi G., Fazi F., di Agostino S., Padula F., Biagioni F., Muti P., Strano S., Blandino G. MicroRNA-128-2 targets the transcriptional repressor E2F5 enhancing mutant p53 gain of function. Cell Death Differ. 2012;19:1038–1048. doi: 10.1038/cdd.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bar J., Gorn-Hondermann I., Moretto P., Perkins T.J., Niknejad N., Stewart D.J., Gross G.D., Dimitroulakos J. MiR profiling identifies cyclin-dependent kinase 6 downregulation as a potential mechanism of acquired cisplatin resistance in non-small-cell lung carcinoma. Clin. Lung Cancer. 2015;16:e121–e129. doi: 10.1016/j.cllc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Zang Y.S., Zhong Y.F., Fang Z., Li B., An J. MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin via negative regulation of Apaf-1 expression. Cancer Gene Ther. 2012;19:773–778. doi: 10.1038/cgt.2012.60. [DOI] [PubMed] [Google Scholar]
- 118.Tang N., Zhang J., Du Y. Curcumin promoted the apoptosis of cisplatin-resistant human lung carcinoma cells A549/DDP through down-regulating miR-186. Zhongguo Feiai Zazhi. 2013;13:301–306. doi: 10.3779/j.issn.1009-3419.2010.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang Y., Liu J., Wan L., Liu F., Wang H., Wang Z. Impact of miRNA-192 for cisplatin resistance of non-small cell lung cancer A549/DDP cells. Linchuang Zhongliuxue Zazhi. 2014;19:481–485. [Google Scholar]
- 120.Lei L., Huang Y., Gong W. MiR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol. Rep. 2013;30:2897–2902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 121.Wang H., Zhu L.J., Yang Y.C., Wang Z.X., Wang R. MiR-224 promotes the chemoresistance of human lung adenocarcinoma cells to cisplatin via regulating G(1)/S transition and apoptosis by targeting p21(WAF1/CIP1) Br. J. Cancer. 2014;111:339–354. doi: 10.1038/bjc.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiu F., Yang L., Ling X., Yang R., Yang X., Zhang L., Fang W., Xie C., Huang D., Zhou Y., Lu J. Sequence variation in mature microRNA-499 confers unfavorable prognosis of lung cancer patients treated with platinum-based chemotherapy. Clin. Cancer Res. 2015;21:1602–1613. doi: 10.1158/1078-0432.CCR-14-1174. [DOI] [PubMed] [Google Scholar]
- 123.Chen X., Lu P., Wu Y., Wang D., Zhou S., Yang S., Shen H., Zhang X., Zhao J., Tang J. MiRNAs-mediated cisplatin resistance in breast cancer. Tumor Biol. 2016 doi: 10.1007/s13277-016-5216-6. [DOI] [PubMed] [Google Scholar]
- 124.Pogribny I.P., Filkowski J.N., Tryndyak V.P., Golubov A., Shpyleva S.I., Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int. J. Cancer. 2010;127:1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 125.Adlakha Y.K., Saini N. MiR-128 exerts pro-apoptotic effect in a p53 transcription-dependent and –independent manner via PUMA-Bak axis. Cell Death Dis. 2013;4:e542. doi: 10.1038/cddis.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He X., Xiao X., Dong L., Wan N., Wan N., Zhou Z., Deng H., Zhang X. MiR-218 regulates cisplatin chemosensitivity in breast cancer by targeting BRCA1. Tumor Biol. 2015;36:2065–2075. doi: 10.1007/s13277-014-2814-z. [DOI] [PubMed] [Google Scholar]
- 127.Tan X., Peng J., Fu Y., An S., Rezaei K., Tabbara S., Teal C.B., Man Y., Brem R.F., Fu S.W. MiR-638 mediated regulation of BRCA1 affects DNA repair and sensitivity to UV and cisplatin in triple-negative breast cancer. Breast Cancer Res. 2014;16:435. doi: 10.1186/s13058-014-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu Y., Wang Y., Ren X., Tsuyada A., Li A., Liu L.J., Wang S.E. Context-dependent bidirectional regulation of the MutS homolog 2 by transforming growth factor β contributes to chemoresistance in breast cancer cells. Mol. Cancer Res. 2010;8:1633–1642. doi: 10.1158/1541-7786.MCR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao S., Tian H., Guo Y., Li Y., Guo Z., Zhu X., Chen X. MiRNA oligonucleotide and sponge for miRNA-21 inhibition mediated by PEI-PLL in breast cancer therapy. Acta Biomater. 2015;25:184–193. doi: 10.1016/j.actbio.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 130.Ye Z., Hao R., Cai Y., Wang X., Huang G. Knockdown of miR-221 promotes the cisplatin-inducing apoptosis by targeting the BIM-Bax/Bak axis in breast cancer. Tumor Biol. 2016;37:4509–4515. doi: 10.1007/s13277-015-4267-4. [DOI] [PubMed] [Google Scholar]
- 131.Ru P., Steele R., Hsueh E.C., Ray R.B. Anti-miR-203 upregulates SOCS3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer. 2011;2:720–727. doi: 10.1177/1947601911425832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaluvally-Raghavan P., Zhang F., Pradeep S., Hamilton M.P., Zhao X., Rupaimoole R., Moss T., Lu Y., Yu S., Pecot C.V., Aure M.R., Peuget S., Rodriguez-Aguayo C., Han H.-D., Zhang D., Venkatanarayan A., Krohn M., Kristensen V.N., Gagea M., Ram P., Liu W., Lopez-Berestein G., Lorenzi P.L., Borresen-Dale A.-L., Chin K., Gray J., Dusetti N.J., McGuire S.E., Flores E.R., Sood A.K., Mills G.B. Copy number gain of has-miR-569 at 3q26.2 leads to loss of TP53INP1 and aggressiveness of epithelial cancers. Cancer Cell. 2014;26:863–879. doi: 10.1016/j.ccell.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 134.Coelho K.R. Challenges of the oral cancer burden in India. J. Cancer Epidemiol. 2012;2012:701932. doi: 10.1155/2012/701932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joshi P., Dutta S., Chaturvedi P., Nair S. Head and neck cancers in developing countries. Rambam Maimonides Med. J. 2014;5:e0009. doi: 10.5041/RMMJ.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 137.Kelsen D.P., Ginsberg R., Pajak T.F., Sheahan D.G., Gunderson L., Mortimer J., Estes N., Haller D.G., Ajani J., Kocha W., Minsky B.D., Roth J.A. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N. Engl. J. Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 138.Ancona E., Ruol A., Santi S., Merigliano S., Sileni V.C., Koussis H., Zaninotto G., Bonavina L., Peracchia A. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. [PubMed] [Google Scholar]
