Abstract
Imaging of a 53-year-old Japanese man revealed two tumors in the liver and a tumor in the head of the pancreas with a swelling lymph node. A needle biopsy for the liver tumors was performed, revealing a neuroendocrine tumor. Enucleation, lymphadenectomy, and partial hepatectomy were performed. The microscopic examination identified many tumor cells with intracytoplasmic inclusions arranged in a nested, cord, or tubular fashion. The intracytoplasmic inclusions displayed densely eosinophilic globules and displaced the nuclei toward the periphery, which constitutes “rhabdoid” features. The tumor cells were positive for synaptophysin and weakly positive for NCAM, but negative for chromogranin A. Epithelial markers (AE1/AE3 and CAM5.2) accentuated intracytoplasmic globules. Pancreatic neuroendocrine tumors with rhabdoid features are very rare. Generally, rhabdoid features are aggressive and dedifferentiated characteristics of various types of tumor. Pancreatic neuroendocrine tumors containing rhabdoid cells tend to display extrapancreatic spread at the time of presentation, although some of these tumors with rhabdoid features are not always associated with aggressive behavior.
Keywords: Neuroendocrine tumor, Rhabdoid feature, Cytoplasmic inclusion, Pancreas
Introduction
Neuroendocrine tumors of the pancreas are rare neoplasms that account for 1–2% of all pancreatic tumors [1]. In the majority of cases, the tumors are characterized by their distinctive “neuroendocrine” appearance, reminiscent of neuroendocrine neoplasms in other organs. Neuroendocrine tumors in the pancreas may show variable morphological alterations, including oncocytic change [2, 3], clear cytoplasm [4, 5], spindle cell morphology [6], and rhabdoid features [7, 8]. Neuroendocrine tumors showing rhabdoid features have been defined based on the cells with deeply eosinophilic cytoplasmic inclusions and a peripherally displaced nucleus that contains a prominent nucleolus [9–11].
We report the case of a patient’s primary neuroendocrine tumor of the pancreas with rhabdoid features, accompanied by multiple liver metastases. We also review the relevant literature.
Case report
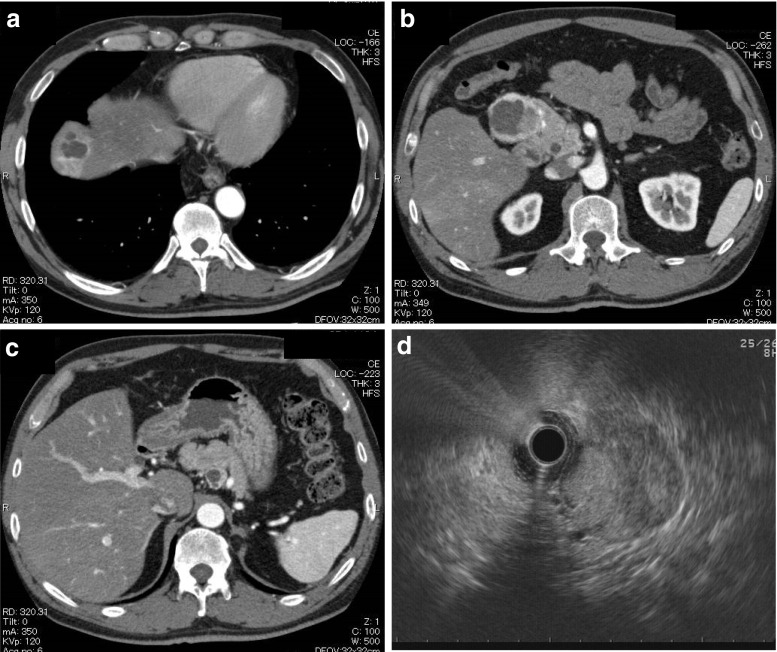
A 53-year-old Japanese man with a liver tumor was referred to our hospital for further investigation. A hypervascular mass had been detected in the left lobule of the liver by computer tomography (CT) for other disease. He had a history of hypertension, but no history of hereditary disease such as Von Hippel-Lindau disease nor family history of the patients. The results of a physical examination were unremarkable, and no notable symptoms were present. The biochemical tests revealed slightly elevated levels of alanine aminotransferase (49 U/L) and lactate dehydrogenase (250 U/L). The serum gastrin level (312 pg/ml) was increased. An abdominal contrast-enhanced CT examination confirmed a 4-cm vascular and cystic mass in the anterosuperior segment of the right hepatic lobe and a 0.5-cm vascular lesion in antero/posterosuperior segment of the right hepatic lobe (Fig. 1a). In addition, a 4-cm vascular and cystic mass in the head of the pancreas and a 2-cm vascular and cystic mass in the posterior portion of the body of the pancreas were found (Fig. 1b, c).
