Sorbitol acts as a signal regulating the expression of an R protein that interacts with its corresponding effector from Alternaria alternata to confer resistance in apple.
Abstract
In plant-microbe interactions, plant sugars produced by photosynthesis are not only a carbon source for pathogens, but may also act as signals that modulate plant defense responses. Here, we report that decreasing sorbitol synthesis in apple (Malus domestica) leaves by antisense suppression of ALDOSE-6-PHOSPHATE REDUCTASE (A6PR) leads to downregulation of 56 NUCLEOTIDE BINDING/LEUCINE-RICH REPEAT (NLR) genes and converts the phenotypic response to Alternaria alternata from resistant to susceptible. We identified a resistance protein encoded by the apple MdNLR16 gene and a small protein encoded by the fungal HRIP1 gene that interact in both a yeast two-hybrid assay and a bimolecular fluorescence complementation assay. Deletion of HRIP1 in A. alternata enables gain of virulence on the wild-type control plant. Overexpression of MdNLR16 in two antisense A6PR lines increases resistance, whereas RNAi suppression of MdNLR16 in the wild-type control decreases resistance against A. alternata. MdWRKY79 transcriptionally regulates MdNLR16 by binding to the promoter of MdNLR16 in response to sorbitol, and exogenous sorbitol feeding partially restores resistance of the antisense A6PR lines to A. alternata. These findings indicate that sorbitol modulates resistance to A. alternata via the MdNLR16 protein that interacts with the fungal effector in a classic gene-for-gene manner in apple.
INTRODUCTION
Sugars produced by photosynthesis not only fuel plant growth and development but may also play a role in plant-microbe interactions. As plant sugars are a carbon source for pathogens, competition for this resource can determine the outcome of plant-pathogen interactions. In apoplastic phloem loading plants, where sucrose efflux from phloem parenchyma cells into the apoplast is mediated by SWEET transporters, transcriptional activation of these transporters by direct binding of bacterial transcriptional activator-like effectors to their promoters allows the pathogens to gain access to sucrose from plants, and interrupting this process makes plants resistant to these pathogens (Antony et al., 2010; Chen et al., 2010, 2012; Cox et al., 2017). Limiting extracellular sugar availability by plants as a resistance mechanism against pathogens has also been demonstrated for hexoses, where upregulation of a hexose transporter via phosphorylation by a coreceptor of flagellin in Arabidopsis thaliana contributes to antibacterial defense (Yamada et al., 2016). However, sugars may also regulate the plant defense system as signals (Bolouri Moghaddam and Van den Ende, 2012). Sucrose, glucose, and fructose induce the expression of several pathogenesis-related (PR) genes (Herbers et al., 1996a, 1996b; Xiao et al., 2000). When a maize (Zea mays) PR gene, PRms, is overexpressed in rice (Oryza sativa), plants accumulate higher levels of sucrose and higher transcript levels of several PR genes and develop resistance to several fungal and bacterial pathogens, including Magnaporthe oryzae, which is confirmed by exogenous feeding of sucrose to wild-type rice (Gómez-Ariza et al., 2007). PR proteins include enzymes and antimicrobial peptides that play important roles in plant disease resistance, but they act at the later stages of plant defense responses. In effector-triggered immunity (ETI), plants deploy disease resistance proteins (R proteins) to interact with pathogen-produced effector proteins in a gene-for-gene manner at the early stage to mount a rapid and robust defense response (Martin et al., 2003; Jones and Dangl, 2006; Cui et al., 2015).The largest group of R genes encode intracellular nucleotide binding domain and leucine-rich repeat (NLR) receptors and these NLRs are highly amplified in plants (McHale et al., 2006; Arya et al., 2014; Zhong et al., 2015), but it is not known whether any of these R genes is regulated by sugars.
Sugar alcohols serve as major end products of photosynthesis and transport carbohydrates in many plants, which make up ∼30% of the primary carbon production on a global scale (Bieleski, 1982). In apple (Malus domestica) and many other tree fruit species in the Rosaceae family, sorbitol accounts for 60 to 80% of photosynthates produced in leaves and transported in the phloem (Bieleski, 1982; Loescher, 1987). Sorbitol synthesis occurs in the cytosol of source leaves via a two-step process. Glucose 6-phosphate is converted to sorbitol 6-phosphate by aldose-6-phosphate reductase (A6PR), and then sorbitol 6-phosphate is dephosphorylated by sorbitol-6-phosphate phosphatase to yield sorbitol (Negm and Loescher, 1981; Zhou et al., 2003). After being transported to sink organs, sorbitol is mainly metabolized to fructose by sorbitol dehydrogenase to support plant growth and development. Sorbitol is also implicated in regulating sugar metabolism, stamen development, and pollen tube growth as a signaling molecule (Archbold, 1999; Zhou et al., 2006; Meng et al., 2018). It has been found that mannitol, a sugar alcohol with antioxidant capability, is used by Alternaria alternata to quench the initial burst of reactive oxygen species generated by plants upon infection for successful colonization (Jennings et al., 1998), and overexpressing a celery (Apium graveolens) mannitol dehydrogenase in tobacco (Nicotiana tabacum), a species that does not synthesize mannitol, increases its resistance to the pathogen (Jennings et al., 2002). However, it is unclear whether any sugar alcohol regulates plant immunity responses to pathogens in sugar alcohol-synthesizing species.
In earlier work we found that suppression of A6PR expression by antisense inhibition in source leaves of ‘Greensleeves’ apple significantly decreased sorbitol synthesis and the transgenic plants had significantly lower sorbitol but higher sucrose levels (Cheng et al., 2005). Neither photosynthesis nor vegetative growth of the transgenic plants was significantly altered due to the compensatory upregulation of starch accumulation during the day and starch breakdown for sucrose synthesis and export at night (Cheng et al., 2005; Zhou et al., 2006). However, we consistently observed that leaves of the transgenic plants with decreased sorbitol levels developed brown spots similar to symptoms caused by A. alternata apple pathotype (Li et al., 2013). In wet years, leaf spot-like symptoms were so severe that early defoliation occurred on these trees. By contrast, leaves of wild-type control plants showed very few symptoms. In this work, we report identification of an NLR gene that confers resistance to an isolate of A. alternata from symptomatic transgenic lines, interaction between the NLR protein and a fungal effector, and regulation of the NLR gene expression by sorbitol via a WRKY transcription factor.
RESULTS
Transgenic Apple Plants with Decreased Sorbitol Synthesis Develop A. alternata Leaf Blotch-Like Symptoms
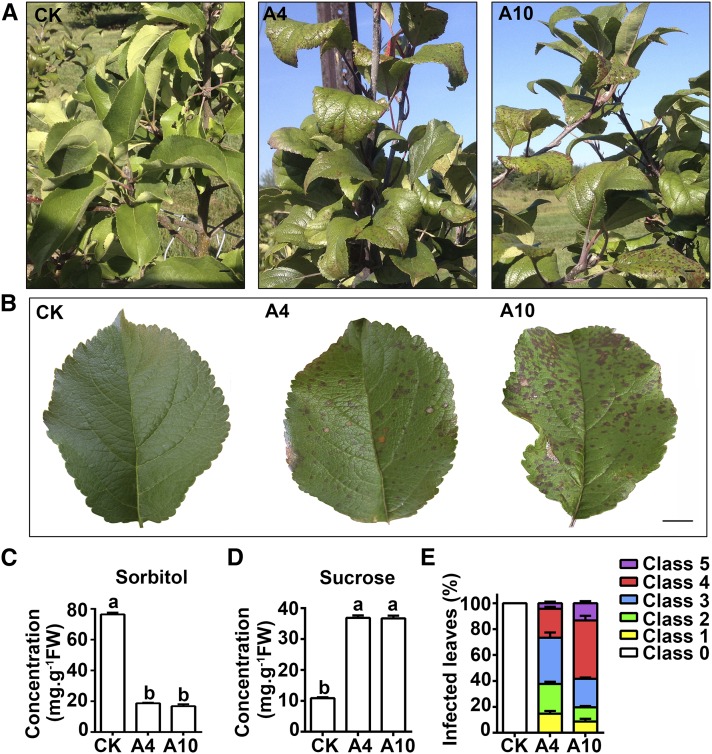
We observed that leaves of A6PR antisense lines, A4 and A10, developed A. alternata leaf blotch-like symptoms (Figures 1A and 1B), i.e., brown spots that led to early defoliation in severe cases in wet years. By contrast, the wild-type control plants showed very few symptoms. All leaves in the A4 and A10 trees had symptoms late in the season, with higher severity observed on A10 than on A4 (Figure 1E). As we reported earlier, A6PR is the key enzyme for sorbitol synthesis in apple leaves and A4 and A10 have lower sorbitol but higher sucrose concentrations in leaves (Cheng et al., 2005) (Figures 1C and 1D).
Figure 1.
Leaf Symptoms and Carbohydrate Levels of the Wild-Type Control and Antisense A6PR Lines of ‘Greensleeves’ Apple.
(A) and (B) Leaf spot-like symptoms on the leaves of antisense lines A4 and A10 late in the season in contrast to no symptoms on the wild-type control (CK). Bars = 1 cm.
(C) and (D) Concentrations of sorbitol and sucrose in the leaves of CK, A4, and A10 measured via gas chromatography-mass spectrometry. Different letters (a and b) indicate significant differences (P < 0.05) between genotypes using Duncan’s multiple range test (MRT) after ANOVA. FW, fresh weight.
(E) Percentage of leaves showing various degrees of severity of the symptoms (% leaf area with symptoms). Class 0, no symptoms; class 1, <5%; class 2, 5 to 15%; class 3, 15 to 25%; class 4, 25 to 35%; and class 5, >35%. Data are mean ± sd of five biological replicates with six leaves pooled from two trees per replicate for sugar analysis or 50 leaves pooled from two trees per replicate for disease evaluation.
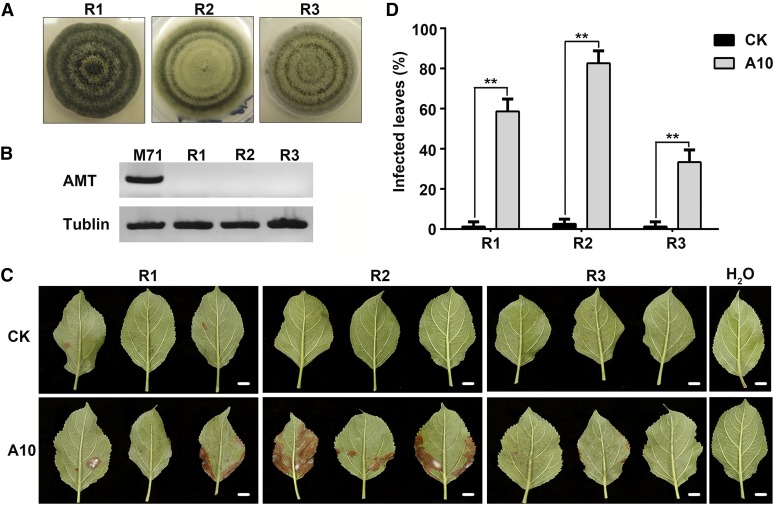
To determine if the leaf spot symptoms in the two antisense lines were indeed caused by the A. alternata apple pathotype (also called A. mali), the causal agent for Alternaria leaf blotch in apple, we isolated and characterized the causal agent(s) from infected leaves. Three morphologically distinct fungal strains, named R1, R2, and R3 were obtained (Figure 2A). None of these was A. alternata apple pathotype because no AM-toxin synthetase (AMT) gene, necessary for AM toxin production (Johnson et al., 2000), was detected in the R1-R3 strains, whereas the control A. alternata apple pathotype reference strain M71 had AMT (Figure 2B). To resolve taxonomic affinity of R1, R2, and R3, we built two different phylogenetic trees (Supplemental Figure 1 and Supplemental Data Set 1 for gene sequence alignments). In the tree built with partial sequences of the gene (HIS3) encoding Histone H3 (Zheng et al., 2015), all three isolates fell into previously described clades of A. alternata (group I-II; Supplemental Figure 1A), with R3 basal to the group. R1 grouped with strain M71 but lacked AMT. In the tree built with sequences of the internal transcribed spacer region (ITS), the glyceraldehyde-3-phosphate dehydrogenase gene (GAPdh), Alternaria major allergen gene (ALTa1), and an anonymous gene region (OPA10-2), R1 grouped in the A. arborescens species complex, R2 grouped with known A. alternata species, while R3 grouped with other Alternaria spp (Woudenberg et al., 2015) (Supplemental Figure 1B). Systematics of Alternaria spp is complex; however, results of our two approaches to tree building support the assignment of the recovered R1, R2, and R3 isolates as members of Alternaria spp, with R2 clearly an A. alternata isolate.
Figure 2.
Isolation of Fungi from Symptomatic Leaves of Transgenic ‘Greensleeves’ Apple Trees and Reinoculation on Apple.
(A) Three fungal strains (R1, R2, and R3, shown to be Alternaria spp; Supplemental Figure 1) isolated from symptomatic transgenic apple leaves.
(B) PCR analysis of the AMT gene encoding AM toxin synthetase for AM toxin biosynthesis. Wild-type strain M-71 of A. alternata apple pathotype (Johnson et al., 2000) was included as a positive control. β-Tubulin was used as a control housekeeping gene. Note lack of an AMT signal in R1-R3 strains.
(C) Disease symptoms on wild-type control (CK) and antisense line A10 after reinoculation with R1, R2, and R3 isolates. Sterile water was used as a negative control. Bars = 1 cm.
