Abstract
Background
Continuous-infusion 5-fluorouracil (5FU) and calcium leucovorin plus nab-paclitaxel and oxaliplatin have been shown to be active in patients with pancreatic cancer. As a protracted low-dose infusion, 5FU is antiangiogenic, and has synergy with bevacizumab. As shown in the treatment of breast cancer, bevacizumab and nab-paclitaxel are also synergetic.
Objective
In this paper we retrospectively analyze the survival of 65 patients with advanced pancreatic cancer who were treated with low-dose continuous (metronomic) chemotherapy given in conjunction with conventional anti-VEGF therapy.
Patients and Methods
Since July of 2008, we have treated 65 patients with 5FU (180 mg/m2/day × 14 days) via an ambulatory pump. Calcium leucovorin (20 mg/m2 IV), nab-paclitaxel (60 mg/m2) IV as a 30-min infusion, and oxaliplatin (50 mg/m2) IV as a 60-min infusion were given on days 1, 8, and 15. Bevacizumab (5 mg/kg) IV over 30 min was administered on days 1 and 15. Cycles were repeated every 28–35 days. There were 42 women and 23 men, and the median age was 59 years. Forty-six patients had stage IV disease.
Results
The median survival was 19 months, with 82% of patients surviving 12 months or longer. The overall response rate was 49%. There were 28 patients who had received prior treatment, 15 of whom responded to therapy. Fifty-two patients had elevated CA 19-9 prior to treatment. Of these, 21 patients had 90% or greater reduction in CA 19-9 levels. This cohort had an objective response rate of 71% and a median survival of 27 months. Thirty patients stopped treatment due to disease progression, and an additional 22 stopped because of toxicity. One patient died while on therapy.
Conclusions
This non-gemcitabine-based regimen resulted in higher response rates and better survival than what is commonly observed with therapy given at conventional dosing schedules. Low-dose continuous (metronomic therapy) cytotoxic chemotherapy combined with antiangiogenic therapy is safe and effective.
Key Points
| In our single institution experience treating 65 patients with advanced pancreatic cancer, one-year survival in our cohort was 82%, substantially higher than has been reported in clinical trials of other accepted therapies (35-48%). |
| The lower toxicity of our approach allowed for longer median duration of treatment compared to other accepted protocols. |
| A reduction in CA19-9 tumor marker levels by 90% or greater on theraphy was associated with significantly longer median survival: 28 months versus 15 months. |
Introduction
Pancreatic ductal adenocarcinoma (PDA) is the third leading cause of cancer-related deaths in the United States. In 2015, an estimated 48,960 new cases were diagnosed, which resulted in 40,000 deaths [1]. Despite improved knowledge regarding the disease and the development of new targeted and immunologic therapies, along with the expanded availability of gene profiling, there has been little progress with respect to improved outcome. High mortality rates and short survival are explained by the lack of effective systemic therapies and the fact that most patients are diagnosed with late-stage disease [2].
Gemcitabine became the standard of care as first-line therapy for patients with metastatic disease, based on the phase III trial reported by Burris et al., which demonstrated a significant increase in clinical benefit rate (CBR, an endpoint that measured overall clinical improvement based on analgesic consumption, pain intensity, performance status, and weight change) and increased survival and time to disease progression when compared to 5-fluorouracil (5FU) [3]. More recently, Conroy et al. reported that a biweekly regimen of infusional 5FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) was superior to single-agent gemcitabine [4]. Patients who received FOLFIRINOX had median survival of 11.1 months, compared with 6.8 months observed for gemcitabine. Gemcitabine has been combined with a wide variety of chemotherapeutic drugs and targeted therapies, only to result in additional side effects and toxicity, without meaningful survival benefit [5–10]. The one exception is the addition of nab-paclitaxel to gemcitabine, as reported in October 2013 by Von Hoff et al., with median survival of 8.5 and 6.7 months for nab-paclitaxel plus gemcitabine and gemcitabine alone, respectively [11]. Apart from FOLFIRINOX and gemcitabine plus nab-paclitaxel, treatments remain largely ineffective.
