Abstract
Background
Clinical trials have recently evaluated safety and efficacy of neoadjuvant therapy among patients with surgically resectable regional melanoma metastases. To capture informative prognostic data connected to pathological response in such trials, it is critical to standardize pathologic assessment and reporting of tumor response after this treatment.
Methods
The International Neoadjuvant Melanoma Consortium meetings in 2016 and 2017 assembled pathologists from academic centers to develop consensus guidelines for pathologic examination and reporting of surgical specimens from AJCC (8th edition) stage IIIB/C/D or oligometastatic stage IV melanoma patients treated with neoadjuvant-targeted or immune therapy. Patterns of pathologic response are provided context to inform these guidelines.
Results
Based on our collective experience and guided by efforts in well-established neoadjuvant settings like breast cancer, procedures directing handling of pre- and post-neoadjuvant therapy–treated melanoma specimens are provided to facilitate comparison of findings across different trials and centers. Definitions of pathologic response are provided together with guidelines for reporting and quantifying the extent of pathologic response. Finally, the spectrum of histopathologic responses observed following neoadjuvant-targeted and immune-checkpoint therapy is described and illustrated.
Conclusions
Standardizing pathologic evaluation of resected melanoma metastases following neoadjuvant-targeted or immune-checkpoint therapy allows more robust stratification of patient outcomes. This includes recognizing the spectrum of histopathologic response patterns to neoadjuvant therapy and a standard approach to grading pathologic responses. Such an approach will facilitate comparison of results across clinical trials and inform ongoing correlative studies into the mechanisms of response and resistance to agents applied in the neoadjuvant setting.
Keywords: melanoma, neoadjuvant therapy, targeted therapy, immune checkpoint blockade, pathology
Key Message
Standardizing pathologic evaluation of resected melanoma metastases and recognizing the spectrum of histopathologic responses patterns after neoadjuvant-targeted or immune-checkpoint therapy will improve stratification of patient outcomes, facilitate comparison of results across clinical trials and inform correlative studies into mechanisms of response and resistance to neoadjuvant therapy.
Introduction
Since 2011, 10 new drug therapies have been approved for treatment of patients with stage IV metastatic melanoma and have dramatically improved outcomes. Many of these agents are being evaluated in trials for patients with earlier disease stages, and recent adjuvant trials demonstrated improvements in relapse-free survival (RFS) after surgery for stage III melanoma [1–3]. In analyses carried out for the 8th edition of the American Joint Committee on Cancer (AJCC) melanoma staging system [4], 5-year melanoma-specific survival (MSS) for stage III patients was as low as 32% (stage IIID), underscoring the critical need to improve their outcomes.
The clinical successes of targeted and immune-checkpoint therapies in patients with advanced disease have also prompted an assessment of their efficacy in the neoadjuvant setting. Neoadjuvant cytotoxic and/or targeted therapy is currently accepted standard of care for many patients with breast [5], colorectal [6], and gastro-esophageal [7] cancers and offers several advantages. First, it provides a unique window to gauge antitumor efficacy of agents via pathologic examination of the definitive resection specimen. Whereas persistence of viable tumor after therapy might indicate the need to alter or explore novel treatments in the adjuvant setting, tumor eradication might lend support to continuation or even cessation of the current therapy. Second, neoadjuvant therapy potentially eradicates clinically occult disease, preventing or delaying disease relapse. Finally, it has the potential to shrink unresectable tumor to enable surgery, which may additionally be less morbid [8].
A recent randomized phase II clinical trial compared safety and efficacy of neoadjuvant and adjuvant dabrafenib and trametinib targeted therapy to standard-of-care (SOC) surgery in patients with surgically resectable stage III or oligometastatic stage IV melanoma. Patients who received neoadjuvant therapy had a >60-fold reduced risk of relapse after surgery compared with those who underwent SOC surgery [9]. Similarly, in a phase II study of 35 patients with resectable stage III or oligometastatic stage IV metastatic melanoma, 17 (49%) patients who received dabrafenib and trametinib had a complete pathologic response (pCR), and no patients progressed during the neoadjuvant period [10]. Early results from the first trials employing neoadjuvant immune-checkpoint blockade in patients with metastatic melanoma have shown higher rates of pCR (45%) in patients receiving combined ipilimumab/nivolumab compared with those who received nivolumab alone (25%), and combination therapy was associated with non-statistically significant improvements in progression-free, recurrence-free, and overall survival (OS) compared with single-agent nivolumab [11]. A prospective study of 10 patients with resectable stage III or oligometastatic stage IV melanoma treated with neoadjuvant combination ipilimumab/nivolumab showed similar rates of pCR (30%) and partial pathologic response (40%; pPR, ≤50% residual viable tumor). Those patients with either pCR or pPR have not recurred with a median follow up of over 24 months [12]. A prospective study of 27 patients with resectable stage III or oligometastatic stage IV melanoma treated with neoadjuvant pembrolizumab showed similar response rates; no patient with a pCR has recurred at a median follow up of over 18 months [13].
