Short abstract
Cancer-induced bone pain is one of the most severe types of pathological pain, which often occurs in patients with advanced prostate, breast, and lung cancer. It is of great significance to improve the therapies of cancer-induced bone pain due to the opioids’ side effects including addiction, sedation, pruritus, and vomiting. Sinomenine, a traditional Chinese medicine, showed obvious analgesic effects on a rat model of chronic inflammatory pain, but has never been proven to treat cancer-induced bone pain. In the present study, we investigated the analgesic effect of sinomenine after tumor cell implantation and specific cellular mechanisms in cancer-induced bone pain. Our results indicated that single administration of sinomenine significantly and dose-dependently alleviated mechanical allodynia in rats with cancer-induced bone pain and the effect lasted for 4 h. After tumor cell implantation, the protein levels of phosphorylated-Janus family tyrosine kinase 2 (p-JAK2), phosphorylated-signal transducers and activators of transcription 3 (p-STAT3), phosphorylated-Ca2+/calmodulin-dependent protein kinase II (p-CAMKII), and phosphorylated-cyclic adenosine monophosphate response element-binding protein (p-CREB) were persistently up-regulated in the spinal cord horn. Chronic intraperitoneal treatment with sinomenine markedly suppressed the activation of microglia and effectively inhibited the expression of JAK2/STAT3 and CAMKII/CREB signaling pathways. We are the first to reveal that up-regulation of microglial JAK2/STAT3 pathway are involved in the development and maintenance of cancer-induced bone pain. Moreover, our investigation provides the first evidence that sinomenine alleviates cancer-induced bone pain by inhibiting microglial JAK2/STAT3 and neuronal CAMKII/CREB cascades.
Keywords: Cancer-induced bone pain, sinomenine, microglia, JAK2/STAT3, CAMKII/CREB
Introduction
As one of the most severe types of chronic pain, cancer-induced bone pain (CIBP) is an intricate condition which involves allodynia, hyperalgesia, and spontaneous pain.1,2 The reported mechanisms underlying CIBP are associated with spinal cord plasticity, glial cell activation, and inflammatory cytokine signaling.3–5 Despite the availability of diverse analgesic agents, including opioids, non-steroidal anti-inflammatory drugs (NSAIDs), and adjuvant drugs, further elucidation of fresh therapy of CIBP is necessary because of their side effects.6–8 Although there have been marked advances in recent years, mechanisms of CIBP are far from clear. Therefore, further exploration of the underlying mechanism for novel therapies is still demanded.
Sinomenine is a morphinan derivative extracted from the traditional Chinese medicine Sinomenium acutum.9 It has been shown to possess a variety of pharmacological effects, such as anti-inflammatory, anti-angiogenesis, antiarrhythmic, and immunosuppressive properties.10–13 A large number of preclinical and clinical studies have confirmed that sinomenine is effectively against rheumatoid arthritis, arrhythmia, and glomerular diseases.14–16 Recently, there was evidence suggesting that sinomenine alleviated mechanical allodynia and heat hyperalgesia in the models of neuropathic and inflammatory pain.17–19 In addition, sinomenine was demonstrated to attenuate brain injury by inhibiting microglial activation in intracerebral hemorrhages.20 However, the potential analgesic effect of sinomenine in CIBP has never been reported.
Considerable research has suggested that numerous extracellular signaling molecules and intracellular transduction pathways are involved in chronic pain.21–24 The Janus family tyrosine kinase (JAK)/signal transducers and activators of transcription (STAT) pathway is one of the most important cell signaling pathways in chronic pain, which includes four types of JAK and seven types of STAT.25,26 Our previous study has demonstrated that STAT1 contributes to CIBP by regulating MHC II expression in spinal microglia.27 Accumulating evidence suggests that the activation of the JAK2/STAT3 pathway contributes to the development of neuropathic and inflammatory pain.28–31 After nerve injury, the expression of p-STAT3 is significantly up-regulated and leads to a neuroinflammatory response.32 In addition, the JAK2/STAT3 pathway was activated after chronic treatment of morphine.33 In the model of ischemic stroke, sinomenine was reported to alleviate neuroinflammatory injury via the STAT3 pathway.34 However, the role of the JAK2/STAT3 pathway has never been demonstrated in model of CIBP.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine kinase which is involved in synaptic plasticity, learning, and memory.35–37 The activation of CAMKII was proven to participate in cyclic adenosine monophosphate response element-binding protein (CREB) phosphorylation.38,39 The activated CaMKII/CREB pathway has been extensively confirmed in different pain models.28,40,41 In an inflammatory pain model, sinomenine was reported to attenuate mechanical allodynia and thermal hyperalgesia by down-regulating the CAMKII/CREB pathway.19 Novel inhibitors targeting the CAMKII/CREB pathway may be an alternative therapy for CIBP.
In the present study, we hypothesized that sinomenine exerts its antinociceptive effects on CIBP through suppressing neuronal CAMKII/CREB and microglial JAK2/STAT3 pathways.
Material and methods
Ethics statement
All experimental protocols were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the guidelines of the International Association for the Study of Pain.42 All procedures were made to minimize the number of animals used and their suffering.
Animals
Because female rats are more susceptible to Walker 256 mammary gland carcinoma cells, all of the experiments were performed on virgin female Sprague Dawley rats (180–200 g, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China) in this study. Rats were kept in controlled conditions (24 ± .5C, 12-h alternating light-dark cycle, with ad libitum access to water and food).
Drug administration
Sinomenine was purchased from Selleck Chemicals (Houston, TX) and dissolved in saline. Different doses were injected intraperitoneally (i.p) based on previous reports.9,43 The drug administration protocol was as follows: for acute treatment, sinomenine (i.p, 10 mg/kg, 20 mg/kg, and 40 mg/kg) or vehicle (i.p) was administrated on day 14 after CIBP model establishment; for chronic treatment, sinomenine (i.p, 10 mg/kg, 20 mg/kg, and 40 mg/kg) or vehicle (i.p) was administrated from day 14 to day 18 once daily after tumor cell implantation (TCI).
Preparation of carcinoma cells
Walker 256 rat mammary gland carcinoma cells (4 ×107 cells/mL, 1 mL) were inoculated into the abdominal cavity of female rats as previously described.44 After seven days, carcinoma cells were extracted from the ascitic fluid and were diluted to the final concentration (4 ×107 cells/mL) using a hemocytometer. The cell suspension was maintained on ice until inoculation.
Cancer-induced bone pain model establishment
The model of CIBP was carried out as described previously.45,46 After anesthesia with pentobarbital sodium (50 mg/kg, i.p), the right leg of rat was shaved and disinfected. Then, a minimal incision was made to expose the proximal tibia and the prepared Walker 256 cells (4×107 cells/ml, 10 µl) were slowly injected into the bone cavity. For the sham group, 10-µl D-Hank’s solution was injected instead. The injected site was immediately closed using bone wax after the syringe was removed. Finally, the incision was sutured using 3-0 silk thread.
Behavioral assessment
Pain-related behaviors were measured on day 0, 3, 7, 14, and 21 after the injection of tumor cells. Mechanical allodynia was assessed using ipsilateral hind paw withdraw threshold (PWT) to von Frey filament as described previously.27,47 Rats were placed in individual plastic boxes with a metal mesh floor and allowed to acclimate for half an hour. A range of von Frey filaments (1, 1.4, 2, 4, 6, 8, 10, and 15 g), with ascending order, were applied to determine the mechanical PWT. Quick paw withdraw and licking were considered positive responses. When a positive response occurred, rats were allowed to have a rest for 5 min before the next descending von Frey filament. The lowest amount of force which induced a positive response was recorded as the PWT.