(D) Percentage of infected leaves in the wild-type control and A10 after reinoculation with R1, R2, and R3, respectively. Data are mean ± sd of five biological replicates with 50 leaves pooled from two trees per replicate. **P < 0.01 (Student’s t test).
To examine virulence of the three recovered strains, each was inoculated onto wild-type (CK) and A10 leaves and the leaves were kept in moist chambers for 96 h. All three strains caused various degrees of browning, with strain R2 inducing the most severe symptoms and the highest infection rate on A10 (Figures 2C and 2D). Insignificant symptoms occurred on the wild-type control leaves. These results indicate that decreasing sorbitol synthesis in ‘Greensleeves’ apple leaves converted the phenotypic response to these Alternaria spp from resistant to susceptible, an outcome that is most likely due to a weakened defense system of the transgenic plants.
Downregulation of Many NLR Genes in Transgenic Leaves with Decreased Sorbitol Synthesis and Identification of Candidate R Genes for A. alternata R2
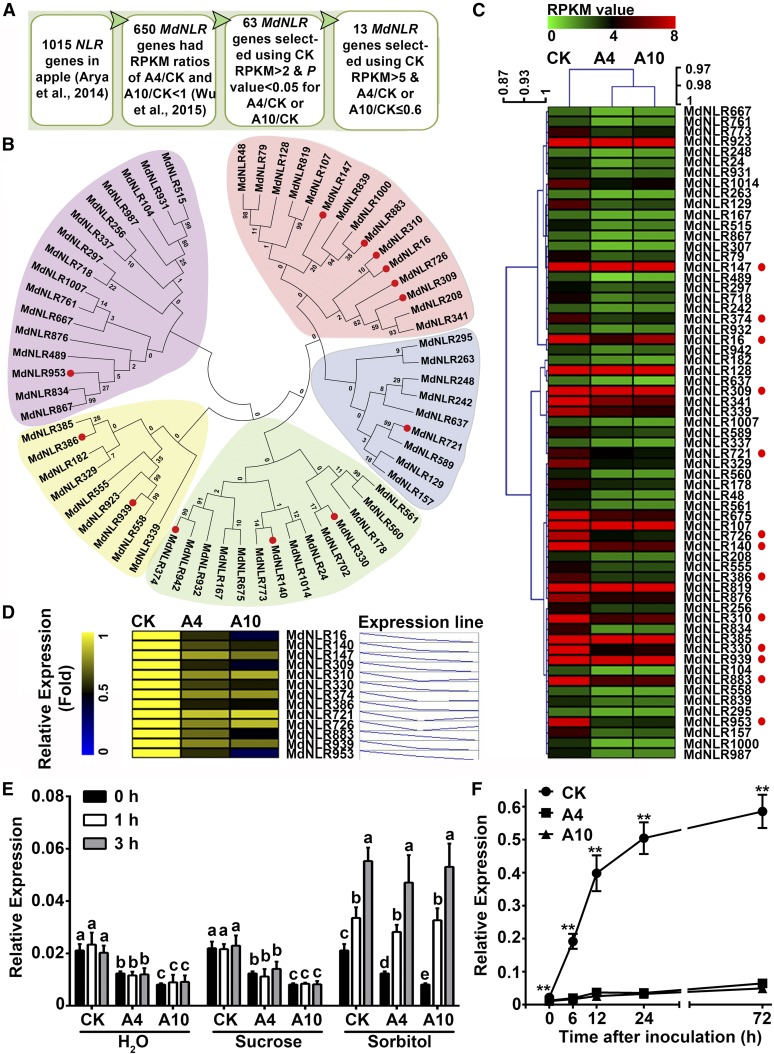
Considering that NLR genes are prominently overrepresented among differentially expressed genes when the two A6PR antisense lines (A4 and A10) are compared with the wild-type control in the RNA-seq data obtained previously on healthy mature leaves (Wu et al., 2015), we hypothesized that one of the downregulated NLR genes was responsible for the resistance phenotype of the wild-type control to A. alternata R2 strain. Of the 1015 NLR genes in the apple genome (Arya et al., 2014), 650 had RPKM (reads per kilobase of exon model per million mapped reads) ratios of A4/CK and A10/CK of <1 in both antisense lines, whereas 70 had ratios of >1 (Supplemental Data Set 2). Using RPKM >2 in CK leaves and the adjusted P value < 0.05 for the RPKM ratio of A4/CK or A10/CK as cutoff values, we identified 63 differentially expressed NLR genes (Supplemental Data Set 3). These proteins fell into five groups in a neighbor-joining tree (Figure 3B; Supplemental Data Set 4 for protein sequences). A heat map showing the transcript levels of the 63 NLR genes was generated based on their RPKM values in the RNA-seq data (Figure 3C). By removing those that only have a nucleotide binding site, this list was reduced to 56. In the first attempt to identify the NLR gene responsible for resistance to A. alternata R2, we selected 13 NLR genes for further analysis based on both the transcript level in CK leaves (RPKM >5) and the degree of differential expression (A4/CK or A10/CK ≤ 0.6), with the only exception being NLR721 that has the highest expression in its group (Figure 3C).
Figure 3.
Phylogenetic Analysis of Differentially Expressed Genes and Selection of 13 NLR Genes for RT-qPCR Analysis in Response to Sorbitol/Sucrose Feeding and A. alternata R2 Inoculation.
(A) Selection strategy.
(B) Phylogenetic analysis of 63 NLR proteins using the neighbor-joining method. The proteins were divided into five groups corresponding to different branches in the neighbor-joining tree. Proteins marked with a red dot were selected for further analysis.
(C) Heat map showing gene expression patterns of the 63 NLR genes in wild-type control CK and antisense A6PR lines A4 and A10 based on RNA-seq data. RPKM range of 0 to 8 was chosen for the color scheme to get the best contrast for most NLR genes, but not all of them. See Supplemental Data Set 3 for actual RPKM values. The NLRs marked with a red dot were the same as the corresponding proteins in (B).
(D) Expression patterns of the 13 NLR genes in the wild-type and antisense lines detected in leaves of in vitro shoots using RT-qPCR.
(E) and (F) Transcript level of MdNLR16 in leaves of the wild-type control and antisense lines A4 and A10 in response to feeding with sorbitol (50 mM) or sucrose (50 mM) via the petiole or A. alternata R2 inoculation, respectively.
In (D) to (F), quantitative RT-PCR was performed using gene-specific primers (Supplemental Data Set 5). For each sample, transcript levels were normalized to that of actin, and the relative expression level of each gene was obtained using the ddCT method. Data are mean ± sd of three biological replicates with 10 leaves pooled from five in vitro shoots (grown in tissue culture) per replicate. Different letters in (E) indicate significant differences between genotypes across sampling points (P < 0.05) using Duncan’s MRT after ANOVA. In (F), asterisks indicate a significant difference (P < 0.01) between CK and the two antisense lines at each sampling point using Student’s t test.
We analyzed transcript levels of the 13 selected NLR genes in leaves of subcultured shoots of the two antisense lines (A4 and A10) and wild-type control using quantitative RT-PCR and found that most of them showed differential expression (Figure 3D) between the antisense lines and wild-type control consistent with the RNA-seq data obtained from the leaves of field-grown trees (Figure 3C). A noticeable difference was detected in NLR16 expression between RT-qPCR and RNA-seq. Both A4 and A10 have significantly lower transcript levels than the wild-type control in the RT-qPCR analysis, with the lowest value detected in A10 (Figure 3D).
As the decrease in resistance to A. alternata R2 in transgenic plants is associated with a decrease in sorbitol level or an increase in sucrose level, we reasoned that the NLR gene conferring the resistance should be upregulated by sorbitol or downregulated by sucrose. To identify the NLR gene, we fed leaves of subcultured CK, A4, and A10 plants with 50 mM sorbitol, 50 mM sucrose, or water. In response to sorbitol feeding (leaf sugar levels shown in Supplemental Figure 2), transcript levels of MdNLR16, MdNLR309, and MdNLR726 in CK, A4, and A10 all increased significantly at 1 and 3 h, whereas transcript level of MdNLR386 increased in A4 and A10 only (Figure 3E; Supplemental Figure 3). In response to sucrose feeding, transcript levels of most of the 13 NLR genes either did not change or increased slightly; those of MdNLR310, MdNLR374, and MdNLR883 decreased in CK, A4, and A10 at 1 and 3 h, whereas the transcript level of MdNLR309 decreased only in CK at 3 h (Figure 3E; Supplemental Figure 3). Of these sorbitol/sucrose-responsive NLRs, MdNLR16 showed the largest change in expression level.
Assuming that any key gene involved in defense responds to pathogen infection and that differential expression of the gene corresponds to a difference in host resistance to the pathogen, we chose differential expression between the two antisense lines and the wild-type control upon fungal challenge as a criterion to identify candidate NLR genes involved in sorbitol/sucrose-modulated resistance to A. alternata strain R2. We inoculated leaves of subcultured CK, A4, and A10 plants with the R2 strain spore suspension and determined transcript levels of the 13 NLR genes via RT-qPCR at 0, 6, 12, 24, and 72 h after inoculation. The type of response MdNLR16 exhibited was of most interest because its expression increased rapidly in the first 24 h in CK but remained largely unchanged in A4 and A10, with the transcript level being significantly higher in CK than in A4 and A10 (Figure 3F). MdNLR147, MdNLR310, and MdNLR374 all showed responses similar to MdNLR16, whereas other NLRs did not (Supplemental Figure 4A). Leaf sorbitol concentrations remained unchanged in each genotype for the 72 h duration (Supplemental Figure 4B). When the results of the sorbitol/sucrose feeding experiment and the R2 strain inoculation experiment were combined, MdNLR16, MdNLR310, and MdNLR374 appeared to be the most likely NLR genes involved in sorbitol/sucrose-modulated resistance to A. alternata strain R2.
Overexpression of MdNLR16 in Transgenic Apple Leaves Enhances Resistance, Whereas RNAi Suppression in Wild-Type Leaves Decreases Resistance to A. alternata Strain R2
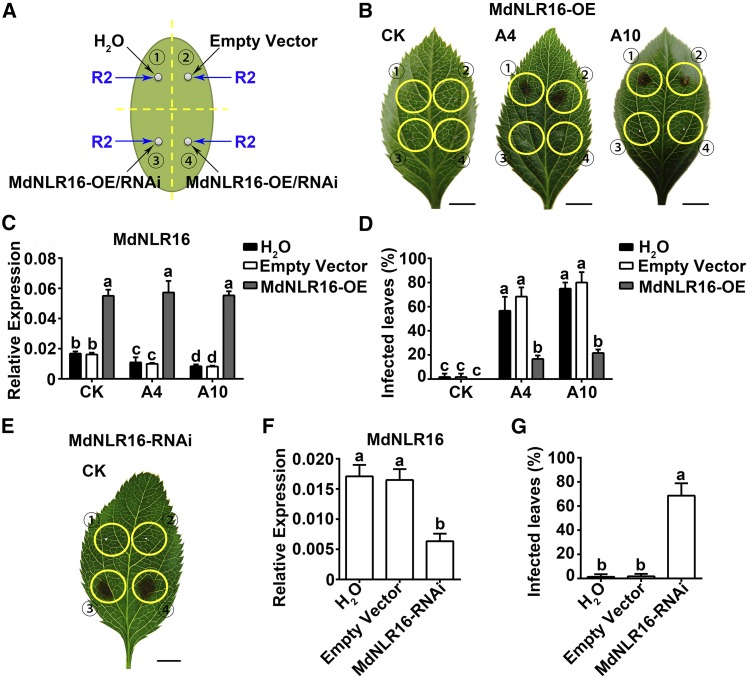
We cloned MdNLR16, MdNLR310, and MdNLR374 using PCR based on their genomic sequences. To determine if any of these NLRs plays a role in defense against A. alternata R2 in apple, we overexpressed them individually in leaves of subcultured A4, A10, and CK plants via agroinfiltration, followed by inoculation with A. alternata R2 (Figures 4A to 4D; Supplemental Figure 5). The transcript level of MdNLR16 was significantly increased at 2 d after agroinfiltration with MdNLR16 (Figure 4C). After 3 d, necrosis was observed around the injection site on A4 and A10 leaves, where water or empty vector was injected prior to inoculation (Figure 4B). In A4 and A10, ∼60 and 80% of the leaves inoculated with A. alternata R2 showed necrosis around the injection site in the controls (water and empty vector injection), respectively, and overexpression of MdNLR16 decreased the infection rates to ∼20% (Figure 4D). However, in CK leaves, little necrosis was observed around the injection site (Figure 4B) and the infection rate was close to 0% regardless of MdNLR16 overexpression (Figure 4D). For both MdNLR310 and MdNLR374, overexpression significantly increased their transcript levels in CK, A4, and A10, but did not alter resistance/susceptibility to A. alternata R2 (Supplemental Figure 5). The resistance conferred by MdNLR16 is specific to the R2 strain as overexpression of MdNLR16 did not alter the disease phenotype of A4 and A10 to strain R1 or R3 (Supplemental Figure 6).
Figure 4.
Overexpression of MdNLR16 in A4 and A10 Leaves Enhances Resistance, Whereas RNAi Suppression of MdNLR16 Expression in Wild-Type Control Leaves Decreases Resistance to A. alternata R2.