The US Food and Drug Administration (FDA) issued the position statement: ‘Pathologic complete response is defined as the absence of residual invasive cancer… after evaluation of the completely resected specimen including …all sampled regional lymph nodes following completion of neoadjuvant systemic therapy’ [14]. The FDA additionally designated pCR as a surrogate end point to justify accelerated approval of a particular agent administered in the neoadjuvant (and adjuvant) setting for breast cancer [14, 15]. With growing application of neoadjuvant systemic therapy in patients with resectable metastatic melanoma, a critical challenge is therefore to establish uniform practices and widely-accepted criteria to determine the extent of pathologic response accurately and reproducibly. Here, we present standardized procedures for gross inspection and tissue submission and describe the continuum of histopathologic patterns of response observed following neoadjuvant-targeted therapy and immunotherapy. Consensus guidelines for recommended reporting parameters and scoring criteria are also presented.
Materials and methods
The International Neoadjuvant Melanoma Consortium meetings in 2016 and 2017 convened pathologists, oncologists, and surgeons from academic centers in multiple countries. Participants were invited by the Chairs of the group according to their experience with targeted and immune-checkpoint blockade therapy neoadjuvant trials in melanoma patients.
The pathology working group subsequently communicated in person, through teleconferences and written communications. This document represents the consensus opinion of its members and serves as an expanded companion to a broader effort summarizing the guidelines for principles of neoadjuvant treatment in melanoma (manuscript in preparation).
Results
Standardized procedures for pathologic assessment in the neoadjuvant setting
Assessment of pretreatment biopsies
Pretreatment pathological confirmation of the diagnosis of metastatic melanoma is essential before initiating neoadjuvant therapy. This specimen often informs BRAF mutation status and/or the expression of immunoactive molecules (PD-L1) and may drive therapeutic selection [16]. It also enables morphologic and immunophenotypic tumor cell assessment and enables comparison with any residual tumor following therapy.
Clinical information provided to the pathologist with the surgical specimen
For proper specimen processing, the pathology team must be aware that it was procured following neoadjuvant systemic therapy. Information regarding the type and duration of neoadjuvant systemic therapy should be provided together with the number of radiographically positive nodes to direct processing.
Initial specimen handling
At present, most patients enrolled in neoadjuvant trials in melanoma have had surgically resectable nodal disease [9–11, 17]. Thus, only the approach to regional lymphadenectomy specimens will be discussed. Gross specimen photography is advised for mapping initial and potential subsequent tissue submission [18]. Sections submitted for histopathologic evaluation should include grossly obvious tumor as well as the entire pre-treatment biopsy site. Placement of a surgical clip during diagnostic biopsy can help direct subsequent localization of this site.
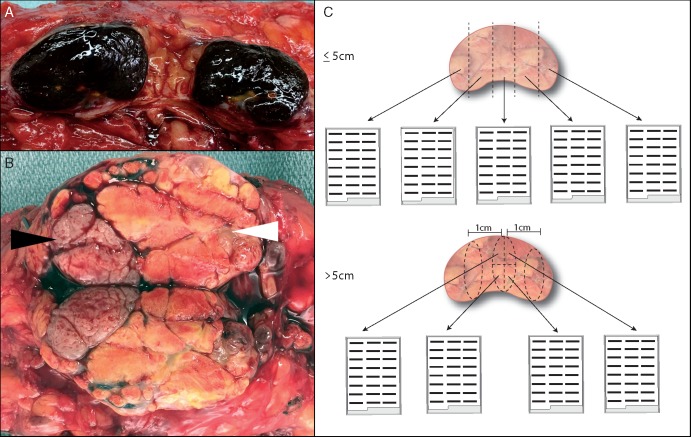
All lymph nodes (grossly positive and negative; Figure 1A and B) or tumor nodules (including necrotic or hemorrhagic areas) should be identified grossly and submitted for histopathologic assessment. Grossly matted nodes or extranodal spread should be documented. Three-dimensional measurements of the largest grossly positive node(s) should be provided. Further assessment is directed according to gross tumor size (see below). Of note, gross measurements may not correspond to viable tumor burden (Figure 1A); thus, histopathologic measurements represent the gold standard for the determination of pathologic response.