Immunohistochemistry
After deep anesthesia with pentobarbital sodium (60 mg/kg, i.p), the rats were perfused intracardially with saline followed by 4% ice-cold paraformaldehyde in 0.1 M phosphate-buffered saline. The enlargement of spinal cord (L4-L6) was removed and postfixed in 4% paraformaldehyde for 4 h and subsequently dehydrated in 30% sucrose for 24 h at 4°C. The segments were sectioned 20 µm thick in a cryostat (CM1900, Leica, Wetzlar, Germany). To confirm the cell type that expressed p-JAK2, the rabbit anti-phospho-JAK2 antibody (1:1000; #3776; Cell Signaling Technology) was mixed with mouse anti-neuronal nuclei (NeuN) antibody (neuronal marker; 1:200; MAB377; EMD Millipore, Billerica, MA), mouse anti-glial fibrillary acidic protein (GFAP) antibody (astrocytic marker; 1:300; 3670; Cell Signaling Technology, Danvers, MA), or goat anti-ionized calcium-binding adapter molecule 1 (Iba1) antibody (microglial marker; 1:300; ab5076; Abcam). Iba1-immunolabeled surface area was quantified from the spinal dorsal horn using Image Pro Plus software version 6.0. Quantification of Iba1immunoreactivity was accomplished by calculating the percentages of immunostaining ((positive immunofluorescent surface area)/(total measured picture area) × 100). Four rats of each group were used for statistical analysis.
Western blot
Under deep anesthesia with pentobarbital sodium (60 mg/kg, i.p), the L4-L6 spinal segments were collected and homogenized in a radioimmunoprecipitation assay lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 1% sodium dodecyl sulfate, 1 mM NaF, and 2 mM ethylenediaminetetraacetic acid. The supernatants were collected and protein concentrations were determined by the Bradford method. The protein samples were heated at 95° for 10 min in a loading buffer. Equal amounts of proteins (40–60 µg) were separated by 10% sodium dodecyl sulfate-PAGE and transferred onto polyvinylidene fluoride membranes (IPVH00010; EMD Millipore). After blocking with 5% BSA in 0.1% TBST for 2 h at room temperature, the membranes were incubated overnight at 4°C with rabbit anti-phospho-CAMKII antibody (1:3000; ab5683; Abcam), rabbit anti-phospho-CREB (pSer133) antibody (1:500; MA5–11192; Thermo), rabbit anti-phospho-JAK2 antibody (1:1000; #3776; CST), rabbit anti-phospho-STAT3 antibody (1:1000; #9145; CST), mouse anti-Iba1 antibody (1;500; ab15690; Abcam), and rabbit anti-GAPDH antibody (1:5000; AS1039; Aspen), respectively. The membranes were washed in TBST and incubated with HRP goat anti-rabbit (1:5000; A21020; Abbkine) or goat anti-mouse secondary antibody (1:5000; A21010; Abbkine) for 2 h at room temperature. The protein bands were finally visualized using SuperLumia ECL Plus HRP Substrate Kit (K22030; Abbkine) and then measured by a computerized image analysis system (BIO-RAD, ChemiDoc XRS+, USA).
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM) and performed using GraphPad Prism version 5.01 for Windows (Graph Pad Software, San Diego, CA). Western blot and immunochemistry data were evaluated via one-way analysis of variance followed by a Bonferroni post hoc test. The differences in the mechanical PWT data were analyzed using two-way analysis of variance with repeated measures, followed by a Bonferroni post hoc test. p < 0.05 was considered statistically significant.
Results
Antinociceptive effects of sinomenine in cancer-induced bone pain
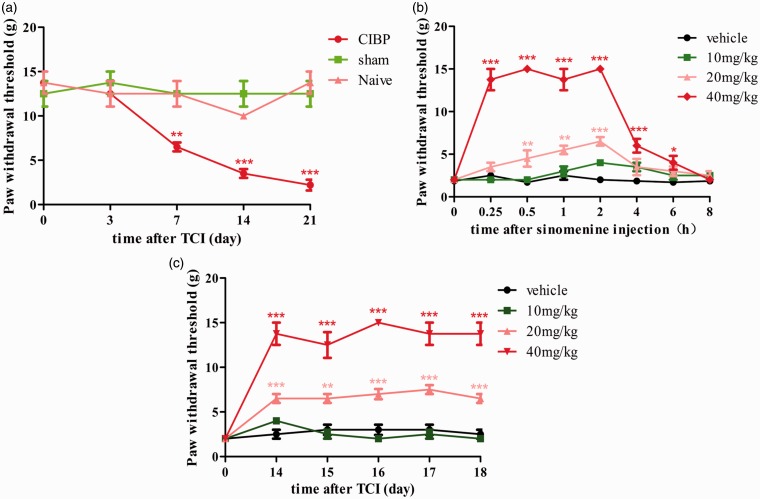
Before cell transplantation, there was no significant difference in pain-related behaviors among the sham and other groups. Consistent with our previous studies, the ipsilateral PWT of CIBP group decreased dramatically from day 7 to day 21 after TCI (Figure 1(a)). The results indicate that TCI leads to the mechanical allodynia.
Figure 1.
Sinomenine attenuated the CIBP-related behaviors. (a) The ipsilateral PWTs were decreased from day 7 after TCI to the last observation on day 21 (**p < 0.01, ***p < 0.001 compared with the naïve group, n = 6 in each group). (b) A single dose of sinomenine (i.p, 20 mg/kg and 40 mg/kg, but not 10 mg/kg, on day 14) significantly reversed the CIBP-induced mechanical allodynia (*p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle group, n = 6 in each group). The behavioral tests were conducted at 0, 0.25, 0.5, 1, 2, 4, 6, and 8 h after sinomenine injection. (c) For chronic treatment, sinomenine (i.p, 10 mg/kg, 20 mg/kg, and 40 mg/kg, once a day) or vehicle was administered for five days (from day 14 to day 18 after TCI). The behavioral tests were conducted 2 h after sinomenine injection. The ipsilateral mechanical allodynia of 20 mg/kg and 40 mg/kg groups was significantly alleviated by sinomenine (**p < 0.01, ***p < 0.001 compared with the vehicle group, n = 6 in each group). TCI: tumor cell implantation; CIBP: cancer-induced bone pain.
To investigate the analgesic effect of single administration, we injected different doses of sinomenine (i.p, 10 mg/kg, 20 mg/kg, and 40 mg/kg) and vehicle on day 14 after TCI and tested the PWT at 0, 0.25, 0.5, 1, 2, 4, 6, and 8 h. Compared to the sham group, i.p injection of sinomenine at a dose of 10 mg/kg had no distinct influence on PWT. However, i.p injection of sinomenine at a dose of 20 mg/kg alleviated the mechanical allodynia, beginning at 0.5 h and peaking at 2 h (Figure 1(b)). As shown in Figure 1(b), the maximum dose of sinomenine (40 mg/kg, i.p) significantly reversed the PWT, beginning at 0.25 h, peaking at 1 h, and lasting for 6 h. For chronic treatment, different doses of sinomenine or vehicle were administered i.p once daily from day 14 until day 18 and the PWTs were conducted 2 h after injection. As shown in Figure 1(c), continuous administration of sinomenine obviously and dose-dependently alleviated TCI-induced pain hypersensitivity, except for the 10 mg/kg sinomenine group. In addition, the PWTs of the 40 mg/kg sinomenine group were higher than the 20 mg/kg group.
Collectively, these results demonstrated that single and repeated sinomenine treatment attenuated the development and maintenance of CIBP, and the 40 mg/kg of sinomenine has a better analgesic effect.
