HSV-1 is a ubiquitous human pathogen that infects the majority of the world's population. Although most infections are asymptomatic, HSV-1 establishes lifelong latency in infected sensory neurons, from which it can reactivate to cause deadly encephalitis or potentially blinding eye disease. No clinically effective vaccine is available. In this study, we tested the protective potential of a neuroattenuated HSV-1 mutant (KOS-NA) as a vaccine in mice. We compared the effects of immunization with KOS-NA to those of two other attenuated viruses, a replication-competent (ICP0−) virus and a replication-incompetent (ICP8−) virus. Our data show that KOS-NA proved superior to the ICP0- and ICP8-null mutants in protecting mice from corneal disease and latent infection. With its significant neuroattenuation, severe impairment in establishing latency, and excellent protective effect, KOS-NA represents a significant discovery in the field of HSV-1 vaccine development.
KEYWORDS: herpes simplex virus, ICP6, cornea, keratitis, vaccine, HSV-1, immunization, mutant, ocular
ABSTRACT
We previously isolated a herpes simplex virus 1 (HSV-1) mutant, KOS-NA, that carries two nonsynonymous mutations in UL39, resulting in L393P and R950H amino acid substitutions in infected cell protein 6 (ICP6). Our published data studying KOS-NA pathogenesis strongly suggest that one of these ICP6 substitutions expressed from KOS-NA, R950H, severely impaired acute viral replication in the eyes and trigeminal ganglia of mice after inoculation onto the cornea and consequently impaired establishment and reactivation from latency. Because of its significant neuroattenuation, we tested KOS-NA as a potential prophylactic vaccine against HSV-1 in a mouse model of corneal infection. KOS-NA stimulated stronger antibody and T cell responses than a replication-competent ICP0-null mutant and a replication-incompetent ICP8-null mutant optimized for immunogenicity. Immunizations with the ICP0−, ICP8−, and KOS-NA viruses all reduced replication of wild-type HSV-1 challenge virus in the corneal epithelium to similar extents. Low immunizing doses of KOS-NA and the ICP8− virus, but not the ICP0− virus, protected mice against eyelid disease (blepharitis). Notably, only KOS-NA protected almost completely against corneal disease (keratitis) and greatly reduced latent infection by challenge virus. Thus, vaccination of mice with KOS-NA prior to corneal challenge provides significant protection against HSV-1-mediated disease of the eye, even at a very low immunizing dose. These results suggest that KOS-NA may be the foundation of an effective prophylactic vaccine to prevent or limit HSV-1 ocular diseases.
IMPORTANCE HSV-1 is a ubiquitous human pathogen that infects the majority of the world's population. Although most infections are asymptomatic, HSV-1 establishes lifelong latency in infected sensory neurons, from which it can reactivate to cause deadly encephalitis or potentially blinding eye disease. No clinically effective vaccine is available. In this study, we tested the protective potential of a neuroattenuated HSV-1 mutant (KOS-NA) as a vaccine in mice. We compared the effects of immunization with KOS-NA to those of two other attenuated viruses, a replication-competent (ICP0−) virus and a replication-incompetent (ICP8−) virus. Our data show that KOS-NA proved superior to the ICP0- and ICP8-null mutants in protecting mice from corneal disease and latent infection. With its significant neuroattenuation, severe impairment in establishing latency, and excellent protective effect, KOS-NA represents a significant discovery in the field of HSV-1 vaccine development.
INTRODUCTION
Infections with herpes simplex virus 1 (HSV-1) can cause a variety of diseases that range from oral-facial and genital sores to life-threatening encephalitis. Recurrent infections with HSV-1 are very common due to the virus' capacity to establish latency in the sensory neurons innervating the primary site of infection and to subsequently reactivate under stress stimuli (1). Recurrent infections of the eye after reactivation from the site of latency in the trigeminal ganglion (TG) can lead to corneal scarring and associated herpes stromal keratitis (HSK), a condition that afflicts more than 450,000 persons annually in the United States alone (2). Notably, recurrent bouts of HSK are a principal cause of nontraumatic corneal blindness (3). While treatment with antiviral drugs such as acyclovir can limit the severity of orofacial infections, acyclovir in combination with other topical therapies does not provide significant benefit to patients in controlling recurrent HSK pathology (4). A prophylactic vaccine to prevent or limit HSV-1 ocular infection could dramatically reduce the incidence and severity of HSK.
Several forms of vaccine have been tested over the years for efficacy against HSV-1 and HSV-2, the latter being the major cause of genital herpes. Initial vaccine candidates for HSV were inactivated virus (either UV inactivated or formalin fixed) (reviewed in references 5 and 6). While these vaccine formulations showed some promise in animal models, they did not provide durable protection in clinical trials. In addition to inactivated virus as a vaccine, three other strategies have been tried. Subunit vaccines have long been studied because of their relative safety. They typically utilize one or more purified, adjuvanted viral glycoproteins alone or in combination with DNA vectors that express viral glycoproteins. A phase 3 trial, the Herpevac Trial for Women, used HSV-2 glycoprotein D (gD) as a subunit vaccine in seronegative women (7). Despite its early promise, the vaccine stimulated an immune response against gD, but it did not prevent HSV-2 genital infection or disease. A second strategy involves using replication-incompetent vaccines (e.g., containing a mutation in an essential viral gene) (8–10) or HSV mutants that replicate for only one lytic cycle (often referred to as disabled-infection single-cycle [DISC] virus) (11). These types of vaccines are attractive given their limited cytopathic effects and potential for broader and more durable immune responses. However, certain of these vaccine candidates have provided no or limited benefits when assessed in humans. For example, an HSV-2 mutant with gH deleted tested as a therapeutic vaccine in individuals who had frequent reactivations did not decrease the incidence of genital lesions (12). It has been hypothesized that subunit (mono- and multivalent) and replication-incompetent vaccines are ineffective in humans because they limit the number and amount of viral antigens being expressed (13). Therefore, a third, more recently assessed strategy is the use of live attenuated strains of HSV-1 and -2 as vaccines. Attenuated viruses are effective vaccines against many other viral pathogens, including the herpesvirus varicella-zoster virus (VZV). Notably, recent studies used HSV-1 and HSV-2 infected cell protein 0 (ICP0) mutants that are replication competent but severely attenuated and can protect against ocular and vaginal challenges, respectively, in animal models (14, 15). Protection by these ICP0 mutant vaccine candidates, in part, appears to be correlated with preexisting humoral immunity (15, 16). The magnitude and breadth of the immune response stimulated by live attenuated viruses, a potential key to successful vaccination, may depend upon the degree to which the live vaccine is attenuated and able to express immunogenic proteins.
HSV-1 encodes numerous enzymes that uniquely facilitate virus replication at specific stages of its complex life cycle. The large subunit of ribonucleotide reductase, ICP6, is encoded by the UL39 gene and assists with viral DNA replication when in a complex with the small subunit encoded by UL40. Although ICP6 is not required for viral growth and replication in dividing cells because HSV can utilize the homologous host enzyme, it is essential for viral replication in quiescent cells, such as neurons (17, 18). In addition, UL39 mutants are severely impaired for replication, establishment of latency, and/or reactivation in vivo (19, 20). Interestingly, ICP6 is a potent immunogen that stimulates cytotoxic T lymphocyte (CTL) responses, with the CTL epitope being mapped to the C-terminal portion of ICP6 (21). Moreover, a recent study reported that CTLs target ICP6 in the infected TG (22) and suggested the potential of ICP6 as a subunit vaccine candidate.
In a previous report, we showed that an HSV-1 ICP6 mutant, KOS-NA, which carries nonsynonymous mutations in the UL39 gene, is severely attenuated for acute replication in vivo and consequently defective in establishment of and reactivation from latency (23). The mutant does not protect infected cells from caspase 8-dependent apoptosis. Because KOS-NA still expresses ICP6, albeit at reduced levels, and is highly attenuated in vivo, we hypothesized that KOS-NA would have potential as the basis for a protective vaccine against HSV-1 ocular infections. Consequently, we tested whether KOS-NA would be an effective vaccine candidate compared to other neuroattenuated or replication-incompetent viruses in an established murine model of vaccination followed by corneal infection with HSV-1. Our data indicate that KOS-NA provides significant prophylactic protection against viral infection and disease, suggesting that KOS-NA may prove useful in the development of a vaccine against ocular HSV-1 infection.
RESULTS
Acute replication of KOS-NA in BALB/c mice.
