Our data reveal an integrin-independent route of KSHV infection and suggest that multiple Eph receptors besides EphA2 can promote and regulate infection. Since integrins and Eph receptors are large protein families with diverse expression patterns across cells and tissues, we propose that KSHV may engage with several proteins from both families in different combinations to negotiate successful entry into diverse cell types.
KEYWORDS: Eph receptors, Kaposi's sarcoma-associated herpesvirus, heparan sulfate, integrins, virus entry, virus receptors, virus-host interactions
ABSTRACT
Host receptor usage by Kaposi's sarcoma-associated herpesvirus (KSHV) has been best studied using primary microvascular endothelial and fibroblast cells, although the virus infects a wide variety of cell types in culture and in natural infections. In these two infection models, KSHV adheres to the cell though heparan sulfate (HS) binding and then interacts with a complex of EphA2, xCT, and integrins α3β1, αVβ3, and αVβ5 to catalyze viral entry. We dissected this receptor complex at the genetic level with CRISPR-Cas9 to precisely determine receptor usage in two epithelial cell lines. Surprisingly, we discovered an infection mechanism that requires HS and EphA2 but is independent of αV- and β1-family integrin expression. Furthermore, infection appears to be independent of the EphA2 intracellular domain. We also demonstrated that while two other endogenous Eph receptors were dispensable for KSHV infection, transduced EphA4 and EphA5 significantly enhanced infection of cells lacking EphA2.
IMPORTANCE Our data reveal an integrin-independent route of KSHV infection and suggest that multiple Eph receptors besides EphA2 can promote and regulate infection. Since integrins and Eph receptors are large protein families with diverse expression patterns across cells and tissues, we propose that KSHV may engage with several proteins from both families in different combinations to negotiate successful entry into diverse cell types.
INTRODUCTION
In the decades since its discovery, it has been observed that Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) has broad tropism and can efficiently infect many types of human primary cells and cell lines (1–3). KSHV entry mechanisms have been most thoroughly studied in primary microvascular endothelial cells and human foreskin fibroblasts (HFFs), which were of particular interest to understand the origin of the KSHV-infected spindle cells that make up the distinct, highly vascularized KS tumors (reviewed in reference 4). Infection of monocytes and dendritic cells has also been observed within KS tumors and in tissue culture models (5–7). B cells are thought to be the latently infected reservoir of KSHV (8, 9), but modeling their infection in a laboratory setting has proven to be technically challenging.
However, it is reasonable to consider that the first cells infected in a new host upon transmission are epithelial cells. While KSHV was first considered to be a sexually transmitted infection because of its pattern of coinfection with HIV, it is now widely recognized that KSHV can be transmitted through saliva and close contact between individuals (reviewed in reference 10). Multiple studies have shown that KSHV infects primary human epithelial cells and cell lines, including oral keratinocytes (1, 3, 11–15), and another clinical report provided compelling clinical evidence that infection of the tonsillar epithelium could provide a gateway through which the virus might access the underlying lymphocytes to establish the reservoir of latently infected B cells (16).
KSHV interacts with a number of receptors on the surface of host cells. Heparan sulfate (HS) is thought to be a major cell attachment factor, and several KSHV glycoproteins have HS-binding activities (17–23). KSHV also coordinates a complex of integrins α3β1, αVβ3, and αVβ5; erythropoietin-producing hepatocellular (Eph) receptor A2 (EphA2); and SLC7A11/xCT to trigger clathrin-mediated endocytosis or macropinocytosis of the virion in HFF cells and primary endothelial cells, respectively (most recently reviewed in references 4 and 24). Some questions have been raised over precisely which integrins are required for infection of individual cell lines (25–28). However, in these two well-characterized infection models, the interaction between KSHV gB and the canonical integrin receptors initiates a signaling cascade of focal adhesion kinase (FAK), Src, and phosphatidylinositol 3-kinase (PI-3K) (29–31; reviewed in reference 4). KSHV gH/gL binds EphA2, which amplifies this cascade and coordinates endocytosis effectors together with c-Cbl and myosin IIA (32–37). Still, there are important differences in the entry mechanisms used during infection of HFF and primary microvascular endothelial cells, such as the form of endocytosis used to ultimately internalize the virion, hinting that KSHV initiates different entry processes in different types of cells while using the same receptors.
A smaller number of studies have examined select receptors on some epithelial cell lines, but such a unified model of KSHV receptor usage and mechanism of entry has not yet been assembled for any individual cell line. Soluble heparin or the enzymatic removal of HS from the cell surface inhibits KSHV infection of human embryonic kidney HEK293 cells and human conjunctival epithelial cells, suggesting that HS is required for epithelial cell infection (17, 25, 38, 39). EphA2 is also clearly important for KSHV infection of several cell lines. Soluble EphA2 or Eph-blocking ligands inhibit infection of HEK293 and SLK cells, and EphA2 becomes phosphorylated upon infection of these two cell lines (32, 40–42). Furthermore, small interfering RNA (siRNA) knockdown of EphA2 significantly reduces infection of SLK cells (32, 41). Soluble EphA2 inhibits infection of two additional epithelial cell lines (HeLa and HepG2), and overexpression of EphA2 enhances infection of HEK293 cells and the human lung epithelial cell line H1299 (32).
The evidence for integrin involvement during infection of epithelial cell lines is mixed. Two groups have reported that integrin ligands, RGD peptides, soluble α3β1, or function-blocking integrin αV and β1 antibodies did not block KSHV infection of a HEK293-derived reporter cell line or HEK293 cells (25, 43). A third group reported that soluble integrins α3β1 and αVβ3 and a function-blocking αVβ3 antibody did not block KSHV infection of SLK cells (32). However, a fourth group reported that soluble integrins α3β1, αVβ3, and αVβ5 reduced infection of HEK293 cells and that the signaling proteins FAK, extracellular signal-regulated kinase 1/2 (ERK1/2), and RhoA were activated upon KSHV infection (30, 38, 44). Finally, a fifth group reported that a HeLa derivative cell line misidentified as human salivary gland epithelial cells, HSG(HeLa), was resistant to KSHV despite expressing all known receptors except integrin β3, and expression of integrin β3 (and restoration of integrin αVβ3) greatly increased the susceptibility of these cells to KSHV infection (28, 45).
Here, we used CRISPR-Cas9 as a tool to comprehensively examine the use of this KSHV receptor complex in two highly infectible epithelial cell lines: Caki-1 kidney epithelial cells and HeLa cervical epithelial cells. Caki-1 cells are significant as they have contaminated all known stocks of the SLK cell line used in KSHV research (46). We found that HS and EphA2 were required for infection of both Caki-1 and HeLa cells, while αV- and β1-family integrins were dispensable. Interestingly, we also found that the intracellular domain of EphA2 was not required for infection of these cells. Moreover, the ectopic expression of EphA5 and overexpression of EphA4 promoted infection in EPHA2 knockout (KO) cells, but knockout of endogenous EphA4 led to elevated infection rates in both wild-type (WT) and EPHA2 KO contexts. Finally, we also found that infection of primary gingival keratinocytes (PGKs) was unaffected by integrin- or Eph-blocking reagents. Together with data from other recent studies, our results point to the existence of another unknown KSHV receptor that could trigger intracellular signaling and virion internalization in all three of the cell types that we investigated. Our studies revealed a novel KSHV infection mechanism in Caki-1 and HeLa cells that is independent of integrins α3β1, αVβ3, and αVβ5 and suggest that Eph receptors may play more diverse and complex roles during infection than previously known.
(This article was submitted to an online preprint archive [47].)
RESULTS
Caki-1 and HeLa cells express most known KSHV receptors.
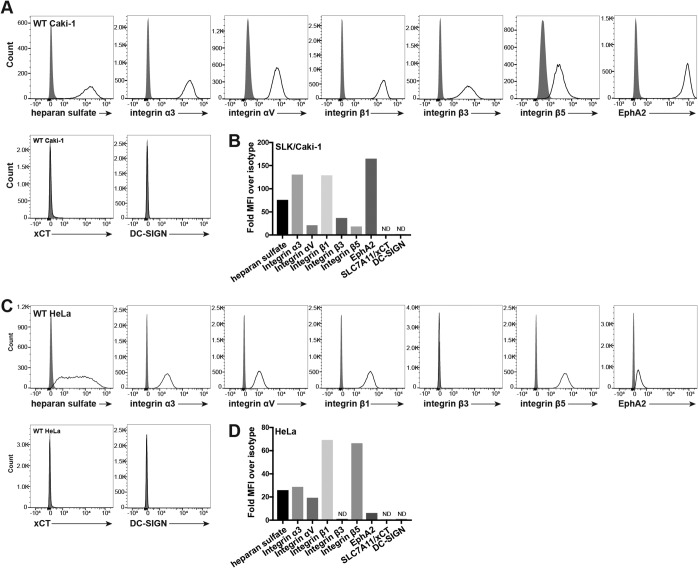
It has been shown that KSHV uses a multimolecular complex of attachment molecules and receptors, including HS, EphA2, xCT, DC-SIGN (in some immune cells), and the integrin heterodimers α3β1, αVβ3, and αVβ5, to enter cells in several different infection models (reviewed in reference 4). The expression of these known KSHV receptors on the surface of Caki-1 and HeLa cells was examined by flow cytometry. Most of the KSHV receptors were expressed on the surface of both cell lines: EphA2, HS, and integrin subunits α3, αV, β1, and β5 (Fig. 1). Integrin β3 was additionally detected on the surface of Caki-1 cells but not HeLa cells (Fig. 1). However, neither the myeloid cell marker DC-SIGN nor xCT was detected on the surface of either cell line (Fig. 1).
