Abstract
The use of monoclonal antibodies that block immunologic checkpoints that would otherwise mediate the adaptive immune resistance have paved the way in cancer treatment. There is evidence that blocking the PD-1/PD-L1 axis is a strategy of overriding importance in the treatment of patients with metastatic melanoma and other solid malignancies, some of which (NSCLC, colorectal cancer, renal cell cancer, head and neck cancer) were not considered to be ‘immune-responsive’ diseases until recently. In this perspective article, the biological and clinical relevance of PD-L1 is summarized in the context of the immune checkpoint inhibitors as a therapeutic strategy in metastatic melanoma patients.
KEYWORDS : immune checkpoint inhibitors, melanoma, PD-1, PD-L1, predictive, prognostic
Practice points.
Blocking the PD-1/PD-L1 axis is a strategy of paramount importance
PD-1/PD-L1 interaction inhibits T-lymphocyte proliferation, survival and effector functions, induces apoptosis of antigen-specific T cells. Blocking the PD-1/PD-L1 axis is a strategy of paramount importance in the treatment of patients with metastatic melanoma (MM) and other solid malignancies, including NSCLC, colorectal cancer and renal cell carcinoma.
Regulation of PD-L1 expression by melanoma is an area of intense investigation
PD-L1 can be induced by microenvironmental signals, including IFN-γ, which is produced by activated CD8+ T lymphocytes. On the other side, PD-L1 may be induced through oncogenic signaling.
The immune-checkpoint inhibitors represents a paradigm shift in the treatment of melanoma
Recent Phase II and III randomized clinical trials with anti-PD-1 antibodies (nivolumab and pembrolizumab) have demonstrated a higher objective response rate and increased overall survival compared with previous standard treatments in melanoma as well as other tumor histotypes. These results lead to the accelerated approval of anti-PD-1 antibodies by regulatory agencies (US FDA and EMA) for MM, NSCLC and renal cell carcinoma.
PD-1 antibodies only for PD-L1-positive patients?
Although there is a doubling of response rate in MM PD-L1-positive patients, a small proportion of PD-L1-negative patients may ultimately benefit from anti-PD-1/PD-L1 therapies. Indeed, a proportion of PD-L1-negative MM patients appear to obtain an objective response to anti-PD-1 therapies. Hence, PD-L1 should not be used as a biomarker to select patients who should receive or not anti-PD-1 antibodies.
Aim
The use of monoclonal antibodies that block immunologic checkpoints that would otherwise mediate the adaptive immune resistance have paved the way in cancer treatment [1]. Among the immune checkpoint targets of clinical importance is PD-1, which is expressed by exhausted T lymphocytes and mediates immunosuppression. PD-1 is expressed in non-naive T cells, including activated and memory T cells as well as senescent T cells. Furthermore, PD-1 is present on activated myeloid lineage cells such as NK cells as well as dendritic cells (DCs) and monocytes. PD-1 can be expressed in mature B cells following stimulation with Toll-like receptor 9 agonists. In mature B cells PD-1 inhibits clonal responses and attenuates their antigen-specific antibody responses [2,3].
PD-1 plays its role not only in peripheral tissues where T cells encounter PD-1 ligands [1], but also during physiological antigen presentation by DC to activate both naive and memory T cells. Specifically, Karwacz et al. [4] demonstrated that during T-cell activation, PD-1 regulates a critical step in T-cell activation. Engagement of PD-L1 on DC to PD-1 in T cells induces TCR downmodulation and limits TCR signal transduction preventing T-cell hyperactivation after antigen presentation by DC. This notion is significant for two main reasons:
It explains the lower expression of TCRs on tumor-infiltrating cells;
By combining PD-L1 silencing with modulators of MAPKs in DC, their antitumor activities can be increased, opening new therapeutic scenarios in human clinical trials.
Recently, it has been shown that murine as well as human melanomas express PD-1 and demonstrated that melanoma cell-intrinsic PD-1 promotes tumorigenesis, even in mice lacking adaptive immunity [5]. Through well-designed experiments it was shown that PD-1 inhibition on melanoma cells by blocking antibodies, RNAi as well as mutagenesis of melanoma-PD-1 signaling pathway suppresses tumor growth in immune-competent, immune-compromised and PD-1-deficient tumor graft recipient mice. These findings suggest that blocking melanoma-PD-1 might contribute to the unprecedented efficacy of anti-PD-1 therapy.