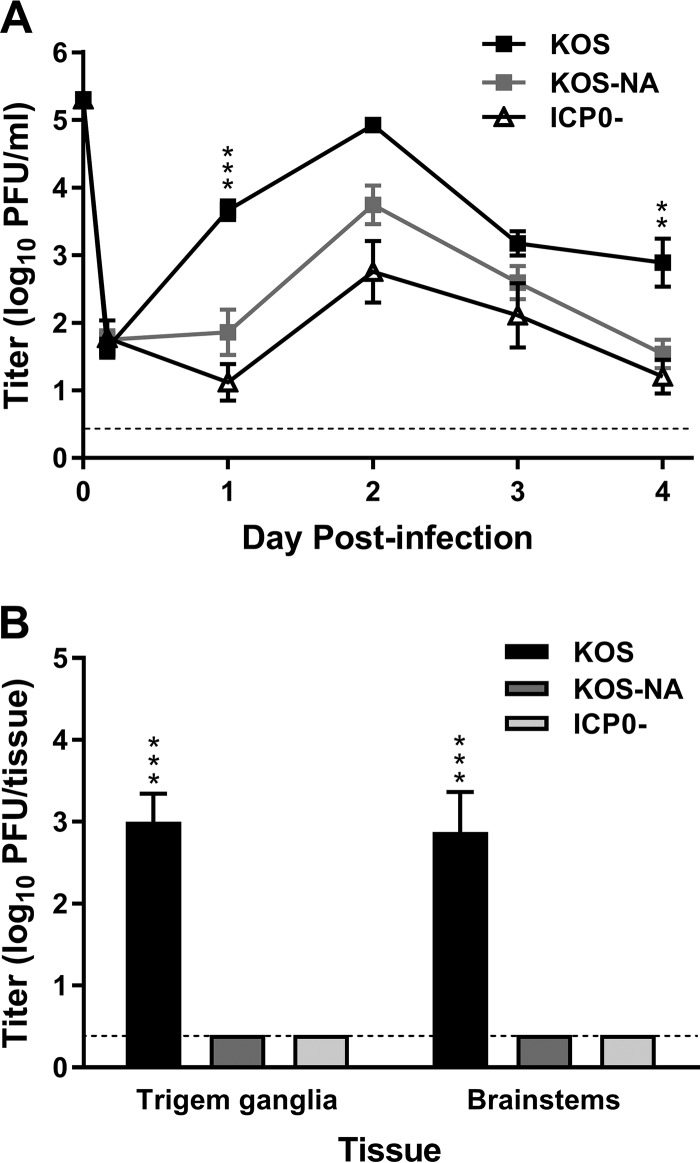
The potential value of KOS-NA as the basis for a vaccine derives from the observation that KOS-NA replication is not detectable in TG during the week after ocular infection of outbred CD-1 mice (23). In addition, KOS-NA-infected CD-1 mice do not show outward signs of HSV pathology compared to mice infected with wild-type HSV-1 (H. H. Mostafa and D. J. Davido, unpublished data). As a prelude to vaccine studies using inbred mice so that T cell responses could be assessed, we determined whether similar results would be obtained in the BALB/c mouse strain. Replication levels of KOS-NA, wild-type HSV-1 (strain KOS), and an ICP0− mutant of KOS (7134) in the corneal epithelium and nervous system were compared after ocular inoculation. KOS-NA replicated with reduced efficiency in the cornea compared to KOS days 1 through 4 postinfection (Fig. 1A). KOS-NA replicated to levels similar to those of the ICP0− mutant in BALB/c mice, in contrast to its greater attenuation than ICP0− virus in CD-1 mice. Day 4 postinfection, KOS had spread to the TG and brainstem, where it replicated to high levels in both tissues (Fig. 1B). In contrast, KOS-NA and ICP0− viruses could not be detected in the nervous system. Thus, KOS-NA is as neuroattenuated as an ICP0− virus after peripheral inoculation in BALB/c mice. This result confirms the neuroattenuation of KOS-NA observed in CD-1 mice and suggests the potential of KOS-NA as a safe means to generate antiviral immunity through vaccination.
FIG 1.
Virus titers in tear film and neural tissues after corneal inoculation. Groups of BALB/c mice were inoculated on their scarified corneas with 2 × 105 PFU per eye of wild-type KOS, KOS-NA, or ICP0− (7134) virus. (A) Titers of virus collected on corneal swabs were determined 4 h and 1 to 4 days postinfection. **, P = 0.0083, and ***, P = 0.0009 for KOS-NA compared with KOS (P > 0.05 for KOS-NA compared with ICP0− virus). (B) Mice were euthanized on day 5 postinfection, and viral titers in TG and brainstems were determined. The values represent means and standard errors of the mean (SEM) of a total of 6 to 10 mice per group compiled from 2 independent experiments. ***, P < 0.0001 for KOS-NA or ICP0− virus compared with KOS. The dashed lines indicate limits of detection.
KOS-NA is more immunogenic than two other viruses with vaccine potential.
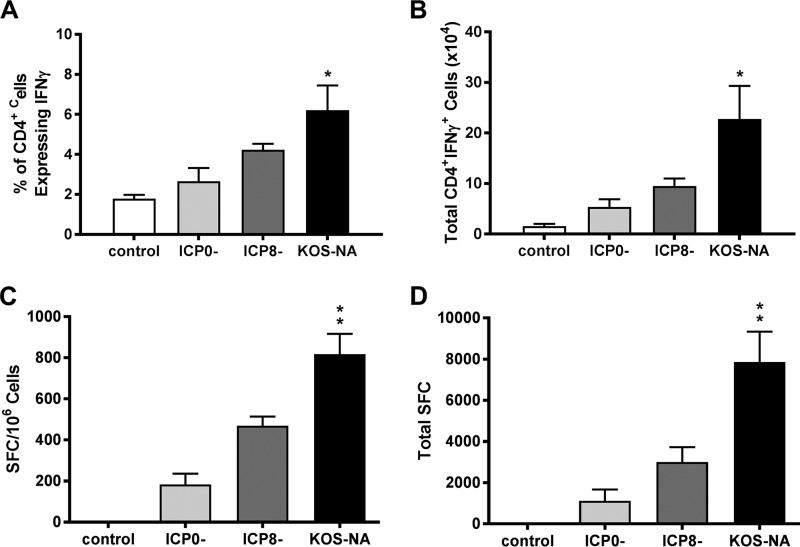
Because KOS-NA consistently showed neuroattenuation, we sought to determine its potential as the basis for an effective prophylactic vaccine against ocular disease caused by HSV-1. We compared the capacity of KOS-NA to stimulate immune responses relative to 7134 (a replication-competent virus lacking ICP0) and to Δ41Δ29B7-2 (a replication-incompetent [ICP8−] form of live virus vaccine optimized for immunogenicity because it also does not express the virion host shutoff protein [vhs] and encodes murine B7-2 costimulation molecules) (24). Because T cell responses are critical to effective immune-mediated inhibition and clearance of HSV infection (25), we first assessed T cell responses induced by the different forms of the vaccines 6 days after subcutaneous (s.c.) immunization. To analyze CD4+ T cell responses, cells from the draining lymph nodes were stimulated with phorbol myristate acetate (PMA) and ionomycin and stained intracellularly for gamma interferon (IFN-γ). A greater percentage (Fig. 2A) and absolute number (Fig. 2B) of CD4+ T cells produced IFN-γ in mice receiving KOS-NA than in those immunized with ICP0− or ICP8− viruses or control supernatant, suggesting that CD4+ T cells were optimally activated in response to the KOS-NA virus vaccine. Response to an immunodominant, Kb-restricted HSV epitope (gB498–505) (26–28) was used to monitor the strength of the CD8+ T cell response to vaccine in congenic BALB.B mice. Cells isolated from the draining lymph nodes were incubated with peptide representing gB498–505, and IFN-γ-producing cells were enumerated by enzyme-linked immunosorbent spot (ELISpot) assay. More HSV-specific CD8+ T cells were found in the draining lymph nodes of KOS-NA-immunized mice than in those of mice immunized with ICP0− or ICP8− virus, whether compared on the basis of spot-forming cells per million lymph node cells (Fig. 2C) or total spot-forming cells in the draining lymph nodes (Fig. 2D). These data indicate that KOS-NA elicits a robust HSV-specific CD8+ T cell response.
FIG 2.
T cell responses to vaccination. Mononuclear cells were isolated from draining lymph nodes 6 days after immunization of BALB.B mice with a low (2 × 104 PFU) dose of the indicated virus or control supernatant. Activated CD4 T cells in draining lymph nodes were quantified by stimulation with PMA and ionomycin, followed by intracellular staining for IFN-γ. (A) Percentages of CD4+ T cells that were IFN-γ+. (B) Total numbers of CD4+ IFN-γ-producing cells in draining lymph nodes. The results are the means of numbers from individual mice compiled from 3 independent experiments (total numbers of mice, 5 for control group and 6 to 9 for vaccine groups). *, P < 0.05 for control or ICP0− virus compared with KOS-NA. HSV-specific CD8 T cell responses were compared using gB498–505 peptide as the stimulus in an IFN-γ ELISpot assay. (C) Numbers of spot-forming cells (SFC) per million lymph node cells. (D) Total numbers of SFC in draining lymph nodes. The results are the means of numbers from individual mice compiled from 3 independent experiments (total numbers of mice, 7 for control group and 8 to 11 for vaccine groups). **, P < 0.001 for control supernatant or ICP0− virus compared with KOS-NA. P < 0.05 to 0.01 for ICP8− compared with KOS-NA. The error bars represent SEM.
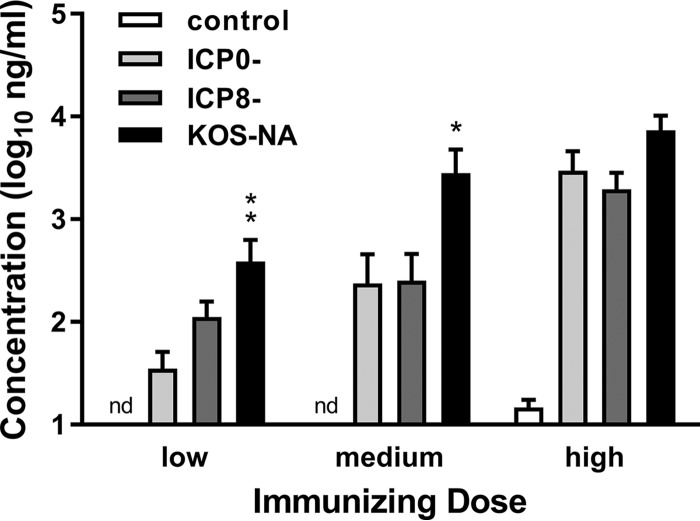
The capacities of the vaccines to elicit HSV-specific antibodies were determined by immunizing groups of mice once s.c. with 5 × 105 PFU (high), 1 × 105 PFU (medium), or 2 × 104 PFU (low) doses of the viruses or control supernatant. Sera were obtained from mice 21 days after immunization, and HSV-1-specific antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) (29). As shown in Fig. 3, KOS-NA induced significantly more HSV-specific IgG than ICP0− or ICP8− virus, with the most pronounced differences at the low (P < 0.01 to 0.05) and medium (P < 0.001) doses. These results, taken together with the T cell responses, demonstrate that KOS-NA elicits a stronger adaptive antiviral immune response than an ICP0− virus or an ICP8− virus.
