Short abstract
Background
Chronic pain is a persistent unpleasant sensation that produces pathological synaptic plasticity in the central nervous system. Both human imaging study and animal studies consistently demonstrate that the anterior cingulate cortex is a critical cortical area for nociceptive and chronic pain processing. Thus far, the mechanisms of excitatory synaptic transmission and plasticity have been well characterized in the anterior cingulate cortex for various models of chronic pain. By contrast, the potential contribution of inhibitory synaptic transmission in the anterior cingulate cortex, in models of chronic pain, is not fully understood.
Methods
Chronic inflammation was induced by complete Freund adjuvant into the adult mice left hindpaw. We performed in vitro whole-cell patch-clamp recordings from layer II/III pyramidal neurons in two to three days after the complete Freund adjuvant injection and examined if the model could cause plastic changes, including transient and tonic type A γ-aminobutyric acid (GABAA) receptor-mediated inhibitory synaptic transmission, in the anterior cingulate cortex. We analyzed miniature/spontaneous inhibitory postsynaptic currents, GABAA receptor-mediated tonic currents, and evoked inhibitory postsynaptic currents. Finally, we studied if GABAergic transmission-related proteins in the presynapse and postsynapse of the anterior cingulate cortex were altered.
Results
The complete Freund adjuvant model reduced the frequency of both miniature and spontaneous inhibitory postsynaptic currents compared with control group. By contrast, the average amplitude of these currents was not changed between two groups. Additionally, the complete Freund adjuvant model did not change GABAA receptor-mediated tonic currents nor the set of evoked inhibitory postsynaptic currents when compared with control group. Importantly, protein expression of vesicular GABA transporter was reduced within the presynpase of the anterior cingulate cortex in complete Freund adjuvant model. In contrast, the complete Freund adjuvant model did not change the protein levels of GABAA receptors subunits such as α1, α5, β2, γ2, and δ.
Conclusion
Our results suggest that the induction phase of inflammatory pain involves spontaneous GABAergic plasticity at presynaptic terminals of the anterior cingulate cortex.
Keywords: Inflammatory pain, anterior cingulate cortex, pyramidal neuron, GABAergic transmission, GABAA receptor-mediated tonic current, vesicular GABA transporter
Introduction
Human imaging studies have shown that the anterior cingulate cortex (ACC) is a critical cortical area for nociception and chronic pain.1,2 Animal studies have also investigated the functional connection between the ACC and nociception and chronic pain.3,4 In vivo electrophysiological studies of mice and rats demonstrate that peripheral nociceptive stimulation and/or injury models produce evoked action potentials or excitatory postsynaptic potentials in the ACC.5–7 So far, the mechanisms of excitatory synaptic transmission have been well characterized in the ACC.4,8–13 Various animal models of chronic pain cause excitatory synaptic plasticity in superficial layers of the ACC. For example, chronic pain induced peripheral inflammation and a nerve injury enhance glutamatergic transmitter releases in layer II/III of the ACC.10,13,14 These models also activate the postsynaptic functions of glutamatergic receptors, N-methyl-D-aspartic acid receptors, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in the ACC.3,10,11,15
The ACC is composed of excitatory and inhibitory neurons which interact to produce microcircuits within the cortex.16 GABAergic transmission in the ACC plays a critical role in regulation of nociception and chronic pain in humans and animals.17,18 Specifically, in the human study, which used optimized spectroscopic sequence for GABA detection, it was demonstrated that GABA levels in the ACC inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain.17 In animal studies, GABAergic synaptic transmission enhancement, by a muscarinic receptor agonist injected into the ACC, produces antinociceptive behaviors.19 Although a chronic inflammatory pain model (in one to three days) obviously enhanced glutamatergic transmission in the superficial layers of the ACC,13,15 whether the early stage of a major chronic inflammatory pain model could produce GABAergic synaptic plasticity is not fully understood in layer II/III of the ACC.
Here, we examined whether a major type of inflammatory pain, complete Freund adjuvant (CFA)-induced chronic pain model (two to three days), could influence inhibitory synaptic plasticity in the adult mouse ACC. Using in vitro whole-cell patch-clamp recordings from brain slices, we recorded both transient and tonic GABAA receptor-mediated currents in the layer II/III pyramidal neurons from the ACC. Transient GABAA receptor-mediated currents were examined through measuring miniature and spontaneous inhibitory postsynaptic currents (mIPSCs and sIPSCs, respectively) from pyramidal neurons in response to peripheral CFA. GABAA receptor-mediated tonic currents were also examined in response to CFA inflammatory pain. In addition, we tested if the CFA model could alter evoked IPSCs in the ACC. Lastly, using methods to separate presynaptic from postsynaptic sites, we tested whether or not GABA-related proteins such as vesicular GABA transporter (vGAT) and GABAA receptor subunits were altered following inflammatory pain.
Materials and methods
Animal, chronic pain model, and mechanical threshold
Male adult C57BL/6 mice (8–12 weeks old) were used throughout the experiment (CLEA, Hirosaki, Japan). Mice were housed at 23 ± 2°C with a 12/12-h light/dark cycle (light on at 07:00 h) and were given free access to commercial food and tap water. Experimental procedures were based on the Guidelines of the Committee for Animal Care and Use of Hirosaki University. Chronic inflammatory pain was induced with CFA, injected into the left hind paw as previously published.12,13 Two to three days after the CFA injection, we tested mechanical hypersensitivity in CFA-chronic inflammatory pain model measured by von Frey filament test.12 Two to three days after CFA injected mice were used for in vitro electrophysiology. Western blot analysis was done in two days after CFA injection.
In vitro whole-cell patch-clamp recording
Coronal brain slices (300 μm) at the level of the ACC were prepared using as previously published.9–13,20 Briefly, brain slices were transferred to a submerged recovery chamber with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid containing (in mM) 124 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 25 NaHCO3, 1 NaH2PO4, and 10 glucose at room temperature for at least 1 h. Experiments were performed in a recording chamber on the stage of an BX50WI microscope (Olympus, Tokyo, Japan) with infrared differential interference contrast optics for visualization. GABAA receptor-mediated IPSCs were recorded from layer II/III neurons of the ACC with an MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Whole-cell recordings were made using voltage-clamp mode (Vh = 0 mV) in the presence of an α-amino-3-hydroxy-5-methyl-4isoxazolyle propionic acid/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM), a N-methyl-D-aspartate receptor antagonist D (–)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 μM), a GABAB receptor antagonist CGP55845 (3 μM). The recording pipettes (3–5 MΩ) were filled with a solution containing (in mM) 120 Cs-MeSO3, 5 NaCl, 1 MgCl2, 0.5 EGTA, 2 Mg-ATP, 0.1 Na3GTP, and 10 HEPES (pH 7.3 with CsOH, 280–300 mOsmol). The [ECl] was –78.1 mV.