- 139.Huang Y., Chuang A., Hao H., Talbot C., Sen T., Trink B., Sidransky D., Ratovitski E. Phospho-ΔNp63α is a key regulator of the cisplatin-induced microRNAome in cancer cells. Cell Death Differ. 2011;18:1220–1230. doi: 10.1038/cdd.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lo A.K.F., To K.F., Lo K.W., Lung R.W.M., Hui J.W.Y., Liao G., Hayward S.D. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16164–16169. doi: 10.1073/pnas.0702896104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang Q., Zhang Y., Zhao M., Li Q., Chen R., Long X., Fang W., Liu Z. MiR-16 induction after CDK4 knockdown is mediated by c-Myc suppression and inhibits cell growth as well as sensitizes nasopharyngeal carcinoma cells to chemotherapy. Tumor Biol. 2016;37:2425–2433. doi: 10.1007/s13277-015-3966-1. [DOI] [PubMed] [Google Scholar]
- 142.Yang G.-D., Huang T.-J., Peng L.-X., Yang C.-F., Liu R.-Y., Huang H.-B., Chu Q.-Q., Yang H.-J., Huang J.-L., Zhu Z.-Y., Qian C.-N., Huang B.-J. Epstein-Barr virus-ncoded LMP1 upregulates microRNA-21 to promote the resistance of nasopharyngeal carcinoma cells to cisplatin-induced apoptosis by suppressing PDCD4 and Fas-L. PLoS One. 2013;8:e78355. doi: 10.1371/journal.pone.0078355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren W., Wang X., Gao L., Li S., Yan X., Zhang J., Huang C., Zhang Y., Zhi K. MiR-21 modulates chemosensitivity of tongue squamous cell carcinoma cells to cisplatin by targeting PDCD4. Mol. Cell. Biochem. 2014;390:253–262. doi: 10.1007/s11010-014-1976-8. [DOI] [PubMed] [Google Scholar]
- 144.Saad M.A., Kuo S.Z., Rahimy E., Zou A.E., Korrapati A., Rahimy M., Kim E., Zheng H., Yu M.A., Wang-Rodriguez J., Ongkeko W.M. Alcohol-dysregulated miR-30a and miR-934 in head and neck squamous cell carcinoma. Mol. Cancer. 2015;14:181. doi: 10.1186/s12943-015-0452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hebert C., Norris K., Scheper M.A., Nikitakis N., Sauk J.J. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol. Cancer. 2007;6:5. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen J.J., Liu S.X., Chen M.Z., Zhao Z.Y. Has-miR-125a and 125b are induced by treatment with cisplatin in nasopharyngeal carcinoma and inhibit apoptosis in a p53-dependent manner by targeting p53 mRNA. Mol. Med. Rep. 2015;12:3569–3574. doi: 10.3892/mmr.2015.3863. [DOI] [PubMed] [Google Scholar]
- 147.Ratovitski E.A. Phospho-ΔNp63α-responsive microRNAs contribute to the regulation of necroptosis in squamous cell carcinoma upon cisplatin exposure. FEBS Lett. 2015;589:1352–1358. doi: 10.1016/j.febslet.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 148.Jiang F., Zhao W., Zhou L., Liu Z., Li W., Yu D. MiR-222 targeted PUMA to improve sensitization of UM1 cells to cisplatin. Int. J. Mol. Sci. 2014;15:22128–22141. doi: 10.3390/ijms151222128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghosh R.D., Ghuwalewala S., Das P., Mandloi S., Alam S.K., Chakraborty J., Sarkar S., Chakrabarti S., Panda C.K., Roychoudhury S. MicroRNA profiling of cisplatin-resistant oral squamous cell carcinoma cell lines enriched with cancer-stem-cell-like and epithelial-mesenchymal transition-type features. Sci. Rep. 2016;6:23923. doi: 10.1038/srep23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng X., Li J., Peng C., Zhao J., Chi J., Meng X., Yun X., Li D., Yu Y., Gao M., Li Y. MicroRNA-24 induces cisplatin resistance by targeting PTEN in human tongue squamous cell carcinoma. Oral Oncol. 2015;51:998–1003. doi: 10.1016/j.oraloncology.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 151.Sun L., Yao Y., Liu B., Lin Z., Lin L., Yang M., Zhang W., Chen W., Pan C., Liu Q., Song E., Li J. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31:432–445. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- 152.Yu Z., Zhong L., Ji T., Zhang P., Chen W., Zhang C. MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma cell lines. Oral Oncol. 2010;46:317–322. doi: 10.1016/j.oraloncology.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 153.Yin W., Wang P., Wang X., Song W., Cui X., Yu H., Zhu W. Identification of microRNAs and mRNAs associated with multidrug resistance of human laryngeal cancer Hep-2 cells. Braz. J. Med. Biol. Res. 2013;46:546–554. doi: 10.1590/1414-431X20131662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sugimura K., Miyata H., Tanaka K., Hamano R., Takahashi T., Kurokawa Y., Yamasaki M., Nakajima K., Takiguchi S., Mori M., Doki Y. Let-7 expression is a significant determinant of response to chemotherapy through the regulation of IL-6/STAT3 pathway in esophageal squamous cell carcinoma. Clin. Cancer Res. 2012;18:5144–5153. doi: 10.1158/1078-0432.CCR-12-0701. [DOI] [PubMed] [Google Scholar]
- 155.Wu K., Yang Y., Zhao J., Zhao S. bAG3-mediated miRNA let-7g and let-7i inhibit proliferation and enhance apoptosis of human esophageal carcinoma cells by targeting the drug transporter ABCC10. Cancer Lett. 2016;371:125–133. doi: 10.1016/j.canlet.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 156.Winter M., Alsner J., Tramm T., Baksgaard L., Holtved E., Nordsmark M. Evaluation of miR-21 and miR-375 as prognostic biomarkers in esophageal cancer. Acta Oncol. 2015;54:1582–1591. doi: 10.3109/0284186X.2015.1064161. [DOI] [PubMed] [Google Scholar]
- 157.Zhang H., Li M., Han Y., Hong L., Gong T., Sun L., Zheng X. Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Dig. Dis. Sci. 2010;55:2545–2551. doi: 10.1007/s10620-009-1051-6. [DOI] [PubMed] [Google Scholar]
- 158.Skinner H.D., Lee J.H., Bhutani M.S., Weston B., Hofstetter W., Komaki R., Shiozaki H., Wadhwa R., Sudo K., Elimova E., Song S., Ye Y., Huang M., Ajani J., Wu X. A validated miRNA profile predicts response to therapy in esophageal adenocarcinoma. Cancer. 2014;120:3635–3641. doi: 10.1002/cncr.28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lindner K., Borchardt C., Schöpp M., Bürgers A., Stock C., Hussey D.J., Haier J., Hummel R. Proton pump inhibitors (PPIs) impact on tumour cell survival, metastatic potential and chemotherapy resistance, and affect expression of resistance-relevant miRNAs in esophageal cancer. J. Exp. Clin. Cancer Res. 2014;33:73. doi: 10.1186/s13046-014-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Imanaka Y., Tsuchiya S., Sato F., Shimada Y., Shimizu K., Tsujimoto G. MicroRNA-141 confers resistance to cisplatin-induced apoptosis by targeting YAP1 in human esophageal squamous cell carcinoma. J. Hum. Genet. 2011;56:270–276. doi: 10.1038/jhg.2011.1. [DOI] [PubMed] [Google Scholar]