FIG 3.
Titers of HSV-specific antibody in immunized mice. Groups of BALB/c mice were immunized with high (5 × 105 PFU), medium (1 × 105 PFU), or low (2 × 104 PFU) doses of the indicated viruses, and 1 group of mice was immunized with control supernatant of uninfected cells. Blood was collected 21 days postimmunization, and HSV-specific serum IgG was quantified by ELISA. The data represent the geometric mean titers for 12 mice per group plus SEM and are the combined results of 2 independent experiments with similar results. *, P < 0.01, and **, P < 0.001 for KOS-NA compared to the ICP0− virus. P < 0.05 for KOS-NA compared to the ICP8− virus. nd, not detected.
KOS-NA vaccination protects mice from subsequent corneal infection with HSV-1.
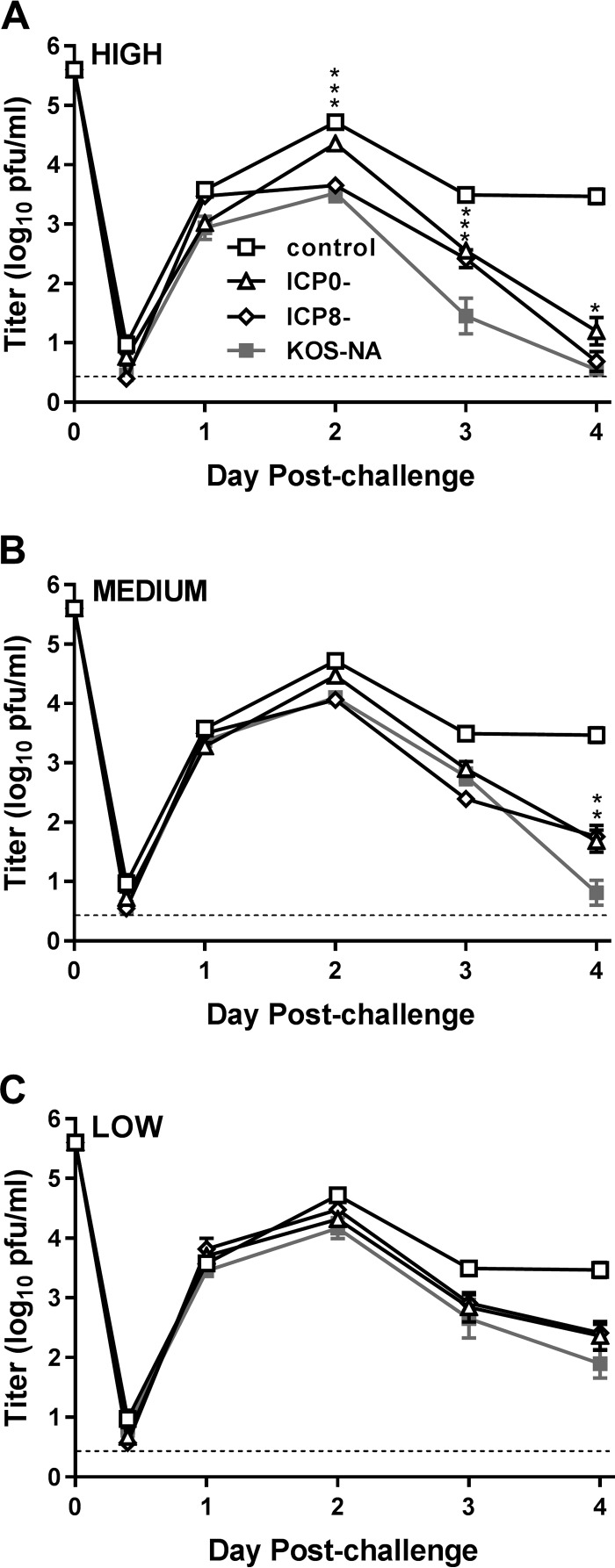
We next assessed how effectively immunization with KOS-NA could protect mice from ocular HSV-1 infection. All the vaccination groups were challenged by inoculation of the virulent, heterologous HSV-1 strain microplaque (mP) onto the scarified corneas 4 weeks after immunization. The corneal epithelia were swabbed over time postchallenge to determine the extent to which immunization with KOS-NA limited replication of the challenge virus. Less challenge virus replication was detected in the eyes of mice immunized with any of the vaccine viruses than in those of mice immunized with control supernatant, regardless of the immunizing dose (Fig. 4). Immunization with the high dose of KOS-NA significantly decreased corneal shedding of the challenge virus from 2 to 4 days postchallenge compared with the ICP0− vaccination group (Fig. 4A). KOS-NA remained more effective than both the ICP0− and ICP8− viruses when given at the medium dose in that it almost completely controlled replication by 4 days postchallenge (Fig. 4B). At the low immunizing dose, the three vaccine strains showed similar capacities to reduce challenge virus replication (Fig. 4C).
FIG 4.
Eye titers of challenge virus shed from the corneal epithelium. Groups of 10 BALB/c mice immunized with the high (A), medium (B), or low (C) dose of virus vaccine or control supernatant as described in the legend to Fig. 3 were challenged with HSV-1 strain mP (4 × 105 PFU/eye) 4 weeks postimmunization. The eyes of 6 mice per group were swabbed at the indicated times, and the titers of challenge virus in them were determined. The experiment was repeated once. The eye swab data represent the geometric means ± SEM of the combined results from the two independent experiments (total number of mice, 12 per group). The control group was the same for all three graphs. *, P = 0.0221; **, P = 0.0044; and ***, P = 0.0002 to 0.0001 for KOS-NA compared with the ICP0− virus. P = 0.0013 on day 3 at the high dose and P = 0.0019 on day 4 at the medium dose for KOS-NA compared with the ICP8− virus. The dashed lines indicate limits of detection.
KOS-NA vaccination reduces morbidity after subsequent corneal infection with HSV-1.
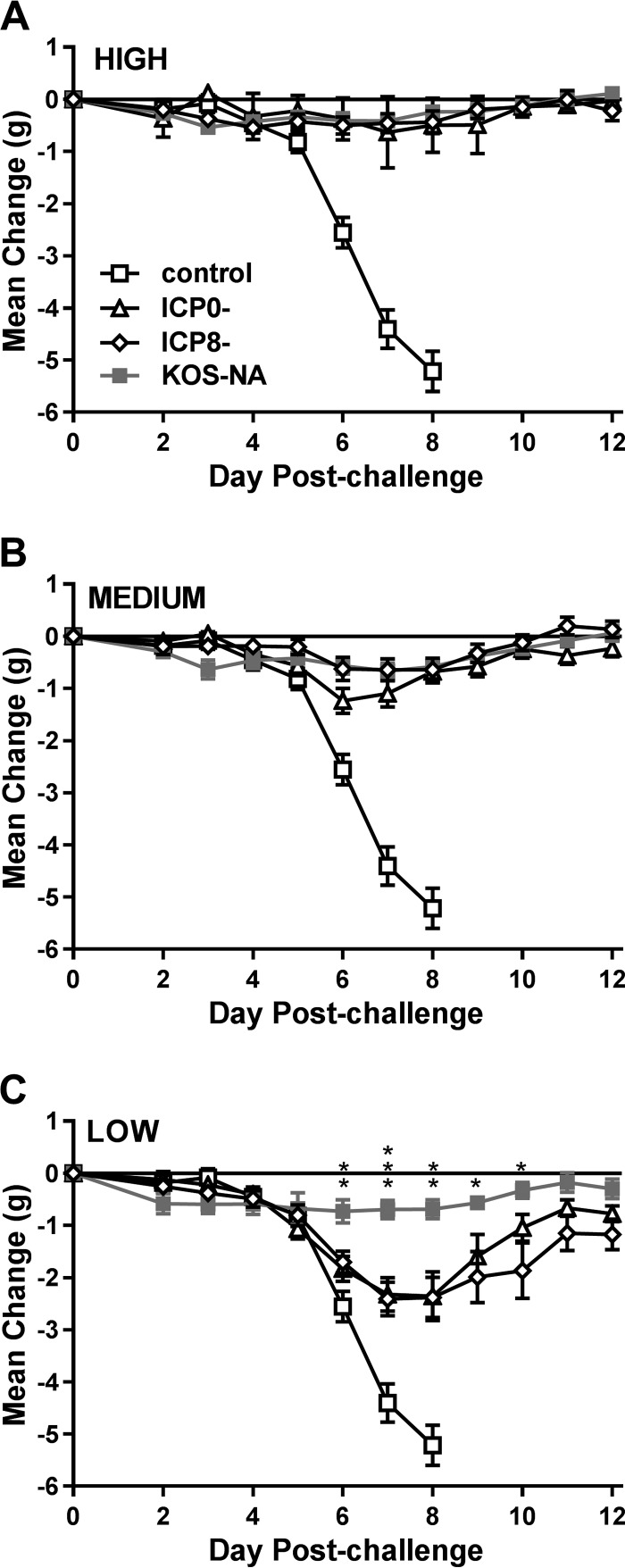
Daily change in body weight was monitored postchallenge to assess the overall fitness of mice vaccinated with KOS-NA versus the other viruses. Mice that were immunized with control supernatant and subsequently challenged with HSV-1 strain mP noticeably lost weight by day 6 postchallenge, which further progressed until day 8, when most of the mice died (Fig. 5). In contrast, mice immunized with the high or medium dose of any of the vaccine viruses maintained their body weight through 12 days postchallenge. Differences among the vaccinated mice became apparent at the low immunizing dose. Mice immunized with KOS-NA generally maintained their weight over the course of the experiment, whereas mice immunized with the ICP0− or ICP8− virus showed significant loss of weight, beginning day 6 postchallenge and extending to days 11 and 12. These results indicate that KOS-NA at the low vaccine dose protected mice better from weight loss than ICP0− or ICP8− virus.