Historically, regimens for pancreatic cancer have employed one, two, or three drugs at their maximum tolerated dose (MTD). Conventional MTD dosing is characterized by the administration of the highest possible dose, followed by a period of rest. In contrast, a low-dose metronomic (LDM) protocol is specifically designed to reduce dose-limiting side effects, is more patient-friendly, and has exhibited substantial rates of disease control in many adult solid tumors. This approach embraces the use of drugs administered at doses that are significantly lower than those considered standard, and usually given over prolonged periods of time, with fewer and shorter breaks [12]. Many examples of effective metronomic therapies have been reported in both pediatric and adult oncology [13–17].
In this paper we report the results of a retrospective analysis of 65 patients with advanced metastatic or locally advanced incurable pancreatic cancer. All patients received a protracted infusion of low-dose 5FU combined with nab-paclitaxel, calcium leucovorin, oxaliplatin, and bevacizumab. This chemotherapeutic regimen was based on rational, scientific principles of cancer chemotherapy.
5FU is approved for the palliative management of patients with pancreatic cancer. Low-dose continuous 5FU infusion is more effective than bolus injections [18]. In a meta-analysis of 1219 cancer patients who received bolus versus infusional 5FU, tumor response was significantly higher in patients treated with continuous infusion versus bolus (22 vs. 14%), as was overall survival. Hematologic grade 3 or 4 toxicity was less common with infusional therapy. By combining oxaliplatin with infusional 5FU, Ducreux et al. observed higher response rates and significantly longer survival than with either infusional 5FU or oxaliplatin alone [19]. Adding irinotecan and oxaliplatin to infusional 5FU improved outcomes in the treatment of patients with pancreatic cancer compared to single-agent gemcitabine [4].
It has been shown that tumor cells that are resistant to chemotherapy at conventional doses will continue to be sensitive to low-dose therapy. This observation was first described by Browder and Klement [20, 21]. 5FU administered continuously at low doses possesses antiangiogenic properties [22, 23] which are potentiated when combined with bevacizumab, as seen in the treatment of colon, gastric, and pancreatic cancers [14, 24–28]. When infusional 5FU, leucovorin, and irinotecan (FOLFIRI) was combined with bevacizumab, the overall survival of 31.3 months compared favorably with that of 20.3 months reported with bolus 5FU, leucovorin, irinotecan, and bevacizumab, and to the 16-month survival reported with FOLFIRI alone [23]. Paclitaxel and docetaxel given weekly on a metronomic schedule are less toxic and more active than on the standard every-three-weeks dosing schedule [29, 30].
Inhibition of tumor angiogenesis by more effective dosing schedules and targeted inhibitors can enhance the therapeutic benefit. Elevated serum and tumor vascular endothelial growth factor (VEGF) levels correlate with poor prognosis in patients with pancreatic cancer, as do other plasma cytokines and pro-angiogenic factors that have been shown to predict drug resistance [31–33]. Bevacizumab has been shown to reverse the angiogenic effects of VEGF, and it can reduce tumor interstitial pressure, leading to enhanced drug delivery [34]. Continuous low-dose 5FU may be more effectively transported than bolus 5FU in an environment of high interstitial pressure [35]. In breast cancer, nab-paclitaxel has been shown to reach higher intratumoral concentrations and to improve survival compared with standard paclitaxel or docetaxel [36–38]. When nab-paclitaxel was combined with bevacizumab, a weekly dosing schedule resulted in higher progression-free survival (PFS) and overall survival (OS), with less toxicity, compared with dosing every 3 weeks or every 2 weeks [39, 40]. The inclusion of bevacizumab in this regimen may effectively prevent the effects of angiogenesis while enhancing the activity of both nab-paclitaxel and continuous-infusion 5FU.
Patients and Methods
Patients
All patients were treated at the University of California, Los Angeles (UCLA), in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation, and were retrospectively selected for analysis. This study was approved by the UCLA Institutional Review Board (approval no. 18–000192), and the requirement for informed consent was waived for the entire study. Eligible patients included those with American Joint Committee on Cancer (AJCC) stage III locally advanced unresectable pancreatic adenocarcinoma or stage IV disease, histologically proven ductal or undifferentiated adenocarcinoma, and measurable or evaluable disease. Patients had Eastern Cooperative Oncology Group Performance Status of 0–2 and were > 18 years of age. All patients underwent baseline laboratory studies that included platelet count > 100,000/mm3, hemoglobin > 9.0 g/dL, absolute neutrophil count > 1500/mm3, ALT/AST < 2.5 times the upper limit of normal (ULN), total bilirubin < 1.5 times the ULN, and creatinine < 1.5 mg/dL. No patient initiated treatment until they were 2 weeks beyond surgical bypass procedure or had recovered from surgery. Patients who previously received prior chemotherapy were included. Patients were excluded if they had a concurrent second malignancy or known history of current or previous central nervous system (CNS) metastatic disease. Pregnant or nursing women were excluded.