FIG 1.
Surface expression of known KSHV receptors on Caki-1 and HeLa cells. (A and C) Live Caki-1 (A) and HeLa (C) cells were immunostained for surface expression of known KSHV receptors and analyzed by flow cytometry. Gray histograms represent the isotype control. (B and D) The mean fluorescence intensity (MFI) of each receptor stain was divided by that of the appropriate primary antibody isotype control and plotted as summarizing bar graphs. ND, not detected.
Heparan sulfate interactions are required for KSHV infection of Caki-1 and HeLa cells.
The role of HS in adhering virions to the cell surface and promoting viral entry is well documented across many virus families. Caki-1 and HeLa cells express HS on the cell surface, and we expected this proteoglycan to play a major role during KSHV infection. We previously showed that a deficiency in the enzyme Ext1 rendered cells unable to synthesize HS (48), so we could use EXT1 KO cells to confirm the requirement for HS during KSHV entry.
An EXT1-specific guide sequence was cloned into px330, a Cas9 and single guide RNA (sgRNA) delivery plasmid, which was then transfected into Caki-1 cells (Table 1). After 4 days, a subpopulation of HS-low mutant cells was discernible by flow cytometry. The mutant population was enriched by fluorescence-activated cell sorting (FACS) and then passaged until the immunostaining of HS in the pool decayed to isotype levels (Fig. 2A). It should be noted that this EXT1 KO pool is polyclonal in nature and presumably contains cells derived from a multitude of individual CRISPR-Cas9-editing events. This approach helps mitigate the chance of off-target effects contributing significantly to any effects on infection.
TABLE 1.
CRISPR-Cas9 guide RNA sequences used to target the indicated genes
| Gene | Exon | Guide sequence (5′–3′) |
|---|---|---|
| EXT1 | 1 | GTGGACGAACGACTACTCCA |
| ITGA3 | 4 | GTCAGAAGACCAGCGGCGCA |
| ITGAV | 2 | GTGACTGGTCTTCTACCCGC |
| ITGB1 | 3 | AATGTAACCAACCGTAGCAA |
| ITGB3 | 3 | GAGCCGGAGTGCAATCCTCT |
| ITGB5 | 1 | GCAGGCGTACAGCGGCGCCG |
| EPHA2 | 4 | GTGTGCAAGGCATCGACGCT |
| EPHA4 | 3 | TCCACTCACAGTCCGCAATC |
| 4 | TCTGAAAAAGCCTCGGTCAC | |
| EPHB2 | 3 | GAACACGATCCGCACGTACC |
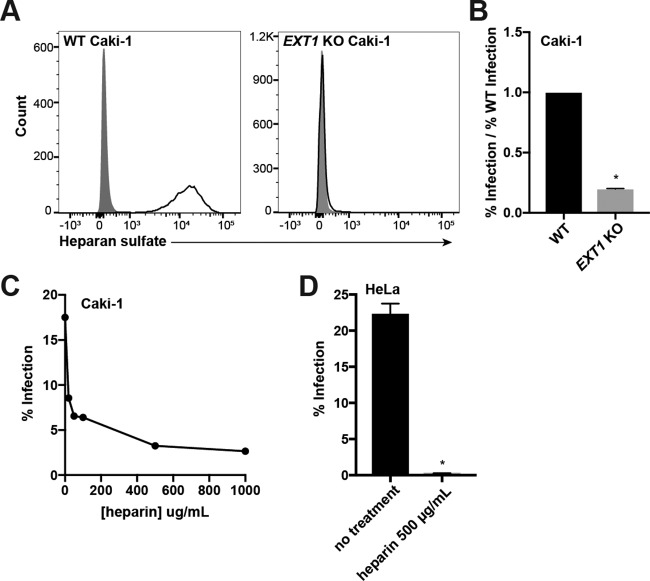
FIG 2.
Heparan sulfate interactions are required for infection of Caki-1 and HeLa cells. (A) WT and EXT1 KO Caki-1 cells were immunostained for surface heparan sulfate (HS) expression. Gray histograms represent isotype controls. (B) WT and EXT1 KO Caki-1 cells were infected with KSHV in duplicate, and infection rates were measured by flow cytometry. The infection rate of the KO was normalized to the average WT infection rate, and data were pooled from multiple experiments. (C) Filtered KSHV was preincubated with the indicated concentrations of soluble heparin at 37°C and then used to infect Caki-1 cells for 2 h at 37°C. Infection percentages were measured by flow cytometry at 2 days postinfection. (D) Filtered KSHV was preblocked with 500 μg/ml of heparin at 37°C and then used to infect WT HeLa cells in triplicate for 2 h at 37°C. The infection percentage was measured by flow cytometry at 2 days postinfection. *, P < 0.05.
The EXT1 KO Caki-1 pool and WT Caki-1 cells were infected with KSHV.BAC16, which encodes a constitutive green fluorescent protein (GFP) reporter (49), and the infection percentage was quantified by measuring GFP-positive (GFP+) cells by flow cytometry after 2 days. As expected, the percent infection of HS-deficient Caki-1 cells was significantly reduced compared to that of WT cells (Fig. 2B).
As an orthogonal approach, we used soluble heparin to competitively block KSHV infection, since this method has been used extensively to investigate HS usage in a variety of cell types (17, 20, 50, 51). KSHV was preincubated with increasing concentrations of heparin and then used to infect Caki-1 cells. In agreement with data in the existing literature and our results with EXT1 KO Caki-1 cells, soluble heparin inhibited infection in a dose-dependent manner but approached a nonzero asymptote (Fig. 2C). Preblocking of KSHV with 500 μg/ml of heparin had an even more dramatic effect on HeLa cells, completely abrogating infection compared to nontreated virus (Fig. 2D). Collectively, these results show that HS interactions are required for infection of both Caki-1 and HeLa cells and demonstrate the value of CRISPR-Cas9 to study viral receptors.
KSHV infection of Caki-1 and HeLa cells is independent of canonical KSHV integrin receptors.
KSHV coordinates several integrin heterodimers to initiate signaling events that are required for infection of HFFs and primary microvascular endothelial cells (reviewed in reference 4). Because we observed the expression of all proposed integrin receptors for KSHV on the surface of Caki-1 cells and, except for integrin β3, HeLa cells (Fig. 1), we investigated whether these integrins were required for KSHV to infect these cell lines. Both the α and β subunits contribute to the unique ligand-binding surface of a given integrin heterodimer. Therefore, we reasoned that infecting cells with reciprocal subunits of KSHV-associated integrins knocked out would reveal precisely which heterodimers were required for infection.
Single-KO pools of integrins α3, αV, β1, β3, and β5 were created by transfecting Caki-1 cells with px330 plasmids containing guide sequences that targeted the genes encoding each integrin subunit (Table 1). The mutant populations were enriched as described above for EXT1 to generate integrin KO Caki-1 cell pools (Fig. 3A). Lacking integrin αV protein, ITGAV KO cells lost the ability to adhere to tissue culture-treated polystyrene dishes, but normal morphology and growth returned when they were plated onto fibronectin-coated dishes. The ITGAV KO cells were grown on fibronectin for passaging and infection experiments. The infection percentages of both WT Caki-1 and HeLa cells were not affected by the presence of a fibronectin coating (data not shown). WT HeLa cells were additionally transfected with px330 plasmids targeting ITGAV or ITGB1, but the transfected populations were not enriched. The cells were passaged until the receptor expression of the mutant population decayed to near-isotype levels, generating mixed WT/KO HeLa pools (Fig. 3E). The mixed ITGAV KO HeLa pool was also grown on fibronectin.
