Abstract
Oxymatrine (OMT) as a type of alkaloids collected from Sophora flavescens Ait exerts some biological functions including anticancer properties. Here, we investigated the therapeutic effects of OMT in gastric cancer cells (HGC 27 and AGS). As a result, the exposure of gastric cancer (GC) cells to OMT contributed to the suppression of cell proliferation and invasion. Interleukin 21 receptor (IL-21R) was identified to be differentially expressed between OMT treatment group (4 mg/mL) and control group (0 mg/mL), and knockdown of IL-21R repressed cell proliferation and invasion via inactivation of the JAK2/STAT3 pathway. The rescue experiment showed that IL-21R overexpression attenuated the anti-tumor effects of OMT through activation of the JAK2/STAT3 pathway. Moreover, the expression of IL-21R was significantly upregulated in GC samples compared with the adjacent normal tissues and associated with overall survival (OS) and tumor recurrence of GC patients. Taken together, in this study, we evaluated the anti-tumor effects of OMT on GC by investigating proliferation and invasion ability changes, and our findings show that OMT exhibits effects via regulation of JAK/STAT signaling pathway. Through the mechanism study, we may enlighten the potential therapeutic target for treatment of GC.
Keywords: gastric cancer, growth, IL-21R, JAK2, oxymatrine, STAT3
Introduction
Gastric cancer (GC) as one of the most common malignant tumors remains a leading cause of cancer mortality worldwide, with an estimated 1,000,000 GC patients per year.1,2 The activity imbalance of oncogenes and anti-oncogenes has been proved to be implicated in gastric carcinogenesis.3,4 Accumulating evidence has shown that targeted inhibition of the oncogenes or regulation of the downstream genes related to cancer progression may provide effective means for intervention of carcinogenesis and metastasis.5,6
Interleukin-21 (IL-21), identified as a member of the IL-2 family, is involved in biological activity in cancer and autoimmunity via binding to its receptor IL-21R,7 which is highly expressed in hematologic malignancies8,9 and enhances the aggressiveness of follicular lymphoma.10 IL-21/IL-21R axis results in the pathogenesis of leukemia and large cell lymphoma through activation of the JAK/STAT signaling pathway11,12 and presents a cancer therapeutic target.
Activation of JAK/STAT signaling is implicated in the development of inflammatory diseases and cancer.13–15 Interleukin-6 (IL-6)-activated JAK/STAT signaling induces angiogenesis,16,17 promotes growth18–20 and cellular invasion,21 and regulates cell apoptosis19 in the tumorigenesis. Inhibition of the JAK/STAT pathway induces cycle arrest and apoptosis in GC cells.22,23 Thus, many studies are focused on the therapeutic efficacy of JAK1/2 inhibitors on tumor growth, proliferation, apoptosis, angiogenesis, and metastasis in various solid tumors,24–26 indicating that JAK/STAT signaling is a promising marker for the diagnosis and therapy for multiple malignancies.
Oxymatrine (OMT) has numerous pharmacological activities27–31 in cardiovascular protection32 and anti-cancer properties33–35 via suppression of tumor growth and invasion, induction of cycle arrest and apoptosis, and reduction of chemotherapy-induced cell toxicity.36 We previously confirmed the inhibitory effects of OMT on vascular and lymph node invasion in GC.37 Herein, we found that OMT displayed anti-tumor effects via regulation of JAK/STAT signaling pathway, suggesting the potential therapeutic target for treatment of GC.
Materials and methods
Materials
The antibodies of IL-21R, p-JAK3, p-STAT3, MMP-2, and MMP-9 used in our experiment were provided by Cell Signaling Technologies (Beverly, MA, USA); OMT (C15H24N2O2, Cas no. 16837-52-8, HPLC ≥ 98%) used in this study was supplied by Shanghai Golden Harvest Biotechnology Co., Ltd (Shanghai, PR China).
Clinical data
The prognostic data (overall survival (OS) or recurrence time and status) for 412 cases of GC patients and 36 adjacent normal tissues as well as the relative expression level of IL-21R were downloaded from The Cancer Genome Atlas (TCGA) 2015 RNA sequencing database (https://genome-cancer.ucsc.edu). The protocols used in our study were approved by the Ethics Committee of Shanghai Sixth People’s Hospital.