Drug Dosing
Treatment consisted of 5FU 180 mg/m2/day as continuous infusion on days 1–15 via an ambulatory chemotherapy pump into a surgically placed central venous line (IV); nab-paclitaxel 60 mg/m2 as a 30-min IV infusion, calcium leucovorin 20 mg/m2 IV bolus injection, and oxaliplatin 50 mg/m2 IV over 60 min on days 1, 8, and 15; and bevacizumab 5 mg/kg IV over 30 min every other week, on days 1 and 15. Cycles were repeated every 28–35 days. Treatment was continued until there was objective evidence of disease progression or unacceptable treatment-related toxicity, or at the patient's or physician's discretion.
Assessments
The primary objective was to quantify the percentage of patients alive at 1 year. Secondary endpoints included median survival, response rate, percentage reduction in carbohydrate antigen (CA)19-9 levels from baseline, and treatment safety.
All patients who completed at least one 15-day cycle of treatment were evaluated for treatment efficacy and safety. Safety was assessed by the incidence of treatment-related adverse events (AE) according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, and included grade 3/4 neutropenia with fever or grade 4 neutropenia lasting ≥ 3 days, any grade 4 hematologic toxicity, grade ≥ 3 thrombocytopenia with bleeding, or any grade 3/4 treatment-related non-hematologic toxicity, including vomiting, diarrhea, neuropathy, or stomatitis.
Response was assessed utilizing Response Evaluation Criteria In Solid Tumors (RECIST) guidelines. Computed tomography (CT) scans were compared against baseline imaging every 8–10 weeks. Serum CA19-9 levels were obtained each cycle. Overall survival was analyzed using the Kaplan–Meier method and a stratified log-rank test. Data were censored for patients who were still alive at the time of analysis. Survival was calculated from the day of initial therapy to the date of death or time of last follow-up.
Results
Cohort Characteristics
A total of 65 consecutive patients were treated and evaluated (Table 1). The median age of the patients was 59 years (range 26–80 years), and 42 patients were women. Nineteen patients had stage III disease; 46 had stage IV disease. Twenty-eight patients had undergone prior surgery for their disease. The median duration of treatment was 29 weeks (range 2–158 weeks). No patients were receiving chemotherapy at the time of assessment. Discontinuation of treatment occurred for the following reasons: all therapy was completed (n = 3), disease progression (n = 30), toxicity (n = 22), patient and/or physician discretion (n = 5), or additional adverse events (n = 5). See Toxicity section for further details.
Table 1.
Demographic and baseline clinical characteristics of patients who were treated
