Chikungunya virus is an emerging pathogen associated with large outbreaks on the African, Asian, European, and both American continents. In most patients, infection results in high fever, rash, and incapacitating (chronic) arthralgia. CHIKV effectively inhibits the first line of defense, the innate immune response. As a result, stimulation of the innate immune response with interferons (IFNs) is ineffective as a treatment for CHIKV disease. The IFN response requires an intact downstream signaling cascade called the JAK/STAT signaling pathway, which is effectively inhibited by CHIKV nonstructural protein 2 (nsP2) via an unknown mechanism. The research described here specifies where in the JAK/STAT signaling cascade the IFN response is inhibited and which protein domain of nsP2 is responsible for IFN inhibition. The results illuminate new aspects of antiviral defense and CHIKV counterdefense strategies and will direct the search for novel antiviral compounds.
KEYWORDS: alphavirus, chikungunya, STAT signaling, innate immunity, interferons, nonstructural protein 2
ABSTRACT
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus that has evolved effective mechanisms to counteract the type I interferon (IFN) response. Upon recognition of the virus, cells secrete IFNs, which signal through transmembrane receptors (IFNAR) to phosphorylate STAT proteins (pSTAT). pSTAT dimers are transported into the nucleus by importin-α5 and activate the transcription of IFN-stimulated genes (ISGs), increasing cellular resistance to infection. Subsequently, STAT proteins are shuttled back into the cytoplasm by the exportin CRM1. CHIKV nonstructural protein 2 (nsP2) reduces ISG expression by inhibiting general host cell transcription and by specifically reducing the levels of nuclear pSTAT1 via an unknown mechanism. To systematically examine where nsP2 acts within the JAK/STAT signaling cascade, we used two well-characterized mutants of nsP2, P718S and KR649AA. Both mutations abrogate nsP2's ability to shut off host transcription, but only the KR649AA mutant localizes exclusively to the cytoplasm and no longer specifically inhibits JAK/STAT signaling. These mutant nsP2 proteins did not differentially affect IFNAR expression levels or STAT1 phosphorylation in response to IFNs. Coimmunoprecipitation experiments showed that in the presence of nsP2, STAT1 still effectively bound importin-α5. Chemically blocking CRM1-mediated nuclear export in the presence of nsP2 additionally showed that nuclear translocation of STAT1 is not affected by nsP2. nsP2 putatively has five domains. Redirecting the nsP2 KR649AA mutant or just nsP2's C-terminal methyltransferase-like domain into the nucleus strongly reduced nuclear pSTAT in response to IFN stimulation. This demonstrates that the C-terminal domain of nuclear nsP2 specifically inhibits the IFN response by promoting the nuclear export of STAT1.
IMPORTANCE Chikungunya virus is an emerging pathogen associated with large outbreaks on the African, Asian, European, and both American continents. In most patients, infection results in high fever, rash, and incapacitating (chronic) arthralgia. CHIKV effectively inhibits the first line of defense, the innate immune response. As a result, stimulation of the innate immune response with interferons (IFNs) is ineffective as a treatment for CHIKV disease. The IFN response requires an intact downstream signaling cascade called the JAK/STAT signaling pathway, which is effectively inhibited by CHIKV nonstructural protein 2 (nsP2) via an unknown mechanism. The research described here specifies where in the JAK/STAT signaling cascade the IFN response is inhibited and which protein domain of nsP2 is responsible for IFN inhibition. The results illuminate new aspects of antiviral defense and CHIKV counterdefense strategies and will direct the search for novel antiviral compounds.
INTRODUCTION
Chikungunya virus (CHIKV) (family Togaviridae; genus Alphavirus) is an emerging pathogen that is transmitted between humans by Aedes species mosquitoes (1). CHIKV is endemic in parts of Africa and southern Asia, where it frequently causes large outbreaks (2, 3). Between 2005 and 2006 a severe outbreak of CHIKV was reported in the island of Reunion and surrounding islands in the Indian Ocean, with over 260,000 estimated human cases, effectively infecting one-third of the island's population (4). In 2007, the first outbreak of CHIKV in Europe was facilitated by the invasive Aedes albopictus mosquito vector, infecting over 200 people in Italy (5). This has since been followed by multiple incidents of CHIKV transmission in France (6, 7). In 2013, CHIKV was introduced into Brazil, from where it rapidly spread across the western hemisphere, infecting over 1.7 million individuals in an ongoing epidemic. At present, CHIKV cocirculates in the Americas with dengue, Zika, and yellow fever viruses (8). CHIKV causes acute febrile illness accompanied by rash and incapacitating joint pain. The infection is generally cleared by a functional innate immune response. However, a substantial percentage of patients experience long-lasting arthralgia, even though the virus can no longer be detected (9, 10).
CHIKV has a single-stranded positive-sense RNA genome of approximately 11 kb. The genome contains two open reading frames (ORFs) that encode a nonstructural and a structural polyprotein. The nonstructural polyprotein is directly translated from the RNA genome and is sequentially cleaved by viral and host factors into nonstructural proteins 1 to 4 (nsP1 to -4) (11). The nsP1 to -3 precursor, together with the RNA-dependent RNA polymerase nsP4, form the replication complex that produces the viral complementary negative-sense RNA. The protease within nsP2 further processes the nsP1 to -3 precursor into individual nsPs, which, together with nsP4, are necessary to produce positive-sense genomic RNA and subgenomic RNA, from which the structural polyprotein is translated (12). All four nsPs are essential for CHIKV replication, but they have additionally evolved a variety of mechanisms to specifically inhibit cellular stress and immune responses, including the shutdown of general host cell gene expression, to further enable viral replication (13–17).
In humans and other vertebrates, the interferon (IFN) response has evolved as the primary innate immune response to viral infection (18). During alphavirus infection, intracellular viral RNA is detected by cytoplasmic RIG-I-like receptors, resulting in the expression of type I IFNs (IFN-α/β) (19–21). Secreted type I IFNs then bind the transmembrane IFN-α/β receptors (IFNAR) on the plasma membranes in an autocrine and paracrine manner. Tyrosine and Janus kinases (Tyk1/2 and JAK1/2) associated with the cytoplasmic tail of IFNAR are activated by phosphorylation and in turn phosphorylate signal transducer and activator of transcription 1 (STAT1) and -2. pSTAT1/2 heterodimers then translocate to the nucleus, together with IFN response factor 9 (IRF9), and bind the IFN-stimulated response element (ISRE), activating the transcription of many antiviral IFN-stimulated genes (ISGs) (22, 23). The heterodimer pSTAT1/2 presents a nuclear localization signal (NLS) that associates with importin-α5 and facilitates active transport across the nuclear pore (24–26). After release from their target promoter elements, STAT proteins are shuttled back into the cytoplasm by the exportin chromosome region maintenance 1 (CRM1) (27). Robust IFNAR-dependent signaling is crucial to limit infection, and treatment with IFNs prior to infection strongly restricts CHIKV propagation and can abolish CHIKV disease in experimental animal models (28–31). In contrast, when CHIKV infection has been established, IFN treatment no longer reduces viral titers or disease in mice, and infection in vertebrate cells becomes resistant to IFNs, effectively inhibiting the expression of ISGs (30, 31).