Cell culture
The human gastric cancer cell lines SGC-7901, MGC-803, BGC-823, HGC-27, AGS, and GES-1 were purchased from Chinese Academy of Sciences Shanghai Cell Bank and were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA).
Vector construction and cell transfection
IL-21R siRNA (siIL-21R), IL-21R-overexpressed vectors, and negative control were synthesized by Shanghai GenePharma Co. Ltd. (Shanghai, PR China) for transfection into the GC cell lines. The following sequence was used to target IL-21R: CCTACACCTGCCACATGGATGTATT. The sequence of the negative control was CCTCCACGTCACGTATAGTGACATT. Target cells were transfected with these vectors using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA). The cells were harvested 48 h after transfection.
Quantitative real-time polymerase chain reaction
To detect the messenger RNA (mRNA) expression level of IL-21R in GC cell lines, quantitative real-time polymerase chain reaction (qRT-PCR) was performed. Total RNA of each clone was extracted with TRIzol according to the manufacturer’s protocol. Reverse transcription was carried out using Moloney murine leukemia virus (M-MLV) Reverse Transcriptase (RT), which is a recombinant DNA polymerase that synthesizes a complementary DNA (cDNA) strand from single-stranded RNA, DNA, or an RNA:DNA hybrid, and cDNA amplification using SYBR Green Master Mix kit (Life Technologies). IL-21R gene was amplified using specific oligonucleotide primer and human GAPDH gene was used as an endogenous control. The polymerase chain reaction (PCR) primer sequences of IL-21R gene were as follows: 5′-CGTGGGAGTCAGCATGCC-3′ and 5′-TGTCGTCGGCCATGAAGTG-3′; GAPDH, 5′-CAACGAATTTGGCTACAGCA-3′ and 5′-AGGGGTCTACATGGCAACTG-3′. The two-step PCR procedure was as follows: 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s and annealing/extension at 60°C for 30 s. The relative expression levels of IL-21R gene were calculated with the 2–ΔΔCT method. And three separate experiments were performed.
Western blotting analysis
Detailed procedure was described elsewhere.38 After treatment, GC cell lines were harvested and extracted using lysis buffer (Tris-HCl, sodium dodecyl sulfate (SDS), mercaptoethanol, glycerol). The primary antibodies against IL-21R, p-JAK3, p-STAT3, MMP-2, and MMP-9 were diluted according to the manufacturer’s instructions and incubated overnight at 4°C. Then, horseradish peroxidase-linked secondary antibodies were added at a dilution ratio of 1:1000 and incubated at room temperature for 2 h. The membranes were washed with phosphate-buffered saline (PBS) for three times and the immunoreactive bands were visualized using ECL-PLUS/Kit according to the manufacturer’s instruction. The relative protein level in different groups was normalized to β-actin concentration.
Cell viability assay
Cell counting kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan), and the detailed procedure was described elsewhere.39 GC cells (2 × 103/well) were seeded in 96-well plates at 37°C, 5% CO2. After treated with different concentrations of OMT (0–10 mg/mL) for 48 h, CCK-8 solution (10 μL) was added into each well, followed by incubation for 2 h. The optical densities at 492 nm were measured using a Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Transwell invasion assay
Corning HTS Transwell 24 Well Permeable Supports (CLS3396) was purchased from the Sigma-Aldrich, and detailed procedure was described elsewhere.38 In the end, the number of cells invading through the matrigel was counted in three randomly selected visual fields from the central and peripheral portion of the filter using an inverted microscope (200× magnification). Each assay was repeated three times.
Statistical analysis
Data were analyzed as mean ± SEM. Kruskal–Wallis H test and chi-square test were used to analyze the differential expression of IL-21R between GC and adjacent normal tissues. The effects of IL-21R on cell growth and invasion in vitro and in vivo were calculated using one-way analysis of variance, and the Student’s t-test was used for cell growth and invasion in vitro and in vivo evaluations. Kaplan–Meier analysis was conducted to analyze the correlation of IL-21R expression with OS and tumor recurrence. Differences were considered significant at P < 0.05 (*P < 0.05, **P < 0.01). NS represents no significance.