To record miniature IPSCs (mIPSCs), tetrodotoxin (TTX, 1 μM) was also applied in the bath solution to block sodium channels. For analyzing GABAA receptor-mediated tonic currents, bicuculline methiodide (10 μM) was applied in the bath solution.19,21,22 Bipolar stimulating electrodes were placed in layer V/VI of the ACC slices to detect evoked IPSCs (eIPSCs).12,20,23,24 The eIPSCs were induced by repetitive stimulations every 30 s, and recorded neurons were voltage clamped at 0 mV. When the firing pattern of ACC neurons was recorded, 124 K-gluconate containing internal solution was used instead of 120 Cs-MeSO3 under current clamp mode (Figure 1(C)).25 Biocytin (0.4%) was included in the pipette solution to label recorded neurons in the ACC (Figure 1(B)). The initial access resistance was 15 to 30 MΩ and was monitored throughout the experiment. Data were discarded if the access resistance changed >15% during experiment. Data were filtered at 2 kHz and digitized at 10 kHz.
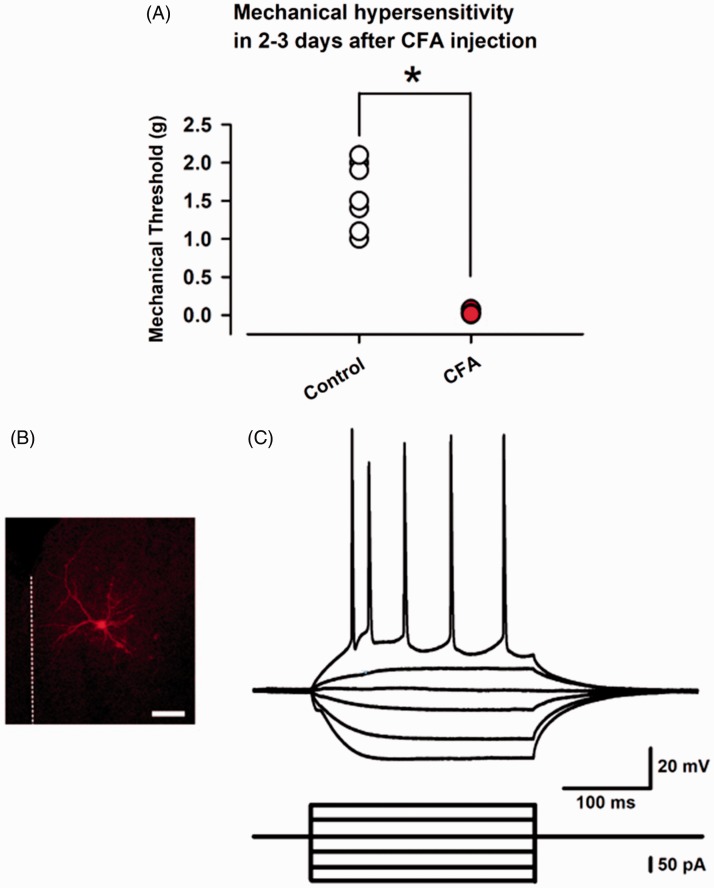
Figure 1.
Two to three days after CFA injection induced mechanical hypersensitivity and whole-cell patch-clamp recording from a layer II/III pyramidal neuron in the ACC. (A) CFA injected into the left hind paw in adult mice clearly produced mechanical hypersensitivity two to three days after the injection. (B) The layer II/III pyramidal neurons in the ACC were recorded in this study. Scale bar = 100 μm. (C) Sample action potential firing pattern of a pyramidal neuron in current-clamp mode by current injection (400 ms, every 15 s). Results are expressed as the mean ± SEM. Asterisks shows P< 0.05.
CFA: complete Freund adjuvant.
Histology and immunohistochemistry
After electrophysiological recordings, slices containing biocytin-filled ACC neurons were fixed overnight in a cold solution containing 4% paraformaldehyde. The slices were washed three times (10 min each time) in 0.01 M phosphate buffered saline (PBS). Next, the slices were applied by PBS containing 30% ethanol for 30 min and then washed by PBS three times. Slices were then incubated in PBS with 1% Triton X-100 and AlexaFluor555-labeled streptavidin (1:500) during overnight at 4°C. The following day, slices were washed in PBS and mounted on glass slides.
Confocal microscopy
Labeled neurons were imaged by a confocal microscope (LSM510 META ZEISS). Optical sections, usually at consecutive intervals of 1 to 2 μm, were imaged through the depth of the labeled neurons and saved as image stacks. Collapsing this stack using z projection on the confocal software onto a single plane generated a two-dimensional reconstruction of the labeled neuron. The image stack was also reconstructed in three dimensional with appropriate software, to define areas of interest in the neuron. Although the effects of laser illumination on fixed tissue are not known, to prevent possible ultrastructural damage we tried to minimize both the scanning time and the laser intensity.
Separation of presynaptic, postsynaptic, and extrasynaptic membrane fraction
Mice were deeply anaesthetized with medetomidine-midazolam-butorphanol combination and transcardially perfused with cold PBS. Brains were quickly removed and cut to 0.5-mm-thick coronal slice with brain matrix. The ACC were punched out from the coronal slice and stored at –80°C for further processing. The pre-, post-, or extrasynaptic membrane was prepared using a modified version of a method.26–28 This modified method makes it possible to extract protein from small amount of tissue (about 15–20 μg of protein of presynaptic membrane from 20 mg tissue). All steps in this method were carried out at 4°C or on crushed-ice. The ACC from five to six mice were combined into one sample to prepare fractionation. The tissue was homogenized in 200 μL of cold synaptosome preparation (SP) buffer at pH 6.0 (SP buffer, 0.32 M sucrose, 20 mM Tris-HCl, 0.1 mM CaCl2, 1 mM MgCl2, and cOmplete™ Protease Inhibitor Cocktail EDTA-free, Roche) using an overhead stirrer with tapered tissue grinder and teflon pestle (WHEATON, #358133) at 1000 r/min for 20 strokes. Further homogenization was performed with Dounce Tissue Grinder (WHEATON, #357538) with a tight pestle for 100 strokes. The homogenate was centrifuged at 1000×g for 10 min to remove nucleus and unhomogenized cells. The pellet (P1) was re-suspended and saved as nuclear (with a little bit contamination of unhomogenized cells). The supernatant (S1) was further centrifuged at 10,000×g for 15 min to obtain a crude membrane fraction. After centrifugation of S1, the supernatant (S2) was centrifuged at 100,000×g for 60 min to obtain cytosolic fraction and intracellular light membranes (ILM). The supernatant was recovered as a cytosolic fraction, and the pellet was lysed. After centrifugation of S1, the pellet (P2) was lysed in extrasynaptic membrane dissociation buffer at pH 6.0 (20 mM Tris-HCl, 0.5% TritonX-100, 0.1 mM CaCl2, 1 mM MgCl2, and cOmplete™ Protease Inhibitor Cocktail EDTA-free) and incubated for 30 min with rotating. After incubation, the homogenates were centrifuged at 32,000×g for 30 min to obtain synaptosome fraction and extrasynaptic membrane fraction. The supernatant was saved as extrasynaptic membrane, and the pellet (P3) was used for further fractionation. P3 was dissolved in synaptosome dissociation buffer at pH 8.0 (20 mM Tris-HCl, 0.5% TritonX-100, 1 mM EGTA, 1 mM EDTA, and cOmplete™ Protease Inhibitor Cocktail EDTA+) and incubated for 30 min while rotating. After incubation, the homogenates were centrifuged at 32,000×g for 30 min, and the supernatant was saved as the presynaptic membrane fraction while the pellet was saved as a postsynaptic membrane. The final pellets of each fraction were resuspended with SP buffer containing 1% sodium dodecyl sulfate and sonicated. To reduce contamination of each fraction, supernatants were centrifuged for three times, and the pellets were washed or rinsed with SP buffer (P1 and P2) or synaptosome dissociation buffer (P3). Protein concentrations were determined using Bradford protein assay (Bio-Rad).