| Characteristics | N = 65 |
|---|---|
| Age (years) | |
| Median | 59 |
| Range | 26–80 |
| Sex | |
| Male | 23 |
| Female | 42 |
| ECOG Performance Status scorea | |
| 0 | 18 |
| 1 | 27 |
| 2 | 20 |
| Site of primary cancer | |
| Head | 29 |
| Body | 24 |
| Tail | 12 |
| Prior surgery | |
| Yes | 28 |
| No | 37 |
| Previous chemotherapy | |
| Yes | 28 |
| No | 37 |
| Disease stage | |
| III | 19 |
| IV | 46 |
| Site of metastatic disease | |
| Liver | 34 |
| Lymph node | 13 |
| Lung | 6 |
| Peritoneal carcinomatosis | 9 |
| Ovary | 1 |
| Carbohydrate antigen 19-9 level | |
| Normal | 13 |
| Elevated | 52 |
aECOG = Eastern Cooperative Oncology Group
Survival and Response
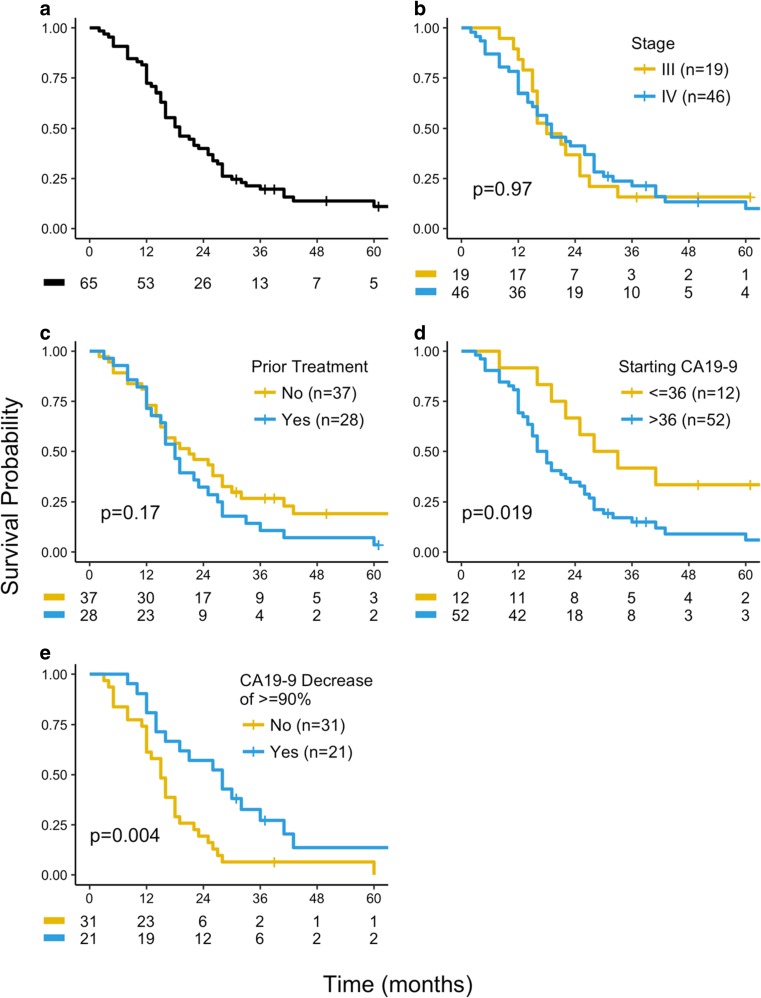
Fifty-three patients (82%) lived 12 months or longer. The overall median survival for the entire cohort was 19 months (range 2–91+; Table 2 and Fig. 1a). According to RECIST criteria on CT or magnetic resonance imaging (MRI), four patients achieved a complete response and 28 achieved a partial response, for a response rate of 49%. Twenty-one patients had stable disease, resulting in a disease control rate of 81% (Table 3). Survival was not influenced by either disease stage at time of diagnosis or whether patients had received prior treatment (Fig. 1b, c; Table 2). Thirteen patients (20%) had normal CA19-9 levels ( ≥ 36 U/mL) at the time of initial therapy, and their median survival was 30.5 months, significantly greater than the overall survival of the 52 patients who had elevated CA19-9 at start of therapy (17 months) (Fig. 1d). Of the 52 patients with elevated pretreatment CA19-9, 21 patients had 90% or greater reduction in CA19-9 levels compared with baseline. This cohort of patients had a response rate of 71%, with median survival of 28 months, versus 15 months in patients with less than 90% reduction in CA19-9 (p = 0.004; Fig. 1e).
Table 2.
Overall survival and survival rates among treated patients
| Overall survival | All patients (N = 65) |
Stage III (n = 19) |
Stage IV (n = 46) |
|
|---|---|---|---|---|
| Median | 19 months | 19 months | 19 months | |
| Range | 2–65+ months | 5–42+ months | 2–65+ months | |
| Survival rate | Number | % | ||
| 6 months | 59 | 90 | ||
| 12 months | 53 | 82 | ||
| 18 months | 35 | 54 | ||
| 24 months | 23 | 41 | ||
| 36 months | 12 | 18 |
Fig. 1.
Overall survival of a all evaluable patients (n = 65), b stage III (n = 19) and stage IV (n = 46) patients, c patients who received prior therapy (n = 28) and those who did not (n = 37), d patients with elevated CA19-9 levels (n = 52) and those with normal CA19-9 (n = 13), and e patients with elevated CA19-9 with a > 90% decrease in CA19-9 (n = 20) and with a < 90% decrease (n = 32)
Table 3.