Fig. 1.
Imaging studies. a–c Dynamic contrast-enhanced CT scan during the artery phase showed a well-enhanced mass with cystic component in an anterosuperior segment (S8) of the liver (a), the head of the pancreas (b), and the posterior portion of the body of the pancreas (c). d Endoscopic ultrasound (EUS) showed a 4.1-cm hypoechoic and well-demarcated mass in the parenchyma of the pancreatic head
Endoscopic ultrasonography (EUS) showed a 4.1-cm well-demarcated hypoechoic mass in the pancreatic head (Fig. 1d) and a 1.9-cm well-demarcated hypoechoic mass in the pancreatic body. High blood flow was detected in the mass by color Doppler ultrasonography. At the previous hospital, a needle biopsy for liver tumors was performed, and it showed that atypical cells with hyperchromatic nuclei and eosinophilic cytoplasm were arranged in a nested fashion. Immunohistochemically, atypical cells were positive for CAM5.2, synaptophysin, chromogranin A, and glucagon and negative for CK7, CK20, AFP, vimentin, CD34, desmin, c-kit, insulin, gastrin, and somatostatin. These results were suggestive of a metastatic neuroendocrine tumor. We performed enucleation for the pancreatic tumor together with lymph node dissection and partial hepatectomy. The postoperative course was uneventful, and the patient was discharged 36 days after the operation. The patient is alive 4.5 years after surgery without evidence of recurrence or metastasis.
Immunohistochemical procedures
For the histological examination, the tissue samples were fixed in 10% formalin and embedded in paraffin, cut into 3-μm-thick sections, and stained with hematoxylin and eosin. A periodic acid Schiff preparation, with and without diastase digestion, and Southgate’s mucicarmine staining were also done. For the immunohistochemical staining, 3-μm-thick tissue sections were deparaffinized in xylene and dehydrated in ethanol. Endogenous peroxidase activity was blocked by incubation in methanol containing 0.3% H2O2 for 30 min. Antigen retrieval was achieved by microwave heating in 10 mM citrate buffer (pH 6.0 or 9.0), or Target Retrieval Solution (DakoCytomation, Glostrup, Denmark). The slides were incubated with primary antibodies for 90 min at room temperature and then incubated with biotin-free horseradish peroxidase enzyme-labeled polymer (Envision+ System, DakoCytomation) for 40 min at room temperature. The labeled antigens were visualized by 3,3′-diaminobenzidine tetrahydrochloride as a chromogen. Counterstaining was done with hematoxylin. All of the antibodies were used with the appropriate positive control.
The primary antibodies used for the immunohistochemical analysis were as follows: synaptophysin (1:50; Dako, Glostrup, Denmark), chromogranin A (1:1500; Dako), AE1/AE3 (1:400; Dako), CAM5.2 (1:20; Becton Dickinson, Lincoln Park, NJ), cytokeratin 7 (1:50; Dako), cytokeratin 19 (1:100; Boehringer Mannheim, Gaithersburg, MD), EMA (1:400; Dako), trypsin (1:1000; Chemicon, Billerica, MA), β-catenin (1:200; Transduction Laboratories, Lexington, KY), NCAM (1:50; Novocastra, Newcastle, UK), insulin (1:1; Nichirei Bioscience, Tokyo), glucagon (1:1; Nichirei Bioscience), gastrin (1:400; Dako), somatostatin (1:100; Biomol, Hamburg, Germany), Ki-67 (1:100; Dako), vimentin (1:25; Dako), hepatocyte (1:200; DakoCytomation), BAF47/INI1 (1:50; BD Bioscience, Franklin Lakes, NJ), BAF155 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), BAF170 (1:100; Santa Cruz Biotechnology), ARID1A (1:500; Sigma, St. Louis, MO), BRG-1 (1:25; Santa Cruz Biotechnology), and p53 (1:500; Calbiochem, Darmstadt, Germany). For the p53 immunolabeling, focal acinar and ductal cells with nuclear p53 immunolabeling served as an internal control. The p53 immunolabeling was interpreted as “high expression” when the neoplastic cells showed robust nuclear accumulation in ≥ 30% of the neoplastic cells. Immunohistochemical labeling of high p53 expression corresponded to mutational inactivation [12]. The MIB-1 labeling index (MIB-1 LI) was determined by counting the positively stained nuclei in at least 500 tumor cells.