- 161.Derouet M.F., Liu G., Darling G.E. MiR-145 expression accelerates esophageal adenocarcinoma progression by enhancing cell invasion and anoikis resistance. PLoS One. 2014;9:e115589. doi: 10.1371/journal.pone.0115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu C., Li M., Hu C., Duan H. Prognostic role of microRNA polymorphisms in patients with advanced esophageal squamous cell carcinoma receiving platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2014;73:335–341. doi: 10.1007/s00280-013-2364-x. [DOI] [PubMed] [Google Scholar]
- 163.Hummel R., Wang T., Watson D.I., Michael M.Z., van der Hoek M., Haier J., Hussey D.J. Chemotherapy-induced modification of microRNA expression in esophageal cancer. Oncol. Rep. 2011;26:1011–1017. doi: 10.3892/or.2011.1381. [DOI] [PubMed] [Google Scholar]
- 164.Hamano R., Miyata H., Yamasaki M., Kuropawa Y., Hara J., Moon J.H., Nakajima K., Takiguchi S., Fujiwara Y., Mori M., Doki Y. Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin. Cancer Res. 2011;17:3029–3038. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- 165.Meng H., Wang K., Chen X., Guan X., Hu L., Xiong G., Li J., Bai Y. MicroRNA-330-3p functions as an oncogene in human esophageal cancer by targeting programmed cell death 4. Am. J. Cancer Res. 2015;5:1062–1075. [PMC free article] [PubMed] [Google Scholar]
- 166.Yamamoto S., Inoue J., Kawano T., Kozaki K., Omura K., Inazawa J. The impact of miRNA-based molecular diagnostics and treatment of NRF2-stabilized tumors. Mol. Cancer Res. 2013;12:58–68. doi: 10.1158/1541-7786.MCR-13-0246-T. [DOI] [PubMed] [Google Scholar]
- 167.Hummel R., Sie C., Watson D.I., Wang T., Ansar A., Michael M.Z., van der Hoek M., Haier J., Hussey D.J. MicroRNA signatures in chemotherapy resistant esophageal cancer cell lines. World J. Gastroenterol. 2014;20:14904–14912. doi: 10.3748/wjg.v20.i40.14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 169.Ali Z., Deng Y., Ma C. Progress of research in gastric cancer. J. Nanosci. Nanotechnol. 2012;12:8241–8248. doi: 10.1166/jnn.2012.6692. [DOI] [PubMed] [Google Scholar]
- 170.Link A., Kupcinskas J., Wex T., Malfertheiner P. Macro-role of microRNA in gastric cancer. Dig. Dis. 2012;30:255–267. doi: 10.1159/000336919. [DOI] [PubMed] [Google Scholar]
- 171.Petrelli F., Zaniboni A., Coinu A., Cabiddu M., Ghilardi M., Sgroi G., Barni S. Cisplatin or not in advanced gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e83022. doi: 10.1371/journal.pone.0083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Du Y., Zhu M., Zhou X., Huang Z., Zhu J., Xu J., Cheng G., Shu Y., Liu P., Zhu W., Wang T. MiR-20a enhances cisplatin resistance of human gastric cancer cell line by targeting NFKBIB. Tumor Biol. 2016;37:1261–1269. doi: 10.1007/s13277-015-3921-1. [DOI] [PubMed] [Google Scholar]
- 173.Li X., Zhang Z., Yu M., Li L., Du G., Xiao W., Yang H. Involvement of miR-20a in promoting gastric cancer progression by targeting early growth response 2 (EGR2) Int. J. Mol. Sci. 2013;14:16226–16239. doi: 10.3390/ijms140816226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang S., Huang C., Li X., Yu M., He Y., Li J. MiR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicol. 2013;306:162–168. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 175.Wang Y., Liu C., Luo M., Zhang Z., Gong J., Li J., You L., Dong L., Su R., Lin H., Ma Y., Wang F., Wang Y., Chen J., Zhang J., Jia H., Kong Y., Yu J. Chemotherapy-induced miRNA-29c/catenin-δ signaling suppresses metastasis in gastric cancer. Cancer Res. 2015;75:1332–1344. doi: 10.1158/0008-5472.CAN-14-0787. [DOI] [PubMed] [Google Scholar]
- 176.Wang L.-L., Zhang X.-H., Chu J.-K. MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT) Eur. Rev. Med. Pharmacol. Sci. 2016;20:1733–1739. [PubMed] [Google Scholar]
- 177.Li G., Yang F., Gu S., Li Z., Xue M. MicroRNA-101 induces apoptosis in cisplatin-resistant gastric cancer cells by targeting VEGF-C. Mol. Med. Rep. 2016;13:572–578. doi: 10.3892/mmr.2015.4560. [DOI] [PubMed] [Google Scholar]
- 178.Zhou X., Su J., Zhu L., Zhang G. Helicobacter pylori modulates cisplatin sensitivity in gastric cancer by down-regulating miR-141 expression. Helicobacter. 2014;19:174–181. doi: 10.1111/hel.12120. [DOI] [PubMed] [Google Scholar]
- 179.Zhou X., Ye F., Yin C., Zhuang Y., Yue G., Zhang G. The interaction between miR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell. Physiol. Biochem. 2015;36:1440–1452. doi: 10.1159/000430309. [DOI] [PubMed] [Google Scholar]
- 180.Chen Y., Zuo J., Liu Y., Gao H., Liu W. Inhibitory effects of miRNA-200c on chemotherapy-resistance and cell proliferation of gastric cancer SGC7901/DDP cells. Chin. J. Cancer. 2010;29:1006–1011. doi: 10.5732/cjc.010.10236. [DOI] [PubMed] [Google Scholar]
- 181.Chang L., Guo F., Wang Y., Lv Y., Huo B., Wang L., Liu W. MicroRNA-200c regulates the sensitivity of chemotherapy of gastric cancer SGC7901/DDP cells by directly targeting RhoE. Pathol. Oncol. Res. 2014;20:93–98. doi: 10.1007/s12253-013-9664-7. [DOI] [PubMed] [Google Scholar]
- 182.Zhang X.-L., Shi H.-J., Wang J.-P., Tang H.-S., Wu Y.-B., Fang Z.-Y., Cui S.-Z., Wang L.-T. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increase chemosensitivity to cisplatin. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i32.11347. 11357–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou X., Jin W., Jia H., Yan J., Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J. Exp. Clin. Cancer Res. 2015;34:28. doi: 10.1186/s13046-015-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xia J., Chen L., Jian W., Wang K.-B., Yang Y., He W., He Y., Chen D., Li W. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J. Transl. Med. 2014;12:33. doi: 10.1186/1479-5876-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou N., Qu Y., Xu C., Tang Y. Upregulation of microRNA-375 increases the cisplatin-sensitivity of human gastric cancer cells by regulation ERBB2. Exp. Ther. Med. 2016;11:625–630. doi: 10.3892/etm.2015.2920. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 186.Ma J., Liu J., Wang Z., Gu X., Fan Y., Zhang W., Xu L., Zhang J., Cai D. NF-kappaB-dependent microRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction. Mol. Cancer. 2014;13:40. doi: 10.1186/1476-4598-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu J., Fang Y., Cao Y., Qin R., Chen Q. MiR-449a regulates proliferation and chemosensitivity to cisplatin by targeting cyclin D1 and BCL2 in SGC7901 cells. Dig. Dis. Sci. 2014;59:336–345. doi: 10.1007/s10620-013-2923-3. [DOI] [PubMed] [Google Scholar]
