Abstract
Background
Patients with diffuse large B-cell lymphoma treated with first-line anthracycline-based immunochemotherapy and remaining in remission at 2 years have excellent outcomes. This study assessed overall survival (OS) stratified by progression-free survival (PFS) at 24 months (PFS24) using individual patient data from patients with DLBCL enrolled in multi-center, international randomized clinical trials as part of the Surrogate Endpoint for Aggressive Lymphoma (SEAL) Collaboration.
Patients and methods
PFS24 was defined as being alive and PFS24 after study entry. OS from PFS24 was defined as time from identified PFS24 status until death due to any cause. OS was compared with each patient’s age-, sex-, and country-matched general population using expected survival and standardized mortality ratios (SMRs).
Results
A total of 5853 patients enrolled in trials in the SEAL database received rituximab as part of induction therapy and were included in this analysis. The median age was 62 years (range 18–92), and 56% were greater than 60 years of age. At a median follow-up of 4.4 years, 1337 patients (23%) had disease progression, 1489 (25%) had died, and 5101 had sufficient follow-up to evaluate PFS24. A total of 1423 assessable patients failed to achieve PFS24 with a median OS of 7.2 months (95% CI 6.8–8.1) after progression; 5-year OS after progression was 19% and SMR was 32.1 (95% CI 30.0–34.4). A total of 3678 patients achieved PFS24; SMR after achieving PFS24 was 1.22 (95% CI 1.09–1.37). The observed OS versus expected OS at 3, 5, and 7 years after achieving PFS24 was 93.1% versus 94.4%, 87.6% versus 89.5%, and 80.0% versus 83.7%, respectively.
Conclusion
Patients treated with rituximab containing anthracycline-based immunochemotherapy on clinical trials who are alive without progression at 24 months from the onset of initial therapy have excellent outcomes with survival that is marginally lower but clinically indistinguishable from the age-, sex-, and country-matched background population for 7 years after achieving PFS24.
Keywords: DLBCL, prognosis, survival, PFS24
Key Message
Newly diagnosed diffuse large B-cell lymphoma patients treated with rituximab and anthracycline-based immunochemotherapy on clinical trials who are alive without progression at 24 months (PFS24) from study entry have survival that is marginally lower but clinically indistinguishable from the age-, sex, and country-matched background population for 7 years after achieving PFS24.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive lymphoma in the USA [1] and Europe [2]. Outcomes have improved with the introduction of immunochemotherapy for DLBCL [3–5] with the majority of patients being cured by front-line therapy. Patients with DLBCL who receive first-line anthracycline-based immunochemotherapy who have not had an event (relapse, re-treatment, or death), at 24 months from diagnosis (EFS24) have excellent outcomes with an overall survival (OS) that is similar to the age- and sex-matched general populations in patient cohorts from observational studies from the USA, France, and Denmark [6, 7].
There is a need for early end points to assess approaches from randomized clinical trials that would not necessitate waiting for OS results in newly diagnosed DLBCL. The Surrogate Endpoint for Aggressive Lymphoma (SEAL) group was assembled to compile a large meta-database as previously described [8]. Progression-free survival (PFS) in patients who had defined evaluation and scan point may offer a more accurate assessment of the time of relapse compared with registry or population-based cohorts. To evaluate the robustness and generalizability of a 24-month end point in the clinical trial setting, this study assessed OS stratified by PFS at 24 months (PFS24) using individual patient data from patients with newly diagnosed DLBCL enrolled in 14 different multi-center, international randomized clinical trials.
Patients and methods
SEAL is an international collaboration of hematologists, oncologists, hematopathologists, statisticians, and scientists. Clinical trials and data in the SEAL database as of 15 July 2016 were utilized for the study [3–5, 9–18]. Individual patient data were pooled as previously described [8]. Central data alignment was carried out by the SEAL statistics and data center at Mayo Clinic Rochester. All patients who were treated with rituximab-containing, anthracycline-based immunochemotherapy as part of initial induction therapy on the trial were included in this analysis. This study was approved by the Institutional Review Board at the Mayo Clinic; research was conducted in accordance with the Declaration of Helsinki.