nsP2 of CHIKV and related alphaviruses is present in both the cytoplasm and the nucleus in infected cells and causes host cell transcriptional shutoff, which also reduces IFN and ISG expression (17, 32, 33). In addition, nsP2 of CHIKV and the related alphaviruses Sindbis virus and Venezuelan equine encephalitis virus (VEEV) disrupts the JAK/STAT signaling pathway, independently of general host cell shutoff (31, 34). A proline-to-serine mutation in CHIKV nsP2 (P718S) renders the protein unable to cause host cell shutoff, while the nuclear translocation of nsP2 is unaffected and JAK/STAT signaling is still effectively inhibited (17, 33). Mutation of the NLS within nsP2 retains the protein in the cytoplasm and hence reduces host cell shutoff (33, 35, 36). Importantly, while a P718S mutant of nsP2 (nsP2P718S) still localizes to the nucleus and inhibits JAK/STAT signaling, mutation of the NLS in a CHIKV KR649AA nsP2 mutant (nsP2KR649AA) results in a strictly cytoplasmic protein that no longer inhibits JAK/STAT signaling (33). This clearly disentangles host transcriptional shutoff from the specific inhibition of JAK/STAT signaling by nsP2. The P718S and KR649AA mutants provide a powerful tool to further investigate the alphavirus nsP2-mediated inhibition of the JAK/STAT signaling pathway. Here, we investigate where in the pathway CHIKV nsP2 acts by systematically examining key steps in the JAK/STAT signaling cascade in the presence of full-length nsP2, the wild type (WT), and the P718S and KR649AA mutants. Further, by expressing truncated nsP2 proteins, either with or without an NLS, we also elucidate the domains and intracellular localization of nsP2 required for the specific inhibition of JAK/STAT signaling.
RESULTS
Expression levels of IFNAR and STAT1.
The IFN response relies on signaling through IFNAR transmembrane receptors and subsequent STAT1/2 phosphorylation to convey the signal across the plasma membrane and finally into the nucleus. In a previous study, we showed by qualitative immunostaining that during CHIKV infection STAT1 could still be activated by phosphorylation but that the subsequent pSTAT1 nuclear translocation was effectively inhibited by nsP2 (31). This inhibition involved a mechanism independent of host shutoff, since one of the host shutoff-deficient mutants, nsP2P718S, could still inhibit JAK/STAT signaling, whereas the mutant nsP2KR649AA could not (33). Together, these results suggest that nsP2 inhibits the JAK/STAT pathway downstream of IFNAR activation and STAT1 phosphorylation. To experimentally confirm that IFNAR receptors are still present at the cell surface and that pSTAT1 is still phosphorylated in cells expressing nsP2, HEK293 cells were transfected with plasmids encoding CHIKV nsP2 (causing both host cell transcriptional shutoff and JAK/STAT inhibition), nsP2P718S (causing only JAK/STAT inhibition), or nsP2KR649AA (causing neither). In two independent experiments, the nsP2 variants were expressed either as previously described enhanced green fluorescent protein (EGFP) fusion proteins (33) or individually with a separate red-fluorescent mCherry marker (31). Twenty-four hours posttransfection (hpt), the expression levels of IFNAR and pSTAT1 were measured using flow cytometry (Fig. 1).
FIG 1.
CHIKV nsP2-mediated specific inhibition of JAK/STAT signaling is not caused by a reduction in IFNAR and pSTAT1 protein levels. HEK293 cells expressing mCherry-2A-nsP2 or mutants were examined by flow cytometry for surface IFNAR1 expression (A) or pSTAT1 (B) following 15-min treatment with 1,000 U/ml IFN-β. The experiments were repeated using nsP2EGFP fusion proteins with identical results (not shown). (C) Quantification of IFNAR1 or pSTAT1 in cells expressing wild-type nsP2, nsP2KR649AA, or nsP2P718S-mCherry. The bars represent the percentages of IFNAR- or pSTAT-positive cells (above the horizontal gate) from either the nsP2-negative population of cells (left of the vertical gate; nsp2−) or the nsP2-positive population (right of the vertical gate; nsp2+). Shown are the mean values of two independent experiments normalized to mock-transfected cells; the error bars represent single standard deviations.
Cells transfected with wild-type nsP2 displayed mildly reduced cell surface IFNAR levels compared to cells expressing the nsP2P718S and nsP2KR649AA mutants that do not cause general host shutoff (Fig. 1A and C). More importantly, no difference in IFNAR expression between nsP2KR649AA and nsP2P718S was observed, indicating no specific inhibition of IFNAR protein expression by nsP2 in the absence of host shutoff. Stimulation of transfected cells with IFN effectively phosphorylated STAT1 (Fig. 1B and C). Similar to the expression of IFNAR, the expression levels of wild-type nsP2 correlated with a reduction in pSTAT1. Large populations of cells that expressed either one of the shutoff-defective nsP2 mutants (nsP2P718S or nsP2KR649AA) allowed effective STAT1 phosphorylation (Fig. 1B and C). General host shutoff caused by wild-type nsP2 may therefore indirectly reduce STAT1 phosphorylation, but these results provide direct evidence that the specific inhibition of JAK/STAT signaling by nsP2 and nsP2P718S takes place downstream of the phosphorylation of STAT proteins in response to IFNs.
nsP2 does not disrupt the nuclear import complex of pSTAT1.
After stimulation with type I IFNs and phosphorylation of STAT1 at the cytoplasmic tail of the IFNAR receptor, pSTAT1 forms a complex with tyrosine-phosphorylated STAT2 and IRF9 (37). Nuclear translocation of these large protein complexes requires active transport across the nuclear pore. Mammalian cells express multiple importin proteins that bind diverse NLSs. After dimerization, STAT1/2 proteins present a nonclassical NLS that is recognized by the cellular protein importin-α5 to mediate nuclear translocation (38). Viral proteins that bind importin-α5, such as Ebola virus VP24, inhibit the IFN response by competitively reducing the nuclear translocation of STATs (39, 40). Interestingly, the NLS in most alphavirus nsP2 proteins resembles the importin-α5-dependent classical monopartite simian virus 40 (SV40)-like NLS, and VEEV nsP2 was shown to specifically bind importin-α5 (41). The mutation KR649AA in nsP2, which renders the protein incapable of inhibiting JAK/STAT signaling, actually alters two of the core residues in the CHIKV nsP2 NLS and results in a cytoplasmic nsP2 variant (33). To investigate whether CHIKV nsP2 can inhibit the interaction between STAT1/2 dimers and importin-α5, wild-type nsP2 and both mutants were transiently expressed in HEK293T cells together with FLAG-tagged importin-α5 (Fig. 2). As controls, either no viral proteins or Ebola virus VP24 was coexpressed with FLAG-tagged importin-α5.
FIG 2.
Interaction of STAT1 with the importin complex is unaffected in the presence of CHIKV nsP2. HEK293 cells were mock transfected or transfected with a plasmid expressing a FLAG-tagged human importin-α5 (impα5) in combination with a plasmid expressing mCherry (mock), Ebola virus VP24 (positive control), or one of the mCherry-2A-nsP2 constructs. Whole-cell lysate was loaded on SDS-PAGE and stained for FLAG and CHIKV nsP2. The coimmunoprecipitation (Co-IP) product was stained for FLAG and STAT1. The numbers at the bottom indicate the importin-α5/STAT1 ratios.