- 188.Wang T., Ge G., Ding Y., Zhou X., Huang Z., Zhu W., Shu Y., Liu P. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin. Med. J. 2014;127:2357–2362. [PubMed] [Google Scholar]
- 189.Huo Z., Chu Z., Hu J., Song B., Lu S. Effects of miRNA765 overexpression on drug resistance of drug-resistant gastric carcinoma cell line BGC-823/CDDP. Shanghai Jiaot. Daxue Xuebao. 2012;32:1581–1586. [Google Scholar]
- 190.Yang M., Shan X., Zhou X., Qiu T., Zhu W., Ding Y., Shu Y., Liu P. MiR1271 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGFR1, IRS1, mTOR and BCL2. Anti-Cancer Agents Med. Chem. 2014;14:884–891. doi: 10.2174/1871520614666140528161318. [DOI] [PubMed] [Google Scholar]
- 191.Kim C.H., Kim H.K., Rettig R.L., Kim J., Lee E.T., Aprelikova O., Choi I.J., Munroe D.J., Green J.E. MiRNA signature associated with outcome of gastric cancer patients following chemotherapy. BMC Med. Genom. 2011;4:79. doi: 10.1186/1755-8794-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhao J., Nie Y., Wang H., Lin Y. MiR-181a suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Gene. 2016;576:828–833. doi: 10.1016/j.gene.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 193.Duffy A., Greten T. Developing better treatments in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2010;4:551–560. doi: 10.1586/egh.10.58. [DOI] [PubMed] [Google Scholar]
- 194.Koul H., Huh J.-S., Rove K.O., Crompton L., Koul S., Meacham R.B., Kim F.J. Molecular aspects of renal cell carcinoma: a review. Am. J. Cancer Res. 2011;1:240–254. [PMC free article] [PubMed] [Google Scholar]
- 195.El-Halawany M.S., Ismail H.M., Zeeneldin A.A., Elfiky A., Tantawy M., Kobaisi M.H., Hamed I., Abdel Wahab A.H.A. Investigating the pretreatment miRNA expression patterns of advanced hepatocellular carcinoma patients in association with response to TACE treatment. Biomed. Res. Int. 2015;2015:649750. doi: 10.1155/2015/649750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mu W., Hu C., Zhang H., Qu Z., Cen J., Qiu Z., Li C., Ren H., Li Y., He X., Shi X., Hui L. MiR-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res. 2015;25:477–495. doi: 10.1038/cr.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tan G., Wu L., Tan J., Zhang B., Tai W.C., Xiong S., Chen W., Yang J., Li H. MiR-1180 promotes apoptotic resistance to human hepatocellular carcinoma via activation of NF-κB signaling pathway. Sci. Rep. 2016;6:22328. doi: 10.1038/srep22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miwa M., Asahiro M., Teppei S., Akiko K., Kiyohito K., Tomoko N., Yuka T., Koji F., Emiko M., Takako N., Joji T., Hisaaki M., Hirohito Y., Hideki K., Shintaro F., Noriko N., Mitsuomi H., Tsutomi M., Hisakazu I., Takashi H. MicroRNA profiles in cisplatin-induced apoptosis of hepatocellular carcinoma cells. Int. J. Oncol. 2015;47:535–542. doi: 10.3892/ijo.2015.3036. [DOI] [PubMed] [Google Scholar]
- 199.Ma J., Wang T., Guo R., Yang X., Yin J., Yu J., Xiang Q., Pan X., Zu X., Peng C., Tang H., Lei X. MicroRNA-133a and microRNA-326 co-contribute to hepatocellular carcinoma 5-fluorouracil and cisplatin sensitivity by directly targeting B-cell lymphoma-extra large. Mol. Med. Rep. 2015;12:6235–6240. doi: 10.3892/mmr.2015.4134. [DOI] [PubMed] [Google Scholar]
- 200.Qin J., Luo M., Qian H., Chen W. Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene. 2014;538:342–347. doi: 10.1016/j.gene.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 201.Yu G., Yao W., Gumireddy K., Li A., Wang J., Xiao W., Chen K., Xiao H., Li H., Tang K., Ye Z., Huang Q., Xu H. Pseudogene PTENP1 functions as a competing endogenous RNA to suppress clear-cell renal cell carcinoma progression. Mol. Cancer Ther. 2014;13:3086–3097. doi: 10.1158/1535-7163.MCT-14-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nordentoft I., Birkenkamp-Demtroder K., Agerbaek M., Theodorescu D., Ostenfeld M.S., Hartmann A., Borre M., Orntoft T.F., Dyrskjot L. MiRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med. Genom. 2012;5:40. doi: 10.1186/1755-8794-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Drayton R.M., Dudziec E., Peter S., Bertz S., Hartmann A., Bryant H.E., Catto J.W.F. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin. Cancer Res. 2014;20:1990–2000. doi: 10.1158/1078-0432.CCR-13-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li H., Yu G., Shi R., Lang B., Chen X., Xia D., Xiao H., Guo X., Guan W., Ye Z., Xiao W., Xu H. Cisplatin-induced epigenetic activation of miR-34a sensitizes bladder cancer cells to chemotherapy. Mol. Cancer. 2014;13:183. doi: 10.1186/1476-4598-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bu Q., Fang Y., Cao Y., Chen Q., Liu Y. Enforced expression of miR-101 enhances cisplatin sensitivity in human bladder cancer cells by modulating the cyclooxygenase-2 pathway. Mol. Med. Rep. 2014;10:2203–2209. doi: 10.3892/mmr.2014.2455. [DOI] [PubMed] [Google Scholar]
- 206.Zhou J., Dai W., Song J. MiR-1182 inhibits growth and mediates the chemosensitivity of bladder cancer by targeting hTERT. Biochem. Biophys. Res. Commun. 2016;470:445–452. doi: 10.1016/j.bbrc.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 207.Lei Y., Hu X., Li B., Peng M., Tong S., Zu X., Wang Z., Qi L., Chen M. MiR-150 modulates cisplatin chemosensitivity and invasiveness of muscle-invasive bladder cancer cells via targeting PDCD4 in vitro. Med. Sci. Monit. 2014;20:1850–1857. doi: 10.12659/MSM.891340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chung K.Y., Saltz L.B. Adjuvant therapy of colon cancer: current status and future directions. Cancer J. 2007;13:192–197. doi: 10.1097/PPO.0b013e318074d26e. [DOI] [PubMed] [Google Scholar]