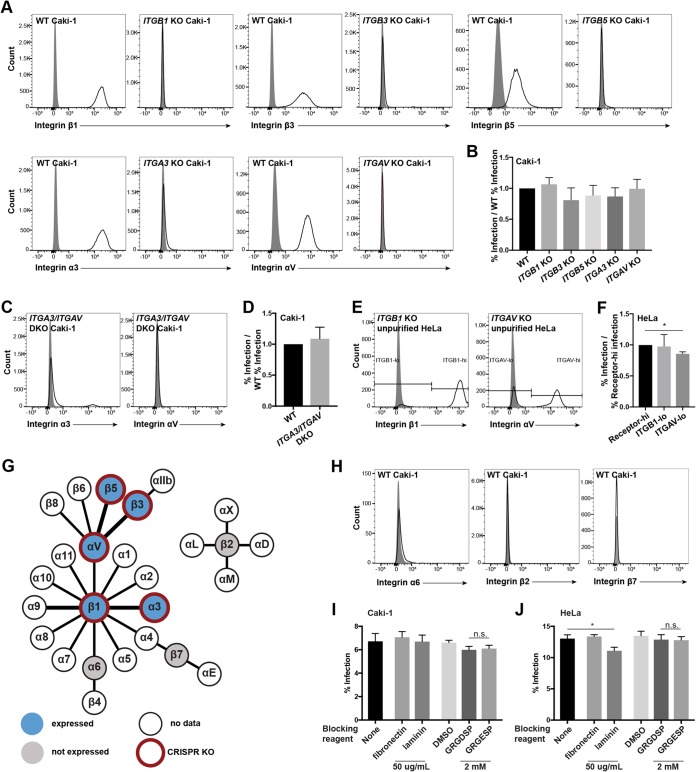
FIG 3.
Canonical KSHV integrin receptors are not required for infection of Caki-1 and HeLa cells. (A and H) WT and the indicated integrin subunit KO Caki-1 cells were immunostained for surface expression of the indicated integrins. Gray histograms represent isotype controls. (B) WT and integrin KO Caki-1 pools were infected with KSHV in duplicate, and infection percentages were measured by flow cytometry at 2 days postinfection. The infection rates of the KO pools were normalized to the average WT infection rate, and data were pooled from multiple experiments. (C) ITGA3/ITGAV double-KO Caki-1 cells were immunostained for surface integrin α3 and αV expression. Gray histograms represent the isotype controls. (D) WT and ITGA3/ITGAV double-KO Caki-1 cells were infected with KSHV in triplicate, and the infection rate was quantified by flow cytometry. The infection rate of the DKO pool was normalized to the average WT infection rate, and data were pooled from multiple experiments. (E) Mixed WT/ITGAV KO and WT/ITGB1 KO HeLa populations were immunostained for surface integrin αV and β1 expression. Gray histograms represent the isotype controls. (F) Mixed integrin KO HeLa pools were infected with KSHV in triplicate, and infection percentages were measured by flow cytometry at 2 days postinfection. The pools were also immunostained for the corresponding integrins and gated on integrin-high or -low populations, as indicated in panel E. The infection rates of integrin-low cells were normalized to integrin-high cells in each well, and data from a representative experiment are shown. *, P < 0.05. (G) Schematic integrin pairing diagram (72) showing expression data measured by surface immunostaining and flow cytometry and subunits targeted by our CRISPR-Cas9 KO approach. Bold connections denote heterodimers previously implicated in KSHV infection. (I and J) WT Caki-1 (I) or HeLa (J) cells were preincubated with medium alone, 50 μg/ml of fibronectin or laminin in medium, 2 mM the peptide GRGDSP or GRGESP, or a control volume of 50% DMSO for 1 h at 4°C. Cells were subsequently washed and infected with KSHV for 2 h. Infection percentages were measured by flow cytometry at 2 days postinfection. n.s., not significant.
WT Caki-1 cells, the single-integrin-KO Caki-1 pools, and the mixed-integrin-KO HeLa cell pools were then infected with KSHV. The mixed-KO HeLa pools were additionally stained for the surface expression of the appropriate integrin at the time of infection analysis to allow for gating on WT and KO subpopulations. Overall, the infection percentages of the integrin KO pools or subpopulations were not significantly reduced compared to that of WT cells for both cell lines (Fig. 3B and F). The slight decline in the infection percentage of the ITGAV KO HeLa subpopulation compared to that of the WT subpopulation reached statistical significance, but the magnitude of the difference was small and similar to those of the other integrin KO Caki-1 pools. Since the KO pools were enriched by FACS, it is likely that there were still a small number of cells that expressed WT levels of each integrin subunit. Nevertheless, these data suggest that KSHV infection of Caki-1 and HeLa cells does not require integrin α3β1, αVβ3, or αVβ5 alone or any other single integrin in the αV and β1 families.
Although targeting single integrin heterodimers has yielded clear infection defects in previous studies (27–29, 38), we considered that our strategy of knocking out individual integrin subunits would not reveal a fully redundant involvement of α3β1, αVβ3, and αVβ5 during infection of Caki-1 cells. To address this, an ITGA3/ITGAV double-KO (DKO) Caki-1 pool was generated to effectively deplete integrin α3β1 and the entire integrin αV family, including αVβ3 and αVβ5, from the cell surface (Fig. 3C). As with previous receptor KO pools, the ITGA3/ITGAV DKO pool was enriched but not purified, since a very small population of cells expressing WT levels of integrin α3 was still visible by flow cytometry (Fig. 3C). WT and ITGA3/ITGAV double-KO Caki-1 cells were then infected with KSHV. Still, the infection percentage of ITGA3/ITGAV double-KO Caki-1 cells was not significantly reduced compared to that of WT cells at either 1 or 2 days postinfection (Fig. 3D; see also Fig. S1 in the supplemental material). These results further indicate that integrins α3β1, αVβ3, and αVβ5 are not required for KSHV infection of Caki-1 cells.
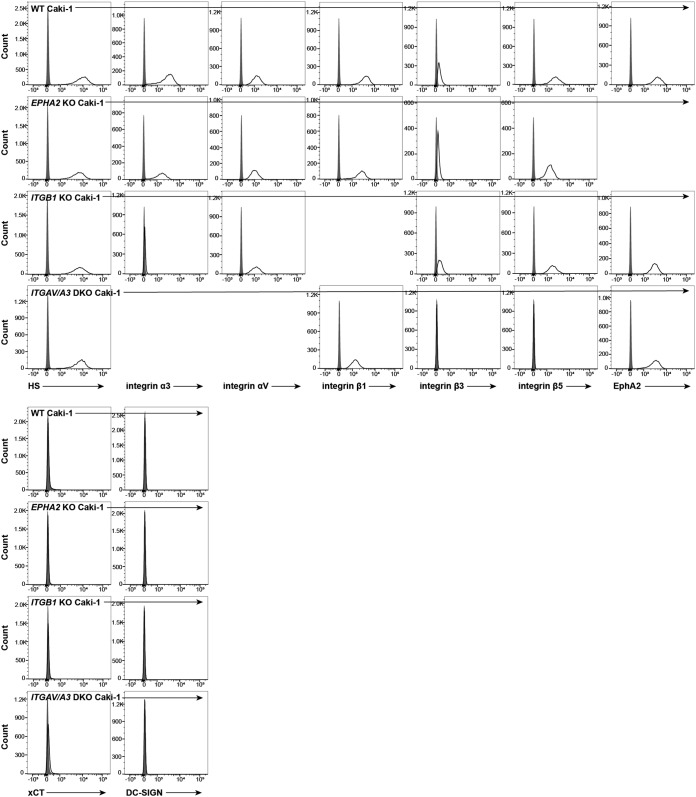
We also considered whether our genetic disruptions in the integrin network were altering the expression levels of other KSHV receptors, potentially obscuring an infection defect in integrin subunit KO cells. To address this, we examined the expression levels of all known KSHV receptors on ITGB1 KO and ITGAV/ITGA3 DKO Caki-1 cells (see Fig. 5). We observed that ITGB1 KO cells lost surface expression of integrin α3, which is not unexpected since integrin α3 does not bind to any other known integrin β subunits (see Fig. 5). Likewise, we found that ITGAV/ITGA3 DKO Caki-1 cells lost surface expression of integrins β3 and β5 (see Fig. 5). Otherwise, we did not observe any large changes in the surface expression levels of unrelated integrin subunits, HS, or EphA2 (see Fig. 5).
FIG 5.
Perturbations in KSHV receptor expression do not unexpectedly affect other known receptors. WT, EPHA2 KO, ITGB1 KO, and ITGAV/ITGA3 DKO Caki-1 cells were concurrently immunostained for surface expression of nontargeted, known KSHV receptors and analyzed by flow cytometry. Gray histograms represent isotype controls.
