Abstract
The epigenetic regulation of cancer cells by small non-coding RNA molecules, the microRNAs (miRNAs), has raised particular interest in the field of oncology. These miRNAs play crucial roles concerning pathogenic properties of cancer cells and the sensitivity of cancer cells towards anticancer drugs. Certain miRNAs are responsible for an enhanced activity of drugs, while others lead to the formation of tumor resistance. In addition, miRNAs regulate survival and proliferation of cancer cells, in particular of cancer stem-like cells (CSCs), that are especially drug-resistant and, thus, cause tumor relapse in many cases. Various small molecule compounds were discovered that target miRNAs that are known to modulate tumor aggressiveness and drug resistance. This review comprises the effects of naturally occurring small molecules (phenolic compounds and terpenoids) on miRNAs involved in cancer diseases.
Keywords: MicroRNA, Polyphenols, Terpenes, Anticancer drugs
Abbreviations: 18-AGA, 18α-glycyrrhetinic acid; AKBA, 3-acetyl-11-keto-β-boswellic acid; CAPE, caffeic acid phenethyl ester; CDODA-Me, methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate; 3,6-DHF, 3,6-dihydroxyflavone; 1,25-D, 1,25-dihydroxyvitamin D3; Dox, doxorubicin; EGCG, (−)-epigallocatechin-3-O-gallate; PEG, polyethylene glycol; PPAP, polycyclic polyprenylated acylphloroglucinol; RA, retinoic acid; ROS, reactive oxygen species; TQ, thymoquinone
1. Introduction
The investigation of the role of microRNAs (miRNAs), short and highly conserved non-coding RNAs of 22-23 nucleotides, for various diseases has risen distinctly over the last years. At the moment, there are more than two thousand miRNAs that regulate about one-third of all human genes [1]. MiRNAs are involved in the regulation of processes such as proliferation, apoptosis, and differentiation of cells [2]. Concerning cancer, miRNAs can exert either tumor suppressor or oncogenic activities [1]. Characteristic patterns of miRNA expression were observed from certain tumor tissues and, thus, miRNAs can also feature valuable diagnostic and prognostic factors [3], [4], [5], [6], [7]. In addition, miRNAs play a crucial role for the formation and survival of cancer stem-like cells (CSCs) [8], [9]. Epithelial-to-mesenchymal transition (EMT) of cancer cells is regulated by miRNAs which, thus, play a crucial role for the formation of metastases [10], [11].
Mature miRNAs bind to target messenger RNAs (mRNAs) and suppress their translation [12]. The biosynthesis of miRNAs generally starts with the transcription of miRNA genes by RNA polymerase II thus generating precursor miRNAs (pri-mRNAs) of several kilobases [13]. Then, the microprocessor complex including the RNase III enzyme Drosha and DGCR8 processes these pri-mRNAs into pre-mRNAs of 65-70 nucleotides that form hairpin structures in the nucleus [13], [14]. These pre-miRNAs are transported from the nucleus to the cytoplasm via the transporter Exportin-5 in a Ran-GTPase dependent way [15], [16], [17]. In the cytoplasm, the pre-miRNAs are processed by the Dicer-TRBP (tar binding protein) complex leading to double-stranded RNAs which comprise the mature miRNA and its complementary strand [1], [15], [16], [17]. After separation from the complementary strand, the mature miRNA binds to the AGO2 (Argonaut 2) protein which recruits further proteins forming miRISC (miRNA-induced silencing complex) [18]. MiRISC is able to bind to target mRNAs leading either to a block of translation or to the degradation of the target mRNAs by miRISC. The seed region of the miRNA (nucleotides 2-7 of the 5′-end of the miRNA) binds to the complementary 3′-untranslated regions (UTR) of target mRNAs leading to inhibition of mRNA translation [9], [19], [20]. Aside negative regulation of mRNA translation, certain miRNAs were also found to promote mRNA translation by recruitment of proteins to the AU-rich elements of mRNA or by interference with translation inhibiting proteins [21], [22].
It was observed that single miRNAs can target various genes while dysregulation of tumor suppressor or oncogenic miRNAs can be found in many human cancer types [23], [24]. Thus, miRNAs with effects on carcinogenesis, cell proliferation and differentiation, apoptosis, and on the formation of metastasis and drug resistance feature a valuable target for the design of potent anticancer drugs [25], [26], [27]. The application of accurate miRNAs (either as mimics or as antagomirs) in therapy is limited due to the low cell permeability and the facile degradation of RNA molecules, however, liposomal formulations such as MRX34 of the tumor suppressor miRNA-34a (Mirnarx Therapeutics) revealed sufficient stability and cell delivery of the anti-cancer active miRNA leading to the initiation of phase I clinical trials with MRX34 [18]. Nevertheless, dietary factors and natural products are easily obtained from natural sources and, thus, usually provide a rich supply of low-cost, safe and easily available miRNA-modulatory drug candidates. Suitable anticancer drugs from this pool of anticancer active dietary factors and natural products should either inhibit the activity of oncogenic miRNAs or promote the activity of tumor suppressor miRNAs. This review provides an overview of anticancer active natural products (phenols, terpenes) and their manifold interactions with miRNAs involved in cancer diseases.
2. Classification of miRNAs as tumor suppressors or oncogenes
2.1. Tumor suppressing miRNAs
Let-7 features one of the most prominent tumor suppressing miRNA families, and let-7 miRNAs are distinctly downregulated or even completely lost in certain cancer types [28]. A connection between let-7 and K-Ras mutation was observed and restored let-7 led to significant tumor growth inhibition in K-Ras lung cancer models [29], [30]. Downregulation of let-7 was shown for breast, colon, lung and pancreas carcinomas and correlated with worse prognosis in lung cancer patients [28], [29], [31], [32]. The first identified tumor suppressing microRNAs were miR-15 and miR-16 in CLL (chronic lymphocytic leukemia) cells [33]. Increased levels of miR-15a and miR-16-1 induced apoptosis while high levels of the anti-apoptotic Bcl-2 protein led to reduced concentrations of miR-15a and miR-16-1 [34]. Restored miR-16 exhibited growth inhibition in prostate cancer cells [35]. In addition, miR-15 and miR-16 sensitized multidrug-resistant gastric cancer cells to chemotherapy [36]. MiR-34 is another important tumor suppressor that attacks tumor-initiating pancreas cancer cells and leads to apoptosis, cell-cycle arrest, and anti-angiogenesis [37], [38], [39]. Singh and coworkers disclosed the tumor suppressing effects of miR-150 in pancreas cancer cells via repression of the oncoprotein MUC4 [40]. In mixed lineage leukemia cells (MLL) miR-495 was suppressed and restoration of this miRNA led to inhibition of leukemia cell growth both in vitro and in vivo [41]. Further examples of tumor suppressor miRNAs with effects on hepatoma and pancreas cancer feature miR-203 and miR-451 [42], [43], [44], [45]. Further to this, there are several miRNAs which explicitly suppress metastasis formation. In breast cancer models, the expression of miRNAs such as miR-126, miR-206, and miR-335 reduced bone and lung metastasis formation [46]. MiR-126 also inhibited the migration and invasion of prostate cancer cells [47]. In lung cancer, miR-126 and miR-183 likewise blocked metastasis formation [48], [49].
2.2. Oncogenic miRNAs
Several oncogenic miRNAs (OncomiRs) were identified which are overexpressed in many cancer types due to gene amplification or upregulated miRNA gene expression [50]. These miRNAs either block tumor suppressor gene expression or regulate epigenetically the expression of pathogenic genes (oncogenes) [51]. MiR-21 features a prominent example of an oncogenic miRNA which inhibited apoptosis in glioblastoma cells [52]. A connection with the anti-apoptotic Bcl-2 protein was observed in breast cancer cells and down-regulation of Bcl-2 occurred after suppression of miR-21 [53]. In pancreas cancer cells, oncogenic miR-27a was overexpressed and targeted the tumor suppressor Sprouty2 [54]. High levels of miR-424-5p in pancreas cancer cells blocked apoptosis and increased cell proliferation via SOCS6 suppression [55]. Another important OncomiR is miR-155 that promoted the proliferation of triple-negative breast cancers and represents a marker of bad prognosis for pancreas cancer patients [56], [57]. Various cancers revealed an overexpression of oncogenic miR-155 [57], [58], [59]. The miR-17-92 cluster plays an oncogenic role in various tumor diseases and upregulation of the miR-17-92 cluster correlated with increased tumor formation and angiogenesis [60], [61], [62]. B-cell lymphoma models exhibited stronger c-myc-dependent tumor growth upon expression of the miR-17-92 cluster [63]. The miRNA miR-10b was upregulated in breast cancer cells and increased migration, invasion and metastasis formation of breast cancer cells [64]. In addition, miR-10b was a marker for bad prognosis in primary breast carcinoma [64]. The miRNAs miR-373 and miR-520c enhanced the migration and invasion of cancer cells by affection of CD44 expression [65]. The metastatic potential of prostate cancer cells was likewise increased by miR-373 and miR-520c [66]. Breast cancer cells were able to form metastases in mice because of miR-373 and miR-520c overexpression [65]. In addition, clinical samples of breast cancer metastases revealed high miR-373 levels [65]. Various miRNAs contribute to drug resistance in cancer cells. Overexpression of miR-221 and miR-222 led to tamoxifen resistance in breast cancer cells and to TRAIL (tumor necrotic factor-related apoptosis-inducing ligand) resistance in non-small cell lung cancer cells (NSCLC) [67], [68]. The cisplatin resistance of ovarian cancer cells correlated with increased miR-214 levels targeting PTEN [69]. The effects of dietary and natural phenolic compounds and terpenoids on these miRNAs as well as on further specific miRNA examples are discussed in the following chapter. A short list of microRNAs with significant oncogenic or tumor suppressing activities in the four main solid tumor diseases of Western peoples [i.e., lung cancer, colorectal cancer, prostate cancer (men), and breast cancer (women)] is given in Table 1 [70], [71], [72], [73].
Table 1.
A selection of important microRNAs as tumor suppressors or oncogenes in breast cancer, prostate cancer, colorectal cancer and lung cancer diseases.
| Compound | Tumor suppressors | Oncogenes |
|---|---|---|
| Breast cancer | miR-31, miR-125b, miR-126, miR-146b, miR-200, miR-205, miR-206, miR-335 | miR-10b, miR-21, miR-155, miR-373, miR-520c |
| Prostate cancer | let-7c, miR-15, miR-16, miR-29b, miR-125b, miR-126, miR-143, miR-145, miR-205, miR-331-3p | miR-20a, miR-21, miR-27a, miR-107, miR-141, miR-221, miR-375 |
| Colorectal cancer | miR-1, miR-17-5p, miR-29a, miR-106a, miR-125b, miR-133b, miR-143, miR-144, miR-145, miR-150, miR-195, miR-203, miR-206, miR-345, mir-365, miR-378 | miR-9, miR-10b, miR-21, miR-31, miR-92a, miR-124, miR-135a/b, miR-139-5p, miR-141, miR-155, miR-181a, miR-183, miR-185, miR-200c, miR-215, miR-219-1, miR-335, miR-372, miR-608 |
| Lung cancer | let-7 family, miR-7, miR-126, miR-145, miR-183 | miR-17-92 cluster, miR-31, miR-146b, miR-155, miR-221, miR-222, miR-630 |
3. Dietary compounds and natural products (phenols and terpenoids) with effects on miRNA expression and activity
3.1. Phenolic compounds
3.1.1. Curcumin and curcuminoids
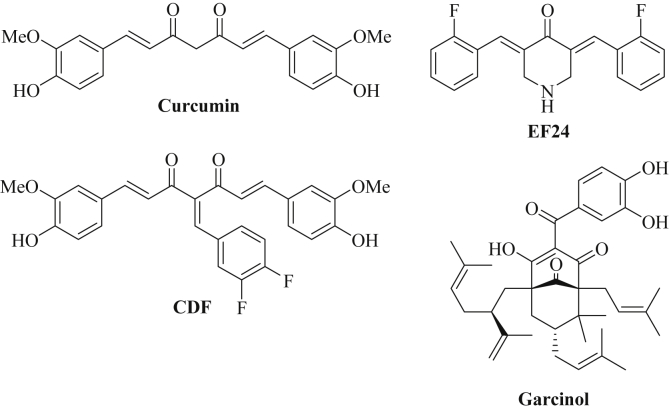
Curcumin is a phytochemical compound isolated from the rhizomes of turmeric (Curcuma longa, haldi) [74]. Turmeric has been applied as a spice for Asian dishes and as a drug in traditional Indian (Ayurveda) and Chinese (TCM) medicine for centuries. Structurally, curcumin is an α,β-unsaturated β-diketone (diferuloylmethane) that shows keto-enol-tautomerism (Fig. 1) [74]. Curcumin has been thoroughly investigated as an anti-inflammatory and anticancer agent and has already undergone several clinical trials [75]. Curcumin features a pleiotropic agent and targets NF-κB (nuclear factor-κB), Akt (protein kinase B) signaling, Wnt signaling, STAT-3 (signal transducers and activators of transcription-3), COX-2 (cyclooxygenase 2), proteasome, MMP (matrix metalloproteinase), etc. [74]. It also exhibited various effects on miRNAs, which contribute to its sound anticancer activity [75]. In MCF-7 breast cancer cells, curcumin induced the expression of the tumor suppressors miR-15a and miR-16 and, thus, suppressed the anti-apoptotic Bcl-2 protein [76]. These miRNAs were also upregulated in K-562 and HL-60 leukemia cells upon treatment with curcumin and WT1 (Wilm's Tumor 1) was identified as a target of miR-15a and miR-16 here [77]. Curcumin also activated the promoter of the miR-203 tumor suppressor gene (by hypomethylation) leading to the overexpression of miR-203 and curcumin-treated bladder cancer cells showed reduced levels of miR-203 targets, the protein kinases Akt and Src, as well as increased apoptosis and decreased proliferation and invasion [78]. Curcumin enhanced the expression of miR-22 in Y79 retinoblastoma cells leading to increased cell growth and migration inhibition via the miR-22 target Erbb3 [79]. In triple-negative breast cancer cells (MDA-MB-231), curcumin induced overexpression of miR-181b leading to inhibition of the formation of MMPs (matrix metalloproteinases) and downregulation of the chemokines CXCL1 and CXCL2 (chemokine C-X-C motif ligand) [80]. These events led to reduced invasion and metastasis formation [76]. Overexpression of miR-9 was observed in ovarian cancer cells (SKOV3) after treatment with curcumin leading to enhanced cytotoxicity [81]. A curcumin diet given to mice suffering from B78H1 murine melanoma revealed distinct tumor regression and a huge overexpression of the murine miRNA mmu-miR-205-5p (135-fold higher expression) in the tumors of curcumin-fed mice [82]. The miRNA miR-205-5p reversed EMT and was downregulated in metastatic melanomas [83], [84]. Curcumin blocked pancreatic cancer cell growth and invasion via induction of miR-7 expression associated with suppression of SET8 expression [85]. In NSCLC cells, curcumin-induced apoptosis was correlated with the curcumin-mediated upregulation of the tumor suppressors miR-192-5p and miR-215 in a p53-dependent way leading to down-regulation of XIAP (X-linked inhibitor of apoptosis) [86]. A major drawback of curcumin is its low bioavailability and aqueous solubility, thus, dendrosomal curcumin formulations were prepared which exhibited improved properties [87]. Indeed, dendrosomal curcumin increased the expression of miR-145 in glioblastoma cells (U87MG) leading to tumor cell growth inhibition via miR-145-mediated suppression of genes involved in the formation of cancer stemness properties such as Nanog, OCT4A (octamer binding protein 4), OCT4B1, and SOX-2 (SRY-box 2) [87]. Dendrosomal curcumin also induced miR-29a and miR-185 expression in hepatocellular carcinoma (HCC) cells associated with suppression of the DNA-methylases DNMT1, DNMT3A and DNMT3b leading to hypomethylation of the MEG3 promoter and increased expression of the tumor suppressing long non-coding RNA MEG3 [88].
Fig. 1.
Chemical structures of curcumin, of the curcuminoids CDF and EF24 and of garcinol.
Curcumin reduced the expression of the oncogenic miR-21 in colon cancer cells (RKO, HCT-116) leading to reduced tumor growth and metastasis formation by enhanced expression of the miR-21 target PDCD4 (programmed cell death protein 4) [89]. In addition, curcumin downregulated miR-17-5p, miR-20a, and miR-27a (targeting SP repressors, zinc finger and BTB-domain proteins such as ZBTB4/10) in colon cancer cells (RKO, SW480) leading to increased growth inhibition [90]. MiR-21 was suppressed in A549 NSCLC cells after treatment with curcumin as well, followed by activation of the miR-21 target PTEN (phosphatase and tensin homolog) [91]. In human esophageal cancer cells (TE-7), curcumin induced apoptosis and cell cycle arrest via suppression of miR-21 and miR-34a, while the tumor suppressor let-7 was induced by curcumin in these cancer cells [92]. In addition, curcumin successfully inhibited lung cancer cell proliferation (both A549 wt and A549/DDP multidrug resistant cells) and induced apoptosis by downregulation of oncogenic miR-186* which targets caspase-10 [93], [94]. Interestingly, there's conflicting data about the role of miR-200 concerning curcumin treatment. In hepatoma cells (HepG2, HepJ5), overexpression of miR-200a and miR-200b led to enhanced curcumin resistance via increased anti-apoptotic Bcl-2 levels and decreased pro-apoptotic Bad and Bax levels [95]. In contrast to that, gemcitabine-resistant pancreas cancer cells (MiaPaCa-E, MiaPaCa-M) showed decreased levels of miR-200b/c while after treatment with curcumin the levels of miR-200b and miR-200c were increased in these cells [96]. Modulation of miR-200c expression (upregulation) also played a role for the curcumin-induced sensitization of colon cancer cells to the approved anticancer drug 5-fluorouracil (5FU) [97]. In prostate cancer cells (PC3), curcumin suppressed oncogenic miR-208 in a dose-dependent way, and, thus, induced the expression of the cell cycle inhibitor and miR-208 target CDKN1A leading to prostate tumor cell growth inhibition [98].
Due to the impressive biological activities of curcumin, the preparation of curcumin derivatives (curcuminoids) with improved properties has become a valuable strategy to obtain sound anticancer drugs. In particular, fluoro-derivatives of curcumin have shown improved anticancer activity when compared with their parent drug curcumin. A remarkable semisynthetic curcuminoid is CDF, the Knoevenagel condensation product of curcumin and 3,4-difluorobenzaldehyde (Fig. 1) [99]. CDF exhibited much higher anticancer activity than curcumin and accumulated in pancreas tissue making it an interesting drug candidate for pancreas cancer diseases [96], [98], [100]. CDF was able to re-sensitize drug-resistant pancreatic cancer cells to gemcitabine and enhanced the expression of tumor suppressor miRNAs (miR-200) while it reduced the levels of oncogenic miR-21 leading to PTEN upregulation [96], [101]. Under hypoxic conditions, prostate cancer cells upregulate miR-21, however, after treatment with CDF miR-21 expression and tumor growth was efficiently reduced [102]. The hypoxia-mediated aggressiveness of pancreas cancer cells was also related to increased expression of miR-21 and miR-210, and treatment with CDF significantly reduced the expression of miR-21 and miR-210 in vitro and in vivo [103]. In addition, CDF reduced miR-21 levels and restored PTEN expression in colon cancer cells, while expression of the tumor suppressor miR-34 was induced by CDF in colon cancer [104], [105]. CDF was also able to modulate miRNAs targeting matrix metalloproteinases (MMPs) which play a crucial role for the invasion competence of cancers. MiR-874 was upregulated by CDF in non-small cell lung cancer cells which led to MMP-2 inhibition [106]. Oncogenic miR-221 was downregulated by CDF in pancreas cancer cells as well, and CDF inhibited proliferation and migration of pancreas cancer cells by upregulation of p27, p57, PTEN, and PUMA (p53 upregulated modulator of apoptosis) [107]. In addition, CDF upregulated let-7 and miR-101 which suppressed the histone methyltransferase EZH2 associated with reduced survival, invasion and cancer stem cell function in pancreatic cancer [108]. The tumor suppressor miR-143 was likewise induced by CDF in pancreatic cancer cells as well as the tumor suppressor miR-146a leading to suppressed EGFR (epidermal growth factor receptor) signaling and inhibition of pancreatic tumor growth [109], [110].
Another interesting synthetic curcuminoid is EF24 [3,5-bis(2-fluorobenzylidene)piperidin-4-one] which is easily prepared by condensation of piperidin-4-one with two equivalents of 2-fluorobenzaldehyde (Fig. 1) [111]. EF24 exhibited distinctly stronger growth inhibition and anti-angiogenic activity than curcumin [111], [112]. Hence, it is not surprising that EF24 efficiently suppressed oncogenic miR-21 in prostate cancer (DU145) and melanoma (B16) cells and upregulated the miR-21 targets PTEN and PDCD4 [112]. A recent study of EF24 in melanoma cells disclosed significant effects on miR-33b expression, and suppression of EMT (=suppression of metastasis formation) as well as HMGA2 (high-mobility group AT-hook 2) inhibition were observed after treatment with EF24 associated with upregulation of miR-33b [113]. The miRNAs (both tumor suppressor and oncogenic ones) regulated by curcumin and its derivatives are presented in Table 2.
Table 2.
Regulation of non-coding RNAs/microRNAs by curcumin, curcumin derivatives (CDF, EF24), and garcinol.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Curcumin | let-7, miR-7, miR-9, miR-15, miR-16, miR-22, miR-29a miR-145, miR-181b, miR-185, miR-192-5p, miR-200b/c, miR-203, miR-205-5p, miR-215, MEG3 | miR-17-5p, miR-20a, miR-21, miR-27a, miR-186*, miR-208 |
| CDF | miR-34, miR-101, miR-146a, miR-200, miR-874 | miR-21, miR-210, miR-221 |
| EF24 | miR-33b | miR-21 |
| Garcinol | let-7, miR-128, miR-200b/c, miR-453, miR-720, miR-1280 | miR-21, miR-494, miR-495, miR-1977 |
| Garcinol + Gemcitabine | miR-453, miR-663, miR-638, miR-720 | miR-196a, miR-495, miR-605, miR-483-3p, miR-1914 |
3.1.2. Garcinol
The phenolic natural product garcinol was isolated from the kokum tree (Garcinia indica) that grows in Western India [114]. Aside its application as a spice for Western Indian dishes, kokum is also applied in Ayurveda medicine for the treatment of infections and edema [115], [116]. Chemically, garcinol (camboginol) is a type B polycyclic polyprenylated acylphloroglucinol (PPAP) featuring a benzophenone derivative with five isoprenyl side chains attached to the phloroglucinol core (Fig. 1) [117]. Garcinol is easily isolated from dried kokum plums, however, there's a total synthetic procedure of garcinol and its close congener isogarcinol available now as well [118], [119]. The chemical structure of garcinol shows some similarities with curcumin (β-diketone, phenol). Garcinol has revealed significant anticancer activity by targeting NF-κB, 5-lipoxygenase (5-LOX), and STAT proteins (for a recent review see ref. [118]). In addition, garcinol is a well-documented HAT inhibitor (histone acetylase inhibitor) and, thus, plays an important role for the epigenetic regulation of gene expression including the expression of oncogenes [120]. Synergistic effects were observed from combinations of garcinol with gemcitabine in pancreas cancer cells (including gemcitabine-resistant Panc-1 cells) due to garcinol-mediated modulation of miRNAs [121]. Garcinol downregulated oncogenic miR-21 (0.56-fold), miR-494 (0.97-fold), miR-495 (1.14-fold), and miR-1977 (0.80-fold) when compared with untreated pancreas cancer cells [121]. The expression of the micro-RNAs miR-453 (4.69-fold), miR-128 (1.82-fold), miR-1280 (0.46-fold) and miR-720 (0.43-fold) was upregulated by garcinol. The combination of garcinol with gemcitabine led to downregulation of miR-196a (0.82-fold), miR-495 (1.26-fold), miR-1914 (1.85-fold), miR-605 (2.42-fold), and miR-483-3p (1.06-fold) as well as upregulation of miR-638 (0.65-fold), miR-720 (0.41-fold), miR-453 (4.78-fold) and miR-663 (0.92-fold) [121]. Further to this, in breast cancer cells (MDA-MB-231, BT-549) garcinol reverted the metastasis-related EMT mechanism to the non-invasive MET (mesenchymal-to-epithelial transition) mechanism by induction of expression of the tumor suppressor miRNAs let-7a, let-7e, let-7f, miR-200b, and miR-200c both in vitro and in vivo in breast cancer xenograft models [122]. The microRNAs regulated by garcinol are presented in Table 2.