FIG 5.
Body weight change after virus challenge. The groups of 10 BALB/c mice described in the legend to Fig. 4 were weighed prior to challenge and at the indicated times postchallenge. The data are the mean changes in weight ± SEM for each group and represent the combined results from two independent experiments (total number of mice, 20 per group). *, P = 0.0300 to 0.0171; **, P = 0.007 to 0.002; ***, P < 0.0001 for KOS-NA compared with the ICP0− virus. P = 0.0189 to 0.0005 at the low dose on days 6 through 10 for KOS-NA compared with the ICP8− virus.
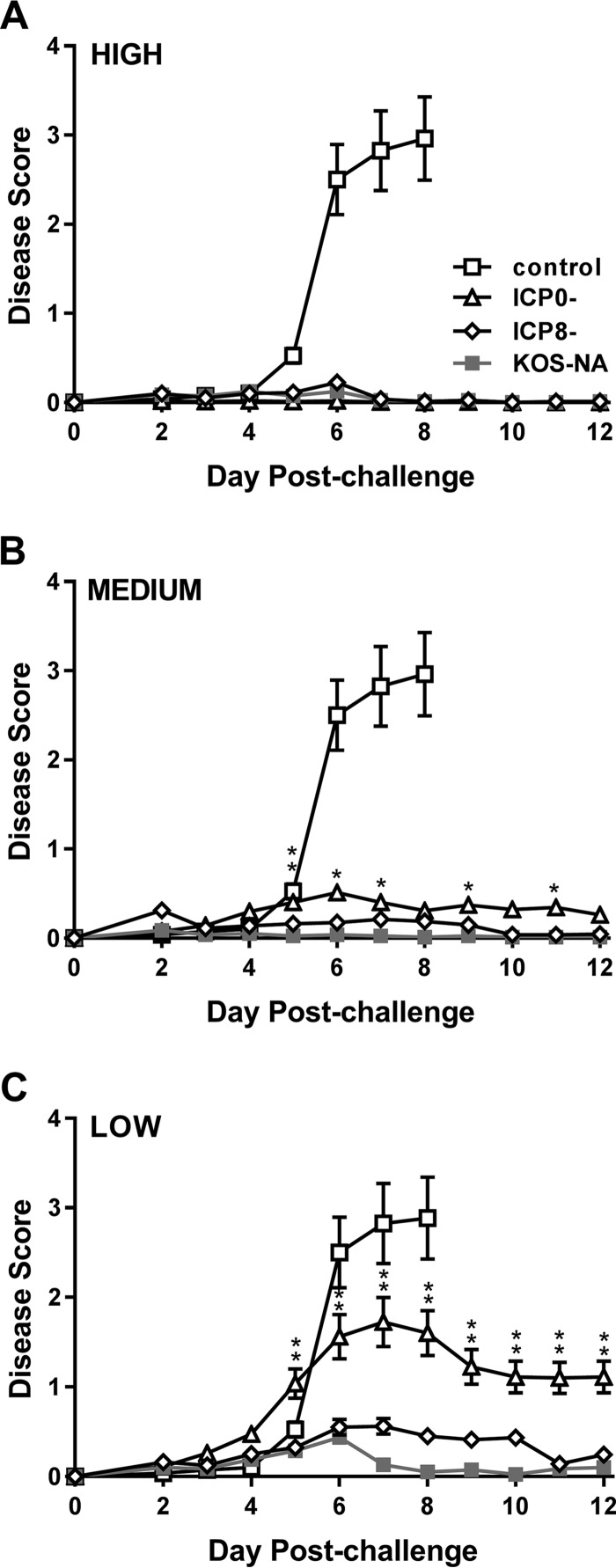
Diseases of the eyelid (blepharitis) and cornea (keratitis) are two prominent pathological features of HSV-1 ocular infection. Blepharitis and keratitis were therefore scored postchallenge to determine the efficacy of ocular protection. At the highest immunization dose tested, the three virus vaccines protected nearly all the mice from blepharitis (Fig. 6A). At the medium dose, KOS-NA and ICP8− viruses prevented most of the mice developing blepharitis. In contrast, protection from periocular disease was slightly less robust in the ICP0−-vaccinated group over several days postchallenge (Fig. 6B). This difference between the mice immunized with ICP8− or KOS-NA virus and the ICP0− virus became most apparent at the low vaccine dose, with the ICP0− vaccine group showing significantly more blepharitis beginning 5 days postchallenge (Fig. 6C).
FIG 6.
Protection of mice from blepharitis after corneal challenge. Groups of mice as described in the legend to Fig. 4 were scored daily for signs of eyelid disease. The values are the means ± SEM of 36 to 40 eyes per group and are the combined results of two independent experiments. *, P = 0.043 to 0.01, and **, P = 0.007 to <0.001 for KOS-NA relative to the ICP0− virus. Differences between KOS-NA and ICP8− virus were not statistically significant.
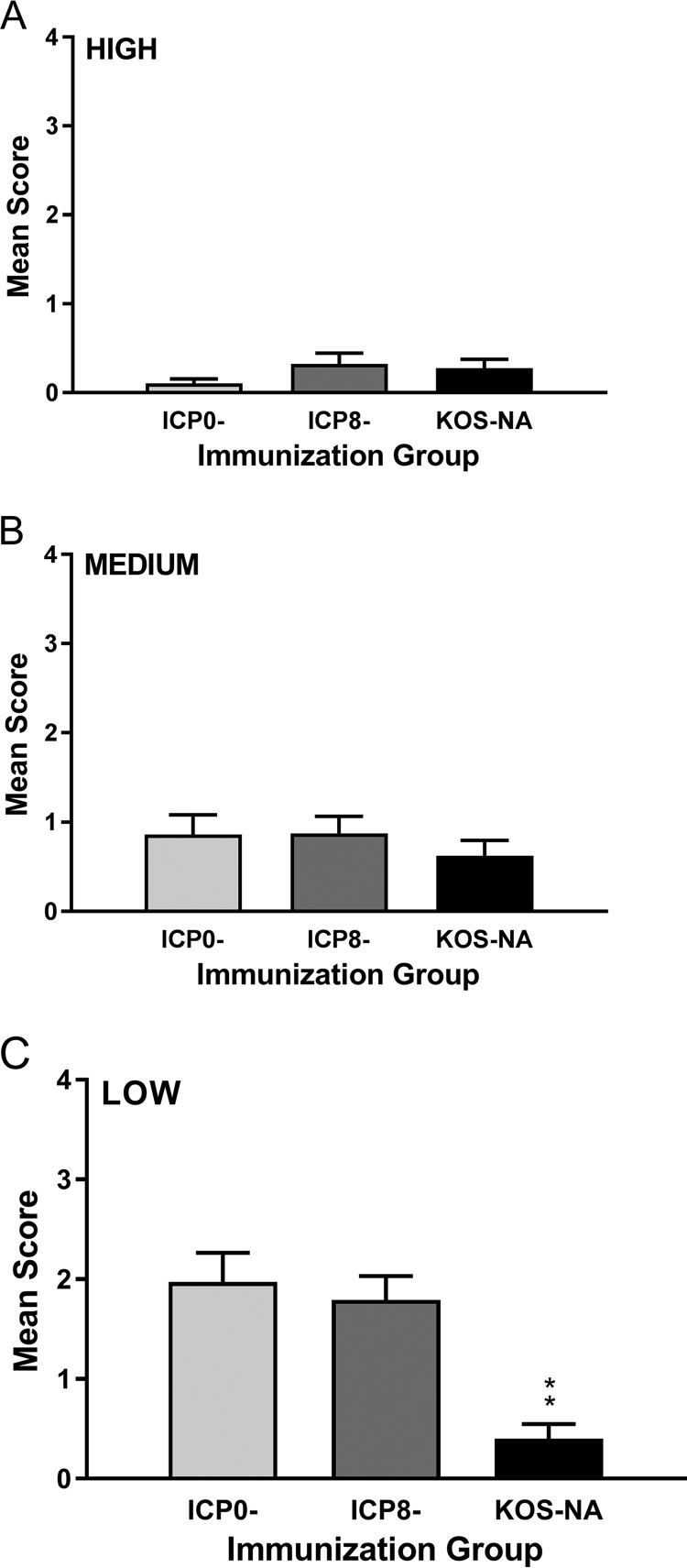
Keratitis was evaluated in all the immunization groups 14 days postchallenge (Fig. 7). All the viruses efficiently protected mice from developing keratitis when given at the high or medium dose (Fig. 7A and B), but only KOS-NA continued to protect the mice from developing keratitis at the low immunizing dose (Fig. 7C). Indeed, among mice in the low-dose immunization groups, only 7.5% of the eyes of mice previously immunized with KOS-NA developed severe keratitis (sight impairment; score of 3 or 4), compared with 45% of the eyes of mice in the ICP0− and ICP8− immunization groups (P < 0.0004). The results were similar at day 9 postchallenge (data not shown). Thus, a single dose of KOS-NA as low as 2 × 104 PFU almost completely protected the corneas from disease.
FIG 7.
Protection of mice from keratitis after corneal challenge. The eyes of the same groups of mice described in the legend to Fig. 4 were examined at 14 days postchallenge for signs of keratitis. The values represent the mean keratitis scores and SEM of eyes from surviving mice (36 to 40 eyes per group). **, P < 0.001 for KOS-NA compared with ICP8− or ICP0− virus and are the combined results of two independent experiments.
KOS-NA vaccination protects mice from subsequent neuronal infection with HSV-1.