Role of the JAK2/STAT3 pathway in CIBP
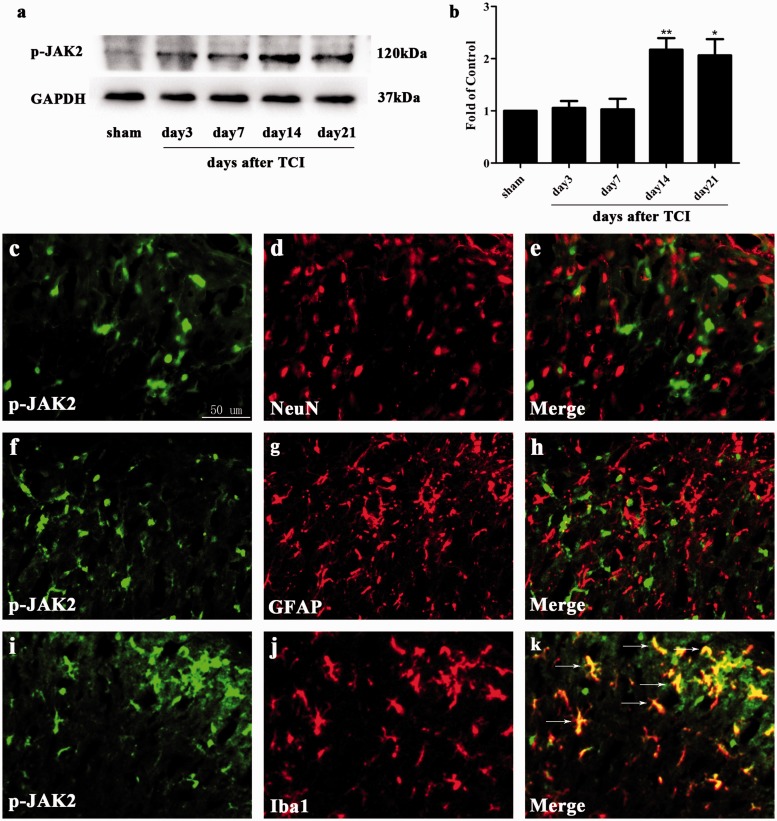
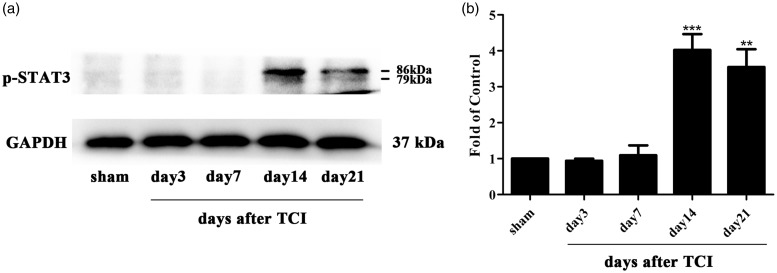
Emerging evidence has reported the involvement of the JAK2/STAT3 pathway in neuropathic pain.48 To explore the expression and distribution of the JAK2/STAT3 pathway in the rat model of CIBP, we conducted Western blot analysis and immunohistochemistry experiments. The expression of spinal p-JAK2 and p-STAT3 was significantly increased in CIBP rats beginning and peaking on day 14 until the final examination on day 21 after TCI (Figure 2(a) and (b) and 3(a) and (b)). To investigate the cellular localization, we conducted double immunofluorescence staining of p-JAK2 with NeuN (neuronal marker), GFAP (astrocytic marker), or Iba1. As illustrated in Figure 2(c) to (k), p-JAK2 was only co-localized with microglia in the spinal cord, but not with the astrocyte or neuron.
Figure 2.
Expression and cellular localization of phosphorylated-Janus family tyrosine kinase 2 (p-JAK2) in spinal cord dorsal horn of CIBP rats. (a) and (b) Western blot analysis showing the time course of p-JAK2 expression in sham and CIBP rats (*p < 0.05, **p < 0.01 compared with the sham group, n = 6 in each group). The fold change for the density of p-JAK2 was normalized to GAPDH for each sample, respectively. The fold change of p-JAK2 in the sham group was set at 1 for quantification. (c to k) Representative photomicrographs of p-JAK2 (green) double fluorescence labeling with NeuN (red) for neurons, GFAP (red) for astrocytes, and Iba1 (red) for microglia in the ipsilateral spinal cord at day 14 after TCI. Photomicrographs were taken from ipsilateral spinal cord dorsal horns of CIBP rats (n = 4 in each group). The results showed that p-JAK2 was co-expressed only with microglia (yellow). TCI: tumor cell implantation; NeuN: neuronal nuclei; GFAP: glial fibrillary acidic protein; GAPDH: glyceraldehyde-3-phosphate dehydrogenase antibody; Iba1: ionized calcium-binding adapter molecule 1; p-JAK2: phosphorylated-Janus family tyrosine kinase 2.
Figure 3.
Expression of phosphorylated-signal transducers and activators of transcription 3 (p-STAT3) in spinal cord dorsal horn of CIBP rats. (a and b) Western blot analysis showing the time course of p-STAT3 expression in sham and CIBP rats (**p < 0.01, ***p < 0.001 compared with the sham group, n = 6 in each group). The fold change for the density of p-STAT3 was normalized to GAPDH for each sample, respectively. The fold change of p-STAT3 in the sham group was set at 1 for quantification. TCI: tumor cell implantation; GAPDH: glyceraldehyde-3-phosphate dehydrogenase antibody; p-STAT3: phosphorylated-signal transducers and activators of transcription 3.
It turns out that the JAK2/STAT3 pathway was phosphorylated and up-regulated in the CIBP group, and p-JAK2 was only distributed in microglia but not with astrocytes or neurons.
Effects of repeated sinomenine on the CIBP-induced activation of JAK2/STAT3 pathway
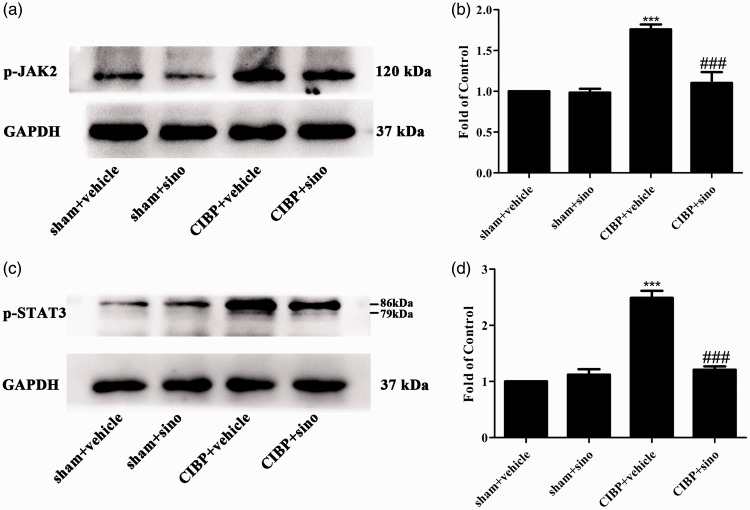
Sinomenine has been shown to alleviate neuroinflammatory injury via the CRYAB/STAT3 pathway after ischemic stroke.49 To explore the specific mechanisms of sinomenine in attenuating TCI-induced pain, we conducted sinomenine (40 mg/kg, i.p) from day 14 to day 18 after TCI and the spinal cords were collected to detect the expression of cellular signaling pathways. As shown in Figure 4(a) to (d), the expression levels of p-JAK2 and p-STAT3 were reduced by sinomenine comparing with the CIBP + vehicle group.
Figure 4.
Repeated i.p administration of sinomenine reversed TCI-induced upregulation of JAK2/STAT3 pathway in the spinal cord. (a to d) Western blot data summary showing that sinomenine decreased the protein expression of p-JAK2 and p-STAT3 (***p < 0.001 compared with the sham + vehicle group; ###p < 0.001 compared with the CIBP + vehicle group, n = 6 in each group). Sinomenine (i.p, 40 mg/kg) or vehicle was administered once a day from day 14 after TCI to day 18. Tissues were collected 2 h after the last injection. GAPDH: glyceraldehyde-3-phosphate dehydrogenase antibody; CIBP: cancer-induced bone pain; p-JAK2: phosphorylated-Janus family tyrosine kinase 2; p-STAT: phosphorylated-signal transducers and activatorsof transcription 3.