Objective response in treated patients
| Number of patients | ||||||
|---|---|---|---|---|---|---|
| Total evaluable (N = 65) |
Stage III (n = 19) |
Stage IV (n = 46) |
||||
| Response results | Number | % | Number | % | Number | % |
| Complete response | 4 | 6 | 2 | 11 | 2 | 4.3 |
| Partial response | 28 | 43 | 8 | 42 | 20 | 43 |
| Stable disease | 21 | 32 | 8 | 42 | 13 | 28.3 |
| Progression of disease | 12 | 18 | 1 | 5 | 11 | 24 |
| Disease control | 53 | 81 | 18 | 95 | 35 | 76 |
Seven patients are alive and being followed off therapy at the time of analysis. Their survival ranges from 39 months to 120 months since their initial diagnosis. Two remain clinically free of disease.
Toxicity
The most frequently observed treatment-related grade 3 or 4 side effects were fatigue (21%), neuropathy (25%), stomatitis (21%), diarrhea (25%), and nausea and vomiting (9%) (Table 4).
Table 4.
Common toxicity as a result of treatment
| Non-hematologic toxicity, grade ≥ 3 | Number | (%) |
| Fatigue | 14 | 21 |
| Neuropathy | 16 | 25 |
| Stomatitis | 14 | 21 |
| Diarrhea | 16 | 25 |
| Nausea/vomiting | 6 | 9 |
| Hypertension (grade ≥ 2) | 13 | 20 |
| Renal failure | 2 | 2 |
| Hematologic toxicity, grade ≥ 3 | Number | (%) |
| Anemia | 7 | 11 |
| Thrombocytopenia | 9 | 14 |
| Neutropenia | 12 | 18 |
The most common non-hematologic toxicity was neuropathy, seen in 32 patients (49%), with 16 patients (25%) experiencing grade 3 or greater. Nineteen patients discontinued therapy because of neuropathic side effects. With time, 31 of 32 patients with neuropathy improved to either grade 1 or 2.
Four patients developed a drug allergy to oxaliplatin, with three additional patients manifesting interstitial pneumonitis. All were successfully treated.
Thirteen patients (25%) developed grade 2 or greater hypertension, a known toxicity of bevacizumab. Of these, two were associated with hemolytic uremic syndrome. Both of these patients recovered.
Anemia was observed in 14 patients (22%), seven of whom had grade 3 or greater. Nine patients (14%) developed grade 3 or 4 thrombocytopenia, and 12 patients (18%) had grade 3 or greater neutropenia.
Additional events included myocardial infarction (n = 1), hemolytic uremic syndrome (n = 2), and small bowel obstruction while on therapy (n = 1); all of these patients recovered. One patient who developed multiple liver abscesses and sepsis secondary to cholangitis died while on therapy.
Discussion
Advanced, inoperable pancreatic cancer is an aggressive and rapidly fatal disease. For decades, there has been no clinically meaningful improvement in survival with therapeutic intervention for patients with metastatic disease. Standard treatments using MTD schedules have been of limited value; thus an urgent oncologic need remains unmet.
In this paper we have retrospectively analyzed the experience of 65 patients with advanced, inoperable pancreatic cancer who were treated with 5FU as a prolonged infusion in conjunction with weekly low-dose leucovorin, nab-paclitaxel, and oxaliplatin. Bevacizumab was added on days 1 and 15. All analyzed and reported parameters compare favorably to those observed with more conventional treatments. Patient survival at 1 year was 82%, with median overall survival of 19 months. Median survival was greater than 27 months for those patients whose serum CA19-9 level decreased by 90% or more from pretreatment values. At 24 and 36 months, survival rates were 41 and 18%, respectively, and 81% of patients had achieved effective disease control.