Previous studies have utilized integrin-blocking reagents to show that certain classes of integrins are required for KSHV entry in a variety of cell types (27, 29, 38, 52). However, at least three publications have reported that several integrin-blocking reagents failed to inhibit KSHV infection in HEK239 and SLK cells (25, 32, 43). To confirm that our results were not unique to the CRISPR-Cas9 KO approach, we replicated key integrin-blocking experiments from those publications. WT Caki-1 and HeLa cells were preincubated with the RGD-containing integrin ligand fibronectin, the integrin ligand laminin, GRGDSP and GRGESP peptides, or a dimethyl sulfoxide (DMSO) control for the peptide resuspension solution for 1 h at 4°C. The cells were then washed and infected with KSHV for 2 h at 37°C. Fibronectin, which contains an RGD sequence and binds αV family integrins, did not significantly alter the percent infection of either cell line (Fig. 3I and J). Laminin, which binds to a different subset of integrins, including α3β1, slightly inhibited infection of HeLa cells but not Caki-1 cells (Fig. 3I and J). Neither the RGD-containing peptide GRGDSP nor the control peptide GRGESP significantly affected KSHV infection of HeLa cells (Fig. 3J). GRGDSP very slightly inhibited infection of Caki-1 cells, but the effect was not significantly different compared to that of GRGESP, suggesting that the inhibitory effect of the peptide was nonspecific, which was noted in a previous publication (25) (Fig. 3I). Overall, we found that these blocking reagents had little or no effect on KSHV infection of Caki-1 and HeLa cells, which was consistent with the results of our CRISPR-Cas9 KO studies.
A non-RGD-binding integrin, α9β1, has been shown to bind a disintegrin-like domain (DLD) in KSHV gB and is important for infection of HFF and primary microvascular endothelial cells but not HEK293 cells (43). Our data demonstrate that KSHV infection of Caki-1 and HeLa cells is independent of the 12 β1-containing integrins and the 5 αV-containing integrins; however, we considered that other integrins could still be required for KSHV infection of Caki-1 cells. There are eight integrins that do not contain the αV or β1 subunits: αIIbβ3, α6β4, α4β7, αEβ7, and four β2-containing integrins (Fig. 3G). Neither integrin α6, integrin β7, nor integrin β2 was detected on the surface of WT Caki-1 cells by flow cytometry (Fig. 3H). Additionally, integrin β3 was lost from the cell surface of ITGAV/ITGA3 DKO Caki-1 cells, implying that integrin αIIbβ3 is not expressed in Caki-1 cells (see Fig. 5). Altogether, these data indicate that none of the eight non-αV, non-β1 integrin heterodimers are expressed in Caki-1 cells, so these integrins are unlikely to play a role in this KSHV infection mechanism in the absence of αV or β1 family integrins.
EphA2 promotes KSHV infection of Caki-1 and HeLa cells.
EphA2 has been well characterized as a receptor for KSHV and binds to the envelope glycoprotein complex gH/gL (32, 33, 37, 42). Together with integrins, EphA2 helps propagate virus-induced signaling and mobilize endocytosis effectors, which leads to viral entry in multiple cell types (32–36, 41). However, we found that KSHV infection of Caki-1 and HeLa cells does not require canonical KSHV integrin receptors, so we investigated whether EphA2 was required for infection in these cell lines.
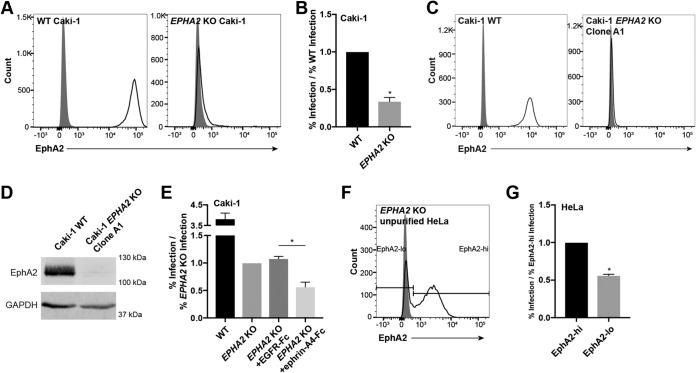
Caki-1 cells were transfected with a px330 plasmid containing a guide sequence targeting EPHA2, and an EPHA2 KO pool was enriched as described above for EXT1 (Table 1 and Fig. 4A). In addition, a mixed WT/EPHA2 KO pool was generated in HeLa cells as described above for ITGAV and ITGB1 (Fig. 4F). WT and EPHA2 KO Caki-1 cells and the mixed WT/KO HeLa pool were then infected with KSHV. The mixed WT/KO HeLa pool was additionally stained for surface EphA2 expression at the time of infection analysis to distinguish the KO and WT subpopulations. The infection percentage of EPHA2 KO cells was significantly reduced compared to that of WT cells in both cell lines, although EPHA2 KO cells were not completely resistant to the virus (Fig. 4B and G). The infection defects in EPHA2 KO cells were similar at both 1 and 2 days postinfection (see Fig. S1 in the supplemental material). These results indicate that EphA2 is necessary for infection of both Caki-1 and HeLa cells.
FIG 4.
EphA2 is required for infection of Caki-1 and HeLa cells. (A) WT and EPHA2 KO Caki-1 cells were immunostained for surface EphA2 expression with the SHM16 antibody and analyzed by flow cytometry. The gray histogram represents the isotype control. (B) WT and EPHA2 KO Caki-1 cells were infected with KSHV in duplicate, and infection rates were quantified by flow cytometry. The rate of infection of the EPHA2 KO pool was normalized to the average WT infection rate, and data were pooled from multiple experiments. (C) WT Caki-1 and EPHA2 KO clone A1 cells were immunostained for surface EphA2 with the antibody AF3035 and analyzed by flow cytometry. The gray histogram represents the isotype control. (D) Fifteen micrograms of whole-cell lysate protein from WT Caki-1 cells and EPHA2 KO clone A1 was run on an SDS-PAGE gel and blotted for EphA2 with AF3035 and GAPDH. (E) EPHA2 KO clone A1 Caki-1 cells were preblocked with EGFR-Fc or ephrin-A4–Fc at 10 μg/ml at 4°C and then infected in triplicate in the presence of EGFR-Fc or ephrin-A4–Fc at 5 μg/ml at 37°C. The infection percentage was measured by flow cytometry at 2 days postinfection, and percent infection was normalized to the average EPHA2 KO infection rate. (D) Mixed EPHA2 KO HeLa cells were immunostained for surface EphA2 expression with the SHM16 antibody and analyzed by flow cytometry. The gray histogram represents the isotype control. (E) Mixed EPHA2 KO HeLa cells were infected with KSHV in triplicate, and the infection percentage was measured by flow cytometry at 2 days postinfection. The cells were also immunostained for surface EphA2 and gated on EphA2-high or -low cells, as indicated in panel D. The rates of infection of EphA2-low cells were normalized to those of EphA2-high cells in each well, and data from a representative experiment are shown. *, P < 0.05.
To ensure that the KO of EPHA2 did not alter the expression of any other known KSHV receptors, WT and EPHA2 KO Caki-1 cells were examined for surface receptor expression by flow cytometry. We did not observe any unexpected changes in the surface expression of any other known receptors (Fig. 5).
A previous study demonstrated that KSHV gH/gL has the ability to bind other Eph receptors besides EphA2 in vitro (40). We hypothesized that EphA2-independent infection could depend on other Eph receptors that may be expressed. A clonal EPHA2 KO Caki-1 cell line was isolated from single-cell clones of the EPHA2 KO pool and was used for this experiment. The clone lacked surface expression of EphA2, and the infection defect compared to WT cells was similar to that of the parent population (Fig. 4E and see Fig. 7E). To ensure that this EPHA2 KO clone did not produce EphA2 protein, surface and total EphA2 were examined by flow cytometry and Western blotting, respectively, using a second EphA2-specific antibody (Fig. 4C and D). To test whether additional Eph receptors were required for infection in these cells, we attempted to block KSHV infection using the A-type Eph ligand ephrin-A4 or epidermal growth factor receptor (EGFR) as a control, as reported previously (32, 40). Clonal EPHA2 KO cells were preincubated with either soluble ephrin-A4–Fc or EGFR-Fc chimeric proteins and then infected with KSHV in the presence of these blocking agents. The infection percentage of EPHA2 KO cells was further reduced in the presence of ephrin-A4–Fc compared to the unrelated EGFR-Fc chimeric protein (Fig. 4E). Since ephrin ligands, including ephrin-A4, can broadly bind to and block interactions with Eph receptors of the same type, these data suggest that another A-type Eph receptor may be required for infection of Caki-1 cells in the absence of EphA2.
FIG 7.