In all samples transfected with FLAG-tagged importin-α5, expression levels of FLAG-tagged importin-α5 were similar, except when coexpressed with wild-type CHIKV nsP2, indicative of general host shutoff (Fig. 2). IFN-β treatment of the cell monolayers transfected with FLAG-tagged importin-α5 expression plasmids resulted in highly similar STAT1 phosphorylation. Immunoprecipitation of FLAG-tagged importin-α5 enriched all the samples with FLAG–importin-α5. Immunostaining for STAT1 revealed that only upon IFN-β stimulation were STAT1 proteins coprecipitated with importin-α5. As expected, Ebola virus VP24 strongly inhibited this interaction between FLAG–importin-α5 and STAT1. Coexpression with wild-type nsP2 also resulted in reduced levels of STAT1 being precipitated: this, however, was equal to the reduction in FLAG–importin-α5 expression. In the presence of the host shutoff-defective nsP2P718S mutant, STAT1 was still able to effectively bind importin-α5, identically to NLS mutant nsP2KR649AA. This indicates that the specific inhibition of JAK/STAT signaling caused by wild-type nsP2 and nsP2P718S is not the result of a reduced interaction between STAT1 and importin-α5. The ratios of importin-α5 to STAT1 band intensities indicate that importin-α5 bound STAT1 with similar efficiency in the presence of either nsP2 WT, nsP2P718S, or nsP2KR649AA (0.84 to 0.86), while a lower ratio was found for the VP24-transfected cells (0.34) and the unstimulated cells (0.28). This confirms that nsP2 does not interfere with the ability of importin-α5 to interact with STAT1 and strongly suggests that nsP2 does not affect the nuclear translocation of STAT1.
Nuclear nsP2 inhibits pSTAT1 nuclear accumulation independently of the nsP2 NLS.
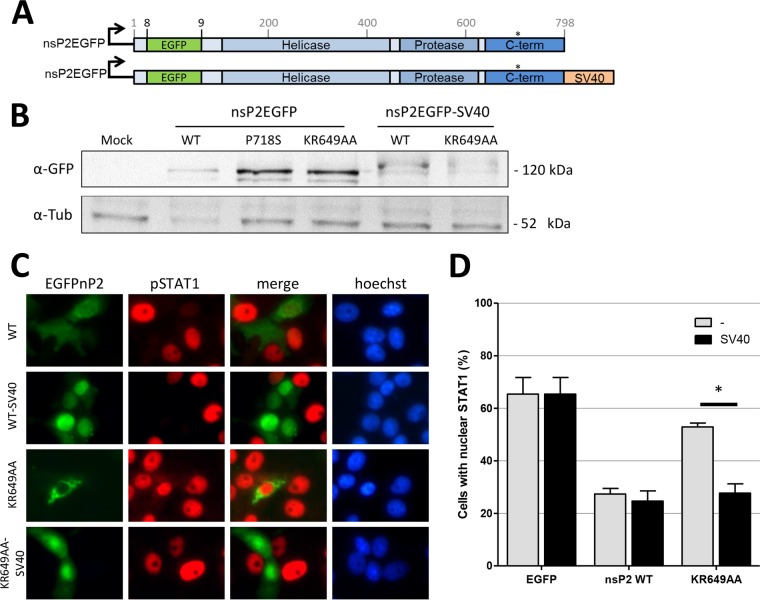
To investigate whether the residues KR649-650 within the NLS of nsP2 are directly involved in JAK/STAT inhibition, in addition to regulating the intracellular localization of nsP2, a second NLS was fused to both wild-type nsP2 and nsP2KR649AA (Fig. 3A). The classical SV40 NLS was fused to the C termini of previously described nsP2EGFP fusion proteins (33), and Vero cells were transfected with the expression plasmids to confirm the intracellular localization of the nsP2 variants. Western blot analysis with anti-green fluorescent protein (α-GFP) antibodies on lysates of transfected cells indicated that both WT and mutant nsP2 proteins were correctly expressed (Fig. 3B). Lower protein expression was observed for nsP2EGFP, nsP2EGFP-SV40, and nsP2EGFPKR649AA-SV40 than for nsP2EGFPP718S and nsP2EGFPKR649AA, suggesting that general host shutoff suppresses protein expression. Despite lower expression levels, nsP2EGFP localizes throughout the cytoplasm and nucleus and effectively inhibits the nuclear accumulation of pSTAT1, whereas nsP2EGFPKR649AA is strictly cytoplasmic and does not abolish the accumulation of nuclear pSTAT1 (Fig. 3C and D). Addition of the SV40 NLS to either wild-type nsP2EGFP or nsP2EGFPKR649AA directs a similarly large percentage of the nsP2 population into the nucleus for both nsP2 variants (Fig. 3C). Importantly, when cells that expressed these nsP2 variants were treated with IFN-β and stained for pSTAT1, it showed that redirecting nsP2KR649AA into the nucleus by addition of the SV40 NLS was sufficient to recover the inhibition of nuclear pSTAT1 accumulation (Fig. 3D). This indicates that only nuclear nsP2 strongly reduces the nuclear accumulation of pSTAT1 independently of the integrity of the amino acid sequence that comprises the original NLS of nsP2.
FIG 3.
Inhibition of IFN signaling by CHIKV nsP2 requires its nuclear localization. (A) Schematic overview of the nsP2EGFP expression plasmids. (B) Vero cells were transfected with nsP2EGFP, nsP2EGFPP718S, nsP2EGFPKR649AA, nsP2EGFP-SV40, or nsP2EGFPKR649AA-SV40. At 24 hpt, the cell monolayers were lysed and assessed by Western blotting using α-GFP and α-tubulin (α-Tub) antibodies. (C and D) Vero cells were transfected with nsP2EGFP, nsP2EGFP-SV40, nsP2EGFPKR649AA, or nsP2EGFPKR649AA-SV40. At 24 hpt, the cells were stimulated with interferon, fixed, and stained for pSTAT1. (C) Immunofluorescence indicating the localization of wild-type nsP2EGFP and nsP2EGFPKR649AA with or without the SV40 NLS and pSTAT1. (D) Cells expressing the various nsP2EGFP constructs were counted based on the presence or absence of nuclear pSTAT1. The bars show the mean percentages of cells with nuclear pSTAT1 and standard errors of the means (SEM). The asterisk indicates a significant difference from the parental protein without SV40 NLS (Student's t test; P < 0.05).
nsP2 promotes CRM1-mediated nuclear export of STAT1.
In the nucleus, STAT dimers bind to the ISRE (STAT1/2) and GAS (STAT1/1) DNA elements. This initiates ISG expression and additionally prevents the nuclear export of STAT dimers mediated by CRM1 (27). A mutation in STAT1 (EE428SA) renders STAT1 unable to bind its DNA elements. This STAT1 mutant is still transported into the nucleus, but its nuclear localization can be visualized only by inhibiting CRM-mediated nuclear export with leptomycin B (LMB) (27). To investigate whether pSTAT1 is still effectively transported across the nuclear pore and into the nucleus in the presence of nsP2, Vero cells were transfected with plasmids that express both mCherry and either CHIKV nsP1 (negative control) or one of the nsP2 variants. Cells were either mock treated or treated with LMB for 3 h prior to 30 min of IFN-β stimulation and staining for nuclear pSTAT1 (Fig. 4). As expected, cells expressing nsP1 or nsP2KR649AA had a high percentage (>85%) of cells displaying nuclear pSTAT1. Correspondingly, expression of wild-type nsP2 or nsP2P718S significantly reduced the nuclear presence of pSTAT1 to 13% and 39% of the cells, respectively (Fig. 4, gray bars). LMB treatment, however, significantly increased nuclear pSTAT1 to 48% in cells expressing wild-type nsP2 and 58% in cells expressing nsP2P718S (Fig. 4, black bars). This is indicative of functional pSTAT1 nuclear translocation upon IFN treatment, but CRM1 mediated nuclear export in the presence of wild-type nsP2 or nsP2P718S. Thus, nsP2 translocates into the nucleus, where it promotes the CRM1-mediated nuclear export of pSTAT1 to inhibit JAK/STAT signaling in infected cells.