We observed that the survival for patients with stage III and stage IV disease were the same, 19 months. In retrospect, patients with stage III disease status at the initiation of therapy may have been inaccurately under-staged, and actually had stage IV cancer. This under-staging results from the inability of current imaging techniques to detect small-volume metastasis. Previous studies utilizing gemcitabine-based therapies have reported a difference in survival between stages III and IV [5–10]. In these studies, there was limited or no therapeutic impact on survival, and hence survival differences between stages may be due to a bias in lead time to the discovery of occult metastases. In our study, median and 1-year survival rates were substantially higher than those achieved in trials of other established therapeutic regimens for pancreatic cancer. In the current work, 1-year survival was 82%, versus 45% reported for FOLFIRINOX, and median overall survival was 19 months, exceeding the 11.1 months for FOLFIRINOX [4]. With regard to the safety profile, FOLFIRINOX was associated with a 45% incidence of ≥ grade 3 neutropenia, compared with 18% in our regimen [4]. We did observe less ≥ grade 3 nausea and vomiting (15 vs. 9%), more ≥ grade 3 diarrhea (25 vs. 13%), and a higher incidence of neuropathy (25 vs. 9%). Because of the high incidence of neutropenia associated with FOLFIRINOX, one could speculate that it would be difficult to build on this regimen. Furthermore, the median duration on therapy with FOLFIRINOX was less than 16 weeks [4], compared with 29 weeks using a metronomic schedule in the present study. Based on response, survival, toxicity, and duration of treatment, metronomic therapy exhibits a more favorable therapeutic index.
The regimen reported here also compares favorably to outcomes achieved with gemcitabine plus nab-paclitaxel. In the Von Hoff study, median overall survival was 8.5 months, and 1-year and 2-year survival was 35 and 9%, respectively, in the gemcitabine plus nab-paclitaxel group [11]. By comparison, median survival, 1-year survival, and 2-year survival reported here are 19 months, 81%, and 41%, respectively. Compared to the 49% response rate and 81% disease control rate achieved in the current study, the response and disease control rates for nab-paclitaxel/gemcitabine were 23 and 48%, respectively. Finally, the median duration on study for nab-paclitaxel and gemcitabine was under 18 weeks, substantially less than the 29 weeks observed in the current study.
In spite of the depressing statistics resulting from mostly negative trials, oncologists continue to accept the marginal benefits of currently sanctioned therapies for pancreatic cancer. Several examples are obvious. One is the establishment of gemcitabine as the standard of care [41]. Others include the frequent use of ineffective combinations such as gemcitabine and erlotinib, gemcitabine and oxaliplatin, or gemcitabine and cisplatin [5, 10, 42, 43]. There is no conclusive evidence that these treatment protocols are better than the 5FU-based regimens which were used in the 1980s and 1990s [44].
As stated previously, metronomic dosing may inhibit tumor angiogenesis, whereas high-dose pulse therapy could potentiate it. Kamen et al. note that the idea of a log-phase dose survival curve developed by Skipper et al. was a consequence of an in vivo model used to study the effect of chemotherapy on cell death [45]. In these models, the dose–response effects were only valid for cells growing during the log phase. Skipper et al. clearly indicate that this model would be of limited value clinically, because growth in solid tumors is not in the log phase, and the majority of cancer cells within the tumor mass are non-proliferating during the time of drug exposure [46]. The focus has been on the “dose”, and has neglected “time”. Experimental evidence shows that endothelial cells are affected by chemotherapy at their MTD [12, 13, 47]. However, prolonged intervals between treatment allow for tumor regrowth and endothelial cell repair. The damage to the tumor endothelium is reversed by a profound rebound in circulating endothelial progenitor cells (CEPs) released from the bone marrow during drug-free intervals [48, 49]. This rebound in CEPs after MTD dosing could explain a potential mechanism for drug resistance, and thus metronomic dosing may inhibit this process [48]. Some of the most profound effects have involved various microtubule inhibitors such as vinblastine, paclitaxel, and docetaxel [50, 51]. Researchers have proposed that the pro-apoptotic properties associated with metronomic chemotherapy may be mediated by the reduction in endothelial cell specific inhibition by thrombospondin 1 (TSP1) [52, 53]. Other therapeutic agents also have angiogenic “side effects” that could work by similar mechanisms. Trastuzumab, a monoclonal antibody against ERBB2, was revealed to have antiangiogenic properties, as it inhibits VEGF expression, and can induce TSP1 in tumor cells [54–56]. On the other hand, supportive growth factors such as erythropoietin and granulocyte colony-stimulating factor (G-CSF) may actually stimulate angiogenesis, contributing to worse survival outcomes in some clinical trials [57, 58].