PFS was defined as the time from study entry to the earliest occurrence of progressive disease, relapse, or death. PFS24 was a binary end point defined as being alive and progression free 24 months (731 days) after initiation of therapy. In patients with progression within 24 months from initiation of therapy (fail to achieve PFS24), OS from PFS24 was defined as time from progression to death from any causes. In patients who were alive and progression free 24 months after initiation of therapy (achieve PFS24), OS from PFS24 was defined as time from achieving PFS24 to death from any cause. Living patients were censored on the date when they were last documented as alive. The OS and PFS24 were derived according to consistent calculation rules across studies. Standardized mortality ratio (SMR) was defined as the ratio of observed deaths to expected deaths in the general population. OS was compared with the age-, sex-, and country-matched general population via SMR and expected survival using a conditional approach [19] via the survexp function in R (package survival), modified to allow country of origin as an additional matching feature in a multinational dataset. Population rate tables for countries were obtained via www.mortality.org where available. A per-study average rate table was used when an individual patient’s country of residence was unavailable. OS beyond 9 years from treatment initiation was only available at the time of analysis for two trials (13% of the cohort); therefore, follow-up from PFS24 was restricted to the first 7 years after PFS24. Analyses were carried out by using SAS version 9.4 (SAS Institute, Inc.) and Rv3.3.1.
Results
From 7975 patients, 5835 (73%) in the SEAL database received rituximab and anthracycline-based immunochemotherapy as part of induction therapy on the trial and were included in this analysis (Table 1). The median age was 62 years (range 18–92), 56% were greater than 60 years of age, 57% were male, 57% had an elevated LDH, 63% had stage III/IV disease, and 13% had an Eastern Cooperative Oncology Group performance status of 2–4. The International Prognostic Index (IPI) was 0–1 in 36%, 2 in 25%, 3 in 23%, and 4–5 in 16%.
Table 1.
Patient’s characteristics
| Variable | N | % |
|---|---|---|
| Sex | ||
| Male | 3148 | 46 |
| Female | 2705 | 54 |
| Age (years) | ||
| ≤60 | 2587 | 44 |
| >60 | 3256 | 56 |
| ECOG Performance Status | ||
| 0–1 | 5079 | 87 |
| 2–4 | 763 | 13 |
| Ann Arbor Stage | ||
| I–II | 2164 | 37 |
| III–IV | 3656 | 63 |
| Number of extranodal sites | ||
| ≤1 | 3660 | 72 |
| ≥2 | 1395 | 28 |
| LDH | ||
| Not elevated | 2490 | 43 |
| Elevated | 3303 | 57 |
| IPI | ||
| 0–1 | 1788 | 36 |
| 2 | 1280 | 25 |
| 3 | 1149 | 23 |
| 4–5 | 809 | 16 |
| Clinical trial | ||
| ANZINTER3 | 224 | 4 |
| ECOG 4494 | 318 | 5 |
| LNH031B | 110 | 2 |
| LNH032B | 380 | 6 |
| LNH036B | 602 | 10 |
| LNH985 | 202 | 3 |
| MAIN | 787 | 13 |
| MEGACHOEP | 262 | 4 |
| MINT | 413 | 7 |
| NHL13 | 741 | 13 |
| PIX203 | 124 | 2 |
| RICOVER60 | 610 | 10 |
| UCL | 1080 | 18 |
| Country of residence | ||
| Austria | 207 | 4 |
| Belgium | 157 | 3 |
| Czech Republic | 74 | 1 |
| France | 1144 | 20 |
| Germany | 861 | 15 |
| Italy | 235 | 4 |
| UK | 1080 | 18 |
| United States | 391 | 7 |
| Other | 504 | 9 |
| Unknown | 1200 | 21 |
At a median follow-up of 4.4 years, 1337 (23%) of patients had disease progression, 1489 patients died; the Kaplan–Meier estimate for achieving PFS24 for the entire cohort of 5835 patient was 73% (95% CI 72% to 74%) and the SMR from diagnosis was 2.42 (95% CI 2.30–2.55). Totally, 5101 patients had sufficient follow-up to be assessable for PFS24; 1423 of assessable patients (28%) did not achieve PFS24 (Figure 1A) with a median OS of 7.2 months (95% CI 6.8–8.1) after progression. The 5-year OS from progression was 19% and the SMR comparing outcomes to expected survival for the age-, sex-, and country-matched general population was 32.1 (95% CI 30.0–34.4). A total of 3678 patients were progression-free at 24 months (achieved PFS24). The subsequent OS after achieving PFS24 approached the age- and sex-matched general population (Figure 1B) with observed versus expected OS at 3, 5, and 7 years of 93.1% versus 94.4%, 87.6% versus 89.5%, and 80.0% versus 83.7%, respectively (Table 2). The SMR after achieving PFS24 was 1.22 (95% CI 1.09–1.37). Higher IPI at diagnosis was associated with greater discrepancies between observed and expected survival (Table 2).