FIG 4.

nsP2 inhibits JAK/STAT inhibition by inducing the nuclear export of pSTAT1. Blocking nuclear export indicates the functional nuclear import of STAT1. Vero cells were transfected with either mCherry-2A-nsP1, mCherry-2A-nsP2, or one of its mutants; 16 hpt, the cells were mock treated or treated with the nuclear exportin CRM1 blocker LMB for 3 h prior to 30-min stimulation with IFN-β. The cells were fixed, permeabilized, and stained for pSTAT1. The bars represent the means from 3 independent experiments, and the error bars indicate SEM. The asterisks indicate significant differences as a result of LMB treatment (Student's t test; P < 0.05).
Inhibition of JAK/STAT signaling is independent of nsP2 helicase and putative MTase activity.
Alphavirus nsP2 can be divided into five domains: an N-terminal domain, two superfamily 1 RNA helicase domains, a papain-like protease, and an Ftsj methyltransferase (MTase)-like domain (42–45). To investigate whether the helicase and putative MTase activities of CHIKV nsP2 are required for JAK/STAT inhibition, amino acid substitutions were introduced into the active sites, either known or modeled from homologous enzymes. We first created active-site mutants in the Walker A and Walker B motifs known to abolish helicase activity (43). To assess the importance of helicase activity for JAK/STAT inhibition, nuclear localization of pSTAT1 following IFN treatment was assessed in cells expressing the nsP2 helicase mutants. Mutations in either the Walker A or Walker B active site did not interfere with JAK/STAT inhibition, as indicated by the absence of nuclear pSTAT1 in cells expressing the helicase mutants of nsP2 (Fig. 5A). This indicates that JAK/STAT inhibition is independent of the helicase activity of nsP2.
FIG 5.
nsP2 helicase and putative MTase active sites are not required for JAK/STAT inhibition. (A) Vero cells were transfected with mCherry-2A-nsP2, mCherry-2A-nsP2P718S, mCherry-2A-nsP2KR649AA, mCherry-2A-nsP2Walker A, or mCherry-2A-nsP2Walker B. At 24 hpt, the cells were stimulated with IFN-β, fixed, and stained for pSTAT1. Shown are the percentages (means and SEM from three independent experiments) of nsP2-expressing cells with nuclear pSTAT1 localization, normalized to the mock (pDEST40-mCherry)-transfected cells. The asterisks indicate significant differences from the mock-transfected control (one-way ANOVA; Dunnett's multiple-comparison test; P < 0.05). (B) 3D protein structure of the nsP2 C-terminal domain. Red and green indicate the mutated amino acids in the predicted SAM binding pocket of the putative MTase domain. Blue indicates the remaining SAM binding pocket. (C) Vero cells were transfected with nsP2EGFP, nsP2EGFPKR649AA, nsP2EGFPNL667AA, or nsP2EGFPR725A. At 24 hpt, the cells were stimulated with IFN-β, fixed, and stained for pSTAT1. Shown are the percentages (means and SEM from three independent experiments) of nsP2-expressing cells with nuclear pSTAT1 localization. The asterisks indicate significant differences from the EGFP control (one-way ANOVA; Dunnett's multiple-comparison test; P < 0.05).
The C-terminal MTase domain has not been shown to have any MTase activity, but it does have a clear S-adenosylmethionine (SAM) binding pocket. We compared the predicted 3-dimensional (3D) structure of the C-terminal MTase domain of nsP2 with that of active MTase from dengue virus NS5, combined with binding-pocket prediction for SAM with 3DLigandsite (46), to identify residues potentially involved in MTase activity. Crucial residues required for MTases to interact with SAM were mutated to alanines (NL667AA and R725A) (Fig. 5B), and these nsP2 mutants were subsequently assessed for JAK/STAT inhibition (Fig. 5C). Both the nsP2NL667AA and nsP2R725A mutants still inhibited JAK/STAT signaling, suggesting that the putative SAM binding pocket is not required for JAK/STAT inhibition.
The C-terminal MTase-like domain is required and sufficient to inhibit JAK/STAT signaling.
To further characterize which domain(s) of CHIKV nsP2 is essential to inhibit nuclear accumulation of pSTAT1, the nsP2EGFP fusion protein was truncated by removal of the C-terminal MTase-like domain to produce nsP2ΔMTase. Conversely, the N-terminal, helicase, and protease domains were removed, and the MTase domain of nsP2 was fused to the C terminus of EGFP, resulting in nsP2MTase (Fig. 6A). Western blotting with α-GFP antibodies on lysates of transfected cells indicated that both WT and mutant nsP2 proteins were correctly expressed (Fig. 6B). Importantly, the expression levels of nsP2ΔMTase and nsP2MTase were similar, indicating that differences in JAK/STAT inhibition are not caused by differences in protein expression. Removal of the C-terminal MTase-like domain also removes the NLS that resides within the C-terminal domain of nsP2. Indeed, nsP2ΔMTase localized exclusively to the cytoplasm of transfected cells (Fig. 6D) and, not surprisingly, did not block JAK/STAT signaling (Fig. 6C). Similar to the experiments presented in Fig. 3, an SV40 NLS was also fused to the truncated nsP2 proteins to redirect the truncated protein into the nucleus. Importantly, addition of the SV40 NLS did not reinstate the inhibition of JAK/STAT signaling for nsP2ΔMTase. This indicates that, even though nsP2ΔMTase-SV40 is able to translocate to the nucleus, the absence of the C-terminal MTase domain abrogates its ability to inhibit nuclear pSTAT1 accumulation; effectively rendering the protein unable to inhibit JAK/STAT signaling. When cells were transfected with plasmids expressing only the nsP2 MTase (Fig. 6A), with or without an additional SV40 NLS, the EGFP fusion protein nsP2MTase readily localized to the nucleus of the cell and effectively inhibited JAK/STAT signaling by significantly reducing levels of nuclear pSTAT1 (Fig. 6C). This indicates that the nuclear localization of the C-terminal MTase-like domain of CHIKV nsP2 is sufficient to antagonize JAK/STAT signaling, independently of any putative MTase activity.
FIG 6.
The nsP2 C-terminal MTase-like domain is essential for JAK/STAT inhibition. (A) Schematic overview of the nsP2EGFP mutant constructs used. (B) Vero cells were transfected with nsP2EGFP, nsP2EGFPΔMTase, or nsP2EGFPMTase with or without an SV40 NLS. After 24 hpt, cell monolayers were lysed and assessed by Western blotting using α-GFP and α-tubulin antibodies. (C and D) Vero cells were transfected with nsP2EGFP, nsP2EGFPΔMTase, or nsP2EGFPMTase with or without an SV40 NLS. At 24 hpt, the cells were stimulated with IFN-β, fixed, and stained for pSTAT1. (C) Percentages of JAK/STAT inhibition. Shown are the percentages of nsP2-expressing cells with nuclear pSTAT1 localization, normalized to the mock (pEGFP-N1)-transfected cells. The bars represent the means and SEM from five independent experiments. The asterisks indicate significant differences from the EGFP control (one-way ANOVA; Dunnett's multiple-comparison test; P < 0.05). (D) Localization of nsP2 mutants (green) and pSTAT1 (red).