(A) Agroinfiltration and inoculation of leaves. Black arrows point to sites of infiltration of pGWB551-MdNLR16 (overexpression)/pGWRNAi-MdNLR16 vector-containing Agrobacterium solution into subcultured ‘Greensleeves’ apple leaves, with water and empty pGWB551/pGWRNAi vector-containing Agrobacterium solution as controls. Two days after infiltration, the R2 spore suspension was applied to the injection site (blue arrows). Numbers represent different injection sites.
(B) Leaf phenotypes of the wild-type control CK, A4, and A10 3 d after inoculation, with the injection site at the center of each yellow circle. Bars = 5 mm.
(C) Transcript levels of MdNLR16 assayed by quantitative RT-PCR just before inoculation.
(D) Percentage of infected leaves in CK, A4, and A10 after inoculation.
(E) Leaf phenotypes after RNAi suppression of MdNLR16 expression in CK leaves.
(F) Transcript levels of MdNLR16 detected via RT-qPCR just before inoculation.
(G) Percentage of infected leaves in CK after RNAi suppression of MdNLR16 expression. Data are mean ± sd of three biological replicates with 10 leaves pooled from five in vitro shoots per replicate for RT-qPCR or 40 leaves pooled from 20 in vitro shoots per replicate for disease evaluation. Different letters (a to d) indicate significant differences (P < 0.05) between treatments across genotypes using Duncan’s MRT after ANOVA.
To further confirm the role of MdNLR16 in conferring resistance to A. alternata R2, we suppressed MdNLR16 expression in CK leaves with the RNAi construct pGWRNAi-MdNLR16 via agroinfiltration, followed by inoculation (Figure 4E). The transcript level of MdNLR16 was significantly decreased in leaves infiltrated with pGWRNAi-MdNLR16, compared with the water and empty vector controls (Figure 4F), which led to a dramatic increase in infection by A. alternata strain R2 (Figure 4G).
Analyses using Target P (http://www.cbs.dtu.dk/services/TargetP/) and TMHMM (http://www.cbs.dtu.dk/services/TMHMM/) showed that MdNLR16 does not have any transmembrane domain. Confocal imaging of protoplasts of apple calli expressing MdNLR16-GFP fusion protein confirmed its cytosolic localization (Supplemental Figure 7).
Hrip1 from A. alternata Strain R2 Interacts with MdNLR16 and Deletion of HRIP1 Enables Virulence on Wild-Type Apple
To identify the factor from A. alternata R2 that interacts with MdNLR16, we first searched the literature for clues. Hrip1, a previously identified protein from Chinese cabbage strain At2004-060 of A. tenuissima (Kulye et al., 2012), came to our attention. It is a small protein that elicits a hypersensitive response in tobacco and is predicted to be an effector (probability of 98%) based on our analysis via EffectorP (http://effectorp.csiro.au/). We cloned and characterized the HRIP1 putative ortholog from A. alternata strain R2; the protein is 164 amino acids long with the first 18 predicted as signal peptide and, like Hrip1 from A. tenuissima, is predicted to be an effector (probability of 98%). The A. alternata strain R2 Hrip1 protein is 98% identical to the previously described one from A. tenuissima. Confocal imaging of apple callus protoplasts expressing the Hrip1-GFP fusion protein indicated cytosolic localization (Supplemental Figure 8).
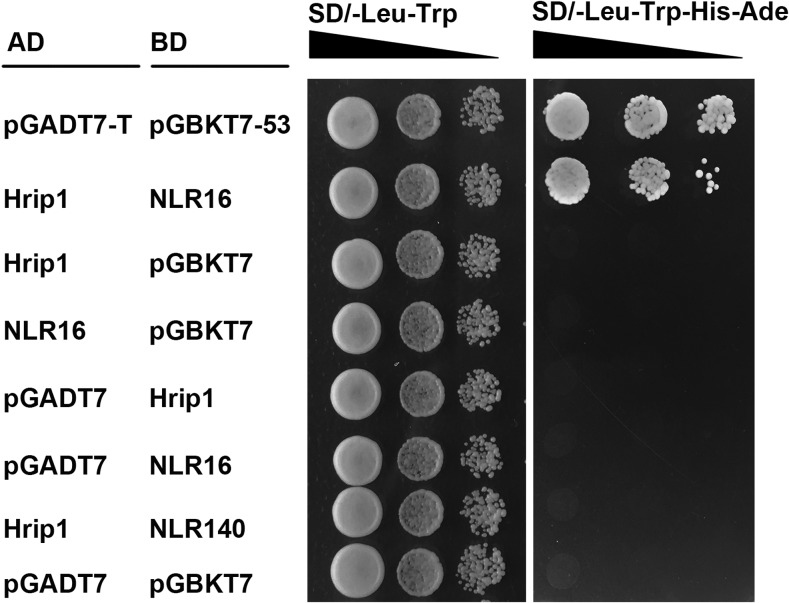
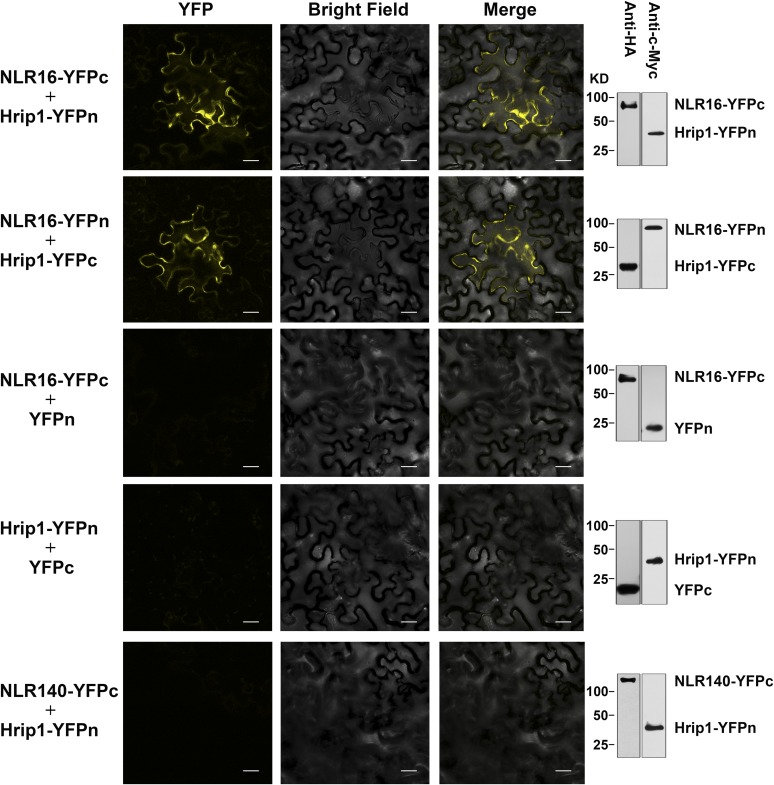
We used a yeast two-hybrid (Y2H) assay and a bimolecular fluorescence complementation (BiFC) assay to determine if Hrip1 interacts with MdNLR16 in vitro and in vivo, respectively. The Y2H assay with MdNLR16 and Hrip1 as bait and prey, respectively, showed a direct interaction between the two proteins (Figure 5). In the BiFC assay, YFP signal was detected in both MdNLR16-YFPn+Hrip1-YFPc and MdNLR16-YFPc+Hrip1-YFPn pairs but was not detected when MdNLR16 or Hrip1 alone or a combination of MdNLR140-YFPc and Hrip1-YFPn was expressed (Figure 6). To determine the specificity of the interaction between Hrip1 and MdNLR16, we tested all five other NLRs (MdNLR310, MdNLR726, MdNLR309, MdNLR208, and MdNLR341) in the same sub-branch of MdNLR16 (Figure 3B) and two more NLRs (MdNLR14 and MdNLR59) closely related to MdNLR16 in the phylogenetic tree built by Arya et al. (2014) for interaction with Hrip1 via both Y2H and BiFC assays; none of the NLRs showed any interaction with Hrip1 in either assay (Supplemental Figures 9 and 10). These results indicate that the interaction between MdNLR16 and Hrip1 is specific. Overexpression of HRIP1 in wild-type apple leaves led to hypersensitive cell death and the percentage of leaves with a hypersensitive response was significantly lower in both A6PR antisense lines than in the wild-type control (Supplemental Figure 11).
Figure 5.
Y2H Assay of the Interaction between Hrip1 (A. alternata R2) and MdNLR16.
The pGADT7-T and pGBKT7-p53 pair was used as a positive control. Pairs of AD-Hrip1 and BD-MdNLR140, and AD-pGADT7 and BD- pGBKT7 were used as negative controls. Each colony was dissolved in 10 μL sterile water and then diluted to 10−1 to 10−3. At least three colonies per combination were tested.
Figure 6.
BiFC Assay of the Interaction between Hrip1 (from Strain R2) and MdNLR16 in Tobacco (Nicotiana benthamiana) Leaves.
HRIP1 and MdNLR16 were introduced into pSYPNE or pSYPCE vectors, respectively, fused with N-terminal or C-terminal YFP. Pairs of NLR16-YFPc+YFPn, Hrip1-YFPn+YFPc, and NLR140-YFPc+Hrip1-YFPn were used as negative control. Immunoblots on the right side obtained using HA or c-Myc antibody for the tag at C-terminal and N-terminal YFP, respectively, showing the expression of various fusion proteins. Bars = 50 µm.
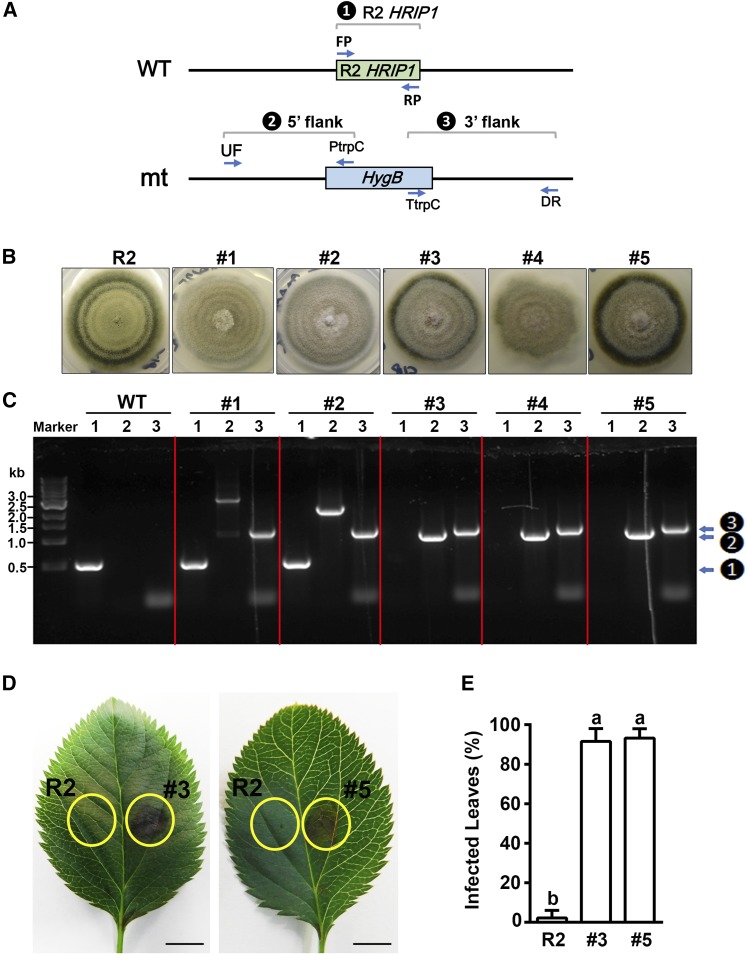
To confirm that Hrip1 is essential for the resistance reaction, we deleted and replaced it in A. alternata strain R2 with the HygB selectable marker (Turgeon et al., 2010; Takao et al., 2016). Of five candidate HygBR strains, three (#3, #4, and #5) were confirmed to lack HRIP1 (Figures 7A to 7C; Supplemental Data Set 5). As #4 mutant did not grow well, we used mutants #3 and #5 to test virulence on CK leaves. Both mutants caused disease on ∼90% of the leaves compared with 0% with wild-type R2 (Figures 7D and 7E), demonstrating that loss of the HRIP1 gene in the fungus enables gain of virulence on wild-type apple. For each of the two A6PR antisense lines (A4 and A10), inoculation of mutants #3 and #5 did not significantly alter the disease rate compared with R2 inoculation (Supplemental Figure 12). We also suppressed MdNLR16 expression in wild-type control leaves with the RNAi construct pGWRNAi-MdNLR16 via agroinfiltration, followed by inoculation, but it did not alter the infection rates by mutants #3 and #5 in A4 or A10 (Supplemental Figure 13). Under these virulence assay conditions, Hrip1 did not exhibit virulence promoting activity.
Figure 7.
HRIP1 Gene Deletion in A. alternata R2 and Virulence Phenotype of Mutants Compared with R2 on Wild-Type ‘Greensleeves’ Apple Leaves.
(A) Scheme of HRIP1 gene deletion from wild-type A. alternata R2 (WT) and three diagnostic PCR reactions (1 to 3, inside black circles, here, and in [C], right side) to identify deletion mutants (mt).
(B) Morphological phenotype of wild-type strain R2 and five HygBR candidate transformants (#1 to #5).
(C) Diagnostic PCR analysis using DNA of wild-type R2 and the five transformants as template. Lanes 1 to 3 in each panel show reactions with primer pairs FP/RP (detects HRIP1), UF/PtrpC (detects targeted insertion into the 5′ flank of HRIP1), and TtrpC/DR (detects insertion into the 3′ flank of HRIP1), respectively. Marker is a 1-kb DNA ladder. Note candidates #1 and #2 still have HRIP1 bands, while #3 to #5 do not (1 to 3, inside black circles, right side).