Results
OMT exerts cytotoxicity to GC cells
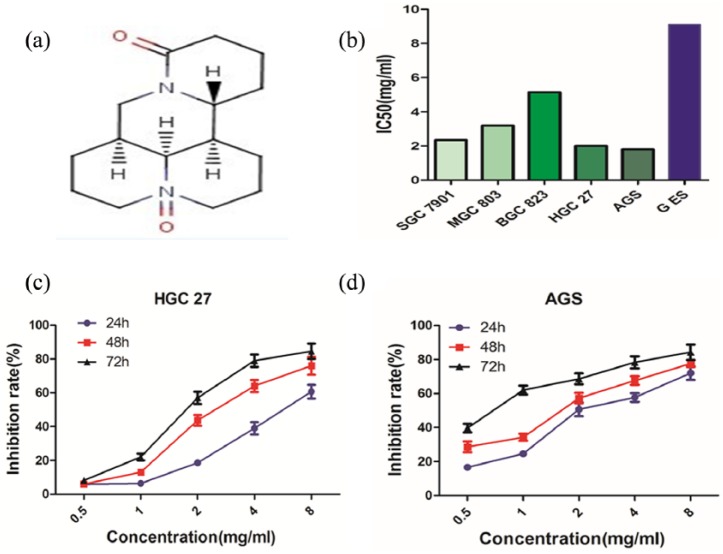
The chemical structure of OMT is illustrated in Figure 1(a). We assessed the effects of OMT on GC cell viability. Five human GC cell lines and GES-1 were exposed to different concentrations of OMT (0–10 mg/mL) for 48 h. Cell viability was examined by the CCK-8 assay. As shown in Figure 1(b), OMT exerted obvious growth inhibition of GC cells with IC50 values of about 1.8–5.4 mg/mL for 48 h, of which HGC-27 and AGS were sensitive to the cytotoxic effect of OMT (IC50 values: 2.2 and 1.8 mg/mL.
Figure 1.
OMT exerts cytotoxicity to GC cells and inhibits cell proliferation. (a) Chemical structure of OMT. (b) OMT displayed a significant growth inhibition in GC cells with IC50 values. (c, d) CCK-8 assay indicated the effects of OMT of different concentrations on cell proliferation in HGC-27 and AGS cells.
OMT inhibits cell proliferation and invasion
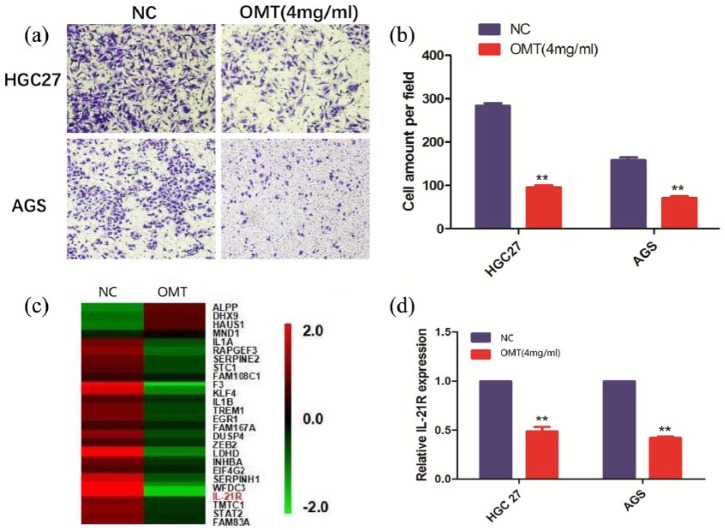
As HGC-27 and AGS cell lines were sensitive to OMT, the results showed that OMT exerted growth inhibition with IC50 values nearly 4 mg/mL at 24 h. Thus, we selected the concentration of 4.0 mg/mL for OMT treatment group and 0 mg/mL for the non-treatment group (NC); the inhibitory efficacy of OMT on cell proliferation was assessed by the CCK-8 assay. The major characteristics of GC are its excessive local invasion and systemic metastasis. Cell invasion was determined by Transwell assay, indicating that OMT exhibited markedly inhibitory effects on cell proliferation (Figure 1(c) and (d)) and invasion (Figure 2(a) and (b); **P < 0.01).
