Abstract
Background
Alterations involving the RET kinase are implicated in the pathogenesis of lung, thyroid and other cancers. However, the clinical activity of multikinase inhibitors (MKIs) with anti-RET activity in RET-altered patients appears limited, calling into question the therapeutic potential of targeting RET. LOXO-292 is a selective RET inhibitor designed to inhibit diverse RET fusions, activating mutations and acquired resistance mutations.
Patients and methods
Potent anti-RET activity, high selectivity, and central nervous system coverage were confirmed preclinically using a variety of in vitro and in vivo RET-dependent tumor models. Due to clinical urgency, two patients with RET-altered, MKI-resistant cancers were treated with LOXO-292, utilizing rapid dose-titration guided by real-time pharmacokinetic assessments to achieve meaningful clinical exposures safely and rapidly.
Results
LOXO-292 demonstrated potent and selective anti-RET activity preclinically against human cancer cell lines harboring endogenous RET gene alterations; cells engineered to express a KIF5B-RET fusion protein −/+ the RET V804M gatekeeper resistance mutation or the common RET activating mutation M918T; and RET-altered human cancer cell line and patient-derived xenografts, including a patient-derived RET fusion-positive xenograft injected orthotopically into the brain. A patient with RET M918T-mutant medullary thyroid cancer metastatic to the liver and an acquired RET V804M gatekeeper resistance mutation, previously treated with six MKI regimens, experienced rapid reductions in tumor calcitonin, CEA and cell-free DNA, resolution of painful hepatomegaly and tumor-related diarrhea and a confirmed tumor response. A second patient with KIF5B-RET fusion-positive lung cancer, acquired resistance to alectinib and symptomatic brain metastases experienced a dramatic response in the brain, and her symptoms resolved.
Conclusions
These results provide proof-of-concept of the clinical actionability of RET alterations, and identify selective RET inhibition by LOXO-292 as a promising treatment in heavily pretreated, multikinase inhibitor-experienced patients with diverse RET-altered tumors.
Keywords: RET, multiknase inhibitor, targeted kinase inhibitor, TKI, gatekeeper
Key Message
Selective RET inhibition with LOXO-292 is highly active in preclinical models of RET-altered cancers in vitro and in vivo. Two patients with tumors that harbor activating RET alterations (RET fusion-positive lung cancer and RET-mutant medullary thyroid cancer) who were heavily pre-treated and progressed on prior multikinase inhibitor therapy received LOXO-292. Dramatic responses were achieved in both, highlighting the utility of this treatment paradigm.
Introduction
The receptor tyrosine kinase RET can be oncogenically activated by gene fusions or point mutations. RET fusions occur in a variety of malignancies, including 1%–2% of lung cancers, up to 10%–20% of papillary thyroid cancers, and rarely in many other solid tumors [1]. RET mutations affect most medullary thyroid cancers (MTCs) [2], and next generation sequencing (NGS) analysis of large numbers of patient tumors has uncovered RET alterations at low frequency in other tumor types [3]. Such alterations possess the hallmarks of cancer drivers: constitutive kinase and signaling activity, transformation of primary cells, and mutual exclusivity from other drivers [1, 4–6].
Until recently, only multikinase inhibitors (MKIs) with nonselective RET inhibitory activity have been available for patients with RET-altered cancers. Clinical experience with these nonselective RET inhibitors has been disappointing, with only modest activity in RET-mutant MTCs [3, 7–9] and RET fusion-positive lung cancers [10–11]. Other MKIs approved for other indications (e.g. sorafenib) possess similar, nonselective anti-RET activity preclinically [12]. In part, this may be due to substantial ‘off-target’ side-effects that limit the degree of RET-specific inhibition and lead to frequent dose reductions. Together with weak anti-RET potency and poor pharmacokinetic (PK) properties, these limitations prevent potent RET pathway inhibition in patients.
LOXO-292 is a novel, highly selective, ATP-competitive small molecule RET inhibitor. In contrast to MKIs, LOXO-292 possesses nanomolar potency against diverse RET alterations (including anticipated acquired resistance mutations), high selectivity for RET, and favorable PK properties, including high bioavailability, predictable exposure, significant central nervous system (CNS) penetration, and a low potential for drug interactions [13]. Here, we describe the preclinical antitumor activity of LOXO-292 and provide clinical proof-of-concept for selective RET inhibition with LOXO-292 in two patients with RET-altered tumors.