Western blot
Western blotting was performed as previously described.29 Immunocomplex were visualized using ImmunoStar® LD (Wako, Tokyo) or Clarity™ Western ECL substrate (Bio-Rad) and ImageQuant LAS 4000 mini system (GE Healthcare). The membranes were stripped with Restore PLUS Western Blot Stripping Buffer (Thermo Scientific) and re-probed with another primary antibody. The antibodies used in this study were described in Table. 1. The expression levels of each protein were normalized to fraction markers.
Table 1.
The list of antibodies used in this study.
| Antibody | Supplier |
|---|---|
| vGAT | Alomone labs (AGT-005) |
| GABAAR-α1 | Alomone labs (AGA-001) |
| GABAAR-α5 | MERCK MILLIPORE (AB9678) |
| GABAAR-β2 | Abcam (ab156000) |
| GABAAR-γ2 | Aviva Systems Biology (OAEB01374) |
| GABAAR-δ | Alomone labs (AGA-014) |
| Synapsin I | Calbiochem (574777) |
| PSD-95 | Thermo Scientific (7E3-1B8) |
| Gephyrin | Synaptic Systems (147 111) |
| Calnexin | Enzo Life Sciences (ADI-SPA-860) |
| Goat anti-rabbit IgG-HRP | Dako (P0448) |
| Rabbit anti-mouse IgG-HRP | Dako (P0260) |
vGAT: vesicular GABA transporter; GABA: γ-aminobutyric acid; PSD-95: postsynaptic density protein-95; IgG: Immunoglobulin G; HRP: horseradish peroxidase.
Statistics
Data are expressed as means ± SEM. Statistical analyses were done with Sigmaplot (12.0) software. Results were analyzed for significance with analysis of variance with post-hoc tests. The differences of behavioral, electrophysiological, and Western blotting data among the groups were analyzed by one way repeated-measures analysis of variance, followed by the Tukey–Kramer test for multiple comparisons or Student’s t test for two comparisons. A value of P < 0.05 was taken to indicate a statistically significant difference. For densitometric analysis of Western blotting, t test was used for statistical comparison.
Results
CFA-induced inflammatory model decreased sIPSCs in the ACC
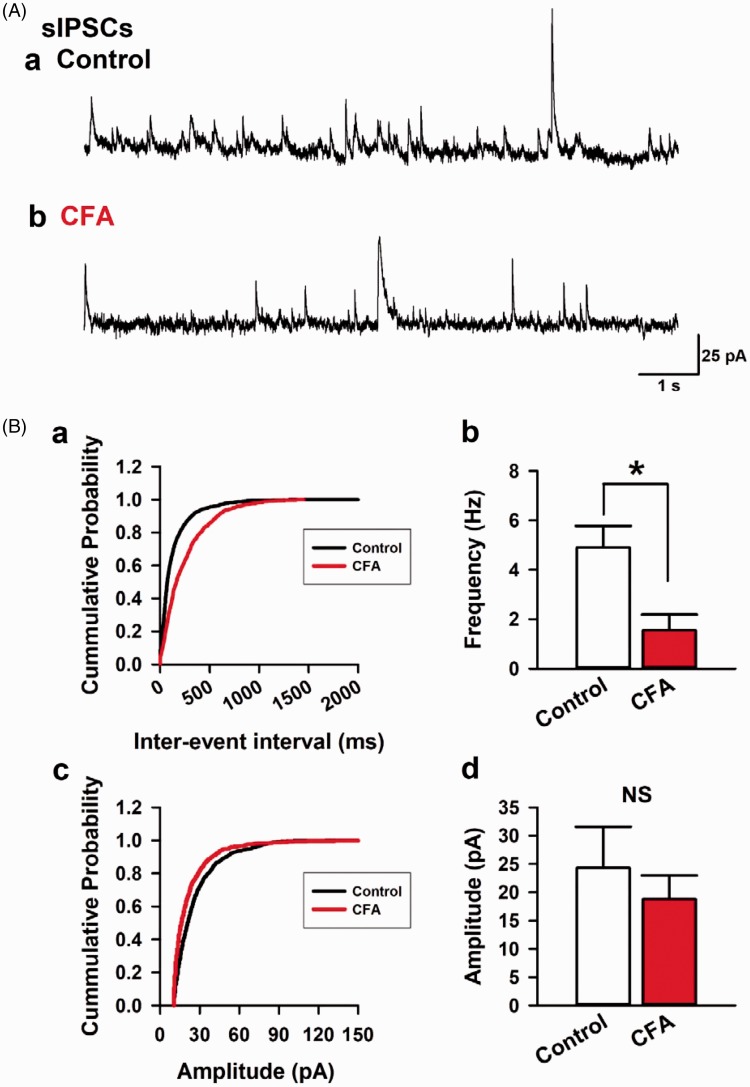
We induced a long-lasting inflammatory pain by injecting CFA into the left hind paw in mice. Two to three days after the CFA or saline injection (as control), we confirmed that CFA produced mechanical hypersensitivity (1.600 ± 0.164 g, n=8, control, and 0.038 ± 0.008 g, n=8, CFA group, P < 0.05; Figure 1(B)). After the assessment of the mechanical hypersensitivity, the animal was sacrificed, and coronal brain slices from the ACC were extracted. We performed whole-cell patch-clamp recordings from layer II/III pyramidal neurons and recorded sIPSCs under voltage-clamp mode at 0 mV. Pyramidal neurons were selected, according to the morphological and electrophysiological properties from layer II/III of the ACC25 (Figure 1(B) and (C)). Current injection into pyramidal neurons elicited a regular spiking firing pattern which is typical of the ACC.25 After a stable recording was obtained, these neurons were electrophysiologically characterized and simultaneously injected with biocytin for histochemical processing to confirm the recording neurons and area (Figure 1(C)). 6-cyano-7-nitroquinoxaline-2,3-dione, a α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/KA receptor antagonist, AP-V, a N-methyl-D-aspartic acid receptor antagonist and CGP35845, and a GABAB receptor antagonist were perfused to isolate GABAA receptor currents (Figure 2(A)). We recorded seven neurons from six control animals and found the average frequency and amplitude of sIPSCs was 4.9 ± 0.7 Hz and 24.3 ± 7.3 pA, respectively (Figure 2(A, a) and (B)). In contrast, in five neurons recorded from four animals of the CFA injected group, the average frequency and amplitude of sIPSCs was 1.6 ± 0.6 Hz and 18.8 ± 4.2 pA, respectively (Figure 2(A, b) and (b)). Statistical analysis revealed that the CFA model reduced the average frequency of sIPSCs compared with control group (P < 0.05; Figure 2(B, b)). In contrast, the average amplitudes of sIPSCs were not altered between two groups (P > 0.05; Figure 2(B, d)). These results suggest that the peripheral and acute CFA induces a reduction in spontaneous synaptic transmitter release of GABA in the ACC.
Figure 2.