3.1.3. Flavanoids
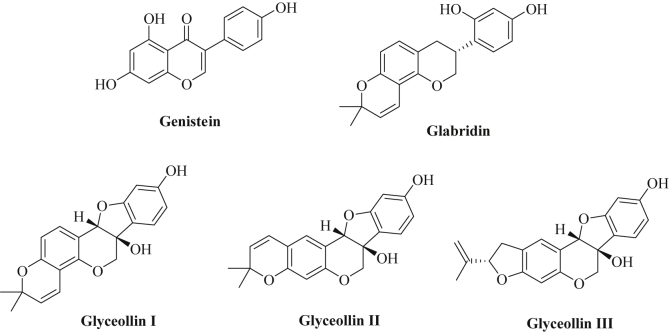
The rich natural product class of the flavanoids (phenylchroman derivatives) comprises numerous bioactive compounds. In particular, soy isoflavones (3-phenylchromon derivatives) such as genistein (Fig. 2) and daidzein have shown promising anticancer effects including tumor growth inhibition and inhibition of metastasis formation by targeting multiple pathways (e.g., NF-κB, Akt, Wnt signaling, Notch signaling, androgen receptor signaling) [123]. Genistein downregulated the expression of oncogenic miR-21 in renal cancer cells (A498) followed by induction of p21 and p38 MAPK (mitogen-activated protein kinase) while cyclin E2 was suppressed by genistein [124]. In addition, numerous other oncogenic miRNAs are modulated by genistein. In renal cancer cells, downregulation of oncogenic miR-23b-3p was observed after treatment with genistein leading to expression of PTEN followed by suppression of PI3K (phosphatidylinositol-3-kinase), Akt and IL-32 (interleukin-32) [125]. Genistein reduced the levels of oncogenic miR-1260b in renal cancer cells (786-O, A498) and, thus, inhibited Wnt signaling via upregulation of the miR-1260b targets sFRP1 (frizzled-related protein 1), Dkk2 (dickkopf 2 homolog) and Smad4 (mothers against decapentaplegic 4) in these cancer cells [126]. Genistein performed analogously in prostate cancer cells (DU-145, PC-3) where suppression of miR-1260b and Wnt signaling was observed as well [127]. Oncogenic miR-27a was suppressed by genistein in various tumors including uveal melanoma (C918), pancreatic, and ovarian cancer (SKOV3) cells followed by induction of ZBTB10 (zinc-finger and BTB domain containing 10) and Sprouty2, the targets of miR-27a [128], [129], [130]. MiR-151, which targets various factors (e.g., N4BP1, CASZ1, SOX17, IL1RAPL1, ARHGDIA), features another miRNA suppressed by genistein in prostate cancer cells (PC-3, DU-145) leading to inhibition of migration and invasion of prostate cancer cells [131]. Further to this, genistein blocked miR-221 and miR-222 expression in prostate cancer cells (PC-3) followed by overexpression of ARH1 (aplysia ras homolog 1) and cell growth, invasion and colony formation inhibition [132]. MiR-223 was likewise suppressed by genistein in pancreatic cancer cells and induction of Fbw7 (F-box and WD-40 domain protein 7) expression was observed leading to cancer cell growth inhibition and apoptosis induction [133]. The G2535 mixture of isoflavones (70.54% genistein, 26.34% daidzein, 0.31% glycitein) reduced oncogenic miR-221 levels in pancreas cancer cells and inhibited proliferation and migration of pancreas cancer cells by induced expression of p27, p57, PTEN, and PUMA [107]. In highly metastatic breast cancer cells (MDA-MB-435), genistein suppressed miR-155 expression accompanied by increased expression of various pro-apoptotic and antiproliferative miR-155 targets (FOXO3, PTEN, casein kinase, p27) [134].
Fig. 2.
Chemical structures of isoflavone derivatives.
In contrast to that, the tumor suppressor miRNAs miR-34a, miR-574-3p and miR-1296 were upregulated in prostate cancer cells (PC-3, DU-145) after treatment with genistein [135], [136], [137]. While genistein-mediated induction of miR-34a knocked down HOTAIR (HOX transcript antisense RNA), overexpression of miR-574-3p suppressed anti-apoptotic Bcl-xL and enhanced caspase-3 and caspase-9 activity. Further targets of miR-574-3p included RAC1, EGFR and EP300 (p300 histone acetyl transferase), while miR-1296 blocks MCM2 (minichromosome maintenance) expression which is a crucial factor for functional DNA replication. However, a differing miRNA modulation by the isoflavones genistein and daidzein was observed in three prostate cancer cell lines [138]. Genistein also upregulated miR-34a in pancreas cancer cells and, thus, induced apoptosis and tumor cell growth inhibition by inhibition of Notch-1 signaling [139]. In addition, let-7 and miR-200 were upregulated in pancreatic cancer after treatment with genistein followed by suppression of miR-200 targets such as ZEB1 (zinc finger E-box-binding homeobox 1), slug and vimentin which are correlated with EMT [140]. Genistein also induced miR-146a expression associated with suppression of pancreatic cancer cell invasion via downregulation of miR-146a targets such as EGFR, MTA-2, IRAK-1, and NF-κB [141]. More recently, genistein exhibited distinct cell growth inhibition of breast cancer cells (MCF-7) by up-regulation of miR-23b expression (56.69-fold compared with untreated cells) [142].
Licorice (Glycyrrhiza glabra) is a well known sweetening plant and has been applied in folk medicine against inflammations, and as an antioxidant, antidote, demulcent and expectorant drug [143]. Extracts of licorice contain significant amounts of the isoflavan glabridin (a phytoestrogen, Fig. 2), which has shown anti-inflammatory, neuro- and cardioprotective activities in addition to distinct anticancer properties (growth inhibition, anti-angiogenic and anti-metastatic effects) [144], [145], [146]. Glabridin suppressed cancer stem cell-like features in hepatocellular carcinoma models (HepG2, Huh-7, MHCC97H) by upregulation of miR-148a and suppression of the miR-148a target SMAD2 (small body size mothers against decapentaplegic) associated with inhibition of TGF(transforming growth factor)-β/SMAD2 signaling [147]. Similar results were obtained from breast cancer cells (MDA-MB-231, Hs-578T) treated with glabridin [148]. In addition, glabridin inhibited angiogenesis of breast tumors (MDA-MB-231, Hs-578T) by upregulation of miR-520a [149]. The expression of NF-κB was blocked by upregulated miR-520a in glabridin-treated breast cancer cells associated with inhibition of NF-κB/IL-6/STAT-3 signaling.
Glyceollins represent further natural isoflavonoids biosynthetically derived from soy isoflavones (e.g., daidzein) which exhibited interesting anticancer properties (Fig. 2) [150]. These anti-estrogenic compounds efficiently stopped estrogen-induced tumor growth of breast and ovarian cancer models (MCF-7 by 53.4%, BG-1 by 73.1%) and suppressed estrogen-mediated expression of the progesterone receptor [151]. In letrozole-resistant breast cancer cells glyceollin I suppressed ZEB1 (zinc-finger E-box binding homeobox 1) expression leading to reversal of EMT in these resistant breast cancer cells [152]. In triple-negative breast cancer cells (MDA-MB-231, MDA-MB-468) a mixture of glyceollin I (68%), II (21%) and III (11%), isolated from soybean seeds incubated with Aspergillus sojae, reduced tumorigenesis by modulation of miRNAs. In particular, tumor suppressor miRNAs (miR181c, miR-181d) as well as EMT reversing miRNAs (miR-22, miR-29b, miR-29c, miR-30d, miR-34a, miR-195) were upregulated by glyceollins while oncogenic miRNAs increasing tumorigenesis (miR-21, miR-193a-5p), metastasis formation (miR-185, miR-224), migration and invasion (miR-486-5p) and mesenchymal cell maintenance (miR-542-5p) were downregulated by glyceollins [153]. The expression of NME1 (non-metastatic cells 1, target of miR-486-5p and miR-542-5p), was increased while vimentin (target of miR-30d) was reduced in glyceollins-treated breast cancer cells [153].
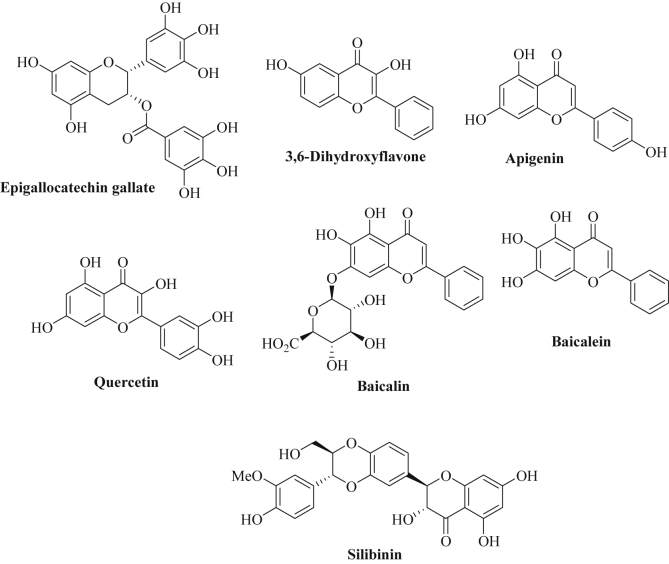
In addition to soy isoflavones, many natural flavanoid derivatives exhibited sound biological activities including antioxidant and anticancer properties. For instance, (-)-epigallocatechin-3-O-gallate (EGCG, Fig. 3), the main catechin flavanol of the tea plant (Camilla sinensis), revealed distinct anticancer activities by modulation of multiple targets [154]. The induction of apoptosis in hepatoma cells after treatment with EGCG was associated with an upregulation of the tumor suppressor miR-16 and inhibition of anti-apoptotic Bcl-2 [155]. In addition, miR-210 was induced by EGCG in lung cancer cells leading to cell growth inhibition [156]. However, the same group disclosed only modest changes of 21 miRNAs after EGCG treatment and that miR-210 played a minor role in vivo, while other miRNAs regulating Akt signaling, MAPK signaling and cell cycle control were of greater importance in lung cancer models in vivo [157]. For instance, down-regulation of miR-449c-5p (targets: ACSL1, elmod1, GAS1, ZFHX4), miR-374c-5p (targets: ART3, cnr1, DCN, EFNA5, GAS1, ELOVL6, rbm24), and miR-450a-2-3p (targets: cd19, IGFBP5, ZFHX4) as well as upregulation of miR-144-3p (targets: AMMECR3, Ccne2, ZCCHC2) and miR-34b-5p (targets: Ccne2, NRN1) was observed in vivo after EGCG treatment [157]. In melanoma cells, increased expression of let-7b via activation of 67LR (67-kDa laminin receptor) was observed after treatment with EGCG [158]. In addition, EGCG inhibited cell growth of osteosarcoma cells via upregulation of miR-1 expression and induced apoptosis in osteosarcoma cells through induction of miR-126 expression [159], [160]. Oncogenic miR-21 and AR (androgen receptor) signaling were suppressed in prostate cancer cells after EGCG treatment while miR-330 was upregulated leading to reduced prostate tumor growth [161]. The microRNAs miR-30* (which targets NF-κB, peroxisome-proliferator activated receptor/PPAR signaling, insulin signaling, glycolysis, gluconeogenesis, oxidative phosphorylation, glutathione, glycerolipids, and mitochondria), miR-453, miR-520-e, miR-608, and miR-629 were downregulated by EGCG in hepatoma cells (HepG2) [162]. Tsang and coworkers showed that 61 miRNAs were modulated by EGCG in hepatoma cells (HepG2) (e.g., upregulation of let-7c, miR-16, miR-18, miR-25, miR-92, and suppression of miR-129, miR-196, miR-200, miR-342, miR-526) [163]. EGCG-mediated suppression of miR-98-5p in NSCLC cells (A549) sensitized these tumor cells to cisplatin treatment [164]. The expression of further miRNAs was either reduced (oncogenic miR-92, miR-93, miR-106b) or increased (tumor suppressors miR-7-1, miR-34a, miR-99a) by a combination of EGCG with N-(4-hydroxyphenyl)retinamide in human neuroblastoma cells (SH-SY5Y, SK-N-DZ) [165]. Protection from UVB irradiation was observed in EGCG-treated dermal fibroblasts via modulation of various miRNAs [166]. Four miRNAs were upregulated (miR-1246, miR-548c-3p, miR-636, miR-933) and 14 miRNAs were downregulated (miR-1202, miR-1207-5p, miR-1225-5p, miR-1227, miR-1271, miR-133a, miR-134, miR-181d, miR-212, miR-362-3p, miR-455-5p, miR-494, miR-513a-5p, miR-660) in EGCG-treated dermal fibroblasts [166]. By the way, EGCG was shown to interact and to bind directly to certain miRNAs (miR-33a, miR-122) in liver cells and the miRNA binding ability of EGCG was determined by 1H NMR spectroscopy [167].
Fig. 3.
Chemical structures of flavanoids.
The screening of a series of flavonoids revealed the outstanding activity of 3,6-dihydroxyflavone (3,6-DHF, Fig. 3) against breast cancer cells [168]. In addition, 3,6-DHF exhibited distinct chemo-preventive effects in 1-methyl-1-nitrosourea treated breast cancer cells by upregulation of miR-34a and downregulation of miR-21 [169]. More recently, the same group disclosed an epigenetic mechanism for the modulation of miR-34a and miR-21 by 3,6-DHF in breast cancer cells [170]. Inhibition of the methyltransferase DNMT1 by 3,6-DHF (probably by binding to the cytosine pocket of the enzyme) blocked the hypermethylation of the miR-34a promoter which led to enhanced miR-34a expression [170]. Concerning miR-21, 3,6-DHF modulated histone modification and depressed H3K9-14ac in the miR-21 promoter region which led to miR-21 suppression and efficient blocking of the PI3K/Akt/mTOR signaling pathway [170].
Apigenin represents another flavone derivative found in fruits, herbs, and vegetables, which induced apoptosis both via the intrinsic and via the extrinsic pathway and suppressed PI3K/Akt and ERK1/2 signaling pathways in various cancer cells (Fig. 3) [171], [172]. Apigenin was shown to inhibit tumor growth both in vitro and in vivo [173], [174]. Decreased expression of miR-138 enhanced the telomerase activity in tumor cells, which is associated with malignant growth [175]. The transfection with hTERT short hairpin RNA (a shRNA, which inhibits hTERT, human telomerase reverse transcriptase) in combination with apigenin treatment increased the expression of miR-138 in neuroblastoma cells [175]. In addition, miR-138 led to enhanced anticancer activity of apigenin and caused increased apoptosis induction and inhibition of tumor cell growth and colony formation of neuroblastomas (SK-N-DZ, SK-N-BE2) by apigenin both in vitro and in vivo [175]. Obesity and diabetes are considered as significant risk factors for various cancer diseases [176]. In particular, miR-103 overexpression is associated with glucose intolerance and apigenin efficiently lowered miR-103 levels by impairing the maturation of this miRNA and by inhibition of ERK-mediated TRBP (trans-activating response RNA-binding protein) phosphorylation [177]. In this way, the miR-103 suppressing properties of apigenin may contribute to the prevention of obesity- and diabetes-induced cancer types.
The consumption of a diet rich in the flavonol quercetin (Fig. 3), which is found in apples, grapes and onions, was associated with the modulation of various miRNAs in lung cancer tissues that play a distinct role for tumorigenesis including miR-146a (upregulation) and miRNAs of the let-7 and miR-26 family (tumor suppressing let-7 and miR-26 miRNAs were significantly expressed upon quercetin consumption) [178]. Quercetin-mediated miR-146a expression which inhibited NF-κB signaling was associated with anti-inflammation and cancer–preventive activity by protection of colonic myofibroblasts (CCD-18Co) from damage by ROS (reactive oxygen species) [179]. In addition, overexpression of let-7 by a combination of quercetin with catechins inhibited K-Ras signaling in pancreatic cancer cells as well as tumor growth inhibition [180]. In lung cancer cells (A549), quercetin induced miR-16 expression and decreased expression of the oncoprotein Claudin-2 in this way [181]. Unlike gemcitabine, quercetin also induced miR-142-3p expression in pancreatic cancer cells [182]. In hepatoma cells (HepG2), quercetin increased the levels of the tumor suppressor miR-34a associated with suppression of SIRT1 deacetylases in a p53-dependent way [183]. In contrast to that, oncogenic miR-27a was suppressed in colon cancer cells by quercetin in combination with resveratrol leading to enhanced apoptosis induction in the colon cancer cells [184]. However, quercetin reduced hepatic miR-125b-3p levels leading to enhanced GGH (γ-glutamyl hydrolase) expression which was linked to methotrexate resistance [185].
Baicalin and baicalein are flavones of the plant Scutellaria baicalensis Georgi (applied in TCM, Traditional Chinese Medicine) that exhibited distinct anti-inflammatory and anti-metastatic activities (Fig. 3) [186], [187]. Baicalin induced expression of miR-23a and suppressed miR-378, miR-199a-3p and miR-181b in UVB-treated mice, all of them miRNAs that probably play a role for DNA repair mechanisms [188]. In addition, oncogenic miR-294 expression was reduced by baicalin in embryonic stem cells (ESCs) associated with suppression of the oncogenes c-jun and c-fos [189]. Baicalein exhibited anti-inflammatory activity by induction of miR-124 expression in neuronal cells (retinal ganglion cells) leading to suppression of the inflammation factor MCP-1 (monocyte chemotactic protein 1), an effect that might contribute to the chemopreventive properties of baicalein as well [190].
The flavolignan silibinin (Fig. 3) represents the active component of the fruits of the milk thistle (Silybum marianum) [191]. Recently, it was shown that silibinin suppressed miR-21 and enhanced miR-200c expression in erlotinib-resistant NSCLC tumors associated with a reversal of EMT and a suppression of mesenchymal markers (SNAIL, ZEB, N-cadherin) [192]. A list of microRNAs regulated by flavanoids is given in Table 3.
Table 3.
Regulation of microRNAs by flavanoids.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Genistein | let-7, miR-23b, miR-34a, miR-146a, miR-200, miR-574-3p, miR-1296 | miR-21, miR-23b, miR-27a, miR-151, miR-155, miR-221, miR-222, miR-223, miR-1260b |
| Glabridin | miR-148a, miR-520a | – |
| Glyceollins | miR-22, miR-29b, miR-29c, miR-30d, miR-34a, miR-181c, miR-181d, miR-195 | miR-21, miR-185, miR-193a-5p, miR-224, miR-486-5p, miR-542-5p |
| EGCG | let-7b, let-7c, miR-1, miR-7-1, miR-16, miR-18, miR-25, miR-34a, miR-34b, miR-92, miR-99a, miR-126, miR-144-3p, miR-210, miR-330 | miR-21, miR-30*, miR-92, miR-93, miR-98-5p, miR-106b, miR-374c-5p, miR-449c-5p, miR-450a-2-3p, miR-453, miR-520-e, miR-608, miR-629 |
| 3,6-DHF | miR-34a | miR-21 |
| Apigenin | miR-183 | miR-103 (glucose intolerance) |
| Quercetin | let-7, miR-16, miR-26, miR-34a, miR-146a, miR-142-3p | miR-27a, miR-125b-3p |
| Baicalin, Baicalein | miR-23a, miR-124 (anti-inflammatory) | miR-181b, miR-199a-3p, miR-294, miR-378 |
| Silibinin | miR-200c | miR-21 |
3.1.4. Stilbenes
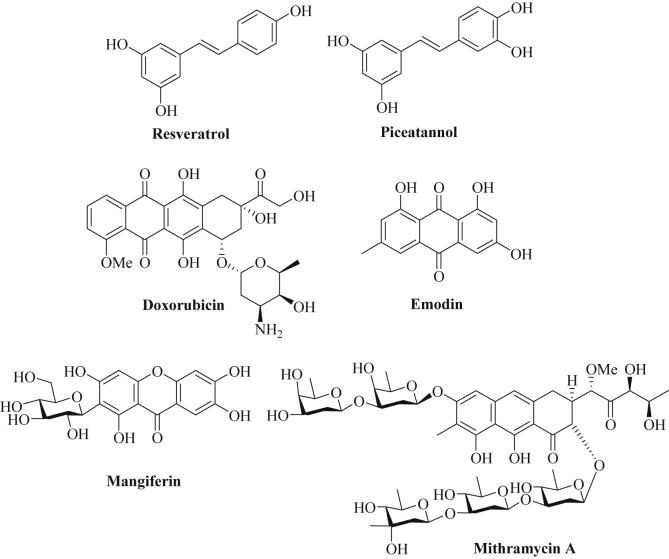
The natural stilbene derivative resveratrol (3,4′,5-trihydroxystilbene) occurs in fruits such as grapes, berries, and plums and its anticancer properties against various cancer diseases were thoroughly investigated (Fig. 4) [193], [194]. In colon cancer cells, resveratrol suppressed oncogenic miRNAs such as miR-17, miR-21, miR-25, miR-92a-2, miR-103-1, and miR-103-2, while tumor suppressor miR-663 (targeting TGF-β) was upregulated by resveratrol [195]. Oncogenic miR-21 was likewise downregulated by resveratrol in pancreatic cancer cells associated with enhanced apoptosis induction via suppression of the anti-apoptotic Bcl-2 protein [196]. Similar effects were observed from resveratrol-treated prostate cancer cells, where miR-21 suppression upregulated the miR-21 targets PDCD4 and maspin, and from gastric cancer cells treated with resveratrol [197], [198]. The oncogenic miRNA clusters miR-17-92 and miR-106ab were suppressed by resveratrol in prostate cancer cells leading to PTEN induction [199]. Resveratrol-mediated downregulation of miR-520h blocked metastasis formation of lung cancer models by inhibition of FOXC2 (forkhead box C2) [200]. The combination of resveratrol and quercetin reduced miR-27a levels leading to ZBTB10 expression and apoptosis induction in HT-29 colon cancer cells [184]. Resveratrol inhibited proliferation of MCF-7 breast cancer cells by upregulation of miR-663 and miR-774 leading to EF1A2 (elongation factor 1A2) inhibition [201]. In addition, decreased cell invasion by induction of miR-141 and reversal of EMT via miR-200c upregulation (leading to ZEB1 suppression and E-cadherin upregulation) was observed after resveratrol treatment [202]. Synergy effects were observed in lung cancer cells from the combination of resveratrol with miR-200c [203]. Further to this, resveratrol-mediated up-regulation of the tumor suppressor miR-34a was associated with increased cell growth inhibition and apoptosis induction in colon cancer cells [204]. In addition, the tumor suppressor miR-34c was induced by resveratrol in colon cancer cells (HT-29, HCT-116) leading to increased apoptosis and suppression of the miR-34c target KITLG (also known as stem cell factor SCF) in vitro and in vivo [205]. The tumor suppressor miR-622 targets K-Ras and was found to be upregulated by resveratrol in transformed bronchial epithelial cells (16HBE-T) leading to cell cycle arrest and suppressed colony formation and tumorigenicity [206]. In hormone-sensitive breast tumors resveratrol inhibited DNA-methyltransferase DNMT3b by up-regulation of miR-129, miR-204, and miR-489 [207]. A general miRNA expression study in A549 lung cancer cells revealed that resveratrol suppressed miR-92a-2 and induced miR-299-5p, miR-194*, miR-338-3p, miR-758, and miR-582-3p which are likely involved in the regulation of proliferation, apoptosis, cell cycle and differentiation processes of lung cancer cells [208]. More recently, a similar study was carried out with triple-negative MDA-MB-231 breast cancer cells and resveratrol strongly upregulated miR-122-5p (37.6-fold) and significantly downregulated miR-542-3p (11-fold) and miR-200c-3p (8-fold) playing a key role for the anticancer effects of resveratrol in these cancer cells [209]. Like EGCG, resveratrol was able bind directly to certain miRNAs (miR-33a, miR-122) in liver cells and the miRNA binding ability of resveratrol was determined by 1H NMR spectroscopy [167].
Fig. 4.
Chemical structures of stilbenes, anthraquinones, xanthone and anthracene derivatives.
Piceatannol is a close stilbene analog of resveratrol (Fig. 4) and occurs in many fruits, in particular, in grapes and red wine which was associated with the so-called “French paradox” together with resveratrol. Indeed, piceatannol exhibited distinct anticancer properties via multiple pathways, and it revealed higher inhibition of COX-1/2 and CSN-associated kinase than resveratrol [210]. In leukemia cells (U937), Akt/Foxp3-mediated miR-183 expression was suppressed by piceatannol leading to inhibition of Sp1-induced metalloprotease ADAM17 expression and suppression of TNFα-induced NF-κB [211]. The expression of the tumor suppressor miR-129 was up-regulated by piceatannol in colon cancer cells (HCT-116, HT-29) and increased apoptosis was observed in piceatannol-treated cells via suppression of Bcl-2 (target of miR-129) [212]. Bone metastasis are difficult to tackle and cause painful and life-threatening bone damages, however, both resveratrol and piceatannol were shown to block osteoclastogenesis by down-regulation of miR-183 via HO-1 (heme oxygenase-1) induction and, thus, feature suitable tools for the management and prevention of bone metastasis [213]. A list of miRNAs regulated by natural stilbenes is given in Table 4.
Table 4.