To investigate whether the nuclear nsP2KR649AA-SV40 fusion protein or the C-terminal MTase expressing truncations also inhibits JAK/STAT signaling by promoting the CRM1-mediated nuclear export of STAT1, cells were transfected with the indicated expression plasmids and treated with the CRM1 inhibitor LMB. Upon stimulation with IFN-β, samples not treated with LMB showed strongly reduced nuclear pSTAT in cells expressing nsP2EGFP WT, nsP2KR649AA-SV40, and nsP2MTase (with or without the SV40 NLS), similar to the results shown in Fig. 3D and 6C. LMB treatment significantly increased nuclear pSTAT in these samples (Fig. 7A). This indicates that CRM1-mediated export of STAT1 occurs more effectively when the MTase-like C-terminal domain of CHIKV nsP2 is present inside the nucleus of the cell.
FIG 7.
The nsP2 C-terminal MTase-like domain promotes CRM1-mediated export of STAT1. (A) Vero E6 cells expressing the indicated nsP2 variants were treated with LMB for 3 h or left untreated prior to 30 min of IFN-β stimulation. The cells were fixed and stained for pSTAT1. Nuclear pSTAT1 was analyzed for cells that expressed nsP2, and the LMB-treated samples were normalized to their untreated controls. The results are depicted as fold change (LMB treated over untreated). The bars represent means ± SEM from three independent experiments. The asterisks indicate significant increases in nuclear pSTAT1 induced by LMB treatment (Student's t test; P < 0.05). (B) In the presence of CHIKV nsP2, STAT1/2 dimers interact effectively with importin-α5 and are transported across the nuclear pore. In the nucleus, the C-terminal domain of nsP2 promotes CRM1-mediated nuclear export of STAT1 (indicated by arrows and plus sign), inhibiting the JAK/STAT signaling pathway and preventing induction of ISG expression (indicated by the dashed arrow and minus sign).
DISCUSSION
The IFN response is an integral component of vertebrate defense against viral infections and plays a major role in limiting CHIKV infections in vivo (28–31). nsP2 of CHIKV and closely related alphaviruses causes the degradation of the RNA polymerase II subunit rpb1 (17), inhibiting host cell transcription and, as a consequence, reducing ISG expression. Additionally, CHIKV nsP2 directly inhibits JAK/STAT signaling, markedly reducing the nuclear accumulation of STAT1 in response to endogenous IFNs (31). Shutoff-defective CHIKV nsP2 mutants (e.g., KR649AA and P718S) and homologous mutants in the related alphaviruses Sindbis virus and Semliki Forest virus additionally reduce the cytopathic effects (CPE) associated with nsP2 expression (33, 35, 36). The expression of the IFN receptor IFNAR1 and presence of phosphorylated STAT1 negatively correlated with the expression levels of wild-type nsP2 (Fig. 1), which is likely the result of transcriptional shutoff and/or the induction of CPE. Both nsP2P718S and nsP2KR649AA did not alter the expression levels of IFNAR and only mildly reduced the levels of pSTAT1. Importantly, there was no difference in IFNAR and pSTAT1 expression levels post-IFN induction between cells that expressed either one of the shutoff-defective nsP2P718S or nsP2KR649AA mutants. This indicates that the specific inhibition of JAK/STAT signaling is not the result of nsP2-mediated differential expression levels and/or the phosphorylation status of IFNAR and STAT1.
Following stimulation with IFNs, both STAT1 and STAT2 are tyrosine phosphorylated by kinases associated with the cytoplasmic domain of the IFNAR subunits promoting dimerization of STAT1/2 (IFN-α/β) or STAT1/1 (IFN-γ). The phosphorylated STAT dimers then display a conditional NLS that binds importin-α5 (24–26). Inhibiting the interaction between importin-α5 and STAT1 through mutagenesis of STAT1 on residue L407 or inhibitory antibodies targeting importin-α5 indeed reduced the nuclear import of STAT1 (25, 26). The ebolavirus protein EV24 has evolved to inhibit this interaction between members of the importin subfamily, NPI-1 (which includes importin-α5) and STAT1 (Fig. 2) (39, 40, 47), resulting in ebolavirus-mediated inhibition of IFN signaling (48). Henipavirus (family Paramyxoviridae) N protein similarly inhibits the nuclear accumulation of STAT1/2 by inhibiting STAT import complex formation in the cytoplasm, though the exact interaction remains to be elucidated (49). In contrast, CHIKV nsP2 only reduced the transient expression of FLAG-tagged importin-α5 and consequently the absolute amount of coimmunoprecipitated STAT1. Both shutoff-defective nsP2 mutants with FLAG-tagged importin-α5 highly enriched coimmunoprecipitated STAT1 upon IFN stimulation, similar to the mock-transfected sample (Fig. 2). The ratios of coimmunoprecipitated STAT1 to purified FLAG-tagged importin-α5 were identical between the samples that expressed wild-type nsP2 and the two shutoff-defective mutants at 0.84 and 0.86 of the mock-transfected IFN-stimulated sample. This indicates that the nsP2-mediated inhibition of JAK/STAT signaling cannot be attributed to interference between importin-α5 and phosphorylated STAT1.
Once in the nucleus, STAT dimers strongly bind specific DNA elements, which likely results in the dissociation of STATs from the importin-α/β complex (24). Binding to DNA masks the nuclear export signal (NES) of STAT, increasing the longevity of STATs in the nucleus. STAT dimers can be released from their DNA binding elements through equilibrium, dephosphorylation by PTPN2, or association with protein inhibitors of activated STAT (PIAS) (50–53). After releasing the DNA, the NES of STAT molecules is exposed and bound by CRM1, which results in the export of STAT from the nucleus (27, 54). Here, we show that blocking CRM1-mediated nuclear export with LMB increases the nuclear fraction of pSTAT1 in the presence of the C-terminal MTase-like domain of nsP2 (Fig. 4 and 7). This suggests that nsP2 induces the nuclear export of STAT1 through other means than the direct dephosphorylation of STAT1.
Mutation of the NLS within nsP2 abolishes both the nuclear translocation of nsP2 and inhibition of the JAK/STAT signaling pathway, which occurred only after the nuclear localization of nsP2 was reinstated by fusing a new SV40 NLS to the nsP2 C terminus. This indicates that nsP2 inhibits the JAK/STAT signaling response in the nucleus, independently of the native NLS. In the nucleus, nsP2 might compete with STAT dimers for binding to the ISRE and GAS DNA elements or recruit PIAS proteins in order to increase its nuclear export (Fig. 7B). Competition for DNA binding most likely depends on nsP2's helicase activity; however, site-directed mutagenesis, which is known to inactivate the active sites of both nsP2 helicase motifs, had no effect on the blockade in JAK/STAT signaling, and removal of the C-terminal domain completely abolished the JAK/STAT inhibition, indicating that the antagonizing function depends on the MTase-like domain. There is functional cross talk between helicase and the MTase domain of nsP2. Truncations and mutations within the C terminus of nsP2 can interfere with its NTPase and helicase activities (43). However, expression of just the C-terminal MTase-like domain of nsP2 also inhibited JAK/STAT signaling, strongly suggesting the NTPase and helicase activities of nsP2 are not required for the specific inhibition of JAK/STAT signaling. Multiple cellular RNA helicases are involved in transcriptional regulation and can additionally disrupt protein-protein interactions (55). Possibly, nsP2's C-terminal domain recruits such helicases to compete with STAT1 binding to the ISRE and GAS promoters.