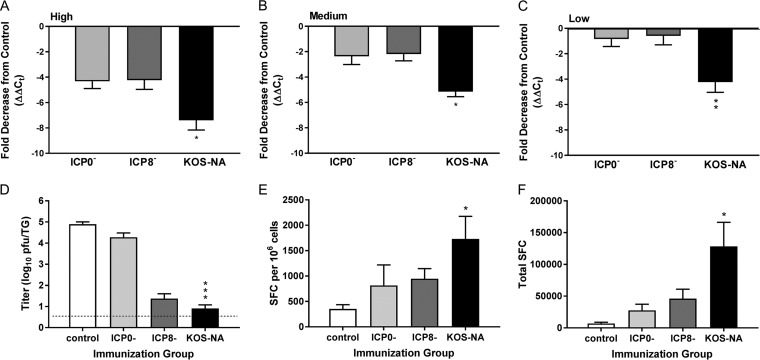
Because vaccination of mice with KOS-NA impaired acute replication of the challenge virus and reduced development of blepharitis and keratitis, we sought to determine whether KOS-NA was effective in limiting challenge virus infection of the nervous system. The TG of latently infected mice were removed 28 days postchallenge, and relative viral genome loads for all samples were quantified by real-time PCR. Prior immunization with the high or medium dose of any of the three vaccine viruses reduced the load of latent virus genomes after challenge relative to the control-immunized mice (Fig. 8A and B). In addition, KOS-NA protected the TG of mice from latent infection by challenge virus markedly (2- to 4-fold) better than either the ICP0− or ICP8− virus at every immunization dose (Fig. 8A to C). These data likely underestimate the differences between virus- and control-immunized animals because the majority of mice immunized with control supernatant did not survive challenge and because HSV-1 DNA in some TG from the KOS-NA high- and medium-dose immunization groups was below the level of detection. These results indicate that immunization with KOS-NA effectively reduces latent infection of the nervous system upon challenge with HSV-1 compared with the ICP0− and ICP8− virus vaccines.
FIG 8.
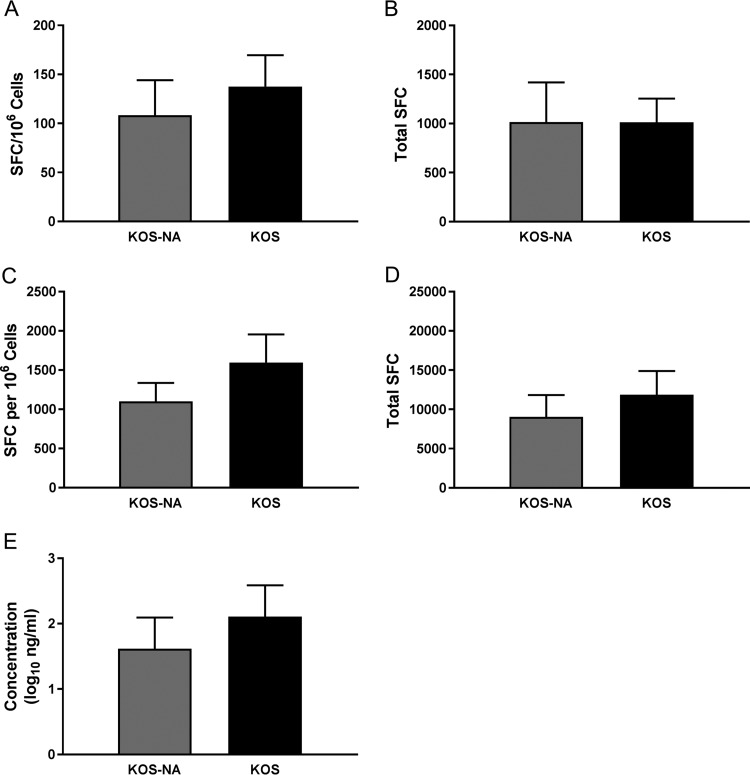
Immunization with KOS-NA impairs the establishment of latency of the challenge virus. Groups of mice immunized with the indicated virus or medium were infected with challenge virus as described in the legend to Fig. 4. (A to C) One month postchallenge, TG were removed and DNA was extracted. The relative viral DNA content was assessed by real-time PCR using primers for the HSV-1 UL50 gene after normalization to the mouse cellular adipsin gene. The data represent the relative mean fold decrease in the latent genome in 5 to 14 TG of virus-immunized mice compared with 3 TG from mice immunized with control supernatant and are the results from one of two experiments performed. HSV-1 DNA in some TG from KOS-NA high- and KOS-NA medium-dose groups were below the limit of detection. The TG were assigned a cycle number at the limit of detection for statistical purposes. *, P < 0.05, and **, P < 0.01 by ANOVA for TG from KOS-NA compared with all other groups. Additional mice were immunized with 2 × 104 PFU of the indicated viruses and challenged 1 month later as described in the legend to Fig. 4. (D) TG were removed 4 days postchallenge, and the challenge virus titer in disrupted tissue was determined. ***, P < 0.001 by ANOVA for KOS-NA compared with ICP0− virus. The data are the means and SEM of 7 to 8 mice (14 to 15 TG) compiled from 2 independent experiments. Also 4 days postchallenge, cervical lymph nodes were removed and used in an IFN-γ ELISpot assay for HSV-specific CD8+ T cells with gB498–505 as the stimulus. (E) Numbers of SFC per 1 × 106 cells. *, P < 0.05 for control compared with KOS-NA. (F) Total numbers of SFC per mouse. *, P < 0.01 for control or ICP0- compared with KOS-NA. P < 0.05 for ICP8- compared with KOS-NA. The results are the means of the results for individual mice compiled from 2 independent experiments (total number of mice, 6 per group).
We next investigated possible mechanisms for reduction in latent infection of the TG. Immunized mice were challenged as before, and infectious virus in the TG was assessed 4 days postchallenge. Mice previously immunized with KOS-NA had much less challenge virus in the TG acutely after challenge compared to mice immunized with ICP0− virus (Fig. 8D). Interestingly, the ICP8− virus vaccine also effectively controlled acute infection of the TG (Fig. 8D). CD8+ T cells have been linked to suppression of latent-virus reactivation from the TG (30, 31) and may be important in preventing establishment of latency (32, 33). We therefore assessed HSV-specific CD8+ T cell responses to corneal challenge. More CD8+ IFN-γ-producing T cells specific for the gB498–505 epitope were observed in the local cervical lymph nodes of all the groups of immunized mice 4 days after challenge than in those of mice immunized with control supernatant, whether evaluated on the basis of spot-forming cells per 106 lymph node cells (Fig. 8E) or total spot-forming cells (Fig. 8F); however, a significantly stronger response was observed in T cells isolated from KOS-NA-immunized mice.
Comparative immunogenicity and efficacy of attenuated KOS-NA and wild-type KOS.
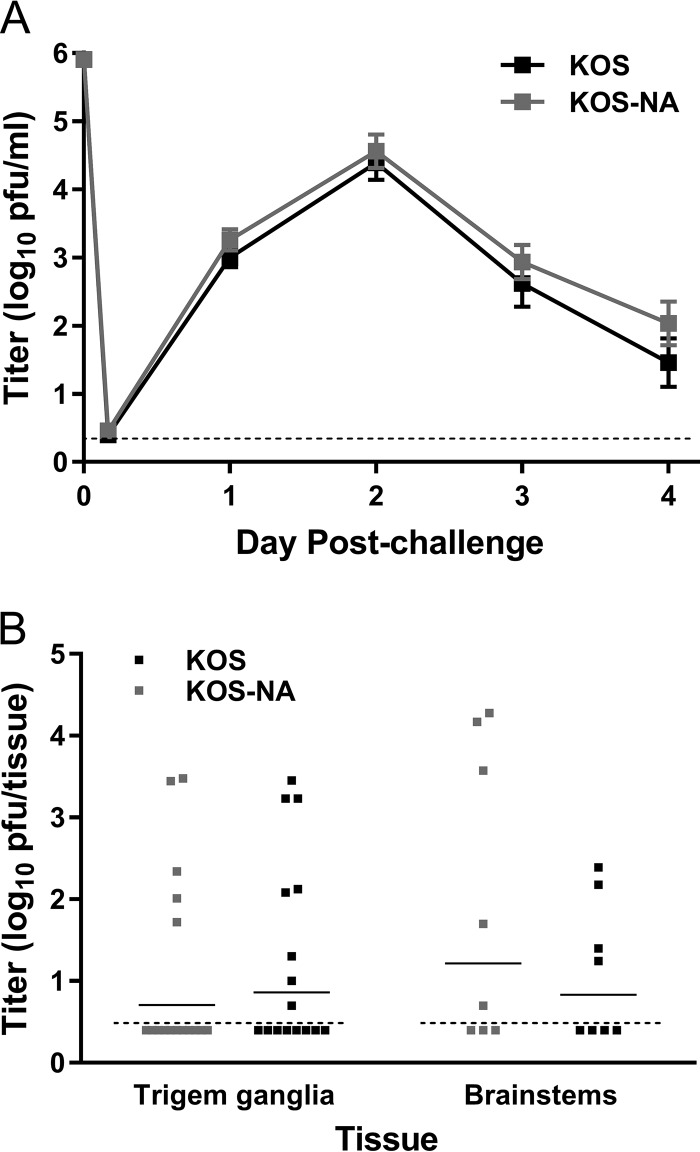
KOS-NA stimulated much stronger immune responses and protective capacity than an ICP0− mutant, even though KOS-NA was nearly as attenuated as ICP0− virus for replication in the cornea and did not replicate in the nervous system after corneal inoculation. We therefore tested the relative immunogenicity and protective capacity of attenuated KOS-NA compared with its wild-type parental strain, KOS. Groups of mice immunized s.c. with 2 × 104 PFU of KOS-NA or the wild-type HSV-1 KOS were evaluated for T cell responses, antibody titer, and capacity to resist HSV-1 challenge. The numbers of IFN-γ-producing CD4+ T cells stimulated by immunization with KOS or KOS-NA were equivalent (Fig. 9A and B). The number of IFN-γ-producing CD8+ T cells per 106 lymph node cells (Fig. 9C) and the total number of spot-forming cells (Fig. 9D) in the draining lymph nodes were slightly, though not statistically significantly, higher in mice immunized with KOS than in those immunized with KOS-NA. HSV-specific antibody levels in the serum were not significantly different between the two immunization groups (Fig. 9E). After challenge of mice immunized with KOS or KOS-NA, no difference was seen in levels of challenge virus replication in the cornea at any time (Fig. 10A). The nervous systems of some mice from each group contained no measurable virus 4 days postchallenge, and titers of challenge virus in the remaining KOS-NA- and KOS-immunized mice were not significantly different (Fig. 10B). Thus, KOS-NA is very nearly as immunogenic and protective as the wild-type virus from which it is derived, although it is comparatively attenuated for replication in the periphery and strongly neuroattenuated.