Figure 2.
OMT inhibits cell invasion and IL-21R was identified to be affected by OMT treatment. (a, b) Transwell assay showed the effects of OMT on cell invasion in HGC-27 and AGS cells. (c) RNA expression profile was used to screen the differentially-expressed gene IL-21R between OMT-treatment group (4 mg/mL) and non-treatment group (0 mg/mL). (d) The effects of OMT on IL-21R expression levels in GC HGC-27 and AGS cells by qRT-PCR analysis. Data are the means ± SEM of three experiments. **P < 0.01.
IL-21R knockdown inhibits cell proliferation and invasion through downregulating JAK/STAT signaling
To determine the downstream target of OMT, we screened out the differentially expressed gene IL-21R between OMT-treatment group and non-treatment group by RNA expression profile (Figure 2(c)) and verified the levels of IL-21R were significantly down-regulated by OMT in HGC-27 and AGS cells by qRT-PCR (Figure 2(d)). Furthermore, we investigated the function and molecular regulation of IL-21R in GC cells, indicating that knockdown of IL-21R gene tremendously attenuated cell proliferation (Figure 3(a)) and invasion (Figure 3(b) and (c)) and reduced the protein expression of p-JAK2 and p-STAT3 (Figure 3(d)).
Figure 3.
L-21R knockdown inhibits cell proliferation and invasion through down-regulating JAK2/STAT3 signaling. (a) CCK-8 assay indicated the effects of IL-21R knockdown on cell proliferation in HGC-27 and AGS cell lines. (b, c) Transwell assay indicated the effects of IL-21R knockdown on cell invasion. (d) The effects of IL-21R knockdown on the protein expression levels of JAK2/STAT3 signaling were determined by Western blotting analysis. Data are the means ± SEM of three experiments. **P < 0.01.
IL-21R overexpression attenuated OMT-mediated JAK2/STAT3 signaling inactivation
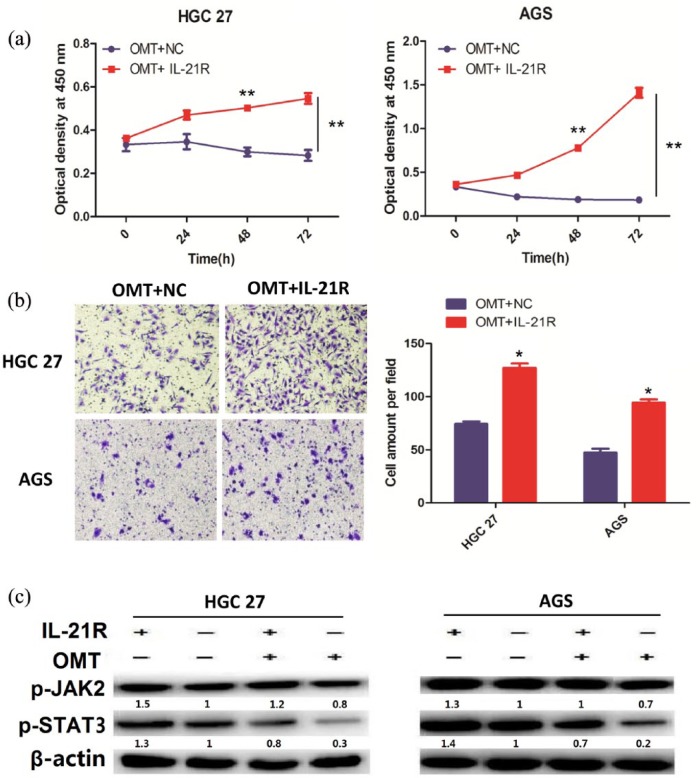
To have a detailed understanding of how IL-21R mediated OMT regulation of JAK/STAT signaling in GC cells, we further constructed the IL-21R vector, which was transfected into the HGC-27 and AGS cells which were pretreated with 4 mg/mL OMT for 24 h, and then cell proliferation activity, cell invasive potential, and the activity of JAK/STAT signaling were respectively assessed. We found that IL-21R overexpression weakened the anti-tumor effects of OMT on cell proliferation (Figure 4(a)) and invasion (Figure 4(b)) and declined the inhibitory effects of OMT on the activity of JAK signaling (Figure 4(c)). These findings suggested that IL-21R attenuated OMT-mediated inactivation of JAK/STAT signaling in GC.