- 209.Liu Y., Gao S., Chen X., Liu M., Mao C., Fang X. Overexpression of miR-203 sensitizes paclitaxel(Taxol)-resistant colorectal cancer cells through targeting the salt-inducible kinase 2 (SIK2) Tumor Biol. 2016 doi: 10.1007/s13277-016-5066-2. [DOI] [PubMed] [Google Scholar]
- 210.Liu L., Chen L, Wu X., Li X., Song Y., Mei Q., Nie J., Han W. Low-dose DANN-demethylating agent enhances the chemosensitivity of cancer cells by targeting cancer stem cells via the upregulation of microRNA-497. J. Cancer Res. Clin. Oncol. 2016;142:1431–1439. doi: 10.1007/s00432-016-2157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Arbyn M., Castellsagué X., de Sanjosé S., Bruni L., Saraiya M., Bray F., Ferlay J. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 212.Fu Y., Reagan J. Major Problems in Pathology. W.B. Saunders and Co; Philadelphia: 1989. Pathology of the uterine cervix, vagina and vulva; pp. 288–335. [Google Scholar]
- 213.Sun H., Xin J., Lu Z., Wang N., Liu N., Guo Q. Potential molecular mechanisms for improved prognosis and outcome with neoadjuvant chemotherapy prior to laparoscopical radical hysterectomy for patients with cervical cancer. Cell. Physiol. Biochem. 2013;32:1528–1540. doi: 10.1159/000356590. [DOI] [PubMed] [Google Scholar]
- 214.Zou Z., Wu L., Ding H., Wang Y., Zhang Y., Chen X., Chen X., Zhang C.-Y., Zhang Q., Zen K. MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing Beclin-1-mediated autophagy. J. Biol. Chem. 2012;287:4148–4156. doi: 10.1074/jbc.M111.307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fan Z., Cui H., Yu H., Ji Q., Kang L., Han B., Wang J., Dong Q., Li Y., Yan Z., Yan X., Zhang X., Lin Z., Hu Y., Jiao S. MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogenesis. 2016;5:e197. doi: 10.1038/oncsis.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li X., Chen C., Wang F., Ding X. MicroRNA-124 and microRNA-520b inhibit proliferation through downregulating Eps8 in Hela cells. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao. 2013;29:1145–1152. [Google Scholar]
- 217.Wen S.-Y., Lin Y., Yu Y.-Q., Cao S.-J., Zhang R., Yang X.-M., Li J., Zhang Y.-L., Wang Y.-H., Ma M.-Z., Sun W.-W., Lou X.-L., Wang J.-H., Teng Y.-C., Zhang Z.-G. MiR-506 acts as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2015;34:717–725. doi: 10.1038/onc.2014.9. [DOI] [PubMed] [Google Scholar]
- 218.Chen Y., Ke G., Han D., Liang S., Yang G., Wu X. MicroRNA-181a enhances the chemoresistance of human cervical squamous cell carcinoma to cisplatin by targeting PRKCD. Exp. Cell Res. 2014;320:12–20. doi: 10.1016/j.yexcr.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 219.Lee J.-W., Choi C.H., Choi J.-J., Park Y.-A., Kim S.-J., Hwang S.Y., Kim W.Y., Kim T.-J., Lee J.-H., Kim B.-G., Bae D.-S. Altered microRNA expression in cervical carcinomas. Clin. Cancer Res. 2008;14:2535–2542. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 220.Jian X., Zhang Y., Dai Y., Mo W., Lu H. Elevated miR-106b/miR-93 contribute to cisplatin resistance in cervical cancer. Fudan Xuebao Ziran Kexueban. 2014;53:689–696. [Google Scholar]
- 221.Yan B., Guo Q., Nan X., Wang Z., Yin Z., Yi L., Wie Y., Gao Y., Zhou K., Yang J. Micro-ribonucleic acid 29b inhibits cell proliferation and invasion and enhances cell apoptosis and chemotherapy effects of cisplatin via targeting of DNMT3b and AKT3 in prostate cancer. OncoTargets Ther. 2015;8:557–565. doi: 10.2147/OTT.S76484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bhatnagar N., Li X., Padi S.K.R., Zhang Q., Tang M., Guo B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1:e105. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pennati M., Lopergolo A., Profumo V., De Cesare M., Sbarra S., Valdagni R., Zaffaroni N., Gandellini P., Folini M. MiR-205 impairs the autophagic flux and enhances cisplatin cytotoxicity in castration-resistant prostate cancer cells. Biochem. Pharmacol. 2014;87:579–597. doi: 10.1016/j.bcp.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 224.Shi X.-B., Xue L., Ma A.-H., Tepper C.G., Kung H.-J., deVere White R.W. MiR-125b promotes growth of prostate cancer xenograft tumor through targeting pro-apoptotic genes. Prostate. 2011;71:538–549. doi: 10.1002/pros.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu L., Lian J., Zhang H., Tan H., Liang M., Yin M., Sun F. MicroRNA-302a sensitizes testicular embryonal carcinoma cells to cisplatin-induced cell death. J. Cell. Physiol. 2013;228:2294–2304. doi: 10.1002/jcp.24394. [DOI] [PubMed] [Google Scholar]
- 226.Huang H., Tian H., Duan Z., Cao Y., Zhang X.-S., Sun F. MicroRNA-383 impairs phosphorylation of H2AX by targeting PNUTS and inducing cell cycle arrest in testicular embryonal carcinoma cells. Cell. Signal. 2014;26:903–911. doi: 10.1016/j.cellsig.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 227.Gao Y., Liu T., Huang Y. MicroRNA-134 suppresses endometrial cancer stem cell by targeting POGLUT1 and Notch pathway proteins. FEBS Lett. 2015;589:207–214. doi: 10.1016/j.febslet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 228.Lee T.S., Jeon H.W., Kim Y.B., Kim Y.A., Kim M.A., Kang S.B. Aberrant microRNA expression in endometrial carcinoma using formalin-fixed paraffin-embedded (FFPE) tissues. PLoS One. 2013;8:e81421. doi: 10.1371/journal.pone.0081421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee J.-W., Park Y.-A., Choi J.-J., Lee Y.-Y., Kim C.-J., Choi C.H., Kim T.-J., Lee N.W., Kim B.-G., Bae D.-S. Gynecol. Oncol. 2011;120:56–62. doi: 10.1016/j.ygyno.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 230.Wu Y., Xiao Y., Ding X., Zhuo Y., Ren P., Zhou C., Zhou J. A miR-200b/200c/429-binding site polymorphism in the 3′ untranslated region of the AP-2α gene is associated with cisplatin resistance. PLoS One. 2011;6:e29043. doi: 10.1371/journal.pone.0029043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Weeraratne S.D., Amani V., Neiss A., Teider N., Scott D.K., Pomeroy S.L., Cho Y.-J. MiR-34a confers chemosensitivity through modulation of MAGE-A and p53 in medulloblastoma. Neuro-Oncol. 2011;13:165–175. doi: 10.1093/neuonc/noq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Creevey L., Ryan J., Harvey H., Bray I.M., Meehan M., Khan A.R., Stallings R.L. MicroRNA-497 increases apoptosis in MYCN amplified neuroblastoma cells by targeting the key cell cycle regulator WEE1. Mol. Cancer. 2013;12:23. doi: 10.1186/1476-4598-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Harvey H., Piskareva O., Creevey L., Alcock L.C., Buckley P.G., O'Sullivan M.J., Segura M.F., Gallego S., Stallings R.L., Bray I.M. Modulation of chemotherapeutic drug resistance in neuroblastoma SK-N-AS cells by the neural apoptosis inhibitory protein and miR-520f. Int. J. Cancer. 2015;136:1579–1588. doi: 10.1002/ijc.29144. [DOI] [PubMed] [Google Scholar]