FIG 9.
Neuroattenuated KOS-NA stimulates immune responses similar to those stimulated by the wild-type strain, KOS. Groups of mice were immunized s.c. with 2 × 104 PFU (low dose) of KOS-NA or KOS, and immune responses were evaluated. Antigen-specific IFN-γ-producing cells in draining lymph nodes were enumerated by ELISpot assay 6 days postvaccination and by HSV-specific IgG in the serum 21 days postvaccination. (A and B) CD4+ IFN-γ-producing cells per 106 lymph node cells from individual BALB/c mice (A) and total CD4+ IFN-γ-producing cells responding to inactivated virus antigen (B) (11 mice per group). (C and D) CD8+ IFN-γ-producing cells specific for the gB498–505 epitope per 106 lymph node cells from individual BALB.B mice (C) and total CD8+ IFN-γ-producing cells (D) (10 mice per group). (E) Concentrations of HSV-specific serum IgG (8 BALB/c mice per group). The error bars represent SEM.
FIG 10.
Mice immunized with KOS-NA or KOS are equivalently protected from challenge. Mice immunized with 2 × 104 PFU of KOS-NA or KOS were challenged with 4 × 105 PFU of HSV-1 strain mP, and protection from acute infection was assessed. (A) Titers of virus shed from the corneal epithelium over time postchallenge. (B) Virus titer in the TG and brainstems 4 days postchallenge. The data are the combined results of two independent experiments with 8 mice per group. The dashed lines indicate the limits of detection. The error bars represent SEM.
DISCUSSION
In this study, we showed that KOS-NA was impaired for replication in the corneal epithelia of BALB/c mice, and it was not detected in the TG or brainstems (Fig. 1), reflecting previous results that demonstrated neuroattenuation in CD-1 mice (23). These observations establish an important aspect of safety required of any HSV live-virus vaccine. KOS-NA was also immunogenic. Compared to the ICP0− and replication-incompetent vaccine groups, mice inoculated with KOS-NA produced the highest number of HSV-1-specific CD4+ and CD8+ IFN-γ-producing T cells (Fig. 2) and generated the highest anti-HSV-1 IgG titers (Fig. 3). Consequently, immunization with KOS-NA at two different doses significantly decreased ocular shedding of the challenge virus 2 to 4 days postinfection (Fig. 4). At the low vaccine dose, KOS-NA protected mice from transient body weight loss (Fig. 5) and from blepharitis (Fig. 6) and keratitis (Fig. 7) better than ICP8− and ICP0− viruses after corneal challenge. An important criterion in evaluating vaccine efficacy must be the capacity of a vaccine to reduce HSV infection and establishment of HSV latency in the peripheral sensory ganglia. Not surprisingly, KOS-NA was able to significantly diminish the establishment of latency by the challenge virus (Fig. 8A to C) compared to the control-vaccinated mice. Less virus reached the TG acutely postinfection (Fig. 8D), and protection was closely associated with larger numbers of CD8+ T cells in the cervical lymph nodes after challenge (Fig. 8E and F). The KOS-NA mutations did not adversely affect the immunogenicity of HSV-1, because the mutant virus was nearly as immunogenic and protective as wild-type HSV-1 when used as a vaccine (Fig. 9 and 10). Taking the data together, we conclude that KOS-NA as a prophylactic vaccine offers better protection from ocular and periocular infections than another attenuated, replication-competent (ICP0−) virus and also a replication-incompetent (ICP8− vhs− B7+) virus engineered to optimize immunogenicity. The basis of this protection is linked to both higher levels of HSV-specific antibody and T cell responses.
We had previously demonstrated that KOS-NA bears nonsynonymous mutations in UL39, encoding ICP6 (23). ICP6, the large subunit of ribonucleotide reductase, functions in a complex with the small subunit of the enzyme, encoded by UL40. The complex catalyzes the formation of deoxyribonucleotides from ribonucleotides, which are used in DNA synthesis. Viral ribonucleotide reductase enzymatic activity becomes essential for viral DNA replication during infection of quiescent cell lines and neurons in vivo (17, 19, 20), where formation of deoxyribonucleotide pools by the host cell enzyme is restricted. In addition to its role in viral replication, ICP6 has chaperone-like activity (34), kinase activity (35), anti- and pronecroptotic activities (36, 37), and an antiapoptotic effect (38, 39). Interestingly, ICP6 also stimulates a specific cytotoxic T cell response that is detected in infected TG (22, 40). Cytotoxic T cells play a central role in controlling lytic and latent HSV-1 infection (41–43) and in maintaining latency in infected neurons (30, 44, 45). ICP6-specific CD8 T cells are prominent in the latently infected TG of C57BL/6 mice (46, 47), suggesting that the T cell response induced by ICP6 is likely important in the maintenance of latency. Thus, expressed but inactive ICP6, as a powerful inducer of CD8+ T cells that is also incapable of facilitating virus replication in neurons or blocking apoptosis, may be a key component of an effective HSV vaccine.
In this study, we showed the substantial protective potential of KOS-NA when used as a vaccine compared to an ICP0− virus and a replication-incompetent (ICP8−) virus. KOS-NA expresses ICP6 protein, albeit at a lower level than that expressed by KOS in cell culture. In contrast, ICP0− mutants express ICP6 at significantly reduced levels (48, 49) because ICP0 potently transactivates the UL39 promoter (50, 51). This suggests an explanation for the greater effectiveness of KOS-NA than the ICP0− mutant, 7134 (21), as a vaccine. Interestingly, however, we found that KOS-NA and an ICP6-null mutant (18) protected equivalently against challenge virus replication in the corneal epithelium, weight loss, blepharitis, and keratitis at the lowest vaccine dose tested (data not shown). It is possible that the reduced levels of ICP6 expressed by KOS-NA (23) are too low for the CD8 T cell response to it to make a discernible difference in overall immunity. Two alternatives are more likely. First, the ICP6 epitope identified in C57BL/6 mice (21, 46, 47) may not be present in the BALB/c mouse haplotype. Second, the T cell response induced by ICP6 may be more important in maintaining latency in neurons than it is in protecting from acute infection. Taken together, our results and those of others suggest that expressed but inactive ICP6 may still be an important component of an effective HSV vaccine strategy, depending on the host genetic capacity to utilize T cell epitopes within it.
KOS-NA's severe neuroattenuation and impaired establishment and reactivation from latency suggest it could be used as the foundation for a vaccine candidate. The facts that KOS-NA replicates as well as KOS in dividing cells in culture (data not shown) and that it does not require a complementary cell line for growth suggest relatively low selective pressure to generate secondary mutations or reversion to wild type that might alter its in vivo phenotype. Nonetheless, an increased measure of safety must be achieved, for example, by combining the KOS-NA mutation with other engineered lesions to avoid the possibility of reversion or development of adventitious mutations within KOS-NA that could negate its neuroattenuation. Accordingly, we are currently examining the effects of combining the KOS-NA mutations with mutation of other attenuating or essential genes.
An HSV-2 kinase activity-deficient mutant of ICP10, the large subunit of HSV-2 ribonucleotide reductase, had previously been evaluated for safety and effectiveness as a vaccine in rodent models. The virus, ICP10ΔPK, replicated to reduced levels after footpad inoculation compared with wild-type virus and effectively protected mice against lesions and latent infection after footpad challenge (52) and guinea pigs after vaginal challenge (53). Thus, multiple lines of evidence indicate that ribonucleotide reductase mutants are effective vaccine prototypes for prevention of HSV-1- and HSV-2-mediated diseases.
Our study is novel in directly comparing three different types of vaccine virus prototypes. We demonstrate that an ICP8− vhs− B7+ replication-incompetent virus engineered to optimize immune responses can indeed generate stronger protective immunity than an attenuated but replication-competent ICP0− virus. Significantly, the attenuated but replication-competent KOS-NA surpassed both of the other vaccine prototypes while being restricted for replication in the nervous system of mice. Our data also demonstrate the importance of testing different vaccine doses to differentiate between vaccine strains. For example, all the vaccine strains struggled to provide substantial protection against acute replication of challenge virus in the cornea, even at the highest immunizing dose, and our ability to discriminate between the strains in assessing this parameter was relatively limited. In contrast, the lowest immunizing dose of vaccine virus allowed us to easily discriminate the protective effect of KOS-NA compared to the other strains in terms of weight loss, blepharitis, and keratitis. Strikingly, KOS-NA at any immunizing dose provided better protection against latent infection of the TG, a critical measure of HSV vaccine efficacy.