Thus, the C-terminal MTase-like domain of nsP2 is crucial for CHIKV to specifically inhibit JAK/STAT signaling. The functions of this Ftsj-like MTase domain, however, remain enigmatic. The MTase activity required for capping of the genomic RNA is provided by nsP1, while enzymatic activity of the alphavirus nsP2 C-terminal domain has never been reported. The nsP2 C-terminal MTase-like domain also lacks crucial amino acid residues required for catalytic activity (43, 56). While we focused our approach on CHIKV nsP2, related alphaviruses might similarly inhibit JAK/STAT signaling. Future research should unravel whether the nsP2s of other alphaviruses are able to inhibit STAT1 nuclear translocation and if they use a mechanism similar to that of CHIKV.
A recent study showed that methylation of lysine 525 on STAT1 by the cellular methyltransferase SETD2 increases STAT phosphorylation and transcriptional activity (57). It is possible that the MTase-like domain of nsP2 has affinity remaining for such targets that are normally methylated by host cell MTases and inhibits these cellular processes. In a first attempt, we mutated residues in the SAM binding pocket of nsP2 that are conserved among viral MTases. These mutations, however, did not alter the nsP2-mediated inhibition of JAK/STAT signaling. Finally, it is possible that nsP2 positively affects CRM1, leading to increased nuclear export of other host proteins in addition to STAT1. The specific interactions between the alphavirus nsP2 C-terminal MTase domain and host factors that result in the CRM1-mediated nuclear export of STAT1 remain the topic of future investigations.
Here, we have shown that the specific inhibition of JAK/STAT signaling by CHIKV nsP2 occurs in the nucleus of the cell. Importantly, the C-terminal MTase domain of nsP2 is sufficient to induce CRM1-mediated nuclear export of pSTAT1. These results increase our understanding of innate immune evasion by alphaviruses, which is important to fully understand the host-pathogen arms race of these widespread pathogenic viruses.
MATERIALS AND METHODS
Cells and reagents.
African green monkey kidney Vero E6 (ATCC CRL-1586) or HEK293 (ATCC CRL-1593) cells were cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco) supplemented with 5% fetal bovine serum (FBS) (Gibco) at 37°C and 5% CO2. One day prior to transfection, the ells were diluted 1:6 in culture medium and seeded into 96- or 24-well plates. Transfections were performed using Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol.
Expression plasmids.
Plasmids expressing mCherry2A-nsP2 and nsP2-EGFP fusion proteins were previously described (33). A 4× SV40 NLS that introduced SacII/AgeI overhangs and an N-terminal stop codon was PCR amplified from pNG72-SV40 (Table 1 lists the primer sequences). The PCR product was SacII (NEB)/AgeI (NEB) cloned into pcDNA-DEST40 to generate pDEST40-SV40. Next, the nsP2EGFP or nsP2EGFPKR649AA ORF was amplified using AttB-containing primers and Gateway (Invitrogen) cloned into pDEST40-SV40 to generate pDEST40-nsP2EGFP and pDEST40-nsP2EGFPKR649AA. The SV40 NLS was fused to the ORFs by SacII digestion to generate pDEST40-nsP2EGFP-SV40 and pDEST40-nsP2EGFPKR649AA-SV40. pDEST40-nsP2EGFPΔMTase was generated by PCR amplification of the pCMV-nsP2EGFP ORF without the MTase domain using AttB-containing primers, followed by Gateway cloning into pDEST40-SV40. The SV40 NLS was ligated to the ORF after SacII digestion, generating pDEST40-nsP2EGFPΔMTase-SV40. pDEST40-nsP2EGFPMTase and pDEST40-nsP2EGFPMTase-SV40 were generated by PCR amplification of the nsP2 MTase domain from either pDEST40-nsP2EGFP or pDEST40-nsP2EGFP-SV40 and subsequently ScaI (NEB)/NotI (NEB) cloned into pDEST40-nsP2EGFP. pDEST40-nsP2EGFPNL667AA and pDEST40-nsP2EGFPR725A were generated by site-directed mutagenesis PCR with mutagenic primers pDEST40-nsP2EGFP as the template. Point mutations in the Walker A and Walker B active sites of the nsP2 helicase domain (K192A and DE252AA, respectively) were introduced into pCMV-mCherry2A-nsP2 using site-directed mutagenesis with mutagenic primers.
TABLE 1.
Primer sequences used in this study
Flow cytometry.
HEK293 cells transfected with the empty vector or various nsP2 expression vectors were treated with 1,000 U/ml IFN-β (I-4151; Sigma) for 15 min, washed twice in cold Dulbecco's phosphate-buffered saline (DPBS), and trypsinized for 10 min at 37°C to dislodge the cells. The cells were resuspended in freshly prepared 2% paraformaldehyde-DPBS and incubated for 10 min at 37°C, followed by permeabilization in 90% methanol for 10 min on ice. The cells were washed once in stain buffer (BD Pharmingen, San Diego, CA), followed by incubation with anti-pY(701)-STAT1 conjugated to peridinin chlorophyll protein (PerCP)-Cy5.5 (BD Pharmingen) or anti-IFNAR 4G8 monoclonal antibody (MAb) for 45 min at room temperature in the dark. PerCP-Cy5.5-conjugated mouse immunoglobulin G2a (IgG2a) was used as an isotype control. The cells were washed once in stain buffer and analyzed using a FACSCalbur or FACSAria flow cytometer (BD Biosciences) and FlowJo software (Tree Star). After mCherry-positive cells were gated, the percentage of cells containing tyrosine-phosphorylated STAT1 (pSTAT1) was determined as the fraction of nsP2-positive cells that were pSTAT1 positive.
Nuclear translocation of reporter proteins.
pDEST40-mCherry NLS-expressing plasmids were cotransfected with pDEST40-nsP2EGFP into Vero cells. At 24 hpt, the cells were fixed for 10 min with 4% paraformaldehyde in PBS, permeabilized for 10 min with 0.1% SDS in PBS, and stained with Hoechst 33258. The cells were visualized by fluorescence microscopy using an Axio Observer Z1m inverted microscope (Zeiss, Jena, Germany) in combination with an X-Cite 120 series lamp.
FLAG-tagged importin-α5 coimmunoprecipitation.
HEK293 cells were mock transfected or transfected with a plasmid expressing FLAG-tagged human importin-α in combination with either a plasmid expressing mCherry or Ebola VP24 or one of the mCherry2A-nsP2-expressing plasmids. Samples were stimulated with IFN-β (25 ng/ml) as indicated. The cells were washed with ice-cold PBS and lysed in lysis buffer (50 mM Tris-HCl [pH 7.4], 280 mM NaCl, 0.1% NP-40, 0.2 mM EDTA, 2 mM EGTA, 10% glycerol, 1 mM dithiothreitol [DTT], and complete protease inhibitor cocktail [Roche]) for 10 min on ice. The cells were spun down in a tabletop centrifuge at 13,000 rpm for 10 min at 4οC. An aliquot of the supernatant was stored as whole-cell lysate, while the remaining supernatant was added to α-FLAG M2 affinity gel resin (Sigma-Aldrich) and gently agitated for 3 h at 4οC. The resin was washed 3 times by adding precooled Tris-buffered saline and spun down for 30 s at 6,000 rpm in a tabletop centrifuge. Finally, samples were prepared for Western blotting by adding SDS loading buffer. Whole-cell lysate was loaded on SDS-PAGE and stained for FLAG, pSTAT1, and CHIKV nsP2. The coimmunoprecipitate was stained for FLAG and STAT1. Band intensities were calculated using Gelanalyzer 2010a.