Two ligands for PD-1, designated PD-L1 and PD-L2, have been identified based on the similarity to other B7 superfamilies. PD-L1 is more broadly expressed than PD-L2. Specifically, PD-L1 is constitutively expressed on T and B cells, macrophages and DC, and may be overexpressed in cancer cells and stromal cells. During T-cell activation PD-L1 regulates a critical step in T-cell activation, downmodulating TCR, limiting TCR signal transduction and preventing T-cell hyperactivation after antigen presentation by DC [4].
In early Phase I trials, antibodies blocking the PD-1, or its ligand (PD-L1), were shown to induce a 30–50% of response in several cancer types, most of them being durable, with a favorable therapeutic ratio. Recent Phase II and III randomized clinical trials with anti-PD-1 antibodies (nivolumab and pembrolizumab) have demonstrated a higher objective response rate and increased overall survival compared with previous standard treatments in metastatic melanoma (MM) as well as in other tumor types including NSCLC and renal cell carcinoma (RCC) [6–10]. These results lead to the accelerated approval of anti-PD-1 antibodies by regulatory agencies (US FDA and EMA) for MM, NSCLC and RCC. Overall, these studies provide evidence that blocking the PD-1/PD-L1 axis is a strategy of overriding importance in the treatment of patients with MM and other solid malignancies, some of which (NSCLC, RCC, colorectal cancer, mesothelioma) were not considered ‘immune-responsive’ diseases until recently.
In this perspective article, the biological and clinical relevance of PD-L1 is summarized in the context of the immune-checkpoint inhibitors as a therapeutic strategy in MM patients.
PD-L1 expression, biological functions & outcomes
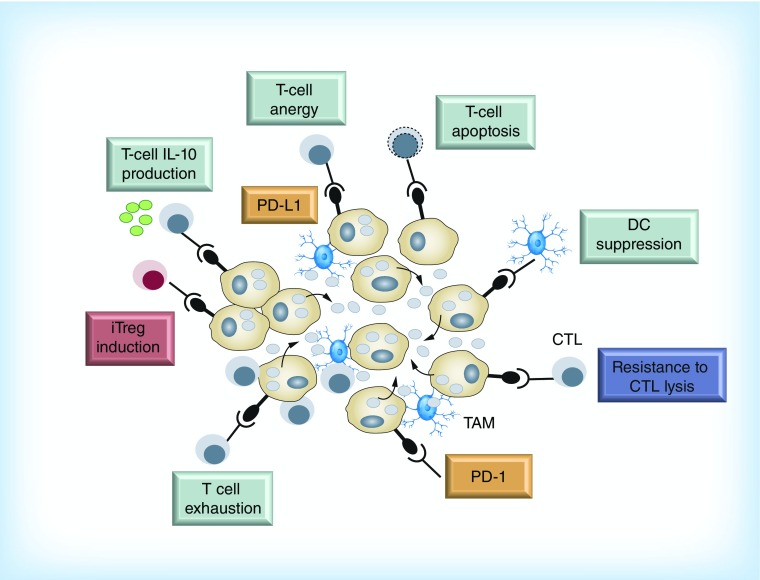
Mechanisms of immunosuppression mediated by the PD-1/PD-L1 interaction include the inhibition of T-lymphocytes’ proliferation, induction of T-cell dysfunction, apoptosis of antigen-specific T cells as well as the promotion of the differentiation of CD4+ T cells into Foxp3+ regulatory T cells (Figure 1). In addition to lymphocytes, PD-1 is also expressed by DCs and plays a role in suppressing production of inflammatory cytokines. PD-1-deficient DCs exhibit enhanced antibacterial function, indicating that PD-1 can also act as an inhibitory receptor on DCs. PD-L1 also serves as a receptor on cancer cells and can induce intrinsic resistance to T-cell killing upon interaction with PD-1 [1].
Figure 1. . Relevant immunosuppressive mechanisms mediated by PD-1/PD-L1 axis.
CTL: Cytotoxic T lymphocyte; DC: Dendritic cell; iTreg: Induced regulatory T cell.
Modified with permission from [3].
Regulation of PD-L1 expression by melanoma is an area of intense investigation [11]. On the one side, PD-L1 can be induced by microenvironmental signals, including IFN-γ, which is produced by activated CD8+ T lymphocytes [12]. On the other side, PD-L1 may be induced through oncogenic signaling. Furthermore, recent data suggest that targeted therapies including BRAFi and MEKi may induce PD-L1 expression by melanoma cells [13,14].