Role of the CAMKII/CREB pathway in CIBP
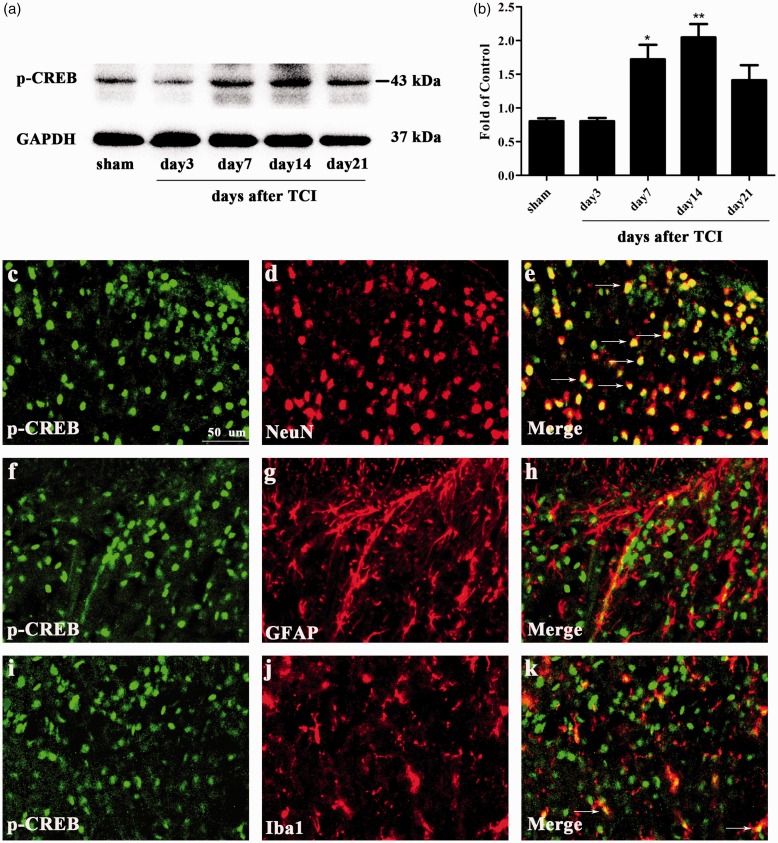
Previous studies have suggested that the activation of the CAMKII/CREB pathway in the spinal cord contributes to neuropathic pain and cognitive impairment.50,51 The CAMKII/CREB signaling pathway participates in the intracellular signal transduction pathway which participates in cell proliferation, cell survival, and metabolism.52,53 The cerebral ischemia-induced injury was attenuated by paeoniflorin via modulation of the Ca2+/CAMKII/CREB signaling pathway.54 In the CIBP group, the protein level of p-CAMKII increased on day 7 and peaked on day 14 (Figure 5(a) and (b)). Consistently, the protein change of p-CREB also raised on day 7, peaking on day 14 (Figure 6(a) and (b)). To investigate the cellular localization, we conducted double immunofluorescence staining of p-CREB with NeuN, GFAP, or Iba1. As shown in Figure 6(c) to (k), p-CREB was predominantly co-localized with neuron and a minority with microglia in the spinal cord.
Figure 5.
Expression of CAMKII in spinal cord dorsal horn of CIBP rats. (a and b) Western blot analysis showing the time course of phosphorylated-Ca2+/calmodulin-dependent protein kinase II (p-CAMKII) expression in sham and CIBP rats (*p < 0.05, ***p < 0.001 compared with the sham group, n = 6 in each group). The fold change for the density of p-CAMKII was normalized to GAPDH for each sample, respectively. The fold change of p-CAMKII in the sham group was set at 1 for quantification. TCI: tumor cell implantation; GAPDH: glyceraldehyde-3-phosphate dehydrogenase antibody; p-CAMKII: phosphorylated-Ca2+/calmodulin-dependent protein kinase II.
Figure 6.
Expression and cellular localization of phosphorylated-cyclic adenosine monophosphate response element-binding protein (p-CREB) in spinal cord dorsal horn of CIBP rats. (a) and (b) Western blot analysis showing the time course of p-CREB expression in sham and CIBP rats (*p < 0.05, **p < 0.01 compared with the sham group, n = 6 in each group). The fold change for the density of p-CREB was normalized to GAPDH for each sample, respectively. The fold change of p-CREB in the sham group was set at 1 for quantification. (c to k) Representative photomicrographs of p-CREB (green) double fluorescence labeling with NeuN (red) for neurons, GFAP (red) for astrocytes, and Iba1 (red) for microglia in the ipsilateral spinal cord at day 14 after TCI. Photomicrographs were taken from ipsilateral spinal cord dorsal horns of CIBP rats (n = 4 in each group). The results showed that p-CREB was predominantly co-expressed with neuron (yellow). TCI: tumor cell implantation; NeuN: neuronal nuclei; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFAP: glial fibrillary acidic protein; Iba1: ionized calcium-binding adapter molecule 1; p-CREB:phosphorylated-cyclic adenosine monophosphate response element-binding protein.
These results indicated that the CAMKII/CREB pathway was activated in the CIBP model and p-CREB was predominantly distributed in neuron.
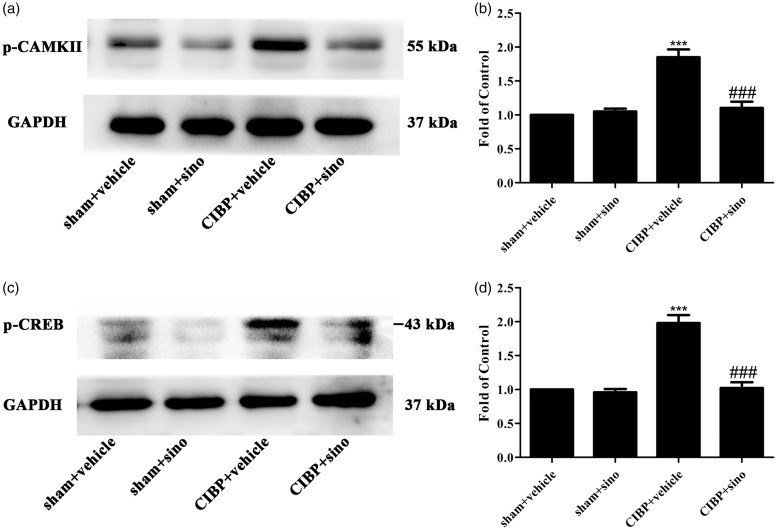
Effects of repeated sinomenine on the CIBP-induced activation of CAMKII/CREB pathway
Sinomenine was reported to suppress inflammatory pain by down-regulating the CAMKII/CREB pathway.19 In order to explore the effect of sinomenine on CIBP model, we conducted sinomenine (40 mg/kg, i.p) from day 14 to day 18. As illustrated in Figure 7(a) to (d), the protein expression of CAMKII/CREB was significantly decreased in the CIBP + sinomenine group.
Figure 7.
Repeated i.p administration of sinomenine reversed TCI-induced upregulation of CAMKII/CREB pathway in the spinal cord. (a to d) Western blot data summary showing that sinomenine restored the protein expression of p-CAMKII and p-CREB (***p < 0.001 compared with the sham + vehicle group; ###p < 0.001 compared with the CIBP + vehicle group, n = 6 in each group). Sinomenine (i.p, 40 mg/kg) or vehicle was administered once a day from day 14 after TCI to day 18. Tissues were collected 2 h after the last injection. GAPDH: glyceraldehyde-3-phosphate dehydrogenase antibody; CIBP: cancer-induced bone pain; p-CAMKII: phosphorylated-Ca2+/calmodulin-dependent protein kinase II; p-CREB: phosphorylated-cyclic adenosinemonophosphate response element-binding protein.