(D) Leaf phenotypes after R2 and mutant #3 or #5 inoculations, with the injection site at the center of each yellow circle. Bars = 5 mm.
(E) Percentages of infected leaves after inoculation. Data are mean ± sd of three biological replicates with 40 leaves pooled from 20 in vitro shoots per replicate. Different letters (a and b) indicate significant differences (P < 0.01) using Duncan’s MRT after ANOVA.
MdWRKY79 Transcriptionally Regulates MdNLR16 in Sorbitol-Modulated Resistance to A. alternata R2
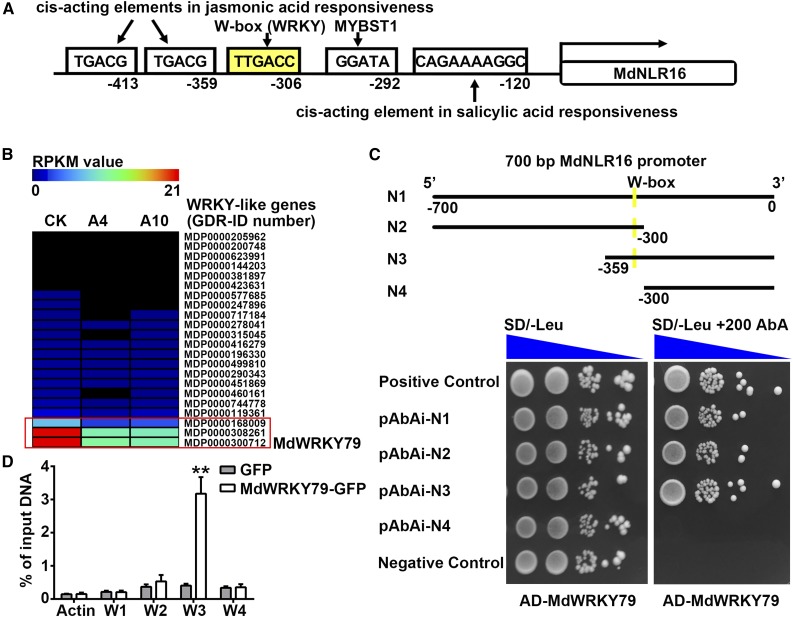
To understand how sorbitol regulates the expression of MdNLR16, we isolated the promoter region (2426 bp) of MdNLR16 from ‘Greensleeves’ apple using specific primers based on the sequence information in the Genome Database for Rosaceae (https://www.rosaceae.org; contig MDC005202.557). This region contained a core promoter at position 340 bp and an enhancer promoter at position 1226 bp (analyzed using the PlantCare website http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). In addition to the promoter elements, the sequence contained cis-acting elements related to jasmonic acid (JA)-responsiveness, salicylic acid (SA) responsiveness, and MYB and WRKY factor binding regions (Figure 8A). As no difference was detected in JA or SA levels among CK, A4, and A10 (Supplemental Figure 14), the presence of cis-acting elements related to JA and SA responsiveness suggests that MdNLR16 may be regulated by JA or SA, but this regulation might not be involved in sorbitol-modulated resistance to A. alternata R2. After searching the RNA-seq data obtained previously on the leaves of A4, A10, and CK, we found that 22 WRKY genes with RPKM ratios of A4/CK and A10/CK of <1 (Figure 8B). Of these, only three genes had RPKM values of >1 with the adjusted P value of <0.01 for the RPKM ratios of A4/CK and A10/CK. MDP0000168009 has only 280 amino acids and does not have a WRKY domain (Supplemental Figure 15). The other two are essentially the same except that the third exon (92 bp) is missing in one of them. We cloned the full length of MdWRKY79 (MDP0000300712) (Meng et al., 2016) and localized the protein to the nucleus of apple leaf cells (Supplemental Figure 16). Subsequently, a yeast one-hybrid (Y1H) assay was conducted to examine the interaction between MdWRKY79 and the MdNLR16 promoter. The results showed that the WRKY protein binds to the promoter region containing the W-box motif (Figure 8C). Chromatin immunoprecipitation (ChIP)-PCR analysis of transgenic apple calli overexpressing MdWRKY79-GFP confirmed the binding between MdWRKY79 and the MdNLR16 promoter region (Figure 8D).
Figure 8.
Binding of MdWRKY79 Transcriptional Factor to the W-Box Motif in MdNLR16 Promoter.
(A) Binding sites in the MdNLR16 promoter; numbers indicate positions of the various cis-acting elements upstream of the MdNLR16 coding sequence with the first nucleotide of its start codon designated as 0; yellow indicates the W-box site.
(B) Expression patterns of 22 WRKY genes in CK, A4 and A10 lines based on RNA-seq data, with the three downregulated WRKY genes outlined in red (RPKM > 1; adjusted P < 0.01 for the RPKM ratios of A4/CK and A10/CK).
(C) Y1H assay on binding of MdWRKY79 protein to the promoter region of MdNLR16. N1 to N4 represent different regions of the MdNLR16 promoter shown in (A). Yellow line shows the location of the W-box site. Blue triangle represents the range of yeast concentrations (100 to 10−3). pGADT7-p53+pAbAi-p53 was used as a positive control and pGADT7+pAbAi-p53 as a negative control.
(D) ChIP-PCR confirming the binding of WRKY79 to the NLR16 promoter. Actin was used as an internal control. W1 to W4 represent the different primers shown in Supplemental Data Set 5. Data are mean ± sd of three biological replicates. **P < 0.01 between MdWRKY79-GFP and GFP alone using Student’s t test.
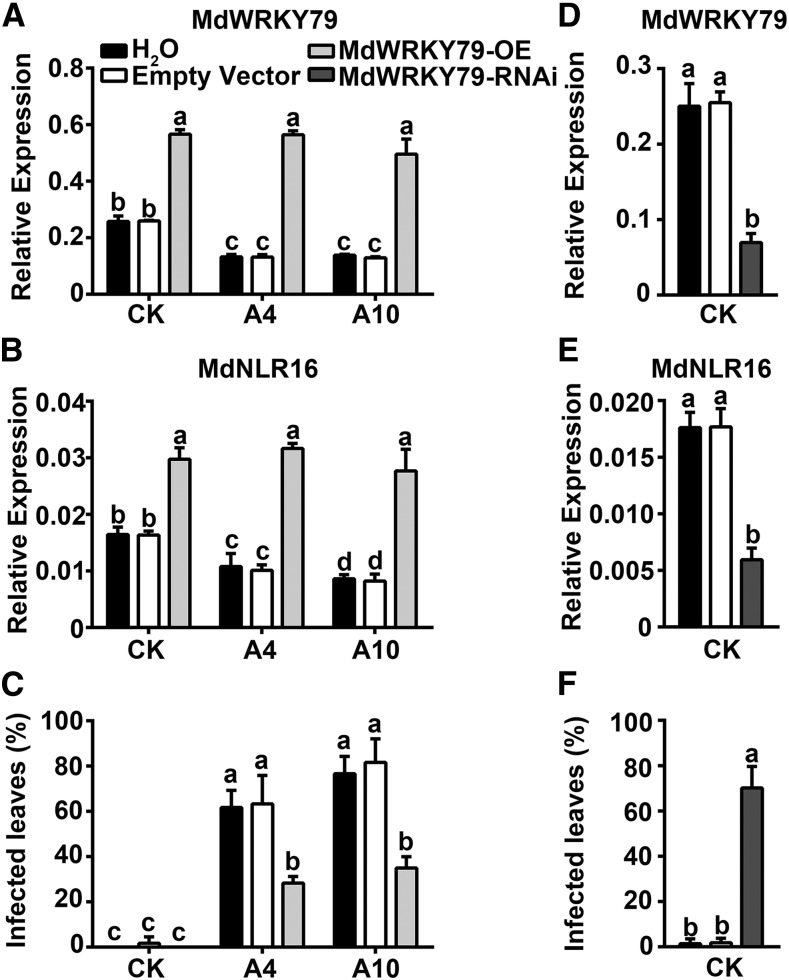
To verify the function of MdWRKY79 in the transcriptional regulation of MdNLR16 in sorbitol-modulated resistance to A. alternata R2, we overexpressed it. Two days after agroinfiltration with the overexpression vector pGWB551-MdWRKY79, the transcript level of WRKY79 was significantly enhanced in leaves overexpressing WRKY79 over water and empty vector controls (Figure 9A). This led to a significant upregulation of the transcript level of MdNLR16 (Figure 9B). In CK, the leaf infection rate was close to zero regardless of MdWRKY79 overexpression. In A4 and A10, ∼60 and 80% leaves were diseased, respectively, in the water and empty vector controls; overexpression of MdWRKY79 decreased the rates to ∼35% (Figure 9C). Simultaneous injections of both pGWB551-MdNLR16 and pGWB551-MdWRKY79 brought the expression of MdNLR16 and resistance against A. alternata R2 in the leaves of A4 and A10 (Supplemental Figure 17) to very similar levels as in the case of overexpressing MdNLR16 alone (Figures 4C and 4D).
Figure 9.
Overexpression of MdWRKY79 in A4 and A10 Leaves Enhances Resistance, Whereas RNAi Suppression of MdWRKY79 Expression in Wild-Type Control Leaves Decreases Resistance to A. alternata R2.
Infiltration of Agrobacterium solution containing pGWB551-MdWRKY79 (overexpression) or pGWRNAi-WRKY79 vector and subsequent inoculation of A. alternata R2 were performed as in Figure 4, with water and empty vector as controls.
(A) and (B) Transcript levels of MdWRKY79 and MdNLR16 in response to overexpression of MdWRKY79 detected via quantitative RT-PCR, respectively, just before the R2 inoculation.
(C) Percentage of infected leaves three days after inoculation with R2 following overexpression of MdWRKY79 in the wild-type CK and antisense lines A4 and A10.
(D) and (E) Transcript levels of MdWRKY79 and MdNLR16 in wild-type control CK in response to RNAi suppression of MdWRKY79 detected via RT-qPCR just before R2 inoculation, respectively.
(F) Percentage of infected leaves after RNAi suppression of MdWRKY79 expression. Data are mean ± sd of three biological replicates with 10 leaves pooled from five in vitro shoots per replicate for RT-qPCR or 40 leaves pooled from 20 in vitro shoots per replicate for disease evaluation. Different letters (a to d) indicate significant differences (P < 0.05) between treatments across genotypes using Duncan’s MRT after ANOVA.
To further confirm the role of MdWRKY79 in transcriptional regulation of MdNLR16 in conferring resistance against A. alternata R2, we suppressed MdWRKY79 expression in wild-type leaves with the RNAi vector pGWRNAi-MdWRKY79 via agroinfiltration, followed by inoculation with an A. alternata R2 spore suspension. Agroinfiltration of pGWRNAi-MdWRKY79 significantly decreased the transcript levels of MdWRKY79 and MdNLR16 and consequently significantly increased the disease rate by A. alternata R2 (Figures 9D to 9F).
Exogenous Sorbitol Feeding Enhances the Expression of Both MdWRKY79 and MdNLR16 and Partially Restores Resistance of Transgenic Apple Leaves to A. alternata Strain R2
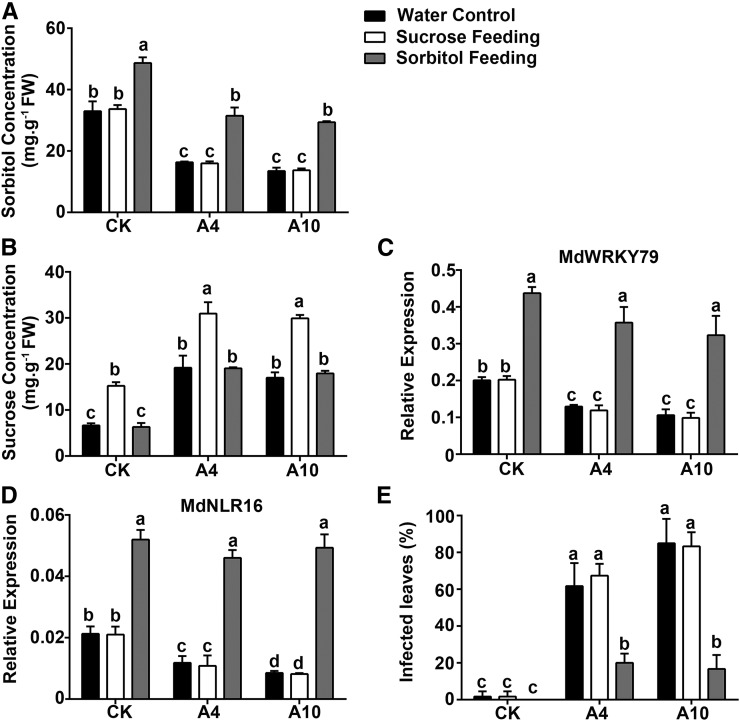
To confirm the role of sorbitol in modulating resistance of apple leaves to A. alternata R2, we fed the leaves of subcultured A4, A10, and CK plants with 50 mM sorbitol, 50 mM sucrose, or water for 3 h, and then inoculated them with A. alternata R2. Sorbitol feeding significantly increased leaf sorbitol concentrations and transcript levels of both MdWRKY39 and MdNLR16 and decreased disease rates in both A4 and A10 (Figure 10). Sucrose feeding significantly increased leaf sucrose concentrations but did not alter MdWRKY39 and MdNLR16 expression or disease rates in A4 or A10. In the wild-type control, sorbitol feeding increased the transcript levels of both MdWRKY39 and MdNLR16, whereas sucrose feeding did not affect their expression; resistance to A. alternata R2 was not altered in either case relative to the water control (Figure 10).