Figure 4.
IL-21R overexpression weakened OMT-mediated inactivation of JAK2/STAT3 signaling pathway. (a) CCK-8 assay indicated that IL-21R overexpression reversed the anti-proliferation effects of OMT in HGC-27 and AGS cell lines. (b) Transwell assay indicated that IL-21R overexpression reversed the anti-invasion effects of OMT. (c) Western blotting indicated that IL-21R overexpression decreased the inhibitory effects of OMT on the protein expression levels of JAK2/STAT3 signaling. Data are the means ± SEM of three experiments. *P < 0.05; **P < 0.01.
NC: the empty vector; IL-21R: overexpression of IL-21R. And the concentration of OMT is 4 mg/mL.
Upregulation of IL-21R expression was associated with OS and tumor recurrence of GC patients
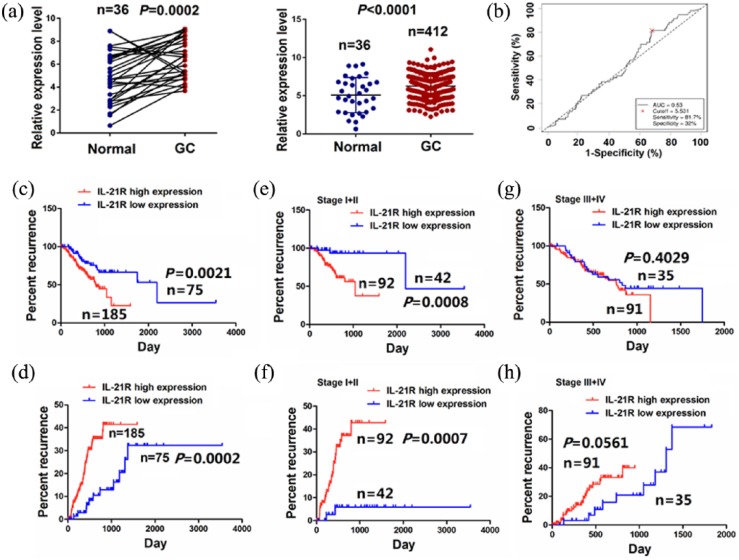
It was found that the expression level of IL-21R was markedly increased in the pair-matched GC samples (n = 36) as well as in total GC samples (n = 412) compared with the adjacent normal tissues using The Cancer Genome Atlas (TCGA) sequencing data (Figure 5(a)). Then, according to the OS time, OS status, and IL-21R expression level, we obtained the cutoff value (5.531) of IL-21R in GC samples (n = 260; Figure 5(b)) and divided the patients into two groups: IL-21R high expression and IL-21R low expression. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to assess the sensitivity and specificity of IL-21R expression, which indicated that the sensitivity, specificity, and AUC of IL-21R were, respectively, 81.7%, 32.0%, and 0.53 (Figure 1(b)), suggesting that IL-21R expression might be a potential marker for GC patients. We further analyzed the correlation of IL-21R expression with the prognosis of GC patients. Kaplan–Meier analysis showed that the patients with IL-21R high expression had shorter survival time (Figure 5(c)) and higher tumor recurrence rate (Figure 5(d)) compared with those with IL-21R low high expression. Besides, the patients in early stage (stage I + II) with IL-21R high expression had shorter survival time (Figure 5(e)) and higher tumor recurrence rate (Figure 5(f)), but those in late stage (stage III + IV) with IL-21R high expression displayed no difference in OS (Figure 5(g)) and tumor recurrence (Figure 5(h)) compared with those with IL-21R low expression.
Figure 5.