A second novel aspect of our study is that the mutant form of ICP6 renders HSV-1 both neuroattenuated and immunogenic. Strikingly, although KOS-NA was attenuated for replication in the periphery compared with wild-type KOS, it rivaled KOS in induction of protective immune responses, suggesting that substitution in ICP6 has a positive impact on immunogenicity. In our previous report, we showed that mutations of UL39 in KOS-NA compromised ICP6 blockade of caspase 8-induced apoptosis (23) and that virus-induced apoptosis enhanced both innate and adaptive immune responses (54). Interestingly, ICP6 can block virus-induced necroptosis as well (36), which is a regulated necrosis process that can largely modulate the inflammatory and T cell responses (55). It is not yet known if this mutant form of ICP6 possesses antinecroptotic activity. The exact mechanism by which the KOS-NA UL39 mutation(s) dramatically affects the in vivo behavior of the virus and enhances its protective potential without detectable replication in sensory neurons will require further investigation.
MATERIALS AND METHODS
Cell lines and viruses.
Viruses for immunizations were produced free of cell debris by isolation from the supernatant of infected cell monolayers using high-speed centrifugation, as previously described (9). HSV-1 strains KOS and mP (56) were propagated in Vero cells. HSV-1 mutants 7134, KOS-NA, and Δ41Δ29B7-2 are all derived from the HSV-1 KOS strain. 7134 is an ICP0-null mutant (48) that was propagated on L7 cells stably expressing ICP0 (57). KOS-NA contains 2 nonsynonymous mutations in the UL39 gene, L393P and R950H, at least one of which is responsible for its neuroattenuated phenotype in mice. Δ41Δ29B7-2 contains deletions in the genes encoding ICP8 and vhs, and an insertion of the gene encoding murine B7-2 costimulation molecules, driven by the human cytomegalovirus immediate early promoter, into the thymidine kinase locus (24). Δ41Δ29B7-2 was propagated in S2 cells, a Vero cell line expressing ICP8 (58). Virus titers were determined on L7, Vero, or S2 cells, as appropriate, by standard plaque assay (59). Here, the HSV-1 mutant 7134 is termed ICP0− and Δ41Δ29B7-2 is referred to as ICP8− virus for simplicity.
Mice.
Female BALB/c mice were purchased from the National Cancer Institute. BALB.B mice (H-2b congenic) were purchased from the Jackson Laboratories and bred at Saint Louis University. All the mice were housed at Saint Louis University under specific-pathogen-free conditions in accordance with institutional and federal guidelines and were used at 6 weeks of age under a protocol approved by Saint Louis University.
Corneal infection.
BALB/c mice were deeply anesthetized and inoculated with 2 × 105 PFU of ICP0−, KOS-NA, or KOS virus in a 5-μl volume of normal saline on their lightly scarified corneas. Days 0 to 4 postinfection, the eyes of the mice were swabbed using a cotton-tipped applicator moistened with phosphate-buffered saline (PBS), and each swab was placed in a vial containing 500 μl of PBS. At 4 days postinfection, the mice were euthanized and TG were dissected. The TG were disrupted individually by bead beating, and virus titers were determined by standard plaque assay. Each experiment was repeated once.
Flow cytofluorometric analyses.
For CD4+ T cell analyses, groups of BALB/b mice were immunized s.c. in the hind flanks with 2 × 104 PFU of virus suspended in a 40-μl total volume of normal saline. Cohorts of mice received an equivalent amount of supernatant concentrated from uninfected cell cultures (control supernatant) as a negative control for immunization. After 6 days, draining para-aortic and inguinal lymph nodes were removed and single-cell suspensions were made. Cells were cultured for 4 h in the presence of PMA (50 ng/ml), calcium ionophore A23187 (1 μg/ml), and GolgiStop (0.67 μl/ml; PharMingen). The cells were then treated with Fc block, followed by anti-CD3 and anti-CD4, and subsequently fixed and permeabilized using a cytostain kit (PharMingen) and stained with anti-IFN-γ. Flow cytofluorometric analysis was performed using an LSRII (Becton Dickinson) and FloJo 8.0 software. This experiment was repeated once.
Immunization of mice for vaccine efficacy studies.
The hind flanks of mice were injected (20 μl per flank) s.c. with 5 × 105 PFU (high dose), 1 × 105 PFU (medium dose), or 2 × 104 PFU (low dose) of KOS-NA, ICP0−, or ICP8− virus or control supernatant.
ELISpot assays.
Groups of BALB/b mice were immunized s.c. with 2 × 104 PFU of the various vaccine strains or an equivalent amount of control supernatant. Draining lymph nodes were removed 6 days later, and single-cell suspensions were made. For CD8 ELISpot assays, 6 × 104 or 2 × 104 cells from individual mice were added per well in duplicate to Milliscreen-HA plates (Millipore) previously coated with antibody to IFN-γ (BD Pharmingen). HSV-1 gB498–505 peptide (17, 35) was added to the cultures at 0.2 μM final concentration. Control wells received medium. After incubation for 20 h, the plates were washed extensively to remove the cells, and captured IFN-γ was detected using a biotinylated anti-IFN-γ antibody (BD Pharmingen), followed by streptavidin conjugated to alkaline phosphatase (BD Pharmingen) and 3-amino-9-ethylcarbazole (AEC) substrate (Sigma). Spots were counted using an Immunospot plate reader (Cellular Technology, Ltd.). The average number of spots in control wells was subtracted from the number of spots in wells containing antigen. For CD4 ELISpot assays, cells were added at 1 × 106 or 3 × 105 cells per well. HSV-1 KOS inactivated by UV light was added at a concentration of 1 × 105 PFU/well prior to inactivation. For postchallenge assessments, cervical lymph nodes were removed 4 days postchallenge and prepared as described above.
Quantitation of serum antibodies.
Blood was collected from the tail veins of mice 21 days after immunization. Serum was prepared by clot retraction and analyzed by ELISA as previously described (34). Anti-mouse IgG-biotin (R&D Systems, Minneapolis, MN) was used as a secondary antibody and detected using streptavidin-horseradish peroxidase (HRP), followed by o-phenylenediamine dihydrochloride (OPD) substrate (Sigma, St. Louis, MO). The plates were read at 490 nm on a Bio-Rad 680 reader. Antibody titers were determined by comparison to standard curves generated with serum containing known concentrations of IgG captured on plates coated with goat anti-kappa light chain antibody (Caltag) as previously described (34).
Challenge and postchallenge assessments.
Four weeks after immunization, mice were anesthetized by intraperitoneal injection of ketamine/xylazine, and infected with 5 μl HSV-1 mP inoculated onto each scarified cornea at a dose of 4 × 105 PFU/eye. Strain mP was chosen because it causes keratitis and has been used extensively in vaccine studies as a heterologous strain that assesses vaccine interstrain cross-protection (8, 24, 60, 61). To measure virus replication in the corneal epithelium, the eyes were gently swabbed with moistened cotton-tipped swabs at 4 h and days 1 through 5 postinfection. The swabs for each mouse were placed together in 1 ml PBS and frozen at −80°C until assayed. The virus yield was quantified on Vero cell monolayers by standard plaque assay. After challenge, body weight, signs of disease, and survival were monitored on a daily basis. The mice were weighed individually, and the mean change from initial body weight was calculated daily for each group. Blepharitis scores were assigned in a masked fashion based on the following scale: 0, no apparent signs of disease; 1, mild swelling and erythema of the eyelid; 2, moderate swelling and crusty exudate; 3, periocular lesions and depilation; and 4, extensive lesions and depilation. A mean daily disease score was calculated for each group. Keratitis was assessed at 9 days and 14 days postchallenge using an ophthalmoscope and scored in a masked fashion based on the following scale: 0, no apparent signs of disease; 1, mild opacity; 2 moderate opacity with discernible iris features; 3, dense opacity; 4, dense opacity with ulceration. Virus replication in neural tissue was analyzed by dissection of TG and brainstems from a cohort of mice 3 days or 5 days after challenge. Tissues were stored frozen until used. For virus titer determination, tissues were thawed and disrupted using a Mini-Bead Beater (BioSpec, Inc.) and then diluted for use in standard plaque assays.
Real-time PCR to determine viral DNA loads.
TG were dissected 4 weeks postchallenge from mice immunized and infected as described above. DNA was isolated from individual TG using a QIAamp DNA minikit (Qiagen). PCRs were run in 25-μl reaction volumes using FastStart SYBR green master mix containing Rox (Roche) and primers at 300 nM final concentration. For adipsin, reaction mixtures contained 10 ng template DNA (62). For HSV-1 UL50 (195-bp product amplified), reaction mixtures contained 125 ng template DNA (24). Reactions were performed using an ABI Prism 7500 real-time PCR system (Applied Biosystems). The specificity was verified by melting curve analysis. Standard curves were created for both UL50 and adipsin. The average of duplicate wells yielded the CT value, and the UL50 signal for each sample was normalized to the adipsin signal content by determination of ΔCT. The fold decrease in the UL50 contents of TG from immunized mice relative to those of mice receiving control (uninfected) supernatant was determined using the 2−ΔΔCT method (63). Samples that contained undetectable amounts of viral DNA relative to the standard curve were given the value of the most dilute sample in the UL50 standard curve to estimate reductions in viral latency.
Statistical analyses.
T cell responses, concentrations of antibodies in sera, keratitis scores, and relative levels of latent viral DNA were compared between immunization groups by one-way analysis of variance (ANOVA) with the Bonferroni post hoc test for multiple groups. Viral titers shed from the cornea were compared between viruses on individual days using ANOVA. Blepharitis scores were compared on individual days using the Kruskall-Wallis test with Dunn's post hoc test for multiple groups. The significance of the difference in the proportions of mice with severe keratitis was determined using the Fisher exact method. Comparisons of KOS-NA and KOS were performed using the Student t test.
ACKNOWLEDGMENTS
This work was supported in part by award R21EY019739 from the National Institutes of Health and by institutional funds (D.J.D. and L.A.M.).