This study is not without limitations. Our work is a retrospective analysis of a single institution’s experience spanning almost 10 years. The lack of randomization precludes a formal comparison to other treatment modalities, as a standard treatment arm is not available. Because this is a single-institution study, there may be additional factors unique to our patient population that could affect patient survival for which we could not control. Furthermore, there is notable heterogeneity in our study population, with a large fraction of patients having received prior therapy (chemotherapy and/or surgery) and a relatively high proportion of confirmed stage IV patients at treatment initiation.
Conclusion
In this report, we demonstrated the feasibility of administering multi-agent chemotherapy to an unselected patient population, giving it frequently and for prolonged periods of time, and observed that it was safe and effective. Metronomic therapy for pancreatic cancer demonstrated a good therapeutic index and non-inferiority to the current standard of care. The clinical experience reported in this retrospective analysis is a safe and effective option.
Funding
This work was supported by the William H. Isacoff, MD Research Foundation for Gastrointestinal Cancer. There was no additional funding for the work or for the preparation of the manuscript. Open access publication of this article was funded by the William H. Isacoff, MD Research Foundation for Gastrointestinal Cancer.
Conflict of Interest
All authors declare that they have no financial interests related to the work described in this paper or its publication.
References
- 1.American Cancer Society . Cancer Facts and Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Weinberg BA, Yabar CS, Brody JR, Pishvaian MJ. Current standards and novel treatment options for metastatic pancreatic adenocarcinoma. Oncology. 2015;29:809–819. [PubMed] [Google Scholar]
- 3.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefits with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. FOLFORINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone I patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group trial EJ 297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 7.Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 8.Philip PA, Benadetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial SO205. J Clin Oncol. 2010;83:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboa-Alfa G, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol. 2006;24:4441–4446. doi: 10.1200/JCO.2006.07.0201. [DOI] [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 11.Von Hoff D, Ervin T, Arena F, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Oncol. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stempak D, Seely D, Baruchel S. Metronomic dosing of chemotherapy: applications in pediatric oncology. Cancer Investig. 2006;24:432–443. doi: 10.1080/07357900600705599. [DOI] [PubMed] [Google Scholar]
- 14.Chue BM. Five-year survival of metastatic pancreatic cancer: a study of courage and hope. Gastrointest Cancer Res. 2009;3:208–211. [PMC free article] [PubMed] [Google Scholar]
- 15.Dellapasqua S, Bertolini F, Bagnardi V, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 16.Hughes S, Garcia AA. Bevacizumab and low dose cyclophosphamide in recurrent ovarian cancer. AM J Hem Oncol. 2008;7:155–156. [Google Scholar]
- 17.Isacoff WH, Reber HA, Hines OJ, et al. Metronomic therapy with 5-FU, weekly nab-paclitaxel, leucovorin, and oxaliplatin, plus bevacizumab for advanced pancreatic cancer: A phase II study. J Clin Oncol. 2012;30(Suppl 15):e14582. [Google Scholar]
- 18.Meta-analysis Group in Cancer Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 19.Ducreux M, Mitry E, Ould-Kaci M, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol. 2004;15:467–473. doi: 10.1093/annonc/mdh098. [DOI] [PubMed] [Google Scholar]
- 20.Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 21.Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuyts RMMA, Eel E, Greve EL. The effects of 5 fluorouracil and Mitomycin C on the corneal epithelium. Curr Eye Res. 1992;11:565–570. doi: 10.3109/02713689209001812. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional bolus, or oral fluoropyrimidines in first-line treatment of colorectal cancer: results from the BICC-study. J Clin Oncol. 2007;25:4776–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz HI, Tebbut NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18:1004–1012. doi: 10.1634/theoncologist.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landry JC, Feug Y, Cohen SJ, et al. Phase 2 study of preoperative radiation with concurrent capecitabine, oxaliplatin and bevacizumab followed by surgery and post-operative 5 fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: ECOG 3204. Cancer. 2013;119:1521–1527. doi: 10.1002/cncr.27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straling M, Watkins D, Cunningham D, et al. Dose finding and early efficacy study of gemcitabine plus capecitabine in combination with bevacizumab plus erlotinib in advanced pancreatic cancer. J Clin Oncol. 