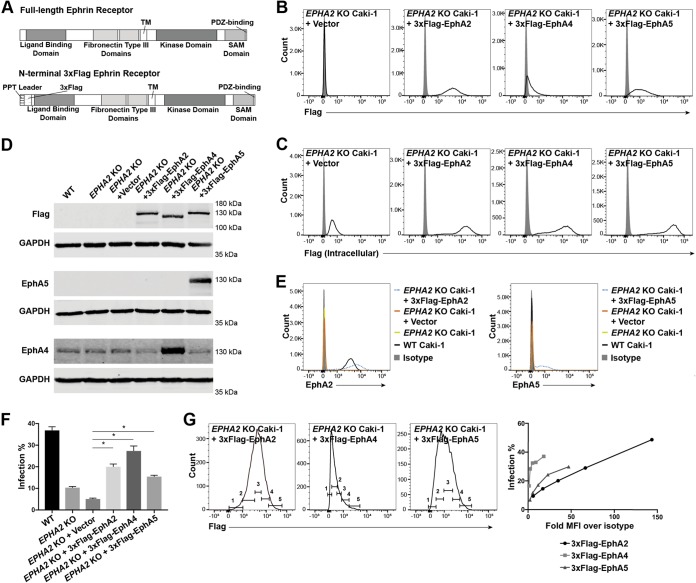
EphA2, EphA4, and EphA5 rescue KSHV infection in EPHA2 KO Caki-1 cells. (A) Diagram of generalized full-length and PPT-3×Flag-mature ephrin receptor constructs. SAM, sterile alpha motif. (B and C) Live (B) or fixed and permeabilized (C) 3×Flag-tagged ephrin receptor-transduced EPHA2 KO cells and a vector control were immunostained for surface (B) or intracellular (C) 3×Flag expression and analyzed by flow cytometry. Gray histograms represent isotype controls. (D) The indicated cell lysates were run on 10% SDS-PAGE gels and blotted for 3×Flag, EphA4, and EphA5 with matched GAPDH as a loading control. For the Flag and EphA5 blots, 15 μg of whole-cell lysate protein was loaded. For the EphA4 blot, 120 μg of whole-cell lysate protein was loaded. (E) The indicated cell lines were immunostained for surface EphA2 or EphA5 expression and analyzed by flow cytometry. Gray histograms represent isotype controls. (F) The indicated cell lines were infected with KSHV in triplicate, and the infection rate was quantified by flow cytometry at 2 days postinfection. Data from a representative experiment are shown. (G) The 3×Flag expression histograms of infected 3×Flag-tagged ephrin receptor-transduced cell lines were divided into five successive gates, as shown. The infection rate within each gate was plotted against the fold MFI over the isotype of each gate. *, P < 0.05.
EphA4 and EphB2 are dispensable for KSHV infection in Caki-1 cells.
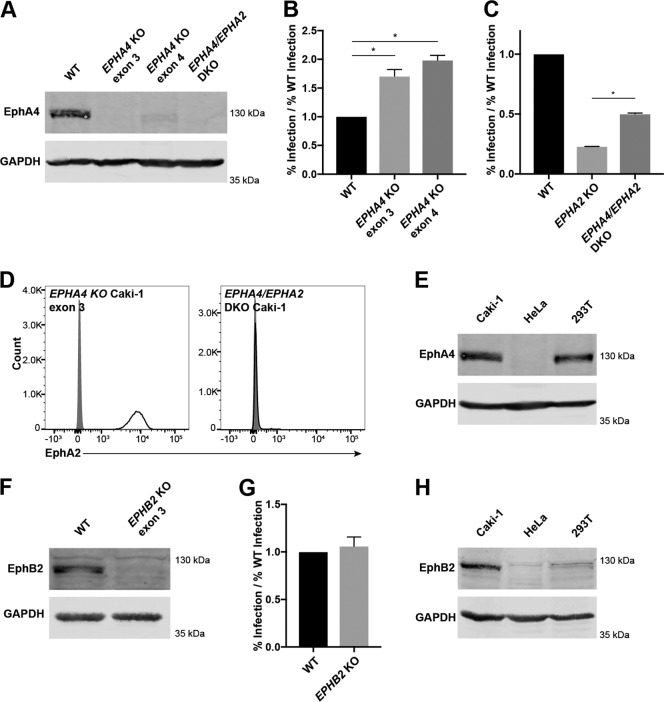
Since we found that KSHV infection in EPHA2 KO Caki-1 cells could be further blocked by a soluble ephrin ligand, we investigated whether additional Eph receptors were expressed by Caki-1 cells and if they were required for KSHV infection of Caki-1 cells. EphA4 and EphB2 were found to be expressed by Caki-1 cells by Western blotting (Fig. 6E and H).
FIG 6.
EphA4 and EphB2 are dispensable for infection of Caki-1 cells. (A, E, F, and H) A total of 120 μg (A, E, and H) or 50 μg (F) of the indicated whole-cell lysate proteins was run on a 10% SDS-PAGE gel and blotted for EphA4 (A and E) or EphB2 (F and H) and GAPDH as a loading control. (B and C) WT and EPHA4 KO Caki-1 cells (B) or WT, EPHA2 KO, and EPHA4/EPHA2 DKO Caki-1 cells (C) were infected with KSHV in triplicate, and the infection percentage was measured by flow cytometry at 2 days postinfection. The infection percentages of KO cell lines were normalized to the average infection percentage of WT cells, and data from a representative experiment are shown. (D) EPHA4 KO and EPHA4/EPHA2 DKO Caki-1 cells were immunostained for surface EphA2 expression. Gray histograms represent isotype controls. (G) WT and EPHB2 KO Caki-1 cells were infected with KSHV in triplicate, and infection percentages were quantified by flow cytometry at 2 days postinfection. The rates of infection of the KO line were normalized to the average WT infection rate, and data from a representative experiment are shown. *, P < 0.05.
WT Caki-1 cells were transfected with px330 plasmids containing guide sequences targeting EPHA4 and EPHB2 (Table 1). We were unable to find an antibody that reliably detected EphA4 or EphB2 by surface immunostaining of live cells, so single-cell clones were derived from the transfected populations and then screened for the loss of EphA4 or EphB2 by Western blotting. Two EPHA4 KO Caki-1 cell lines and one EPHB2 KO Caki-1 cell line were isolated (Fig. 6A and F). WT, EPHA4 KO, and EPHB2 KO Caki-1 cell lines were then infected with KSHV. Surprisingly, the infection percentage of EPHA4 KO cells was elevated compared to that of WT cells, while the infection percentage of EPHB2 KO cells was not significantly different (Fig. 6B and G). Similar results were observed in EPHA4 and EPHB2 KO Caki-1 cells created with a lentiviral CRISPR-Cas9 system (data not shown).
To further understand the infection phenotype of EPHA4 KO cells, EPHA4 single-KO Caki-1 cells (exon 3) were transfected with the EPHA2-targeted px330 plasmid (Table 1), and a pool of EPHA2/EPHA4 DKO cells was isolated by FACS (Fig. 6A and D). When these cells were infected with KSHV, the infection percentage was reduced compared to that of WT cells but significantly elevated compared to that of EPHA2 KO cells (Fig. 6C). These data suggest that either EphA4 is a negative regulator of KSHV infection or Caki-1 cells compensate for the loss of EphA4 in a way that enhances KSHV infection.
EphA4 and EphB2 were detected in Caki-1 lysates, and EphA4 was additionally found in HEK293T lysates. Importantly, both of these proteins were lacking in HeLa cell lysates, even though EphA2-independent infection was observed in both Caki-1 and HeLa cells (Fig. 6E and H). Furthermore, these results show that EphA4 and EphB2 are dispensable for KSHV infection and are unlikely to be the functional targets of ephrin-A4–Fc blocking during infection of EPHA2 KO Caki-1 cells.
Multiple Eph receptors rescue KSHV infection of EphA2 KO cells.
Although we found that two endogenous Eph receptors besides EphA2 were not required for KSHV infection of WT Caki-1 cells, the significant infection defect of EPHA2 KO Caki-1 cells provided an ideal platform to test the effects of transduced Eph receptors on KSHV infection. The clonal EPHA2 KO Caki-1 cell line, described above, was used for these experiments.
To ensure that expression levels of different Eph receptors could be compared, mature forms of EPHA2, EPHA4, and EPHA5 lacking endogenous signal peptides were cloned into p3xFlag-CMV-9 following the preprotrypsin (PPT) leader sequence and a 3×Flag tag (Fig. 7A). This cloning scheme ensured that the proteins would be properly oriented in the membrane during translation and ultimately be N-terminally tagged with 3×Flag. The 3×Flag-tagged Eph receptor constructs were cloned into a retroviral vector and transduced into EPHA2 KO Caki-1 cells.
The 3×Flag tag was detected on the surface of each cell line by flow cytometry, although the magnitude of expression varied with each receptor (Fig. 7B). However, when the cell lines were stained for intracellular 3×Flag, the overall expression levels of the receptors appeared to be similar to each other (Fig. 7C). Additionally, when the expression of the receptors was examined by Western blotting, the intensities of the 3×Flag-tagged bands were similar across the three transduced cell lines (Fig. 7D). These data show that all three constructs were expressed to similar degrees but that cell surface trafficking of the three receptors was different.