Figure 1.
Gross assessment and sampling of lymphadenectomy specimens following neoadjuvant therapy. (A) Bisected lymph node with extensive melanin deposition grossly. (B) Bisected lymph node with morphologically distinct regions: viable tumor (black arrowhead) adjacent to necrotic tumor (white arrowhead). (C) Schema for processing grossly positive lymph nodes according to size. Lymph nodes ≤5 cm in greatest dimension are submitted entirely (top panel), whereas for lymph nodes >5 cm in greatest dimension, one tissue slice representing a complete cross section of the lymph node is submitted per cm (bottom panel; submission of one complete cross section is represented).
Tissue submission for lymphadenectomy specimens with tumor burden ≤5 cm
Provided each lymph node grossly measures ≤5 cm in greatest dimension, each lymph node should be serially sectioned at ∼3–4 mm intervals and submitted entirely, (Figure 1C, upper panel). Complete submission facilitates the most accurate assessment of residual viable tumor.
Tissue submission for lymphadenectomy specimens with tumor burden >5 cm
When the largest grossly positive lymph node exceeds 5 cm in greatest dimension, representative sections are considered sufficient. Specifically, sections representing one full slice (itself encompassing a complete cross section of the entire surface area), per 1 cm of each grossly involved lymph node are recommended (Figure 1C, bottom panel). This approach mirrors that advocated by an international consortium of breast pathologists [18, 19]. Ultimately, the amount of tissue submitted for histopathologic evaluation should reflect an integrated assessment of the complete clinical, radiographic, and gross findings specific to a given case—particularly when there is evidence of grossly residual viable tumor [18]. Tissue procured from specimens resected after neoadjuvant therapy offer a critical resource for correlative studies determining pathways of response and/or resistance. While some studies (whole exome sequencing and T-cell receptor sequencing) may utilize FFPE, others (RNA sequencing and fresh tissue for cell dissociates) are ideally carried out on fresh or fresh frozen tissue. Institutions with tissue banking protocols are encouraged to develop policies for procurement of fresh tumor tissue, including areas of regression and viable tumor (if grossly evident). Verification of cellularity/viability with frozen section review of fresh tissue is encouraged. In rare cases, gross evidence of a tumor bed (treated or otherwise) is lacking in the surgical specimen. Placement of a surgical clip at the time of the diagnostic biopsy would be particularly useful in this situation to identify the tumor bed. Thorough sampling is advised to identify any residual or regressed tumor; it may be necessary to submit additional tissue depending on the findings of the initial histopathologic assessment.
Histopathologic assessment of the excised lymph nodes
In addition to standard AJCC staging parameters [4, 20], evidence of therapy-specific effects should be described and quantified, including the relative percentage of tumor bed occupied by viable tumor, tumoral necrosis and/or melanosis, inflammatory infiltrate, and fibrosis (Table 1). These features are used to calculate the percent residual viable tumor:
Table 1.
Quick reference guide to pathologic assessment of neoadjuvant treated melanoma specimens
| Working definitions |
|
| Gross evaluation of the surgical specimen after neoadjuvant therapy |
|
| Microscopic templates: |
| (1) For viable melanoma |
| MELANOMA, METASTATIC TO XX OF YY LYMPH NODES (XX/YY). |
| Largest tumor deposit size:____×____mma |
| Location: Subcapsular/Intraparenchymal |
| Extracapsular extension: Present/Not identified |
| See comment. |
|
a
For the measurements, we recommend including the following:
|
| (2) If no viable melanoma |
| FIBROSIS AND/OR NODULAR AGGREGATES OF PIGMENTED MACROPHAGES AND/OR (COMPLETELY) NECROTIC TUMOR CONSISTENT WITH MELANOMA (COMPLETELY REGRESSED WITH TREATMENT EFFECT), METASTATIC TO XX OF YY LYMPH NODES (XX/YY). |
| Largest tumor deposit size: ____×____mm (CORRESPONDS TO LARGEST AREA OF REGRESSED/NECROTIC MELANOMA—Gross measurement preferred over microscopic) |
| Location: Subcapsular/Intraparenchymal |
| Extracapsular extension: Present/not identified (corresponds to pigmented macrophages/fibrosis consistent with completely regressed melanoma) |
| See comment. |
| Comment: |
| Sections reveal (viable/partially viable/completely regressed) melanoma involving XX of YY lymph nodes. An evaluation of the complete tumor bed revealsa, b: |
|
The sum of these three elements (% viable tumor, % tumoral melanosis/necrosis, and fibrosis/fibrinflammatory stroma should equal 100%).