Chronic inflammation model mice reduced the frequency of sIPSCs in the ACC. (A) Recorded sIPSCs holding potential at 0 mV in a layer II/III pyramidal neuron from control (a) or CFA group (b). (B) The cumulative probability in inter-event intervals between control and CFA group (a). CFA group reduced the average frequency of sIPSCs compared with control group (b). The cumulative probability in amplitude between control and CFA group (c). There was no difference in average amplitude of sIPSCs between control and CFA group (d). Results are expressed as the mean ± SEM. Asterisks shows P< 0.05.
sIPSCs: spontaneous inhibitory postsynaptic currents; CFA: complete Freund adjuvant; NS: not significant.
CFA model reduced the frequency of mIPSCs in the ACC
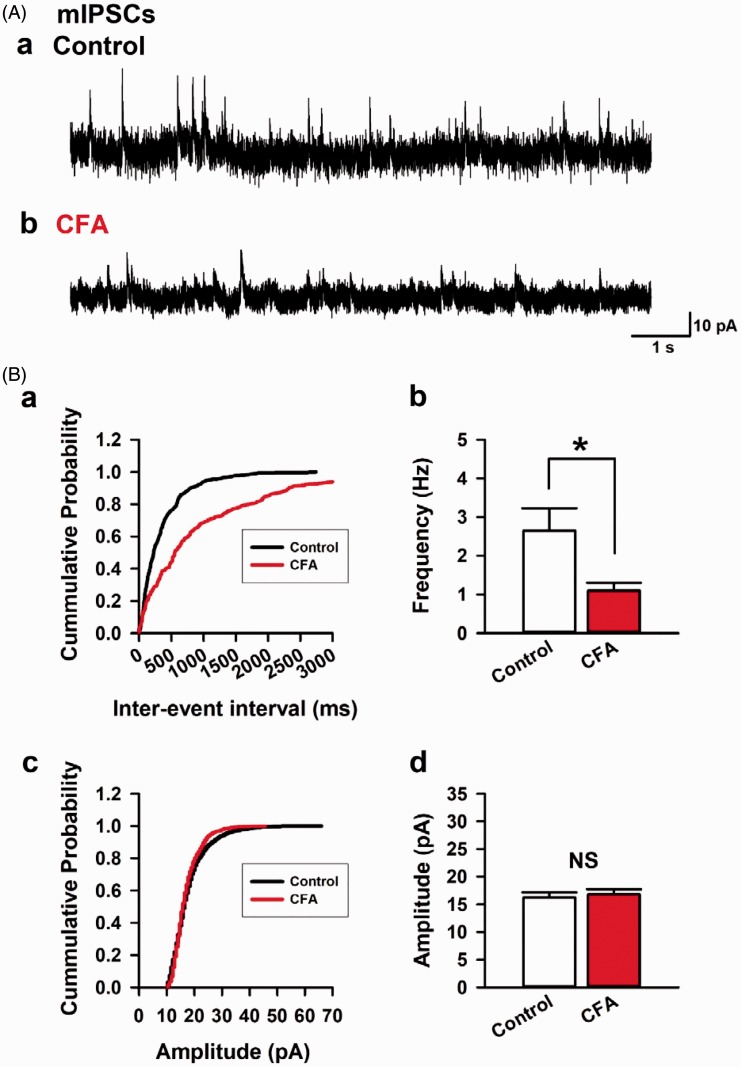
We next recorded mIPSCs in the layer II/III pyramidal neurons from the ACC in the presence of (TTX, 1 μM) by blocking sodium channels mediated transmitter release (Figure 3). We compared the electrophysiological property of mIPSCs in both control and CFA group (Figure 3). We recorded 11 neurons from 8 control mice and 11 neurons from 7 animals of the CFA group. In the CFA group, there was a significant reduction in the average frequency of mIPSCs compared with control group (2.6 ± 0.6 Hz, control and 1.1 ± 0.2 Hz, CFA group, P < 0.05; Figure 3(B, a–b)). In contrast, the average amplitudes of mIPSCs were not changed between two groups (16.3 ± 0.9 Hz, control and 16.8 ± 0.9 Hz, CFA group, P > 0.05; Figure 3(B, c–d)). These results suggest that CFA model reduces miniature synaptic transmitter release of GABA in layer II/III pyramidal neurons of the ACC.
Figure 3.
Chronic inflammation model mice reduced the frequency of mIPSCs in the ACC. (A) Recorded mIPSCs holding potential at 0 mV in a layer II/III pyramidal neuron from control (a) or CFA model in the presence of TTX (1 μM) (b). (B) The cumulative probability in inter-event intervals between control and CFA group (a). CFA group reduced the average frequency of mIPSCs compared with control group (b). The cumulative probability in amplitude between control and CFA group (c). There was no difference in average amplitudes of mIPSCs between control and CFA group (d). Results are expressed as the mean ± SEM. Asterisks shows P< 0.05.
mIPSCs: miniature inhibitory postsynaptic currents; CFA: complete Freund adjuvant; NS: not significant.
CFA model did not alter GABAA receptor-mediated tonic currents
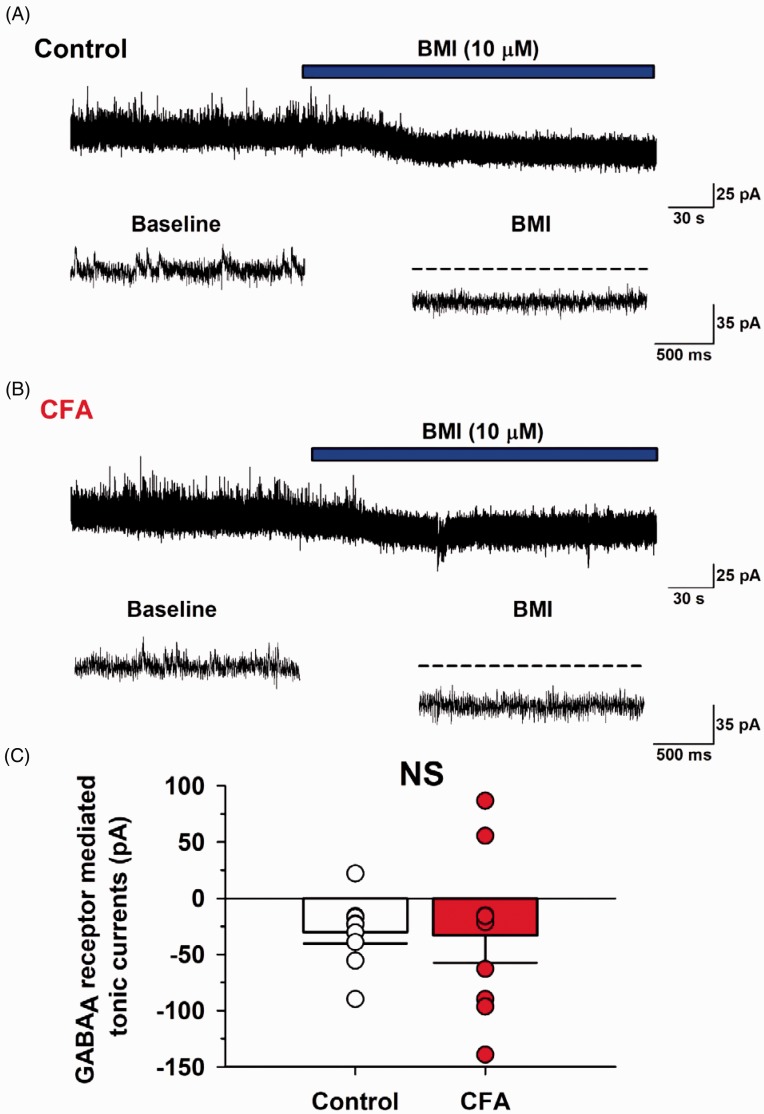
Next, we tested if CFA model could affect extrasynaptic GABAA receptor-mediated currents in the ACC (Figure 4). First, we recorded mIPSCs in the presence of TTX (1 μM) and then applied bicuculline methiodide (10 μM) in the bath solution to analyze GABAA receptor-mediated tonic currents.19,21,22 In the control group, the neurons produced tonic currents (average currents: –30.2 ± 10.2 pA, n=9/6, control; Figure 4(A)). In the CFA group, the average GABAA receptor-mediated tonic current was –33.2 ± 24.2 pA (n=9/6, CFA group; Figure 4(B)). Statistical analysis revealed that the CFA group did not differ from the control group when GABAA receptor-mediated tonic currents were compared (P > 0.05; Figure 4(C)). These results indicate that CFA injection does not result in extrasynaptic GABAA receptor activation in the ACC.