IL-21R expression was associated with OS and tumor recurrence of GC patients. (a) TCGA cohort analysis of the differentially expressed levels of IL-21R in pair-matched tumor tissues as well as in total GC samples. P < 0.001. (b) The cutoff value, sensitivity and specificity of IL-21R was assessed in GC samples (n = 260). (c, d) Kaplan–Meier analysis of the correlation of IL-21R high or low expression with the OS and tumor recurrence of the patients with GC. P < 0.01. Kaplan–Meier analysis of the correlation of IL-21R high or low expression with the OS and tumor recurrence of GC patients (e, f) in early stage (stage I + II; P < 0.01) and (g, h) late stage (stage III + IV; P > 0.05).
Discussion
The JAK/STAT signaling pathway participates in the carcinogenesis and metastasis of various cancers. The expression levels of JAK/STAT signaling compounds such as JAK3 and STAT-3 are correlated with the lymph node metastasis and OS of patients with lung adenocarcinoma, colorectal cancer,40 and esophageal squamous cell carcinoma.41 Importantly, IL-21/IL-21R axis activates JAK/STAT signaling participating in the pathogenesis of disease including GC.42 In our study, IL-21R expression was found upregulated and correlated with OS and tumor recurrence of the patients with GC in a large sample size from TCGA data. Our results were supported by other studies42 and suggested that IL-21R might be involved in the GC tumorigenesis.
Despite advances in chemotherapies, radiotherapies, and bio-therapies for treatment of GC in the decades, the mortality of GC patients remains exceedingly high. Some traditional Chinese medicines have been widely applied for GC treatment in China. We dedicated to developing new, effective, and safe drugs from natural products for GC therapy.43 OMT from traditional Chinese herbal medicine Sophora flavescens Ait reveals multiple pharmacological activities including anti-tumor activities.36 We have also reported that OMT combined with low-dose 5-fluorouracil (FU) or angiogenesis inhibitor NM-3 exhibits anti-tumor activity in GC cells.37,44 Guo et al.45 have found that OMT inhibits the proliferation and invasion of GC cells via blockade of EGFR/AKT pathway. Here, we found that OMT exerted obvious anticancer effects on GC cells, which was related to inhibiting cell growth and invasion, arresting cell cycle, and inducing cell apoptosis, showing that OMT might be a novel candidate for adjuvant treatment of GC.
Disruption of the activated JAK/STAT signaling by JAK inhibitors or novel small molecular inhibitors can inhibit the tumorigenicity in multiple cancer cells.24–26 IL-21 and IL-21R are involved in promoting growth, migration, and invasion in breast cancer through activation of JAK/STAT signaling.46 Interestingly, OMT protects septic shock-induced myocardial injury through inhibition of the JAK/STAT signaling. Whether IL-21R mediates the OMT regulation on JAK2/STAT3 signaling in GC cells is still elusive. Here, we found that IL-21R was differentially expressed in OMT treatment group and non-treatment group, and knockdown of IL-21R by siRNA suppressed GC cell growth and invasion, while IL-21R over-expression counteracted the anti-tumor activity of OMT on GC via inactivation of JAK2/STAT3 signaling. These findings indicated that OMT might impede the development of GC cells via inhibition of the IL-21R-activated JAK2/STAT3 signaling.
In conclusion, our findings demonstrate that OMT derived from Sophora flavescens Ait exhibits potential anti-tumor activity through inactivation of IL-21R-mediated JAK2/STAT3 signaling. Although the pharmacologic and toxicologic experiments are not performed, our results indicate OMT as a chemotherapeutic agent for the treatment of GC and other JAK/STAT signaling-related tumors.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our project was supported by China National Nature Science Foundation (No. 81573747) and Hong Kong Scholars Program (No. XJ2015033).