- 234.Chen W., Yang Y., Chen B., Lu P., Zhan L., Yu Q., Cao K., Li Q. MiR-136 targets E2F1 to reverse cisplatin chemosensitivity in glioma cells. J. Neurooncol. 2014;120:43–53. doi: 10.1007/s11060-014-1535-x. [DOI] [PubMed] [Google Scholar]
- 235.Schreiber R., Mezencev R., Matyunina L.V., McDonald J.F. Evidence for the role of microRNA 374b in acquired cisplatin resistance in pancreatic cancer cells. Cancer Gene Ther. 2016 doi: 10.1038/cgt.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ji Q., Hao X., Zhang M., Tang W., Meng Y., Li L., Xiang D., DeSano J.T., Bommer G.T., Fan D., Fearon E.R., Lawrence T.S., Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhan M., Zhao X., Wang H., Chen W., Xu S., Wang W., Shen H., Huang S., Wang J. MiR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumor Biol. 2016 doi: 10.1007/s13277-016-4957-6. [DOI] [PubMed] [Google Scholar]
- 238.Zhang Y., Yang W.Q., Zhu H., Qian Y.Y., Zhou L., Ren Y.J., Ren X.C., Zhang L., Liu X.P., Liu C.G., Ming Z.J., Li B., Chen B., Wang J.R., Liu Y.B., Yang J.M. Regulation of autophagy by miR-30d impacts sensitivity of anaplastic thyroid carcinoma to cisplatin. Biochem. Pharmacol. 2014;87:562–570. doi: 10.1016/j.bcp.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fujiwara T., Katsuda T., Hagiwara K., Kosaka N., Yoshioka Y., Takahashi R.-U., Takeshita F., Kubota D., Kondo T., Ichikawa H., Yoshida A., Kobayashi E., Kawai A., Ozaki T., Ochiya T. Clinical relevance and therapeutic significance of microRNA-133a expression profiles and functions in malignant osteosarcoma-initiating cells. Stem Cells. 2014;32:959–973. doi: 10.1002/stem.1618. [DOI] [PubMed] [Google Scholar]
- 240.Huang J.W., Wang Y., Dhillon K.K., Calses P., Villegas E., Mitchell P.S., Tewari M., Kemp C.J., Taniguchi T. Systematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivity. Mol. Cancer Res. 2013;11:1564–1573. doi: 10.1158/1541-7786.MCR-13-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang Y., Huang J.-W., Li M., Cavenee W.K., Mitchell P.S., Zhou X., Tewari M., Furnari F.B., Taniguchi T. MicroRNA-138 modulates DNA damage response by repressing histone H2AX expression. Mol. Cancer Res. 2011;9:1100–1111. doi: 10.1158/1541-7786.MCR-11-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xu S., Xue C., Li J., Bi Y., Cao Y. Marek's Disease Virus Type 1 microRNA miR-M3 suppresses cisplatin-induced apoptosis by targeting SMAD2 of the transforming growth factor beta signal pathway. J. Virol. 2011;85:276–285. doi: 10.1128/JVI.01392-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Petrillchini M., Zannoni G.F., Beltrame L., Martinelli E., DiFeo A., Paracchini L., Craparotta I., Mannarino L., Vizzielli G., Scambia G., D'Incalci M., Romualdi C., Marchini S. Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: a retrospective longitudinal analysis using matched tumor biopsies. Ann. Oncol. 2016;27:625–634. doi: 10.1093/annonc/mdw007. [DOI] [PubMed] [Google Scholar]
- 244.Benson E.A., Skaar T.C., Liu Y., Nephew K.P., Matei D. Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating miRNAs concentrations: a pilot study. PLoS One. 2015;10:e0141279. doi: 10.1371/journal.pone.0141279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ziliak D., Gamazon E.R., LaCroix B., Im H.K., Wen Y., Huang R.S. Genetic variation that predicts platinum sensitivity reveals the role of miR-193b* in chemotherapeutic susceptibility. Mol. Cancer Ther. 2012;11:2054–2061. doi: 10.1158/1535-7163.MCT-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Brozovic A., Duran G.E., Wang Y.C., Francisco E.B., Sikic B.I. The miR-200 family differentially regulates sensitivity to paclitaxel and carboplatin in human ovarian carcinoma OVCAR-3 and MES-OV cells. Mol. Oncol. 2015;9:1678–1693. doi: 10.1016/j.molonc.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Leskelä S., Leandro-García L.J., Mendiola M., Barriuso J., Inglada-Pérez L., Munoz I., Martínez-Delgado B., Redondo A., de Santiago J., Robledo M., Hardisson D., Rodríguez-Antona C. The miR-200 family controls β-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocrine-Rel. Cancer. 2011;18:85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 248.Kuhlmann J.D., Baraniskin A., Hahn S.A., Mosel F., Bredeschneider M., Wimberger P., Kimmig R., Kasimir-Bauer S. Circulating U2 small nuclear RNA fragments as a novel diagnostic tool for patients with epithelial ovarian cancer. Clin. Chem. 2014;60:206–213. doi: 10.1373/clinchem.2013.213066. [DOI] [PubMed] [Google Scholar]
- 249.Frederick P.J., Green H.N., Huang J.S., Egger M.E., Friboes H.B., Grizzle W.E., McNally L.R. Chemoresistance in ovarian cancer linked to expression of microRNAs. Biotech. Histochem. 2013;88:403–409. doi: 10.3109/10520295.2013.788736. [DOI] [PubMed] [Google Scholar]
- 250.Lu L., Schwartz P., Scarampi L., Rutherford T., Canuto E.M., Yu H., Katsaros D. MicroRNA let-7a: a potential marker for selection of paclitaxel in ovarian cancer management. Gynecol. Oncol. 2011;122:366–371. doi: 10.1016/j.ygyno.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 251.Wu C., Cao Y., He Z., He J., Hu C., Duan H., Jiang J. Serum levels of miR-19b and miR-146a as prognostic biomarkers for non-small cell lung cancer. Tohoku J. Exp. Med. 2014;232:85–95. doi: 10.1620/tjem.232.85. [DOI] [PubMed] [Google Scholar]