If multiple nodes or nodal basins are involved by disease (whether completely or partially necrotic), a summary statement should estimate the combined percentages of viable tumor cells, necrosis/melanosis and fibrosis occupying the surface area encompassed by the tumor bed comprising each of the involved nodes.
The ‘tumor bed’ is defined as the area of the tissue occupied by viable tumor or tumoral regression (necrosis with or without clusters/sheets of pigmented macrophages and fibrosis/fibroinflammatory stroma). If multiple lymph nodes are/were involved by melanoma, the percent residual viable tumor should be calculated by summing the surface areas occupied by the multiple residual viable tumor deposits, and dividing that by the sum of the surface areas of the tumor beds in the histopathologically involved nodes.
Finally, routine application of immunohistochemistry (IHC) to identify/exclude viable tumor cells in neoadjuvant-treated specimens is not required. IHC should be applied based on a histopathologic suspicion for viable tumor, rather than as a reflex to secure the designation of pCR. When IHC is required, Sox-10 is recommended, since Sox-10 is a highly sensitive melanocytic antigen, and its nuclear localization renders it less susceptible to non-specific cytoplasmic staining caused by excessive pigmentation often encountered with Melan-A and HMB-45.
Defining pathologic response
A continuum of pathologic responses may be observed in patients treated with neoadjuvant-targeted or immune-checkpoint therapy. pCR is defined as the complete absence of residual viable tumor. In the early trials for patients with melanoma, pPR was empirically defined as ≤50% of the tumor bed occupied by viable tumor cells, and pathologic non-response (pNR) was defined as >50% of the tumor bed occupied by viable tumor cells [9, 21]. Some neoadjuvant immunotherapy studies have also utilized a category of ‘near pCR/major PR’ that was defined as >0% but ≤10% viable tumor cells [13, 22]. Newer grading systems supporting scoring of residual viable tumor as a continuous variable have also been proposed [22]. Long-term outcome studies are required to determine the extent to which pathologic response is associated with improved patient survival in distinct neoadjuvant settings.
Histopathologic patterns observed in melanoma specimens from patients treated with neoadjuvant BRAF/MEK inhibition
Two primary patterns were observed in patients with pCR in neoadjuvant-targeted therapy trials: (1) hyalinized fibrosis pattern (Figure 2A and B) and (2) tumoral melanosis pattern (Figure 2C and D). A mixture of these patterns was also seen. The hyalinized fibrosis pattern consists of homogeneous pale eosinophilic collagen, effacing the lymph node architecture and replacing the previous metastatic deposit(s). The tumoral melanosis pattern of pCR is characterized by effacement of the lymph node by sheets of pigmented macrophages (melanophages). A variable extent of coagulative tumoral necrosis and fibrosis is usually associated with these melanophages.
Figure 2.