Figure 4.
CFA model did not alter GABAA receptor-mediated tonic currents in the ACC. (A) A recorded mIPSCs holding potential at 0 mV in a layer II/III pyramidal neuron from the control group. Bicuculline methiodide (BMI, 10 μM) into the bath solution produced GABAA receptor-mediated tonic currents. (B) In CFA group, BMI also produced the tonic currents. (C) There were no difference in GABAA receptor-mediated tonic currents between control and CFA group. Results are expressed as the mean ± SEM.
CFA: complete Freund adjuvant; GABA: γ-aminobutyric acid; BMI: body mass index; NS: not significant.
CFA model did not change the electrophysiological property of evoked inhibitory synaptic transmission
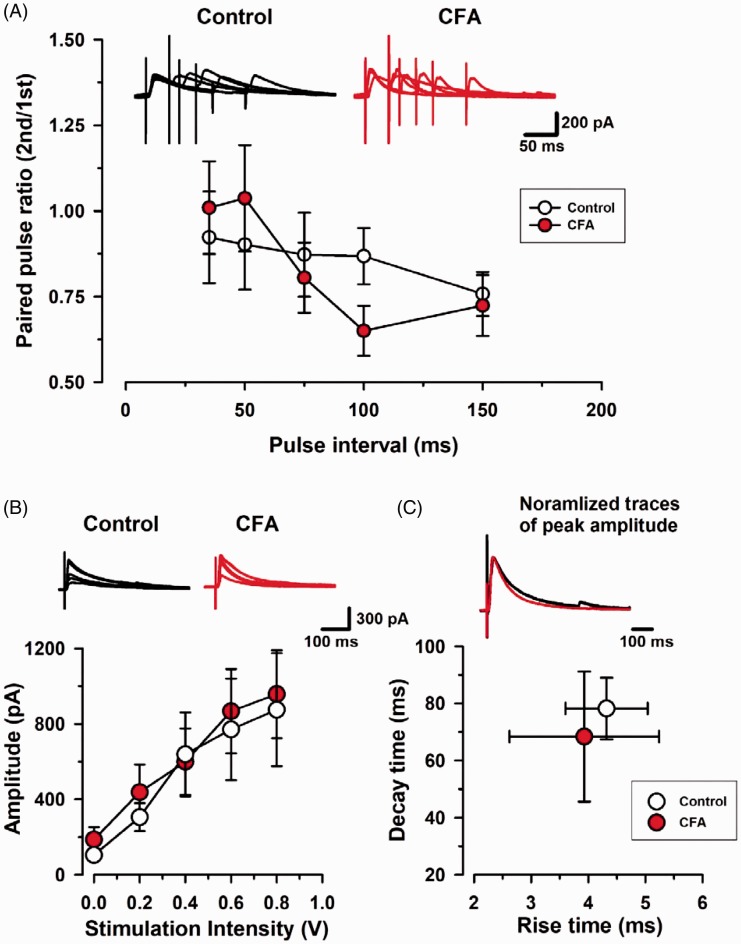
We tested if two to three days after CFA injection could alter evoked IPSCs (eIPSCs) including paired-pulse ratio, input-output, and kinetics delivered by the stimulation electrode in layer V/VI (Figure 5). Paired-pulse ratio among 35, 50, 75, 100, and 150 ms interval were not changed between control and CFA group (n=5, control; n=5, CFA group, P= 0.841; Figure 5(A)). The input–output relationship of neurons for the stimulation intensity, ranging from 0.0 to 1.0 V, was not altered between these groups (n=5, control; n=5, CFA group, P= 0.735; Figure 5(B)). We further analyzed the kinetics of eIPSCs in control and CFA groups. The averaged rise time and decay time of normalized eIPSCs were similar between control and CFA groups (n=9, control; n=9, CFA group, P= 0.657 in rise time, P= 0.596 in decay time; Figure 5(C)). These results suggest that the early stage of peripheral inflammation may not alter evoked inhibitory synaptic transmission in layer II/III of the ACC.
Figure 5.
Paired-pulse ratio (PPR) in mice with CFA injection. (A) (Upper) Representative traces of PPR with an interval of 35, 50, 75, 100, and 150 ms recorded in the ACC. (Bottom) PPR was not changed at each interval in CFA group. White circles from saline control mice; Red circles from CFA group. (B) Potentiation of input–output relationship in CFA group. (Upper) Representative traces show averages of six IPSCs at 0.0 and 1.0 V stimulation intensity in the ACC. (Bottom) Plots of input–output curves show no significant enhancement of evoked inhibitory synaptic transmission in the ACC of CFA group. White circles from control group; Red circles from CFA group. (C) (Upper) Scaled traces showing the similar kinetics for control and CFA group. (Bottom) Statistical results for the rise time and decay time constant of control and CFA group. Results are expressed as the mean ± SEM.
CFA: complete Freund adjuvant.