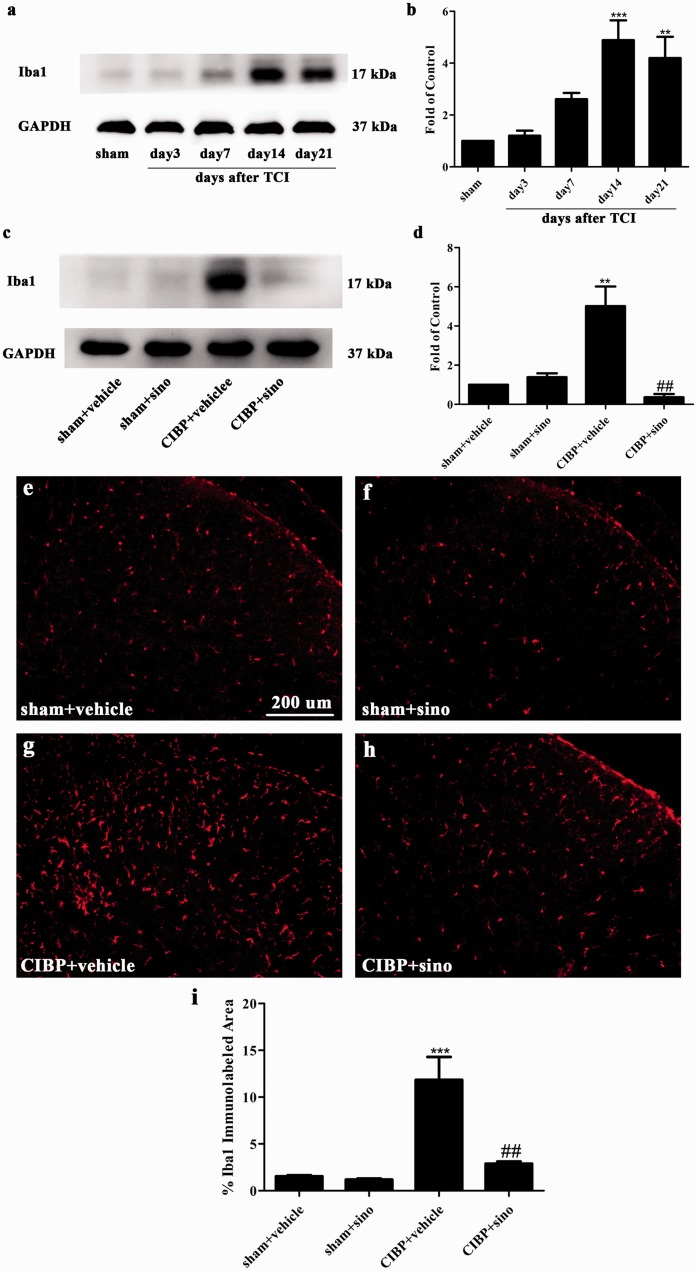
Involvement of spinal microglia in the analgesic effect of sinomenine
In our previous investigations, the inhibition of microglia was proven to relieve cancer-induced allodynia and hyperalgesia.55–57 Expression level of Iba1 has been used as an indicator of microglial activity. As shown in Figure 8(a) and (b), the expression of Iba1 was significantly increased in CIBP rats beginning on day 14 until the final examination on day 21 after TCI. It was reported that sinomenine alleviates brain injury through inhibiting the activation of microglia.20 In our study, we found that chronic treatment of sinomenine (40 mg/kg, once a day, i.p) suppressed the protein expression of microglia (Figure 8(c) and (d)). Furthermore, the immunofluorescence staining of Iba1 had demonstrated the results. As illustrated in Figure 8(e) to (i), microglia was distinctly activated in CIBP + vehicle group and the activation of Iba1 was significantly suppressed in CIBP + sinomenine group.
Figure 8.
Repeated i.p administration of sinomenine reversed TCI-induced activation of microglia in the spinal cord. (a) and (b) Western blot analysis showing the time course of Iba1 (microglia maker) in sham and CIBP rats (**p < 0.01, ***p < 0.001 compared with the sham group, n = 6 in each group). The fold change for the density of Iba1 was normalized to GAPDH for each sample, respectively. The fold change of Iba1 in the sham group was set at 1 for quantification. (c) and (d) Western blot data showing that sinomenine restored the protein expression of Iba1 (**p < 0.01 compared with the sham + vehicle group; ##p < 0.01 compared with the CIBP + vehicle group, n = 6 in each group). Sinomenine (i.p, 40 mg/kg) or vehicle was administered once a day from day 14 after TCI to day 18. Tissues were collected 2 h after the last injection. (e to h) Representative photomicrographs of Iba1 (red) for microglia in the spinal cord. Photomicrographs were taken from ipsilateral spinal cord dorsal horns of sham + vehicle group, sham + sino group, CIBP + vehicle group, and CIBP + sino group (n = 4 in each group). (i) Iba1-immunolabeled surface area was quantified from the spinal dorsal horn using Image Pro Plus software version 6.0. Quantification of Iba1immunoreactivity was accomplished by calculating the percentages of immunostaining ((positive immunofluorescent surface area)/(total measured picture area) × 100). Four rats of each group were used for statistical analysis (***p < 0.001 compared with sham + vehicle group. ##p < 0.01 compared with the CIBP + vehicle group). TCI: tumor cell implantation; CIBP: cancer-induced bone pain; GAPDH: glyceraldehyde-3-phosphate dehydrogenase antibody; Iba1: ionized calcium-binding adapter molecule 1.
We have demonstrated that the JAK2 pathway was co-localized with microglia in this study. These results suggest that one of the mechanisms of sinomenine relieving TCI-related pain was down-regulating the JAK2/STAT3 pathway through inhibiting the activation of microglia. In addition, sinomenine may suppress the expression of the CAMKII/CREB pathway to weaken cancer-induced mechanical allodynia.
Discussion
In the current study, the principal findings were as follows: (1) acute and chronic intraperitoneal administration of sinomenine significantly alleviated TCI-induced mechanical allodynia; (2) transplanting of Walker 256 mammary gland carcinoma cells resulted in the activation of the JAK2/STAT3 pathway and p-JAK2 was only distributed in microglia; (3) the CAMKII/CREB signaling pathway in the spinal cord was activated in the rat model of CIBP; (4) chronic treatment of sinomenine distinctly suppressed the activation of microglia and significantly reduced the expression of both the JAK2/STAT3 pathway and the CAMKII/CREB pathway. To the best of our knowledge, we provide the first evidence that the JAK2/STAT3 signaling pathway is involved in CIBP. Moreover, we are the first to find that sinomenine can attenuate TCI-induced pain by inhibiting the microglial JAK2/STAT3 pathway and the CAMKII/CREB pathway.
CIBP-induced allodynia and hyperalgesia significantly compromise the quality of life in advanced cancer patients.58,59 In the CIBP rats, the activation of glia cells, including microglia and astrocyte, in the spinal segments is distinctly confirmed.60,61 Our previous studies showed that inhibition of microglia by minocycline significantly relieved mechanical allodynia in CIBP rats.44 Sinomenine, a traditional Chinese medicine, has emerged as a potent inhibitor of microglial activation. Sinomenine was reported to prevent oligomeric Aβ-induced microglia activation and to show protection against Alzheimer’s disease.62 In diabetic retinopathy, sinomenine reduced the release of TNF-α, IL-1β, and IL-6 in microglia via ROS production and NF-kB translocation.63 In addition, a series of studies have proven the analgesic effect of sinomenine on chronic inflammatory pain and neuropathic.43,64 However, there is no evidence confirming the analgesic effect of sinomenine on CIBP. In this study, we found that sinomenine alleviated the PWT after TCI through inhibiting microglial activation.