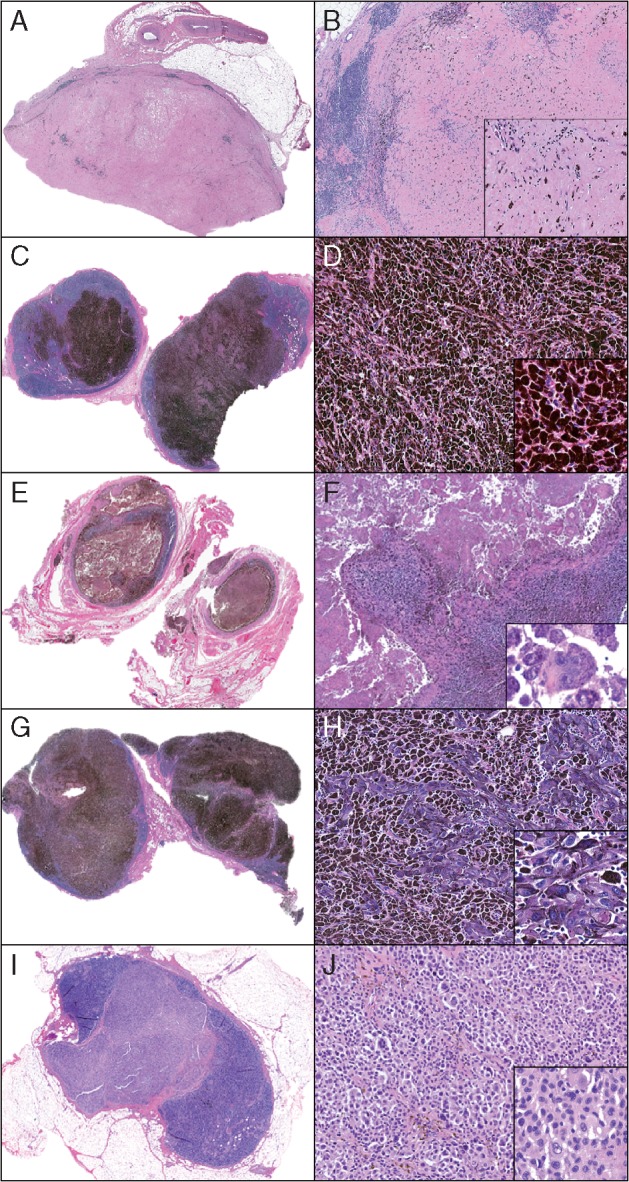
Spectrum of pathologic responses observed following treatment with neoadjuvant dabrafinib-trametinib. Patterns of pathologic complete response (pCR) include (A, B) hyalinized fibrosis pattern in which lymph node parenchyma is replaced by dense hyalinized collagen (A, 40×; B, 100×; inset: 200×) with no residual viable tumor and (C, D) tumoral melanosis pattern where lymph node parenchyma is replaced by melanophages (C, 40×; D, 100×; inset: 200×). Patterns of incomplete pathologic response range from partial pathologic response (pPR) to pathologic non-response (pNR) (E–H) with variable densities of viable tumor cells in a background of necrosis, tumoral melanosis, fibrosis and lymphohistiocytic inflammation. Rare tumor cells evident in a background of necrosis and melanophages (E, 40×; F, 100×; inset: 400×), and clusters of tumor cells evident in a background of tumoral melanosis (G, 40×; H, 100×; inset: 200×). pNR (I, J) characterized by mostly viable tumor cells (I, 40×; J, 100×; inset: 200×). H&E staining, all panels.
Patterns of pPR and pNR include a continuum occupied by a variable density of viable tumor cells admixed with a similar spectrum of changes observed in pCR, including an admixture of tumoral necrosis and melanosis, lymphohistiocytic inflammation and/or fibrosis (Figure 2E–J).
Histopathologic patterns observed in melanoma specimens from patients treated with neoadjuvant checkpoint inhibition
Specimens from patients receiving immune-checkpoint blockade demonstrated a response spectrum ranging from pCR to pNR (Figure 3A–F). Features of immune-mediated tumor regression were most often observed around the periphery of residual tumor, underscoring the importance of obtaining full cross-sections of the tumor mass during grossing. Pathologic features of response include an immune infiltrate (moderate-high densities of tumor infiltrating lymphocytes, plasma cells, lymphoid aggregates and macrophages) together with features of wound healing/repair (immature, proliferative fibrosis and neovascularization) (supplementary Figures S1 and S2, available at Annals of Oncology online). For the assessment of neoadjuvant immune-checkpoint blockade specimens, it remains to be determined whether additional studies, including assessments of CD3+ or CD8+ T-cell densities or an assessment of mutational load, might provide improved predictive value over routine histologic assessment.
Figure 3.