Figure 10.
Exogenous Sorbitol Feeding Partially Restores Resistance of A4 and A10 Leaves to A. alternata Strain R2.
(A) and (B) Levels of sorbitol and sucrose in leaves of the wild-type control CK and two A6PR antisense lines A4 and A10 at 3 h of feeding with exogenous sorbitol (50 mM) or sucrose (50 mM) via the petiole, respectively.
(C) and (D) Transcript levels of MdWRKY79 and MdNLR16 detected via quantitative RT-PCR at 3 h of sorbitol or sucrose feeding, respectively.
(E) Percentage of infected leaves 3 d after inoculation with R2 following sorbitol or sucrose feeding. Data are mean ± sd of three biological replicates with 10 leaves pooled from five in vitro shoots per replicate for RT-qPCR or 40 leaves pooled from 20 in vitro shoots per replicate for disease evaluation. Different letters (a to d) indicate significant differences (P < 0.05) between treatments across genotypes using Duncan’s MRT after ANOVA.
DISCUSSION
As a primary photosynthetic product and a major transport carbohydrate in many plant species including all the pome and stone fruits in the Rosaceae family, sorbitol plays an important role in carbohydrate metabolism for fueling plant growth and development (Cheng et al., 2005; Zhou et al., 2006). In addition, sorbitol has been suggested to act as a signal molecule in regulating carbohydrate metabolism in both fruit and shoot tips (Archbold, 1999; Zhou et al., 2006) and flower development and pollen tube growth (Meng et al., 2018). Here, we show that sorbitol modulates resistance against an isolate of A. alternata by regulating the expression of MdNLR16. Leaves of wild-type ‘Greensleeves’ apple with normal sorbitol levels are resistant, whereas transgenic apples with decreased sorbitol levels are susceptible. Exogenous sorbitol feeding partially restores resistance of the transgenic apples to A. alternata. Thus, our study revealed that a sugar (alcohol) can act as a signal regulating the expression of an R gene to alter plant resistance in plant-microbe interactions.
MdNLR16 plays a key role in sorbitol-modulated resistance against A. alternata by interacting with the Hrip1 protein from A. alternata in a classic gene-for-gene manner. The expression of MdNLR16 is responsive to decreased sorbitol synthesis in vivo (Figures 3C and 3D), exogenous sorbitol feeding in vitro (Figure 3E), and inoculation with A. alternata (Figure 3F). Overexpression of MdNLR16 in the leaves of antisense lines A4 and A10 significantly increases resistance against A. alternata, whereas RNAi suppression of MdNLR16 expression in leaves of the wild-type control decreases the resistance (Figure 4). MdNLR16 interacts with Hrip1 in both a Y2H assay and a BiFC assay (Figures 5 and 6), and this interaction is specific (Supplemental Figures 9 and 10). Deletion of HRIP1 in A. alternata R2 enables gain of virulence on wild-type ‘Greensleeves’ apple (Figure 7) but does not affect the susceptibility of antisense lines A4 and A10 (Supplemental Figure 12). In addition, susceptibility of the wild-type control to the A. alternata R2 HRIP1 mutants is not altered by RNAi suppression of MdNLR16 expression (Supplemental Figure 13). These results confirm that Hrip1 is the effector recognized by MdNLR16 in the apple-A. alternata interaction.
As MdNLR16 does not have a transmembrane domain and localizes to the cytosol (Supplemental Figure 7), recognition of Hrip1 from A. alternata by MdNLR16 must take place inside apple leaf cells. Considering that A. alternata is classified as a necrotroph (Thomma, 2003; Chung, 2012), our findings raise an interesting question as to the role of R gene-mediated resistance in plant-necrotrophic pathogen interactions. It has been widely recognized that R gene-mediated resistance is effective against biotrophic and hemibiotrophic pathogens, but not against necrotrophic pathogens (Glazebrook, 2005; Wang et al., 2014). Some necrotrophic pathogens, however, apparently exploit the plant NLR-based resistance mechanism against biotrophs to promote virulence. For example, the fungal necrotrophs Stagonospora nodorum and Pyrenophora tritici-repentis, causal agents of S. nodorum blotch and tan spot of wheat, respectively, produce ToxA, a proteinaceous host-selective toxin that interacts indirectly with the product of the NLR gene Tsn1 that has both a protein kinase domain and an NLR domain to confer susceptibility and elicit severe necrosis (Faris et al., 2010). Cochliobolus victoriae, a fungal necrotroph and causal agent of Victoria blight of oats (Avena sativa), secretes victorin, a low molecular weight, chlorinated cyclic pentapeptide host-selective toxin, that repurposes R gene (Pc2 in oats and LOV1 in Arabidopsis)-mediated ETI against the biotrophic fungus Puccinia coronata for pathogenesis (Navarre and Wolpert, 1999; Lorang et al., 2007, 2012). These examples support an inverse gene-for-gene model for plant-necrotrophic pathogen interactions. To our knowledge, the ETI mechanism that we have discovered between apple and the necrotroph A. alternata has not been reported previously. It is possible that the fungus is actually a hemibiotroph. As pointed out by Oliver et al. (2012), although necrotrophs are defined as pathogens that derive energy from dying or dead plant tissues, they differ significantly in the progression of pathogenesis, ranging from rapidly killing host cells such as Botrytis cinerea to having an extended asymptomatic phase before killing host cells. Arguably, the latter group is closer to a hemibiotrophic lifestyle; therefore, R gene-mediated resistance via gene-for-gene interaction could be operating. The facts that most Alternaria spp are saprophytes or opportunistic pathogens and strains that are pathogenic generally cause relatively slow destruction of plant tissues and many have a quiescent infection period (Thomma, 2003) might explain why R gene-mediated resistance is still effective in apple against A. alternata. Alternatively, R gene-mediated resistance may actually operate in plant-necrotroph interactions to a much larger extent than currently known. Although its corresponding effector has not been identified, RLM3, an NLR gene in Arabidopsis, has been found to confer resistance against a wide range of necrotrophic pathogens including not only two Alternaria spp, A. brassicicola and A. brassicae, but also B. cinerea that quickly kills host tissues (Staal et al., 2008).
Unlike Arabidopsis and most other herbaceous plants, transfer of both sorbitol and sucrose from mesophyll cells to the sieve element-companion cell complex in apple leaves is by diffusion through plasmodesmata and no apoplastic step is involved (Reidel et al., 2009; Fu et al., 2011). Thus, most of the sorbitol stays inside the leaf cells rather than effluxing into the apoplast, although nonspecific leakage is expected to occur to some degree. In wild-type ‘Greensleeves’ apple, due to the presence of high concentrations of sorbitol inside leaf cells, expression of MdNLR16 stays at a high level and increases rapidly in response to inoculation of A. alternata (Figures 3E and 3F). Presumably, the MdNLR16 protein in high abundance recognizes the effector delivered by A. alternata, triggering a set of defense responses to render immunity against the pathogen. However, in the antisense A6PR lines with decreased sorbitol synthesis, sorbitol-modulated expression of MdNLR16 is decreased to such a low level that it severely impairs its upregulation in response to A. alternata infection (Figures 3E and 3F), and the resulting low level of MdNLR16 protein is not adequate for effective recognition of the effector to initiate an effective defense response, leading to susceptibility. The reduced hypersensitive response to overexpression of the HRIP1 gene in the two antisense A6PR lines relative to the wild-type control (Supplemental Figure 11) is consistent with this proposed mechanism.
Although how the sorbitol signal is perceived and transduced in regulating the expression of MdNLR16 is not entirely clear, our data demonstrate that MdWRKY79 is involved in transcriptional regulation of MdNLR16. The promoter region of MdNLR16 has a WRKY binding site, W-box, and the binding of MdWRKY79 to the W-box site in the promoter of MdNLR16 is confirmed by a Y1H assay in vitro (Figures 8A and 8C) and ChIP-PCR in vivo (Figure 8D). The transcript level of MdWRKY79 is responsive to decreased sorbitol levels in the antisense lines (Figure 8B) and exogenous sorbitol feeding (Figure 10C). Overexpression of MdWRKY79 in leaves of the antisense lines significantly increased their resistance to A. alternata infection (Figures 9A to 9C), whereas RNAi suppression of MdWRKY79 expression in leaves of the wild-type control decreased the resistance (Figures 9D to 9F). It has been shown that some WRKY transcription factors are phosphorylated by mitogen-activated protein kinase to regulate downstream defense responses such as phytoalexin synthesis (Mao et al., 2011) and burst of reactive oxygen species (Adachi et al., 2015) in ETI as well as pattern-triggered immunity (Rasmussen et al., 2012). Our data show that MdWRKY79 regulates MdNLR16 expression upstream in the signal transduction process in response to sorbitol level, consequently altering the resistance of apple leaves to A. alternata R2.
Modulation of MdNLR16 expression and resistance to A. alternata R2 by sorbitol was uncovered by genetically altering sorbitol synthesis in the leaves of ‘Greensleeves’ apple in this work. Currently, it is not known if natural variations in leaf sorbitol levels in different Malus species or accessions would cause a difference in resistance to A. alternata R2 as no screening work has been conducted in this regard. However, sorbitol modulation of resistance to A. alternata R2 is relevant to apple-Alternaria interactions in at least two aspects. As the sorbitol level is much lower in apple fruit than in mature leaves and is developmentally regulated (Loescher, 1987; Zhang et al., 2010), sorbitol-modulated resistance to A. alternata might be operating in the fruit. There are two types of diseases caused by A. alternata on apple fruit, core rot (moldy core) and fruit spot. The A. alternata that causes apple core rot does not have the AMT gene found in the A. alternata apple pathotype (Reuveni et al., 2007; Gur et al., 2017); A. alternata strains without AMT can cause apple fruit spot as well as those with AMT (Rotondo et al., 2012; Harteveld et al., 2014; Gur et al., 2017). From an evolutionary perspective, our finding on sorbitol-modulated resistance to A. alternata R2 would be consistent with a scenario where, prior to gaining sorbitol-synthesizing capacity, apple leaves were susceptible to A. alternata R2, but gaining the capacity has allowed apples to fend off its attack on the leaves. On the Alternaria side, presence of abundant sorbitol in the leaves of apple might have prompted the evolution of new pathogenicity factors such as AM-toxin as found in A. alternata apple pathotype, a major pathogen causing leaf blotch of many apple varieties worldwide (Johnson et al., 2000; Rotondo et al., 2012; Li et al., 2013; Gur et al., 2017). As deletion of HRIP1 in A. alternata R2 enables gain of virulence on the leaves of wild-type ‘Greensleeves’ apple with normal sorbitol levels, this could be another evolutionary route for A. alternata to cope with sorbitol-modulated resistance in apple leaves. It has been reported that, in the rice bacterial blight pathosystem, three avirulence (avr) genes avrxa5, avrXa7, and avrXa10 from Xanthomonas oryzae pv oryzae (Xoo) correspond to the rice bacterial blight R genes xa5, Xa7, and Xa10 (Hopkins et al., 1992) and natural mutations of avrXa7 in Xoo overcame the resistance conferred by Xa7 but at the cost of reduced aggressiveness (Vera Cruz et al., 2000). Many A. alternata/tenuissima/arborescerns isolates that do not belong to A. alternata apple pathotype were recently reported to cause leaf blotch-like symptoms and tree defoliation in many apple varieties in Italy (Rotondo et al., 2012) and Australia (Harteveld et al., 2013, 2014), but it remains to be seen if HRIP1 is lost in any of these isolates, similar to mutants #3 and #5 generated in this study, or any mutation is present in the gene such that it evades recognition by MdNLR16.
A significant number of NLR genes (56) had lower transcript levels in the antisense lines of ‘Greensleeves’ apple with decreased sorbitol synthesis (Figure 3). Some of these NLR genes were most likely downregulated by sucrose accumulation in the transgenic plants as shown in the sucrose feeding experiment, but decreases in transcript levels of others were a response to lower sorbitol concentrations in these plants as demonstrated in the sorbitol feeding experiment (Figure 3E; Supplemental Figure 3). This, along with the confirmation of MdNLR16 in conferring resistance to A. alternata via its interaction with Hrip1 (Figures 4 to 7; Supplemental Figures 9 and 10) suggests that sorbitol may play a role in modulating resistance against a range of pathogens in sorbitol-synthesizing species. In any plant species, pattern-triggered immunity confers broad spectrum resistance against a diverse group of pathogens by recognizing conserved microbe/pathogen-associated molecular patterns, but this resistance can be overcome by pathogen effectors (Jones and Dangl, 2006; Bigeard et al., 2015). If sorbitol provides a “baseline” resistance to a range of pathogens by regulating the expression of a number of NLR genes, this would have the advantage of achieving broad spectrum resistance while maintaining the specificity of ETI in combating various diseases (Martin et al., 2003; Jones and Dangl, 2006; Cui et al., 2015). Clearly, the molecular network of sorbitol perception/signal transduction, virulence mechanism of A. alternata, and identification of other sorbitol-modulated R genes conferring resistance to other pathogens warrant further work as this may not only allow us to gain a better understanding of plant-microbe interactions in sugar alcohol-synthesizing species, but also open the door for engineering broad spectrum resistance to pathogens in species that do not produce sugar alcohols.