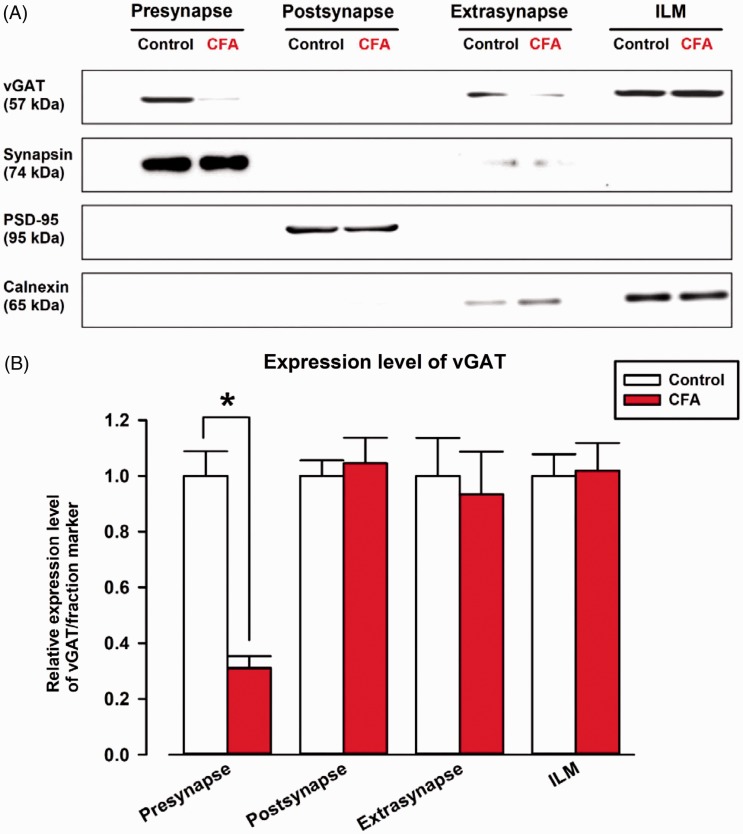
CFA reduced the protein expression of vGAT but not GABAA receptor subunits in the ACC
Since CFA-induced inflammation changed the GABAA receptor-mediated synaptic transmission in the ACC, we tested if CFA could alter the expression levels of proteins related to GABAergic synaptic transmission such as GABA-releasing protein and GABAA receptor subunits in the ACC (Figures 6 and 7). We focused on the protein expressions of vGAT because vGAT plays a critical role in storing GABA transmitter in the synaptic vesicles on GABAergic synaptic terminals.30,31 Thus, we studied whether CFA could alter the protein expression of vGAT in the ACC (Figure 6). Notably, CFA reduced the protein expression of vGAT in the presynaptic membrane fraction (0.31 ± 0.04 of control, P < 0.05; Figure 6(B)). The vGAT also exists in the extrasynaptic fraction and ILMs; however, no difference was found between control and CFA groups (Extrasynaptic fraction: 0.93 ± 0.15 of control group; ILM: 1.02 ± 0.10 of control, P > 0.05; Figure 6(B)). These results indicate that the fusion of GABAergic synaptic vesicle to presynaptic membrane was disrupted.
Figure 6.
The protein expression of vGAT at presynaptic sites was reduced in the ACC by CFA. (A) The protein expressions of vGAT were measured by Western blotting. Synapsin I, PSD-95, and calnexin were used as fraction markers for presynaptic, postsynaptic, extrasynaptic, and ILM, locations respectively. (B) Comparison of relative expression of vGAT between control and CFA model in each fraction. vGAT expression was normalized to fraction markers expression and is represented as relative expression. vGAT in presynaptic fraction was decreased in CFA mice. vGAT in postsynaptic, extrasynaptic fraction, and ILM did not change between control and CFA group (CFA group; postsynaptic fraction: 1.05 ± 0.09 of control group, extrasynaptic fraction: 0.93 ± 0.15 of control group, ILM: 1.02 ± 0.10 of control group). Three independent experiments were performed, and the results are expressed as the mean ± SEM. Asterisks indicate statistically significant differences from the control (P< 0.05).
CFA: complete Freund adjuvant; ILM: intracellular light membrane; vGAT: vesicular GABA transporter; PSD: postsynaptic density protein-95.
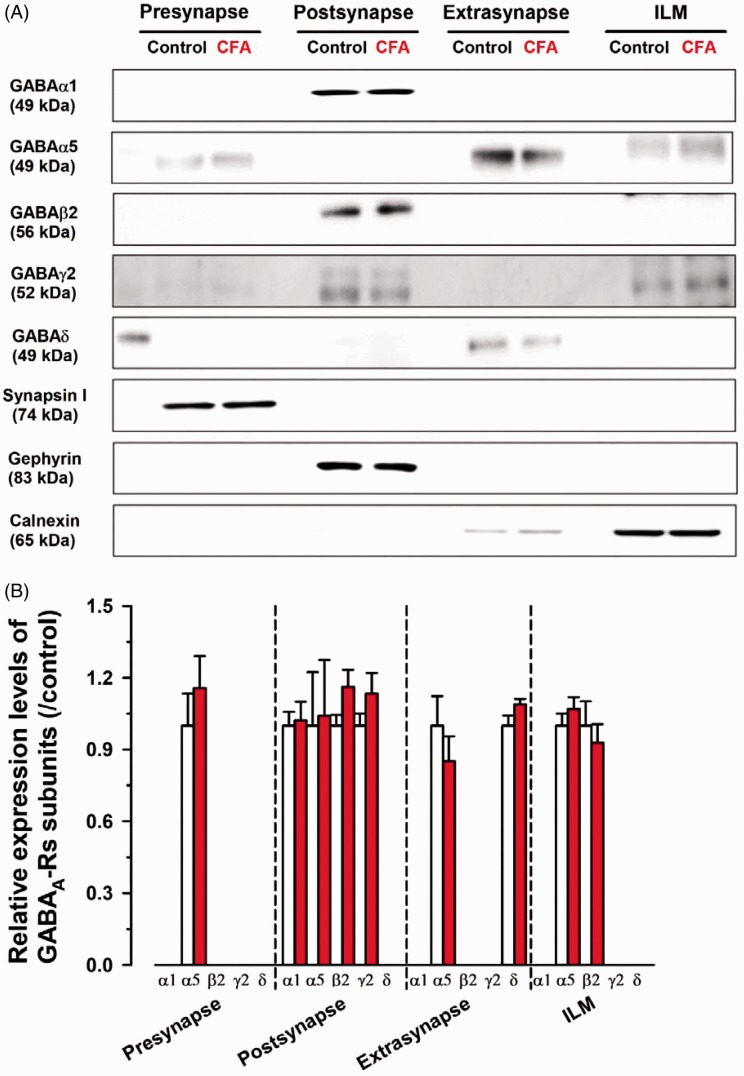
Figure 7.
The protein expression of GABAA receptor subunits was not altered in the ACC. (A) Western blotting analysis of GABAA receptor subunit expression. Synapsin I, gephyrin, and calnexin were used as presynaptic, postsynaptic (GABAergic), extrasynaptic, and ILM fraction markers, respectively. (B) Comparison of relative expression of GABAA receptor subunits between control and CFA in all fractions. In the presynaptic fraction of CFA group, the relative value of α5 was 1.15 ± 0.13 of control group. In the postsynaptic fraction of CFA group, the relative values of α1, α5, β2, and γ2 were 1.02 ± 0.08, 1.04 ± 0.23, 1.16 ± 0.07, and 1.13 ± 0.09 of control, respectively. In the extrasynaptic fraction of CFA group, the relative values of α5 and δ were 0.85 ± 0.10 and 1.10 ± 0.02 of control group. In ILM of CFA group, the relative values of α5 and β2 were 1.07 ± 0.05 and 0.93 ± 0.08 of control group. Three independent experiments were performed, and the results are expressed as the mean ± SEM.
CFA: complete Freund adjuvant; ILM: intracellular light membrane; GABA: γ-aminobutyric acid.
Next, we tested whether CFA could affect the protein expression of GABAA receptor subunits (Figure 7(A)). Importantly, GABAA receptor subunits including α1, α5, β2, γ2, and δ were not changed between CFA and control group in all fractions (P > 0.05; Figure 7(B)). These results suggest that two to three days after chronic inflammatory induction can alter presynaptic vesicular GABAergic transporter without altering GABAA receptor subunits in the ACC.