The interferon-inducible expression of PD-L1 seems to be more common than the constitutive expression in melanoma, and results in a restricted PD-L1 expression in T-cell-rich areas of tumors, in particular at the invasive peripheral margin, at the interphase between melanoma growth and adjacent stromal component. This pattern of expression suggests that PD-L1 is expressed as a mechanism of immune evasion, an indirect consequence of the presence of tumor antigen-specific T cells that recognized the cancer cells [12].
The importance of PD-L1 expression for immune homeostasis in the melanoma microenvironment is underlined by two recently reported studies, which shed the light on the biological and the clinical significance of PD-L1 in melanoma. In the first study, Taube et al. evaluated the immunoarchitectural features, the patterns of immune-cell infiltration and the lymphocyte subpopulation pattern, in 41 patients with different malignant diseases, including melanoma, which had been treated with an anti-PD-1 antibody (nivolumab) [15]. The main finding of that work is that PD-L1 expression was geographically associated with infiltrating immune cells. Furthermore, expression of PD-L1 by tumor cells was significantly associated with expression of PD-1 on neighboring lymphocytes. Finally, tumor cell PD-L1 expression correlated with objective response to anti-PD-1 therapy.
In the second study, Spranger et al. found that melanoma lesions showing a correlation between the presence of a CD8+ T-cell infiltrate and the recruitment of CD4+/CD25+/FOXP3+ Treg cells [16]. The latter finding was also correlated to expression of IDO and PD-L1 by melanoma cells [16]. Of these two findings, the first was mainly attributed to the production of CCR4-binding chemokines (CCL22), while the latter to IFN-γ secretion. These results suggest that tumor cells use different immunosuppressive mechanisms to skew immune system toward tolerance, preventing optimal T-cell activation.
In stage III melanoma patients, outside of PD-1 treatment, tumor-negative PD-L1 status was found to be a marker of worse patient survival and is associated with a poor immune-response gene signature [17]. An immune-related gene expression signature was found in PD-L1-positive tumors, particularly a marked increase in cytotoxic T-cell and macrophage-specific genes. While, lower nonsynonymous mutation levels were associated with PD-L1-negative status, suggesting differences in somatic mutation profiles is a determinant of PD-L1-associated antitumor immunity in stage III melanoma.
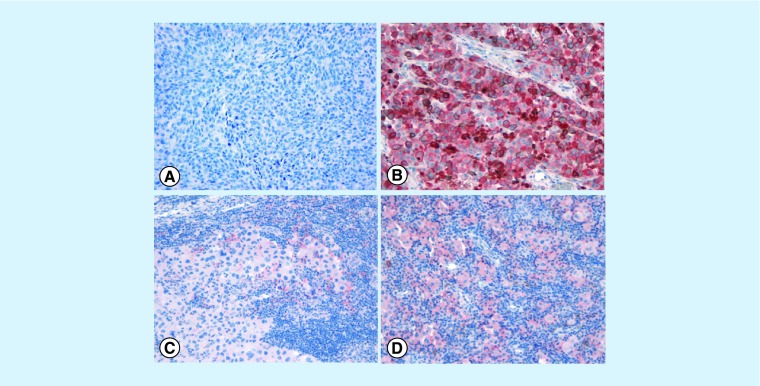
Overall, in the context of the tumor microenvironment, PD-L1 can be expressed in tumor cells in association or in absence of infiltrating T cells or, alternatively, tumor cells can be PD-L1 negative, in presence or absence of infiltrating T cells, thus featuring four different subgroups (Figure 2) [18,19]. The exact proportion of human melanomas that can be classified into these four categories is still an open issue although rough estimates suggest the following: PD-L1+/TILS+ 35%; PD-L1-/TILs- 40%; PD-L1+/TILs- 5% and PD-L1-/TILs+ 20% [18]. In tumors that do not express PD-L1, the function and specificity of T cells is unclear. It is not yet understood whether the lack of T-cell infiltrate results from the absence of tumor antigen-specific responses or an as-yet unknown biological process that excludes mononuclear immune cells from the tumor microenvironment.
Figure 2. . PD-L1 status and immune cell infiltrates in the context of the melanoma microenvironment.
(A) PD-L1- melanoma in absence of immune cells; (B) strong and diffuse PD-L1 immunohistochemical staining in melanoma cells in absence of immune cells; (C) PD-L1 is expressed only focally in immune cells at the periphery of tumor aggregates but the tumor is PD-L1 negative; (D) PD-L1+ melanoma cells associated with prominent immune-cell infiltrate (original magnification ×20). The exact proportion of human melanomas that can be classified into these four categories is still an open issue although rough estimates suggest the following: PD-L1+/TILs+ 35%; PD-L1-/TILs- 40%; PD-L1+/TILs- 5% and PD-L1-/TILs+ 20% [18].