Regulation of microRNAs by stilbenes.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Resveratrol | miR-34a/c, miR-122-5p, miR-141, miR-194*, miR-200c, miR-299-5p, miR-338-3p, miR-582-3p, miR-622, miR-663, miR-758, miR-774 | miR-17, miR-21, miR-25, miR-27a, miR-92a-2, miR-103-1, miR-103-2, miR-183, miR-200-3p, miR-520h, miR-542-3p, miR-17-92 cluster, miR-106ab cluster |
| Piceatannol | miR-129 | miR-183 |
3.1.5. Anthraquinones, xanthone and anthracene derivatives
Doxorubicin (Adriamycin, Dox) is a natural anthracycline anticancer drug (inhibitor of topoisomerase II, isolated from Streptomyces bacteria) widely applied for the treatment of various advanced cancer diseases including metastatic breast cancer (Fig. 4) [214]. Resistance factors concerning doxorubicin treatment include the expression of ABC-transporters (increased drug efflux), enhanced drug metabolism, reduced topoisomerase II levels, altered p53 function, and apoptosis inhibition [214]. Restored miR-128 expression in breast cancer stem-like cells (bCSCs) enhanced the activity of doxorubicin and increased apoptosis and DNA damage in these drug resistant cells [215]. In addition, miR-451 regulated MDR1 (multidrug-resistance-protein 1) gene expression and played a crucial role for doxorubicin resistance in MCF-7 breast cancer cells [216]. Transfection of resistant breast cancer cells with miR-451 re-sensitized these cancer cells to doxorubicin treatment [216]. In addition, resistant MCF-7/DOX cells revealed down-regulated let-7 (target: K-Ras), miR-27b (target: CYP1B1), miR-34a (target: Notch-1, E2F3), miR-127 (target: Bcl-6) and miR-200c (target: TCF8), while miR-21 (target: PTEN), miR-28 (target: BRCA1), miR-106a (target: RB1) and miR-206 (target: ERα) were upregulated in the doxorubicin-resistant MCF-7 cells [216]. Eroles and coworkers investigated the miRNA profile of doxorubicin-treated breast cancer cells and differences between MCF-7 cells and triple-negative breast cancer (TNBC) cells were observed (25 altered miRNAs were specific to TNBC cells, including miR-21-3p, miR-222-5p, miR-181a-3p, and miR-92a-1-5p) [217]. In addition, the miRNA miR-548c-3p mediated doxorubicin-resistance in resistant MCF-7 cells [217]. Further to this, receptor tyrosine kinase TrkB and repressor Bmi1 (B-cell-specific Moloney murine leukemia virus integration site 1) were identified as targets of miR-200c that mediate doxorubicin-resistance in breast cancer cells (BT474) upon down-regulation of miR-200c [218]. Doxorubicin-resistant MCF-7 breast cancer cells also exhibited reduced miR-125b and mir-193b expression associated with upregulated anti-apoptotic Mcl1 (myeloid cell leukemia 1) and inhibition of apoptosis [219], [220]. In addition, drug-resistant breast cancer cells showed reduced miR-205 levels and restored miR-205 expression sensitized these breast cancer cells to TAC (docetaxol, doxorubicin, cyclophosphamide) treatment again via suppression of VEGFA and FGF2 and increased apoptosis induction [221]. Low levels of miR-218 were also observed in drug-resistant breast cancer cells, and re-expression of miR-218 broke the resistance to doxorubicin via inhibition of surviving [222]. MiR-450b-3p expression was significantly lowered in HER2/HER3-positive breast cancer cells as well as in patients with poor prognosis, while overexpression of miR-450b-3p directly inhibited HER3 expression leading to increased sensitivity to doxorubicin [223]. Expression of oncogenic miR-181a upon genotoxic treatment induced doxorubicin-resistance in metastatic triple-negative breast cancers (TNBC) via STAT-3 activation and inhibition of pro-apoptotic Bax proteins [224]. Interestingly, the combination of a miR-10b-inhibitor (miR-10b is overexpressed in metastatic breast cancer) with low dose doxorubicin exhibited long-lasting remissions of metastatic breast cancer because of significant growth inhibition and apoptosis induction [225]. Another promising strategy to tackle TNBC was observed from targeted co-delivery of doxorubicin and miR-542-3p via hyaluronic acid-modified polyethyleneimine-poly(d,l-lactide-co-glycolide) (HA-PEI-PLGA) nanoparticles [226]. The restoration of miR-542-3p in TNBC cells treated with doxorubicin in this way enhanced apoptosis induction via p53 activation and survivin suppression [226]. The tumor suppressor miR-101 was downregulated in hepatocellular carcinoma (HCC) cells, and transfection of HCC cells with miR-101 sensitized these cells to treatment with doxorubicin via suppression of anti-apoptotic Mcl-1 [227]. Doxorubicin-resistant colon cancer cells (HT-29/Dox) exhibited low miR-522 levels and overexpression of miR-522 restored doxorubicin sensitivity via suppression of ABCB5 expression [228]. In addition, the mTOR-inhibitor sirolimus distinctly increased miR-34b expression in osteosarcoma cells leading to enhanced doxorubicin activity via suppressed PAK1 and ABCB1 expression [229]. In contrast to that, TGF-β1-mediated miR-202 expression blocked apoptosis induction in osteosarcoma cells and induced doxorubicin-resistance via downregulation of PDCD4 [230]. In glioblastoma cells (U87, LN229), the combination of doxorubicin with a miR-21 inhibitor (miR-21i) revealed increased anticancer effects by up-regulation of E-cadherin, RECK (reversion-inducing-cysteine-rich protein with kazal motifs), PTEN, VHL (Von Hippel-Lindau tumor suppressor), p21 and of tumor suppressor miRNAs (miR-200a/b, miR-429, miR-181d) while the tumor suppressor gene silencing DNA methyltransferase 1 (DNMT1) was suppressed by doxorubicin [231]. The application of a doxorubicin-conjugated miR-221 molecular beacon suppressed miR-221 expression efficiently while increased anticancer efficacy was observed in treated glioblastoma cells [232]. In diffuse large B-cell lymphoma (DLBCL) cells, the expression of miR-199a and miR-497 distinctly sensitized these lymphoma cells to doxorubicin treatment. In addition, better overall survival was observed in DLBCL patients with high miR-199a and miR-497 expression [233]. A list of miRNAs involved in doxorubicin-resistance is given in Table 5.
Table 5.
MicroRNAs involved in doxorubicin-resistance of cancer cells.
| Tumor suppressors (down-regulated) | Oncogenes (up-regulated) |
|---|---|
| let-7, miR-27b, miR-34a, miR-34b, miR-101, miR-125b, miR-127, miR-128, miR-193b, mir-199a, miR-200c, miR-205, miR-218, miR-450b-3p, miR-451, miR-497, miR-522, miR-542-3p | miR-10b, miR-21, miR-28, miR-106a, miR-181a, miR-202, miR-206, miR-221, miR-548c-3p |
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) features another natural anthraquinone derivative which was isolated from the roots of the Turkish rhubarb Rheum palmatum, a plant applied in TCM for the treatment of several diseases (Fig. 4) [234]. Emodin exhibited distinct anticancer activities via inhibition of tyrosine kinases [235]. Oncogenic miRNAs miR-221 and miR-222 were suppressed by emodin in leukemia cells (K562) leading to the induction of erythroid differentiation by upregulation of ALAS2 (aminolevulinate synthase) and of the tyrosine kinase c-KIT [235]. The growth inhibitory effects of emodin in lung cancer cells (A549) was associated with upregulation of miR-211 and miR-429 expression and suppression of their targets SATB2 (special AT-rich sequence-binding protein 2) and CRKL (Crk-like protein) [236]. The combination of emodin with curcumin revealed synergistic effects concerning the inhibition of breast cancer cell proliferation and invasion via upregulation of miR-34a and suppression of the miR-34 targets Bcl-2 and Bmi-1 [237]. In addition, emodin was able to suppress Wnt4/Dvl-1/β-catenin signaling by induction of miR-126 expression [238]. Angiogenesis of pancreatic cancer was blocked by emodin via upregulation of miR-20b and downregulation of miR-155 and miR-210 leading to reduced TGF-β levels and inhibition of the TGF-β/Smad pathway [239].
Mangiferin represents an important bioactive component of the Mango plant (Mangifera indica) [240]. Chemically, mangiferin features a C-glucosidic xanthone (2-C-β-d-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone, Fig. 4) with potent antioxidant, anti-inflammatory, anti-diabetic, and anticancer activities (e.g., with activity against breast, colon, lung and prostate cancer as well as anti-leukemic activity) [240]. Inhibition of NF-κB and PPARγ was observed for mangiferin [240]. In prostate cancer cells (PC-3), mangiferin stopped cell growth and induced apoptosis via upregulated miR-182 expression associated with suppressed Bcl-2 expression [241]. In addition, apoptosis induction and levels of the tumor suppressor miR-15b were increased upon mangiferin treatment in glioma cells (U87) leading to reduced expression of MMP-9 which suggests an efficient inhibition of metastasis formation by mangiferin as well [242]. The anthracene derivative mithramycin A (aureoilic acid, plicamycin) was isolated from soil bacteria and revealed cell differentiation effects in myeloid leukemia [243]. In erythroleukemia cells (K562), mithramycin A induced leukemia cell differentiation by induction of miR-210 expression and γ-globin expression [244]. A list of microRNAs regulated by emodin, mangiferin and mithromycin A is shown in Table 6.
Table 6.
Regulation of microRNAs by emodin, mangiferin, and mithramycin A.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Emodin | miR-20b, miR-34a, miR-126, miR-211, miR-429 | miR-155, miR-210, miR-221, miR-222 |
| Mangiferin | miR-15b, miR-182 | – |
| Mithramycin A | miR-210 | – |
3.1.6. Miscellaneous phenols
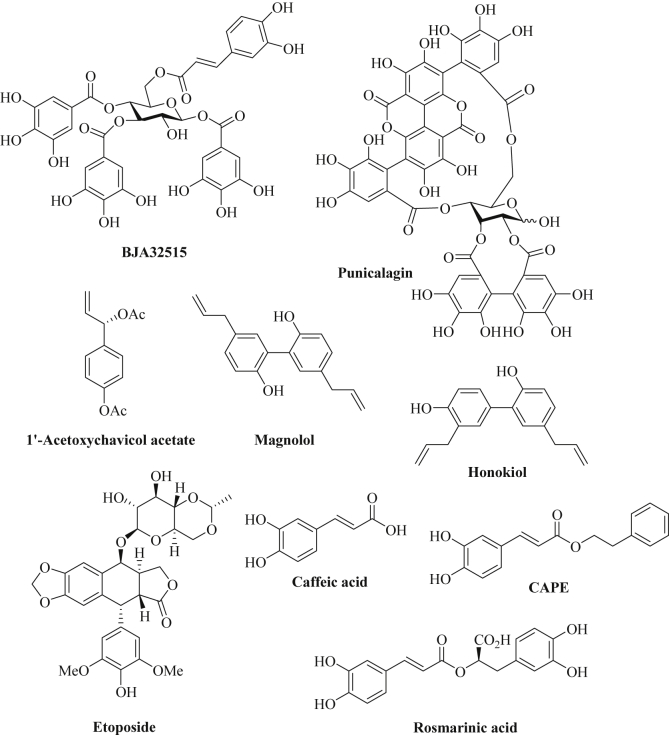
Ellagitannins represent natural galloyl and caffeoyl esters of glucose isolated from the parasitic plant Balanophora japonica that grows on symploco plants in southern China and Japan and which is applied as antidote and hemostatic agent in TCM (Traditional Chinese Medicine) [245]. Derivative BJA3121 (1,3-di-O-galloyl-4,6-hexahydroxydiphenoyl-β-d-glucopyranose) revealed growth inhibitory effects on hepatoma cells (HepG2) and modulated the expression of various miRNAs (e.g., upregulation of let-7e), however, tumor suppressors were also downregulated (let-7a) and oncomiRs were upregulated (miR-373*) by BJA3121 in these cells [246]. In contrast to that, HepG2 hepatoma cells treated with its close analog BJA32515 (1,3,4-tri-O-galloyl-6-O-caffeoyl-β-d-glucopyranose) exhibited increased expression of the tumor suppressors let-7a and miR-29a while oncogenic miR-373 and miR-197 were suppressed (Fig. 5) [247]. Pomegranate (Punica granatum L.) is rich in polyphenolic compounds and pomegranate extracts exhibited distinct anticancer and anti-inflammatory activities. These properties correlated with the inhibition of NF-κB and further inflammatory factors in various cancer cells [248]. A pomegranate extract with significant contents of the ellagitannins punicalagin A, punicalagin B (Fig. 5), and of the anthocyanins delphinidin-3-glucoside and cyanidin-3-glucoside was tested against breast cancer cells (BT-474, MDA-MB-231) [248]. Indeed, the pomegranate extract reduced miR-27a levels in these breast cancer cells associated with increased ZBTB10 (=Sp-repressor) expression and reduced levels of Sp, VEGF, VEGFR (vascular endothelial growth factor receptor) and survivin [248]. In addition, pomegranate extract suppressed miR-155 leading to upregulation of inositol phosphatase SHIP-1 and inhibition of PI3K/Akt signaling [248]. Pomegranate extract also suppressed DNA repair and led to double-strand breaks (DSBs) accompanied by increased apoptosis in MCF-7 breast cancer cells via upregulation of miR-24 and miR-183 [249]. Pomegranate extracts likewise induced miR-34a expression depending on activated p53 leading to suppression of c-Myc and CD44 and increased apoptosis induction in EJ bladder cancer cells [250].
Fig. 5.
Miscellaneous phenolic derivatives.
1′-Acetoxychavicol acetate (Fig. 5), a phenolic compound isolated from Alpinia conchigera (wild ginger), modulated the expression of miRNAs in cervix carcinoma cells (Ca Ski, HeLa) [251]. More precisely, the tumor suppressor miRNAs miR-138, miR-210 and miR-744 were upregulated by 1′-acetoxychavicol acetate in combination with cisplatin [251]. In addition, oncogenic miR-23a was suppressed in head-and-neck squamous cells (HN4) associated with PTEN upregulation and increased apoptosis induction after treatment with 1′-acetoxychavicol acetate [252].
The biphenol magnolol, [4-allyl-2-(5-allyl-2-hydroxyphenyl)phenol], was isolated from Magnolia obovata which has been applied in the Japanese folk medicine for the treatment of cough and gastrointestinal diseases (Fig. 5) [253]. Magnolol exhibited growth inhibitory and anti-metastatic activity against various cancer types and suppressed Wnt signaling in colon cancer cells [253]. In breast cancer cells (MCF-7), the expression of the tumor suppressor miR-200c was increased after treatment with magnolol leading to ZEB1 inhibition and E-cadherin upregulation [254]. Honokiol, [2-(4-hydroxy-3-prop-2-enyl-phenyl)-4-prop-2-enyl-phenol], features a close analog of magnolol which was isolated from various Magnolia species (M. officinalis, M. obovata, M. grandiflora) of Southeast and East Asia (Fig. 5) [255]. The biphenol honokiol revealed distinct anti-oxidative, anti-inflammatory and anticancer activities by targeting multiple factors including signaling pathways of NF-κB, EGFR, STAT-3 and mTOR (mammalian target of rapamycin) [256]. In breast tumors, honokiol inhibited tumor growth via increased expression of miR-34a and STAT-3 inhibition associated with suppression of the Wnt1-MTA-β-catenin signaling pathway [257]. Honokiol also inhibited the growth of bladder tumors by increased expression of the tumor suppressor miR-143 leading to suppression of EZH2, MMP-9, CD44, and Sox2 [258].
Caffeic acid (3,4-dihydroxycinnamic acid) and its phenethyl ester CAPE belong to the active constituents of the propolis extract, a honey bee hive extract, and also occur in various plants (Fig. 5) [259]. Caffeic acid and CAPE exhibited distinct antioxidant, anti-inflammatory and anticancer properties [260], [261]. A significant suppression of angiogenesis was observed after treatment with caffeic acid and CAPE [259]. In hepatocarcinoma cells (HepG2, MHCC97H), caffeic acid increased miR-124 levels via DNA-demethylation associated with suppression of NF-κB/p65, STAT-3, and IL-6 (interleukin-6) [262]. In addition, caffeic acid suppressed cancer stem cell-like features by induction of miR-148a leading to inhibition of the TGF-β/SMAD2 signaling pathway and blocking of SMAD2 expression by miR-148a that binds to the SMAD2-3′UTR [263]. CAPE downregulated pro-inflammatory miRNAs (miR-9, miR-125b, miR-146a, and miR-155) in neuronal cells exposed to Alzheimer's disease derived extracellular fluid via inhibition of NF-κB which might play a role for the anticancer properties of CAPE as well [264]. Rosmarinic acid (isolated from rosemary, Rosmarinus officinalis) features another caffeate derivative with distinct anti-oxidant, anti-inflammatory and anticancer effects (Fig. 5) [265]. In gastric cancer cells (MKN45), rosmarinic acid revealed an anti-Warburg effect, i.e., it suppressed glycolytic biosynthesis of ATP which is a common feature of many cancer types [266]. Downregulation of the microRNAs miR-155, miR-146a and miR-223 was observed after treatment with rosmarinic acid as well as suppression of STAT-3, IL-6 and HIF-Iα (hypoxia inducible factor) [266]. Table 7 provides a list of miRNAs modulated by various phenolic natural compounds.
Table 7.
Regulation of microRNAs by miscellaneous phenols.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| BJA32515 | let-7a, miR-29a | miR-197, miR-373 |
| Pomegranate extract | miR-24, miR-183 | miR-27a, miR-155 |
| 1′-Acetoxychavicol acetate | miR-138, miR-210, miR-744 | miR-23a |
| Magnolol | miR-200c | – |
| Honokiol | miR-34a, miR-143 | – |
| Caffeic acid, CAPE, Rosmarinic acid | miR-124, miR-148a | miR-9, miR-125b, miR-146a, miR-155, miR-223 |
The topoisomerase II inhibitor and approved anticancer agent etoposide is derived from the natural product podophyllotoxin and is applied in advanced stages of breast cancer, lung cancer, leukemia and lymphoma diseases (Fig. 5) [267], [268]. Dean and coworkers investigated the influence of miRNAs on etoposide sensitivity and etoposide resistance of breast cancer cells (sensitive MCF-7, resistant MCF-7/VP) [269]. A significant upregulation of miR-382, miR-23b and miR-885-5p (>2-fold) and suppression of miR-218, miR-758 and miR-548d-5p (>2-fold) was observed from etoposide-resistant MCF-7/VP cells when compared with sensitive MCF-7 cells [269]. MiR-218 was particularly down-regulated (7.79-fold) in the resistant MCF-7/VP cells. The authors assumed a link between the modulation of these miRNAs with the significant upregulation of ABC transporters such as ABCC1 and ABCC6 in the resistant MCF-7/VP cells, which remove etoposide from the cancer cells again [269]. Recently, increased levels of miR-124 upregulated the CDK (cyclin-dependent kinase) inhibitor p27 leading to G1 arrest and sensitized breast and ovarian cancer tumors to etoposide treatment [270]. In hepatoma carcinoma, increased miR-23a expression enhanced the activity of etoposide by suppression of topoisomerase 1, the target of miR-23a, and miR-23a expression depended on functional p53 [271]. HepG2 hepatoma cells were turned into etoposide-resistant cells by upregulation of miR-210 (induced by hypoxia) while upregulation of miR-196b sensitized hepatoma cells to etoposide via suppression of IGF2BP1 (insulin-like growth factor 2 RNA binding protein 1) [272]. Expression of miR-1915-3p in lung cancer cells inhibited etoposide-induced apoptosis by suppression of DRG2 (developmentally regulated GTP-binding protein 2) and PBX2 (pre-B cell leukemia homeobox 2) [273]. In contrast to that, expression of miR-1469 enhanced apoptosis induction by etoposide in lung cancer cells via suppression of STAT5A [274]. The induction of miR-153 reduced the anticancer activity of etoposide in HCC cells via upregulation of survivin and Bcl-2, and inhibition of p21, PTEN and FOXO1 [275]. In addition, osteosarcoma cells responded to etoposide treatment by activation of the tumor suppressor miR-34a in a p53-dependent way [276]. A list of miRNAs involved in etoposide-resistance and –sensitivity is provided in Table 8.
Table 8.
MiRNAs involved in etoposide resistance and sensitivity.
| Etoposide-resistance | Etoposide-sensitivity |
|---|---|
| miR-23b, miR-153, miR-210, miR-382, miR-885-5p, miR-1915-3p | miR-23a, miR-34a, miR-124, miR-196b, miR-218, miR-548d-5p, miR-758, miR-1469 |
3.2. Terpenoids
3.2.1. Triterpenes
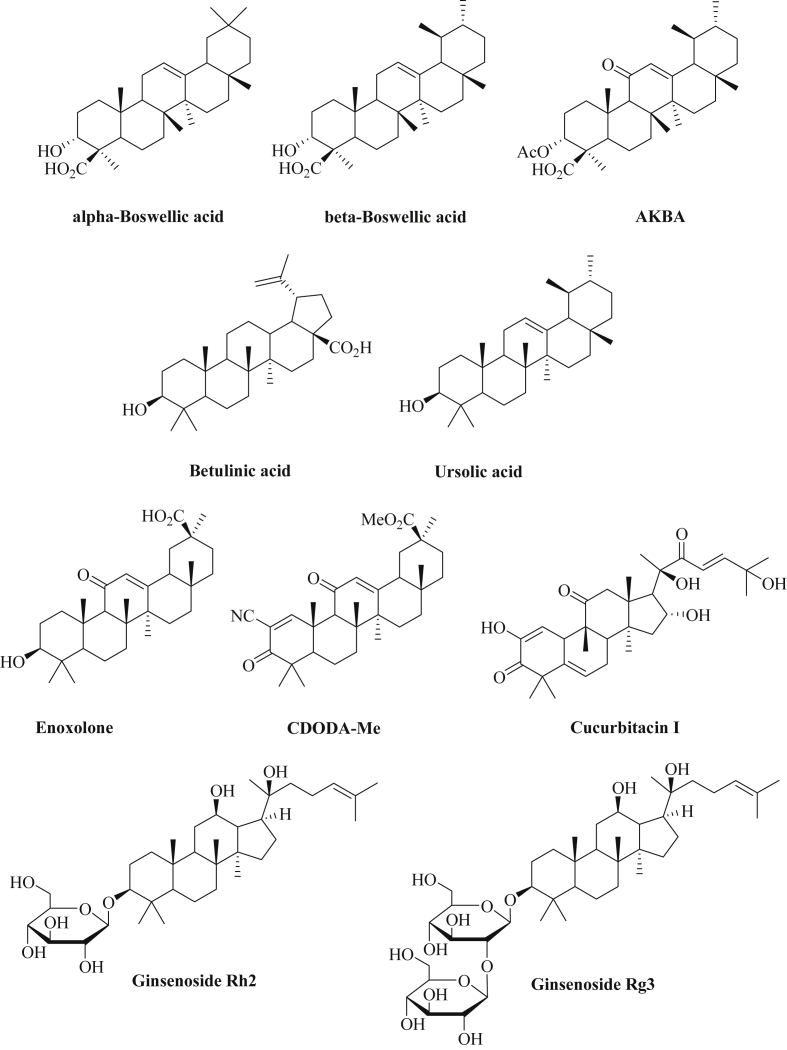
Boswellic acids (Fig. 6) represent the major triterpene constituents of the resin of the plant Boswellia serrata and were found to be potent anti-inflammatory agents (5-LOX inhibitors) [277]. In addition, significant anticancer properties were observed for boswellic acids including the complete remission of brain metastases in a breast cancer patient after consumption of commercially available Boswellia extract [278]. The most active boswellic acid is AKBA (3-acetyl-11-keto-β-boswellic acid) which induced apoptosis and blocked invasion of cancer cells by inhibition of NF-κB and STAT-3 signaling [279], [280]. In colon cancer models, AKBA enhanced the expression of the tumor suppressors let-7 and miR-200 both in vitro and in vivo leading to reduced CDK6 (cyclin-dependent kinase 6) and vimentin levels [281]. In combination with curcumin, AKBA upregulated the tumor suppressor miR-34a in colon cancer cells, while miR-27a was suppressed by AKBA and curcumin. The modulation of these miRNAs was also observed from colon cancer xenografts (HCT-116) upon treatment with AKBA and curcumin associated with tumor growth inhibition when compared with untreated tumors [282].
Fig. 6.
Chemical structures of triterpene derivatives.
The bioactive triterpene betulinic acid (Fig. 6) occurs in birch and plane trees and is easily isolated in significant amounts from the fallen bark of plane trees (Platanus acerifolia) [283]. Betulinic acid has revealed topoisomerase I inhibition and exhibited distinct and selective anticancer activity in mouse xenografts (ovarian cancer, melanoma) while it was non-toxic to the organism at doses up to 500 mg/kg [284], [285]. In colon cancer cells (RKO, SW480), betulinic acid exhibited growth inhibitory and pro-apoptotic effects via suppression of miR-27a and induction of the Sp repressor ZBTB10 (miR-27a target) [286]. Similar effects were observed from estrogen-negative breast cancer cells (MDA-MB-231) after treatment with betulinic acid [287]. Interestingly, the usually oncogenic miR-21 was upregulated by betulinic acid in hepatoma cells in a p53-dependent way and contributed to the pro-apoptotic properties of betulinic acid by suppression of Sod2 [288]. In this special case, miR-21 revealed clear tumor suppressor properties upon activation by betulinic acid. In addition, betulinic acid suppressed miR-33 and NF-κB in lipopolysaccharide-treated macrophages and promoted the expression of the ABC-transporter ABCA1 that is responsible for the transport of cholesterol and phospholipids [289].
Ursolic acid features another natural triterpene found in herbal drug plants such as Radix actinidiae and Oldenlandia diffusa and revealed significant anti-inflammatory and anti-cancer activities (Fig. 6) [290], [291]. In U251 glioma cells, ursolic acid inhibited tumor cell growth via reduced miR-21 expression associated with increased expression of apoptotic PDCD4 and caspase-3 activation as well as blocked TGF-β/SMAD signaling [292].