Methods-patients
Case 1: RET M918T-mutant MTC with an acquired RET V804M gatekeeper resistance mutation
A healthy 49-year-old man presented with neck swelling. A diagnostic work-up led to total thyroidectomy and bilateral neck dissection, and a diagnosis of MTC with lymph node spread. NGS (FoundationOne®) analysis of the resected tumor identified a RET M918T mutation. Additional imaging identified liver metastases. He was sequentially treated with six MKI regimens: sorafenib [best response of progressive disease (PD)], vandetanib [stable disease (SD)], cabozantinib (SD), MGCD-516 (PD), RXDX-105 [partial response (PR)], and vandetanib plus everolimus (PD). Each treatment was discontinued for disease progression in the liver, most recently with hepatomegaly, large-volume ascites, severe fatigue and markedly decreased performance status. Molecular analysis of cell-free DNA (Guardant360®) isolated from blood taken before vandetanib plus everolimus treatment identified the founder RET M918T mutation together with a RET V804M gatekeeper mutation; the latter mutation was not detectable in the initial surgical tumor specimen.
Case 2: KIF5B-RET fusion-positive non-small-cell lung cancer metastatic to the brain
A 44-year-old woman with multiple comorbidities presented with cough and worsening dyspnea [14]. Imaging revealed bilateral lung nodules and mediastinal lymphadenopathy. A bronchoscopic biopsy of a right lung mass revealed lung adenocarcinoma. Molecular testing for EGFR, ALK and ROS1 gene alterations was negative. She received five lines of systemic therapy, including chemotherapy and immunotherapy (best response of PR to first line chemotherapy and no response to immunotherapy), and she required whole-brain radiotherapy to control new brain metastases. Analysis of the initial biopsy by NGS (FoundationOne®) identified a KIF5B-RET fusion. Compassionate use treatment with alectinib, an MKI with modest anti-RET activity but significant CNS penetration [15], achieved an extracranial PR. However, her cancer progressed in the brain. An increased dose of alectinib led to an intracranial response that was consolidated with stereotactic radiosurgery. However, despite continuing the increased dose of alectinib, she developed further intracranial progression, difficulty walking, and short-term memory loss.
In vitro and in vivo studies
Cell target inhibition was assessed using engineered HEK-293 cells treated with each agent. All DNA research was carried out in accordance with the National Institutes of Health Guidelines for Research Involving Recombinant DNA Molecules. For cell proliferation studies, cell lines were treated with each inhibitor for 72 h followed by cell counting. Animal studies were carried out in accordance with the 2011 Guide for the Care and Use of Laboratory Animals and AAALAC-International. Tumor cells were injected subcutaneously (or intracranially) into immunodeficient mice and treated by oral gavage. Body weight, tumor size and animal condition were monitored at regular intervals.
PK–pharmacodynamic analyses
Cellular phosphorylated-KIF5B-RET−/+ V804M and RET M918T inhibitory concentrations for each agent were corrected for plasma protein binding and estimated CNS penetration, and displayed as the percent RET inhibition achieved at the maximum and minimum (Cmax/Cmin) concentrations of each agent measured in patient plasma samples.
Treatment protocols
Each patient provided written informed consent to participate in a single-patient clinical trial of LOXO-292 after Institutional Review Board (IRB) approval from treating sites (The University of Texas MD Anderson Cancer Center, Cleveland Clinic Foundation) approved the treatment plan, and the U.S. FDA allowed treatment to proceed.
Rapid, intrapatient, PKs-guided dose-escalation was carried out. LOXO-292 was administered orally as a powder-in-capsule. Drug plasma levels were measured pre-dose and at defined post-dose intervals on days 1 and 8 of the starting dose and each dose escalation. Escalation was allowed only after ≥7 days, in the absence of grade 3 and 4 toxicity, and in defined increments (1.5–3× the previous dose depending on the predicted AUC0–24 at the new dose). Dose modifications and interruptions followed a prescribed algorithm. Adverse events were graded using the Common Terminology Criteria for Adverse Events version 4.03 [16]. Response was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) [17, 18].
Additional methodologic details are included in supplementary Appendix, available at Annals of Oncology online.