Figure 1.
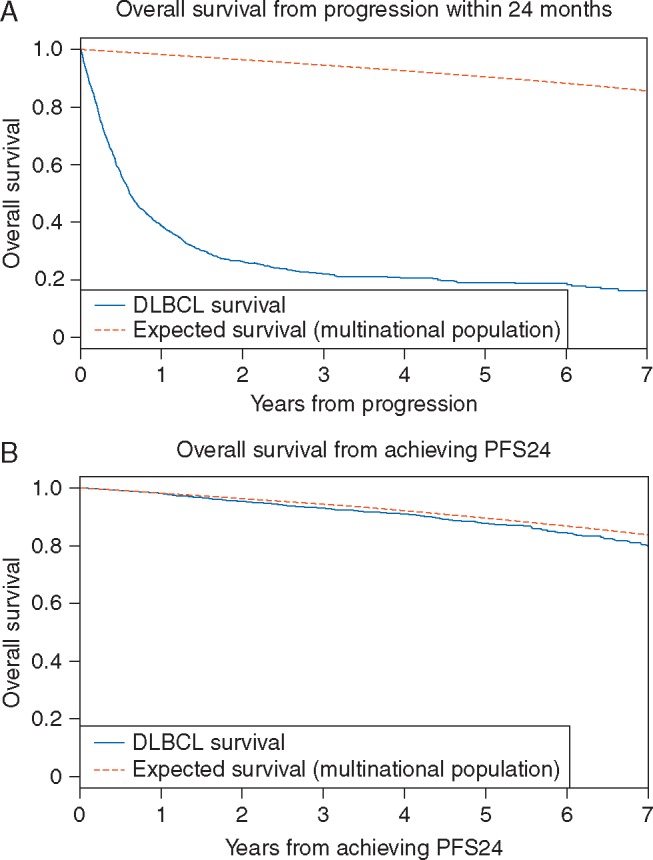
(A) OS from progression of the 1423 patients who failed to achieve PFS24 versus the expected survival from age-, sex-, and country-matched general population data. (B) OS from PFS24 of the 3678 SEAL patients who were progression free at 24 months after initiating treatment versus expected survival from age-, sex-, and country-matched general population data.
Table 2.