Discussion
In this study, we studied whether the induction phase of long-lasting inflammatory pain could alter GABAergic transmissions in the adult mice ACC. A major type of chronic inflammatory pain, induced by CFA, reduced the frequencies of both sIPSCs and mIPSCs in layer II/III pyramidal neurons, suggesting that the CFA model decreased the transmitter release probability of GABA in the ACC. This is consistent with the Western blot analysis in which protein expression of vGAT was reduced in CFA group. By contrast, GABAA receptor-mediated tonic currents were not altered between the two groups and GABAA receptor subunits including α1, α5, β2, γ2, and δ did not change between CFA and control group. Collectively, the results indicate that presynaptic but not postsynaptic changes occur in the GABA system during early phases in this chronic pain model.
CFA model reduced spontaneous GABAergic transmitter release in the layer II/III pyramidal neurons in the ACC
In this study, we used CFA-induced inflammatory pain model because this model is one of major types to show long-lasting chronic pain.32 In general, the CFA model stability starts to represent severe hypersensitivity to mechanical and thermal stimulation from one to three days.12,13,15,32 We predict that the induction phase lays a neural foundation (observed as plastic changes) for the chronic pain experienced over weeks and years. Accordingly, the early phase of this chronic pain model could cause synaptic plasticity of inhibitory transmissions within the ACC. Our results show that superficial layer pyramidal neurons from the ACC decreased the neurotransmitter release of GABA following the early stage of chronic pain induction. This result is corroborated by our finding that there was a coincident change in inhibitory currents and protein expression of vGAT on the presynaptic membrane. By contrast, the amplitude of mIPSCs and sIPSCs did not change between control and CFA groups. Moreover, the protein levels of GABAA receptor subunits in postsynaptic and extrasynaptic membrane fractions were not changed between control and CFA groups. These results suggest that the early stage of this chronic inflammatory pain model mainly influences the presynaptic function in GABA neurons, but not postsynaptic function, resulting in a reduction of vesicular GABA release in layer II/III pyramidal neurons of the ACC.
It has been previously reported that GABAergic transmission is altered in deep layers of the ACC following the induction of chronic pain.18 Blom et al.18 reported that chronic constriction injury of the sciatic nerve (7–14 days after the surgery) altered GABAergic transmissions in layer V pyramidal neurons from the ACC. Consistent with our findings, nerve injury also reduced the release of GABA in the deep layer of the ACC without affecting the GABAA receptor-mediated function. We extended this research by showing that GABAA receptor-mediated tonic currents were unaltered in the ACC in response to CFA-induced inflammatory pain. Consistently, the CFA model may not change the functional roles of GABAA receptors and protein expressions of subsynaptic and extrasynaptic GABAA receptors.
CFA model did not alter evoked inhibitory synaptic transmission in the ACC
CFA model reduced the frequency of miniature and spontaneous inhibitory synaptic transmissions. However, evoked inhibitory synaptic transmission, including paired-pulse ratio, and the input–output relationships were not changed in CFA group. The differences between spontaneous and evoked neurotransmissions in the ACC are still unknown. However, one possibility might be that spontaneous and evoked neurotransmissions have different transmitter release mechanisms. For example, spontaneous and evoked fusion events may be mediated by separate pools of vesicles within the same synapse.33 Alternatively, some synapses may have a strong propensity for spontaneous fusion, whereas other synapses may preferentially release neurotransmitter in response to action potentials.33 So the early stage of chronic inflammation might alter spontaneous but not evoked inhibitory transmissions in the ACC. Further studies are needed to reveal the different mechanisms between spontaneous and evoked inhibitory synaptic transmission in the ACC.
A possible mechanism to reduce spontaneous GABA release in CFA model
Our electrophysiological analysis demonstrated that CFA reduces the release of GABA from terminals in layer II/III onto pyramidal neurons of the ACC. Since vGAT is expressed in GABA neurons in cortex and plays an important role in the storage of GABA at the GABAergic synaptic terminal,30,31,34 we examined whether it might be influenced by chronic pain. Our Western blot analysis demonstrates that chronic inflammatory reduced the protein expression of vGAT in the ACC. This finding is consistent with a previous study which genetically manipulated vGAT in mice.35 In that study, heterozygous vGAT knockout enhanced formalin-induced inflammatory nociceptive behaviors. Taken together, the CFA model may reduce transmitter release of GABA by decreasing the expressions of presynaptic vGAT in the ACC.
The question remains as to how GABAergic synaptic transmitter release was decreased in the ACC following CFA-induced inflammatory pain. However, one possibility might be that enhanced glutamatergic transmission or glutamatergic transmitter release induced by chronic pain condition might influence on GABAergic transmissions in the ACC. So far, various animal models of chronic pain including CFA, nerve injury, cancer pain, and visceral pain produced synaptic plasticity on glutamatergic neurons in the ACC.12,14,36–38 Chronic pain facilitated glutamatergic transmitter release in the layer II/III pyramidal neurons from the ACC.10,13,14,36 Glutamatergic GluK receptors are located on presynaptic terminals and the postsynaptic membrane in the ACC.20,39–41 It is interesting that GluK1 receptors are expressed at the GABAergic axon terminals in layer II/III of the ACC20,40 since activating the GluK1 receptors have biphasic effects on the inhibitory transmissions in a dose-dependent manner in the ACC.20 At a low dose of the GluK1 agonist ATPA, GABAergic transmission is increased, while at a high dose GABAergic transmission in the layer II/III of the ACC is decreased.20 Therefore, it might be possible that persistent pain facilitates the release of glutamate in the ACC, and the enhanced release of glutamate could act as a switch to downregulate GABAergic transmission via presynaptic GluK1 receptors. We will need further study to reveal the molecular mechanisms in detail.
Functional roles of GABAergic transmission in the ACC
At the cortical network level, layer II/III pyramidal neurons project to layer V output neurons in the ACC. Animal models of chronic pain facilitate excitatory transmission in layer II/III.4,12,42 In this study, we found that the CFA model reduced spontaneous GABAergic transmissions in layer II/III. These abnormal balances of the enhanced excitatory transmission and loss of GABAergic inhibition in layer II/III affect layer V pyramidal neurons. Indeed, in the nerve injury model, pyramidal neurons are facilitated in layer V which project to spinal cord indirectly and directly.43,44 The facilitation of layer V pyramidal neurons can enhance behavioral sensitization.
The functional roles of GABAergic transmission in the ACC have been reported for nociceptive behaviors.19 Blocking GABAA receptors with bicuculline infused into the ACC showed enhanced nociceptive behaviors.19 On the other hand, stimulating GABAA receptor-mediated synaptic transmission by muscarinic M1 receptor agonist into the ACC produces antinociceptive effect.19 Importantly, Kang et al.45 reported that selective activations of parvalbumin GABAergic interneurons in the ACC by optogenetic stimulation can reduce the CFA-induced mechanical hypersensitivity. Therefore, regulations of GABAergic transmissions in the ACC can impact nociceptive responses to mechanical stimulation for acute and chronic pain. Since GABAergic transmissions cause synaptic plasticity in the induction phase of chronic inflammatory pain within the ACC, rescuing the GABAergic plasticity in the ACC may be a target to alleviate the maintenance stage of chronic pain.4,42
Acknowledgments
The authors thank Dr. H Steenland (NeuroTek Innovative Technology) for critical comments and editing the manuscript.