Results
Characterization of RET inhibitor selectivity
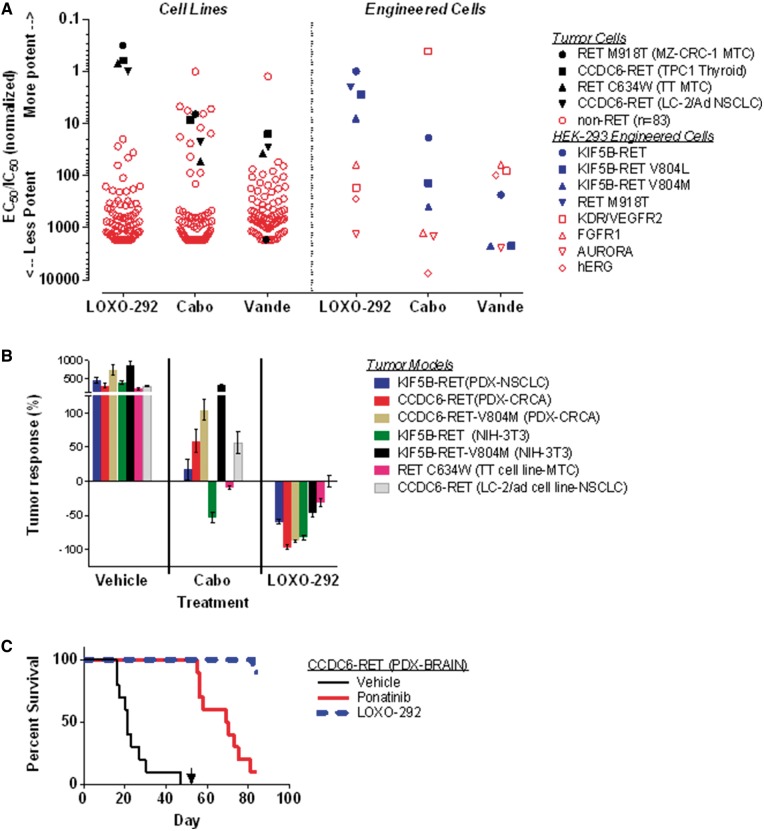
Consistent with high selectivity for RET compared with other kinases [13], LOXO-292 potently inhibited cell proliferation in four RET fusion-positive or RET-mutant cell lines, but caused 20- to 1700-fold less growth inhibition in 83 cell lines that did not contain a RET alteration (Figure 1A and supplementary Figure S1, available at Annals of Oncology online). In contrast, the inhibitory activity of cabozantinib and vandetanib in cell lines without RET alterations significantly overlapped with the RET-altered cell lines, indicative of broad inhibition of proliferation due to inhibition of multiple cellular targets (Figure 1A, left). In engineered cells, LOXO-292 was 60- to 1300-fold more effective in inhibiting KIF5B-RET (−/+V804L/M) than other kinase and nonkinase targets commonly inhibited by MKIs (Figure 1A, right). In contrast, the MKIs cabozantinib and vandetanib were more potent against cellular KDR/VEGFR2 (both agents) or FGFR1 and hERG (vandetanib) than against KIF5B-RET, and possessed less inhibitory activity against KIF5B-RET-V804L/M gatekeeper mutant cells (Figure 1A).
Figure 1.
Pre-clinical characterization of RET inhibitor selectivity and antitumor activity. As shown in the left portion of (A), four RET-altered (filled black symbols) and 83 RET-wild-type (open red symbols) human cancer cell lines were treated with LOXO-292, cabozantinib or vandetanib for 72 h. Cell survival was determined by DAPI staining and cell counting. Half-maximal effective concentration (EC50) values were normalized to the EC50 value for LOXO-292-treated LC-2/ad (CCDC6-RET) non-small-cell lung cancer cells. For each cell line, ratios equal to 1 correspond to the same cytotoxicity as for LC-2/ad, ratios <1 correspond to greater cytotoxicity than for LC-2/ad and ratios >1 correspond to lower cytotoxicity than for LC-2/ad. As shown in the right portion of Panel A, HEK-293 cells harboring different mutant versions of RET (filled blue symbols) or other targets (open red symbols) were treated with each inhibitor, followed by determination of RET kinase or target activity in cell lysates as described in supplementary Appendix, available at Annals of Oncology online. Half-maximal inhibitory concentration (IC50) values were normalized to the IC50 value for LOXO-292-treated KIF5B-RET cells. For each target, ratios equal to 1 correspond to the same cellular potency as for KIF5B-RET cells, ratios less than 1 correspond to greater potency than for KIF5B-RET cells, and ratios greater than 1 correspond to lower potency than for KIF5B-RET cells. As shown in (B), immunodeficient mice xenografted with the indicated RET-altered tumor models were treated orally with vehicle, cabozantinib (40-60 mg/kg daily) or LOXO-292 (30 mg/kg twice daily) for 14 (NIH-3T3 allografts), 22 (cell line xenografts) or 