Gross and histologic features
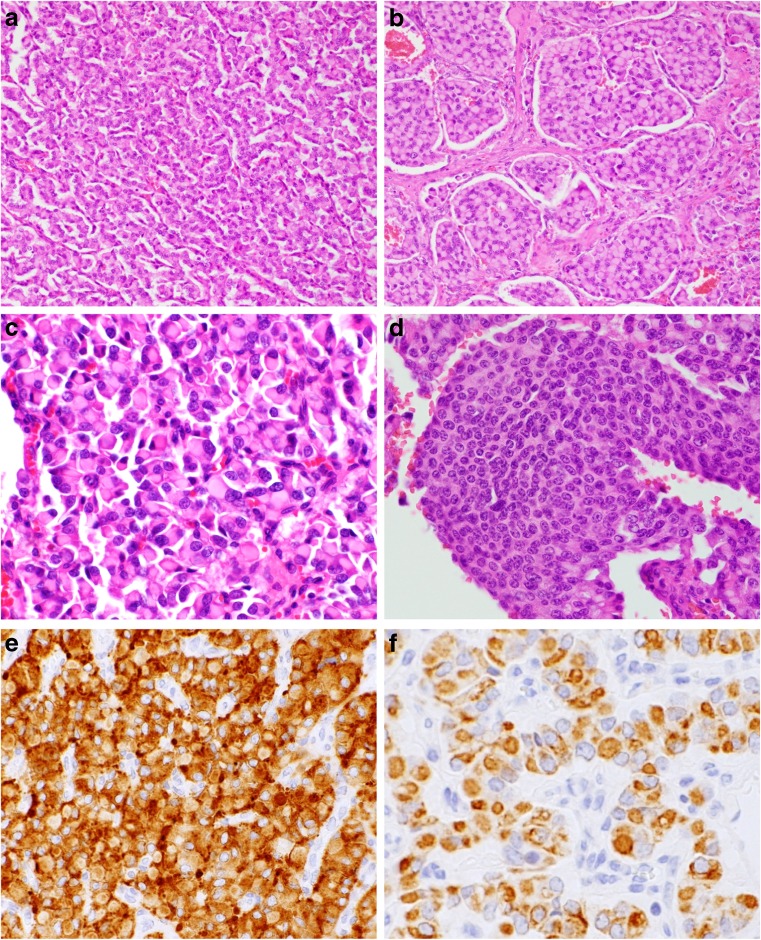
Resected specimens showed solid, partly cystic and brown/tan to white color with hemorrhage. The size of the pancreatic tumor was 57 × 40 mm, and the sizes of the three liver tumors were 50 × 20 mm, 10 × 6 mm, and 5 × 4 mm. Cystic component was also found in a lymph node and the liver. In both the pancreas and liver, the tumor cells were arranged in a nested, cord, or tubular fashion (Fig. 2a, b). Tumor cells had abundant cytoplasm and lightly or densely eosinophilic globular inclusions that displaced the nuclei toward the periphery (Fig. 2c). The nuclei were round to oval with dispersed (“salt and pepper”) chromatin and nuclear pleomorphism (Fig. 2c). Tumor cells showed mitoses at the rate of one per 10 high-power fields (HPF) in the pancreatic tumor, while six per 10 HPF in the liver tumor. There was an area of moderate necrosis in the pancreatic tumor, but not in the liver tumor. Vessel invasion of tumor cells was observed in the pancreatic tumor and the liver. In part, there were also typical features of a pancreatic neuroendocrine tumor, characterized by a nesting arrangement of uniform cells (Fig. 2d), but tumor cells with intracytoplasmic globules accounted for 80% of the total tumor cell population in the pancreas and 90% of the total tumor cell population in the liver. The rhabdoid features between primary and metastatic tumors were not changed. Hyaline inclusions were negative with the periodic acid Schiff, with and without diastase digestion, and mucin stains.
Fig. 2.