Aside glabridin isoflavans, licorice extracts also contain significant amounts of the triterpene glycyrrhetinic acid (enoxolone, up to 24% in licorice root extracts) which showed strong apoptosis induction in cancer cells (Fig. 6) [293]. Enoxolone blocked tumor cell invasion via upregulated miR-200c expression in breast cancer cells accompanied by ZEB1 suppression and E-cadherin upregulation [254]. The semi-synthetic glycyrrhetinic acid derivative CDODA-Me (methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate, Fig. 6) inhibited colon cancer growth (RKO, SW480) in vitro and in vivo via reduction of miR-27a levels associated with increased expression of the Sp repressor ZBTB10 and upregulation of Myt-1 which causes G2/M arrest by cdc2 phosphorylation [294]. Interestingly, the gap junction blocker 18α-glycyrrhetinic acid (18-AGA) was able to inhibit the intercellular transfer of miRNAs efficiently [295].
Cucurbitacins represent further triterpenes with amazing biological properties. Cucurbitacin I (Fig. 6), for instance, inhibited JAK/STAT-3 signaling accompanied with strong anticancer activity in mice [296]. CD4+ T cells (cutaneous T-cell lymphoma, Sézary syndrome) treated with cucurbitacin I showed reduced IL-21-mediated oncogenic miR-21 expression and, thus, IL-21 promoted miR-21 expression strongly depended on STAT-3 activation [297]. Apoptosis induction in CD4+ T cells by cucurbitacin I was in parts mediated by miR-21 suppression. Similarly, the IL-21-induced and STAT-3 dependent expression of miR-155 was downregulated by cucurbitacin I [298]. In addition, STAT-3 targeting/suppressing miRNAs such as miR-337-3p revealed synergistic effects in combination with cucurbitacin I in NCI-H1155 lung cancer cells and the IC50 values of cucurbitacin I were improved in these cells when co-treated with miR-337-3p (IC50 = 0.37 μM in miR-337-3p co-treated cells, IC50 = 1.14 μM for cucurbitacin I alone) [299].
Ginsenosides are triterpene saponins with a terpenoid steroidal nucleus connected to a sugar fragment and were isolated from the ginseng plant that is widely applied as a herbal drug both in East Asia and in the West [300]. In glioma cells (U251), ginsenoside Rh2 (Fig. 6) induced miR-128 expression and suppressed miR-21 associated with tumor cell growth inhibition and apoptosis induction in these tumor cells [301]. The Rh2-mediated modulation of microRNA expression of A549 non-small cell lung cancer cells was determined and miR-148a was distinctly upregulated (9.59-fold) by Rh2 in these lung cancer cells [302]. MiR-148a expression revealed significant antitumor effects in various cancer models and, thus, likely contributes to the anticancer effects of Rh2 as well. In addition, oncogenic miR-21, miR-23b, miR-27b, miR-100, miR-221 (4-9-fold) and miR-424 (12.4-fold) were downregulated by Rh2 in A549 lung cancer cells [302]. Ginsenoside Rg3 features another triterpene saponin derivative of red ginseng that efficiently blocked angiogenesis via upregulation of miR-520h leading to suppression of the ephrin receptors EphB2 and EphB3 (Fig. 6) [303]. A list of microRNAs regulated by triterpenes is presented in Table 9.
Table 9.
Interaction with and regulation of microRNAs by triterpenes.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Boswellic acids (AKBA) | let-7, miR-34a, miR-200 | miR-27a |
| Betulinic acid | miR-21(!) | miR-27a, miR-33 |
| Ursolic acid | – | miR-21 |
| Glycyrrhetinic acid derivatives | miR-200c | miR-27a |
| Cucurbitacin I | (synergism with miR-337-3p) | miR-21, miR-155 |
| Ginsenosides (Rh2, Rg3) | miR-148a, miR-520h | miR-21, miR-23b, miR-27b, miR-100, miR-221, miR-424 |
3.2.2. Diterpenes, sesquiterpenes and monoterpenes
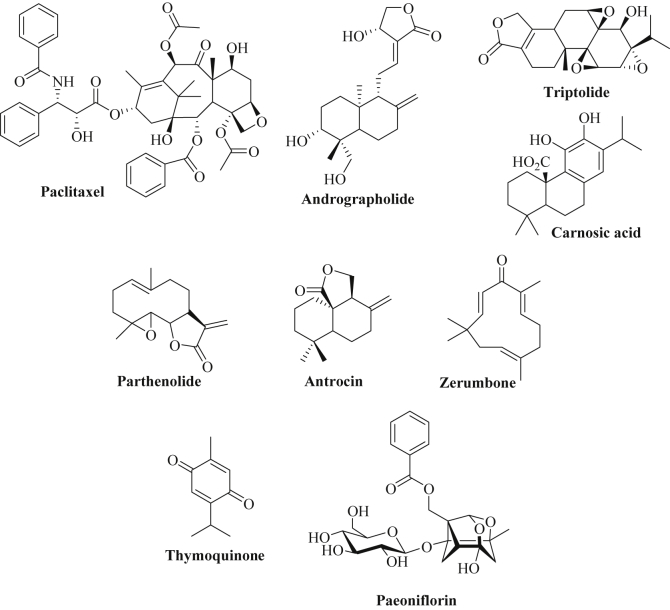
Paclitaxel (taxol) is a clinically approved natural diterpene anticancer drug initially isolated from the Pacific yew (Taxus brevifolia) that induces cell death via microtubule stabilization (Fig. 7) [304], [305]. The formation of paclitaxel resistance is a serious problem in the clinics and miRNAs are involved in sensitivity or resistance to paclitaxel. Forty human cancer cell lines were investigated for miRNAs involved in in vitro sensitivity to paclitaxel and two miRNAs, miR-30a-5p and miR-367, were identified to play a crucial role [306]. While expression of miR-367 increased paclitaxel activity, expression of miR-30a-5p reduced paclitaxel efficacy in cancer cells [306]. In ovarian cancer cells (A2780), paclitaxel sensitivity was associated with miR-149 downregulation and enhanced MyD88 expression [307]. In addition, increased expression of miR-320a, miR-22 and miR-129-5p as well as reduced expression of miR-9, miR-155 and miR-640 were observed in ovarian cancer cells resistant to paclitaxel (ST30 cells) [308]. The miR-9 target RAB34 (a GTPase) might play a role for the observed paclitaxel-resistance [308]. MiR-433 features another oncogenic miRNA that is upregulated in paclitaxel-resistant ovarian cancer cells (A2780) leading to suppression of CDK6 and to cellular senescence [309]. In addition, expression of miR-130b and of miR-197 contributed to paclitaxel-resistance in ovarian cancer cells [310], [311]. Paclitaxel-resistant epithelial ovarian cancer revealed high levels of miR-1307, which was associated with drug resistance [312]. Mimics of miR-200c and miR-141 sensitized drug-resistant ovarian carcinoma cells (MES-OV) to paclitaxel treatment via reversal of EMT (induction of E-cadherin, suppression of vimentin) [313]. Further to this, expression of miR-873 downregulated the drug efflux transporter ABCB1 and increased the anticancer activity of paclitaxel in ovarian cancer cells [314]. Distinctly increased expression of miR-622 and miR-663 featured bad prognosis factors for patients suffering from taxol-resistant ovarian cancer, while expression of miR-647 indicated good prognosis for taxol-sensitive ovarian cancer patients [315]. The expression of miR-125a sensitized cervical cancer to paclitaxel treatment via suppression of STAT3, while miR-375 expression reduced paclitaxel sensitivity by induction of EMT processes [316], [317]. MiR-490-3p features another oncomir in ovarian cancer cells that mediates paclitaxel resistance via upregulation of Pgp and GST-π [318]. Induction of miR-134 in endometrial cancer cells (ECS) promoted paclitaxel activity by inhibition of POGLUT1 (protein O-glucosyltransferase 1) and Notch signaling [319]. Reduced levels of miR-218 were found in taxol-resistant breast cancer cells, and re-expression of miR-218 broke the resistance to taxol via inhibition of survivin [222]. Upregulation of the RNA-processing enzyme Dicer induced the expression of miR-494 and increased the activity of paclitaxel in breast cancer via suppression of AXL kinase [320]. In contrast to that, expression of miR-520h promoted paclitaxel-resistance in breast cancer cells associated with inhibition of DAPK2 (death-associated protein kinase 2) expression [321]. Breast cancer cells expressing miR-621 inhibited FBXO11 expression and activated p53 leading to enhanced sensitivity to paclitaxel treatment [322]. Patients suffering from luminal A subtype breast cancer resistant to neoadjuvant chemotherapy (NAC, paclitaxel plus epirubicin) exhibited distinctly stronger expression of miR-19a and miR-205 than drug-sensitive patients [323]. In addition, breast cancer patients who received NAC exhibited increased expression of miR-34a and miR-122, which may contribute to the anticancer activity of paclitaxel and epirubicn [324]. In paclitaxel-resistant lung cancer cells, miR-16 and miR-17 were downregulated leading to induction of anti-apoptotic Bcl-2, and expression of miR-16 and miR-17 enhanced apoptosis induction by paclitaxel via suppression of Bcl-2 as well as autophagy inhibition via Beclin-1 downregulation [325]. In addition, miR-17-5p was downregulated in paclitaxel-resistant lung cancer cells accompanied by Beclin-1 upregulation [326]. A pH-dependent liposomal formulation of a combination of antagomir-10b and paclitaxel released antagomir-10b and paclitaxel at lower pH values and inhibited lung metastasis formation and tumor growth inhibition of 4T1 breast tumors by suppression of miR-10b [327]. Another formulation of a special copolymer including polyethylene glycol 5000, vitamin E and diethylenetriamine (PEG5K-VE4-DET20) was loaded with a let-7b mimic and paclitaxel and exhibited increased anticancer activity (increased growth inhibition and apoptosis induction as well as reduced invasiveness) in KRAS-mutant lung cancer (A549) both in vitro and in vivo [328]. In gastric cancer cells (SGC7901), miR-21 expression mediated paclitaxel-resistance and inhibition of miR-21 restored paclitaxel activity in these cancer cells via suppression of the drug efflux transporter P-gp [329]. Patients suffering from metastatic and recurrent gastric cancer with low levels of miR-1 and miR-27a revealed better responses to a combination of paclitaxel and fluoropyrimidine than patients with high expression of miR-1 and miR-27a [330]. Downregulation of miR-203 was observed in paclitaxel-resistant colon cancer cells, and induced expression of miR-203 suppressed SIK2 (salt-inducible kinase 2) expression leading to sensitization to paclitaxel [331]. In HCT-116 colon cancer cells, upregulation of miR-497 suppressed the oncogene Smurf1 leading to increased paclitaxel activity [332]. In paclitaxel-resistant prostate cancer cells, miR-130a was downregulated and re-expression of miR-130a promoted the activity of paclitaxel via apoptosis activation [333]. Paclitaxel-resistant nasopharyngeal carcinoma (NPC) cells (CNE-1/Taxol) showed low expression both of miR-634 and of miR-1204, and restored miR-634 and miR-1204 expression sensitized NPC cells to paclitaxel treatment in vitro and in vivo again [334], [335]. The expression of miR-153 diminished the anticancer activity of paclitaxel in HCC cells via upregulation of survivin and Bcl-2, and inhibition of p21, PTEN and FOXO1 [275]. In addition, treatment of HCC cells with paclitaxel upregulated the tumor suppressor miR-877 and induced tumor cell growth inhibition by suppression of FOXM1 [336]. Inhibition of miR-133a/b and miR-361-3p induced cell cycle arrest (S phase arrest) and apoptosis (activation of caspase-3/7) and enhanced distinctly the anti-proliferative effects of paclitaxel in NSCLC cells (H1993) [337]. A list of miRNAs involved in paclitaxel-resistance and –sensitivity is provided in Table 10.
Fig. 7.
Chemical structures of di-, sesqui- and monoterpenes.
Table 10.
MiRNAs involved in paclitaxel resistance and sensitivity.
| Paclitaxel-resistance | Paclitaxel-sensitivity |
|---|---|
| miR-19a, miR-21, miR-22, miR-30a-5p, miR-129-5p, miR-130b, miR-149, miR-153, miR-133a/b, miR-197, miR-205, miR-320a, miR-361-3p, miR-375, miR-433, miR-490, miR-520h, miR-622, miR-663, miR-1307 | let-7b, miR-9, miR-10b, miR-16, miR-17, miR-17-5p, miR-34a, miR-122, miR-125a, miR-130a, miR-134, miR-141, miR-155, miR-200c, miR-218, miR-367, miR-494, miR-497, miR-647, miR-873, miR-877, miR-1204 |
The labdane diterpene andrographolide of Andrographis paniculata has revealed chemopreventive effects and protected A549 alveolar epithelial cells from damages induced by cigarette smoke (Fig. 7) [338]. In these cells, andrographolide increased the expression of miR-218 accompanied by reduced NF-κB signaling (reduced IκB-α phosphorylation and lowered p65 accumulation in the nucleus) leading to reduced inflammation [338]. In addition, andrographolide increased the expression of miR-23a-5p, miR-30b-5p, miR-106b-5p and miR-222-3p in hepatoma cells leading to hepatoma growth inhibition [339].
Triptolide features an anticancer active diterpene triepoxide lactone of the Chinese plant Tripterygium wilfordii (thunder god vine, applied in TCM) and suppressed the heat shock protein Hsp70 in cancer cells associated with cancer cell growth inhibition (Fig. 7) [182]. Unlike the approved anti-pancreatic cancer drug gemcitabine, triptolide also induced miR-142-3p expression (a miRNA repressor of Hsp70) in pancreatic cancer cells, while minnelide, the water-soluble triptolide prodrug, increased miR-142-3p expression in vivo in a pancreatic cancer xenograft model [182]. Further to this, triptolide augmented the MDM2-suppressing effects of miR-339-5p both in MCF-7 breast cancer and in HCT-116 colon cancer cell lines after induction of the p53 inhibitor MDM2 by the approved anticancer drug 5-FU [340].
Aside rosmarinic acid, rosemary (Rosmarinus officinalis L.) extracts also contain bioactive diterpenes such as carnosol and carnosic acid (Fig. 7) [341]. Both carnosol and carnosic acid showed antitumor activity against pancreatic and colon cancer both in vitro and in vivo by suppression of miR-15b and induction of glycosyltransferase GCNT3 [342]. In addition, carnosic acid in combination with 1,25-dihydroxyvitamin D3 reduced miR-181a levels in leukemia cells (HL-60, U937) leading to cell cycle arrest and cell differentiation [343].
Sesquiterpenes have shown potent anticancer activities, for instance, the fungal compound class of illudins exhibited strong tumor growth inhibitory activity via unique DNA binding properties [344]. The sesquiterpene lactone parthenolide (Fig. 7) isolated from feverfew (Tanacetum parthenium) has also shown distinct anticancer activity in various cancer cell lines and parthenolide showed NF-κB inhibition and DNA hypomethylation via DNMT1 suppression [345]. Thus, miRNA modulation by parthenolide was assumed and in acute myelogenous leukemia cells (MOLM-13), parthenolide indeed increased the expression of the known tumor suppressors miR-15a and miR-16 [346].
The medicinal fungus Antrodia camphorata contains the sesquiterpene lactone antrocin (Fig. 7) that has revealed strong anticancer activity in triple-negative breast cancer cells (MDA-MB-231) [347]. In addition, antrocin revealed growth inhibition and apoptosis induction in lung cancer cells (H441, gefitinib-resistant H1975) via increased let-7c expression associated with STAT3 inhibition [348]. Another Southeast Asian ginger sesquiterpene, zerumbone (Fig. 7), induced apoptosis in Panc-1 pancreatic cancer cells via increased expression of miR-34 accompanied by p53 and p21 activation [349].
Monoterpenes such as menthol have shown antitumor activity against various cancer cells, and monoterpene conjugates even overcame cisplatin-resistance [350], [351], [352]. Thymoquinone (TQ, Fig. 7) represents the active monoterpene component of the oil of black seeds (Nigella sativa), a frequently applied plant (kalonji) in traditional Indian medicine (Ayurveda, Unani), and exhibited significant antineoplastic effects [353]. Among others, polo-like kinases (Plk) were identified as tumor targets for thymoquinone [354]. Recently, a PEGylated thymoquinone nanoparticle (PEG4000-TQ-Np, PEG = polyethylene glycol) with improved aqueous solubility and tumor selectivity was prepared and investigated for its anticancer properties in breast cancer cells [355]. This nanoparticle exhibited increased breast tumor cell killing and anti-migratory activity by upregulation of miR-34a and downregulation of its target Rac1 (a Rac GTPase) leading to actin depolymerisation [355].
Paeoniflorin represents a major monoterpene glucoside of the medicinal plant Paeonia lactiflora Pall. and blocked tumor cell invasion and metastasis (Fig. 7) [356]. Paeoniflorin also inhibited glioma cell growth (U87) and induced apoptosis in glioma cells via upregulation of miR-16 and suppressed MMP9 expression [357]. In addition, paeoniflorin showed protective effects on doxorubicin-treated cardiomyocetes via suppressed miR-1 expression and inhibition of doxorubicin-induced apoptosis in cardiomyocetes [358]. A list of microRNAs regulated by di-, sesqui- and monoterpenes is presented in Table 11.
Table 11.
MicroRNAs regulated or influenced by di-, sesqui- and monoterpenes.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Andrographolide | miR-23a-5p, miR-30b-5p, miR-106b-5p, miR-218, miR-222-3p | – |
| Triptolide | miR-142-3p, miR-339-5p (synergism) | – |
| Carnosic acid | – | miR-15b, miR-181a |
| Parthenolide | miR-15a, miR-16 | – |
| Antrocin | let-7c | – |
| Zerumbone | miR-34 | – |
| PEG-Thymoquinone | miR-34a | – |
| Paeoniflorin | miR-16 | – |
3.2.3. Terpenoidal vitamins A, D, and E
Retinoic acid (RA, vitamin A, Fig. 8) is an essential natural diterpene for the regulation of cell growth and differentiation through binding to retinoic acid receptors (RARs) and revealed anticancer effects in various cancer models including breast, prostate, lung, liver, and pancreatic cancer [359]. In acute promyelocytic leukemia cells, retinoic acid induced the expression of several miRNAs such as let-7a-3, let-7c, miR-15a, miR-15b, miR-16-1, miR-107, miR-186, miR-215, miR-223, and miR-342, while five miRNAs (miR-17, miR-25, miR-93, miR-193, miR-181b) were suppressed after treatment with retinoic acid [360], [361]. Though the inhibition of breast cancer cell growth by retinoic acid was counteracted by RA-induced expression of miR-21, RA-induced expression of miR-21 also inhibited the motility of estrogen-receptor(ER)-positive breast cancer cells (MCF-7) and suggested a beneficial effect concerning the anti-metastatic properties of retinoic acid via suppression of maspin [362]. In neuroblastoma cells (SH-SY5Y), retinoic acid induced differentiation via upregulation of miR-10a and miR-10b [363]. The SR-family splicing factor SFRS1 was identified as an important target of miR-10a and miR-10b upon treatment with retinoic acid. In addition, miR-10a and miR-10b mediated the anti-migratory and anti-metastatic activity of retinoic acid in these neuroblastoma cells [363]. In addition, retinoic acid induced neuroblastoma differentiation by upregulation of miR-9 and miR-103 associated with suppression of the expression of the differentiation inhibitor ID2 (inhibitor of DNA binding-2) [364]. In breast cancer, levels both of RARβ and of the tumor suppressor miR-10a were reduced and a positive correlation between miR-10a and RARβ was assumed [365]. Indeed, retinoic acid was able to upregulate miR-10a expression (2-fold) in breast cancer cells either alone (in SK-BR-3 cells) or in combination with thyroid hormone receptor/THRα-binding l-thyroxine (in T47D cells) [365]. Retinoic acid also downregulated c-Myc and miR-130a in cancer cells leading to increased HOXA5 expression and cell death [366]. The differentiation of embryonic stem cells (ESCs) was induced by retinoic acid via suppression of miR-200b and miR-200c and of pluripotent genes such as Oct4 and Nanog [367]. Hence, in this special case the tumor suppressors miR-200b and miR-200c act as promoters of stem-cell properties such as pluripotency and immortality which can be suppressed by retinoic acid. In addition, the tumor suppressor miR-663 was upregulated by retinoic acid in leukemia cells (HL-60) leading to leukemia cell differentiation [368]. By the way, miR-10a was downregulated in these HL-60 leukemia cells after treatment with retinoic acid [368]. In glioblastoma cells, retinoic acid enhanced the anticancer effects of transfected miR-124-3p by formation of gap junctions and simplified intercellular transfer of miR-142-3p [369]. In addition, retinoic acid induced glioma cell death by induction of miR-302b leading to suppression of the transcriptional glioma growth regulator E2F3 [370]. Retinoids are derived from carotenoids and lycopene (Fig. 8) features an interesting carotenoid antioxidant from tomatoes that inhibited tumor growth [371]. In prostate cancer (PC3 cells), lycopene revealed growth inhibition and apoptosis induction via upregulation of let-7f-1 associated with suppression of Akt2 (let-7f-1 target) [372].
Fig. 8.
Chemical structures of lipophilic vitamins and of their derivatives.
Vitamin D and its active metabolite 1,25-dihydroxyvitamin D3 (1,25-D, Fig. 8) are essential natural 9,10-seco-steroids that bind to the vitamin D receptor (VDR) and, thus, modulate various signaling pathways leading to cell cycle arrest (G0/G1), apoptosis and cell differentiation [373]. 1,25-D induced miR-22 expression in colon cancer cells (SW480-ADH, HCT-116) and suppressed target genes such as NELL2, OGN, HNRPH1, RERE and NFAT5 associated with anti-proliferative and anti-migratory effects of 1,25-dihydroxyvitamin D3 [374]. On the other hand, 1,25-D inhibited AraC-induced apoptosis of acute myeloid leukemia cells by induction of miR-32 via Bim [375]. 1,25-D increased miR-627 expression in colon cancer models associated with suppressed JMJD1A (a histone demethylase) and growth and differentiation factor 15 leading to colon tumor growth inhibition [376]. The tumor suppressors miR-22, miR-296-3p and miR-498 were induced by 1,25-D in cervical cancer cells (SiHa) accompanied by increased Dicer expression [377]. Dicer expression was increased by 1,25-D because of the vitamin D response element in the Dicer promoter [377]. In addition, the tumor suppressor let-7a-2 was induced by 1,25-D in lung cancer cells (A549), while the tumor suppressors miR-100 and miR-125b were upregulated by 1,25-D in prostate cancer cells [378], [379]. However, in MCF-7 breast cancer cells, miR-125b acted as a repressor for VDR and, thus, induced resistance to 1,25-D via reduced levels of VDR [380]. In human myeloid leukemia cells, 1,25-D suppressed miR-181a and miR-181b leading to cell cycle arrest by upregulation of p27KIP1 and p21CIP1 [381]. In addition, downregulation of the miR-17–92 cluster contributed to 1,25-D anticancer activity in NSCLC cells [382]. 1,25-D also upregulated miR-15a, miR-20b, and miR-181C in HUVECs (human umbilical vein endothelial cells) which contributed to the anti-angiogenic activity of vitamin D [383].
Tocopherol (vitamin E, Fig. 8) is a natural 6-chromanol with an isoprenoid side-chain that possesses antioxidant properties [384]. Vitamin E deficiency led to decreased levels of miR-122a and miR-125b (i.e., microRNAs involved in lipid metabolism, cancer and inflammation) in the liver of rats [385]. Downregulated miR-122a and miR-125b were observed from various cancer types and it is assumed that induction of these miRNAs contributes to the anticancer effects of tocopherol [385]. Tocotrienols are closely related to tocopherol and exhibited apoptosis induction via inhibition of signaling pathways such as PI3K/Akt and NF-κB [386]. In non-small cell lung cancer cells, delta-tocotrienol (Fig. 8) increased the expression of the tumor suppressor miR-34a leading to inhibition of Notch-1 signaling [387]. Delta-tocotrienol-induced expression of miR-34a suppressed the expression of Notch-1 and of its downstream factors (Hes-1, Cyclin-D1, Survivin, Bcl-2) associated with lung tumor cell growth inhibition, apoptosis induction and inhibition of lung cancer cell invasion [387]. Antroquinonol is a structurally related ubiquinone derivative of the camphor tree mushroom Antrodia camphorata which is applied in TCM for the treatment of several diseases (Fig. 8) [388]. Antroquinonol inhibited cell growth of hepatocellular carcinoma cell lines via AMPK (AMP-activated protein kinase) activation and mTOR inhibition [389]. In non-small cell lung cancer cells, antroquinonol revealed anti-proliferative activity and suppressed the expression of Bcl-2, PI3K and mTOR proteins [390]. Seven microRNAs (miR-15a, miR-18a, miR-2861, miR-302b, miR-382, miR-487a, miR-760) were upregulated in A549 lung cancer cells treated with antroquinonol, while twelve miRNAs were downregulated (miR-10a, miR-135a, miR-299-3p, miR-3126-5p, miR-3153, miR-411, miR-4321, miR-486-5p, miR-505, miR-598, miR-601, miR-939) after antroquinonol treatment [390]. A list of microRNAs regulated by terpenoidal vitamins is presented in Table 12.
Table 12.