28 (patient-derived xenografts/PDX) days. For each model, tumor volume from 8 to 10 animals at the end of the treatment period was normalized to tumor volume before the first treatment; 0% corresponds to no net effect of treatment on tumor size, <0% corresponds to net tumor regression with treatment, and >0% corresponds to net tumor growth despite treatment. As shown in (C), immunodeficient mice (10 per treatment) were injected intracranially with CCDC6-RET fusion-positive PDX tumor suspensions and treated orally with vehicle, ponatinib (40 mg/kg daily) or LOXO-292 (30 mg/kg twice daily). Animals were sacrificed if they displayed significant morbidity, and survival was compared with Kaplan–Meier analysis before and after dose reduction by 10-fold on day 52 (indicated with a black arrow). EC50, half-maximal effective concentration; IC50, half maximal inhibitory concentration; Cabo, cabozantinib; Vande, vandetanib; PDX, patient-derived xenograft; MTC, medullary thyroid cancer; NSCLC, non-small cell lung cancer; CRCA, colorectal cancer.
Determining RET inhibitor antitumor activity preclinically
The antitumor activity of LOXO-292 was compared with the MKI cabozantinib in engineered and patient-derived RET fusion-positive and RET-mutant mouse tumor models, including two RET fusion-positive models harboring the V804M acquired resistance gatekeeper mutation [19]. At the maximum tolerated dose (MTD), cabozantinib caused only mild regression or tumor growth inhibition and was inactive against models harboring RET V804M (Figure 1B). In contrast, LOXO-292 caused significant regression in all models, including those harboring RET V804M, and was well-tolerated (Figure 1B and supplementary Figure S2, available at Annals of Oncology online).
As brain metastases are present in a substantial proportion of RET fusion-positive lung cancer patients (∼45% lifetime incidence) [20], the intracranial antitumor activity of LOXO-292 was investigated in an orthotopic RET fusion-positive mouse tumor model. Animals injected intracranially with CCDC6-RET fusion-positive PDX cell suspensions were treated orally with LOXO-292, the MKI ponatinib (at the MTD), or vehicle. Both LOXO-292 and ponatinib significantly prolonged survival (median not reached) compared with vehicle-treated animals (median 21 days) (Figure 1C). At proportionately reduced doses, LOXO-292 significantly prolonged survival (median not reached) compared with ponatinib (median 18.5 days) (Figure 1C).
Clinical experience with LOXO-292
Although a LOXO-292 phase I study had been initiated (NCT03157128), because of the clinical urgency and desire to achieve meaningful clinical exposures in a time frame that could help each patient, a real-time, PKs-guided intrapatient dose escalation approach was undertaken using IRB-approved single-patient protocols (supplementary Figure S3, available at Annals of Oncology online) [21].