In conclusion, the decreased sorbitol level in transgenic apple trees with antisense suppression of A6PR makes the trees susceptible to A. alternata, a fungus that is not virulent on the untransformed control with normal sorbitol levels. MdNLR16 plays a key role in sorbitol-modulated resistance to A. alternata by interacting with the fungal effector Hrip1 in a gene-for-gene manner. The MdWRKY79 transcription factor regulates MdNLR16 expression in response to sorbitol. These findings indicate that sorbitol acts as a signal in regulating disease resistance in sorbitol-synthesizing plants.
METHODS
Plant and Fungal Materials
The wild-type control (CK) and two antisense lines (A4 and A10) of ‘Greensleeves’ apple (Malus domestica) with antisense suppression of A6PR were used in this study. The mature leaves of both A4 and A10 had ∼10% transcript level and 15% activity of A6PR in CK, with the sorbitol level being slightly lower in A10 than in A4 (Cheng et al., 2005; Wu et al., 2015).
Four-year-old trees of CK, A4, and A10 on M.26 rootstock were grown in 20-liter pots containing medium comprising 1 part sand:2 parts MetroMix360 (v/v) (Scotts) under natural conditions in Ithaca, NY. All trees received standard horticultural and insect control practices, but fungicide sprays for microbial disease control were primarily focused on apple scab (caused by Venturia inaequalis) in the first 8 weeks after budbreak. Healthy mature leaves were taken during active shoot growth in late June, frozen in liquid nitrogen, and stored at −80°C for extraction of sugars and plant hormones.
Mature leaves with brown spots were harvested in late summer for isolation of disease-causing organisms. The leaves were first surface sterilized with ethanol and rinsed several times with sterile distilled water, then pieces of leaf with diseased spots were cultured on plates containing complete medium with xylose (CMX) (Tzeng et al., 1992). Three fungal strains, R1, R2, and R3 were isolated and subcultured on potato dextrose agar (PDA) plates for DNA extraction and also stored as spore suspensions in CMX liquid with 15% glycerol at −80°C. Aliquots from glycerol stocks were plated on PDA and spores from mycelia were used for inoculation experiments. Wild-type Alternaria alternata apple pathotype M-71 described previously (Johnson et al., 2000) was grown on PDA plates for DNA extraction.
Initially, to confirm that R1, R2, and R3 were the causal organisms, they were inoculated back to youngest fully expanded leaves taken from the field-grown trees of CK, A4, and A10 following the procedure of Johnson et al. (2000). Briefly, spores at a concentration of 5 × 105/mL were sprayed onto leaves which were then kept in moist chambers in the dark for 4 d before evaluation of disease.
Tissue-cultured plantlets of CK, A4, and A10 were subsequently used in sugar feeding, leaf agroinfiltration, and fungal inoculation experiments. They were grown on MS medium with 30 g/L sucrose, 0.3 mg/L 6-benzylaminopurine (6-BA), and 0.03 mg/L indole-3-butyric acid at 23°C under fluorescent lights at ∼50 μmol photons m−2 s−1 in a 16-h photoperiod.
Apple calli derived from ‘Orin’ apple were used for ChIP-PCR assays. They were cultured on MS medium with 30g/L sucrose, 1.5 mg/L 6-BA, and 0.5 mg/L indole-3-acetic acid at 25°C in the dark.
Identification of R1, R2, and R3 as Alternaria spp
Two phylogenetic approaches, a single gene analysis (Zheng et al., 2015) and a multigene analysis (Woudenberg et al., 2015), were used to identify taxonomic affinity of the R1-R3 isolates (Supplemental Data Set 1 for gene sequence alignments).
For the single gene analysis, a partial sequence of the gene (HIS3) encoding Histone H3 was used as by Zheng et al. (2015). For the multigene analysis, ITS, GAPdh, ALTa1, and OPA10-2 were used as by Woudenberg et al. (2015). Accession numbers provided by Zheng et al. (2015) were used to acquire HIS3 nucleotide sequences and those provided by Woudenberg et al. (2015) were used to acquire ITS, GAPdh, ALTa1, and OPA10-2 nucleotide sequences from GenBank (Benson et al., 2013) via the SeqinR package v3.3-6 (Charif and Lobry, 2007) in R v3.2.1 (R Core Team, 2015) using the RStudio v0.99.451 environment (R Studio Team, 2015).
Partial HIS3 sequences were amplified and sequenced from R1, R2, R3, and M71 strains and aligned to those from Zheng et al. (2015) using MAFFT v7 (Katoh and Standley, 2013). The total numbers of taxa and characters in the analysis were 91 and 549, respectively. For the multigene analysis, ITS, GAPdh, ALTa1, and OPA10-2 sequences were amplified and sequenced from R1, R2, and R3 then individually aligned to those of a subset of isolates used by Woudenberg et al. (2015). Care was taken to avoid selecting isolates with missing data. Four isolates used by Woudenberg et al. (2015) that were formerly recognized as Alternaria tenuissima were included in the subset. The individual nucleotide alignments were manually adjusted using Mesquite v3.03 (Madison and Maddison, 2017) and concatenated using SequenceMatrix v1.8 (Vaidya et al., 2011). The final data set included 43 taxa and 2211 characters.
For both the HIS3 and multi-gene analysis, trees were constructed by Bayesian inference using MrBayes v3.2.6 (Ronquist et al., 2012), implementing the BEAGLE library (Ayres et al., 2012) through the CIPRES Science Gateway (Miller et al., 2010). For the HIS3 gene analysis, the tree was constructed using the best fit model of molecular evolution, HKY+I, selected by jModelTest v2.1.7 (Darriba et al., 2012), following the Bayesian information criterion. For the multigene analysis, the tree was constructed using the Bayesian model averaging approach of rjMCMC (Huelsenbeck et al., 2004), with each gene considered a separate partition, and allowing for independent estimation of substitution rates, character state frequencies, gamma shape distributions, and proportions of invariable sites per partition. Posterior probabilities at branch nodes were inferred using two runs of 1,000,000 generations, with 25% burn-in. Phylogenetic trees were visualized in R using the Ape (v4.1), and pBrackets (v1.0) packages (Paradis et al., 2004; Schulz, 2014).
Phylogenetic Analysis of NLR Genes and Heat Map Visualization of Gene Expression Data
The RNA-seq data for leaves of the wild-type control and antisense A6PR lines A4 and A10 of ‘Greensleeves’ apple were described previously (Wu et al., 2015). The selection of NLR genes is shown in Figure 3A, and the selected genes are listed in Supplemental Data Sets 2 and 3. A phylogenetic tree of 63 downregulated NLRs was constructed via the neighbor-joining method using MEGA6.0 software (Tamura et al., 2013), with 1000 bootstrap replicates performed for each analysis (Supplemental Data Set 4 for protein sequence alignments). A heat map showing the expression of the selected 63 NLR genes was constructed using MeV software (http://mev.tm4.org/) based on the RPKM value of each gene obtained in the RNA-seq.
Feeding Leaves with Exogenous Sorbitol and Sucrose
Leaves of subcultured CK, A4, and A10 plants were fed with exogenous sorbitol or sucrose via the transpiration stream according to Zhou et al. (2006) with modifications. The youngest fully expanded leaves were cut from the shoots with a razor blade, floated on water, and then randomly assigned to 50 mM sorbitol, 50 mM sucrose, or water control. Each treatment was replicated three times with 10 leaves from five shoots per replicate in a Petri dish. In each Petri dish, only the leaf petioles were sandwiched between two pieces of filter paper (1.5 cm in width) that was soaked with either sugar or water and placed along the Petri dish diameter. Two additional pieces of filter paper (in the shape of circular segment) soaked with water were placed into the Petri dish in clear separation with the first two pieces of filter paper. The Petri dishes were capped and randomly arranged in a fume hood (2 m × 1.2 m × 1.5 m) under fluorescent lights at ∼50 μmol photons m−2 s−1 at 23°C. Leaf samples were collected at 0, 1, and 3 h after initiation of sugar feeding, frozen in liquid nitrogen and stored at −80°C for use.
For the experiment with exogenous sorbitol/sucrose feeding followed by A. alternata strain R2 inoculation (Figure 10), sugar feeding was done as described above with each treatment replicated three times, but each replicate had five Petri dishes of detached leaves (10 leaves/Petri dish): One Petri dish was sampled for RNA extraction and subsequent RT-qPCR at 3 h, and the other four Petri dishes were inoculated with the R2 strain as described below for disease evaluation 3 d later.
Extraction and Analysis of Leaf Sugars
Leaf soluble sugars were extracted in 75% methanol with ribitol as an internal standard, derivatized sequentially with methoxyamine hydrochloride and N-methyl-N-trimethylsilyl-trifluoroacetamide, and analyzed with an Agilent 7890A GC/5975C MS system (Agilent Technologies) on a DB-5MS capillary column (20 m × 0.18 mm × 0.18 µm) with a 5-m Duraguard column (Agilent Technologies) as described (Wang et al., 2010).
Leaf RNA Extraction and RT-qPCR
Leaf RNA was extracted using the CTAB method (Gasic et al., 2004). After treatment with RQ1 DNase (Promega), RNA was quantified using a NanoDrop spectrophotometer and RNA integrity was confirmed by agarose gel electrophoresis. One microgram of total RNA was reverse-transcribed to cDNA using an iScript cDNA Synthesis kit (Bio-Rad). Detection of gene transcript levels was performed in triplicate using gene-specific primers (listed in Supplemental Data Set 5) on an iQ5 Multicolor real-time PCR detection system (Bio-Rad) with the SYBR Green Supermix Kit (Bio-Rad). The expression of actin was used as an internal standard. The data were analyzed using iQ5 2.0 software (Bio-Rad) with the ddCT method.
Gene Cloning and Construction of Expression Vectors
cDNAs of MdNLR14, MdNLR16, MdNLR59, MdNLR140, MdNLR208, MdNLR309, MdNLR310, MdNLR341, MdNLR726, and HRIP1(R2) were cloned into the entry vector pDONR207 via BP reactions using BP Clonase II enzyme mix following the Gateway Cloning Protocols (Thermo Fisher Scientific). Then, through LR reactions using LR Clonase II enzyme mix, the cDNAs of MdNLR14, MdNLR16, MdNLR59, MdNLR140, MdNLR208, MdNLR309, MdNLR310, MdNLR341, MdNLR726 in pDOR207 were transferred into the destination vector pGWB551 with a fused GFP protein at the C-terminal, and the HRIP1 (R2) cDNA was transferred into the destination vector pGWB417 with a fused 4xMyc tag for expression in apple leaves. cDNA fragments of 200 and 225 bp in regions specific to MdNLR16 and MdWRKY79 were also cloned into pDONR207 vector, respectively, and then transferred into the destination vector pGWRNAi, which was modified from binary vector pPZP221 with a 35S RNAi Gateway cassette (attR1-CmR-ccdB-aatR2 and its reverse on both sides of CHSA intron) inserted at the SmaI site in the multiple cloning sites, for RNAi suppression of MdNLR16 and MdWRKY79 expression in apple leaves.
Subcellular Localization of MdNLR16 and Hrip1
The coding sequences of MdNLR16 and HRIP1 were cloned into the pBI221-GFP vector and the recombined plasmids were transformed into protoplasts isolated from ‘Orin’ apple calli as described (Hu et al., 2016). Fluorescence images of transformed protoplasts were obtained using a Zeiss LSM710 confocal microscope. A membrane dye, FM4-64, was used to delineate the cell membrane with red fluorescence (excitation/emission maxima 515/640 nm).
Y2H and Y1H Assays
For the Y2H assay, the cDNAs of MdNLR14, MdNLR16, MdNLR59, MdNLR140, MdNLR208, MdNLR309, MdNLR310, MdNLR341, and MdNLR726 were cloned into the pGBKT7 vector as a bait, and the cDNA of HRIP1 from strain R2 was cloned into the pGADT7 vector as a prey. The pGADT7-T and pGBKT7-p53 pair was used as a positive control whereas the pGADT7-MdNLR140 and pGBKT7-HRIP1 pair as well as empty vectors were used as negative controls. Various combinations of activation domain (AD) and binding domain (BD) vectors were cotransformed into the yeast strain AH109. After growth on SD/-Leu-Trp medium for 4 to 6 d at 30°C, the clones were transferred into the selective medium (SD/-Leu-Trp-His-Ade) at 30°C for 3 to 4 d. At least three independent experiments were performed, and the results from a single representative experiment were presented.
The Y1H assay was performed using the Yeast One-Hybrid System-Matchmaker Gold Kit (Clontech) following the manufacturer’s instructions. The MdWRKY79 gene was fused in-frame with the GAL4 AD in a pGADT7 vector (prey plasmid). The fragments of the promoter region of MdNLR16 were ligated to the pAbAi vector (Bait vector). After linearizing the bait vector plasmids using BstBI, the pAbAi-MdNLR16 promoter was transformed into Y1HGold yeast strain and selected on an SD/-Ura plate. The pGADT7-MdWRKY79 constructs were transformed into strain Y1HGold harboring pAbAi-bait and screened on an SD/-Ura/AbA (Aureobasidin A) plate (200 ng/mL AbA). To confirm the results, positive clones (cotransformed) were spotted in serial dilutions of yeast (1:1, 1:10, 1:100, and 1:1000) and cultured on the SD/-Leu/AbA medium at 30°C for 4 d. pGADT7-p53+pAbAi-p53 was used as a positive control and pGADT7+pAbAi-p53 as a negative control.