We thank Josh Hilliard for technical assistance, Jane Schrimpf for advice on the real-time PCR assay, and members of the Morrison and Davido laboratories for their comments and input related to this project.
D.J.D. and L.A.M. are coinventors of U.S. patent 9616119, in which KOS-NA is used as a vaccine to limit HSV-1 infections.
The content of this article is solely our responsibility and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Roizman R, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601. In Knipe DM, Howley PM (ed), Fields virology, vol 2 Lippincott Williams & Wilkins, New York, NY. [Google Scholar]
- 2.Dana MR, Qian Y, Hamrah P. 2000. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Wilhelmus KR, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting D, Asbell PA. 1994. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101:1871–1882. [DOI] [PubMed] [Google Scholar]
- 5.Koelle DM, Corey L. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev 16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitley RJ. 2001. Herpes simplex viruses, p 2461–2509. In Knipe DM, Howley PM, Griffin DE (ed), Fields virology, 4th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 7.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison LA, Knipe DM. 1994. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol 68:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison LA, Knipe DM. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 220:402–413. doi: 10.1006/viro.1996.0328. [DOI] [PubMed] [Google Scholar]
- 10.van Lint AL, Torres-Lopez E, Knipe DM. 2007. Immunization with a replication-defective herpes simplex virus 2 mutant reduces herpes simplex virus 1 infection and prevents ocular disease. Virology 368:227–231. doi: 10.1016/j.virol.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean CS, Erturk M, Jennings R, Challanain DN, Minson AC, Duncan I, Boursnell ME, Inglis SC. 1994. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis 170:1100–1109. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- 12.de Bruyn G, Vargas-Cortez M, Warren T, Tyring SK, Fife KH, Lalezari J, Brady RC, Shahmanesh M, Kinghorn G, Beutner KR, Patel R, Drehobl MA, Horner P, Kurtz TO, McDermott S, Wald A, Corey L. 2006. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 24:914–920. doi: 10.1016/j.vaccine.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 13.Halford WP. 2014. Antigenic breadth: a missing ingredient in HSV-2 subunit vaccines? Expert Rev Vaccines 13:691–710. doi: 10.1586/14760584.2014.910121. [DOI] [PubMed] [Google Scholar]
- 14.Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. 2011. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One 6:e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royer DJ, Gurung HR, Jinkins JK, Geltz JJ, Wu JL, Halford WP, Carr DJ. 2016. A highly efficacious herpes simplex virus 1 vaccine blocks viral pathogenesis and prevents corneal immunopathology via humoral immunity. J Virol 90:5514–5529. doi: 10.1128/JVI.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halford WP, Geltz J, Messer RJ, Hasenkrug KJ. 2015. Antibodies are required for complete vaccine-induced protection against herpes simplex virus 2. PLoS One 10:e0145228. doi: 10.1371/journal.pone.0145228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein DJ, Weller SK. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol 62:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DJ, Weller SK. 1988. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology 166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 19.Brandt CR, Kintner RL, Pumfery AM, Visalli RJ, Grau DR. 1991. The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J Gen Virol 72:2043–2049. doi: 10.1099/0022-1317-72-9-2043. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson JG, Leib DA, Goldstein DJ, Bogard CL, Schaffer PA, Weller SK, Coen DM. 1989. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 173:276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 21.Salvucci LA, Bonneau RH, Tevethia SS. 1995. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J Virol 69:1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Velzen M, Jing L, Osterhaus AD, Sette A, Koelle DM, Verjans GM. 2013. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog 9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mostafa HH, Thompson TW, Konen AJ, Haenchen SD, Hilliard JG, Macdonald SJ, Morrison LA, Davido DJ. 2018. Herpes simplex virus 1 mutant with point mutations in UL39 is impaired for acute viral replication in mice, establishment of latency, and explant-induced reactivation. J Virol 92:e01654-17. doi: 10.1128/JVI.01654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrimpf JE, Tu EM, Wang H, Wong YM, Morrison LA. 2011. B7 costimulation molecules encoded by replication-defective, vhs-deficient HSV-1 improve vaccine-induced protection against corneal disease. PLoS One 6:e22772. doi: 10.1371/journal.pone.0022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghiasi H, Perng G, Nesburn AB, Wechsler SL. 1999. Either a CD4(+) or CD8(+) T cell function is sufficient for clearance of infectious virus from trigeminal ganglia and establishment of herpes simplex virus type 1 latency in mice. Microb Pathog 27:387–394. doi: 10.1006/mpat.1999.0314. [DOI] [PubMed] [Google Scholar]
- 26.Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. 1993. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology 195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 27.Hanke T, Graham FL, Rosenthal KL, Johnson DC. 1991. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol 65:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace ME, Keating R, Heath WR, Carbone FR. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol 73:7619–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison LA, Da Costa XJ, Knipe DM. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan BS, Knickelbein JE, Hendricks RL. 2007. CD8 T cells and latent herpes simplex virus type 1: keeping the peace in sensory ganglia. Expert Opin Biol Ther 7:1323–1331. doi: 10.1517/14712598.7.9.1323. [DOI] [PubMed] [Google Scholar]
- 32.Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virol 83:2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakim LM, Jones CM, Gebhardt T, Preston CM, Carbone FR. 2008. CD8(+) T-cell attenuation of cutaneous herpes simplex virus infection reduces the average viral copy number of the ensuing latent infection. Immunol Cell Biol 86:666–675. doi: 10.1038/icb.2008.47. [DOI] [PubMed] [Google Scholar]
- 34.Chabaud S, Lambert H, Sasseville AM, Lavoie H, Guilbault C, Massie B, Landry J, Langelier Y. 2003. The R1 subunit of herpes simplex virus ribonucleotide reductase has chaperone-like activity similar to Hsp27. FEBS Lett 545:213–218. doi: 10.1016/S0014-5793(03)00547-7. [DOI] [PubMed] [Google Scholar]
- 35.Cooper J, Conner J, Clements JB. 1995. Characterization of the novel protein kinase activity present in the R1 subunit of herpes simplex virus ribonucleotide reductase. J Virol 69:4979–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. 2015. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe 17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong CQ, Xia W, Zhou R, Zheng C, Han J. 2015. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Dufour F, Bertrand L, Pearson A, Grandvaux N, Langelier Y. 2011. The ribonucleotide reductase R1 subunits of herpes simplex virus 1 and 2 protect cells against poly(I.C)-induced apoptosis. J Virol 85:8689–8701. doi: 10.1128/JVI.00362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufour F, Sasseville AM, Chabaud S, Massie B, Siegel RM, Langelier Y. 2011. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFalpha- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis 16:256–271. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- 40.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, Elliott M, Grabstein K, Posavad C, Corey L. 2006. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol 80:5509–5515. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonneau RH, Jennings SR. 1989. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol 63:1480–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonneau RH, Jennings SR. 1990. Herpes simplex virus-specific cytolytic T lymphocytes restricted to a normally low responder H-2 allele are protective in vivo. Virology 174:599–604. doi: 10.1016/0042-6822(90)90113-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith PM, Wolcott RM, Chervenak R, Jennings SR. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 44.Decman V, Kinchington PR, Harvey SA, Hendricks RL. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol 79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St Leger AJ, Hendricks RL. 2011. CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation. J Neurovirol 17:528–534. doi: 10.1007/s13365-011-0062-1. [DOI] [PubMed] [Google Scholar]
- 47.St Leger AJ, Jeon S, Hendricks RL. 2013. Broadening the repertoire of functional herpes simplex virus type 1-specific CD8+ T cells reduces viral reactivation from latency in sensory ganglia. J Immunol 191:2258–2265. doi: 10.4049/jimmunol.1300585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai W, Schaffer PA. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol 65:4078–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samaniego LA, Neiderhiser L, DeLuca NA. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol 72:3307–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai P, Ramakrishnan R, Lin ZW, Osak B, Glorioso JC, Levine M. 1993. The RR1 gene of herpes simplex virus type 1 is uniquely trans activated by ICP0 during infection. J Virol 67:6125–6135. (Erratum, 68:1264, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sze P, Herman RC. 1992. The herpes simplex virus type 1 ICP6 gene is regulated by a ‘leaky’ early promoter. Virus Res 26:141–152. doi: 10.1016/0168-1702(92)90153-Z. [DOI] [PubMed] [Google Scholar]
- 52.Aurelian L, Kokuba H, Smith CC. 1999. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase (ICP10). Vaccine 17:1951–1963. doi: 10.1016/S0264-410X(98)00470-8. [DOI] [PubMed] [Google Scholar]
- 53.Wachsman M, Kulka M, Smith CC, Aurelian L. 2001. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine 19:1879–1890. doi: 10.1016/S0264-410X(00)00446-1. [DOI] [PubMed] [Google Scholar]
- 54.Restifo NP. 2000. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immuni.ty. Curr Opin Immunol 12:597–603. doi: 10.1016/S0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaczmarek A, Vandenabeele P, Krysko DV. 2013. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Hoggan MD, Roizman B. 1959. The isolation and properties of a variant of herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg 70:208–219. [DOI] [PubMed] [Google Scholar]
- 57.Samaniego LA, Wu N, DeLuca NA. 1997. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol 71:4614–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao M, Knipe DM. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol 63:5258–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knipe DM, Spang AE. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol 43:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geiss BJ, Smith TJ, Leib DA, Morrison LA. 2000. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J Virol 74:11137–11144. doi: 10.1128/JVI.74.23.11137-11144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrison LA, Knipe DM. 1997. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology 239:315–326. doi: 10.1006/viro.1997.8884. [DOI] [PubMed] [Google Scholar]
- 62.Strand SS, Leib DA. 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J Virol 78:13562–13572. doi: 10.1128/JVI.78.24.13562-13572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]