Inhibition of CRM1-mediated nuclear export.
Vero cells were transfected with either mCherry2A-nsP1, mCherry2A-nsP2, or one of its mutants; 16 hpt, cells were mock treated or treated with 20 nM LMB (Cell Signaling Technology) for 3 h prior to 30-min stimulation with IFN-β (25 ng/ml). The cells were fixed, permeabilized, and stained for STAT1 and nuclear Hoechst 33342.
Immunofluorescence analysis of pSTAT1 nuclear translocation.
At 24 hpt, cells expressing nsP2 or a mutated/truncated form of nsP2 were stimulated with 25 ng/ml of IFN-β for 30 min at 37°C and 5% CO2. The cells were fixed with 4% paraformaldehyde solution in PBS for 10 min at room temperature (RT) and subsequently permeabilized with 0.1% SDS in PBS for 10 min at RT. The monolayers were stained with primary rabbit anti-pSTAT1 (pTyr701; number 700349; Thermo Scientific) diluted 1:500 in a solution of 5% FBS in PBS for 1 h at 37°C. Subsequently, the cells were stained with secondary goat anti-rabbit Alexa 568 (ab175471; Abcam) diluted 1:2,000 in a solution of 5% FBS in PBS for 1 h at 37°C, and the nuclei were stained with a solution of 10 ng/ml Hoechst 33258 in PBS for 2 min at RT. A fixed exposure time for pSTAT1 was used for all the samples in each replicate experiment. The relative pSTAT1 nuclear translocation was calculated as follows: number of pSTAT1-positive nuclei divided by the total number of cells expressing nsP2.
Western blotting of GFP fusion constructs.
Cell monolayers were lysed in 2× SDS loading buffer containing 10% β-mercaptoethanol and heated to 95°C for 10 min before loading 10 μl onto an SDS-10% PAGE gel. Proteins were size separated by electrophoresis and semidry blotted onto Immobilin-P membranes (Merck Millipore). The membranes were blocked in 5% milk powder-PBS-Tween 20 (0.05%) overnight at 4°C, stained with primary rabbit α-GFP (1:2,000 in 1% milk powder-PBS-Tween 20; A6455 Molecular Probes) or mouse α-tubulin (1:2,000 in 1% milk powder-PBS-Tween; A11126; Molecular Probes), stained with secondary alkaline phosphatase-conjugated antibodies (α-mouse, A5153 [Sigma-Aldrich]; α-rabbit, D0487 [Dako]), and developed with nitroblue tetrazolium (NBT)/BCIP (5-bromo-4-chloro-3-indolylphosphate) (Roche) until the desired signal was achieved.
Statistical analysis.
One-way analysis of variance (ANOVA) with Dunnett's multiple-comparison test and Student t tests were performed using GraphPad Prism 5 (α = 0.05).
In silico models.
3DLigandsite (46) was used to predict the possible interaction between the nsP2 C-terminal domain and S-adenosyl methionine. The chikungunya virus nsP2 “protease” (Protein Data Bank accession no. 3TRK) was used as a template to predict ligand binding sites. The modulations were performed with standard software settings.
ACKNOWLEDGMENTS
We thank Ann C. Palmenberg, University of Wisconsin, Madison, WI, USA, for sharing plasmid pNG72-SV40 and Christopher Basler, Georgia State University, Atlanta, GA, USA, for sharing the plasmids that express FLAG-tagged hKPNAs. We also thank Just Vlak and Corinne Geertsema, Laboratory of Virology, Wageningen UR, for their advice and technical support.
REFERENCES
- 1.Vega-Rua A, Lourenco-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yebakima A, Gustave J, Girod R, Dusfour I, Leparc-Goffart I, Vanlandingham DL, Huang YJ, Lounibos LP, Mohamed Ali S, Nougairede A, de Lamballerie X, Failloux AB. 2015. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis 9:e0003780. doi: 10.1371/journal.pntd.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers AM, Logue CH. 2007. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 3.Townson H, Nathan MB. 2008. Resurgence of chikungunya. Trans R Soc Trop Med Hyg 102:308–309. doi: 10.1016/j.trstmh.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Josseran L, Paquet C, Zehgnoun A, Caillere N, Le Tertre A, Solet JL, Ledrans M. 2006. Chikungunya disease outbreak, Reunion Island. Emerg Infect Dis 12:1994–1995. doi: 10.3201/eid1212.060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A, CHIKV Study Group. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 6.Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, Tolou HJ, Budelot M, Cosserat D, Leparc-Goffart I, Despres P. 2011. Chikungunya virus, southeastern France. Emerg Infect Dis 17:910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L'Ambert G, Cochet A, Prat C, Foulongne V, Ferre JB, Catelinois O, Flusin O, Tchernonog E, Moussion IE, Wiegandt A, Septfons A, Mendy A, Moyano MB, Laporte L, Maurel J, Jourdain F, Reynes J, Paty MC, Golliot F. 2015. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill 20:21108. doi: 10.2807/1560-7917.ES2015.20.17.21108. [DOI] [PubMed] [Google Scholar]
- 8.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. 2014. Chikungunya in the Americas. Lancet 383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 9.Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, de Lamballerie X. 2013. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antivir Res 99:345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suhrbier A, Jaffar-Bandjee MC, Gasque P. 2012. Arthritogenic alphaviruses—an overview. Nat Rev Rheumatol 8:420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 11.Strauss JH, Strauss EG. 1994. The alphaviruses: gene-expression, replication, and evolution. Microbiol Rev 58:491–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jose J, Snyder JE, Kuhn RJ. 2009. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol 4:837–856. doi: 10.2217/fmb.09.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fros JJ, Pijlman GP. 2016. Alphavirus infection: host cell shut-off and inhibition of antiviral responses. Viruses 8:E166. doi: 10.3390/v8060166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fros JJ, Geertsema C, Zouache K, Baggen J, Domeradzka N, van Leeuwen DM, Flipse J, Vlak JM, Failloux AB, Pijlman GP. 2015. Mosquito Rasputin interacts with chikungunya virus nsP3 and determines the infection rate in Aedes albopictus. Parasites Vectors 8:464. doi: 10.1186/s13071-015-1070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fros JJ, Major LD, Scholte FE, Gardner J, van Hemert MJ, Suhrbier A, Pijlman GP. 2015. Chikungunya virus non-structural protein 2-mediated host shut-off disables the unfolded protein response. J Gen Virol 96:580–589. doi: 10.1099/vir.0.071845-0. [DOI] [PubMed] [Google Scholar]
- 16.Fros JJ, Domeradzka NE, Baggen J, Geertsema C, Flipse J, Vlak JM, Pijlman GP. 2012. Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci. J Virol 86:10873–10879. doi: 10.1128/JVI.01506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhrymuk I, Kulemzin SV, Frolova EI. 2012. Evasion of the innate immune response: the Old World alphavirus nsP2 protein induces rapid degradation of Rpb1, a catalytic subunit of RNA polymerase II. J Virol 86:7180–7191. doi: 10.1128/JVI.00541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.tenOever BR. 2016. The evolution of antiviral defense systems. Cell Host Microbe 19:142–149. doi: 10.1016/j.chom.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez David RY, Combredet C, Sismeiro O, Dillies MA, Jagla B, Coppee JY, Mura M, Guerbois Galla M, Despres P, Tangy F, Komarova AV. 2016. Comparative analysis of viral RNA signatures on different RIG-I-like receptors. Elife 5:e11275. doi: 10.7554/eLife.11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhrymuk I, Frolov I, Frolova EI. 2016. Both RIG-I and MDA5 detect alphavirus replication in concentration-dependent mode. Virology 487:230–241. doi: 10.1016/j.virol.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke CW, Gardner CL, Steffan JJ, Ryman KD, Klimstra WB. 2009. Characteristics of alpha/beta interferon induction after infection of murine fibroblasts with wild-type and mutant alphaviruses. Virology 395:121–132. doi: 10.1016/j.virol.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy DE, Marie IJ, Durbin JE. 2011. Induction and function of type I and III interferon in response to viral infection. Curr Opin Virol 1:476–486. doi: 10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider WM, Chevillotte MD, Rice CM. 2014. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagerlund R, Melen K, Kinnunen L, Julkunen I. 2002. Arginine/lysine-rich nuclear localization signals mediate interactions between dimeric STATs and importin alpha 5. J Biol Chem 277:30072–30078. doi: 10.1074/jbc.M202943200. [DOI] [PubMed] [Google Scholar]