MicroRNAs regulated by lipophilic vitamins and vitamin derivatives.
| Compound | Tumor suppressors (up-regulated) | Oncogenes (down-regulated) |
|---|---|---|
| Retinoic acid | let-7a-3, let-7c, miR-9, miR-10a, miR-10b, miR-15a, miR-15b, miR-16-1, miR-21(!), miR-103, miR-107, miR-124-3p, miR-186, miR-215, miR-223, miR-302b, miR-342, miR-663 | miR-17, miR-25, miR-93, miR-130a, miR-181b, miR-193, miR-200b(!), miR-200c(!) |
| Lycopene | let-7f-1 | – |
| 1,25-Dihydroxy-vitamin D3 | let-7a-2, miR-15a, miR-20b, miR-22, miR-100, miR-125b (prostate cancer), miR-181C, miR-296-3p, miR-498, miR-627 | miR-17–92 cluster, miR-181a, miR-181b |
| Tocopherol, delta-Tocotrienol | miR-34a, miR-122a, miR-125b | – |
| Antroquinonol | miR-15a, miR-18a, miR-2861, miR-302b, miR-382, miR-487a, miR-760 | miR-10a (lung cancer), miR-135a, miR-299-3p, miR-3126-5p, miR-3153, miR-411, miR-4321, miR-486-5p, miR-505, miR-598, miR-601, miR-939 |
4. Conclusions
Various natural phenolic and terpenoidal compounds of known anticancer activity exerted their anticancer activity via regulation of microRNAs involved in proliferation, cell death and differentiation, angiogenesis and metastasis formation. Quite a few of these natural compounds (e.g., curcumin, boswellic acid, etc.) have shown low toxicity both in animal studies and in clinical trials and appear to be suitable additives to conventional cancer therapies. In addition, the sensitivity and resistance of cancer cells to approved phenolic and terpenoidal anticancer agents such as doxorubicin, etoposide and taxol include modified expression of tumor suppressor and oncogenic miRNAs. A detailed understanding of the miRNA regulation by natural phenols and terpenes will provide essential information for improved therapy regimens including combination therapies with approved anticancer drugs or other conventional therapies associated with improved prognoses, reduced side-effects, increased quality of life of cancer patients, and breach or prevention of drug resistance.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Srivastava S.K., Arora S., Averett C., Singh S., Singh A. Modulation of microRNAs by phytochemicals in cancer: underlying mechanisms and translational significance. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/848710. Article ID 848710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarnieri D.J., DiLeone R.J. MicroRNAs: a new class of gene regulators. Ann. Med. 2008;40:197–208. doi: 10.1080/07853890701771823. [DOI] [PubMed] [Google Scholar]
- 3.Jeong H.C., Kim E.K., Lee J.H., Yoo H.N., Kim J.K. Aberrant expression of let-7a miRNA in the blood of non-small cell lung cancer patients. Mol. Med. Rep. 2011;4:383–387. doi: 10.3892/mmr.2011.430. [DOI] [PubMed] [Google Scholar]
- 4.Mallick R., Patnaik S.K., Yendamuri S. Micro RNAs and lung cancer: biology and applications in diagnosis and prognosis. J. Carcinog. 2010;9:8. doi: 10.4103/1477-3163.67074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patnaik S.K., Mallick R., Yendamuri S. Detection of microRNAs in dried serum blots. Anal. Biochem. 2010;407:147–149. doi: 10.1016/j.ab.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothe F., Ignatiadis M., Chaboteaux C., Haibe-Kains B., Kheddoumi N., Majjaj S., Badran B., Fayyad-Kazan H., Desmedt C., Harris A.L., Piccart M., Sotiriou C. Global microRNA expression profiling identifies miR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enerly E., Steinfeld I., Kleivi K., Leivonen S.-K., Aure M.R., Russnes H.G., Ronneberg J.A., Johnsen H., Navon R., Rodland E., Mäkelä R., Naume B., Perälä M., Kallioniemi O., Kristensen V.N., Yakhini Z., Borresen-Dale A.-L. MiRNA-mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS One. 2011;6:e16915. doi: 10.1371/journal.pone.0016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang-Verslues W.W., Chang P.-H., Wei P.-C., Yang C.-Y., Huang C.-K., Kuo W.-H., Shew J.-Y., Chang K.-J., Lee E.Y.-H.P., Lee W.-H. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via down-regulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 9.Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., Dirbas F.M., Somlo G., Pera R.A., Lao K., Clarke M.F. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory P.A., Bracken C.P., Bert A.G., Godall G.J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 11.Yu F., Jiao Y., Zhu Y., Wang Y., Zhu J., Cui X., Liu Y., He Y., Park E.-Y., Zhang H., Lv X., Ma K., Su F., Park J.H., Song E. MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J. Biol. Chem. 2012;287:465–473. doi: 10.1074/jbc.M111.280768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y., Kim M., Han J., Yeom K.-H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denli A.M., Tops B.B., Plasterk R.H., Ketting R.F., Hannon G.J. Processing of microRNAs by the microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 15.Lund E., Guttinger S., Calado A., Dahlberg J.E., Kutay U. Nuclear export of miRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 16.Zeng Y., Wagner E.J., Cullen B.R. Both natural and designed microRNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell. 2002;9:1327–1333. doi: 10.1016/s1097-2765(02)00541-5. [DOI] [PubMed] [Google Scholar]
- 17.Hutvagner G., McLachlan J., Pasquinelli A.E., Bálint E., Tuschl T., Zamore P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M.F. Drug target miRNAs: chances and challenges. Trends Biotechnol. 2014;32:578–585. doi: 10.1016/j.tibtech.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Jackson A.L., Bartz S.R., Schelter J., Kobayashi S.V., Burchard J., Mao M., Li B., Cavet G., Linsley P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 20.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targtets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Vasudevan S., Tong Y., Steitz J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 22.Eiring A.M., Harb J.G., Neviani P., Garton C., Oaks J.J., Spizzo R., Liu S., Schwind S., Santhanam R., Hickey C.J., Becker H., Chandler J.C., Andino R., Cortes J., Hokland P., Huettner C.S., Bhatia R., Roy D.C., Liebhaber S.A., Caligiuri M.A., Marcucci G., Garzon R., Croce C.M., Calin G.A., Perrotti D. MiR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic cells. Cell. 2010;140:652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S., Huang S., Ding J., Zhao Y., Liang L., Liu T., Zhan R., He X. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene. 2010;29:2302–2308. doi: 10.1038/onc.2010.34. [DOI] [PubMed] [Google Scholar]
- 24.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 25.di Leva G., Garofolo M., Croce C.M. MicroRNAs in cancer. Ann. Rev. Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouyssou J.M., Manier S., Huynh D., Issa S., Roccaro A.M., Ghobrial I.M. Regulation of microRNAs in cancer metastasis. Biochim. Biophys. Acta. 2014;22:255–265. doi: 10.1016/j.bbcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King H., Fabbri M., Calin G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu F., Yao H., Zhu P., Zhang X., Pan Q., Gong C., Huang Y., Hu X., Su F., Liebeman J., Song E. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 29.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Kumar M.S., Erkeland S.J., Pester R.E., Chen C.Y., Ebert M.S., Sharp P.A., Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B., Pan X., Cobb G.P., Anderson T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Takamizawa J., Konishi H., Yanagisawa K., Tomida S., Osada H., Endoh H., Harano T., Yatabe Y., Nagino M., Nimura Y., Mitsudomi T., Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened post-operative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 33.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., Rassenti L., Kipps T., Negrini M., Bullrich F., Croce C.M. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcil S.E., Aqeilan R.I., Zupo S., Dono M., Rassenti L., Aldler H., Volinia S., Liu C., Kipps T.J., Negrini M., Croce C.M. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeshita F., Patrawala L., Osaki M., Takahashi R.U., Yamamoto Y., Kosaka N., Kawamata M., Kelnar K., Bader A.G., Brown D., Ochiya T. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via down-regulation of multiple cell-cycle genes. Mol. Ther. 2010;18:181–187. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia L., Zhang D., Du R., Pan Y., Zhao L., Sun S., Hong L., Liu J., Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 37.Ji Q., Hao X., Zhang M., Tang W., Yang M., Li L., Xiang D., DeSano J.T., Bommer G.T., Fan D., Fearon E.R., Lawrence T.S., Xu L. MicroRNA miR-34 inhibits human pancreatic cancer tumor initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang T.C., Wentzel E.A., Kent O.A., Ramachandran K., Mullendore M., Lee K.H., Feldmann G., Yamakuchi M., Ferlito M., Lowenstein C.J., Arking D.E., Beer M.A., Maitra A., Mendell J.T. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javeri A., Ghaffarpour M., Taha M.F., Houshmand M. Downregulation of miR-34a in breast tumors is not associated with either p53 mutations or promoter hypermethylation while it correlates with metastasis. Med. Oncol. 2013;30:413. doi: 10.1007/s12032-012-0413-7. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava S.K., Bhardwaj A., Singh S., Arora S., Wang B., Grizzle W.E., Singh A.P. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang X., Huang H., Li Z., He C., Li Y., Chen P., Gurbuxani S., Arnovitz S., Hong G.-M., Price C., Ren H., Kunjamma R.B., Neilly M.B., Salat J., Wunderlich M., Slany R.K., Zhang Y., Larson R., Le Beau M.M., Mulloy J.C., Rowley J.D., Chen J. MiR-495 is a tumor-suppressor microRNA down-regulated in MLL-rearranged leukemia. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19397–19402. doi: 10.1073/pnas.1217519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D., Liu C., Wang X., Ingvarsson S., Chen H. MicroRNA-451 suppresses tumor cell growth by down-regulating IL6R gene expression. Cancer Epidemiol. 2014;38:85–92. doi: 10.1016/j.canep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Liu X., Zhang X., Xiang J., Lu Y., Shi J. miR-451: potential role as tumor suppressor of human hepatoma cell growth and invasion. Int. J. Oncol. 2014;45:739–745. doi: 10.3892/ijo.2014.2446. [DOI] [PubMed] [Google Scholar]
- 44.Lv G., Hu Z., Tie Y., Du J., Fu H., Gao X., Zheng X. MicroRNA-451 regulates activating transcription factor 2 expression and inhibits liver cancer cell migration. Oncol. Rep. 2014;32:1021–1028. doi: 10.3892/or.2014.3296. [DOI] [PubMed] [Google Scholar]
- 45.Xu D., Wang Q., An Y., Xu L. MiR-203 regulates the proliferation, apoptosis and cell cycle progression of pancreatic cancer cells by targeting Survivin. Mol. Med. Rep. 2013;8:379–384. doi: 10.3892/mmr.2013.1504. [DOI] [PubMed] [Google Scholar]
- 46.Tavazoie S.F., Alarcon C., Oskarsson T., Padua D., Wang Q., Bos P.D., Gerald W.L., Massagué J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musiyenko A., Bitko V., Barik S. Ectopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates protein translation and invasiveness of prostate cancer LNCaP cells. J. Mol. Med. 2008;86:313–322. doi: 10.1007/s00109-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G., Mao W., Zheng S. MicroRNA-183 regulates Ezrin expression in lung cancer cells. FEBS Lett. 2008;582:3663–3668. doi: 10.1016/j.febslet.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 49.Crawford M., Brawner E., Batte K., Yu L., Hunter M.G., Otterson G.A., Nuovo G.J., Marsh C.B., Nana-Sinkam S.P. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem. Biophys. Res. Commun. 2008;373:607–612. doi: 10.1016/j.bbrc.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 50.Ha T.Y. MicroRNAs in human diseases: from cancer to cardiovascular disease. Immune Netw. 2011;11:135–154. doi: 10.4110/in.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., Coukos G. MicroRNAs: a new insight into cancer genome. Cell Cycle. 2006;5:2216–2219. doi: 10.4161/cc.5.19.3319. [DOI] [PubMed] [Google Scholar]
- 52.Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 53.Si M.L., Zhu S., Wu H., Lu Z., Wu F., Mo Y.Y. MiR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 54.Ma Y., Yu S., Zhao W., Lu Z., Chen J. MiR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298:150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Wu K., Hu G., He X., Zhou P., Li J., He B., Sun W. MicroRNA-424-5p suppresses the expression of socs6 in pancreatic cancer. Pathol. Oncol. Res. 2013;19:739–748. doi: 10.1007/s12253-013-9637-x. [DOI] [PubMed] [Google Scholar]
- 56.Jiang S., Zhang H.W., Lu M.H., He X.H., Li Y., Gu H., Liu M.F., Wang E.D. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010;70:3119–3127. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 57.Greither T., Grochola L.F., Udelnow A., Lautenschlager C., Wurl P., Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int. J. Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 58.Iorio M.V., Ferracin M., Liu C.G., Veronese A., Spizzo R., Sabbioni S., Magri E., Pedriali M., Fabbri M., Campiglio M., Ménard S., Palazzo J.P., Rosenberg A., Musiani P., Volinia S., Nenci I., Calin G.A., Querzoli P., Negrini M., Croce C.M. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 59.Lawrie C.H. MicroRNA expression in lymphoma. Expert Opin. Biol. Ther. 2007;7:1363–1374. doi: 10.1517/14712598.7.9.1363. [DOI] [PubMed] [Google Scholar]
- 60.Diosdado B., van de Wiel M.A., Terhaar Sive Droste J.S., Mongera S., Postma C., Meijerink W.J., Carvalho B., Meijer G.A. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br. J. Cancer. 2009;101:707–714. doi: 10.1038/sj.bjc.6605037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashita Y., Osada H., Tatematsu Y., Yamada H., Yanagisawa K., Tomida S., Yatabe Y., Kawahara K., Sekido Y., Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 62.Dews M., Homayouni A., Yu D., Murphy D., Sevignani C., Wentzel E., Furth E.E., Lee W.M., Enders G.H., Mendell J.T., Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He L., Thomson J.M., Hemann M.T., Hernando-Monge E., Mu D., Goodson S., Powers S., Cordon-Cardo C., Lowe S.W., Hannon G.J., Hammond S.M. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 65.Huang Q., Gumireddy K., Schrier M., Ie S.C., Nagel R., Nair S., Egan D.A., Li A., Huang G., Klein-Szanto A.J., Gimotty P.A., Katsaros D., Coukos G., Zhang L., Puré E., Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 66.Yang K., Handorean A.M., Iczkowski K.A. MicroRNAs 373 and 520c are down regulated in prostate cancer, suppress CD44 translation and enhance invasion of prostate cancer cells in vitro. Int. J. Clin. Exp. Pathol. 2009;2:361–369. [PMC free article] [PubMed] [Google Scholar]
- 67.Miller T.E., Goshal K., Ramaswamy B., Roy S., Datta J., Shapiro C.L., Jacob S., Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garofalo M., Quintavalle C., Di L.G., Zanca C., Romano G., Taccioli C., Liu C.G., Croce C.M., Concorelli G. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;19:3845–3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- 69.Yang H., Kong W., He L., Zhao J.J., O'Donnell J.D., Wang J., Wenham R.M., Coppola D., Kruk P.A., Nicosia S.V., Cheng J.Q. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 70.van Schooneveld E., Wildiers H., Vergote I., Vermeulen P.B., Dirix L.Y., van Laere S.J. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015;17:21. doi: 10.1186/s13058-015-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson B.L., Grabowska A., Ratan H.L. MicroRNA in prostate cancer: functional importance and potential as circulating biomarkers. BMC Cancer. 2014;14:930. doi: 10.1186/1471-2407-14-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazeh H., Mizrahi I., Ilyayev N., Halle D., Brücher B.L.D.M., Bilchik A., Protic M., Daumer M., Stojadinovic A., Avital I., Nissan A. The diagnostic and prognostic role of microRNA in colorectal cancer – a comprehensive review. J. Cancer. 2013;4:281–295. doi: 10.7150/jca.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin P.-Y., Yu S.-L., Yang P.-C. MicroRNA in lung cancer. Br. J. Cancer. 2010;103:1144–1148. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vyas A., Dandawate P., Padhye S., Ahmad A., Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013;19:2047–2069. [PMC free article] [PubMed] [Google Scholar]
- 75.Phuah N.H., Nagoor N.H. Regulation of microRNAs by natural agents: new strategies in cancer therapies. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/804510. Article ID 804510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., Cao Y., Sun J., Zhang Y. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR16 in MCF-7 cells. Med. Oncol. 2010;27:1114–1118. doi: 10.1007/s12032-009-9344-3. [DOI] [PubMed] [Google Scholar]
- 77.Gao S., Yang J., Chen C., Chen J., Ye L., Wang L., Wu J., Xing C., Yu K. Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells. J. Exp. Clin. Cancer Res. 2012;31 doi: 10.1186/1756-9966-31-27. Article 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saini S., Arora S., Majid S., Shahryari V., Chen Y., Deng G., Yamamura S., Ueno K., Dahiya R. Curcumin modulates micrRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev. Res. 2011;4:1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sreenivasan S., Thirumalai K., Danda R., Krishnakumar S. Effect of curcumin on miRNA expression in human Y79 retinoblastoma cells. Curr. Eye Res. 2012;37:421–428. doi: 10.3109/02713683.2011.647224. [DOI] [PubMed] [Google Scholar]
- 80.Kronski E., Fiori M.E., Barbieri O., Astigiano S., Mirisola V., Killian P.H., Bruno A., Pagani A., Rovera F., Pfeffer U., Sommerhoff C.P., Noonan D.M., Nerlich A.G., Fontana L., Bachmeier B.E. MiR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 2014;8:581–595. doi: 10.1016/j.molonc.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S.F., Zhang X., Zhang X.J., Shi X.Q., Yu Z.J., Kan Q.C. Induction of microRNA-9 mediates cytotoxicity of curcumin against SKOV3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2014;15:3363–3368. doi: 10.7314/apjcp.2014.15.8.3363. [DOI] [PubMed] [Google Scholar]
- 82.Dahmke I.N., Backes C., Rudzitis-Auth J., Laschke M.W., Leidinger P., Menger M.D., Meese E., Mahlknecht U. Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS One. 2013;8:e81122. doi: 10.1371/journal.pone.0081122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell. Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y., Brenn T., Brown E.R., Doherty V., Melton D.W. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br. J. Cancer. 2012;106:553–561. doi: 10.1038/bjc.2011.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma J., Fang B., Zeng F., Pang H., Zhang J., Shi Y., Wu X., Cheng L., Ma C., Xia J., Wang Z. Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol. Lett. 2014;231:82–91. doi: 10.1016/j.toxlet.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 86.Ye M., Zhang J., Zhang J., Miao Q., Yao L., Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357:196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 87.Mirgani M.T., Isacchi B., Sadeghizadeh M., Marra F., Bilia A.R., Mowla S.J., Najafi F., Babaei E. Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int. J. Nanomed. 2014;9:403–417. doi: 10.2147/IJN.S48136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zamani M., Sadeghizadeh M., Behmanesh M., Najafi F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine. 2015;22:961–967. doi: 10.1016/j.phymed.2015.05.071. [DOI] [PubMed] [Google Scholar]
- 89.Mudduluru G., George-William J.N., Muppala S., Asangani I.A., Kumarswamy R., Nelson L.D., Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cells. Biosci. Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 90.Gandhy S.U., Kim K., Larsen L., Rosengren R.J., Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W., Bai W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin. Transl. Oncol. 2014;16:708–713. doi: 10.1007/s12094-013-1135-9. [DOI] [PubMed] [Google Scholar]
- 92.Subramaniam D., Ponnurangam S., Ramamoorthy P., Standing D., Battafarano R.J., Anant S., Sharma P. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One. 2012;7:e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J., Du Y., Wu C., Ren X., Ti X., Shi J., Zhao F., Yin H. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol. Rep. 2010;24:1217–1223. doi: 10.3892/or_00000975. [DOI] [PubMed] [Google Scholar]
- 94.Zhang J., Zhang T., Ti X., Shi J., Wu C., Ren X., Yin H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem. Biophys. Res. Commun. 2010;399:1–6. doi: 10.1016/j.bbrc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 95.Liang H.H., Wei P.L., Hung C.S., Wu C.T., Wang W., Huang M.T., Chang Y.J. MicroRNA-200a/b influenced the therapeutic effects of curcumin in hepatocellular carcinoma (HCC) cells. Tumor Biol. 2013;34:3209–3218. doi: 10.1007/s13277-013-0891-z. [DOI] [PubMed] [Google Scholar]
- 96.Ali S., Ahmad A., Banerjee S., Padhye S., Dominiak K., Schaffert J.M., Wang Z., Philip P.A., Sarkar F.H. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Toden S., Okugawa Y., Jascur T., Wodarz D., Komarova N., Buhrmann C., Shakibaei M., Boland C.R., Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo H., Xu Y., Fu Q. Curcumin inhibits growth of prostate carcinoma via miR-208-mediated CDKN1A activation. Tumor Biol. 2015;36:8511–8517. doi: 10.1007/s13277-015-3592-y. [DOI] [PubMed] [Google Scholar]
- 99.Padhye S., Yang H., Jamadar A., Cui Q.C., Chavan D., Dominiak K., McKinney J., Banerjee S., Dou Q.P., Sarkar F.H. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009;26:1874–1880. doi: 10.1007/s11095-009-9900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padhye S., Banerjee S., Chavan D., Chavan S., Pandye S., Swamy K.V., Ali S., Li J., Dou Q.P., Sarkar F.H. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm. Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao B., Ali S., Kong D., Sarkar S.H., Wang Z., Banerjee S., Aboukameel A., Padhye S., Philip P.A., Sarkar F.H. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Bao B., Ahmad A., Kong D., Ali S., Azmi A.S., Li Y.-, Banerjee S., Padhye S., Sarkar F.H. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One. 2012;7:e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bao B., Ali S., Ahmad A., Azmi A.S., Li Y., Banerjee S., Kong D., Sethi S., Aboukameel A., Padhye S.B., Sarkar F.H. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One. 2012;7:e50165. doi: 10.1371/journal.pone.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy S., Yu Y., Padhye S.B., Sarkar F.H., Majumdar A.P.N. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS One. 2013;8:e68543. doi: 10.1371/journal.pone.0068543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy S., Levi E., Majumdar A.P.N., Sarkar F.H. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J. Hematol. Oncol. 2012;5:58. doi: 10.1186/1756-8722-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmad A., Sayed A., Ginnebaugh K.R., Sharma V., Suri A., Saraph A., Padhye S., Sarkar F.H. Molecular docking and inhibition of matrix metalloproteinase-2 by novel difluorinated benzylidene curcumin analog. Am. J. Transl. Res. 2015;7:298–308. [PMC free article] [PubMed] [Google Scholar]
- 107.Sarkar S., Dubaybo H., Ali S., Goncalves P., Kollepara S.L., Sethi S., Philip P.A., Li Y. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27kip1, p57kip2, and PUMA. Am. J. Cancer Res. 2013;3:465–477. [PMC free article] [PubMed] [Google Scholar]