Observed versus expected survival in at 3, 5, and 7 years after achieving PFS24
| Subset | N | 3 years from PFS24 |
5 years from PFS24 |
7 years from PFS24 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N at risk | % alive | % expected per population | N at risk | % alive | % expected per population | N at risk | % alive | % expected per population | ||
| All | 3678 | 1617 | 93.1 | 94.4 | 753 | 87.6 | 89.5 | 269 | 80.0 | 83.7 |
| Age <60 | 1597 | 492 | 97.7 | 98.5 | 195 | 96.7 | 97.5 | 46 | 96.7 | 95.9 |
| Age ≥60 | 2080 | 1125 | 90.3 | 91.9 | 558 | 83.4 | 85.9 | 223 | 74.4 | 79.1 |
| Male | 1948 | 836 | 92.8 | 93.4 | 387 | 86.1 | 87.7 | 123 | 77.8 | 81.2 |
| Female | 1730 | 780 | 93.4 | 95.5 | 366 | 89.3 | 91.5 | 146 | 82.2 | 86.3 |
| Stage I/II | 1528 | 678 | 94.3 | 94.8 | 309 | 88.8 | 90.1 | 94 | 83.1 | 84.5 |
| Stage III/IV | 2137 | 935 | 92.2 | 94.1 | 443 | 86.7 | 89.1 | 175 | 78.1 | 83.1 |
| LDH<ULN | 1723 | 771 | 94.2 | 94.3 | 362 | 89.6 | 89.2 | 123 | 81.5 | 83.1 |
| LDH>ULN | 1934 | 846 | 92.1 | 94.5 | 391 | 85.9 | 89.8 | 146 | 78.6 | 84.2 |
| ECOG PS 0–1 | 3309 | 1432 | 93.4 | 94.5 | 662 | 88.4 | 89.8 | 235 | 81.3 | 84.1 |
| ECOG PS 2–4 | 367 | 185 | 90.7 | 93.2 | 91 | 81.4 | 87.6 | 34 | 71.1 | 80.4 |
| IPI 0–1 | 1297 | 533 | 96.5 | 96.3 | 228 | 93.9 | 92.7 | 53 | 89.5 | 88.3 |
| IPI 2–3 | 1653 | 848 | 92.3 | 93.6 | 413 | 85.7 | 88.3 | 164 | 77.8 | 82.1 |
| IPI 4–5 | 427 | 232 | 87.5 | 92.5 | 111 | 80.4 | 86.8 | 52 | 71.2 | 80.2 |
Discussion
In this large series of patients on randomized clinical trials, the PFS24 from the start of initial induction therapy stratifies DLBCL patients treated with the international standard of care, rituximab-containing anthracycline-based immunochemotherapy into populations with distinct outcomes. DLBCL patients who are alive without progression at 24 months from the onset of initial therapy have excellent survival. The OS for this group was marginally lower than, but clinically indistinguishable from the age-, sex-, and country-matched background population for at least 5 to 7 years after achieving PFS24. Survival without an event at this timepoint provides clinicians, patients, and caregivers with a clear benchmark for evaluating the success of initial treatment in the modern era.
The strengths of this study include the large numbers of patients treated prospectively on protocol who were followed at protocol defined time intervals and evaluation criteria to prospectively document progression. This is the largest series of patients with DLBCL studied in the immunochemotherapy era world-wide with prospectively collected data of treatment and outcomes at prespecified follow-up intervals and should have excellent generalizability. Combining data from multiple randomized controlled clinical trials added substantial increases in sample size and statistical power. The limitations of this analysis include the following. There is an under-representation of patients older than 85 years of age. Outcomes for older individuals with DLBCL have been previously assessed using registry data linked to a claims database [20]. In this analysis, there was a lack of long-term follow-up >10 years and only two trials, 13% of the cohort, had OS data beyond 9 years. The patients included in these studies may not reflect the general population of DLBCL since they needed to qualify for the study and there may have been a time to treatment bias [21]. Patients with significant end-organ disease, central nervous system disease, HIV, and other contraindications to clinical trials would not have been included in this analysis. The impact of management was not assessed after relapse, which is beyond the scope of this dataset, and such information is not routinely collected in clinical trials. Finally, analysis was carried out using the PFS definition from the individual clinical trials. EFS, where change in therapy was considered an event, was only available on a small subset of trials in the SEAL database at the time of this study. The evaluation of EFS24 versus PFS24 for patients being treated on trials may be explored in a future study as more EFS data becomes available in the SEAL database.