References
- 1. Hu XT, He C. (2013) Recent progress in the study of methylated tumor suppressor genes in gastric cancer. Chinese Journal of Cancer 32: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao JJ, Huang CY, Yang XB, et al. (2013) Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS ONE 8: e78158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hecht SS, Felton JS, Wogan GN, et al. (2004) Environmental and chemical carcinogenesis. Seminars in Cancer Biology 14: 473–486. [DOI] [PubMed] [Google Scholar]
- 4. Tijssen M, El-Bchiri J, Buffart TE, et al. (2009) NMD inhibition fails to identify tumour suppressor genes in microsatellite stable gastric cancer cell lines. BMC Medical Genomics 2: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friday BB, Adjei AA. (2005) K-ras as a target for cancer therapy. Biochimica et Biophysica Acta 1756: 127–144. [DOI] [PubMed] [Google Scholar]
- 6. Maillet L, Ryan J, Bah N, et al. (2014) Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death & Diseases 5: e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan MJ, Wang T. (2016) Advances of the interleukin-21 signaling pathway in immunity and angiogenesis. Biomedical Reports 5: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cha Z, Gu H, Guo H, et al. (2014) Effect of interleukin 21 and its receptor on CD8+ T cells in the pathogenesis of diffuse large B-cell lymphoma. Oncology Letters 8: 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Browning RL, Mo X, Muthusamy N, et al. (2015) CpG oligodeoxynucleotide CpG-685 upregulates functional interleukin-21 receptor on chronic lymphocytic leukemia B cells through an NF-κB mediated pathway. Oncotarget 6: 15931–15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood B, Sikdar S, Choi SJ, et al. (2013) Abundant expression of interleukin-21 receptor in follicular lymphoma cells is associated with more aggressive disease. Leukemia & Lymphoma 54: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 11. Zhang M, Mathews Griner LA, Ju W, et al. (2015) Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proceedings of the National Academy of Sciences of the United States of America 112: 12480–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao Y, Chapuy B, Monti S, et al. (2014) Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clinical Cancer Research 20: 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quintás-Cardama A, Verstovsek S. (2013) Molecular pathways: Jak/STAT pathway: Mutations, inhibitors, and resistance. Clinical Cancer Research 19: 1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vainchenker W, Constantinescu SN. (2013) JAK/STAT signaling in hematological malignancies. Oncogene 32: 2601–2613. [DOI] [PubMed] [Google Scholar]
- 15. Proia DA, Foley KP, Korbut TP, et al. (2011) Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling. PLoS ONE 6: e18552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu MS, Shun CT, Huang SP, et al. (2004) Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. Journal of Biomedical Science 11: 517–527. [DOI] [PubMed] [Google Scholar]
- 17. Qiao D, Meyer K, Yang X, et al. (2009) Signal transducers and activators of transcription mediate fibroblast growth factor-induced vascular endothelial morphogenesis. Cancer Research 69: 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Navarro A, Ferrer G, Diaz T, et al. (2011) Lestaurtinib inhibition of the Jak/STAT signaling pathway in Hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS ONE 6: e18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rachagani S, Gupta S, Macha MA, et al. (2013) Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Letters 341: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin H, Xu R, Wang Z, et al. (2009) Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Experimental and Molecular Medicine 41: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao D, Sun Y, Chen P, et al. (2012) Protein inhibitor of activated STAT-1 is downregulated in gastric cancer tissue and involved in cell metastasis. Oncology Reports 28: 2D149–2D155. [DOI] [PubMed] [Google Scholar]
- 22. Hu XM, Xu SQ, Yu RX, et al. (2011) Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma M-803 cells via JAK/STAT signal pathway. European Journal of Pharmacology 657: 10–19. [DOI] [PubMed] [Google Scholar]
- 23. Yan S, Li Z, Thiele CJ. (2013) Inhibition of STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor growth in neuroblastoma and pediatric sarcomas in vitro and in vivo. Oncotarget 4: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nam HJ, Kim HP, Kim MJ, et al. (2013) OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Letters 335: 145–152. [DOI] [PubMed] [Google Scholar]