- 108.Bao B., Ali S., Banerjee S., Wang Z., Logna F., Azmi A.S., Kong D., Ahmad A., Li Y., Padhye S., Sarkar F.H. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ali S., Ahmad A., Aboukameel A., Bao B., Padhye S., Philip P.A., Sarkar F.H. Increased Ras GTPase activity is regulated by miRNAs that can be attenuated by CDF treatment in pancreatic cancer cells. Cancer Lett. 2012;319:173–181. doi: 10.1016/j.canlet.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Ali S., Ahmad A., Aboukameel A., Ahmed A., Bao B., Banerjee S., Philip P.A., Sarkar F.H. Deregulation of miR-146a expression in a mouse model of pancreatic cancer affecting EGFR signaling. Cancer Lett. 2014;351:134–142. doi: 10.1016/j.canlet.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adams B., Ferstl E., Davis M., Herold M., Kurtkaya S., Camalier R.F., Hollingshed M.G., Kaur G., Sausville E.A., Rickles F.R., Snyder J.P., Liotta D.C., Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Yang C.H., Yue J., Sims M., Pfeffer L.M. The curcumin analog EF24 targets NF-κB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS One. 2013;8:e71130. doi: 10.1371/journal.pone.0071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang P., Bai H., Liu G., Wang H., Chen F., Zhang B., Zeng P., Wu C., Peng C., Huang C., Song Y., Song E. MicroRNA-33b, upregulated by EF24, a curcumin analog, suppresses the epithelial-to-mesenchymal transition (EMT) and migratory potential of melanoma cells by targeting HMGA2. Toxicol. Lett. 2015;234:151–161. doi: 10.1016/j.toxlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 114.Hemshekhar M., Sunitha K., Santhosh M.S., Devaraja S., Kemparaju K., Vishwanath B.S., Niranjana S.R., Girish K.S. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochem. Rev. 2011;10:325–351. [Google Scholar]
- 115.Padhye S., Ahmad A., Oswal N., Sarkar F.H. Emerging role of garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J. Hematol. Oncol. 2009;2:38. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saadat N., Gupta S.V. Potential role of garcinol as an anticancer agent. J. Oncol. 2012;2012:647206. doi: 10.1155/2012/647206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ciochina R., Grossman R.B. Polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2006;106:3963–3986. doi: 10.1021/cr0500582. [DOI] [PubMed] [Google Scholar]
- 118.Biersack B. Effects of garcinol from kokum (Garcinia indica) on the prevention and treatment of cancer. In: Ullah M.F., Ahmad A., editors. Critical Dietary Factors in Cancer Chemoprevention. Springer International Publishing Switzerland; Cham: 2016. pp. 253–271. [Google Scholar]
- 119.Socolsky C., Plietker B. Total synthesis and absolute configuration assignment of MRSA active garcinol and isogarcinol. Chem. Eur. J. 2015;21:3053–3061. doi: 10.1002/chem.201406077. [DOI] [PubMed] [Google Scholar]
- 120.Balasubramaniam K., Altaf M., Varier R.A., Swaminathan V., Ravindran A., Sadhale P.P., Kundu T.K. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 2004;279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 121.Parasramka M.A., Ali S., Banerjee S., Deryavoush T., Sarkar F.H., Gupta S.V. Garcinol sensitizes human pancreatic adenocarcinoma cells to gemcitabine in association with microRNA signatures. Mol. Nutr. Food Res. 2013;57:235–248. doi: 10.1002/mnfr.201200297. [DOI] [PubMed] [Google Scholar]
- 122.Ahmad A., Sarkar S.H., Bitar B., Ali S., Aboukameel A., Sethi S., Li Y., Bao B., Kong D., Banerjee S., Padhye S.B., Sarkar F.H. Garcinol regulates EMT and Wnt signaling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells. Mol. Cancer Ther. 2012;11:2193–2201. doi: 10.1158/1535-7163.MCT-12-0232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarkar F.H., Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat. Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zaman M.S., Shahryari V., Deng G., Thamminana S., Saini S., Majid S., Chang I., Hirata H., Ueno K., Yamamura S., Singh K., Tanaka Y., Tabatabai Z.L., Dahiya R. Up-regulation of microRNA-21 correlates with lower kidney cancer survival. PLoS One. 2012;7:e31060. doi: 10.1371/journal.pone.0031060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaman M.S., Thamminana S., Shahryari V., Chiyomaru T., Deng G., Saini S., Majid S., Fukuhara S., Chang I., Arora S., Hirata H., Ueno K., Singh K., Tanaka Y., Dahiya R. Inhibition of PTEN gene expression by oncogenic miR-23b-3p in renal cancer. PLoS One. 2012;7:e50203. doi: 10.1371/journal.pone.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hirata H., Ueno K., Nakajima K., Tabatabai Z.L., Hinoda Y., Ishii N., Dahiya R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signaling in renal cancer cells. Br. J. Cancer. 2013;108:2070–2078. doi: 10.1038/bjc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirata H., Hinoda Y., Sharyari V., Deng G., Tanaka Y., Tabatabai Z.L., Dahiya R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer. 2014;110:1645–1654. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun Q., Cong R., Yan H., Gu H., Zeng Y., Liu N., Chen J., Wang B. Genistein inhibits growth of human uveal melanoma cells and affects microRNA-27ba and target gene expression. Oncol. Rep. 2009;22:563–567. doi: 10.3892/or_00000472. [DOI] [PubMed] [Google Scholar]
- 129.Xu L., Xiang J., Shen J., Zou X., Zhai S., Yin Y., Li P., Wang X., Sun Q. Oncogenic microRNA-27a is a target for genistein in ovarian cancer cells. Anti-Cancer Agents Med. Chem. 2013;13:1126–1132. doi: 10.2174/18715206113139990006. [DOI] [PubMed] [Google Scholar]
- 130.Xia J., Cheng L., Mei C., Ma J., Shi Y., Zeng F., Wang Z., Wang Z. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr. Pharm. Des. 2014;20:5348–5353. doi: 10.2174/1381612820666140128215756. [DOI] [PubMed] [Google Scholar]
- 131.Chiyomaru T., Yamamura S., Zaman M.S., Majid S., Deng G., Shahryari V., Saini S., Hirata H., Ueno K., Chang I., Tanaka Y., Tabatabai Z.L., Enokida H., Nakagawa M., Dahiya R. Genistein suppresses prostate cancer growth through inhibition of oncogenic MicroRNA151. PLoS One. 2012;7:e43812. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen Y., Zaman M.S., Deng G., Majid S., Saini S., Liu J., Tanaka Y., Dahiya R. MicroRNAs 221/222 and genistein-mediated regulation of ARH1 tumor suppressor gene in prostate cancer. Cancer Prev. Res. 2011;4:76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma J., Cheng L., Liu H., Zhang J., Shi Y., Zeng F., Miele L., Sarkar F.H., Xia J., Wang Z. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr. Drug Targets. 2013;14:1150–1156. doi: 10.2174/13894501113149990187. [DOI] [PubMed] [Google Scholar]
- 134.de la Parra C., Castillo-Picchardo L., Cruz-Collazo A., Cubano L., Redis R., Calin G.A., Dharmawardhane S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of Genistein. Nutr. Cancer. 2016;68:154–164. doi: 10.1080/01635581.2016.1115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiyomaru T., Yamamura S., Fukuhara S., Yoshino H., Kinoshita T., Majid S., Saini S., Chang I., Tanaka Y., Enokida H., Seki N., Nakagawa M., Dahiya R. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chiyomaru T., Yamamura S., Fukuhara S., Hidaka H., Majid S., Saini S., Arora S., Deng G., Shahryari V., Chang I., Tanaka Y., Tabatabai Z.L., Enokida H., Seki N., Nakagawa M., Dahiya R. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS One. 2013;8:e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Majid S., Dar A.A., Saini S., Chen Y., Sharyiari V., Liu J., Zaman M.S., Hirata H., Yamamura S., Ueno K., Tanaka Y., Dahiya R. Regulation of minichromosome maintenance gene family by MicroRNA-1296 and genistein in prostate cancer. Cancer Res. 2010;70:2809–2818. doi: 10.1158/0008-5472.CAN-09-4176. [DOI] [PubMed] [Google Scholar]
- 138.Rabiau N., Trraf H.-K., Adjakly M., Bosviel R., Guy L., Fontana L., Bignon Y.-J., Bernard-Gallon D.J. miRNAs differentially expressed in prostate cancer cell lines after soy treatment. Vivo. 2011;25:917–922. [PubMed] [Google Scholar]
- 139.Xia J., Duan Q., Ahmad A., Bao B., Banerjee S., Shi Y., Ma J., Geng J., Chen Z., Wahidur Rahman K.M., Miele L., Sarkar F.H., Wang Z. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr. Drug Targets. 2012;13:1750–1756. doi: 10.2174/138945012804545597. [DOI] [PubMed] [Google Scholar]
- 140.Li Y., Vandenboom T.G., Kong D., Wang Z., Ali S., Philip P.A., Sarkar F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Y., Vandenboom T.G., Wang Z., Kong D., Ali S., Philip P.A., Sarkar F.H. MiR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Avci C.B., Susluer S.Y., Caglar H.O., Balci T., Aygunes D., Dodurga Y., Gunduz C. Genistein-induced miR-23b expression inhibits the growth of breast cancer cells. Contemp. Oncol. 2015;19:32–35. doi: 10.5114/wo.2014.44121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vaya J., Belinky P.A., Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic. Biol. Med. 1997;23:302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 144.Kwon H.S., Oh S.M., Kim J.K. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 2008;151:165–173. doi: 10.1111/j.1365-2249.2007.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu X.Q., Xue C.C., Zhou Z.W., Li C.G., Du Y.M., Liang J., Zhou S.F. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhyza glabra (licorice) Life Sci. 2008;82:68–78. doi: 10.1016/j.lfs.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 146.Hsu Y.L., Wu L.Y., Hou M.F., Tsai E.M., Lee J.N., Liang H.L., Jong Y.J., Hung C.H., Kuo P.L. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol. Nutr. Food Res. 2011;55:318–327. doi: 10.1002/mnfr.201000148. [DOI] [PubMed] [Google Scholar]
- 147.Jiang F., Mu J., Wang X., Ye X., Su L., Ning S., Li Z., Li Y. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS One. 2014;9:e96698. doi: 10.1371/journal.pone.0096698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang F., Li Y., Hu C., Zhou M., Wang X., Si L., Ning S., Li Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: an epigenetic regulation of miR-148a/SMAD2 signaling. Mol. Carcinog. 2016;55:929–940. doi: 10.1002/mc.22333. [DOI] [PubMed] [Google Scholar]
- 149.Mu J., Ning S., Wang X., Si L., Jiang F., Li Y., Li Z. The repressive effect of miR-520a on NF-kB/IL-6/STAT-3 signal involved in the glabridin-induced anti-angiogenesis in human breast cancer cells. RSC Adv. 2015;5:34257–34264. [Google Scholar]
- 150.Zimmermann M.C., Tilghman S.L., Boué S.M., Salvo V.A., Elliott S., Williams K.Y., Skripnikova E.V., Ashe H., Payton-Stewart F., Vanhoy-Rhodes L., Fonseca J.P., Corbitt C., Collins-Burow B.M., Howell M.H., Lacey M., Shih B.Y., Carter-Wientjes C., Cleveland T.E., McLachlan J.A., Wiese T.E., Beckman B.S., Burow M.E. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J. Pharmacol. Exp. Ther. 2010;332:35–45. doi: 10.1124/jpet.109.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salvo V.A., Boué S.M., Fonseca J.P., Elliott S., Corbitt C., Collins-Burow B.M., Curiel T.J., Srivastav S.K., Shih B.Y., Carter-Wientjes C., Wood C.E., Erhardt P.W., Beckman B.S., McLachlan J.A., Cleveland T.E., Burow M.E. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin. Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 152.Carriere P.P., Llopis S.D., Naiki A.C., Nguyen G., Phan T., Nguyen M.M., Preyan L.C., Yearby L., Pratt J., Burks H., Davenport I.R., Nguyen T.A., Parker-Lemieux K., Payton-Stewart F., Williams C.C., Boué S.M., Burow M.E., Collins-Burow B., Hillard A., Davidson A.M., Tilghman S.L. Glyceollin I reverses epithelial to mesenchymal transition in letrozole resistant breast cancer through ZEB1. Int. J. Environ. Res. Public Health. 2016;13:10. doi: 10.3390/ijerph13010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rhodes L.V., Tilghman S.L., Boué S.M., Wang S., Khalili H., Muir S.E., Bratton M.R., Zhang Q., Wang G., Burow M.E., Collins-Burow B.M. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol. Lett. 2012;3:163–171. doi: 10.3892/ol.2011.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang C.S., Wang X., Lu G., Picinich S.C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsang W.P., Kwok T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 156.Wang H., Bian S., Yang C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. 2011;32:1881–1889. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou H., Chen J.X., Yang C.S., Yang M.Q., Deng Y., Wang H. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC Genomics. 2014;15(Suppl. 11):S3. doi: 10.1186/1471-2164-15-S11-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yamada S., Tsukamoto S., Huang Y., Makio A., Kumazoe M., Yamashita S., Tachibana H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci. Rep. 2016;6:19225. doi: 10.1038/srep19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu K., Wang W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2015 doi: 10.1007/s13277-015-4187-3. Article ID 26499783. [DOI] [PubMed] [Google Scholar]
- 160.Jiang L., Tao C., He A., He X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J. Surg. Oncol. 2014;12:383. doi: 10.1186/1477-7819-12-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Siddiqi I.A., Asim M., Hafeez B.B., Adhami V.M., Tarapore R.S., Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arola-Arnal A., Bladé C. Proanthocyanidins modulate microRNA expression in human HepG2 cells. PLoS One. 2011;6:e25982. doi: 10.1371/journal.pone.0025982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tsang J.S., Ebert M.S., van Oudenaarden A. Genome-wide dissection of microRNA functions and cotargeting networks using genes et signatures. Mol. Cell. 2010;38:140–153. doi: 10.1016/j.molcel.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou D.-H., Wang X., Feng Q. EGCG enhances the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC A549 cells. Nutr. Cancer. 2014;66:636–644. doi: 10.1080/01635581.2014.894101. [DOI] [PubMed] [Google Scholar]
- 165.Chakrabarti M., Ai W., Banik N.L., Ray S.K. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem. Res. 2013;38:420–432. doi: 10.1007/s11064-012-0936-5. [DOI] [PubMed] [Google Scholar]
- 166.An I.-S., An S., Park S., Lee S.N., Bae S. Involvement of microRNAs in epigallocatechin gallate-mediated UVB protection in human dermal fibroblasts. Oncol. Rep. 2013;29:253–259. doi: 10.3892/or.2012.2083. [DOI] [PubMed] [Google Scholar]
- 167.Baselga-Escudero L., Blade C., Ribas-Latre A., Casanova E., Suárez M., Torres J.L., Salvadó M.J., Arola L., Arola-Arnal A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucl. Acids Res. 2014;42:882–892. doi: 10.1093/nar/gkt1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang H., Mi M., Ling W., Zhu J., Zhang Q., Wie N., Zhou Y., Tang Y., Yuan J. Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro. Arch. Pharm. Res. 2008;31:1137–1144. doi: 10.1007/s12272-001-1280-8. [DOI] [PubMed] [Google Scholar]
- 169.Chang H., Hu Y., Yuan L., Xu H., Zhou Y., Zhu J., Zhang Q., Mi M. MicroRNA-34a and microRNA-21 play roles in the chemopreventive effects of 3,6-dihydroxyflavone on 1-methyl-1-nitrosourea-induced breast carcinogenesis. Breast Cancer Res. 2012;14:R80. doi: 10.1186/bcr3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peng X., Chang H., Gu Y., Chen J., Yi L., Xie Q., Zhu J., Zhang Q., Mi M. 3,6-Dihydroxyflavone suppresses breast carcinogenesis by epigenetically regulating miR-34a and miR-21. Cancer Prev. Res. 2015;8:509–517. doi: 10.1158/1940-6207.CAPR-14-0357. [DOI] [PubMed] [Google Scholar]
- 171.Karmakar S., Davis K.A., Choudhury S.R., Deeconda A., Banik N.L., Ray S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009;388:705–710. doi: 10.1016/j.bbrc.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chao S.-C., Huang S.-C., Hu D.-N., Lin H.-Y. Subtoxic levels of apigenin inhibit expression and secretion of VEGF by uveal melanoma cells via suppression of ERK1/2 and PI3K/Akt pathways, Evidence-Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/817674. Article ID 817674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm. Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patel D., Shukla S., Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int. J. Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 175.Chakrabarti M., Banik N.L., Ray S.K. miR-138 overexpression is more powerful than hTERT knockdown to potentiate apigenin for apoptosis in neuroblastoma in vitro and in vivo. Exp. Cell Res. 2013;319:1575–1585. doi: 10.1016/j.yexcr.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bao B., Oraha A., Ahmad A., Ali S., Li Y., Azmi A.S., Banerjee S., Sarkar F.H. The biology of the deadly love connection between obesity, diabetes and breast cancer. In: Ahmad A., editor. Breast Cancer Metastasis and Drug Resistance. Springer Science + Business Media; New York: 2013. pp. 117–142. [Google Scholar]
- 177.Ohno M., Shibata C., Kishikawa T., Yoshikawa T., Takata A., Kojima K., Akanuma M., Kang Y.J., Yoshida H., Otsuka M., Koike K. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA 103 transgenic mice. Sci. Rep. 2013;3:2553. doi: 10.1038/srep02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lam T.K., Shao S., Zhao Y., Marincola F., Pesatori A., Bertazzi P.A., Caporaso N.E., Wang E., Landi M.T. Influence of quercetin-rich food intake on miRNA expression in lung cancer tissues. Cancer Epidemiol. Biomark. Prev. 2012;21:2176–2184. doi: 10.1158/1055-9965.EPI-12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Noratto G., Kim Y., Talcott S., Mertens-Talcott S. Flavonol-rich fractions of yaupon holly leaves (Ilex vomitora, Aquifoliaceae) induce microRNA-146a and have anti-inflammatory and chemopreventive effects in intestinal myofibroblast CCD-18Co cells. Fitoterapia. 2011;82:557–569. doi: 10.1016/j.fitote.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 180.Appari M., Babu K., Kaczorowski A., Gross W., Herr I. Sulphoraphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int. J. Oncol. 2014;45:1391–1400. doi: 10.3892/ijo.2014.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sonoki H., Sato T., Endo S., Matsunaga T., Yamaguchi M., Yamazaki Y., Sugatani J., Ikari A. Quercetin decreases Claudin-2 expression mediated by up-regulation of microRNA miR-16 in lung adenocarcinoma A549 cells. Nutrients. 2015;7:4578–4592. doi: 10.3390/nu7064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mackenzie T.N., Mujumdar N., Banerjee S., Sangwan V., Sarver A., Vickers S., Subramaniam S., Saluja A.K. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol. Cancer Ther. 2013;12:1266–1275. doi: 10.1158/1535-7163.MCT-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lou G., Liu Y., Wu S., Xue J., Yang F., Fu H., Zheng M., Chen Z. The p53/miR-34a/SIRT1 positive feedback loop in quercetin-induced apoptosis. Cell. Physiol. Biochem. 2015;35:2192–2202. doi: 10.1159/000374024. [DOI] [PubMed] [Google Scholar]
- 184.del Follo-Martinez A., Banerjee N., Li X., Safe S., Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer. 2013;65:494–504. doi: 10.1080/01635581.2012.725194. [DOI] [PubMed] [Google Scholar]
- 185.Wein S.A., Laviano A., Wolffram S. Quercetin induces hepatic γ-glutamyl hydrolase expression in rats by suppressing hepatic microRNA rno-miR-125b-3p. J. Nutr. Biochem. 2015;26:1660–1663. doi: 10.1016/j.jnutbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 186.Lin C.C., Shieh D.E. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein, and wogonin. Am. J. Chin. Med. 1996;24:31–36. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]
- 187.Chen K., Zhang S., Ji Y., Li J., An P., Ren H., Liang R., Yang J., Li Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu Y., Zhou B., Wu D., Yin Z., Lou D. Baicalin modulates microRNA expression in UVB irradiated mouse skin. J. Biomed. Res. 2012;26:125–134. doi: 10.1016/S1674-8301(12)60022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang J., Masika J., Zhou J., Wang J., Zhu M., Luo H., Hu X., Zhang L., Tang M., Gao L., Hescheler J., Liang H. Traditional Chinese medicine baicalin suppresses mESCs proliferation through inhibition of miR-294 expression. Cell. Physiol. Biochem. 2015;35:1868–1876. doi: 10.1159/000373997. [DOI] [PubMed] [Google Scholar]
- 190.Dong N., Xu B., Shi H., Tang X. Baicalein inhibits Amadori-glycated albumin-induced MCP-1 expression in retinal ganglion cells via a microRNA-124-dependent mechanism. Invest. Ophthalmol. Vis. Sci. 2015;56:5844–5853. doi: 10.1167/iovs.15-17444. [DOI] [PubMed] [Google Scholar]
- 191.Agarwal R., Agarwal C., Ichikawa H., Singh R.P., Aggarwal B.B. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 192.Cufí S., Bonavia R., Vasquez-Martin A., Oliveras-Ferraros C., Corominas-Faja B., Cuyàs E., Martin-Castillo B., Barrajón-Catalán E., Sequra-Carretero A., Joven J., Bosch-Barrera J., Micol V., Menendez J.A. Silibinin suppresses EMT-driven erlotinib resistance by reversing the high miR-21/low miR-200c signature in vivo. Sci. Rep. 2013;3:2459. doi: 10.1038/srep02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Athar M., Back J.H., Tang X., Kim K.H., Kopelovich L., Bickers D.R., Kim A.L. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tili E., Michaille J.-J. Resveratrol, microRNAs, inflammation, and cancer. J. Nucl. Acids. 2011;2011 doi: 10.4061/2011/102431. Article ID 102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tili E., Michaille J.J., Alder H., Volinia S., Delmas D., Latruffe N., Croce C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGF-beta signaling pathway in SW480 cells. Biochem. Pharmacol. 2010;80:2057–2065. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu P., Liang H., Xia Q., Li P., Kong H., Lei P., Wang S., Tu Z. Resveratrol induces apoptosis of pancreatic cancer cells by inhibiting miR-21 regulation of Bcl-2 expression. Clin. Transl. Oncol. 2013;15:741–746. doi: 10.1007/s12094-012-0999-4. [DOI] [PubMed] [Google Scholar]
- 197.Shet S., Jajoo S., Kaur T., Mukherjea D., Sheehan K., Rybak L.P., Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/microRNA-21 pathway. PLoS One. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cao Z., Yoon J.H., Nam S.W., Lee J.Y., Park W.S. PDCD4 expression inversely correlated with miR-21 levels in gastric cancers. J. Cancer Res. Clin. Oncol. 2012;138:611–619. doi: 10.1007/s00432-011-1140-8. [DOI] [PubMed] [Google Scholar]
- 199.Dhar S., Hicks C., Levenson A.S. Resveratrol and prostate cancer: promising role for microRNAs. Mol. Nutr. Food Res. 2011;55:1219–1229. doi: 10.1002/mnfr.201100141. [DOI] [PubMed] [Google Scholar]
- 200.Yu Y.H., Chen H.A., Chen P.S., Cheng Y.J., Hsu W.H., Chang Y.W., Chen Y.H., Jan Y., Hsiao M., Chang T.Y., Liu Y.H., Jeng Y.M., Wu C.H., Huang M.T., Su Y.H., Hung M.C., Chien M.H., Chen C.Y., Kuo M.L., Su J.L. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene. 2013;32:431–443. doi: 10.1038/onc.2012.74. [DOI] [PubMed] [Google Scholar]
- 201.Vislovukh A., Kratassiouk G., Porto E., Gralievska N., Beldiman C., Pinna G., El’skaya A., Harel-Benan A., Negrutskii B., Groisman I. Proto-oncogenic isoform A2 of eukaryotic translation elongation factor eEF1 is a target of miR-663 and miR-774. Br. J. Cancer. 2013;108:2304–2311. doi: 10.1038/bjc.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hagiwara K., Kosaka N., Yoshioka Y., Takahashi R.U., Takeshita F., Ochiya T. Stilbene derivatives promote AGO2-dependent tumour-suppressive microRNA activity. Sci. Rep. 2012;2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bai T., Dong D.S., Pei L. Synergistic antitumor activity of resveratrol and miR-200c in human lung cancer. Oncol. Rep. 2014;31:2293–2297. doi: 10.3892/or.2014.3090. [DOI] [PubMed] [Google Scholar]
- 204.Kumazaki M., Noguchi S., Yasui Y., Iwasaki J., Shinohara H., Yamada N., Akao Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 2013;24:1849–1858. doi: 10.1016/j.jnutbio.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 205.Yang S., Li W., Sun H., Wu B., Ji F., Sun T., Chang H., Shen P., Wang Y., Zhou D. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Cancer. 2015;15:969. doi: 10.1186/s12885-015-1958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han Z., Yang Q., Liu B., Wu J., Li Y., Yang C., Jiang Y. MicroRNA-622 functions as a tumor suppressor by targeting K-Ras and enhancing the anticarcinogenic effect of resveratrol. Carcinogenesis. 2012;33:131–139. doi: 10.1093/carcin/bgr226. [DOI] [PubMed] [Google Scholar]
- 207.Qin W., Zhang K., Clarke K., Weiland T., Sauter E.R. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr. Cancer. 2014;66:270–277. doi: 10.1080/01635581.2014.868910. [DOI] [PubMed] [Google Scholar]