BiFC Assay
Coding sequences of MdNLR14, MdNLR16, MdNLR59, MdNLR140, MdNLR208, MdNLR309, MdNLR310, MdNLR341, MdNLR726, and HRIP1 were cloned into pSYPNE and pSYPCE vectors harboring a YFP coding sequence to generate either N-terminal or C-terminal fusion proteins, respectively (Walter et al., 2004). The plasmids were introduced into Agrobacterium tumefaciens GV3101, and infiltration of Nicotiana benthamiana was performed as described previously (Walter et al., 2004; Witte et al., 2004). Infected tissues were imaged at 48 h after infiltration using a Zeiss LSM710 confocal microscope. Immunoblotting was performed to confirm the expression of various fusion proteins using HA (product no. PA1-985, lot no. RI237644) or c-Myc antibody (product no. PA1-981, lot no. SB247983) for the tag at C-terminal and N-terminal YFP, respectively (Invitrogen).
Transformation of A. alternata Strain R2 for Generation of HRIP1 Deletion Mutants
To obtain HRIP1 deletion mutants, A. alternata strain R2 was transformed using the PCR-based targeted gene replacement strategy as described (Turgeon et al., 2010), except the fungus was grown in potato dextrose broth or on PDA. Briefly, HRIP1 flanking regions and the HygB cassette (for resistance to hygromycin B) were amplified using primer pairs described in Supplemental Data Set 5 (FP1/RP1 [Aa175FP1/Aa175RP1] for the 5′ flanking region, FP2/RP2 [Aa175FP2/Aa175RP2] for 3′ flanking region, and HygB-F/HygB-R for the HygB cassette in pUCATPH). The PCR products were added to strain R2 protoplasts prepared as described (Turgeon et al., 2010). Candidate transformants were selected after two rounds of plating on PDA plus 50 μg hygromycin B/mL. Genomic DNA was extracted from candidate transformants and the wild-type R2 strain using an UltraClean Microbial DNA Isolation Kit (catalog no. 12224; Qiagen) and diagnostic PCR reactions were performed to confirm deletion of HRIP1 and targeted integration of the HygB marker into the HRIP1 flanking regions. PCR primer pairs FP/RP (Aa175-F1/Aa175-R1) were used to detect the presence of HRIP1, while primer pairs UF(Aa175UF)/PtrpC and TtrpC/DR(Aa175DR) were used to confirm insertion into the HRIP1 5′ and 3′ flanks respectively (Supplemental Data Set 5; Figures 7A to 7C). Two confirmed mutant strains (#3 and #5) were selected and used for pathogenicity tests.
Apple Callus Transformation and ChIP-qPCR Assay
Apple callus was transformed as described (An et al., 2012) with slight modifications. MdWRKY79 (MDP0000300712) was cloned and introduced into the pCAMBIA1300 vector with a GFP fused to the C terminus, and the construct was transformed into Agrobacterium LBA4404. After being cultured in liquid medium with shaking at 140 rpm at 25°C for ∼5 d in the dark, ‘Orin’ apple calli were collected using gauze and suspended in the Agrobacterium harboring pCAMBIA1300-MdWRKY79 vector for 15 min. The calli were then cocultured on MS solid medium with 1.5 mg/L 6-BA and 0.5 mg/L indole-3-acetic acid at 25°C for 2 d in the dark. After being washed three to five times with sterile water, the calli were cultured on the solid MS medium above supplemented with 250 mg/L carbenicillin and 30 mg/L kanamycin for selection.
ChIP-PCR was performed using an EpiTect ChIP OneDay Kit (Qiagen; catalog no. 334471). Approximately 2 g of MdWRKY79-GFP or GFP (control) transgenic calli was cross-linked in 1% formaldehyde. Subsequently, the immunoprecipitate was used to isolate the protein-DNA complex using a GFP antibody (Invitrogen; product no. PA-980A, lot no. RH236759). The DNA was purified from the protein-DNA complex using the Qiagen kit, and the abundance of each DNA fragment was quantified as the percentage of total input DNA by RT-qPCR (Hsieh et al., 2012).
Leaf Agroinfiltration and Fungal Inoculation
Healthy and uniform leaves in the upper portion of 4-week-old, subcultured plantlets of CK, A4, and A10 were selected for agroinfiltration following an injection procedure used in Nicotiana benthamiana (Walter et al., 2004)(; (; Bai et al., 2011) and apple (Meng et al., 2014) with some modifications. Positive colonies of Agrobacterium GV3101 harboring pGWB551-MdNLR16, pGWRNAi-MdNLR16, pGWB551-MdNLR310, pGWB551-MdNLR374, pGWB551-MdWRKY79, pGWRNAi-MdWRKY79, or empty vectors were cultured in 5 mL YEP liquid medium plus 50 mg/L kanamycin and 20 mg/L rifampicin with shaking (200 rpm) at 28°C to an OD600 value of 2.0, followed by centrifugation at 8000 rpm for 10 min at room temperature. The Agrobacterium was resuspended to an OD600 of 0.1 in MES buffer (10 mM MES-KOH, pH 5.2, 10 mM MgCl2, and 100 µM acetosyringone) and incubated at 4°C for 3 h. Twenty microliters of Agrobacterium suspension was injected into attached apple leaves per site using a syringe, with MES buffer and empty vector-containing Agrobacterium solution as controls (Figure 4A). Two days after injection, transcript levels of MdNLR16, MdNLR310, MdNLR374, and MdWRKY79 in the leaves were assessed using RT-qPCR. Ten microliters of spore suspension (5 × 105/mL) of A. alternata strain R2 was placed on each injection site after the leaves were detached, and they were incubated in moist Petri dishes at 26°C in the dark for 3 d before evaluation of disease.
Inoculation of A. alternata strain R2 was performed as described above for the experiment characterizing transcript levels of the 13 selected NLR genes in response to the inoculation (Figure 3F; Supplemental Figure 4), but a sterile needle was used to create four wounding sites per leaf and no agroinfiltration was involved.
Extraction and Analysis of SA and JA
SA and JA were extracted from healthy mature leaves of field-grown trees with d4-SA and d5-JA (CDN Isotopes) as internal standards and analyzed on a triple-quadrupole LC-MS/MS system (Quantum Access; Thermo Fisher Scientific) with a C18 reversed-phase HPLC column (Gemini-NX, 3-μm particle size, 150 × 2.00 mm; Phenomenex) as described (Thaler et al., 2010).
Localization of MdWRKY79 in Apple Leaves
pGWB551-MdWRKY79 was introduced into leaves of subcultured ‘Greensleeves’ apple via agroinfiltration as described above and fluorescence images were obtained using a Zeiss LSM710 confocal microscope.
Accession Numbers
Sequence data from this article can be found in the Genome Database for Rosaceae (https://www.rosaceae.org) and the GenBank/EMBL data libraries under the following accession numbers: MdNLR16, MDP0000257201; MdNLR59, MDP0000902193; MdNLR140, MDP0000158225; MdNLR147, MDP0000157225; MdNLR208, MDP0000218445; MdNLR309, MDP0000241462; MdNLR310, MDP0000243301; MdNLR330, MDP0000292810; MdNLR341, MDP0000304378; MdNLR374, MDP0000207438; MdNLR386, MDP0000269116; MdNLR721, MDP0000214360; MdNLR726, MDP0000129374; MdNLR883, MDP0000259527; MdNLR939, MDP0000212932; MdNLR953, MDP0000198228; MdWRKY79, MDP0000300712; MdActin, MDP0000921834; HRIP1, MH384396; and AMT, AF184074.1.
Supplemental Data
Supplemental Figure 1. Identification of R1, R2 and R3 as Alternaria spp via phylogenetic analyses.
Supplemental Figure 2. Concentrations of sugars in the leaves of sub-cultured shoots of the wild-type control (CK) and A6PR antisense lines (A4 and A10) of ‘Greensleeves’ apple in response to feeding with sorbitol (50 mM) or sucrose (50 mM).
Supplemental Figure 3. Transcript levels of 12 NLR genes in the leaves of the wild-type control (CK) and A6PR antisense lines (A4 and A10) of ‘Greensleeves’ apple in response to feeding with sorbitol (50 mM) or sucrose (50 mM).
Supplemental Figure 4. Transcript levels of 12 NLR genes and sugar concentrations in leaves of the wild-type control (CK) and A6PR antisense lines (A4 and A10) of ‘Greensleeves’ apple in response to inoculation with A. alternata R2.
Supplemental Figure 5. Overexpression of MdNL310 or MdNLR374 does not alter disease phenotype of wild-type ‘Greensleeves’ apple (CK) and two A6PR antisense lines (A4 and A10) to A. alternata R2.
Supplemental Figure 6. Overexpression of MdNL16 does not alter disease phenotype of two antisense lines A4 and A10 of ‘Greensleeves’ apple to Alternaria strain R1 or R3.
Supplemental Figure 7. Subcellular localization of MdNLR16 in apple callus protoplast.
Supplemental Figure 8. Subcellular localization of A. alternata R2 Hrip1 in apple callus protoplast.
Supplemental Figure 9. Yeast two-hybrid assay of the interaction between Hrip1 (A. alternata R2) and seven other MdNLRs.
Supplemental Figure 10. Bimolecular fluorescence complementation assay of the interaction between Hrip1 (from strain R2) and seven other MdNLRs in tobacco (Nicotiana benthamiana) leaves.
Supplemental Figure 11. Hypersensitive cell death caused by overexpression of A. alternata R2 HRIP1 in ‘Greensleeves’ apple leaves.
Supplemental Figure 12. Percentage of infected leaves in two antisense lines A4 and A10 of ‘Greensleeves’ apple after inoculation with A. alternata R2 or HRIP1 knockout mutants #3 and #5.
Supplemental Figure 13. Disease phenotypes of wild-type ‘Greensleeves’ apple with RNAi suppression of MdNLR16 expression after inoculation with A. alternata R2 HRIP1 knockout mutants #3 and #5.
Supplemental Figure 14. Levels of salicylic acid and jasmonic acid in healthy mature leaves of the wild-type (CK) and antisense lines (A4 and A10) of ‘Greensleeves’ apple.
Supplemental Figure 15. Sequence alignments of three candidate WRKY proteins in apple by DNAman software.
Supplemental Figure 16. Subcellular localization of MdWRKY79 in ‘Greensleeves’ apple leaves.
Supplemental Figure 17. Effects of simultaneous overexpression of MdWRKY79 and MdNLR16 in the leaves of wild-type control (CK) and two antisense lines (A4 and A10) of ‘Greensleeves’ apple on disease phenotype after inoculation with A. alternata R2.
Supplemental Data Set 1. Gene sequence alignments used in phylogenetic analyses of Alternaria isolates.
Supplemental Data Set 2. Expression of 1015 NLR genes in the leaves of wild-type control (CK) and two antisense A6PR lines (A4 and A10) of ‘Greensleeves’ apple from RNA-seq analysis (Wu et al., 2015).
Supplemental Data Set 3. Differentially expressed NLR genes between the wild-type control (CK) and two antisense A6PR lines (A4 and A10) of ‘Greensleeves’ apple from RNA-seq analysis (Wu et al., 2015).
Supplemental Data Set 4. Protein sequence alignments used in phylogenetic analyses of apple NLR proteins.
Supplemental Data Set 5. List of primers used in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This work was supported by Cornell University Agricultural Experiment Station. We thank Shunwen Lu and Patrick Inderbitzin for help in isolating the three Alternaria strains, Motoichiro Kodama (Tottori University, Japan) for sharing the A. alternata apple pathotype M71 strain, Takaya Moriguchi (National Institute of Fruit Tree Science, Japan) for providing ‘Orin’ apple calli, and Alan Collmer, Adam Bogdanove, Robert Turgeon, Awais Khan, Hailei Wei, and Shien Lu for critical reading of the manuscript. The Agilent gas chromatography-mass spectrometry system used in this work was generously donated by David Zimerman (Cornell Pomology PhD 1954).
AUTHOR CONTRIBUTIONS
L.C., D.M., B.G.T., and A.M.D. planned and designed the experiments. D.M., C.L., H.-J.P., J.G., and J.W. performed the experiments and analyzed the data. D.M., L.C., and B.G.T. wrote the manuscript with inputs from all the other authors.
Footnotes
Articles can be viewed without a subscription.