STAT3, a member of the JAK/STAT signaling pathway, is phosphorylated and activated after CNS damage and participates in the microglial inflammatory response.65,66 Previous evidence suggested that JAK2 played a major role in STAT3 phosphorylation, and intrathecal injection of the JAK2 inhibitor AG490 markedly inhibits the accumulation of p-STAT3 in the dorsal spinal cord of spinal nerve ligation rats.67 Furthermore, microglial JAK/STAT3 pathway activity directly impacted the characteristics of astrocytes and neurons, which participated in spinal cord plasticity and remodeling.68 The JAK2/STAT3 pathway was shown to contribute to cell apoptosis in rats with transient global cerebral ischemia.69 In rats with neuropathic pain and inflammatory pain, the JAK2/STAT3 signaling pathway was found to be up-regulated.34,70 Sinomenine was discovered to inhibit A549 human lung cancer cell invasion by mediating the STAT3 pathway.71 In addition, sinomenine attenuated ischemic stroke-induced neuroinflammation by the CRYAB/STAT3 pathway.49 Hence, we determined to prove whether inhibiting the microglial JAK2/STAT3 pathway was one of the mechanisms underlying the analgesic effect of sinomenine on CIBP. As shown in Figures 2(a) and (b) and 3(a) and (b), the expression of the JAK2/STAT3 pathway was significantly up-regulated in the rat model of CIBP, peaking on day 14 and lasting until day 21. The changes were the same as expression of microglia. Consistent with previous studies, our results showed that p-JAK2 was co-localized with microglia. Compared with the CIBP + vehicle rats, the activation of the JAK2/STST3 pathway was inhibited by the chronic treatment of sinomenine.
CAMKII was mainly distributed in the superficial laminae of the spinal cord dorsal horn, which played a vital role in transmission and processing of nociceptive signals.37,72,73 Autocamtide 2-related inhibitor peptide, a highly specific and potent inhibitor of CAMKII, was identified to reduce the protein level of p-CREB and showed analgesic effects.74 Accumulating evidence has established that up-regulation of the CAMKII/CREB signaling pathway participates in models of pathological pain.40,41 As shown in Figures 5(a) and (b) and 6(a) and (b), our study demonstrated that the activation of the CAMKII/CREB pathway began on day 7 and peaked on day 14. Our study confirmed the role of the CAMKII/CREB signaling pathway in CIBP. In addition, our results showed that the expression of CAMKII/CREB was restored by chronic treatment of sinomenine.
Our investigation provides the first evidence that sinomenine alleviates CIBP by inhibiting microglial JAK2/STAT3 and neuronal CAMKII/CREB cascades. Sinomenine has significant analgesic effects against CIBP and may be considered as an alternative therapy for the clinical management of CIBP.
Authors’ contribution
Da-Wei Ye, Yu-Ke Tian, and Fang Luo designed the research study; Shu-Ping Chen, Ya-Qun Zhou, Jia Sun, Fei Cao, and Cody Braun performed the study; Shu-Ping Chen prepared the manuscript; all authors approved the final version of manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from National Natural Science Foundation of P.R. China (Grant No. 81400917, 81371250, 81571053, and 81771196).
References
- 1.Zhou YQ, Chen SP, Liu DQ, Manyande A, Zhang W, Yang SB, Xiong BR, Fu QC, Song ZP, Rittner H, Ye DW, Tian YK. The role of spinal GABAB receptors in cancer-induced bone pain in rats. J Pain 2017; 18: 933–946. DOI: 10.1016/j.jpain.2017.02.438. [DOI] [PubMed] [Google Scholar]
- 2.Ke CB, He WS, Li CJ, Shi D, Gao F, Tian YK. Enhanced SCN7A/Nax expression contributes to bone cancer pain by increasing excitability of neurons in dorsal root ganglion. Neuroscience 2012; 227: 80–89. DOI: 10.1016/j.neuroscience.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflamm 2016; 13: 141. DOI: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu XF, He XT, Zhou KX, Zhang C, Zhao WJ, Zhang T, Li JL, Deng JP, Dong YL. The analgesic effects of triptolide in the bone cancer pain rats via inhibiting the upregulation of HDACs in spinal glial cells. J Neuroinflamm 2017; 14: 213. DOI: 10.1186/s12974-017-0988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou YQ, Gao HY, Guan XH, Yuan X, Fang GG, Chen Y, Ye DW. Chemokines and their receptors: potential therapeutic targets for bone cancer pain. Curr Pharm Des 2015; 21: 5029–5033. [DOI] [PubMed] [Google Scholar]
- 6.Christo PJ, Mazloomdoost D. Interventional pain treatments for cancer pain. Ann N Y Acad Sci 2008; 1138: 299–328. DOI: 10.1196/annals.1414.034. [DOI] [PubMed] [Google Scholar]
- 7.Boland JW, Ziegler L, Boland EG, McDermid K, Bennett MI. Is regular systemic opioid analgesia associated with shorter survival in adult patients with cancer? A systematic literature review. Pain 2015; 156: 2152–2163. DOI: 10.1097/j.pain.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, Zhou Y, Peng Y, Su P, Li Z, Xu Q, Tu Y, Tian X, Yang H, Wu Z, Mei W, Gao F. Endoplasmic reticulum stress in spinal cord contributes to the development of morphine tolerance. Front Mol Neurosci 2018; 11: 72. DOI: 10.3389/fnmol.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Q, Sun Y, Mao L, Liu C, Jiang B, Zhang W, Li JX. Antinociceptive effects of sinomenine in a rat model of postoperative pain. Br J Pharmacol 2016; 173: 1693–1702. DOI: 10.1111/bph.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kok TW, Yue PY, Mak NK, Fan TP, Liu L, Wong RN. The anti-angiogenic effect of sinomenine. Angiogenesis 2005; 8: 3–12. DOI: 10.1007/s10456-005-2892-z. [DOI] [PubMed] [Google Scholar]
- 11.Isik S, Karaman M, Micili SC, Caglayan-Sozmen S, Bagriyanik HA, Arikan-Ayyildiz Z, Uzuner N, Karaman O. Sinomenine ameliorates the airway remodelling, apoptosis of airway epithelial cells, and Th2 immune response in a murine model of chronic asthma. Allergol Immunopath 2018; 46: 67–75. DOI: 10.1016/j.aller.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Jiang C, Chen X, He K, Hu Y. Protective effects of sinomenine against LPS-induced inflammation in piglets. Microb Pathog 2017; 110: 573–577. DOI: 10.1016/j.micpath.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Liu JX, Luo JF, Cheng CS, Leung EL, Li Y, Su XH, Liu ZQ, Chen TB, Duan FG, Dong Y, Zuo YH, Li C, Lio CK, Li T, Luo P, Xie Y, Yao XJ, Wang PX, Liu L. Suppressing mPGES-1 expression by sinomenine ameliorates inflammation and arthritis. Biochem Pharmacol 2017; 142: 133–144. DOI: 10.1016/j.bcp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med 2008; 74: 1423–1429. DOI: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 15.Zhao XX, Peng C, Zhang H, Qin LP. Sinomenium acutum: a review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharmaceut Biol 2012; 50: 1053–1061. DOI: 10.3109/13880209.2012.656847. [DOI] [PubMed] [Google Scholar]