In the setting of BRAFi and MEKi, it has been shown [20] that CD4+ and CD8+ lymphocytes increased markedly following BRAFi treatment and there was a correlation between the degree of tumor infiltration by CD8+ and Granzyme B-expressing lymphocytes in post-iBRAF-treated biopsies. Increased intratumoral CD8+ lymphocyte expression correlated with a reduction in tumor size and an increase in necrosis in post-treatment biopsies.
In the context of anti PD-1 antibodies preclinical and clinical data show that patients who respond contain tumor-specific CD8+ T cells expressing PD-1 in close proximity to PD-L1-expressing cells. The challenge is to understand which aspects of cancer immunity need to be targeted by novel immunotherapies to provide benefit for patients without CD8+ infiltration in the context of otherwise pathologic mechanism known as immunoediting escape [18].
Finally, besides its role as an immunomodulatory molecule, recent data in ovarian tumor models suggest that PD-L1 might per se determine a more aggressive clinical course [21]. Preliminary data, in A375 melanoma cell lines, suggest that constitutive PD-L1 expression may define a subset of melanoma cell line characterized by a highly invasive phenotype and by enhanced ability to grow in vivo [22]. Whether PD-L1 is only an epiphenomenon or is mechanistically involved in this phenotype is far to be elucidated.
Immunohistochemical expression of PD-L1 in melanoma
PD-L1 immunohistochemistry has previously suffered from poor standardization and harmonization since reliable PD-L1-specific antibodies for paraffin embedded tissues have been difficult to develop. In addition, PD-L1 has been evaluated in different subcellular localization (membranous vs cell surface vs cytoplasmic) and in different context (immune cells and melanoma cells), with different methods and reagents and this adds further complexity and variability in the interpretation of results in PD-L1 expression in the different datasets [11].
Several studies investigated the prognostic role of PD-L1 immunohistochemical overexpression. However, it is clear that the prognostic role of PD-L1 and PD-1 in the context of different tumor types requires further investigation for several reasons, including:
Heterogeneity of expression in primary and metastatic melanoma samples;
Heterogeneity in PD-L1 expression in the same melanoma sample;
The retrospective design of all reported studies;
Different types of tissues have been evaluated (frozen vs paraffin-embedded samples);
Melanoma cells and/or the tumor microenvironment have been evaluated;
Monoclonal and/or polyclonal antibodies have been used;
Both cytoplasmic and/or membranous immunostaining have been evaluated to define positivity;
Different scores of PD-L1 and/or PD-1 positivity have been reported.
In regards to heterogeneity, the expression of PD-L1 in immunotherapy-naive metastatic melanoma patients was evaluated to assess longitudinal intrapatient concordance [23]. PD-L1 expression was found frequently discordant between primary tumors and metastases and between intrapatient metastases, such that 23/46 longitudinal patient specimens were discordant.
In summary, there are still considerable areas of uncertainties about the interpretation, reliability in scoring and clinical significance of PD-L1 immunohistochemical testing in view of the dynamic nature of this marker and tumor environment. The difficulties with PD-L1 determination in clinical practice are also due to the lack of robust comparability data between the different assays. In terms of interpretation, one of the main limitations may be the challenge in distinguishing cytoplasmic from clear membranous staining, a critical issue at low percentages of positivity in pigmented tissue specimens or with low numbers of tumor cells available. Further data on the reliability of this assay in a real-life setting as well as data to compare the different assays are highly needed.
PD-L1: predictive, correlative or prognostic marker?
Identifying biomarkers to tailor therapy with immune-checkpoint inhibitors is highly needed. Recently, we conducted a systematic literature search and meta-analysis to investigate whether the PD-L1 status, detected by immunohistochemistry, is associated with clinical response and mortality in patients in MM patients receiving anti PD-1 antibodies that received approval by regulatory agencies (FDA and EMA) [24].
Our analysis led to three main findings:
The expression of PD-L1 in tumor tissues correlates with the overall response to antibodies targeting the PD-1/PD-L1 axis in MM;
PD-L1 is a predictive marker of clinical response, since PD-L1 expression was significantly associated with clinical response rates in anti-PD1 treatment but not in other treatments;
PD- L1 expression was associated with risk reduction in mortality of 53% in MM patients receiving anti-PD-1 antibodies. Notably, the prognostic role was obtained in 1274 MM patients under a remarkable absence of study heterogeneity and unbiased results.