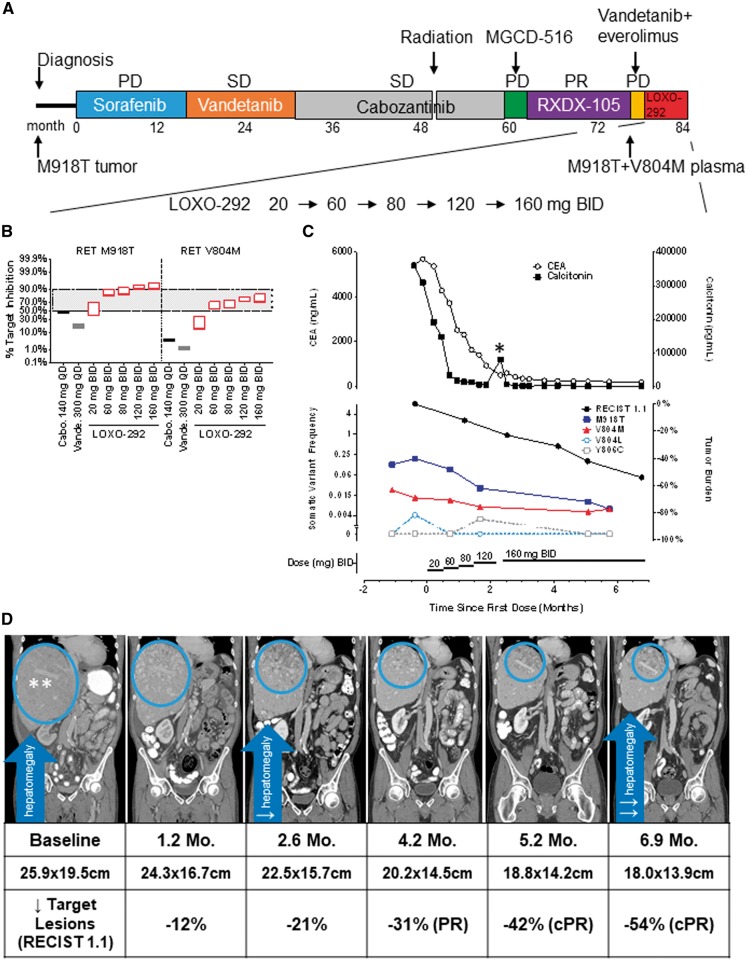
Case 1 was highly symptomatic before therapy (Figure 2A), with right upper quadrant pain from tumor infiltration of the liver, ascites and severe tumor-related diarrhea (30 bowel movements/day). LOXO-292 was initiated at a dose of 20 mg twice daily. Real-time PK analysis revealed that the estimated RET-M918T and RET-V804M target coverage might be insufficient at this dose. Therefore, the patient was dose-escalated stepwise (if no grade 3 and 4 adverse events were experienced after a minimum of 14 days on the prior dose) to a final dose of 160 mg twice daily, resulting in dose-proportional systemic drug exposure consistent with continuous and significant RET-M918T and RET-V804M target inhibition (Figure 2B and supplementary Figure S4, available at Annals of Oncology online). Consistent with this pharmacodynamic RET inhibition, serum carcinoembryonic antigen and calcitonin levels decreased dramatically, together with resolution of diarrhea, abdominal pain and fatigue, and a deepening radiographic tumor response, reaching 54% (by RECIST) after 6.9 months of treatment (Figure 2C and D; supplementary Figure S5, available at Annals of Oncology online). Plasma allele fractions encoding RET-M918T and RET-V804M in cell-free DNA decreased over the first 8 weeks of treatment and remained suppressed (Figure 2C). Additional gatekeeper mutations (RET V804L and RET Y806C) detected just above the lower limit of assay detection also decreased to undetectable levels (Figure 2C). LOXO-292 was well-tolerated. The only treatment-emergent adverse events observed (fatigue, dyspnea, joint pain, insomnia and aspartate aminotransferase elevation) were grade 1, and none were attributed to therapy. The patient remains on LOXO-292 for more than 7 months.
Figure 2.
Clinical activity of LOXO-292 in medullary thyroid cancer after acquired resistance to multikinase inhibitors. (A) The various treatments the patient received for metastatic RET M918T-mutated medullary thyroid cancer, together with the duration of and best response to each treatment. (B) Estimated RET target inhibition based on measured LOXO-292 plasma levels determined at the indicated times after 8 days of treatment with each dose of LOXO-292, compared with cabozantinib and vandetanib. The lower edge of each rectangle corresponds to the steady-state minimum concentration (Cmin) of each inhibitor/target, the upper edge corresponds to the maximum concentration (Cmax). The degree of target inhibition was modelled using actual patient (LOXO-292) or published (cabozantinib, vandetanib) human pharmacokinetic parameters (see supplementary Appendix, available at Annals of Oncology online). (C) Serial monitoring of (upper) carcinoembryonic antigen (CEA, open circles), calcitonin (filled squares), and (lower) plasma cell-free DNA allelic fraction of the founder M918T mutation and V804M, V804L and Y806C gatekeeper mutations during LOXO-292 treatment. (D) Computed tomographic images of the patient's metastatic liver disease before and at the indicated times after he initiated treatment with LOXO-292. A radiologic PR by RECIST was achieved after 4.2 months that deepened with treatment (best objective tumor regression 54% after 6.9 months of treatment), with concurrent resolution of hepatomegaly. PD, progressive disease; SD, stable disease; PR, partial response; Cabo, cabozantinib; Vande, vandetanib; mg, milligrams; QD, once daily; BID, twice daily; CEA, carcinoembryonic antigen; ng, nanograms; ml, milliliters; pg, picograms; Mo, month; PR, partial response; cPR, confirmed partial response. *The patient had an interruption in the dosing of LOXO-292 during ‘Hurricane Harvey’, the unprecedented 1000-year flood that affected Houston, Texas. There was a transient elevation in calcitonin and CEA that decreased when LOXO-292 was resumed. **Note infiltrative pattern of liver involvement by tumor.