These data confirm other recently published data in newly diagnosed DLBCL. In a cohort of 767 patients from the USA with a median follow-up of 60 months, the SMR after achieving EFS24 (freedom from progression, second therapy, or death) was 1.18 (0.89–1.57) [6]. In the same publication, a Lyon/Groupe d’Etude des Lymphomes de l’Adulte (GELA) cohort of 820 patients with a median follow-up of 42 months had an SMR after achieving EFS24 of 1.09 (0.69–1.74). In a Danish population-based cohort of 1621 patients with a median follow-up of 85 months, those who achieved EFS24 from the completion of frontline immunochemotherapy had a subsequent SMR of 1.27 (1.12–1.44) [7]. Combined with our study, these results support a small but clinically insignificant reduction in survival for DLBCL survivors in the first 5–7 years after achieving EFS24 or PFS24. Additional follow-up is needed on these and other cohorts to evaluate longer outcomes and assess for impact of late relapses, quality of life and other factors that may negatively affect survivorship beyond the time periods that have been studied.
Our results and the findings of these corroborating studies have significant implications for lymphoma survivorship. Given that the vast majority of DLBCL events occur in the first 24 months, individuals treated with R-CHOP who survive to this time point without relapse are highly likely to experience a near normal life expectancy. Surveillance imaging with computed tomography has been demonstrated to be of limited value [22, 23]. Novel approach for surveillance assessment utilizing circulating tumor DNA are in development [22–25]. Our data suggest that studies evaluating novel surveillance strategies for disease relapse are likely to be of greatest benefit in the first 2 years following immunochemotherapy. Following achievement of PFS24, the critical research questions for DLBCL survivors should focus on quality of life, secondary malignancies, and other outcomes.
It is important to caution that extension of these results in the settings of relapsed DLBCL or maintenance after immunochemotherapy in DLBCL is not supported at this time. A number of randomized clinical trials have failed to show either a PFS or OS benefit for maintenance therapy after immunochemotherapy [5, 18, 26, 27] in DLBCL while a recent trial showing a benefit of PFS for maintenance after completion of immunochemotherapy failed to show an OS benefit [28]. It is important to note that both the pattern of treatment failure and overall prognosis in the maintenance setting are distinct from diagnosis. The rate of progression or relapse for newly diagnosed DLBCL is highest in the first 6 months after diagnosis, reduces slightly between 6 and 12 months, and then shows further decreases between 12 and 24 months and beyond 24 months [6]. In regard to prognosis, the SMR of patients in CR or CRu after immunochemotherapy was 1.75 in a recent Danish study [7] that was markedly lower than the SMR at diagnosis of 2.42 from our study and SMRs of 2.88 and 4.99 reported in USA and French patients at diagnosis [6]. In addition, there are limited data on the validity of PFS24 in the current relapsed and refractory setting in DLBCL. A recent study evaluated 1617 patients with relapsed DLBCL or Hodgkin lymphoma who were 2-year survivors after autologous hematopoietic transplant [29]. At a median follow-up of 10.6 years of the cohort, the 5-year OS was 89% and the SMR for relapsed DLBCL was 3.4 (95%: 2.9–4.1) compared with the age- and sex-matched general population. However, the marked clinical heterogeneity of outcomes in patients with relapsed DLBCL [30] make adaptation of PFS24 challenging in the broader relapsed setting. Further evaluation is needed before utilization of the PFS24 end point can be extended to the relapsed setting.
In summary, patients treated with rituximab containing anthracycline-based immunochemotherapy who are alive without progression at 24 months from the onset of initial therapy on clinical trials have excellent outcomes with survival that is marginally lower but clinically indistinguishable from the age-, sex-, and country-matched background population for 7 years after achieving PFS24. Further follow-up will be needed to assess any long-term impact of late relapse and survivorship issues in this population.