References
- Adachi H., Nakano T., Miyagawa N., Ishihama N., Yoshioka M., Katou Y., Yaeno T., Shirasu K., Yoshioka H. (2015). WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell 27: 2645–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X.H., Tian Y., Chen K.Q., Wang X.F., Hao Y.J. (2012). The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. J. Plant Physiol. 169: 710–717. [DOI] [PubMed] [Google Scholar]
- Antony G., Zhou J., Huang S., Li T., Liu B., White F., Yang B. (2010). Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 22: 3864–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold D.D. (1999). Carbohydrate availability modifies sorbitol dehydrogenase activity of apple fruit. Physiol. Plant. 105: 391–395. [Google Scholar]
- Arya P., Kumar G., Acharya V., Singh A.K. (2014). Genome-wide identification and expression analysis of NBS-encoding genes in Malus x domestica and expansion of NBS genes family in Rosaceae. PLoS One 9: e107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres D.L., Darling A., Zwickl D.J., Beerli P., Holder M.T., Lewis P.O., Huelsenbeck J.P., Ronquist F., Swofford D.L., Cummings M.P., Rambaut A., Suchard M.A. (2012). BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Syst. Biol. 61: 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Kasai A., Yamada K., Li T., Harada T. (2011). A mobile signal transported over a long distance induces systemic transcriptional gene silencing in a grafted partner. J. Exp. Bot. 62: 4561–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D.A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W. (2013). GenBank. Nucleic Acids Res. 41: D36–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R.L. (1982). Sugar alcohols. In Encyclopedia of Plant Physiology, New Series. Vol. 13A, Loewus F.A., Tanner W., eds (New York: Springer-Verlag; ), pp. 158–192. [Google Scholar]
- Bigeard J., Colcombet J., Hirt H. (2015). Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 8: 521–539. [DOI] [PubMed] [Google Scholar]
- Bolouri Moghaddam M.R., Van den Ende W. (2012). Sugars and plant innate immunity. J. Exp. Bot. 63: 3989–3998. [DOI] [PubMed] [Google Scholar]
- Charif D., Lobry J.R. (2007). SeqinR 1.0-2: a contributed package to the R project for statistical computing devoted to biological sequences retrieval and analysis. In Structural Approaches to Sequence Evolution, Bastolla U., Porto M., Eduardo Roman H., Vendruscolo M., eds (Berlin: Springer-Verlag; ), pp. 207–232. [Google Scholar]
- Chen L.Q., et al. (2010). Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Q., Qu X.Q., Hou B.H., Sosso D., Osorio S., Fernie A.R., Frommer W.B. (2012). Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211. [DOI] [PubMed] [Google Scholar]
- Cheng L., Zhou R., Reidel E.J., Sharkey T.D., Dandekar A.M. (2005). Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta 220: 767–776. [DOI] [PubMed] [Google Scholar]
- Chung K.R. (2012). Stress response and pathogenicity of the necrotrophic fungal pathogen Alternaria alternata. Scientifica 2012: 635431–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K.L., et al. (2017). TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nat. Commun. 8: 15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H., Tsuda K., Parker J.E. (2015). Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol. 66: 487–511. [DOI] [PubMed] [Google Scholar]
- Darriba D., Taboada G.L., Doallo R., Posada D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris J.D., et al. (2010). A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc. Natl. Acad. Sci. USA 107: 13544–13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Cheng L., Guo Y., Turgeon R. (2011). Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiol. 157: 1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic K., Hernandez A., Korban S. (2004). RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol. Biol. Report. 22: 437a–437g. [Google Scholar]
- Glazebrook J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43: 205–227. [DOI] [PubMed] [Google Scholar]
- Gómez-Ariza J., Campo S., Rufat M., Estopà M., Messeguer J., San Segundo B., Coca M. (2007). Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Mol. Plant Microbe Interact. 20: 832–842. [DOI] [PubMed] [Google Scholar]
- Gur L., Reuveni M., Cohen Y. (2017). Occurrence and etiology of Alternaria leaf blotch and fruit spot of apple caused by Alternaria alternata f. sp. mali on cv. Pink lady in Israel. Eur. J. Plant Pathol. 147: 695–708. [Google Scholar]
- Harteveld D.O.C., Akinsanmi O.A., Drenth A. (2013). Multiple Alternaria species groups are associated with leaf blotch and fruit spot diseases of apple in Australia. Plant Pathol. 62: 289–297. [Google Scholar]
- Harteveld D.O.C., Akinsanmi O.A., Drenth A. (2014). Pathogenic variation of Alternaria species associated with leaf blotch and fruit spot of apple in Australia. Eur. J. Plant Pathol. 139: 789–799. [Google Scholar]
- Herbers K., Meuwly P., Frommer W.B., Metraux J.P., Sonnewald U. (1996a). Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8: 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K., Meuwly P., Métraux J.P., Sonnewald U. (1996b). Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 397: 239–244. [DOI] [PubMed] [Google Scholar]
- Hopkins C.M., White F.F., Choi S.-H., Guo A., Leach J.E. (1992). Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 5: 451–459. [DOI] [PubMed] [Google Scholar]
- Hsieh W.P., Hsieh H.L., Wu S.H. (2012). Arabidopsis bZIP16 transcription factor integrates light and hormone signaling pathways to regulate early seedling development. Plant Cell 24: 3997–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D.G., Sun C.H., Zhang Q.Y., An J.P., You C.X., Hao Y.J. (2016). Glucose sensor MdHXK1 phosphorylates and stabilizes MdbHLH3 to promote anthocyanin biosynthesis in apple. PLoS Genet. 12: e1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Larget B., Alfaro M.E. (2004). Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol. Biol. Evol. 21: 1123–1133. [DOI] [PubMed] [Google Scholar]
- Jennings D.B., Ehrenshaft M., Pharr D.M., Williamson J.D. (1998). Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. USA 95: 15129–15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings D.B., Daub M.E., Pharr D.M., Williamson J.D. (2002). Constitutive expression of a celery mannitol dehydrogenase in tobacco enhances resistance to the mannitol-secreting fungal pathogen Alternaria alternata. Plant J. 32: 41–49. [DOI] [PubMed] [Google Scholar]
- Johnson R.D., Johnson L., Itoh Y., Kodama M., Otani H., Kohmoto K. (2000). Cloning and characterization of a cyclic peptide synthetase gene from Alternaria alternata apple pathotype whose product is involved in AM-toxin synthesis and pathogenicity. Mol. Plant Microbe Interact. 13: 742–753. [DOI] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulye M., Liu H., Zhang Y., Zeng H., Yang X., Qiu D. (2012). Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence-related genes and systemic acquired resistance in tobacco. Plant Cell Environ. 35: 2104–2120. [DOI] [PubMed] [Google Scholar]
- Li Y., Aldwinckle H.S., Sutton T., Tsuge T., Kang G., Cong P.H., Cheng Z.M. (2013). Interactions of apple and the Alternaria alternata apple pathotype. Crit. Rev. Plant Sci. 32: 141–150. [Google Scholar]
- Loescher W.H. (1987). Physiology and metabolism of sugar alcohols in higher plants. Physiol. Plant. 70: 553–557. [Google Scholar]
- Lorang J.M., Sweat T.A., Wolpert T.J. (2007). Plant disease susceptibility conferred by a “resistance” gene. Proc. Natl. Acad. Sci. USA 104: 14861–14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorang J., Kidarsa T., Bradford C.S., Gilbert B., Curtis M., Tzeng S.C., Maier C.S., Wolpert T.J. (2012). Tricking the guard: exploiting plant defense for disease susceptibility. Science 338: 659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison W.P., Maddison D.R. (2017). Mesquite: a modular system for evolutionary analysis. Version 3.03. http://mesquiteproject.org.
- Mao G., Meng X., Liu Y., Zheng Z., Chen Z., Zhang S. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.B., Bogdanove A.J., Sessa G. (2003). Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 54: 23–61. [DOI] [PubMed] [Google Scholar]
- McHale L., Tan X., Koehl P., Michelmore R.W. (2006). Plant NBS-LRR proteins: adaptable guards. Genome Biol. 7: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D., Gu Z., Li W., Wang A., Yuan H., Yang Q., Li T. (2014). Apple MdABCF assists in the transportation of S-RNase into pollen tubes. Plant J. 78: 990–1002. [DOI] [PubMed] [Google Scholar]
- Meng D., Li Y., Bai Y., Li M., Cheng L. (2016). Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress. Plant Physiol. Biochem. 103: 71–83. [DOI] [PubMed] [Google Scholar]
- Meng D., He M., Bai Y., Xu H., Fei Z., Dandekar A.M., Cheng L. (2018). Decreased sorbitol synthesis leads to abnormal stamen development and reduced pollen tube growth via a MYB transcription factor, MdMYB39L, in apple (Malus domestica). New Phytol. 217: 641–656. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop, IEEE (New Orleans, LA), pp. 1–8. [Google Scholar]
- Navarre D.A., Wolpert T.J. (1999). Victorin induction of an apoptotic/senescence-like response in oats. Plant Cell 11: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm F.B., Loescher W.H. (1981). Characterization and partial purification of aldose-6-phosphate reductase (alditol-6-phosphate: NADP 1-oxidoreductase) from apple leaves. Plant Physiol. 67: 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver R.P., Friesen T.L., Faris J.D., Solomon P.S. (2012). Stagonospora nodorum: from pathology to genomics and host resistance. Annu. Rev. Phytopathol. 50: 23–43. [DOI] [PubMed] [Google Scholar]
- Paradis E., Claude J., Strimmer K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.W., Roux M., Petersen M., Mundy J. (2012). MAP kinase cascades in Arabidopsis innate immunity. Front. Plant Sci. 3: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing; ), http://www.R-project.org/. [Google Scholar]
- Reidel E.J., Rennie E.A., Amiard V., Cheng L., Turgeon R. (2009). Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol. 149: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M., Sheglov N., Eshel D., Prusky D., Ben-Arie R. (2007). Virulence and the production of endo-1, 4-β-glucanase by isolates of Alternaria alternata involved in the moldy-core disease of apples. J. Phytopathol. 155: 50–55. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotondo F., Collina M., Brunelli A., Pryor B.M. (2012). Comparison of Alternaria spp. collected in Italy from apple with A. mali and other AM-toxin producing strains. Phytopathology 102: 1130–1142. [DOI] [PubMed] [Google Scholar]
- R Studio Team (2015). RStudio: Integrated Development for R. (Boston: RStudio; ), http://www.rstudio.com/. [Google Scholar]
- Schulz A. (2014). pBrackets: Plot Brackets. R package version 1.0. http://CRAN.R-project.org/package=pBrackets.
- Staal J., Kaliff M., Dewaele E., Persson M., Dixelius C. (2008). RLM3, a TIR domain encoding gene involved in broad-range immunity of Arabidopsis to necrotrophic fungal pathogens. Plant J. 55: 188–200. [DOI] [PubMed] [Google Scholar]
- Takao K., Akagi Y., Tsuge T., Harimoto Y., Yamamoto M., Kodama M. (2016). The global regulator LaeA controls biosynthesis of host-specific toxins, pathogenicity and development of Alternaria alternata pathotypes. J. Gen. Plant Pathol. 82: 121–131. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J.S., Agrawal A.A., Halitschke R. (2010). Salicylate-mediated interactions between pathogens and herbivores. Ecology 91: 1075–1082. [DOI] [PubMed] [Google Scholar]
- Thomma B.P.H.J. (2003). Alternaria spp.: from general saprophyte to specific parasite. Mol. Plant Pathol. 4: 225–236. [DOI] [PubMed] [Google Scholar]
- Turgeon B.G., Condon B., Liu J., Zhang N. (2010). Protoplast transformation of filamentous fungi. In Molecular and Cell Biology Methods for Fungi: Methods in Molecular Biology, Series 638, Sharon A., ed (New York: Humana Press; ), pp. 3–19. [DOI] [PubMed] [Google Scholar]
- Tzeng T.H., Lyngholm L.K., Ford C.F., Bronson C.R. (1992). A restriction fragment length polymorphism map and electrophoretic karyotype of the fungal maize pathogen Cochliobolus heterostrophus. Genetics 130: 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya G., Lohman D.J., Meier R. (2011). SequenceMatrix: concatenation software for the fast assembly of multi‐gene datasets with character set and codon information. Cladistics 27: 171–180. [DOI] [PubMed] [Google Scholar]
- Vera Cruz C.M., Bai J.F., Oña I., Leung H., Nelson R.J., Mew T.W., Leach J.E. (2000). Predicting durability of a disease resistance gene based on an assessment of the fitness loss and epidemiological consequences of avirulence gene mutation. Proc. Natl. Acad. Sci. USA 97: 13500–13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438. [DOI] [PubMed] [Google Scholar]
- Wang H., Ma F., Cheng L. (2010). Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 232: 511–522. [DOI] [PubMed] [Google Scholar]
- Wang X., Jiang N., Liu J., Liu W., Wang G.L. (2014). The role of effectors and host immunity in plant-necrotrophic fungal interactions. Virulence 5: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte C.P., Noël L.D., Gielbert J., Parker J.E., Romeis T. (2004). Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol. Biol. 55: 135–147. [DOI] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Seidl M.F., Groenewald J.Z., de Vries M., Stielow J.B., Thomma B.P.H.J., Crous P.W. (2015). Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 82: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Wang Y., Zheng Y., Fei Z., Dandekar A.M., Xu K., Han Z., Cheng L. (2015). Suppressing sorbitol synthesis substantially alters the global expression profile of stress response genes in apple (Malus domestica) leaves. Plant Cell Physiol. 56: 1748–1761. [DOI] [PubMed] [Google Scholar]
- Xiao W., Sheen J., Jang J.C. (2000). Multiple sugar signal transduction pathways in plants. Plant Mol. Biol. 44: 451–461. [DOI] [PubMed] [Google Scholar]
- Yamada K., Saijo Y., Nakagami H., Takano Y. (2016). Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 354: 1427–1430. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li P., Cheng L. (2010). Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 123: 1013–1018. [Google Scholar]
- Zheng H.H., Zhao J., Wang T.Y., Wu X.H. (2015). Characterization of Alternaria species associated with potato foliar diseases in China. Plant Pathol. 64: 425–433. [Google Scholar]
- Zhong Y., Yin H., Sargent D.J., Malnoy M., Cheng Z.M. (2015). Species-specific duplications driving the recent expansion of NBS-LRR genes in five Rosaceae species. BMC Genomics 16: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Cheng L., Wayne R. (2003). Purification and characterization of sorbitol-6-phosphate phosphatase from apple leaves. Plant Sci. 165: 227–232. [Google Scholar]
- Zhou R., Cheng L., Dandekar A.M. (2006). Down-regulation of sorbitol dehydrogenase and up-regulation of sucrose synthase in shoot tips of the transgenic apple trees with decreased sorbitol synthesis. J. Exp. Bot. 57: 3647–3657. [DOI] [PubMed] [Google Scholar]