Histological findings for the tumor located in the pancreas head (a, c–f) and liver (b). a Tumor cells arranged in a cord fashion. b Tumor cells arranged in a nested fashion. c Tumor cells with abundant cytoplasm and densely eosinophilic globular inclusions that displaced the nuclei toward the periphery. d Uniform cells arranged in a nested fashion, demonstrating typical features of a pancreatic neuroendocrine tumor. e Tumor cells were positive for synaptophysin. f Immunohistochemically, intracytoplasmic globular inclusions were positive for AE1/AE3. Original magnification × 200 (a, b), × 400 (c–f)
Immunohistochemically, pancreatic tumor cells were positive for epithelial markers (AE1/AE3 and CAM5.2), CK19, EMA, synaptophysin (Fig. 2e), somatostatin, INI1, BAF155, BAF170, and ARID1A; weakly positive for NCAM, gastrin, and BRG-1; and negative for chromogranin A, β-catenin, trypsin, glucagon, insulin, and vimentin. The p53 immunolabeling for the pancreatic tumor tissue was low expression. AE1/AE3 (Fig. 2f) and CAM5.2 highlighted intracytoplasmic globules. In addition, tumor cells in the liver were positive for chromogranin A and focally positive for HepPar-1. The MIB-1 labeling index was approx. 3% in the pancreas tumor and 2% in the liver tumor. We diagnosed the tumor as a primary pancreatic neuroendocrine tumor, G2, accompanied by metastatic liver tumors and lymph nodes.
Discussion
The term “rhabdoid” has been used to describe tumors composed of cells with intensely eosinophilic/acidophilic globular cytoplasmic inclusions which are similar to rhabdomyoblasts [9–11]. Electron microscopy studies have shown that the rhabdoid inclusions of pancreatic endocrine tumors are composed of whorls of intermediate filaments that entrapped variable numbers of neurosecretory granules and that in some inclusions, the intermediate filaments and neurosecretory granules are accompanied by rough and smooth endoplasmic reticulum profiles [8]. The whorls of intermediate filaments are mainly keratin filaments, as demonstrated by the positive immunohistochemical staining for keratin and not vimentin [8].
In follicular thyroid carcinomas, the rhabdoid inclusions are vimentin-positive but lack immunoreactivity for cytokeratins, thyroglobulin, smooth-muscle actin, and desmin [13]. By electron microscopy, the eosinophilic inclusions were revealed to be composed of whorls of intermediate filaments that displaced cytoplasmic organelles and the nuclei to the periphery [13].
To the best of our knowledge, there have been only four previous reports regarding such a characteristic morphology of inclusions in a pancreatic neuroendocrine tumor. One report indicated that large intracytoplasmic inclusions occur in approx. 16% of pancreatic neuroendocrine tumors [14]. We conducted a search of the records of the Department of Surgery and Oncology, Kyushu University (1991–2010) for cases of pancreatic neuroendocrine tumor or carcinoma, and we retrieved a total of 72 cases. We reviewed the hematoxylin-eosin-stained slides of all cases, but no case of neuroendocrine tumor with rhabdoid features was found.
It has been proposed that rhabdoid morphology is associated with an aggressive biologic behavior based on lymph node metastasis, local invasion, and early recurrence [10, 13, 15–17]; however, we were unable to find any report regarding other neuroendocrine tumors other than those in the lung and pancreas. We identified only two cases of a lung neuroendocrine tumor with rhabdoid features. One patient had local invasion and died due to the tumor within 1.5 months after the operation [16]. The other patient died because of the tumor 3 months post-surgery [17]. In other neuroendocrine tumors except for those in the lung and pancreas, the biologic behavior of neuroendocrine tumors with rhabdoid features may be unclear.
The clinical features of the cases in the four previous reports and the present case are summarized in Table 1. The patients were six men and six women 37–79 years of age. Eight of the 12 cases (66.7%) showed evidence of metastatic spread (either peripancreatic fat, lymph nodes, or liver), although the tumors in the metastatic cases were classified as a neuroendocrine tumor, G1 or G2, according to the current WHO classification [1]. Six of the 12 cases (50%) were alive and free of disease 2–5 years after surgery. Though the follow-up periods were not available in three cases, for the two patients who died due to tumor, the overall survival periods were 24 and 73 months after diagnosis (Table 1). Not many of the pancreatic neuroendocrine tumor patients containing rhabdoid cells died due to the tumor early after the operation. Pancreatic endocrine tumors containing rhabdoid cells seem to display extrapancreatic spread at the time of presentation while lacking the histomorphological features associated with aggressive tumors (large size, necrosis, high mitotic count, and high proliferate rate) [14]. In our case, the comparatively low Ki-67 index for the proliferation marker may be consistent with an indolent nature of the lesion.