- 16.Feng S, Zhu L, Huang Z, Wang H, Li H, Zhou H, Lu L, Wang Y, Liu Z, Liu L. Controlled release of optimized electroporation enhances the transdermal efficiency of sinomenine hydrochloride for treating arthritis in vitro and in clinic. Drug Des Devel Ther 2017; 11: 1737–1752. DOI: 10.2147/DDDT.S136313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Yoon SY, Won J, Kim HB, Kang Y, Oh SB. Sinomenine produces peripheral analgesic effects via inhibition of voltage-gated sodium currents. Neuroscience 2017; 358: 28–36. DOI: 10.1016/j.neuroscience.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Rao S, Liu S, Zou L, Jia T, Zhao S, Wu B, Yi Z, Wang S, Xue Y, Gao Y, Xu C, Li G, Xu H, Zhang C, Liang S. Erratum to: the effect of sinomenine in diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Purinergic Signal 2017; 13: 237. DOI: 10.1007/s11302-017-9560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Han J, Wang DS, Yang Q, Feng B, Kang WB, Yang L, Liu G, Zhao MG. Sinomenine attenuates chronic inflammatory pain in mice. Metab Brain Dis 2017; 32: 211–219. DOI: 10.1007/s11011-016-9889-8. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Liu Y, Yuan F, Li Z, Huang S, Shen H, Yuan B. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol Immunol 2014; 60: 109–114. DOI: 10.1016/j.molimm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhou YQ, Liu Z, Liu HQ, Liu DQ, Chen SP, Ye DW, Tian YK. Targeting glia for bone cancer pain. Expert Opin Ther Targets 2016; 20: 1365–1374. DOI: 10.1080/14728222.2016.1214716. [DOI] [PubMed] [Google Scholar]
- 22.Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 2017; 10: 174. DOI: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SP, Zhou YQ, Liu DQ, Zhang W, Manyande A, Guan XH, Tian YK, Ye DW, Omar DM. PI3K/Akt pathway: a potential therapeutic target for chronic pain. Curr Pharm Des 2017; 23: 1860–1868. DOI: 10.2174/1381612823666170210150147. [DOI] [PubMed] [Google Scholar]
- 24.Yang F, Luo WJ, Sun W, Wang Y, Wang JL, Yang F, Li CL, Wei N, Wang XL, Guan SM, Chen J. SDF1-CXCR4 signaling maintains central post-stroke pain through mediation of glial-neuronal interactions. Front Mol Neurosci 2017; 10: 226. DOI: 10.3389/fnmol.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol 2000; 37: 1–11. [DOI] [PubMed] [Google Scholar]
- 26.Li YY, Li H, Liu ZL, Li Q, Qiu HW, Zeng LJ, Yang W, Zhang XZ, Li ZY. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol Pain 2017; 13: 174480691774742. DOI: 10.1177/1744806917747425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Z, Xiong B, Zheng H, Manyande A, Guan X, Cao F, Ren L, Zhou Y, Ye D, Tian Y. STAT1 as a downstream mediator of ERK signaling contributes to bone cancer pain by regulating MHC II expression in spinal microglia. Brain Behav Immun 2017; 60: 161–173. DOI: 10.1016/j.bbi.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Mi WL, Li Q, Zhang MT, Han P, Hu S, Mao-Ying QL, Wang YQ. Spinal IL-33/ST2 signaling contributes to neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3 cascades in mice. Anesthesiology 2015; 123: 1154–1169. DOI: 10.1097/ALN.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Li Q, Zhang MT, Mao-Ying QL, Hu LY, Wu GC, Mi WL, Wang YQ. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1beta via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep 2016; 6: 28956. DOI: 10.1038/srep28956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Yan Y, Yu L, Duan Y. Procaine attenuates pain behaviors of neuropathic pain model rats possibly via inhibiting JAK2/STAT3. Biomol Ther 2016; 24: 489–494. DOI: 10.4062/biomolther.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao J, Tian Y, Mao-Ying QL, Jiang JW, Ma HJ, Wang YQ, Wu GC. Aspirin-triggered lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience 2014; 273: 65–78. DOI: 10.1016/j.neuroscience.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 32.Peppin JF, Webster L. Letter to the editor in response to “The evidence for pharmacological treatment of neuropathic pain,” by Finnerup et al. Pain 2011; 152: 1440. DOI: 10.1016/j.pain.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Tao R, Wang J, Xia L. Upregulation of miR-375 level ameliorates morphine analgesic tolerance in mouse dorsal root ganglia by inhibiting the JAK2/STAT3 pathway. J Pain Res 2017; 10: 1279–1287. DOI: 10.2147/JPR.S125264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang J, Li ZH, Ge SN, Wang W, Mei XP, Wang W, Zhang T, Xu LX, Li JL. The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med 2012; 2012: 1. DOI: 10.1155/2012/185167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukushima H, Maeda R, Suzuki R, Suzuki A, Nomoto M, Toyoda H, Wu LJ, Xu H, Zhao MG, Ueda K, Kitamoto A, Mamiya N, Yoshida T, Homma S, Masushige S, Zhuo M, Kida S. Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J Neurosci 2008; 28: 9910–9919. DOI: 10.1523/JNEUROSCI.2625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tetenborg S, Yadav SC, Hormuzdi SG, Monyer H, Janssen-Bienhold U, Dedek K. Differential distribution of retinal Ca(2+)/calmodulin-dependent kinase II (CaMKII) isoforms indicates CaMKII-beta and -delta as specific elements of electrical synapses made of connexin36 (Cx36). Front Mol Neurosci 2017; 10: 425. DOI: 10.3389/fnmol.2017.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Zeng J, Wan X, Yao Y, Zhao N, Yu Y, Yu C, Xia Z. Enhancement of spinal dorsal horn neuron NMDA receptor phosphorylation as the mechanism of remifentanil induced hyperalgesia: roles of PKC and CaMKII. Mol Pain 2017; 13: 174480691772378. DOI: 10.1177/1744806917723789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Cheng X, Xu J, Liu Z, Wan Y, Ma D. Anti-hyperalgesic effect of CaMKII inhibitor is associated with downregulation of phosphorylated CREB in rat spinal cord. J Anesth 2011; 25: 87–92. DOI: 10.1007/s00540-010-1068-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Luo F, Tian YK, Ye DW. Cellular and molecular mechanisms of calcium/calmodulin-dependent protein kinase II in chronic pain. J Pharmacol Exp Ther 2017; 363: 176–183. DOI: 10.1124/jpet.117.243048. [DOI] [PubMed] [Google Scholar]
- 40.Yao CY, Weng ZL, Zhang JC, Feng T, Lin Y, Yao S. Interleukin-17A acts to maintain neuropathic pain through activation of CaMKII/CREB signaling in spinal neurons. Mol Neurobiol 2016; 53: 3914–3926. DOI: 10.1007/s12035-015-9322-z. [DOI] [PubMed] [Google Scholar]
- 41.Hu XM, Zhang H, Xu H, Zhang HL, Chen LP, Cui WQ, Yang W, Shen W. Chemokine receptor CXCR4 regulates CaMKII/CREB pathway in spinal neurons that underlies cancer-induced bone pain. Sci Rep 2017; 7: 4005. DOI: 10.1038/s41598-017-04198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 43.Gao T, Hao J, Wiesenfeld-Hallin Z, Wang DQ, Xu XJ. Analgesic effect of sinomenine in rodents after inflammation and nerve injury. Eur J Pharmacol 2013; 721: 5–11. DOI: 10.1016/j.ejphar.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 44.Song ZP, Xiong BR, Guan XH, Cao F, Manyande A, Zhou YQ, Zheng H, Tian YK. Minocycline attenuates bone cancer pain in rats by inhibiting NF-kappaB in spinal astrocytes. Acta Pharmacol Sin 2016; 37: 753–762. DOI: 10.1038/aps.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Q, Shi D, Zhou Y, Zheng H, Xiang H, Tian X, Gao F, Manyande A, Cao F, Tian Y, Ye D. MHC-I promotes apoptosis of GABAergic interneurons in the spinal dorsal horn and contributes to cancer induced bone pain. Exp Neurol 2016; 286: 12–20. DOI: 10.1016/j.expneurol.