The expression of the 3×Flag-tagged Eph receptors was also compared to that of the corresponding endogenous protein. The peak level of surface expression of transduced 3×Flag-EphA2 was slightly higher than that of endogenous EphA2 as measured by flow cytometry, but the range of EphA2 expression levels in the population of 3×Flag-EphA2-transduced cells was much higher than that in WT cells (Fig. 7E). EphA5 was not naturally expressed by Caki-1 cells, but EphA5 was readily detected in 3×Flag-EphA5-transduced cells as a wide peak by flow cytometry and also as a strong band by Western blotting (Fig. 7E and D). In contrast, EphA4 was found to be expressed endogenously by Caki-1 cells by Western blotting, and the EphA4 band became more pronounced in the 3×Flag-EphA4-transduced cell lysate (Fig. 7D).
WT Caki-1, EPHA2 KO Caki-1, and 3×Flag-tagged Eph receptor-transduced EPHA2 KO cell lines and a vector control were infected with KSHV. Surface 3×Flag expression was measured concurrently with the infection percentage by flow cytometry. 3×Flag-EphA2, 3×Flag-EphA4, and 3×Flag-EphA5 all significantly rescued the infection percentage to various degrees compared to the vector control (Fig. 7F). Because of the broad range of Flag-tagged receptor expression within the populations of the transduced cell lines, we also examined how the infection percentage changed with the surface protein level. The histograms of 3×Flag expression from one replicate well of the experiment were divided into five successive gates (Fig. 7G). The percentage of GFP+ cells in each bin was plotted against the fold geometric mean fluorescence intensity (MFI) over the isotype MFI of each bin (Fig. 7G). Surprisingly, we found that both EphA4 and EphA5 promoted higher KSHV infection rates at smaller amounts of surface protein than EphA2. However, at very high surface expression levels that were attained only by EphA2, the infection percentage surpassed that of WT cells from the same experiment (Fig. 7F).
Altogether, these data show that EphA2, EphA4, and EphA5 can rescue the infection rate phenotype of EPHA2 KO cells, which suggests that the function of EphA2 in this infection mechanism may not be specific to EphA2. Moreover, the overexpression of EphA4 in this context strongly enhanced KSHV infection, which is not what we expected based on our EPHA4 KO experiments. The precise role of endogenous EphA4 cannot be discerned from these studies alone.
The ectodomain of EphA2 is sufficient to rescue KSHV infection in EphA2 KO cells.
For primary microvascular endothelial cells and HFFs, several studies have reported that downstream effector proteins coimmunoprecipitate and colocalize with EphA2 during infection, implying an involvement of the cytoplasmic tail of EphA2, which contains a kinase and several protein-protein interaction domains (33–36, 41). EphA2 is also phosphorylated upon KSHV infection in HEK293 and SLK cells (32, 41). Here we have shown that KSHV infection of Caki-1 and HeLa cells is independent of canonical KSHV integrin receptors but still dependent on EphA2. Eph receptors can naturally trigger endocytosis in response to ephrin ligand binding by several mechanisms (reviewed in reference 53). Since KSHV gH/gL binds to the ephrin-binding domain of EphA2 (37, 42), we hypothesized that the signaling domains in the cytoplasmic tail of EphA2 would be necessary for infection and would provide clues about how the virus might use EphA2 to enter cells without utilizing canonical integrin receptors.
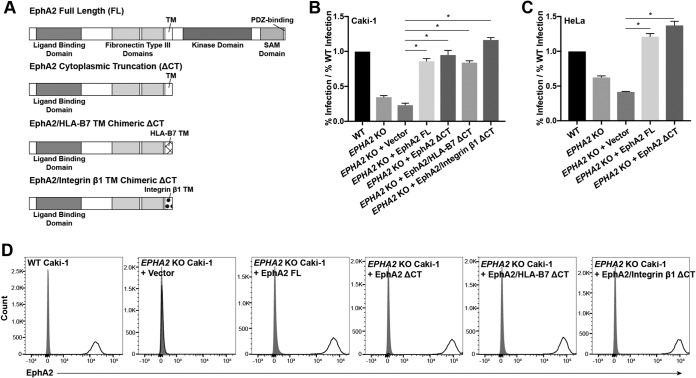
To this end, a truncation mutant of EphA2 was generated which lacked all cytoplasmic signaling domains. This cytoplasmic truncation (ΔCT) contained the entire peptide signal and ectodomain (amino acids [aa] 1 to 537) and the transmembrane (TM) domain (aa 538 to 558) (Fig. 8A). Full-length EPHA2 (EPHA2 FL) and EPHA2 ΔCT were cloned into retroviral vectors and stably transduced into EPHA2 KO Caki-1 and HeLa cells. Both EphA2 FL and EphA2 ΔCT were expressed on the cell surface to a slightly higher degree than endogenous EphA2 on Caki-1 cells (Fig. 8D) and HeLa cells (data not shown). When infected with KSHV, both EphA2 FL and EphA2 ΔCT significantly rescued infection to nearly identical levels compared to the vector control (Fig. 8B and C).
FIG 8.
The EphA2 ectodomain is sufficient to rescue the rate of infection of EPHA2 KO cells. (A) Diagram of EphA2 truncation and domain swap constructs. (B and C) WT, EPHA2 KO, and EPHA2 KO cells transduced with the EphA2 constructs indicated in panel A were infected with KSHV in triplicate, and the infection rate was quantified by flow cytometry at 2 days postinfection. The infection rates were normalized to the average rate of infection of WT cells, and data from a representative experiment are shown. (D) WT, EPHA2 KO, and the indicated transduced EPHA2 KO Caki-1 cells were immunostained for surface EphA2 expression and analyzed by flow cytometry. Gray histograms represent the isotype controls. *, P < 0.05.
Eph receptor clustering is essential for natural signaling events, and a homodimerization region has been found within the transmembrane domain of EphA1 by nuclear magnetic resonance spectroscopy (54). To test whether the transmembrane domain was required for EphA2 ectodomain function during KSHV infection, we performed additional domain swaps with the EphA2 ΔCT construct and replaced the EphA2 TM domain with that of integrin β1 or HLA-B7, two unrelated single-pass transmembrane proteins (Fig. 8A). These domain-swapped constructs were also cloned into retroviral vectors and transduced into EPHA2 KO Caki-1 cells. The EphA2/HLA-B7 chimeric CT and EphA2/integrin β1 chimeric ΔCT constructs were expressed at the cell surface to the same degree as the FL and CT constructs, and they also significantly rescued KSHV infection of EPHA2 KO cells (Fig. 8B and D). Together, these data show that only the ectodomain of EphA2 is required to rescue KSHV infection in EPHA2 KO Caki-1 cells.
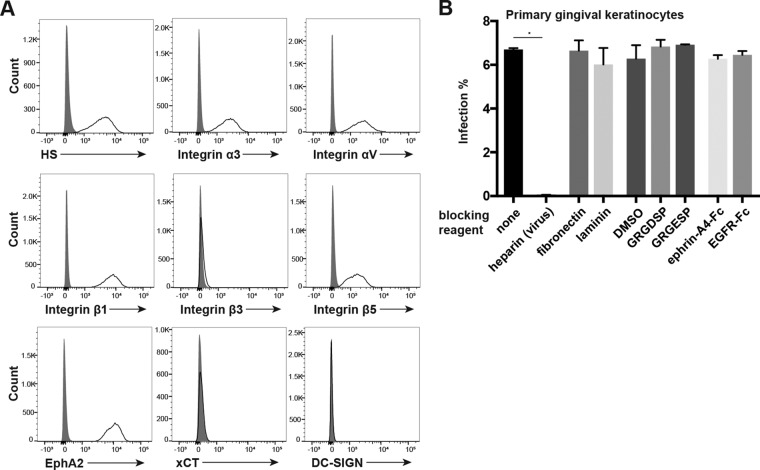
Infection of primary gingival keratinocytes requires HS interactions.
Since transmission through saliva is thought to be a major route of KSHV infection, we examined the expression and use of known KSHV receptors during KSHV infection of primary gingival keratinocytes (PGKs). First, surface expression of known KSHV receptors on PGKs was examined by flow cytometry. We found that HS, EphA2, and the integrin subunits α3, αV, β1, and β5 were readily detected at the cell surface (Fig. 9A). Like HeLa cells, PGKs did not express integrin β3 at the cell surface, nor did we detect xCT or DC-SIGN (Fig. 9A).
FIG 9.
KSHV infection of PGKs depends on HS interactions but is not inhibited by integrin- or Eph-blocking agents. (A) PGKs were immunostained for surface expression of known KSHV receptors and analyzed by flow cytometry. Gray histograms represent isotype controls. (B) PGK cells were preincubated with fibronectin or laminin at 50 μg/liter, the GRGDSP or GRGESP peptide at 2 mM, or an appropriate control volume of DMSO and ephrin-A4–Fc or EGFR-Fc as a control at 5 μg/ml for 1 h at 4°C. For the no-treatment and heparin conditions, cells were preincubated in normal medium at 4°C. For heparin block conditions, virus was blocked with heparin at 500 μg/ml for 1 h at 37°C. Cells were then washed and infected in triplicate with KSHV or heparin-blocked KSHV for 2 h at 37°C. Ephrin-A4–Fc and EGFR-Fc concentrations were maintained during the infection. The infection percentage was quantified by flow cytometry at 2 days postinfection. *, P < 0.05.