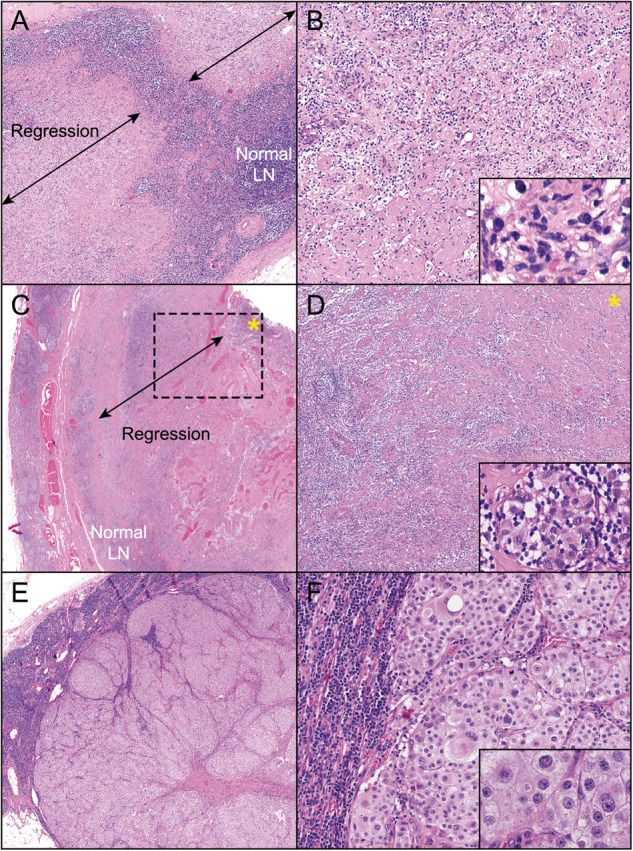
Spectrum of pathologic responses observed following treatment with neoadjuvant ipilimumab-nivolumab. (A, B) pCR characterized by replacement of lymph node with histologic features of immune-mediated regression with no residual viable tumor present (A, 100×; B, 200×). Inset shows multiple immune cell subsets, including foamy macrophages and plasma cells (600×). (C, D) pPR characterized by partial replacement of the lymph node architecture with histologic features of regression, including fibrosis, neovascularization, and lymphocytic infiltrate. Residual viable tumor is present (marked by asterisk). A cuff of normal lymph node architecture is seen adjacent to the area of regression (C, 50×; D, 100×). Inset shows residual viable tumor (Inset, D, 600×). (E, F) pNR characterized by mostly viable tumor cells abutting normal lymph node without any accompanying features of response (E, 20×; F, 100×; inset: 600×). H&E staining, all panels.
Discussion
Here, we provide standardized procedures directing assessment of metastatic melanoma specimens and provide criteria to quantify the extent of pathologic response following neoadjuvant therapy. The premise of pCR as a surrogate end point is that the ability of a neoadjuvant therapy to eradicate tumor at local and regional sites correlates with eradication of distant micrometastatic disease and mirrors adjuvant therapy trials where impacts on survival (OS or DFS) are determined. However, such phase III adjuvant trials require significantly more time and patients to determine survival outcomes. The correlation between pCR and improved OS and/or DFS has been confirmed in neodjuvant trials for breast [23], bladder [24], rectal [25], esophageal [26], and gastric carcinomas [27]. In breast cancer, the extent of residual cancer following neoadjuvant therapy robustly predicts RFS within phenotypic subsets and even within discrete stage categories [28]. However, while correlation between neoadjuvant pCR findings and adjuvant survival outcomes has not been absolute, preliminary data from neoadjuvant melanoma trials suggest a correlation of pCR with improved RFS among patients treated with neoadjuvant-targeted [29] and immune-checkpoint [13, 17, 21] therapies. Whether the survival correlates associated with pCR in targeted therapy are different from that seen with immune-checkpoint therapy requires further investigation. Specifically, either pCR or ‘near pCR/major PR’ (e.g. ≤10% viable tumor cells) may associate with improved survival indices in patients treated with neoadjuvant immune-checkpoint blockade. In support of this concept, the Residual Cancer Burden (RCB) model largely confirms that pCR patients (RCB = 0) have improved RFS compared with patients with non-pCR for neoadjuvant-treated breast cancer patients, particularly those with triple negative and Her2 positive tumours. However, in certain scenarios, patients with minimal viable tumor following neoadjuvant therapy (RCB-I) actually have better RFS than patients with pCR (RCB-0) [28]. This suggests either that residual tumor persists in occasional cases designated as pCR or that the difference between pCR and some minimal percentage of viable tumor may not be clinically significant. Furthermore, other organ systems, including lung and some early studies in melanoma, designate ‘major pathologic response’ to indicate ≤10% residual viable tumor [13, 17, 30]. In one melanoma study, patients with either a pCR or ≤10% residual viable tumor after neoadjuvant PD-1 blockade remained cancer free with a median follow up of 18 months [13]. It is therefore possible that the percentage of residual viable tumor following neoadjuvant therapy that correlates with improved melanoma survival may depend on the agent used. For this reason, we advocate recording the percentage residual viable tumor as a continuous variable until more definitive evidence becomes available.