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Bu H, Shu B, Gao F, Liu C, Guan X, Ke C, Cao F, Hinton AO, Jr., Xiang H, Yang H, Tian X, Tian Y. Spinal IFN-gamma-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat 2014; 143: 255–263. DOI: 10.1007/s10549-013-2807-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Rittner H, Mei W, Tian YK, Zhang HX, Chen F, Ye DW. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol 2018; 14: 391–397. DOI: 10.1016/j.redox.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 2011; 134: 1127–1139. DOI: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu J, Yan Z, Tao K, Li Y, Li Y, Li J, Dong Y, Feng D, Chen H. Sinomenine activates astrocytic dopamine D2 receptors and alleviates neuroinflammatory injury via the CRYAB/STAT3 pathway after ischemic stroke in mice. J Neuroinflamm 2016; 13: 263. DOI: 10.1186/s12974-016-0739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crown ED, Gwak YS, Ye Z, Yu Tan H, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Calcium/calmodulin dependent kinase II contributes to persistent central neuropathic pain following spinal cord injury. Pain 2012; 153: 710–721. DOI: 10.1016/j.pain.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu X, Bo J, Zhang W, Sun X, Zhang J, Yang Y, Ma Z. Intrathecal administration of cyclic AMP response element-binding protein-antisense oligonucleotide attenuates neuropathic pain after peripheral nerve injury and decreases the expression of N-methyl-D-aspartic receptors in mice. Oncol Rep 2013; 30: 391–398. DOI: 10.3892/or.2013.2437. [DOI] [PubMed] [Google Scholar]
- 52.Takano H, Fukushi H, Morishima Y, Shirasaki Y. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain Res 2003; 962: 41–47. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Du F, Shi M, Ye R, Cheng H, Han J, Ma L, Cao R, Rao Z, Zhao G. Ginsenoside Rd protects neurons against glutamate-induced excitotoxicity by inhibiting ca(2+) influx. Cell Mol Neurobiol 2012; 32: 121–128. DOI: 10.1007/s10571-011-9742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Qiao L, Xu W, Wang X, Li H, Xu W, Chu K, Lin Y. Paeoniflorin attenuates cerebral ischemia-induced injury by regulating Ca2+/CaMKII/CREB signaling pathway. Molecules 2017; 22: E359. DOI: 10.3390/molecules22030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain 2009; 13: 138–145. DOI: 10.1016/j.ejpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, Ji RR, Zhang YQ. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci 2015; 35: 7950–7963. DOI: 10.1523/JNEUROSCI.5250-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song H, Han Y, Pan C, Deng X, Dai W, Hu L, Jiang C, Yang Y, Cheng Z, Li F, Zhang G, Wu X, Liu W. Activation of adenosine monophosphate-activated protein kinase suppresses neuroinflammation and ameliorates bone cancer pain: involvement of inhibition on mitogen-activated protein kinase. Anesthesiology 2015; 123: 1170–1185. DOI: 10.1097/ALN.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 58.Miao XR, Fan LC, Wu S, Mao Q, Li Z, Lutz B, Xu JT, Lu Z, Tao YX. DNMT3a contributes to the development and maintenance of bone cancer pain by silencing Kv1.2 expression in spinal cord dorsal horn. Mol Pain 2017; 13: 174480691774068. DOI: 10.1177/1744806917740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao P, Ding Y, Han Z, Mu Y, Hong T, Zhu Y, Li H. Suppression of asparaginyl endopeptidase attenuates breast cancer-induced bone pain through inhibition of neurotrophin receptors. Mol Pain 2017; 13: 174480691770812. DOI: 10.1177/1744806917708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo CH, Bai L, Wu HH, Yang J, Cai GH, Wang X, Wu SX, Ma W. The analgesic effect of rolipram is associated with the inhibition of the activation of the spinal astrocytic JNK/CCL2 pathway in bone cancer pain. Int J Mol Med 2016; 38: 1433–1442. DOI: 10.3892/ijmm.2016.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao M, Chang XY, Chu YX, Yang JP, Wang LN, Cao HQ, Liu MJ, Xu QN. Antiallodynic effects of propentofylline elicited by interrupting spinal glial function in a rat model of bone cancer pain. J Neurosci Res 2011; 89: 1877–1886. DOI: 10.1002/jnr.22711. [DOI] [PubMed] [Google Scholar]
- 62.Shukla SM, Sharma SK. Sinomenine inhibits microglial activation by Abeta and confers neuroprotection. J Neuroinflamm 2011; 8: 117. DOI: 10.1186/1742-2094-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang AL, Li Z, Yuan M, Yu AC, Zhu X, Tso MO. Sinomenine inhibits activation of rat retinal microglia induced by advanced glycation end products. Int Immunopharmacol 2007; 7: 1552–1558. DOI: 10.1016/j.intimp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 64.Zhang MY, Li P, Wang DQ, Niu XH, Wang Y, Wang ZG, Zhang Y, XU S, Xu XJ. Analgesic effect of sinomenine on SSNI model rats and monoamine neurotransmitters in striatal extracellular fluid. Zhongguo Zhong Yao Za Zhi 2013; 38: 597–604. [PubMed] [Google Scholar]
- 65.Kim OS, Park EJ, Joe EH, Jou I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem 2002; 277: 40594–40601. DOI: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- 66.Kang MJ, Park SY, Han JS. Hippocalcin is required for astrocytic differentiation through activation of Stat3 in hippocampal neural precursor cells. Front Mol Neurosci 2016; 9: 110. DOI: 10.3389/fnmol.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem 2008; 107: 50–60. DOI: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- 68.Molet J, Mauborgne A, Diallo M, Armand V, Geny D, Villanueva L, Boucher Y, Pohl M. Microglial Janus kinase/signal transduction and activator of transcription 3 pathway activity directly impacts astrocyte and spinal neuron characteristics. J Neurochem 2016; 136: 133–147. DOI: 10.1111/jnc.13375. [DOI] [PubMed] [Google Scholar]
- 69.Kim HC, Kim E, Bae JI, Lee KH, Jeon YT, Hwang JW, Lim YJ, Min SW, Park HP. Sevoflurane postconditioning reduces apoptosis by activating the JAK-STAT pathway after transient global cerebral ischemia in rats. J Neurosurg Anesthesiol 2017; 29: 37–45. DOI: 10.1097/ANA.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 70.Busch-Dienstfertig M, Labuz D, Wolfram T, Vogel NN, Stein C. JAK-STAT1/3-induced expression of signal sequence-encoding proopiomelanocortin mRNA in lymphocytes reduces inflammatory pain in rats. Mol Pain 2012; 8: 1744-8069-8-83. DOI: 10.1186/1744-8069-8-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang S, Gao Y, Hou W, Liu R, Qi X, Xu X, Li J, Bao Y, Zheng H, Hua B. Sinomenine inhibits A549 human lung cancer cell invasion by mediating the STAT3 signaling pathway. Oncol Lett 2016; 12: 1380–1386. DOI: 10.3892/ol.2016.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlton SM. Localization of CaMKIIalpha in rat primary sensory neurons: increase in inflammation. Brain Res 2002; 947: 252–259. [DOI] [PubMed] [Google Scholar]
- 73.Melgarejo da Rosa M, Yuanxiang P, Brambilla R, Kreutz MR, Karpova A. Synaptic GluN2B/CaMKII-alpha signaling induces synapto-nuclear transport of ERK and jacob. Front Mol Neurosci 2016; 9: 66. DOI: 10.3389/fnmol.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bian H, Yu LC. Intra-nucleus accumbens administration of the calcium/calmodulin-dependent protein kinase II inhibitor AIP induced antinociception in rats with mononeuropathy. Neurosci Lett 2015; 599: 129–132. DOI: 10.1016/j.neulet.2015.05.048. [DOI] [PubMed] [Google Scholar]