- 25.McBride KM, Banninger G, McDonald C, Reich NC. 2002. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-alpha. EMBO J 21:1754–1763. doi: 10.1093/emboj/21.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. 1997. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J 16:7067–7077. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride KM, McDonald C, Reich NC. 2000. Nuclear export signal located within the DNA-binding domain of the STAT1transcription factor. EMBO J 19:6196–6206. doi: 10.1093/emboj/19.22.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog 4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schilte C, Couderc T, Chretien F, Sourisseau M, Gangneux N, Guivel-Benhassine F, Kraxner A, Tschopp J, Higgs S, Michault A, Arenzana-Seisdedos F, Colonna M, Peduto L, Schwartz O, Lecuit M, Albert ML. 2010. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med 207:429–442. doi: 10.1084/jem.20090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. 2010. Chikungunya virus arthritis in adult wild-type mice. J Virol 84:8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fros JJ, Liu WJ, Prow NA, Geertsema C, Ligtenberg M, Vanlandingham DL, Schnettler E, Vlak JM, Suhrbier A, Khromykh AA, Pijlman GP. 2010. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol 84:10877–10887. doi: 10.1128/JVI.00949-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peranen J, Rikkonen M, Liljestrom P, Kaariainen L. 1990. Nuclear localization of Semliki Forest virus-specific nonstructural protein nsP2. J Virol 64:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fros JJ, van der Maten E, Vlak JM, Pijlman GP. 2013. The C-terminal domain of chikungunya virus nsP2 independently governs viral RNA replication, cytopathicity, and inhibition of interferon signaling. J Virol 87:10394–10400. doi: 10.1128/JVI.00884-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons JD, White LJ, Morrison TE, Montgomery SA, Whitmore AC, Johnston RE, Heise MT. 2009. Venezuelan equine encephalitis virus disrupts STAT1 signaling by distinct mechanisms independent of host shutoff. J Virol 83:10571–10581. doi: 10.1128/JVI.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frolova EI, Fayzulin RZ, Cook SH, Griffin DE, Rice CM, Frolov I. 2002. Roles of nonstructural protein nsP2 and alpha/beta interferons in determining the outcome of Sindbis virus infection. J Virol 76:11254–11264. doi: 10.1128/JVI.76.22.11254-11264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamm K, Merits A, Sarand I. 2008. Mutations in the nuclear localization signal of nsP2 influencing RNA synthesis, protein expression and cytotoxicity of Semliki Forest virus. J Gen Virol 89:676–686. doi: 10.1099/vir.0.83320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melen K, Fagerlund R, Franke J, Kohler M, Kinnunen L, Julkunen I. 2003. Importin alpha nuclear localization signal binding sites for STAT1, STAT2, and influenza A virus nucleoprotein. J Biol Chem 278:28193–28200. doi: 10.1074/jbc.M303571200. [DOI] [PubMed] [Google Scholar]
- 39.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. 2006. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol 80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, Pappu RV, Leung DW, Basler CF, Amarasinghe GK. 2014. Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microbe 16:187–200. doi: 10.1016/j.chom.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery SA, Johnston RE. 2007. Nuclear import and export of Venezuelan equine encephalitis virus nonstructural protein 2. J Virol 81:10268–10279. doi: 10.1128/JVI.00371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. 1989. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res 17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das PK, Merits A, Lulla A. 2014. Functional cross-talk between distant domains of chikungunya virus non-structural protein 2 is decisive for its RNA-modulating activity. J Biol Chem 289:5635–5653. doi: 10.1074/jbc.M113.503433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karpe YA, Aher PP, Lole KS. 2011. NTPase and 5′-RNA triphosphatase activities of Chikungunya virus nsP2 protein. PLoS One 6:e22336. doi: 10.1371/journal.pone.0022336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez de Cedron M, Ehsani N, Mikkola ML, Garcia JA, Kaariainen L. 1999. RNA helicase activity of Semliki Forest virus replicase protein NSP2. FEBS Lett 448:19–22. doi: 10.1016/S0014-5793(99)00321-X. [DOI] [PubMed] [Google Scholar]
- 46.Wass MN, Kelley LA, Sternberg MJ. 2010. 3DLigandSite: predicting ligand-binding sites using similar structures. Nucleic Acids Res 38:W469–W473. doi: 10.1093/nar/gkq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. 2007. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J Virol 81:13469–13477. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mateo M, Reid SP, Leung LW, Basler CF, Volchkov VE. 2010. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J Virol 84:1169–1175. doi: 10.1128/JVI.01372-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugai A, Sato H, Takayama I, Yoneda M, Kai C. 2017. Nipah and Hendra virus nucleoproteins inhibit nuclear accumulation of STAT1 and STAT2 by interfering with their complex formation. J Virol 91:e01136-. doi: 10.1128/JVI.01136-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reich NC. 2013. STATs get their move on. Jak-Stat 2:e27080. doi: 10.4161/jkst.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reich NC, Liu L. 2006. Tracking STAT nuclear traffic. Nat Rev Immunol 6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 52.ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, Zhu W, Tremblay M, David M, Shuai K. 2002. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol 22:5662–5668. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haspel RL, Salditt-Georgieff M, Darnell JE Jr. 1996. The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO J 15:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- 54.Begitt A, Meyer T, van Rossum M, Vinkemeier U. 2000. Nucleocytoplasmic translocation of Stat1 is regulated by a leucine-rich export signal in the coiled-coil domain. Proc Natl Acad Sci U S A 97:10418–10423. doi: 10.1073/pnas.190318397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jankowsky E, Fairman ME. 2007. RNA helicases: one fold for many functions. Curr Opin Struct Biol 17:316–324. doi: 10.1016/j.sbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Mayuri Geders TW, Smith JL, Kuhn RJ. 2008. Role for conserved residues of Sindbis virus nonstructural protein 2 methyltransferase-like domain in regulation of minus-strand synthesis and development of cytopathic infection. J Virol 82:7284–7297. doi: 10.1128/JVI.00224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen K, Liu J, Liu S, Xia M, Zhang X, Han D, Jiang Y, Wang C, Cao X. 2017. Methyltransferase SETD2-mediated methylation of STAT1 is critical for interferon antiviral activity. Cell 170:492–506 e414. doi: 10.1016/j.cell.2017.06.042. [DOI] [PubMed] [Google Scholar]