Resection specimens from patients treated with immune-checkpoint blockade showed tumor beds that were more densely inflamed. In contrast, specimens from patients treated with targeted therapy were more often characterized by necrosis and hyalinized fibrosis. These differences likely reflect the varying mechanisms of drug action, but their clinical significance remains unclear. Additional studies in a larger number of patients correlating degree of pathologic response and individual histologic features with long-term outcomes will be required to establish an RCB model predictive of survival for each neoadjuvant therapy. The standardization of processing and reporting criteria as described herein maximizes our capacity to accomplish this goal, and is well suited for integration into future planned prognostic models that include patients treated in the neoadjuvant arena [4, 20].
Funding
Supported in part by The University of Texas MD Anderson Cancer Center Melanoma Moon Shots Program. This work was also supported by the Melanoma Research Alliance (JMT), Harry J. Lloyd Trust (JMT), National Cancer Institute R01 CA142779 (JMT), and The Bloomberg-Kimmel Institute for Cancer Immunotherapy. Support from colleagues at Melanoma Institute Australia is also gratefully acknowledged. GVL and RAS are supported by the Australian National Health and Medical Research Council Practitioner Fellowship program (no grant number applies). JES was supported by a T32 training grant to Johns Hopkins (grant number NIH T32 CA193145).
Disclosure
MTT (Advisory Board with Myriad Genetics, Novartis, Seattle Genetics); RNA (research funding from Bristol Myers Squibb); CB (advisory board with Bristol Myers Squibb, Merck, Roche, Novartis, GlaskoSmithKline, Pfizer, Lilly; Research grants: Bristol Meyers Squibb, Novartis); MIR (consultancy with Merck and AMGEN; Speaker for AMGEN and Provectus; Advisopry Board Merck, AMGEN, Provectus, Castle Biosciences, GlaskoSmithKline); RPMS (education honoraria BMS, advisory board Novartis); TM (honorarium from Merck for scientific advisory committee, advisory for Bristol Myers Squibb and Incyte); GVL (Consultant Advisor: Amgen, Bristol Myers Squibb, Merck MSD, Novartis, Roche, Pierre Fabre, Array and Honoraria from Bristol Myers Squibb, Merck MSD, Novartis, Roche); MAD (PI of research grants MDACC from GlaskoSmithKline, Roche-Genentech, Bristol Myers Squibb, Merck, Astrazeneca, Sanofi-Aventis, Oncothyreon, Myriad; Consultant for Novartis, GlaskoSmithKline, Roche-Genentech, Bristol Myers Squibb, Sanofi-Aventis, Vaccinex, and Syndax); VGP (Advisory Board with Myriad and Novartis); JAW (Compensation for speaker’s bureau and honoraria from Dava Oncology, Bristol-Myers Squibb and Illumina; Advisory committees for GlaxoSmithKline, Roche/Genentech, Novartis and AstraZeneca); JMT (Advisory board/consultant for Bristol Myers Squibb, Astra-Zeneca, Merck, Amgen Investigator-initiated research funding from Bristol Myers Squibb); JEG (Advisory board/consultant for Merck, Syndax, Castle Biosciences, Novartis, and Bristol Myers Squibb). All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Eggermont AMM, Blank CU, Mandala M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018; 378(19): 1789–1801. [DOI] [PubMed] [Google Scholar]
- 2. Long GV, Hauschild A, Santinami M. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017; 377(19): 1813–1823. [DOI] [PubMed] [Google Scholar]
- 3. Weber J, Mandala M, Del Vecchio M. et al. Adjuvant Nivolumab versus Ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377(19): 1824–1835. [DOI] [PubMed] [Google Scholar]
- 4. Gershenwald JE, Scolyer RA, Hess KR. et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67(6): 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zardavas D, Piccart M.. Neoadjuvant therapy for breast cancer. Annu Rev Med 2015; 66: 31–48. [DOI] [PubMed] [Google Scholar]
- 6. Aklilu M, Eng C.. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol 2011; 8(11): 649–659. [DOI] [PubMed] [Google Scholar]
- 7. Kelsen DP. Adjuvant and neoadjuvant therapy for gastric cancer. Semin Oncol 1996; 23(3): 379–389. [PubMed] [Google Scholar]