- 252.Wei J., Gao W., Zhu C.-J., Liu Y.-Q., Mei Z., Cheng T., Shu Y.-Q. Identification of plasma microRNA-21 as a biomarker for early detection and chemosensitivity of non-small cell lung cancer. Chin. J. Cancer. 2011;30:407–414. doi: 10.5732/cjc.010.10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Saito M., Shiraishi K., Matsumoto K., Schetter A.J., Ogata-Kawata H., Tsuchiya N., Kunitoh H., Nokihara H., Watanabe S., Tsuta K., Kumamoto K., Takenoshita S., Yokota J., Harris C.C., Kohno T. A three-microRNA signature predicts responses to platinum-based doublet chemotherapy in patients with lung adenocarcinoma. Clin. Cancer Res. 2014;20:4784–4793. doi: 10.1158/1078-0432.CCR-14-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zarogoulidis P., Petanidis S., Kioseoglou E., Domvri K., Anestakis D., Zarogoulidis K. MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and survivin in lung cancer cells. Cell. Signal. 2015;27:1576–1588. doi: 10.1016/j.cellsig.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 255.Zhuo L., Liu J., Wang B., Gao M., Huang A. Differential miRNA expression profiles in hepatocellular carcinoma cells and drug-resistant sublines. Oncol. Rep. 2013;29:555–562. doi: 10.3892/or.2012.2155. [DOI] [PubMed] [Google Scholar]
- 256.Xue J., Chi Y., Chen Y., Huang S., Ye X., Niu J., Wang W., Pfeffer L.M., Shao Z., Wu Z.-H., Wu J. MiRNA-621 sensitizes breast cancer to chemotherapy by suppressing FBXO11 and enhancing p53 activity. Oncogene. 2016;35:448–458. doi: 10.1038/onc.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dong R., Qiu H., Du G., Wang Y., Yu J., Mao C. Restoration of microRNA-218 increases cellular chemosensitivity to cervical cancer by inhibiting cell-cycle progression. Mol. Med. Rep. 2014;10:3289–3295. doi: 10.3892/mmr.2014.2622. [DOI] [PubMed] [Google Scholar]
- 258.Liu K., Huang J., Xie M., Yu Y., Zhu S., Kang R., Cao L., Tang D. MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy. 2014;10:442–452. doi: 10.4161/auto.27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhou J., Zhou Y., Yin B., Hao W., Zhao L., Ju W., Bai C. 5-Fluorouracil and oxaliplatin modify the expression profiles of microRNAs in human colon cancer cells in vitro. Oncol. Rep. 2010;23:121–128. [PubMed] [Google Scholar]
- 260.Zhou Y., Wan G., Spizzo R., Ivan C., Marthur R., Hu X., Ye X., Lu J., Fan F., Xia L., CAlin G.A., Ellis L.M., Lu X. MiR-203 induces oxaliplatin resistance in colorectal cancer by negatively regulating ATM kinase. Mol. Oncol. 2014;8:83–92. doi: 10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang L., Pickard K., Jenei V., Bullock M.D., Bruce A., Mitter R., Kelly G., Paraskeva C., Strefford J., Primrose J., Thomas G.J., Packham G., Mirnezami A.H. MiR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res. 2013;73:6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chai H., Liu M., Tian R., Li X., Tang H. MiR-20a targets BNIP2 and contributes chemotherapeutic resistance in colorectal adenocarcinoma SW480 and SW620 cell lines. Acta Biochim. Biophys. Sin. 2011;43:217–225. doi: 10.1093/abbs/gmq125. [DOI] [PubMed] [Google Scholar]
- 263.Bullock M.D., Pickard K.M., Nielsen B.S., Sayan A.E., Jenei V., Mellone M., Mitter R., Primrose J.N., Thomas G.J., Packham G.K., Mirnezami A.H. Cell Death Dis. 2013;4:e684. doi: 10.1038/cddis.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nangia-Makker P., Yu Y., Vasudevan A., Farhana L., Rajendra S.G., Levi E., Majumdar A.P.N. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014;9:e84369. doi: 10.1371/journal.pone.0084369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kalimutho M., Minutolo A., Grelli S., Formosa A., Sancesario G., Valentini A., Federici G., Bernardini S. Satraplatin (JM-216) mediates G2/M cell cycle arrest and potentiates apoptosis via multiple death pathways in colorectal cancer cells thus overcoming platinum chemo-resistance. Cancer Chemother. Pharmacol. 2011;67:1299–1312. doi: 10.1007/s00280-010-1428-4. [DOI] [PubMed] [Google Scholar]
- 266.Kiyonari S., Iimori M., Matsuoka K., Watanabe S., Morikawa-Ichinose T., Miura D., Niimi S., Saeki H., Tokunaga E., Oki E., Morita M., Kadomatsu K., Maehara Y., Kitao H. The 1,2-diaminocyclohexane carrier ligand in oxaliplatin induces p53-dependent transcriptional repression of factors involved in thymidylate biosynthesis. Mol. Cancer Ther. 2015;14:2332–2342. doi: 10.1158/1535-7163.MCT-14-0748. [DOI] [PubMed] [Google Scholar]
- 267.Pang M., Wu G., Hou X., Hou N., Liang L., Jia G., Shuai P., Luo B., Wang K., Li G. LIN28B promotes colon cancer migration and recurrence. PLoS One. 2014;9:e109169. doi: 10.1371/journal.pone.0109169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dong Y., Zhao J., Wu C.-W., Zhang L., Liu X., Kang W., Leung W.-W., Zhang N., Chan F.K.L., Sung J.J.Y., Ng S.S.M., Yu J. Tumor suppressor functions of miR-133a in colorectal cancer. Mol. Cancer Res. 2013;11:1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 269.Li Q., Liang X., Wang Y., Meng X., Xu Y., Cai S., Wang Z., Liu J., Cai G. MiR-139-5p inhibits the epithelial-mesenchymal transition and enhances the chemotherapeutic sensitivity of colorectal cancer cells by downregulating BCL2. Sci. Rep. 2016;6:27157. doi: 10.1038/srep27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xu K., Liang X., Shen K., Cui D., Zheng Y., Xu J., Fan Z., Qiu Y., Li Q., Ni L., Liu J. MiR-297 modulates multidrug resistance in human colorectal carcinoma by down-regulating MRP-2. Biochem. J. 2012;446:291–300. doi: 10.1042/BJ20120386. [DOI] [PubMed] [Google Scholar]
- 271.Xu K., Liang X., Cui D., Wu Y., Shi W., Liu J. MiR-1915 inhibits Bcl-2 to modulate multidrug resistance by increasing drug-sensitivity in human colorectal carcinoma cells. Mol. Carcinog. 2013;52:70–78. doi: 10.1002/mc.21832. [DOI] [PubMed] [Google Scholar]
- 272.Hu J., Cai G., Cai S. The plasma microRNA miR-1914* and -1915 suppresses chemoresistant in colorectal cancer patients by down-regulating NFIX. Curr. Mol. Med. 2016;16:70–82. doi: 10.2174/1566524016666151222144656. [DOI] [PubMed] [Google Scholar]