Acknowledgements
We would like to sincerely thank and dedicate this manuscript in memory of Dr Daniel Sargent, who was a principal leader in integrating his expertise in biostatistics and oncology with vision and foresight for establishing the importance of identifying early surrogate markers of prolonged survival outcomes. Dan was vital in bringing global investigators together to build data sharing programs with large metadatabases across academic and industry teams for purposes of answering advanced research questions across multiple, diverse studies, and with the continuation of these studies, his legacy continues to live on. We thank all the patients, families, and caregivers who participated in each of these studies. We thank all of the investigators and contributors for providing study data: FM, TMH, CR, RD, CH, BC, PF, JFS, NS, MP, UJ, RH, MP, and DC. We also thank all the SEAL group co-investigators and study groups who contributed data for this analysis, and the Mayo Clinic. SEAL Group Membership: The SEAL group (in alphabetical order) consists of: Bertrand Coiffier, David Cunningham, Jocelyne Flament, Christopher R. Flowers, Tommy Fu, Herve Ghesquieres, Thomas M. Habermann, Corinne Haioun, Raoul Herbrecht, Ulrich Jaeger, Matthew J. Maurer, Francesco Merli, Tina Nielsen, Michael Pfreundschuh, Norbert Schmitz, John F. Seymour, Qian Shi, Hervé Tilly, Lixia Wang, and Marita Ziepert.
Funding
This work was supported by grants from Celgene Corporation. Data were transmitted directly from original study cooperative groups to the Mayo Clinic. Celgene Corporation supported organization and meetings of the SEAL group. The National Institutes of Health University of Iowa/Mayo Clinic Lymphoma SPORE (CA97274) grant provided support for MJM and TMH.
Disclosure
MJM reports research funding to his institution from Celgene and Morphosys. DC reports research funding from Amgen, Astra-Zeneca, Bayer, Celgene, Merrimack, and Medimmune. JS reports consultancy, honoraria, and membership on an entity’s Board of Directors or advisory committee for AbbVie, Clegene, Genentech, Gilead, Janssen, Roche, and Takeda. UJ reports honoraria and research funding from Celgene and Roche. CH reports honoraria and membership on an entity’s Board of Directors or advisory committee for Celgene, Gilead, Janssen, Roche, and Sandoz. HT reports research funding and membership on an advisory board for Celgene. HG reports consultancy for Celgene and Mundlpharma, advisory committee for Celgene, and research funding from Roche, France. FM reports membership on an entity’s Board of Directors or advisory committee for Celgene, Roche, and Teva Pharmaceuticals Industries, and research funding from Roche. RH reports membership on an entity’s Board of Directors or advisory committee for Cell Therapeutics, Inc., Gilead, and Servier. JF and TF are employed by and have received equity ownership in Celgene Corporation. CF reports consultancy for Genetech, Gilead, and Roche; research funding from AbbVie, Acerta, ECOG, Genentech, Gilead, Infinity, Millenium/Takeda, NIH, Pharmacyclics, LLC(an AbbVie company), Roche, and TG Therapeutics. BC reports consultancy for Celgene, Celltrion, Gilead, MorphoSys, Novartis, Pfizer, and Roche; membership on an entities Board or advisory committee for Celgene, Celltrion, Gilead, Novartis, and Pfizer. QS, TH, NS, and MZ have declared no conflicts of interest.
Footnotes
Note: This study was previously presented at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, USA, 3–6 December 2016.
References
- 1. Al-Hamadani M, Habermann TM, Cerhan JR. et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 2015; 90(9): 790–795. [DOI] [PubMed] [Google Scholar]
- 2. Sant M, Allemani C, Tereanu C. et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010; 116(19): 3724–3734. [DOI] [PubMed] [Google Scholar]
- 3. Coiffier B, Lepage E, Briere J. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346(4): 235–242. [DOI] [PubMed] [Google Scholar]
- 4. Pfreundschuh M, Trümper L, Österborg A. et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7(5): 379–391. [DOI] [PubMed] [Google Scholar]
- 5. Habermann TM, Weller EA, Morrison VA. et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006; 24(19): 3121–3127. [DOI] [PubMed] [Google Scholar]
- 6. Maurer MJ, Ghesquières H, Jais J-P. et al. Event-free survival at 24 months is a Robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 2014; 32(10): 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jakobsen LH, Bøgsted M, Brown PDN. et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: a Danish Population-Based Study. J Clin Oncol 2017; 35(7): 778–784. [DOI] [PubMed] [Google Scholar]