Next, we utilized the blocking experiments that we had replicated from data in the existing KSHV receptor literature to test whether these known receptors were utilized during infection of PGKs. To test whether HS interactions were required for infection, KSHV was preblocked with heparin before infection. To investigate whether any canonical integrin receptors were required for infection, cells were preblocked with fibronectin, laminin, the RGD-containing peptide GRGDSP, the control peptide GRGESP, or a control volume of DMSO. Finally, to investigate whether Eph receptor interactions were required for infection, cells were preblocked with ephrin-A4–Fc or EGFR-Fc as a control. The cells were then washed and infected with KSHV (or heparin-blocked KSHV under HS test conditions) for 2 h.
We found that heparin block of virions completely abrogated the infection of PGKs, as was the case for WT HeLa cells (Fig. 2D and 9B). Similar to our results with Caki-1 and HeLa cells, the integrin ligands and RGD peptide had no significant effects on infection percentages of PGKs (Fig. 9B). Surprisingly, the ephrin-A4 ligand also had no effect on infection in these cells (Fig. 9B). These results suggest that KSHV infection of PGKs does not require interactions with the laminin-binding integrin α3β1, the RGD-binding integrin αVβ5, or EphA2, which is competitively blocked by ephrin-A4 (32, 40). However, infection of PGKs clearly requires heparan sulfate interactions.
DISCUSSION
Here we describe a novel KSHV infection mechanism in Caki-1 and HeLa cells that requires HS and the ectodomain of EphA2 but is independent of the canonical KSHV integrin receptors α3β1, αVβ3, and αVβ5. We also present evidence that infection of primary gingival keratinocytes (PGKs) is dependent on HS but not EphA2 or the canonical KSHV integrin receptors. Finally, we found that EphA4 and EphA5 may regulate KSHV infection in various contexts. CRISPR-Cas9 proved to be a valuable tool to dissect the roles of individual receptors during KSHV infection.
It is thought that HS broadly acts as an attachment factor for many viruses, but some publications indicate that HS may have additional functions during KSHV infection of several cell types. One study reported that HS was required on target HEK293, Chinese hamster ovary (CHO), and human conjunctival epithelial cells in a virus-free fusion assay with effector cells that expressed KSHV gB, gH, and gL, suggesting that HS is involved in the interactions between KSHV glycoproteins and entry receptors (39). A second study used advanced imaging to reveal that upon initial binding to HT1080 fibrosarcoma cells, KSHV colocalized with HS only about half of the time, while colocalization with canonical integrin receptors was much more robust (28). However, soluble heparin still inhibited KSHV binding to these cells (55). Our experiments clearly demonstrated that HS interactions are required for KSHV to infect Caki-1, HeLa, and PGK cells, but the precise role of HS during infection remains an open question. Interestingly, the blocking effect of heparin appeared to be more severe on HeLa cells and PGKs, both of which lacked surface integrin β3. A possible explanation for this is that integrin αVβ3 could play a role in attachment during the infection of Caki-1 cells, especially in the EXT1 KO context. Likewise, our integrin subunit KO studies were performed in the context of HS-expressing cells, which could mask a functionally redundant role shared by HS and certain integrin heterodimers.
Surprisingly, we found that KSHV infection was unaffected by perturbations in the integrin network in Caki-1 and HeLa cells despite the well-characterized roles that integrins α3β1, αVβ3, and αVβ5 play during infection of HFF and primary endothelial cells (reviewed in reference 4). However, these results are in agreement with those of several studies in which integrin-blocking reagents failed to inhibit KSHV infection of HEK293 and SLK cells (25, 32, 43). We found that Caki-1 and HeLa cells lacking either integrin αV or β1, abolishing the expression of 5 and 12 integrin heterodimers, respectively, were infected at a similar percentage as WT cells. We also found no infection defect in Caki-1 cells knocked out for both integrin subunits α3 and αV, effectively lacking integrins α3β1, αVβ3, and αVβ5. Furthermore, a panel of integrin ligands and RGD peptides had little or no effect on the percentage of Caki-1 cells, HeLa cells, or PGKs infected by KSHV.
Our CRISPR-Cas9 KO studies covered 16 of the 24 known integrin heterodimers, and we further determined that the remaining 8 heterodimers were not expressed in Caki-1 cells. It is still conceivable that an αV family and one or more β1 family integrins besides α3β1 are fully redundant receptors of KSHV in this system, although such a situation would not be consistent with several previous studies where a KSHV infection phenotype was recorded after targeting only a single integrin heterodimer with a blocking antibody (27, 29, 38).
It should be noted that the results of our experiments with HeLa cells may not be in agreement with those of a recent KSHV receptor study on a HeLa-derivative cell line that was misidentified as human salivary gland epithelial cells [HSG(HeLa)] (28; corrected in reference 45). That study reported that HSG(HeLa) cells were mostly resistant to infection, despite expressing HS, EphA2, xCT, and integrins α3β1 and αVβ5 (28, 45). Like our HeLa CCL-2 cells, HSG(HeLa) cells did not express integrin αVβ3. The rate of infection of HSG(HeLa) cells was greatly increased upon the expression of integrin β3, leading the authors of that study to conclude that integrin αVβ3 was a crucial receptor for KSHV in these cells (28, 45). The differing conclusions from that study and ours may be attributed to the experimental approaches used, since our work focused on depleting receptors from WT cells instead of overexpressing them. It is also possible that HSG(HeLa) cells and our HeLa CCL-2 cells are too divergent to be comparable, as it is unclear how far removed HSG(HeLa) cells were from parental HeLa strains.
The KSHV glycoprotein gB binds integrins through an RGD domain that mimics natural integrin ligands as well as a DLD (27, 29, 43, 52). In HFF and primary microvascular endothelial cells, this gB-integrin interaction is required to initiate the KSHV-induced signaling cascade through the activation of FAK and other downstream effectors that eventually lead to virion endocytosis (29–31). This leads to the outstanding question of how KSHV internalizes in Caki-1 and HeLa cells without the involvement of canonical integrin receptors. We hypothesized that KSHV might directly induce endocytosis through EphA2, mimicking natural ephrin ligand-receptor-binding events. Several studies reported the phosphorylation of EphA2 during KSHV infection and suggested that the cytoplasmic domain of EphA2 is essential to propagate KSHV-induced signaling events and recruit effectors of macropinocytosis and clathrin-mediated endocytosis, but this idea has never been directly tested in the context of EPHA2 KO cells (32–36, 41). While we found that infection of Caki-1 and HeLa cells required EphA2, remarkably, an EphA2 construct truncated after the TM domain rescued infection in EPHA2 KO cells as efficiently as the full-length EphA2 construct. Furthermore, infection of PGK cells was unaffected by competitive blocking with ephrin-A4, which was previously shown to efficiently inhibit infection in multiple cell types (32, 40–42). It is possible that in certain cellular contexts, EphA2-mediated signaling is not necessary for infection but could be activated or recruit effector proteins as a bystander effect.
Together, our results suggest that in Caki-1 cells, HeLa cells, and PGKs, KSHV does not trigger the same integrin-EphA2 signaling axis that is so crucial for entry into HFF and primary microvascular endothelial cells. However, this conclusion must be rectified with the strong infection defect that we observed in EPHA2 KO Caki-1 and HeLa cells. The KSHV membrane glycoprotein gH/gL binds strongly to EphA2 (32, 37, 40, 42), so one interpretation of our data is simply that the ectodomain of EphA2 acts as an adhesion receptor in the cellular context of Caki-1 and HeLa cells. EphA2 could also have this function in PGKs, but functional redundancy with another protein may mask its role.
Taken together with data from our experiments investigating potential roles for different Eph receptors during KSHV infection, more speculative hypotheses can also be made. The result that we were able to further inhibit infection of EPHA2 KO cells with ephrin-A4 suggests that another factor that is blocked by ephrin-A4, most likely an Eph receptor, promotes KSHV infection. We ruled out that endogenous EphA4 and EphB2 were necessary for infection of Caki-1 cells but also found that these Eph receptors were not expressed by HeLa cells. This is important because we found that HeLa cells exhibited even more EphA2-independent KSHV infection than Caki-1 cells. It is possible that additional Eph receptors are expressed by both cell lines and affect KSHV infection in EPHA2 KO and WT contexts.
In support of this idea, we demonstrated that transduced EphA4 and EphA5 constructs rescued infection rates in EPHA2 KO cells to levels comparable to those with transduced EphA2. In fact, at small amounts of surface expression, EphA4 and EphA5 constructs outperformed EphA2 in this assay. It is unclear why endogenous EphA4 is dispensable for and perhaps even inhibits infection in the endogenous setting, while it promoted KSHV infection in the overexpression context. Spliced or modified forms of EphA4 produced from the endogenous gene could account for this discrepancy. Alternatively, EphA4 may be part of a homeostatic network that ultimately impacts KSHV infection efficiency, and cellular adaptation to the loss of EphA4 could be responsible for the KO phenotype. Whether EphA4, EphA5, and other Ephs act as true cellular receptors for KSHV or otherwise regulate KSHV infection should be further investigated.