Table 1.
The clinicopathologic features of reported cases of neuroendocrine tumor of the pancreas with rhabdoid features
| Case | Age | Sex | Location | Size (cm) | Gross appearance | Metastases | Mitosis (HPF) | Ki-67 labeling index (%) | MEN-1 | Treatment | Additional treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | M | Tail | 3.4 | Poorly circumscribed, infiltrative mass | Periaortic lymph nodes | Very scant | ND | NA | Partial pancreatectomy + lymphadenectomy | No | Alive, no recurrence, 2 years after surgery | [7] |
| 2 | 55 | F | Head | 2.5 | Well-circumscribed mass | Multiple liver metastases | NA | ND | NA | Pancreaticoduodenectomy + partial hepatectomy | – | Dead, peritonitis 1 month after surgery | [7] |
| 3 | 58 | M | Head | 4 | Well-circumscribed mass | No | Rare | ND | NA | Pancreaticoduodenectomy | No | Alive, no recurrence, 4 years after surgery | [7] |
| 4 | 79 | F | Tail | 4.5 | Well-circumscribed mass | No | < 2/10 | ND | NA | Distal pancreatectomy + splenectomy | No | Alive, no recurrence, 5 years after surgery | [7] |
| 5 | 60 | M | Head | NA | NA | Bilobar liver metastases | ND | < 1% | NA | Radiation + chemotherapy + alcohol injection | NA | Dead, 73 months after the initial diagnosis | [8] |
| 6 | 35 | M | NA | 3.2 | NA | 3 lymph nodes, invasion of peripancreatic soft tissue | ≤ 4/10 | < 5% | Yes | NA | NA | Alive, no recurrence, after 2 years | [14] |
| 7 | 55 | M | NA | 11 | NA | 9 lymph nodes, invasion of peripancreatic soft tissue | ≤ 4/10 | < 5% | No | NA | NA | Alive, no recurrence, after 5 years | [14] |
| 8 | 45 | F | NA | 5 | NA | No | ≤ 4/10 | < 5% | Yes | NA | NA | No follow-up available | [14] |
| 9 | 41 | F | NA | 8 | NA | 1 lymph node | ≤ 4/10 | < 5% | No | NA | NA | No follow-up available | [14] |
| 10 | 67 | F | NA | 6 | NA | 1 lymph node | ≤ 4/10 | < 5% | No | NA | NA | Dead, 2 years after diagnosis | [14] |
| 11 | 50 | F | Head | 4.4 | Well-circumscribed mass | No | 4–9/10 | 6% | NA | NA | NA | NA | [13] |
| 12 | 53 | M | Head | 5.7 | Well-circumscribed mass | Multiple liver metastases 4 lymph nodes | 1/10 | 3% | No | Enucleation + lymphadenectomy + partial hepatectomy | No | Alive, no recurrence, 4.5 years after surgery | Present case |
NA not available, ND not detected
For both biopsy and resected specimens in the liver, the tumor cells were positive for chromogranin A, but pancreatic tumor cells were negative for chromogranin A. There were discrepant results of chromogranin A between resected specimens in the pancreas and liver. The reason for discrepancy is that there may be possibility of genetic heterogeneity in the primary pancreatic tumor as pancreatic cancer [18].