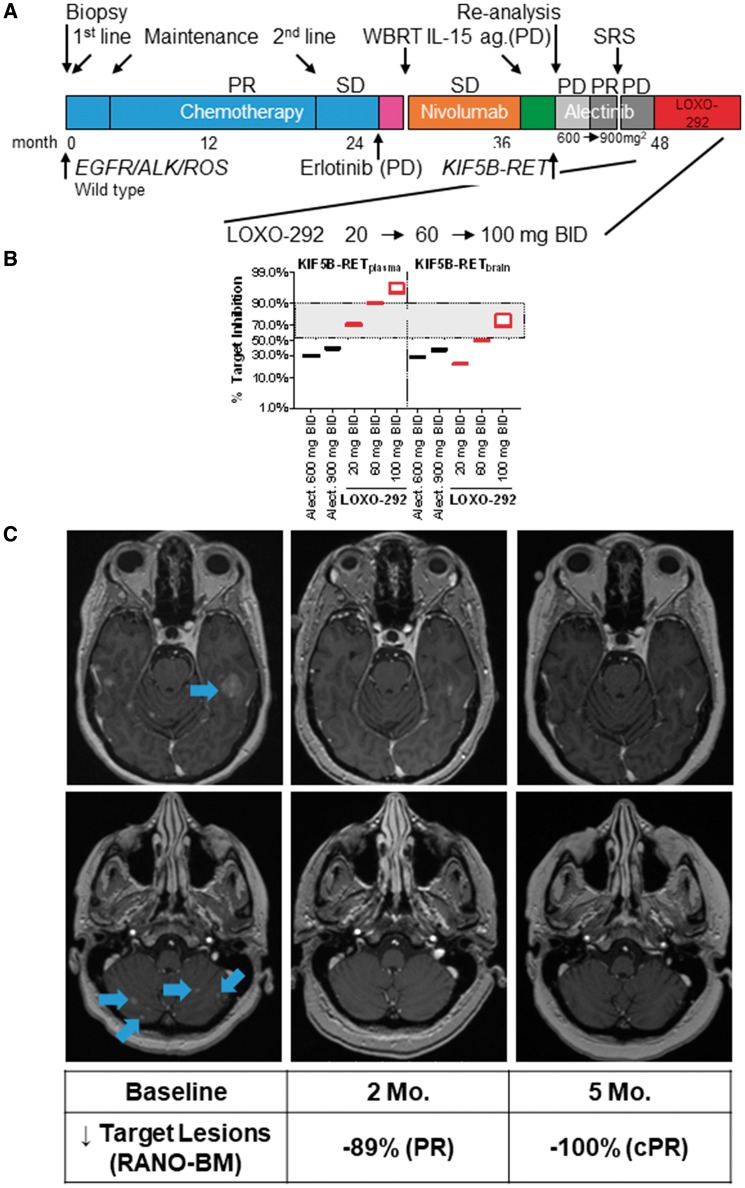
Case 2 was also highly symptomatic, with confusion and unsteady gait due to progressive brain metastases. She initiated treatment with LOXO-292 at a starting dose of 20 mg twice daily (Figure 3A). Although real-time PK analysis revealed adequate KIF5B-RET target coverage in plasma, steady-state levels were thought to be sub-optimal for inhibition of brain metastases. Therefore, the dose of LOXO-292 was escalated stepwise (after a minimum of 14 days on the prior dose, in the absence of grade 3 and 4 adverse events) to 100 mg twice daily, resulting in LOXO-292 exposure consistent with continuous and significant KIF5B-RET fusion target coverage in the brain (Figure 3B and supplementary Figure S4, available at Annals of Oncology online). LOXO-292 treatment resulted in a rapid clinical response to therapy, with resolution of confusion and gait imbalance, together with rapid tumor regression (−57% by RECIST) and substantial shrinkage or resolution of multiple brain metastases (−89% by RANO-BM) after 2 months of treatment. These responses deepened and were confirmed with continued treatment (best objective responses at 5 months, −67% extracranially, with residual foci of nonspecific enhancement and no measurable target lesions in the brain) (Figure 3C and supplementary Figure S6, available at Annals of Oncology online). However, the patient’s complex comorbidities contributed to unrelated hospitalizations and treatment-emergent adverse events that were not attributed to LOXO-292. Initially, treatment with LOXO-292 was continued with evidence of continued tumor response and clinical benefit, but after 5 months, the patient chose to discontinue LOXO-292 during a recurrent depressive episode, against medical advice.