Although this analysis showed a doubling of response rate in MM PD-L1-positive patients, a proportion of 20–25% of PD-L1-negative MM patients appear to obtain an objective response to anti-PD-1 therapies. Hence, PD-L1 should not be used as a biomarker to select patients who should receive or not anti-PD-1 antibodies.
Reasons explaining the response in these ‘negative patients’ may include intratumor and intrapatient heterogeneity of PD-L1 (false negative), the discrepancies between primary and metastatic samples and the technically challenging issues related to immunohistochemical analysis in formalin-fixed and paraffin-embedded samples. Since PD-L1 plays an important role during physiological T-cell activation [4], it cannot be excluded that interfering with the engagement during normal antigen presentation in secondary lymphoid organs we may activate tumor-specific T cells away from the tumor itself, whether the tumor expresses PD-L1 or not.
Indeed, the optimal strategy for PD-L1 testing in tumor tissues remains an open question. There is an critical need to direct research toward additional targets, such as mutation rates, immune scores/cytokine profiling, CD8+ T-cell ratios or neoepitopes, gene signatures or RNA expression profiles, in melanoma cells and in the microenvironment. Next-generation sequencing to broadly characterize the neoantigens and transcriptional landscape within cancers will also be necessary to clarify the mechanisms of evasion from the immune system.
Conclusion
The past decade has seen essential advances in the comprehension of melanoma biology. New and innovative strategies to manipulate the immune response to cancer have been developed, through the identification of negative regulators of the immune system, called immunologic checkpoints, including PD-1 and the axis PD-1/PD-L1. Several clinical trials have reported unprecedented favorable clinical responses and outcome with anti PD-1 antibodies. These results led to the approval of anti-PD-1 antibodies by regulatory agencies for MM, NSCLC and RCC. Although PD-L1 expression appears to correlate with response to treatment, additional biomarkers such as immune scores/cytokine profiling, CD8+ T-cell ratios or neoepitopes, gene signatures or RNA expression profiles, in melanoma cells and in the microenvironment are needed, to minimize unnecessary exposure of patients to potentially severe toxicities and reduce the financial burden for health systems due to these expensive treatments
PD-L1: future perspective
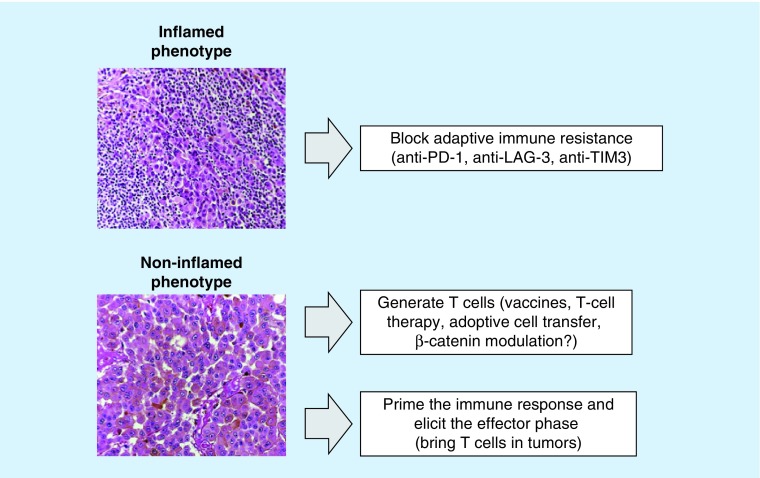
PD-L1 status should be evaluated in light of the complexity and the plasticity of the immune response. There are three areas of priority research in this field (Figure 3). The first is to explore the translational relevance of other inhibitory molecules in T cells such as LAG-3 or TIM-3. LAG-3 or TIM-3 expression by PD-1+ T cells may directly modulate the size of the T-cell response supporting the rational use of multiple blockade regimens to enhance CD8+ T-cell responses. In this perspective, the development of combination strategies to achieve increased clinical benefit is based on the recognition of the ligands and their receptors expressed in the tumor microenvironment by tumor cells, T cells, myeloid cells and other immune cells [25].
Figure 3. . Potential therapeutic strategies in inflamed and noninflamed melanoma subtypes.