The term “rhabdoid,” meaning rhabdomyoblast-like, implies a resemblance to rhabdomyoblasts that is characteristic of aggressive, dedifferentiated malignancies. Pancreatic neuroendocrine tumors containing rhabdoid cells seem to display extrapancreatic spread at the time of presentation, but they are not always associated with aggressive behavior compared to other neoplasms with rhabdoid features.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Klimstra DS, Arnold R, Capella C, et al. et al. Neuroendcrine neoplasm of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al.et al., editors. WHO classification of tumours of the digestive system. 4. Lyon: IARC; 2010. pp. 281–291. [Google Scholar]
- 2.Gotchall J, Traweek ST, Stenzel P. Benign oncocytic endocrine tumor of the pancreas in a patient with polyarteritis nodosa. Hum Pathol. 1987;18:967–969. doi: 10.1016/S0046-8177(87)80278-2. [DOI] [PubMed] [Google Scholar]
- 3.Pacchioni D, Papotti M, Macri L, et al. Pancreatic oncocytic endocrine tumours. Cytologic features of two cases. Acta Cytol. 1996;40:742–746. doi: 10.1159/000333950. [DOI] [PubMed] [Google Scholar]
- 4.Lubensky IA, Pack S, Ault D, Vortmeyer AO, Libutti SK, Choyke PL, Walther MCM, Linehan WM, Zhuang Z. Multiple neuroendocrine tumors of the pancreas in von Hippel-Lindau disease. Am J Pathol. 1998;153:223–231. doi: 10.1016/S0002-9440(10)65563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang MP, Hruban R, Albores-Saavedra J. Clear cell endocrine pancreatic tumor mimicking renal cell carcinoma: a distinctive neoplasm of Von Hippel-Lindau disease. Am J Surg Pathol. 2001;25:602–609. doi: 10.1097/00000478-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Terada R, Ito S, Akama F, Kashima K, Kidogawa H, Ooe H. Small nonfunctioning islet cell tumor in the body of the pancreas: report of a case. Surg Today. 2004;34:177–180. doi: 10.1007/s00595-003-2650-z. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Montiel MD, Frankel WL, Suster S. Neuroendocrine carcinomas of the pancreas with “rhabdoid” features. Am J Surg Pathol. 2003;27:642–649. doi: 10.1097/00000478-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Shia J, Erlandson RA, Klimstra DS. Whorls of intermediate filaments with entrapped neurosecretory granules correspond to the “rhabdoid” inclusions seen in pancreatic endocrine neoplasms. Am J Surg Pathol. 2004;28:271–273. doi: 10.1097/00000478-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Nappi O, Ferrara G, Wick MR. Neoplasms composed of eosinophilic polygonal cells: an overview with consideration of different cytomorphologic patterns. Semin Diagn Pathol. 1999;16:82–90. [PubMed] [Google Scholar]
- 10.Wick MR, Ritter JH, Denhner LP. Malignant rhabdoid tumors: a clinicopathologic review and conceptual discussion. Semin Diagn Pathol. 1995;12:233–248. [PubMed] [Google Scholar]
- 11.Chetty R, Asa SL. Pancreatic endocrine tumour with cytoplasmic keratin whorls. Is the term “rhabdoid” appropriate? J Clin Pathol. 2004;57:1106–1110. doi: 10.1136/jcp.2004.018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baas IO, Mulder JW, Offerhaus GJ, et al. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 13.Albores-Saavedra J, Sharma S. Poorly differentiated follicular thyroid carcinoma with rhabdoid phenotype: a clinicopathologic, immunohistochemical and electron microscopic study of two cases. Mod Pathol. 2001;14:98–104. doi: 10.1038/modpathol.3880263. [DOI] [PubMed] [Google Scholar]
- 14.Serra S, Asa SL, Chetty R. Intracytoplasmic inclusions (including the so-called “rhabdoid” phenotype) in pancreatic endocrine tumors. Endocr Pathol. 2006;17:75–81. doi: 10.1385/EP:17:1:75. [DOI] [PubMed] [Google Scholar]
- 15.Mojghan A, Ro JY, Ordonez NG, et al. Adenocarcinomas of the gastrointestinal tract with prominent rhabdoid features. Ann Diagn Pathol. 2002;6:357–363. doi: 10.1053/adpa.2002.36657. [DOI] [PubMed] [Google Scholar]
- 16.Shimazaki H, Aida S, Sato M, Deguchi H, Ozeki Y, Tamai S. Lung carcinoma with rhabdoid cells: a clinicopathological study and survival analysis of 14 cases. Histopathology. 2001;38:425–434. doi: 10.1046/j.1365-2559.2001.01145.x. [DOI] [PubMed] [Google Scholar]
- 17.Chetty R, Bhana B, Batitang S, Govender D. Lung carcinomas composed of rhabdoid cells. Eur J Surg Oncol. 1997;23:432–434. doi: 10.1016/S0748-7983(97)93725-2. [DOI] [PubMed] [Google Scholar]
- 18.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]