- 273.Della Vitoria Scarpati G., Falcetta F., Carlomagno C., Ubezia P., Marchini S., De Stefano A., Singh V.K., S'Incalci M., De Placido S., Pepe S. A specific miRNA signature correlates with complete pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1113–1119. doi: 10.1016/j.ijrobp.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 274.Hansen T.F., Sorensen F.B., Lindebjerg J., Jakobsen A. The predictive value of microRNA-126 in relation to first line treatment with capecitabine and oxaliplatin in patients with metastatic colorectal cancer. BMC Cancer. 2012;12:83. doi: 10.1186/1471-2407-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Molina-Pinelo S., Carnero A., Rivera F., Estevez-Garcia P., Bozada J.M., Limon M.L., Benavent M., Gomez J., Pastor M.D., Chaves M., Suarez R., Paz-Ares L., de la Portilla F., Carranza-Carranza A., Sevilla I., Vicioso L., Garcia-Carbonero R. MiR-107 and miR-99a-3p predict chemotherapy response in patients with advanced colorectal cancer. BMC Cancer. 2014;14:656. doi: 10.1186/1471-2407-14-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Takahashi M., Cuatrecasas M., Balaguer F., Hur K., Toiyama Y., Castells A., Boland C.R., Goel A. The clinical significance of miR-148a as a predictive biomarker in patients with advanced colorectal cancer. PLoS One. 2012;7:e46684. doi: 10.1371/journal.pone.0046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mekenkamp L.J., Tol J., Dijkstra J.R., de Krijger I., Vink-Borger M.E., van Sliet S., Teerenstra S., Kamping E., Verwiel E., Koopman M., Meijer G.A., Han J., van Krieken J.M., Kuiper R., Punt C.J.A., Nagtegaal I.D. Beyond KRAS mutation status: influence of KRAS copy number status and microRNAs on clinical outcome to cetuximab in metastatic colorectal cancer patients. BMC Cancer. 2012;12:292. doi: 10.1186/1471-2407-12-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Boisen M.K., Dehlendorff C., Linnemann D., Nielsen B.S., Larsen J.S., Osterlind K., Nielsen S.E., Tarpqaard L.S., Qvortrup C., Pfeiffer P., Holländer N.H., Keldsen N., Hansen T.F., Jensen B.B., Hogdal E.V., Jensen B.V., Johansen J.S. Tissue microRNAs as predictors of outcome in patients with metastatic colorectal cancer treated with first-line capecitabine and oxaliplatin with or without bevacizumab. PLoS One. 2014;9:e109430. doi: 10.1371/journal.pone.0109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhang J., Zhang K., Bi M., Jiao X., Zhang D., Dong Q. Circulating microRNA expressions in colorectal cancer as predictors of response to chemotherapy. Anticancer Drugs. 2014;25:346–352. doi: 10.1097/CAD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 280.Pagliuca A., Valvo C., Fabrizi E., di Martino S., Biffoni M., Runci D., Forte S., de Maria R., Ricci-Vitani L. Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene. 2013;32:4806–4813. doi: 10.1038/onc.2012.495. [DOI] [PubMed] [Google Scholar]
- 281.Kjersem J.B., Ikdahl T., Lingjaerde O.C., Guren T., Tveit K.M., Kure E.H. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol. Oncol. 2014;8:59–67. doi: 10.1016/j.molonc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Perez-Carbonell L., Sinicrope F.A., Alberts S.R., Oberg A.L., Balaguer F., Castells A., Boland C.R., Goel A. MiR-320e is a novel prognostic biomarker in colorectal cancer. Br. J. Cancer. 2015;113:83–90. doi: 10.1038/bjc.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rasmussen M.H., Jensen N.F., Tarpgaard L.S., Qvortrup C., Romer M.U., Stenvang J., Hansen T.P., Christensen L.L., Lindebjerg J., Hansen F., Jensen B.V., Hansen T.F., Pfeiffer P., Brünner N., Orntoft T.F., Andersen C.L. High expression of micro-RNA-625-3p is associated with poor response to first-line oxaliplatin based treatment of metastatic colorectal cancer. Mol. Oncol. 2013;7:637–646. doi: 10.1016/j.molonc.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jiang J., Zheng X., Xu X., Zhou Q., Yan H., Zhang X., Lu B., Wu C., Ju J. Prognostic significance of miR-181b and miR-21 in gastric cancer patients treated with S-1/oxaliplatin or doxifluridine/oxaliplatin. PLoS One. 2011;6:e23271. doi: 10.1371/journal.pone.0023271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Huang D., Wang H., Liu R., Li H., Ge S., Bai M., Deng T., Yao G., Ba Y. MiRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J. Cell. Biochem. 2014;115:549–556. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 286.Danza K., Silvestris N., Simone G., Signorile M., Saragoni L., Brunetti O., Monti M., Mazzotta A., de Summa S., Mangia A., Tommasi S. Role of miR-27a, miR-181a and miR-20b in gastric cancer hypoxia-induced chemoresistance. Cancer Biol. Ther. 2016;17:400–406. doi: 10.1080/15384047.2016.1139244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu J., Shi W., Xu B., Zheng X., Eang Q., Lu B., Ju J., Wu C., Jiang J. Expression and clinical significance of microRNA-129 in advanced gastric cancer. Zhonghua Shiyan Waike Zazhi. 2014;31:495–498. [Google Scholar]
- 288.Smid D., Kubackova D., Dolezal J., Treska V., Skalicky T., Kulda V., Srbecka K., Daum O., Kucera R., Topolcan O., Pesta M. Tissue microRNAs as predictive markers for gastric cancer patients undergoing palliative chemotherapy. Int. J. Oncol. 2016;48:2693–2703. doi: 10.3892/ijo.2016.3484. [DOI] [PubMed] [Google Scholar]
- 289.Yu G., Wang J., Xu K., Dong J. Dynamic regulation of uncoupling protein 2 expression by microRNA-214 in hepatocellular carcinoma. Biosci. Rep. 2016;36:e00335. doi: 10.1042/BSR20160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yang T., Zheng Z.-M., Zhang X.-B., Li Z.-F., Zhang G.-S. MiR-93 renders the drug resistance of hepatoma cells to oxaliplatin through targeting PTEN. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao. 2012;28:926–934. [Google Scholar]
- 291.Ali S., Almhanna K., Chen W., Philip P.A., Sarkar F.H. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am. J. Transl. Res. 2011;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 292.Schmidt M.F. Drug target miRNAs: chances and challenges. Trends Biotechnol. 2014;32:578–585. doi: 10.1016/j.tibtech.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 293.Biersack B. Non-coding RNA/microRNA-modulatory dietary factors and natural products for improved cancer therapy and prevention: alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins. Non-coding RNA Res. 2016 doi: 10.1016/j.ncrna.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