Figure 3.
Clinical activity of LOXO-292 in KIF5B-RET fusion-positive lung cancer metastatic to the brain. (A) The various treatments the patient received for metastatic KIF5B-RET fusion-positive non-small-cell lung cancer together with the best response to each treatment. (B) Estimated target inhibition based on steady-state LOXO-292 Cmin and Cmax plasma levels determined at the indicated times after 8 days of treatment with the indicated doses of LOXO-292, compared with alectinib. The degree of target inhibition was modelled using actual patient (LOXO-292) or published (alectinib) human pharmacokinetic parameters (see supplementary Appendix, available at Annals of Oncology online). (C) T1, contrast-enhanced magnetic resonance imaging images of the patient’s metastatic brain disease before and at the indicated times after she initiated treatment with LOXO-292. A radiologic partial response was achieved extracranially and intracranially after 2 months of treatment that was sustained and deepened at 5 months of treatment (with tiny foci of nonspecific enhancement but no measurable target lesions remaining in the brain indicating a confirmed PR). See supplementary Figure S6, available at Annals of Oncology online for additional brain and extracranial imaging. PR, partial response; SD, stable disease; PD, progressive disease; WBRT, whole brain radiation; SRS, stereotactic radiosurgery; ag, agonist; Alect, alectinib; mg, milligrams; BID, twice daily; Mo, month; cPR, confirmed partial response.
Discussion
The limited clinical activity of MKIs with activity against RET in patients with RET-altered cancers has led to preliminary conclusions that RET fusions are not highly actionable [22]; and the clinical activity of such MKIs results from concurrent inhibition of other targets, such as VEGFR2/KDR [23].
Here we report two patients with advanced, highly symptomatic RET-altered cancers who progressed on such MKIs. In both patients, significant RET target coverage was achieved with LOXO-292, following real-time, PKs-guided intrapatient dose escalation. Consistent with its high selectivity for RET, LOXO-292 was well-tolerated, including at escalated doses. This contrasts with MKIs, and further suggests that on-target toxicities from potent RET inhibition are unlikely to limit the development of selective RET inhibitors [24].
To our knowledge, this is the first clinical report of the predicted RET V804M gatekeeper mutation arising in a patient previously treated with MKIs. Germline RET gatekeeper mutations, which occur in patients with hereditary MTC [25], cause markedly diminished anti-RET potency for most MKIs [19, 26]. LOXO-292 was optimized to retain activity in this setting. This case also illustrates the potential value of plasma cell-free DNA in detecting RET mutations at the time of clinical progression, and the opportunity, through potent RET inhibition, to control symptomatic diarrhea caused by cancer in patients with MTC.
To our knowledge, this is also the first report of the reestablishment of extracranial and intracranial disease control after progression on alectinib, often considered the most brain-penetrant of the currently available MKIs. A previous case series described a RET fusion-positive lung cancer patient who experienced improvement of brain metastases with alectinib at a dose escalated above the recommended phase II dose [27], although intracranial disease control was short-lived. Given the excellent brain penetration of alectinib, this case further highlights the lack of clinically sufficient RET target coverage of repurposed MKIs currently used off-label.
These results provide preliminary evidence that diverse RET alterations are actionable therapeutic targets, and indicate that selective RET inhibition by LOXO-292 is a promising treatment approach in patients with RET-altered cancers. A phase I trial of LOXO-292 for patients with advanced, RET-altered cancers, treated with any prior MKIs, is currently accruing (NCT03157128).
Supplementary Material
Acknowledgements
The authors express their gratitude to the patients and their families and caregivers for participation in these trials, and to Alturas Analytics for ‘real-time’ analysis of plasma levels of LOXO-292.
Funding
The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health Cancer Center Support Grant CA016672. The University of Texas MD Anderson Cancer Center clinical trials program is supported in part by Cancer Prevention Research Institute of Texas Grant RP110584 and National Center for Advancing Translational Sciences Grant UL1 TR000371 (Center for Clinical and Translational Sciences).