- 8. Sargent DJ, Shi Q, Flowers CR. et al. The search for surrogate endpoints in trials in diffuse large B‐cell lymphoma: the surrogate endpoints for aggressive lymphoma project. Oncologist 2017; 22(12): 1415–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfreundschuh M, Schubert J, Ziepert M. et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9(2): 105–116. [DOI] [PubMed] [Google Scholar]
- 10. Haioun C, Mounier N, Emile JF. et al. Rituximab versus observation after high-dose consolidative first-line chemotherapy with autologous stem-cell transplantation in patients with poor-risk diffuse large B-cell lymphoma. Ann Oncol 2009; 20(12): 1985–1992. [DOI] [PubMed] [Google Scholar]
- 11. Récher C, Coiffier B, Haioun C. et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet 2011; 378(9806): 1858–1867. [DOI] [PubMed] [Google Scholar]
- 12. Merli F, Luminari S, Rossi G. et al. Cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly “fit” patients with diffuse large B-cell lymphoma: results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma 2012; 53(4): 581–588. [DOI] [PubMed] [Google Scholar]
- 13. Schmitz N, Nickelsen M, Ziepert M. et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol 2012; 13(12): 1250–1259. [DOI] [PubMed] [Google Scholar]
- 14. Delarue R, Tilly H, Mounier N. et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013; 14(6): 525–533. [DOI] [PubMed] [Google Scholar]
- 15. Herbrecht R, Cernohous P, Engert A. et al. Comparison of pixantrone-based regimen (CPOP-R) with doxorubicin-based therapy (CHOP-R) for treatment of diffuse large B-cell lymphoma. Ann Oncol 2013; 24(10): 2618–2623. [DOI] [PubMed] [Google Scholar]
- 16. Cunningham D, Hawkes EA, Jack A. et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013; 381(9880): 1817–1826. [DOI] [PubMed] [Google Scholar]
- 17. Seymour JF, Pfreundschuh M, Trnĕný M. et al. R-CHOP with or without bevacizumab in patients with previously untreated diffuse large B-cell lymphoma: final MAIN study outcomes. Haematologica 2014; 99(8): 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaeger U, Trneny M, Melzer H. et al. Rituximab maintenance for patients with aggressive B-cell lymphoma in first remission: results of the randomized NHL13 trial. Haematologica 2015; 100(7): 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verheul HA, Dekker E, Bossuyt P. et al. Background mortality in clinical survival studies. Lancet 1993; 341(8849): 872–875. [DOI] [PubMed] [Google Scholar]
- 20. Williams JN, Rai A, Lipscomb J. et al. Disease characteristics, patterns of care, and survival in very elderly patients with diffuse large B-cell lymphoma. Cancer 2015; 121(11): 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maurer MJ, Ghesquières H, Link BK. et al. Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol 36(16): 1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson CA, Ghesquieres H, Maurer MJ. et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol 2014; 32(31): 3506–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen JB, Behera M, Thompson CA. et al. Evaluating surveillance imaging for diffuse large B-cell lymphoma and Hodgkin lymphoma. Blood 2017; 129(5): 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurtz DM, Green MR, Bratman SV. et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 2015; 125(24): 3679–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherer F, Kurtz DM, Newman AM. et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med 2016; 8(364): 364ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crump M, Leppä S, Fayad L. et al. Randomized, double-blind, phase III trial of enzastaurin versus placebo in patients achieving remission after first-line therapy for high-risk diffuse large B-cell lymphoma. J Clin Oncol 2016; 34(21): 2484–2492. [DOI] [PubMed] [Google Scholar]
- 27. Witzig TE, Tobinai K, Rigacci L. et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol 2018; 29(3): 707–714. [DOI] [PubMed] [Google Scholar]
- 28. Thieblemont C, Tilly H, Gomes da Silva M. et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2017; 35(22): 2473–2481. [DOI] [PubMed] [Google Scholar]
- 29. Myers RM, Hill BT, Shaw BE. et al. Long‐term outcomes among 2‐year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large b‐cell lymphoma. Cancer 2018; 124(4): 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farooq U, Maurer MJ, Thompson CA. et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol 2017; 179(1): 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