- 208.Bae S., Lee E., Cha H.J., Kim K., Yoon Y., Lee H., Kim J., Kim Y.J., Lee H.G., Jeung H.K., Min Y.H., An S. Resveratrol alters microRNA expression profiles in A549 human non-small cell lung cancer cells. Mol. Cells. 2011;32 doi: 10.1007/s10059-011-1037-z. 243–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Venkatadri E., Muni T., Iyer A.K.V., Yakisich J.S., Azad N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016;7:e2104. doi: 10.1038/cddis.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Seyed M.A., Jantan I., Bukhari S.N.A., Vijayaraghavan K. A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J. Agric. Food Chem. 2016;64:725–737. doi: 10.1021/acs.jafc.5b05993. [DOI] [PubMed] [Google Scholar]
- 211.Liu W.-H., Chang L.-S. Suppression of Akt/Foxp3-mediated miR-183 expression blocks Sp1-mediated ADAM17 expression and TNFα-mediated NFκB activation in piceatannol-treated human leukemia cells. Biochem. Pharmacol. 2012;84:670–680. doi: 10.1016/j.bcp.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 212.Zhang H., Jia R., Wang C., Hu T., Wang F. Piceatannol promotes apoptosis via up-regulation of microRNA-129 expression in colorectal cancer cell lines. Biochem. Biophys. Res. Commun. 2014;452:775–781. doi: 10.1016/j.bbrc.2014.08.150. [DOI] [PubMed] [Google Scholar]
- 213.Ke K., Sul O.-J., Rajasekaran M., Choi H.-S. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone. 2015;81:237–246. doi: 10.1016/j.bone.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 214.Edwardson D., Chewchuk S., Parissenti A.M. Resistance to Anthracyclines and Taxanes in breast cancer. In: Ahmad A., editor. Breast Cancer Metastasis and Drug Resistance. Springer Science + Business Media; New York: 2013. pp. 227–247. [Google Scholar]
- 215.Singh S. Breast cancer stem cells and miRNAs. In: Ahmad A., editor. Breast Cancer Metastasis and Drug Resistance. Springer Science + Business Media; New York: 2013. pp. 367–383. [Google Scholar]
- 216.Kovalchuk O., Filkowski J., Meservy J., Ilnytskyy Y., Tryndyak V.P., Chekhun V.F., Pogribny I.P. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 217.Tormo E., Pineda B., Serna E., Guijarro A., Ribas G., Fores J., Chirivella E., Climent J., Lluch A., Eroles P. MicroRNA profile in response to doxorubicin treatment in breast cancer. J. Cell. Biochem. 2015;116:2061–2073. doi: 10.1002/jcb.25162. [DOI] [PubMed] [Google Scholar]
- 218.Kopp F., Oak P.S., Wagner E., Roidl A. miR-200c sensitizes breast cancer cells to doxorubicin treatment by decreasing TrkB and Bmi1 expression. PLoS One. 2012;7:e50469. doi: 10.1371/journal.pone.0050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xie X., Hu Y., Xu L., Fu Y., Tu J., Zhao H., Zhang S., Hong R., Gu X. The role of miR-125b-mitochondria-caspase-3 pathway in doxorubicin resistance and therapy in human breast cancer. Tumor Biol. 2015;36:7185–7194. doi: 10.1007/s13277-015-3438-7. [DOI] [PubMed] [Google Scholar]
- 220.Long J., Ji Z., Jiang K., Wang Z., Meng G. MiR-193b modulates resistance to doxorubicin in human breast cancer cells by downregulating Mcl-1. Biomed. Res. Int. 2015 doi: 10.1155/2015/373574. Article ID 373574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu Y., Qiu Y., Yagüe E., Ji W., Liu J., Zhang J. MiRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis. 2016;7:e2291. doi: 10.1038/cddis.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hu Y., Xu K., Yagüe E. MiR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res. Treat. 2015;151:269–280. doi: 10.1007/s10549-015-3372-9. [DOI] [PubMed] [Google Scholar]
- 223.Zhao Z., Li R., Sha S., Wang Q., Mao W., Liu T. Targeting HER3 with miR-450b-3p suppresses breast cancer cells proliferation. Cancer Biol. Ther. 2014;15:1404–1412. doi: 10.4161/cbt.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Niu J., Xue A., Chi Y., Xue J., Wang W., Zhao Z., Fan M., Yang C.H., Shao Z.-M., Pfeffer L.M., Wu J., Wu Z.-H. Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene. 2016;35:1302–1313. doi: 10.1038/onc.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yoo B., Kavishwar A., Ross A., Wang P., Tabassum D.P., Polyak K., Barteneva N., Petkova V., Pantazopoulos P., Tena A., Moore A., Medarova Z. Combining miR-10b-targeted nanotherapy with low-dose doxorubicin elicits durable regressions of metastatic breast cancer. Cancer Res. 2015;75:4407–4415. doi: 10.1158/0008-5472.CAN-15-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang S., Zhang J., Wang Y., Chen M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomed. 2016;12:411–420. doi: 10.1016/j.nano.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 227.He H., Tian W., Chen H., Deng Y. MicroRNA-101 sensitizes hepatocellular carcinoma cells to doxorubicin-induced apoptosis via targeting Mcl-1. Mol. Med. Rep. 2016;13:1923–1929. doi: 10.3892/mmr.2015.4727. [DOI] [PubMed] [Google Scholar]
- 228.Yang G., Jiang O., Ling D., Jiang X., Yuan P., Zeng G., Zhu J., Tian J., Weng Y., Wu D. MicroRNA-522 reverses drug resistance of doxorubicin-induced HT29 colon cancer cell by targeting ABCB5. Mol. Med. Rep. 2015;12:3930–3936. doi: 10.3892/mmr.2015.3890. [DOI] [PubMed] [Google Scholar]
- 229.Zhou Y., Zhao R.-H., Tseng K.-F., Li K.-P., Lu Z.-G., Liu Y., Han K., Gan Z.-H., Lin S.-C., Hu H.-Y., Min D.-I. Sirolimus induces apoptosis and reverses multidrug resistance in human osteosarcoma cells in vitro via increasing microRNA-34b expression. Acta Pharmacol. Sin. 2016;37:519–529. doi: 10.1038/aps.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lin Z., Song D., Wei H., Yang X., Liu T., Yan W., Xiao J. TGF-b1-induced miR-202 mediates drug resistance by inhibiting apoptosis in human osteosarcoma. J. Cancer Res. Clin. Oncol. 2016;142:239–246. doi: 10.1007/s00432-015-2028-9. [DOI] [PubMed] [Google Scholar]
- 231.Zhang S., Han L., Wei J., Shi Z., Pu P., Zhang J., Yuan X., Kang C. Combination treatment with doxorubicin and microRNA-21 inhibitor synergistically augments anticancer activity through upregulation of tumor supressing genes. Int. J. Oncol. 2015;46:1589–1600. doi: 10.3892/ijo.2015.2841. [DOI] [PubMed] [Google Scholar]
- 232.Lee J., Choi K.-J., Moon S.U., Kim S. Theragnosis-based combined cancer therapy using doxorubicin-conjugated microRNA-221 molecular beacon. Biomaterials. 2016;74:109–118. doi: 10.1016/j.biomaterials.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 233.Troppan K., Wenzl K., Pichler M., Pursche B., Schwarzenbacher D., Feichtinger J., Thallinger G.G., Beham-Schmid C., Neumeister P., Deutsch A. MiR-199a and miR-497 are associated with better overall survival due to increased chemosensitivity in diffuse large B-cell lymphoma patients. Int. J. Mol. Sci. 2015;16:18077–18095. doi: 10.3390/ijms160818077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ma Y.-N., Chen M.-T., Wu Z.-K., Zhao H.-L., Yu H.-C., Yu J., Zhang J.-W. Emodin can induce K562 cells to erythroid differentiation and improve the expression of globin genes. Mol. Cell. Biochem. 2013;382:127–136. doi: 10.1007/s11010-013-1726-3. [DOI] [PubMed] [Google Scholar]
- 235.Zhang L., Lau Y.K., Xi L., Hong R.L., Kim D.S., Chen C.F., Hortobagyi G.N., Chang C., Hung M.C. Tyrosine kinase inhibitors, emodin and its derivative repress HER-2/neu-induced cellular transformation and metastasis-associated properties. Oncogene. 1998;16:2855–2863. doi: 10.1038/sj.onc.1201813. [DOI] [PubMed] [Google Scholar]
- 236.Ren Z., Tong H., Chen L., Yao Y., Huang S., Zhu F., Liu W. miR-211 and miR-429 are involved in emodin's anti-proliferative effects on lung cancer. Int. J. Clin. Med. 2016;9:2085–2093. [Google Scholar]
- 237.Guo J., Li W., Shi H., Xie X., Li L., Tang H., Wu M., Kong Y., Yang L., Gao J., Liu P., Wei W., Xie X. Synergistic effects of curcumin with emodin against the proliferation and invasion of breast cancer cells through upregulation of miR-34a. Mol. Cell. Biochem. 2013;382:103–111. doi: 10.1007/s11010-013-1723-6. [DOI] [PubMed] [Google Scholar]
- 238.Hua J., He Y., Xu Y., Jiang X., Ye W., Pan Z. Emodin prevents intima thickness via Wnt4/Dvl-1/β-catenin signaling pathway mediated by miR-126 in balloon-injured carotid artery rats. Exp. Mol. Med. 2015;47:e170. doi: 10.1038/emm.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lin S.-Z., Xu J.-B., Ji X., Chen H., Xu H.-T., Hu P., Chen L., Guo J.-Q., Chen M.-Y., Lu D., Wang Z.-H., Tong H.-F. Emodin inhibits angiogenesis in pancreatic cancer by regulating the transforming growth factor-β/drosophila mothers against decapentaplegic pathway and angiogenesis-associated microRNAs. Mol. Med. Rep. 2015;12:5865–5871. doi: 10.3892/mmr.2015.4158. [DOI] [PubMed] [Google Scholar]
- 240.Vyas A., Syeda K., Ahmad A., Padhye S., Sarkar F.H. Perspectives on medicinal properties of mangiferin. Mini-Rev. Med. Chem. 2012;12:412–425. doi: 10.2174/138955712800493870. [DOI] [PubMed] [Google Scholar]
- 241.Li M., Ma H., Yang L., Li P. Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol. Lett. 2016;11:817–822. doi: 10.3892/ol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xiao J., Liu L., Zhong Z., Xiao C., Zhang J. Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression. Oncol. Rep. 2015;33:2815–2820. doi: 10.3892/or.2015.3919. [DOI] [PubMed] [Google Scholar]
- 243.Lombo F., Menendez N., Salas J.A., Mendez C. The aureolic acid family of antitumor compounds: structure, mode of action, biosynthesis, and novel derivatives. Appl. Microbiol. Biotechnol. 2006;73:1–14. doi: 10.1007/s00253-006-0511-6. [DOI] [PubMed] [Google Scholar]
- 244.Bianchi N., Zuccato C., Lampronti I., Borgatti M., Gambari R. Expression of miR-210 during erythroid differentiation and induction of gamma-globin gene expression. BMP Rep. 2009;42:493–499. doi: 10.5483/bmbrep.2009.42.8.493. [DOI] [PubMed] [Google Scholar]
- 245.Jiang Z.H., Hirose Y., Iwata H., Sakamoto S., Tanaka T., Kouno I. Caffeoyl, coumaroyl, galloyl, and hexahydroxydiphenoyl glucoses from Balanophora japonica. Chem. Pharm. Bull. 2001;49:887–892. doi: 10.1248/cpb.49.887. [DOI] [PubMed] [Google Scholar]
- 246.Wen X.-Y., Wu S.-Y., Li Z.-Q., Liu Z.-Q., Zhang J.-J., Wang G.-F., Jiang Z.-H., Wu S.-G. Ellagitannin (BJA3121), an antiproliferative natural polyphenol compound, can regulate the expression of miRNAs in HepG2 cancer cells. Phytother. Res. 2009;23:778–784. doi: 10.1002/ptr.2616. [DOI] [PubMed] [Google Scholar]
- 247.Ai R., Wu S., Wen X., Xu W., Lü L., Rao J., Wu S. 1,3,4-tri-O-galloyl-6-O-caffeoyl-β-D-glucopyranose, a new anti-proliferative ellagitannin, regulates the expression of microRNAs in HepG2 cancer cells. J. South Med. Univ. 2011;31:1641–1648. [PubMed] [Google Scholar]
- 248.Banerjee N., Talcott S., Safe S., Mertens-Talcott S.U. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: potential role of miRNA-27a and miR-155 in cell survival and inflammation. Breast Cancer Res. Treat. 2012;136:21–34. doi: 10.1007/s10549-012-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shirode A.B., Kovvuru P., Chittur S.V., Henning S.M. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014;53:458–470. doi: 10.1002/mc.21995. [DOI] [PubMed] [Google Scholar]
- 250.Zhou B., Yi H., Tan J., Wue Y., Liu G., Qiu Z. Anti-proliferative effects of polyphenols from pomegranate rind (Punica granatum L.) on EJ bladder cancer cells via regulation of p53/miR-34a axis. Phytother. Res. 2015;29:415–422. doi: 10.1002/ptr.5267. [DOI] [PubMed] [Google Scholar]
- 251.Phuah N.H., In L.L.A., Azmi M.N., Ibrahim H., Awang K., Nagoor N.H. Alterations of microRNA expression patterns in human cervical carcinoma cells (Ca Ski) toward 1’S-1′-acetoxychavicol acetate and cisplatin. Reprod. Sci. 2013;20:567–578. doi: 10.1177/1933719112459220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang H., Shen L., Li X., Sun M. MicroRNAs contribute to the anticancer effect of 1′-acetoxychavicol acetate in human head and neck squamous cell carcinoma cell line HN4. Biosci. Biotechnol. Biochem. 2013;77:2348–2355. doi: 10.1271/bbb.130389. [DOI] [PubMed] [Google Scholar]
- 253.Kang Y.-J., Park H.J., Chung H.-J., Min H.-Y., Park E.J., Lee N.A., Shin Y., Lee S.K. Wnt/β-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol. Pharmacol. 2012;82:168–177. doi: 10.1124/mol.112.078535. [DOI] [PubMed] [Google Scholar]
- 254.Hagiwara K., Gailhouste L., Yasukawa K., Kosaka N., Ochiya T. A robust screening method for dietary agents that activate tumour-suppressor microRNAs. Sci. Rep. 2015;5:14697. doi: 10.1038/srep14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Esumi T., Makado G., Zhai H., Shimizu Y., Mitsumoto Y., Fukuyama Y. Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorg. Med. Chem. Lett. 2004;14:2621–2625. doi: 10.1016/j.bmcl.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 256.Arora S., Singh S., Piazza G.A., Contreras C.M., Panyam J., Singh A.P. Honokiol: a novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2012;12:1244–1252. doi: 10.2174/156652412803833508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Avtanski D.B., Nagalingam A., Kuppusamy P., Bonner M.Y., Arbiser J.L., Saxena N.K., Sharma D. Honokiol abrogates leptin-induced tumor progression by inhibiting Wnt1-MTA1-β-catenin signaling axis in a microRNA-34a dependent manner. Oncotarget. 2015;6:16396–16410. doi: 10.18632/oncotarget.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang Q., Zhao W., Ye C., Zhuang J., Chang C., Li Y., Huang X., Shen L., Li Y., Cui Y., Song J., Shen B., Eliaz I., Huang R., Ying H., Guo H., Yan J. Honokiol inhibits bladder tumor growth by suppressing EZH2/miR-143 axis. Oncotarget. 2015;6:37335–37348. doi: 10.18632/oncotarget.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jung J.E., Kim H.S., Lee C.S., Park D.H., Kim Y.N., Lee M.J., Lee J.W., Park J.W., Kim M.S., Ye S.K., Chung M.H. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis. 2007;28:1780–1787. doi: 10.1093/carcin/bgm130. [DOI] [PubMed] [Google Scholar]
- 260.Touabia M., Jean-Francois J., Doiron J. Caffeic acid, a versatile pharmacophore: an overview. Mini-Rev. Med. Chem. 2011;11:695–713. doi: 10.2174/138955711796268750. [DOI] [PubMed] [Google Scholar]
- 261.Guerriero E., Sorice A., Capone F., Costantini S., Palladino P., D'Ischia M., Castello G. Effect of lipoic acid, caffeic acid and a synthesized lipoyl-caffeic conjugate on human hepatoma cell lines. Molecules. 2011;16:6365–6377. doi: 10.3390/molecules16086365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang L., Lu M., Yi M., Chen L., Shen J., Li Z., Li L., Yang Y., Zhang J., Li Y. Caffeic acid attenuates the autocrine IL-6 in hepatocellular carcinoma via the epigenetic silencing of the NF-κB-IL-6-STAT-3 feedback loop. RSC Adv. 2015;5:52952–52957. [Google Scholar]
- 263.Li Y., Jiang F., Chen L., Yang Y., Cao S., Ye Y., Wang X., Mu J., Li Z., Li L. Blockade of TGFβ-SMAD2 by demethylation-activated miR-148a is involved in caffeic acid-induced inhibition of cancer stem cell-like properties in vitro and in vivo. FEBS Open Bio. 2015;5:466–475. doi: 10.1016/j.fob.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Alexandrov P.N., Dua P., Hill J.M., Bhattacharjee S., Zhao Y. MicroRNA (miRNA) speciation in Alzheimer's disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF) Int. J. Biochem. Mol. Biol. 2012;3:365–373. [PMC free article] [PubMed] [Google Scholar]
- 265.Hossan M.S., Rahman S., Bashar A.B.M.A., Jahan R., Al-Nahain A., Rahmatullah M. Rosmarinic acid: a review of its anticancer action. World J. Pharm. Pharm. Sci. 2014;3:57–70. [Google Scholar]
- 266.Han S., Yang S., Cai Z., Pan D., Li Z., Huang Z., Zhang P., Zhu H., Lei L., Wang W. Anti-Warburg effect of rosmarinic acid via miR-155 in gastric cancer cells. Drug Des. Dev. Ther. 2015;9:2695–2703. doi: 10.2147/DDDT.S82342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Henwood J.M., Brogden R.N. Etoposide. A review of its pharmacodynamics and pharmacokinetic properties, and therapeutic potential in combination chemotherapy of cancer. Drugs. 1990;39:438–490. doi: 10.2165/00003495-199039030-00008. [DOI] [PubMed] [Google Scholar]
- 268.Hande K.R. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 269.Moitra K., Im K., Limpert K., Borsa A., Sawitzke J., Robey R., Yuhki N., Savan R., Huang D.W., Lempicki R.A., Bates S., Dean M. Differential gene and microRNA expression between etoposide resistant and etoposide sensitive MCF7 breast cancer cell lines. PLoS One. 2012;7:e45268. doi: 10.1371/journal.pone.0045268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Seviour E.G., Sehgal V., Lu Y., Luo Z., Moss T., Zhang F., Hill S.M., Liu W., Maiti S.N., Cooper L., Azencot R., Lopez-Berestein G., Rodriguez-Aguayo C., Roopaimoole R., Pecot C.V., Sood A.K., Mukherjee S., Gray J.W., Mills G.B., Ram P.T. Functional proteomics identifies miRNAs to target a p27/Myc/phosphor-Rb signature in breast and ovarian cancer. Oncogene. 2016;35:691–701. doi: 10.1038/onc.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang N., Zhu M., Tsao S.-W., Man K., Zhang Z., Feng Y. MiR-23a-mediated inhibition of topoisomerase 1 expression potentiates cell response to etoposide in human hepatocellular carcinoma. Mol. Cancer. 2013;12:119. doi: 10.1186/1476-4598-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rebucci M., Sermeus A., Leonard E., Delaive E., Dieu M., Fransolet M., Arnould T., Michiels C. MiRNA-196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1. Mol. Cancer. 2015;14:79. doi: 10.1186/s12943-015-0349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Xu C., Li H., Zhang L., Jia T., Duan L., Lu C. MicroRNA-1915-3p prevents the apoptosis of lung cancer cells by downregulating DRG2 and PBX2. Mol. Med. Rep. 2016;13:505–512. doi: 10.3892/mmr.2015.4565. [DOI] [PubMed] [Google Scholar]
- 274.Xu C., Zhang L., Li H., Liu Z., Duan L., Lu C. MiRNA1469 promotes lung cancer cells apoptosis through targeting STAT5a. Am. J. Cancer Res. 2015;5:1180–1189. [PMC free article] [PubMed] [Google Scholar]
- 275.Chen Y., Feng F., Gao X., Wang C., Sun H., Zhang C., Zheng Z., Lu Y., An L., Qu J., Wang F., Yang Y. MiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cells. Curr. Cancer Drug Targets. 2015;15:176–187. doi: 10.2174/1568009615666150225122635. [DOI] [PubMed] [Google Scholar]
- 276.Novello C., Pazzaglia L., Conti A., Quattrini I., Pollino S., Perrego P., Picci P., Benassi M.S. p53-dependent activation of microRNA-34a in response to etoposide-induced DNA damage in osteosarcoma cell lines not impaired by dominant negative p53 expression. PLoS One. 2014;9:e114757. doi: 10.1371/journal.pone.0114757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Safayhi H., Mack T., Sabieraj J., Anazodo M.I., Subramanian L.R., Ammon H.P. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992;261:1143–1146. [PubMed] [Google Scholar]
- 278.Flavin D.F. A lipoxygenase inhibitor in breast cancer brain metastases. J. Neurooncol. 2007;82:91–93. doi: 10.1007/s11060-006-9248-4. [DOI] [PubMed] [Google Scholar]
- 279.Takada Y., Ichikawa H., Badmaev V., Aggarwal B.B. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J. Immunol. 2006;176:3127–3140. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- 280.Kunnumakkara A.B., Nair A.S., Sung B., Pandey M.K., Aggarwal B.B. Boswellic acid blocks signal transduction and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol. Cancer Res. 2009;7:118–128. doi: 10.1158/1541-7786.MCR-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 281.Takahashi M., Sung B., Shen Y., Hur K., Link A., Boland C.R., Aggarwal B.B., Goel A. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis. 2012;33:2441–2449. doi: 10.1093/carcin/bgs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Toden S., Okugawa Y., Buhrmann C., Nattamai D., Anguiano E., Baldwin N., Shakibaei M., Boland C.R., Goel A. Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev. Res. 2015;8:431–443. doi: 10.1158/1940-6207.CAPR-14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Galgon T., Höke D., Dräger B. Identification and quantification of betulinic acid. Phytochem. Anal. 1999;10:187–190. [Google Scholar]
- 284.Chowdhury A.R., Mandal S., Mittra B., Sharma S., Mukhopahyay S., Majumder H.M. Betulinic acid, a potent inhibitor of eukaryotic topoisomerase I: identification of the inhibitory step, the major functional group responsible and development of more potent derivatives. Med. Sci. Monit. 2002;8:BR245–260. [PubMed] [Google Scholar]
- 285.Cichewicz R.H., Kouzi S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- 286.Chintharlapalli S., Papineni S., Lei P., Pathi S., Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and –independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11:371. doi: 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mertens-Talcott S.U., Noratto G.D., Li X., Angel-Morales G., Bertoldi M.C., Safe S. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: role of Sp transcription factors and microRNA-27a:ZBTB10. Mol. Carcinog. 2013;52:591–602. doi: 10.1002/mc.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yang J., Qiu B., Li X., Zhang H., Liu W. p53-p66shc/miR-21-Sod2 signaling is critical for the inhibitory effect of betulinic acid on hepatocellular carcinoma. Toxicol. Lett. 2015;238:1–10. doi: 10.1016/j.toxlet.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 289.Zhao G.-J., Tang S.-L., Lv Y.-C., Ouyang X.-P., He P.-P., Yao F., Chen W.-J., Lu Q., Tang Y.-Y., Zhang M., Fu Y., Zhang D.-W., Yin K., Tang C.-K. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-kappaB signaling pathway and miR-33 expression. PLoS One. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ikeda Y., Murakami A., Ohigashi H. Ursolic acid: an anti-and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008;52:26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- 291.Shanmugam M.K., Rajendran P., Li F., Nema T., Vali S., Abbasi T., Kapoor S., Sharma A., Kumar A.P., Ho P.C., Hui K.M., Sethi G. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011;89:713–727. doi: 10.1007/s00109-011-0746-2. [DOI] [PubMed] [Google Scholar]
- 292.Wang J., Li Y., Wang X., Jiang C. Ursolic acid inhibits proliferation and induces apoptosis in human glioblastoma cell lines U251 by suppressing TGF-β1/miR-21/PDCD4 pathway. Basic Clin. Pharmacol. Toxicol. 2012;111:106–112. doi: 10.1111/j.1742-7843.2012.00870.x. [DOI] [PubMed] [Google Scholar]
- 293.Baltina L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003;10:155–171. doi: 10.2174/0929867033368538. [DOI] [PubMed] [Google Scholar]
- 294.Chintharlapalli S., Papineni S., Abdelrahim M., Abudayyeh A., Jutooru I., Chadalapaka G., Wu F., Mertens-Talcott S., Vanderlaag K., Cho S.D., Smith R., III, Safe S. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18b-olean-1,12-dien-30-oate in colon cancer cells. Int. J. Cancer. 2009;125:1965–1974. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zong L., Zhu Y., Liang R., Zhao H.-B. Gap junction mediated miRNA intercellular transfer and gene regulation: a novel mechanism for intercellular genetic communication. Sci. Rep. 2015;6:19884. doi: 10.1038/srep19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Blaskovich M.A., Sun J., Cantor A., Turkson J., Jove R., Sebti S.M. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 297.van der Fits L., van Kester M.S., Qin Y., Out-Luiting J.J., Smit F., Zoutman W.H., Willemze R., Tensen C.P., Vermeer M.H. MicroRNA-21 expression in CD4+ T cells is regulated by STAT3 and is pathologically involved in Sézary syndrome. J. Invest. Dermatol. 2011;131:762–768. doi: 10.1038/jid.2010.349. [DOI] [PubMed] [Google Scholar]
- 298.Rasmussen T.K., Andersen T., Bak R.O., Yiu G., Sorensen C.M., Stengaard-Pedersen K., Mikkelsen J.G., Utz P.J., Holm C.K., Deleuran B. Overexpression of microRNA-155 increases IL-21 mediated STAT3 signaling and IL-21 production in systemic lupus erythematosus. Arthritis Res. Ther. 2015;17:154. doi: 10.1186/s13075-015-0660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Du L., Subauste M.C., DeSevo C., Zhao Z., Baker M., Borkowski R., Schageman J.J., Greer R., Yang C.-R., Suraokar M., Wistuba I.I., Gazdar A.F., Minna J.D., Pertsemlidis A. miR-337–3p and its targets STAT3 and RAP1A modulate taxane sensitivity in non-small cell lung cancer. PLoS One. 2012;7:e39167. doi: 10.1371/journal.pone.0039167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean. Med. Sci. 2001;16:S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wu N., Wu G.C., Hu R., Li M., Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol. Sin. 2011;32:345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.An I.-S., An S., Kwon K.J., Kim Y.J., Bae S. Ginsenoside Rh2 mediates changes in the microRNA expression profile of human non-small cell lung cancer A549 cells. Oncol. Rep. 2013;29:523–528. doi: 10.3892/or.2012.2136. [DOI] [PubMed] [Google Scholar]