- 25. Herrmann A, Reckamp K, Xin H, et al. (2011) Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Research 71: 6601–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma JY, Langford CP, McFarland BC, et al. (2011) Therapeutic potential of AZD1480 for the treatment of human glioblastoma. Molecular Cancer Therapeutics 10: 2384–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yin R, Cong Y, Yang Z, et al. (2014) Oxymatrine lightened the inflammatory response of LPS-induced mastitis in mice through affecting NF-κB and MAPKs signaling pathways. Inflammation 37: 2047–2055. [DOI] [PubMed] [Google Scholar]
- 28. Koo JS, Goldsmith JR, Guzman JR, et al. (2013) Oxymatrine prevents NF-κB nuclear translocation and ameliorates acute intestinal inflammation. Scientific Reports 3: 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao W, Xue R, Wang YP, et al. (2011) Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antiviral Research 89: 227–231. [DOI] [PubMed] [Google Scholar]
- 30. Fu Q, Shi H, Chai NL, et al. (2012) Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World Journal of Gastroenterology 18: 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Wang T, Wu J, et al. (2014) Liposomal oxymatrine in hepatic fibrosis treatment: Formulation, in vitro and in vivo assessment. AAPS Pharmscitech 15: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang YY, Zhang Y, Xiao TT, et al. (2014) Similar to spironolactone, oxymatrine is protective in aldosterone-induced cardiomyocyte injury via inhibition of calpain and apoptosis-inducing factor signaling. PLoS ONE 9: e88856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang T, Gu J, Wu XS, et al. (2014) Effects of oxymatrine on the apoptosis and proliferation of gallbladder cancer cells. Anticancer Drugs 25:1007–1015. [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Luo J, Chen H, et al. (2013) Antiangiogenic effects of oxymatrine on pancreatic cancer by inhibition of the NF-κB-mediated VEGF signaling pathway. Oncology Reports 30: 589–595. [DOI] [PubMed] [Google Scholar]
- 35. Zhu Y, Xu G, Wei J, et al. (2014) Oxymatrine extracted from Sophora flavescens inhibited cell growth and induced apoptosis in human osteosarcoma MG-63 cells in vitro. Cell Biochemistry and Biophysics 70: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 36. Xu Y, Ji W, Liu Y, et al. (2014) Anti-tumor activities of matrine and oxymatrine: Literature review. Tumour Biology 35: 5111–5119. [DOI] [PubMed] [Google Scholar]
- 37. Zhu JS, Xu ZP, Song MQ, et al. (2011) Effect of oxymatrine combined with low dose 5-FU on lymphatic vessel and microvascular endothelial cell growth of gastric cancer in a severe combined immunodeficient mouse orthotopic implantation model. European Journal of Inflammation 9: 45–53. [Google Scholar]
- 38. Zhang J, Wang G, Chu SJ, et al. (2016) Loss of large tumor suppressor 1 promotes growth and metastasis of gastric cancer cells through upregulation of the YAP signaling. Oncotarget 7: 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du Y, Gong J, Tian X, et al. (2015) Japonicone A inhibits the growth of non-small cell lung cancer cells via mitochondria-mediated pathways. Tumour Biology 36: 7473–7482. [DOI] [PubMed] [Google Scholar]
- 40. Lundgreen A, Kadlubar SA, Slattery ML, et al. (2013) JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Molecular Carcinogenesis 52: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu D, Ji J, You Z, et al. (2012) JAK/STAT signal pathway activation promotes progression and survival of human oesophageal squamous cell carcinoma. Clinical & Translation Oncology 14: 143–149. [DOI] [PubMed] [Google Scholar]
- 42. Netchiporouk E, Litvinov IV, Moreau L, et al. (2014) Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 13: 3331–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song MQ, Wang L, Zhu JS, et al. (2006) Immunoregulation and short-term therapeutic effects of super-selective intra-arterial chemotherapy combined with traditional Chinese drugs on gastric cancer patients. Zhong xi yi jie he xue bao: Journal of Chinese Integrative Medicine 4: 478–481. [DOI] [PubMed] [Google Scholar]
- 44. Zhu JS, Chen JL, Song MQ, et al. (2007) Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cells. World Journal of Gastroenterology 13: 1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo B, Zhang T, Su J, et al. (2015) Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Cancer Chemotherapy and Pharmacology 75: 353–363. [DOI] [PubMed] [Google Scholar]
- 46. Pascutti MF, Jak M, Tromp JM, et al. (2013) IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood 122: 3010–3019. [DOI] [PubMed] [Google Scholar]