Disclosure
BT, KE, SC-T, DH, BB, SA and SMR are employees of Loxo Oncology, Inc. MB, RH, KB, SW, RDW, DH, SR and MR are employees of Array BioPharma, Inc. All remaining authors have declared no conflicts of interest.
References
- 1. Stransky N, Cerami E, Schalm S.. The landscape of kinase fusions in cancer. Nat Commun 2014; 5: 4846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ji JH, Oh YL, Hong M. et al. Identification of driving ALK fusion genes and genomic landscape of medullary thyroid cancer. PLoS Genet 2015; 11(8): e1005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kato S, Subbiah V, Marchlik E. et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res 2017; 23(8): 1988–1997. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi M, Ritz J, Cooper GM.. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985; 42(2): 581–588. [DOI] [PubMed] [Google Scholar]
- 5. Acton DS, Velthuyzen D, Lips CJM, Höppener JWM.. Multiple endocrine neoplasia type 2B mutation in human RET oncogene induces medullary thyroid carcinoma in transgenic mice. Oncogene 2000; 19(27): 3121–3125. [DOI] [PubMed] [Google Scholar]
- 6. Saito M, Ishigame T, Tsuta K. et al. A mouse model of KIF5B-RET fusion-dependent lung tumorigenesis. Carcinogenesis 2014; 35(11): 2452–2456. [DOI] [PubMed] [Google Scholar]
- 7. Kurzrock R, Sherman SI, Ball DW. et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. JCO 2011; 29(19): 2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elisei R, Schlumberger MJ, Muller SP. et al. Cabozantinib in progressive medullary thyroid cancer. JCO 2013; 31(29): 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells SA Jr, Robinson BG, Gagel RF. et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012; 30(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drilon A, Rekhtman N, Arcila M. et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016; 17(12): 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoh K, Seto T, Satouchi M. et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2016. [DOI] [PubMed] [Google Scholar]
- 12. Hong D, Ye L, Gagel R. et al. Medullary thyroid cancer: targeting the RET kinase pathway with sorafenib/tipifarnib. Mol Cancer Ther 2008; 7(5): 1001–1006. [DOI] [PubMed] [Google Scholar]
- 13. Brandhuber BB, Haas J, Tuch BB. et al. ENA-0490 The development of LOXO-292, a potent, KDR/VEGFR2-sparing RET kinase inhibitor for treating patients with RET-dependent cancers. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2016; Poster No. 441.
- 14. Velcheti V, Ahluwalia M.. Intracranial and systemic response to alectinib in a patient with RET-KIF5B oncogenic fusion. J Thorac Oncol 2017; 12(7): e98–e99. [DOI] [PubMed] [Google Scholar]
- 15. Kodama T, Hasegawa M, Takanashi K. et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014; 74(5): 1023–1028. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03; 2010.
- 17. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 18. Lin NU, Lee EQ, Aoyama H. et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 2015; 16(6): e270–e278. [DOI] [PubMed] [Google Scholar]
- 19. Yang M, Cai J, Guo S. et al. Rapid conversion to resistance, of a colon PDW with ret-fusion, by Ponatinib treatment could potentially be attributed to the introduction of the gate keeper mutation, V804M. Cancer Res 2015; 75(15 Suppl): 3581; Poster 3581. [Google Scholar]
- 20. Drilon AE, Filleron T, Bergagnini I. et al. Baseline frequency of brain metastases and outcomes with multikinase inhibitor therapy in patients with RET-rearranged lung cancers. J Clin Oncol 2017; 35: 9069–9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drilon A, Nagasubramanian R, Blake JF. et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 2017; 7(9): 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrara R, Auger N, Auclin E, Besse B.. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol 2018; 13(1): 27–45. [DOI] [PubMed] [Google Scholar]
- 23. Romei C, Ciampi R, Elisei R.. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 2016; 12(4): 192–202. [DOI] [PubMed] [Google Scholar]
- 24. Drilon A, Hu ZI, Lai GGY, Tan DSW.. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018; 15(3): 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wells SA Jr, Asa SL, Dralle H. et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25(6): 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carlomagno F, Guida T, Anaganti S. et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 2004; 23(36): 6056–6063. [DOI] [PubMed] [Google Scholar]
- 27. Lin JJ, Kennedy E, Sequist LV. et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J Thorac Oncol 2016; 17(12): 1653–1660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.