- 303.Keung M.-H., Chan L.-S., Kwok H.-H., Wong R.N.-S., Yue P.Y.-K. Role of microRNA-520h in 20(R)-ginsenoside-Rg3-mediated angiosuppression. J. Ginseng Res. 2016;40:151–159. doi: 10.1016/j.jgr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. The isolation and structure of Taxol. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 305.Schiff P.B., Horwitz S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chen N., Chon H.S., Xiong Y., Marchion D.C., Judson P.L., Hakam A., Gonzales-Bosquet J., Permuth-Wey J., Wenham R.M., Apte S.M., Cheng J.Q., Sellers T.A., Lancaster J.M. Human cancer cell line microRNAs associated with in vitro sensitivity to paclitaxel. Oncol. Rep. 2014;31:376–383. doi: 10.3892/or.2013.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhan Y., Xiang F., Wu R., Xu J., Ni Z., Jiang J., Kang X. MiRNA-149 modulates chemosensitivity of ovarian cancer A2780 cells to paclitaxel by targeting MyD88. J. Ovarian Res. 2015;8:48. doi: 10.1186/s13048-015-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Li X., Lu Y., Chen Y., Lu W., Xie X. MicroRNA profile of paclitaxel-resistant serous ovarian carcinoma based on formalin-fixed paraffin-embedded samples. BMC Cancer. 2013;13:216. doi: 10.1186/1471-2407-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Weiner-Gorzel K., Dempsey E., Milewska M., McGoldrick A., Toh V., Walsh A., Lindsay S., Gubbins L., Cannon A., Sharpe D., O'Sullivan J., Murphy M., Madden S.F., Kell M., McCann A., Furlong F. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med. 2015;4:745–758. doi: 10.1002/cam4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zong C., Wang J., Shi T.-M. MicroRNA 130b enhances drug resistance in human ovarian cancer cells. Tumor Biol. 2015;35:12151–12156. doi: 10.1007/s13277-014-2520-x. [DOI] [PubMed] [Google Scholar]
- 311.Zou D., Wang D., Li R., Tang Y., Yuan L., Long X., Zhou Q. MiR-197 induces taxol resistance in human ovarian cancer cells by regulating NLK. Tumor Biol. 2015;36:6725–6732. doi: 10.1007/s13277-015-3365-7. [DOI] [PubMed] [Google Scholar]
- 312.Zhou Y., Wang M., Wu J., Wu Z., Jie Z., Chang S., Shuang T. The clinicopathological significance of miR-1307 in chemotherapy resistant epithelial ovarian cancer. J. Ovarian Res. 2015;8:23. doi: 10.1186/s13048-015-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Brozovic A., Duran G.E., Wang Y.C., Francisco E.B., Sikic B.I. The miR-200 family differentially regulates sensitivity to paclitaxel and carboplatin in human ovarian carcinoma OVCAR-3 and MES-OV cells. Mol. Oncol. 2015;9:1678–1693. doi: 10.1016/j.molonc.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wu D., Li X., Meng X., Yan J., Zong Z. MicroRNA-873 mediates multidrug resistance in ovarian cancer cells by targeting ABCB1. Tumor Biol. 2016 doi: 10.1007/s13277-016-4944-y. [DOI] [PubMed] [Google Scholar]
- 315.Kim Y.-W., Kim E.Y., Jeon D., Liu J.-L., Kim H.S., Choi J.W., Ahn W.S. Differential microRNA expression signatures and cell type-specific association with taxol resistance in ovarian cancer cells. Drug Des. Dev. Ther. 2014;8:293–314. doi: 10.2147/DDDT.S51969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Fan Z., Cui H., Yu H., Ji Q., Kang L., Han B., Wang J., Dong Q., Li Y., Yan Z., Yan X., Zhang X., Lin Z., Hu Y., Jiao S. MiR-125a promotes paclitaxel sensitivity in cervical cancer through altering STAT3 expression. Oncogenesis. 2016;5:e197. doi: 10.1038/oncsis.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Shen Y., Zhou J., Li Y., Ye F., Wan X., Lu W., Xie X., Cheng X. MiR-375 mediated acquired chemo-resistance in cervical cancer by facilitating EMT. PLoS One. 2014;9:e109299. doi: 10.1371/journal.pone.0109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chen S., Chen X., Xiu Y.-L., Sun K.-X., Zong Z.-H., Zhao Y. MicroRNA 490-3p enhances the drug-resistance of human ovarian cancer cells. J. Ovarian Res. 2014;7:84. doi: 10.1186/s13048-014-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gao Y., Liu T., Huang Y. MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins. FEBS Lett. 2015;589:207–214. doi: 10.1016/j.febslet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 320.Chang T.-Y., Chen H.-A., Chiu C.-F., Chang Y.-W., Kuo T.-C., Tseng P.-C., Wang W., Hung M.-C., Su J.-L. Dicer elicits paclitaxel chemosensitization and suppresses cancer stemness in breast cancer by repressing AXL. Cancer Res. 2016;76:3916–3928. doi: 10.1158/0008-5472.CAN-15-2555. [DOI] [PubMed] [Google Scholar]
- 321.Su C.-M., Wang M.Y., Hong C.-C., Chen H.-A., Su Y.-H., Wu C.-H., Huang M.-T., Chang Y.-W., Jiang S.-S., Sung S.-Y., Chang J.-Y., Chen L.-T., Chen P.-S., Su J.-L. MiR-520h is crucial for DAPK2 regulation and breast cancer progression. Oncogene. 2016;35:1134–1142. doi: 10.1038/onc.2015.168. [DOI] [PubMed] [Google Scholar]
- 322.Xue J., Chi Y., Chen Y., Huang S., Ye X., Niu J., Wang W., Pfeffer L.M., Shao Z.-M., Wu Z.-H., Wu J. MiRNA-621 sensitizes breast cancer to chemotherapy by suppressing FBXO11 and enhancing p53 activity. Oncogene. 2016;35:448–458. doi: 10.1038/onc.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Li Q., Liu M., Ma F., Luo Y., Cai R., Wang L., Xu N., Xu B. Circulating miR-19a and miR-205 in serum may predict the sensitivity of luminal A subtype of breast cancer patients to neoadjuvant chemotherapy with epirubicin plus paclitaxel. PLoS One. 2014;9:e104870. doi: 10.1371/journal.pone.0104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Frères P., Josse C., Bovy N., Boukerroucha M., Struman I., Bours V., Jerusalem G. Neodjuvant chemotherapy in breast cancer patients induces miR-34a and miR-122 expression. J. Cell. Physiol. 2015;230:473–481. doi: 10.1002/jcp.24730. [DOI] [PubMed] [Google Scholar]
- 325.Chatterjee A., Chattopadhyay D., Chakrabarti G. MiR-16 targets Bcl-2 in paclitaxel-resistant lung cancer cells and overexpression of miR-16 along with miR-17 causes unprecedented sensitivity by simultaneously modulating autophagy and apoptosis. Cell. Signal. 2015;27:189–203. doi: 10.1016/j.cellsig.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 326.Chatterjee A., Chattopadhyay D., Chakrabarti G. MiR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering Beclin 1 expression. PLoS One. 2014;9:e95716. doi: 10.1371/journal.pone.0095716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang Q., Ran R., Zhang L., Liu Y., Mei L., Zhang Z., Gao H., He Q. Simultaneous delivery of therapeutic antagomirs with paclitaxel fort he management of metastatic tumors by a pH-responsive anti-microbial peptide-mediated liposomal delivery system. J. Contr. Release. 2015;197:208–218. doi: 10.1016/j.jconrel.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 328.Dai X., Fan W., Wang Y., Huang L., Jiang Y., Shi L., Mckinley D., Tan W., Tan C. Combined delivery of let-7b micro-RNA and paclitaxel via biodegradable nanoassemblies for the treatment of KRAS mutant cancer. Mol. Pharm. 2016;13:520–533. doi: 10.1021/acs.molpharmaceut.5b00756. [DOI] [PubMed] [Google Scholar]
- 329.Jin B., Liu Y., Wang H. Antagonism of miR-21 sensitizes human gastric cancer cells to paclitaxel. Cell. Biochem. Biophys. 2015;72:275–282. doi: 10.1007/s12013-014-0450-2. [DOI] [PubMed] [Google Scholar]
- 330.Huang D., Wang H., Liu R., Li H., Ge S., Bai M., Deng T., Yao G., Ba Y. MiRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J. Cell. Biochem. 2014;115:549–556. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 331.Liu Y., Gao S., Chen X., Liu M., Mao C., Fang X. Overexpression of miR-203 sensitizes paclitaxel (taxol)-resistant colorectal cancer cells through targeting the salt-inducible kinase 2 (SIK2) Tumor Biol. 2016 doi: 10.1007/s13277-016-5066-2. [DOI] [PubMed] [Google Scholar]
- 332.Liu L., Chen L., Wu X., Li X., Song Y., Mei Q., Nie J., Han W. Low-dose DANN-demethylating agent enhances the chemosensitivity of cancer cells by targeting cancer stem cells via the upregulation of microRNA-497. J. Cancer Res. Clin. Oncol. 2016;142:1431–1439. doi: 10.1007/s00432-016-2157-9. [DOI] [PubMed] [Google Scholar]
- 333.Fujita Y., Kojima T., Kawakami K., Mizutani K., Kato T., Deguchi T., Ito M. MiR-130a activates apoptotic signaling through activation of caspase-8 in taxane-resistant prostate cancer cells. Prostate. 2015;75:1568–1578. doi: 10.1002/pros.23031. [DOI] [PubMed] [Google Scholar]
- 334.Peng X., Cao P., He D., Han S., Zhou J., Tan G., Li W., Yu F., Yu J., Li Z., Cao K. MiR-634 sensitizes nasopharyngeal carcinoma cells to paclitaxel and inhibits cell growth both in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014;7:6784–6791. [PMC free article] [PubMed] [Google Scholar]
- 335.Peng X., Cao P., Li J., He D., Han S., Zhou J., Tan G., Li W., Yu F., Yu J., Li Z., Cao K. MiR-1204 sensitizes nasopharyngeal carcinoma cells to paclitaxel both in vitro and in vivo. Cancer Biol. Ther. 2015;16:261–267. doi: 10.1080/15384047.2014.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Huang X., Qin J., Lu S. Up-regulation of miR-877 induced by paclitaxel inhibits hepatocellular carcinoma cell proliferation though targeting FOXM1. Int. J. Clin. Exp. Pathol. 2015;8:1515–1524. [PMC free article] [PubMed] [Google Scholar]
- 337.Du L., Borkowski R., Zhao Z., Ma X., Yu X., Xie X.-J., Pertsemlidis A. A high-throughput screen identifies miRNA inhibitors regulating lung cancer cell survival and response to paclitaxel. RNA Biol. 2013;10:1700–1713. doi: 10.4161/rna.26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li Y., Yu C., Li J., Wu X. Andrographolide antagonizes cigarette smoke extract-induced inflammatory response and oxidative stress in human alveolar epithelial A549 cells through induction of microRNA-128. Exp. Lung Res. 2013;39:463–471. doi: 10.3109/01902148.2013.857443. [DOI] [PubMed] [Google Scholar]
- 339.Lu B., Sheng Y., Zhang J., Zheng Z., Ji L. The alteres microRNA profile in andrographolide-induced inhibition of hepatoma tumor growth. Gene. 2016;588:124–133. doi: 10.1016/j.gene.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 340.Jansson M.D., Damas N.D., Lees M., Jacobsen A., Lund A.H. MiR-339-5p regulates the p53 tumor-suppressor pathway by targeting MDM2. Oncogene. 2015;34:1908–1918. doi: 10.1038/onc.2014.130. [DOI] [PubMed] [Google Scholar]
- 341.Aruoma O.I., Haliwell B., Aeschbach R., Löligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 342.González-Vallinas M., Molina S., Vicente G., Zarza V., Martín-Hernández R., García-Risco M.R., Fornani T., Reglero G., Ramírez de Molina A. Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of rosemary diterpenes in colon and pancreatic cancer. PLoS One. 2014;9:e98556. doi: 10.1371/journal.pone.0098556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Duggal J., Harrison J.S., Studzinski G.P., Wang X. Involvement of microRNA181a in differentiation and cell cycle arrest induced by a plant-derived antioxidant carnosic acid and vitamin D analog doxercalciferol in human leukemia cells. MicroRNA. 2012;1:26–33. doi: 10.2174/2211536611201010026. [DOI] [PubMed] [Google Scholar]
- 344.Schobert R., Knauer S., Seibt S., Biersack B. Anticancer active illudins: recent developments of a potent alkylating compound class. Curr. Med. Chem. 2011;18:790–807. doi: 10.2174/092986711794927766. [DOI] [PubMed] [Google Scholar]
- 345.Liu Z., Liu S., Xie Z., Pavlovicz R.E., Wu J., Chen P., Aimiuwu J., Pang J., Bhasin D., Neviani P., Fuchs J.R., Plass C., Li P.-K., Li C., Huang T.H.-M., Wu L.-C., Rush L., Wang H., Perrotti D., Marcucci G., Chan K.K. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J. Pharmacol. Exp. Ther. 2009;329:505–514. doi: 10.1124/jpet.108.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Pickering B.F., Yu D., Van Dyke M.W. Nucleolin protein interacts with microprocessor complex to affect biogenesis of microRNAs 15a and 16. J. Biol. Chem. 2011;286:44095–44103. doi: 10.1074/jbc.M111.265439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rao Y.K., Wu A.T., Geethangili M., Huang M.T., Chao W.J., Wu C.H., Deng W.P., Yeh C.T., Tzeng Y.M. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signaling in metastatic breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011;24:238–245. doi: 10.1021/tx100318m. [DOI] [PubMed] [Google Scholar]
- 348.Yeh C.-T., Huang W.-C., Rao Y.K., Ye M., Lee W.H., Wang L.-S., Tzeng D.T.W., Wu C.-H., Shieh Y.-S., Huang C.-Y.F., Chen Y.-J., Hsiao M., Wu A.T.H., Yang Z., Tzeng Y.-M. A sesquiterpene lactone antrocin from Antrodia camphorata negatively modulates JAK2/STAT3 signaling via microRNA let-7c and induces apoptosis in lung cancer cells. Carcinogenesis. 2013;34:2918–2928. doi: 10.1093/carcin/bgt255. [DOI] [PubMed] [Google Scholar]
- 349.Zhang S., Liu Q., Liu Y., Qiao H., Liu Y. Zerumbone, a Southeast Asian ginger sesquiterpene, induced apoptosis of pancreatic carcinoma cells through p53 signaling pathway, evidence-based complement. Altern. Med. 2012;2012 doi: 10.1155/2012/936030. Article ID 936030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Thebault S., Lemonnier L., Bidaux G., Flourakis M., Bavenkoffe A., Gordienko D., Roudbaraki M., Delcourt P., Panchin Y., Shuba Y., Skryma R., Prevarskaya N. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J. Biol. Chem. 2005;280:39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- 351.Lu H.-F., Hsueh S.-C., Yu F.-S., Yang J.-S., Tang N.-Y., Chen S.-C., Chung J.-G. The role of Ca2+ in (-)-menthol-induced human promyelocytic leukemia HL-60 cell death. In Vivo. 2006;20:69–76. [PubMed] [Google Scholar]
- 352.Schobert R., Biersack B., Dietrich A., Grotemeier A., Müller T., Kalinowski B., Knauer S., Voigt W., Paschke R. Monoterpenes as drug shuttles: cytotoxic (6-aminomethylnicotinate)-dichloridoplatinum(II) complexes with potential to overcome cisplatin resistance. J. Med. Chem. 2007;50:1288–1293. doi: 10.1021/jm061379o. [DOI] [PubMed] [Google Scholar]
- 353.Worthen D., Ghosheh G., Crooks P. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18:1527–1532. [PubMed] [Google Scholar]
- 354.Reindl W., Yuan J., Krämer A., Strebhardt K., Berg T. Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein-protein interactions. Chem. Biol. 2008;15:459–466. doi: 10.1016/j.chembiol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 355.Bhattacharya S., Ahir M., Patra P., Mukherjee S., Ghosh S., Mazumdar M., Chattopadhyay S., Das T., Chattopadhyay D., Adhikary A. PEGylated-thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a. Biomaterials. 2015;51:91–107. doi: 10.1016/j.biomaterials.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 356.Li J.T., He W., Song S.S., Wei W. Paeoniflorin inhibited the tumor invasion and metastasis in human hepatocellular carcinoma cells. Bratisl. Lek. Listy. 2014;115:427–433. doi: 10.4149/bll_2014_084. [DOI] [PubMed] [Google Scholar]
- 357.Li W., Qi Z., Wei Z., Liu S., Wang P., Chen Y., Zhao Y. Paeoniflorin inhibits proliferation and induces apoptosis of human glioma cells via microRNA-16 upregulation and matrix metalloproteinase-9 downregulation. Mol. Med. Rep. 2015;12:2735–2740. doi: 10.3892/mmr.2015.3718. [DOI] [PubMed] [Google Scholar]
- 358.Li J-Z., Tang X.-N., Li T.-T., Liu L.-J., Yu S.-Y., Zhou G.-Y., Shao Q.-R., Sun H.-P., Wu C., Yang Y. Paeoniflorin inhibits doxorubicin-induced cardiomyocete apoptosis by downregulating microRNA-1 expression. Exp. Ther. Med. 2016;11:2407–2412. doi: 10.3892/etm.2016.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sun Y., Zuo L., Xu C., Shen T., Pan H., Zhang Z. Apoptosis and differentiation induced by sodium selenite combined with all-trans retinoic acid (ATRA) in NB4 cells. Zhonghua Xue Ye Xue Za Zhi. 2002;23:628–630. [PubMed] [Google Scholar]
- 360.Rossi A., D’urso O.F., Gatto G., Poltronieri P., Ferracin M., Remondelli P., Negrini M., Caporaso M.G., Bonatti S., Mallardo M. Non-coding RNAs change their expression profile after retinoid induced differentiation of the promyelocytic cell line NB4. BMC Res. 2010;3:24. doi: 10.1186/1756-0500-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Garzon R., Pichiorri F., Palumbo T., Visentini M., Aqeilan R., Cimmino A., Wang H., Sun H., Volinia S., Alder H., Calin G.A., Liu C.G., Andreeff M., Croce C.M. MicroRNA gene expression during retinoid acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 362.Terao M., Fratelli M., Kurosaki M., Zanetti A., Guarnaccia V., Paroni G., Tsykin A., Lupi M., Gianni M., Goodall G.J., Garattini E. Induction of miR-21 by retinoic acid in estrogen receptor-positive breast carcinoma cells: biological correlates and molecular targets. J. Biol. Chem. 2011;286:4027–4042. doi: 10.1074/jbc.M110.184994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Meseguer S., Mudduluru G., Escamilla J.M., Allgayer H., Barettino D. MicroRNAs-10a and -10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF) J. Biol. Chem. 2011;286:4150–4164. doi: 10.1074/jbc.M110.167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Annabali D., Gioia U., Savino M., Laneve P., Caffarelli E., Nasi S. A new module in neural differentiation control: two microRNAs upregulated by retinoic acid, miR-9 and miR-103, target the differentiation inhibitor ID2. PLoS One. 2012;7:e40269. doi: 10.1371/journal.pone.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Khan S., Wall D., Curran C., Newell J., Kerin M.J., Dwyer R.M. MicroRNA-10a is reduced in breast cancer and regulated in part through retinoic acid. BMC Cancer. 2015;15:345. doi: 10.1186/s12885-015-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Yang F., Miao L., Mei Y., Wu M. Retinoic acid-induced HOXA5 expression is co-regulated by HuR and miR-130a. Cell. Signal. 2013;25:1476–1485. doi: 10.1016/j.cellsig.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 367.Zhang J., Gao Y., Yu M., Wu H., Ai Z., Wu Y., Liu H., Du J., Guo Z., Zhang Y. Retinoic acid induces embryonic stem cell differentiation by altering both encoding RNA and miRNA expression. PLoS One. 2015;10:e0132566. doi: 10.1371/journal.pone.0132566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Jian P., Li Z.W., Fang T.Y., Jian W., Zhuan Z., Mei L.X., Yan W.S., Jian N. Retinoic acid induces HL-60 cell differentiation via the upregulation of miR-663. J. Hematol. Oncol. 2011;4:20. doi: 10.1186/1756-8722-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Zhang S., Tao L., Peng Y., Liu L., Hong X., Zhang Y., Wang Q. Gap junctions enhance the antiproliferative effect of microRNA-124-3p in glioblastoma cells. J. Cell. Physiol. 2015;230:2476–2488. doi: 10.1002/jcp.24982. [DOI] [PubMed] [Google Scholar]
- 370.Chen P.-H., Shih C.-M., Chang W.-C., Cheng C.-H., Lin C.-W., Ho K.-H., Su P.-C., Chen K.-C. MicroRNA-302b-inhibited E2F3 transcription factor is related to all trans retinoic acid-induced glioma cell apoptosis. J. Neurochem. 2014;131:731–742. doi: 10.1111/jnc.12820. [DOI] [PubMed] [Google Scholar]
- 371.van Breemen R.B., Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008;269:339–351. doi: 10.1016/j.canlet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Li D., Chen L., Zhao W., Hao J., An R. MicroRNA-let-7f-1 is induced by lycopene and inhibits cell proliferation and triggers apoptosis in prostate cancer. Mol. Med. Rep. 2016;13:2708–2714. doi: 10.3892/mmr.2016.4841. [DOI] [PubMed] [Google Scholar]
- 373.Fleet J.C., Desmet M., Johnson R., Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem. J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Alvares-Díaz S., Valle N., Ferrer-Mayorga G., Lombardía L., Herrera M., Domínguez O., Segura M.F., Bonilla F., Hernando E., Munoz A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Gen. 2012;21:2157–2165. doi: 10.1093/hmg/dds031. [DOI] [PubMed] [Google Scholar]
- 375.Gocek E., Wang X., Liu X., Liu C.G., Studzinski G.P. MicroRNA-32 up-regulation by 1,25-dihydroxyvitamin D3 in human myeloid leukemia cells leads to Bim targeting and inhibition of AraC-induced apoptosis. Cancer Res. 2011;71:6230–6239. doi: 10.1158/0008-5472.CAN-11-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Padi S.K.R., Zhang Q., Rustum Y.M., Morrison C., Guo B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145:437–446. doi: 10.1053/j.gastro.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.González-Duarte R.J., Cázares-Ordonez V., Romero-Córdoba S., Díaz L., Ortíz V., Freyre-González J.A., Hidalgo-Miranda A., Larrea F., Avila E. Calcitriol increases Dicer expression and modifies the microRNAs signature in SiHa cervical cancer cells. Biochem. Cell Biol. 2015;93:376–384. doi: 10.1139/bcb-2015-0010. [DOI] [PubMed] [Google Scholar]
- 378.Guan H., Liu C., Chen Z., Wang L., Li C., Zhao J., Yu Y., Zhang P., Chen W., Jiang A. 1,25-Dihydroxyvitamin D3 up-regulates expression of hsa-let-7a-2 through the interaction of VDR/VDRE in human lung cancer A549 cells. Gene. 2013;522:142–146. doi: 10.1016/j.gene.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 379.Giangreco A.A., Vaishnav A., Wagner D., Finelli A., Fleshner N., van der Kwast T., Vieth R., Nonn L. Tumor suppressor microRNAs, miR-100 and miR-125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev. Res. 2013;6:483–494. doi: 10.1158/1940-6207.CAPR-12-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Mohri T., Nakajima M., Takagi S., Komagata S., Yokoi T. MicroRNA regulates human vitamin D receptor. Int. J. Cancer. 2009;125:1328–1333. doi: 10.1002/ijc.24459. [DOI] [PubMed] [Google Scholar]
- 381.Wang X., Gocek E., Liu C.G., Studzinski G.P. MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle. 2009;8:736–741. doi: 10.4161/cc.8.5.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Borkowski R., Du L., Zhao Z., McMillan E., Kosti A., Yang C.-R., Suraokar M., Wistuba I.I., Gazdar A.F., Minna J.D., White M.A., Pertsemlidis A. Genetic mutation of p53 and suppression of the miR-17∼92 cluster are synthetic lethal in non-small cell lung cancer due to upregulation of vitamin D signaling. Cancer Res. 2014;75:666–675. doi: 10.1158/0008-5472.CAN-14-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zitman-Gal T., Green J., Pasmanik-Chor M., Golan E., Bernheim J., Benchetrit S. Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment. Cardiovasc. Diabetol. 2014;13:8. doi: 10.1186/1475-2840-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Rimbach G., Moehring J., Huebbe P., Lodge J.K. Gene-regulatory activity of α-tocopherol. Molecules. 2010;15:1746–1761. doi: 10.3390/molecules15031746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gaedicke S., Zhang X., Schmelzer C., Lu Y., Doering F., Frank J., Rimbach G. Vitamin E dependent microRNA regulation in rat liver. FEBS Lett. 2008;582:3542–3546. doi: 10.1016/j.febslet.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 386.Shirode A.B., Sylvester P.W. Synergistic anticancer effects of combined gamma-tocotrienol and celecoxib treatment are associated with suppression of Akt and NFκB signaling. Biomed. Pharmacother. 2010;64:327–332. doi: 10.1016/j.biopha.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Ji X., Wang Z., Geamanu A., Goja A., Sarkar F.H., Gupta S.V. Delta-tocotrienol suppresses Notch-1 pathway by upregulating miR-34a in non-small cell lung cancer cells. Int. J. Cancer. 2012;131:2668–2677. doi: 10.1002/ijc.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Chen C.C., Shiao Y.J., Lin R.D., Shao Y.Y., Lai M.N., Lin C.C., Ng L.T., Kuo Y.H. Neuroprotective diterpenes from the fruiting body of Antrodia camphorate. J. Nat. Prod. 2006;69:689–691. doi: 10.1021/np0581263. [DOI] [PubMed] [Google Scholar]
- 389.Chiang P.C., Lin S.C., Pan S.L., Kuo C.H., Tsai I.L., Kuo M.T., Wen W.C., Chen P., Guh J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: a crucial role of AMPK and mTOR pathways. Biochem. Pharmacol. 2010;79:162–171. doi: 10.1016/j.bcp.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 390.Kumar V.B., Yuan T.-C., Liou J.-W., Yang C.-J., Sung P.-J., Weng C.-F. Antroquinonol inhibits NSCLC proliferation by altering PI3K/mTOR proteins and miRNA expression profiles. Mut. Res. 2011;707:42–52. doi: 10.1016/j.mrfmmm.2010.12.009. [DOI] [PubMed] [Google Scholar]