Acknowledgments
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by JSPS KAKENHI Grant Number JP17H05074 and JP17K19879 to KK, and JP26460388 to SU. KK and SS were supported by Hirosaki University Grant for exploratory research by young scientists and newly appointed scientists. A part of this research was supported by Karouji memorial, CREST, The Nakatomi Foundation, The Kato Memorial Bioscience Foundation, The Novartis Foundation, Brain Science Foundation, and GSK Japan Research Grant 2016 (to KK).
Author Contributions
KK and SS designed the experiments and wrote the draft of the manuscript. KK, AY, and TF performed electrophysiological analysis. KK and SS performed behavioral analysis. YN, HF, KN, and SU participated in experimental conception and design and edited the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
References
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Euro J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 2.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 5.Wu MF, Pang ZP, Zhuo M, Xu ZC. Prolonged membrane potential depolarization in cingulate pyramidal cells after digit amputation in adult rats. Mol Pain 2005; 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga K, Li X, Chen T, Steenland H, Descalzi G, Zhuo M. In vivo whole-cell patch-clamp recording of sensory synaptic responses of cingulate pyramidal neurons to noxious mechanical stimuli in adult mice. Mol Pain 2010; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei F and, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol 2001; 532: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 9.Zhao MG, Toyoda H, Lee Y, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005; 47: 859–872. [DOI] [PubMed] [Google Scholar]
- 10.Xu HM, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 2008; 28: 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 12.Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao MG, Kaang BK, Collingridge GL, Zhuo M. Co-existence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, Li J, Jia Y, Ren M, Xu ZC, Zhuo M. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 2006; 26: 8923–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyoda H, Zhao MG, Zhuo M. Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol Pain 2009; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 2005; 25: 11107–11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth A. Principles of neural science. 5th ed. New York: McGraw-Hill Professional, 2012. [Google Scholar]
- 17.Reckziegel D, Raschke F, Cottam WJ, Auer DP. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol Pain 2016; 12: 1744806916650690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom SM, Pfister JP, Santello M, Senn W, Nevian T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci 2014; 34: 5754–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga K, Matsuzaki Y, Honda K, Eto F, Furukawa T, Migita K, Irie K, Mishima K, Ueno S. Activations of muscarinic M1 receptors in the anterior cingulate cortex contribute to the antinociceptive effect via GABAergic transmission. Mol Pain 2017; 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu LJ, Xu H, Ren M, Zhuo M. Genetic and pharmacological studies of GluR5 modulation of inhibitory synaptic transmission in the anterior cingulate cortex of adult mice. Dev Neurobiol 2007; 67: 146–157. [DOI] [PubMed] [Google Scholar]
- 21.Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex 2007; 17: 1782–1787. [DOI] [PubMed] [Google Scholar]
- 22.Migita K, Tomiyama M, Yamada J, Fukuzawa M, Kanematsu T, Hirata M, Ueno S. Phenotypes of pain behavior in phospholipase C-related but catalytically inactive protein type 1 knockout mice. Mol Pain 2011; 7: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koga K, Liu MG, Qiu S, Song Q, O’Den G, Chen T, Zhuo M. Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knockout mice. J Neurosci 2015; 35: 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koga K, Yao I, Setou M, Zhuo M. SCRAPPER selectively contributes to spontaneous release and presynaptic long-term potentiation in the anterior cingulate cortex. J Neurosci 2017; 37: 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao XY, Xu H, Wu LJ, Li XY, Chen T, Zhuo M. Characterization of intrinsic properties of cingulate pyramidal neurons in control and neuropathic pain adult mice. Mol Pain 2009; 5: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcao P, Oliveira CR, Cunha RA, Agostinho P. Subsynaptic localization of nicotinic acetylcholine receptor subunits: a comparative study in the mouse and rat striatum. Neurosci Lett 2014; 566: 106–110. [DOI] [PubMed] [Google Scholar]
- 27.Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron 2001; 32: 63–77. [DOI] [PubMed] [Google Scholar]
- 28.Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 2009; 158: 1446–1459. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa T, Shimoyama S, Miki Y, Nikaido Y, Koga K, Nakamura K, Wakabayashi K, Ueno S. Chronic diazepam administration increases the expression of Lcn2 in the CNS. Pharmacol Res Perspect 2017; 5: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature 1997; 389: 870–876. [DOI] [PubMed] [Google Scholar]
- 31.Aubrey KR. Presynaptic control of inhibitory neurotransmitter content in VIAAT containing synaptic vesicles. Neurochem Int 2016; 98: 94–102. [DOI] [PubMed] [Google Scholar]
- 32.Wall PD, Melzack R. Textbook of pain. New York: Elsevier Academic Press, 2006. [Google Scholar]
- 33.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 2015; 16: 5–16. [DOI] [PubMed] [Google Scholar]
- 34.Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y, Kawaguchi Y. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex 2008; 18: 315–330. [DOI] [PubMed] [Google Scholar]
- 35.Yamada MH, Nishikawa K, Kubo K, Yanagawa Y, Saito S. Impaired glycinergic synaptic transmission and enhanced inflammatory pain in mice with reduced expression of vesicular GABA transporter (VGAT). Mol Pharmacol 2012; 81: 610–619. [DOI] [PubMed] [Google Scholar]
- 36.Liu SB, Zhang MM, Cheng LF, Shi J, Lu JS, Zhuo M. Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain. Mol Brain 2015; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang WB, Yang Q, Guo YY, Wang L, Wang DS, Cheng Q, Li XM, Tang J, Zhao JN, Liu G, Zhuo M, Zhao MG. Analgesic effects of adenylyl cyclase inhibitor NB001 on bone cancer pain in a mouse model. Mol Pain 2016; 12: 1744806916652409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu LJ, Steenland HW, Kim SS, Isiegas C, Abel T, Kaang BK, Zhuo M. Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Mol Pain 2008; 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI, Ren M, Knifed E, Shum F, Quan J, Zhang XH, Zhuo M. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS One 2007; 3: e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu LJ, Zhao MG, Toyoda H, Ko SW, Zhuo M. Kainate receptor-mediated synaptic transmission in the adult anterior cingulate cortex. J Neurophysiol 2005; 94: 1805–1813. [DOI] [PubMed] [Google Scholar]
- 41.Wu LJ, Kim SS, Zhuo M. Molecular targets of anxiety: from membrane to nucleus. Neurochem Res 2008; 33: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 42.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci 2013; 369: 20130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen T, Koga K, Descalzi G, Qiu S, Wang J, Zhang LS, Zhang ZJ, He XB, Qin X, Xu FQ, Hu J, Wei F, Huganir RL, Li YQ, Zhuo M. Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Mol Pain 2014; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen T, Wang W, Dong YL, Zhang MM, Wang J, Koga K, Liao YH, Li JL, Budisantoso T, Shigemoto R, Itakura M, Huganir RL, Li YQ, Zhuo M. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol Brain 2014; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SJ, Kwak C, Lee J, Sim SE, Shim J, Choi T, Collingridge GL, Zhuo M, Kaang BK. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol Brain 2015; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]