- 8. Mauri D, Pavlidis N, Ioannidis JP.. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005; 97(3): 188–194. [DOI] [PubMed] [Google Scholar]
- 9. Amaria RN, Prieto PA, Tetzlaff MT. et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol 2018; 19(2): 181–193. [DOI] [PubMed] [Google Scholar]
- 10. Menzies AM, Gonzalez M, Guminski A. et al. Phase 2 study of neoadjuvant dabrafenib plus trametinib (D plus T) for resectable stage IIIB/C BRAF V600 mutant melanoma. Ann Oncol 2017; 28(Suppl 5): v428–v448. [Google Scholar]
- 11. Reddy SMAR, Spencer CN, Tetzlaff MT. et al. Neoadjuvant nivolumab versus combination ipilimumab and nivolumab followed by adjuvant nivolumab in patients with resectable stage III and oligometastatic stage IV melanoma: preliminary findings. J Immunother Cancer 2017; 5: 86. [Google Scholar]
- 12. Rozeman EA, Blank CU, Akkooi ACJV. et al. Neoadjuvant ipilimumab + nivolumab (IPI+NIVO) in palpable stage III melanoma: updated data from the OpACIN trial and first immunological analyses. J Clin Oncol 2017; 35: 9586–9586. [Google Scholar]
- 13. Huang ACXX, Orlowski RJ, George SM. et al. Safety, activity, and biomarkers for neoadjuvant anti-PD-1 therapy in melanoma. Chicago, IL: American Association for Cancer Research; 2018.
- 14. US Center for Drug Evaluation and Research. Guidance for industry: pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf May 2012.
- 15. Fumagalli D, Bedard PL, Nahleh Z. et al. A common language in neoadjuvant breast cancer clinical trials: proposals for standard definitions and endpoints. Lancet Oncol 2012; 13(6): e240–e248. [DOI] [PubMed] [Google Scholar]
- 16. Taube JM, Klein A, Brahmer JR. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20(19): 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rozeman EA, Fanchi L, van Akkooi ACJ. et al. (Neo-)adjuvant ipilimumab plus nivolumab (IPI plus NIVO) in palpable stage 3 melanoma – updated relapse free survival (RFS) data from the OpACIN trial and first biomarker analyses. Ann Oncol 2017; 28(Suppl 5): v428–v448. [Google Scholar]
- 18. Provenzano E, Bossuyt V, Viale G. et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol 2015; 28(9): 1185–1201. [DOI] [PubMed] [Google Scholar]
- 19. Bossuyt V, Provenzano E, Symmans WF. et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol 2015; 26(7): 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gershenwald JE, Scolyer RA, Hess KR. et al. Melanoma of the skin In Amin MB, Edge S, Greene FL. et al. (eds) AJCC Cancer Staging Manual. 8th ed Switzerland: Springer; 2017; 563–585. [Google Scholar]
- 21. Amaria RN, Reddy SM, Tawbi HA. et al. Neoadjuvant (neo) immune checkpoint blockade (ICB) in patients (Pts) with high-risk resectable metastatic melanoma (MM). J Clin Oncol 2018; 36(Suppl): abstr 9510. [Google Scholar]
- 22. Cottrell TR, Thompson ED, Forde PM. et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018; 29(8): 1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortazar P, Zhang L, Untch M. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384(9938): 164–172. [DOI] [PubMed] [Google Scholar]
- 24. Petrelli F, Coinu A, Cabiddu M. et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 2014; 65(2): 350–357. [DOI] [PubMed] [Google Scholar]
- 25. Park IJ, You YN, Agarwal A. et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012; 30(15): 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lorenzen S, Thuss-Patience P, Al-Batran SE. et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol 2013; 24(8): 2068–2073. [DOI] [PubMed] [Google Scholar]
- 27. Ajani JA, Mansfield PF, Crane CH. et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol 2005; 23(6): 1237–1244. [DOI] [PubMed] [Google Scholar]
- 28. Symmans WF, Wei C, Gould R. et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 2017; 35(10): 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menzies AM, Rozeman EA, Amaria RN. et al. Preliminary results from the international neoadjuvant melanoma consortium (INMC). J Clin Oncol 2017; 35. [Google Scholar]
- 30. Hellmann MD, Chaft JE, William WN Jr. et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014; 15(1): e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.