In melanoma with T-cell infiltrates that trigger an adaptive immune-resistant response, defining the specific mechanism of this reactive tumor protection would allow tailoring the treatment to block that particular escape mechanism. Furthermore, LAG-3 or TIM-3 expression by PD-1+ T cells may directly modulate the size of the T-cell response supporting the rational use of multiple blockade regimens to enhance CD8+ T-cell responses. For treatment of melanomas without T-cell infiltrates there are two different areas of research: to generate T cells (vaccines, T-cell therapy, adoptive cell transfer, β-catenin modulation?); to combine anti-PD-1 with anti-CTLA-4 antibodies in order to prime the immune response and then elicit the effector phase.
Alternatively, in BRAFV600-mutated melanomas, to explore the better combination of BRAFi e MEKi with anti-PD-1 or anti-PD-L1 antibodies concomitantly or sequentially, or anti-PD-1 e anti-CTLA-4 followed by BRAFi and MEKi and vice versa. Finally combine immune-checkpoint inhibitors with VEGFR inhibitors or poly-ADP ribose polymerase inhibitors could be another option.
The second area of research is to explore new strategies in melanoma without tumor-infiltrating lymphocytes and PD-L1 expression (immune ignorance). This group represents a large fraction of melanoma patients (41%) with poor prognosis based on their lack of detectable immune reaction. In this group of patients, single-agent checkpoint blockade would most likely not to be successful given the lack of pre-existing T-cell infiltrates. Combination therapy that is designed to bring T cells into tumors and then avoid them being turned off, such as the combination of anti-CTLA-4 and anti-PD-1, would be considered in this scenario.
The third area of investigation is to explore the best combination of immunomodulatory antibodies with target therapies. Preclinical data demonstrated that oncogenic BRAF contributes to immune escape and that targeting this mutation can increase melanoma immunogenicity. Treatment with BRAFi affects tumor–host interactions by inducing increased melanoma antigen expression and stimulating immune response [26]. Exploring the best synergy between targeted therapy and immune-modulating agents could represent a new strategy to overcome resistance and ultimately to improve survival of MM patients.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An outstanding review on immune-checkpoint inhibitors and immunotherapy.
- 2.Sui X, Ma J, Han W, et al. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget. 2015;6(23):19393–19404. doi: 10.18632/oncotarget.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karwacz K, Arce F, Bricogne C, Kochan G, Escors D. PD-L1 co-stimulation, ligand-induced TCR down-modulation and anti-tumor immunotherapy. Oncoimmunology. 2012;1(1):86–88. doi: 10.4161/onci.1.1.17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]; •• The first trials that support the use of nivolumab as first choice in BRAF wild-type T metastatic melanoma patients.
- 7.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]; •• The first evidence that pembrolizumab is better than ipilimumab in metastatic melanoma patients.
- 8.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J. Clin. Oncol. 2015;33:2013–2020. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 10.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merelli B, Massi D, Cattaneo L, Mandalà M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Crit. Rev. Oncol. Hematol. 2014;89:140–165. doi: 10.1016/j.critrevonc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin. Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 13.Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin. Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The interferon-inducible expression of PD-L1 seems to be more common than the constitutive expression in melanoma, and results in a restricted PD-L1 expression in T-cell-rich areas of tumors, in particular at the invasive margin, in the interphase between melanoma growth and the immune system.
- 15.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madore J, Strbenac D, Vilain R, Menzies AM, Yang JYH, Thompson JF. PD-L1 negative status is associated with lower mutation burden, differential expression of immune-related genes, and worse survival in stage-III melanoma. Clin. Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1714. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Teng MWL, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]; • With regards to the PD-L1 expression in the context of melanoma, four distinct groups of tumors can be identified and described as having the presence of both PD-L1 and tumor infiltrating T cells, presence of T cells without PD-L1, PD-L1 expression without T cells or absence of both T cells and PD-L1 expression.
- 19.Massi D, Brusa D, Merelli B, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann. Oncol. 2015;26:1980–1987. doi: 10.1093/annonc/mdv255. [DOI] [PubMed] [Google Scholar]
- 20.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012;18(5):1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 21.Massi D, Brusa D, Merelli B, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann. Oncol. 2014;25:2433–2442. doi: 10.1093/annonc/mdu452. [DOI] [PubMed] [Google Scholar]; • In the context of BRAFi the four groups described by Teng et al. [18] could be prognostic in metastatic melanoma.
- 22.Abiko K, Mandai M, Hamanishi J, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin. Cancer Res. 2013;19:1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 23.Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28(3):245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 24.Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Sakuishi K, Apetoh L, Sullivan JM, et al. Anderson targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]