2009;33:5499–5505. doi: 10.1200/JCO.2008.21.5384. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsu A, Shah MA, Van Custom E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double blind placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3972. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 29.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase II trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment of trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- 32.Buchler P, Reber HA, Buchler MW, et al. VEGF-RII influences the prognosis of pancreatic cancer. Ann Surg. 2002;236:738–749. doi: 10.1097/00000658-200212000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahbari N, Schmidt T, Falk C, et al. Expression and prognostic value of circulating angiogenic cytokines in pancreatic cancer. BMC. 2011;11:280–296. doi: 10.1186/1471-2407-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumour edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inge RH, Sleijfer S, Mathijssen RH, et al. Increasing tumoral 5-fluorouracil concentration during a 5-day continuous infusion: a microanalysis study. Cancer Chemother Pharmacol. 2011;67:1055–1062. doi: 10.1007/s00280-010-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increase antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bond paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 37.ten Tije AJ, Verweij J, Los WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 38.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 39.Seidman AD, Conlin AK, Bach A, Moynahan ME, Lake D, Forero A, et al. Randomized phase II trial of weekly vs. every 2 weeks vs. every 3 weeks nanoparticle albumin-bound paclitaxel with bevacizumab as first-line chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2003;13:239–246. doi: 10.1016/j.clbc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Rugo HS, Barry WT, Moreno-Aspitia A, Lyss A, Cirrincione C, Toppmeyer D, et al. CALGB 40502/NCCTG N063H: randomized phase III trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nab-paclitaxel or ixabepilone ± bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer. J Clin Oncol. 2012;30(Suppl 15):CRA1002. doi: 10.1200/jco.2012.30.18_suppl.cra1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology V. 1. 2013. [DOI] [PubMed]
- 42.Kulke MH, Tempero MA, Niedzwiecki D, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with pancreatic cancer: CALGB 89904. J Clin Oncol. 2009;27:5506–5512. doi: 10.1200/JCO.2009.22.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinemann V, Quietzsch D, Giesler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 44.Sultana A, Tudur Smith C, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607–2615. doi: 10.1200/JCO.2006.09.2551. [DOI] [PubMed] [Google Scholar]
- 45.Kamen BA, Rubin E, Aisner J, Glatstein E. High-time chemotherapy or high time for low dose. J Clin Oncol. 2000;18:2935–2937. doi: 10.1200/JCO.2000.18.16.2935. [DOI] [PubMed] [Google Scholar]
- 46.Skipper HE, Schabal FM, Mellet LB. Implications of biochemical, cytokinetic, pharmacologic and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970;54:431–450. [PubMed] [Google Scholar]
- 47.Kerbel RS. Improving conventional or low dose metronomic chemotherapy with targeted antiangiogenic drugs. Cancer Res Treat. 2007;39:150–159. doi: 10.4143/crt.2007.39.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philips C. A new “target” for chemotherapy? Natl Cancer Inst Bull. 2006;Vol 3:#26. [Google Scholar]
- 49.Bertotolini F, Saki P, Mancuso P, et al. Maximum tolerable dose and low dose metronomic chemotherapy have opposite side effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;65:4342–4346. [PubMed] [Google Scholar]
- 50.Wang J, Lou P, Leseniewski R, Henkin J. Paclitaxel at ultra low concentrations inhibits angiogenesis without affecting cellular microtubule assembly. Anticancer Drug Des. 2003;14:13–19. doi: 10.1097/00001813-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Lau D, Guo L, Gandara D, et al. Is inhibition of cancer angiogenesis and growth by paclitaxel schedule-dependent? Anti-Cancer Drugs. 2004;15:871–875. doi: 10.1097/00001813-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Hamano Y. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–1574. doi: 10.1158/0008-5472.CAN-03-3126. [DOI] [PubMed] [Google Scholar]
- 53.Bocci G, Francia G, Man S, et al. Thrombospondin-1, a mediator of the angiogenic effects of low dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viloria-Petit A. Neutralizing antibodies against EGF and ErbB-2/neu receptor tyrosine kinases down-regulate VEGF production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 55.Izami Y, Xu L, Citaniaso E, et al. Tumor biology: Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi T, et al. Ischemia and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 57.Heeschen C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 58.Brower V. Epoetin for cancer patients: a boom or a danger? J Natl Cancer Inst. 2004;95:1820–1821. doi: 10.1093/jnci/95.24.1820. [DOI] [PubMed] [Google Scholar]