A striking property of Eph receptors is that they form heterotetramers with their ligands as well as large oligomerized arrays through Eph-Eph interactions in their ectodomains, which are critical to trigger forward signaling in response to ligands (56–59). These signaling arrays can contain multiple types of Eph receptors, Eph receptors that are not bound to ligands, and Eph receptor ectodomains (56, 57, 59–61). Moreover, Eph cluster size and composition and the presence of alternatively spliced Eph receptor forms may all influence the cellular outcomes of Eph signaling (62–64). Importantly, one study showed that the ectodomain of EphA2 was sufficient to localize the protein to cell-cell contacts (56), and another study of chimeric EphA2 and EphA4 constructs suggested that the ectodomain may be a stronger determinant of cellular responses than the attached cytoplasmic domain (58). Thus, it is conceivable that in the presence of other signaling-competent Eph receptors, the ectodomain of EphA2 could promote clustering and signaling during KSHV infection just as well as the full-length receptor, as we observed in our experiments.
However, it is also possible that an unknown factor—not an Eph receptor—is responsible for virion internalization and EphA2-independent infection in Caki-1 and HeLa cells. In support of this, a new study identified the motif in gH of KSHV and rhesus rhadinovirus (RRV) that is required for Eph receptor binding (42). When this motif was mutated, de novo KSHV infection of HFF and endothelial cells was severely attenuated at the postattachment stage but was not completely blocked (42). Additionally, infection of HFFs, endothelial cells, and SLK/Caki-1 cells with this mutant could no longer be blocked with soluble forms of EphA2 or ephrin-A4 (42). Not only are data from that study consistent with our EPHA2 KO data in Caki-1 and HeLa cells, the existence of another KSHV receptor may explain why infection of PGK cells was not inhibited by soluble ephrin-A4. The hypothesis of an unknown receptor is also not necessarily exclusive to the potential involvement of other Eph receptors.
It is still unclear why targeting EphA2 with either CRISPR-Cas9 or ephrin-A4 had such differential effects on Caki-1 and HeLa cells versus PGKs. Eph receptor signaling is known to be cell type dependent, and therefore, the functional availability of EphA2 in the cell membrane or its intracellular signaling outcomes may naturally differ in PGKs. However, EphA2 has also been found to be upregulated in many types of solid tumors, and its intracellular signaling functions may also be dysregulated in this context, possibly complicating the interpretation of our results in the Caki-1 and HeLa cell lines (reviewed in references 65 and 66).
Interestingly, two independent groups recently discovered that EphA2 is a receptor for the gammaherpesvirus Epstein-Barr virus (EBV) on epithelial cells (67, 68). While integrins αVβ5, αVβ6, and αVβ8 had previously been identified as epithelial cell receptors for EBV (69, 70), one group demonstrated with CRISPR-Cas9 KO cells that αV family integrins were not required for EBV glycoprotein-mediated fusion with HEK293 cells (67). Furthermore, those studies demonstrated that the kinase activity of EphA2 and, indeed, the entire intracellular domain were dispensable for EBV glycoprotein fusion and infection, respectively (67, 68). These results are strikingly similar to the findings that we report here, and further characterization of both infection mechanisms may reveal informative parallels in receptor use between these two related gammaherpesviruses.
Given the importance of epithelial cell infection for host colonization, it will be valuable to further characterize KSHV entry in the absence of canonical integrin receptors and its impact on the viral life cycle. Our data support the notion that KSHV receptor usage and entry mechanisms vary widely between cell types. We propose that KSHV infection is not restricted by integrin and EphA2 expression and that the virus may utilize several members of both the integrin and Eph receptor families in various combinations for entry into a broad variety of cell types throughout the body. Modern gene-editing technologies such as CRISPR-Cas9 will facilitate detailed studies of KSHV receptors in the future and have the potential to rapidly expand the field of virus-host interactions.
MATERIALS AND METHODS
Cell lines and culture.
SLK/Caki-1 (ATCC HTB-46) cells were a gift from D. Ganem. HeLa cells (ATCC CCL-2) were obtained from the UC Berkeley BDS Cell Culture Facility. HEK293T cells (ATCC CRL-1573), Phoenix cells (ATCC CRL-3213), and primary gingival keratinocytes (ATCC PCS-200-014) were purchased from the ATCC. Primary gingival keratinocytes were grown in dermal cell basal medium (ATCC PCS-200-030) supplemented with a keratinocyte growth kit (ATCC PCS-200-040) at 37°C with 5% CO2. All other cells were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 5% fetal bovine serum (FBS; SeraDigm) at 37°C with 5% CO2.
KSHV production and infection.
iSLK.Bac16 (gift from J. U. Jung, USC) (71) cells harboring latent KSHV.BAC16 infection were cultured under selection with 1.2 mg/ml of hygromycin B (Invitrogen). The cells were induced to produce virus with 1 mM sodium butyrate (Alfa Aesar) and 1 μg/ml doxycycline (Sigma-Aldrich). Three days after reactivation, the supernatant was collected and filtered through a 0.45-μm syringe filter. The unconcentrated viral supernatant was stored at 4°C and diluted with standard culture medium for use in infection experiments. The dilution was calculated for each batch for an infection percentage of ∼30% on WT Caki-1 cells after 24 h of infection, measured in GFP+ events by flow cytometry. Cells were incubated with virus for 12 to 24 h, and the viral supernatant was then removed and replaced with fresh medium until analysis at 2 days postinfection. For blocking experiments, cells were incubated with virus for only 2 h and then washed and grown in fresh medium.
CRISPR-Cas9 genome editing.
Guide sequences were designed by using the CRISPR Design online tool (http://crispr.mit.edu/) and are provided in Table 1. A 5′ G was added to sequences that did not already contain one, and the appropriate adaptors were then appended to both forward and reverse oligonucleotides to facilitate cloning into px330 (Addgene plasmid 42230) according to the protocol provided at the Zhang laboratory website (http://genome-engineering.org/). Assembled px330 plasmids were transfected into cells of interest, and mutant cells were sorted by FACS or subcloned to obtain genetic KO cell pools or cell lines, respectively.
Antibodies.
Heparan sulfate antibody (F58-10E4) was purchased from Amsbio; integrin α3 antibody (P1B5) was purchased from Calbiochem; integrin αV, integrin β7, EphA2, and EphA5 antibodies (MAB12191, MAB4669, AF3035, and MAB541, respectively) were obtained from R&D Systems; integrin β1 and integrin β3 antibodies (T2S/16 and PM6/13, respectively) were obtained from Novus Biologicals; integrin β5 and EphA2 antibodies (AST-3T and SHM16, respectively) were obtained from BioLegend; xCT and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (ab37185 and ab181602, respectively) were obtained from Abcam; DC-SIGN antibody (DCN47.5) was obtained from Miltenyi Biotec; EphA4 antibody (4C8H5) was obtained from ThermoFisher; EphB2 antibody (2D12C6) was obtained from Santa Cruz Biotech; and Flag antibody (M2) was obtained from Sigma-Aldrich. Purified isotype control antibodies (MAB002, MAB003, MAB004, AB-105-C, and MAB006) were purchased from R&D Systems, except that mouse IgMκ (MM-30) was obtained from BioLegend.
Blocking reagents.
Ephrin-A4–Fc and EGFR-Fc were purchased from R&D Systems. GRGDSP and GRGESP peptides were purchased from Anaspec. Human fibronectin and mouse laminin were purchased from Corning.
Constructs and cloning.
Eph receptors were amplified from BJAB (EphA4 and EphA5) or Caki-1 (EphA2) cDNA and directly cloned into pQCXIN (Clontech) or cloned into p3xFlag-CMV-9 (Sigma-Aldrich) and subsequently cloned into pQCXIN to add an N-terminal 3×Flag tag preceded by the preprotrypsin leader sequence. Truncation mutants were amplified with a reverse primer in the indicated position containing an artificial stop codon. Chimeric TM domain EphA2 constructs were made using splicing by overhang extension PCR (SOEing PCR).
Transfection and transduction.
Caki-1 and HeLa cells were transfected with px330, and Phoenix cells were transfected with pQCXIN-based constructs using Fugene transfection reagent (Promega) and Opti-MEM (Gibco) according to the manufacturers' instructions. After 2 to 3 days, retrovirus was collected from the Phoenix cell supernatant and filtered through a 0.45-μm filter. The filtered retroviral supernatant was applied to target cells with 6 μg/ml Polybrene (Santa Cruz) and spinfected at 500 × g for 2 h at room temperature. Transduced cells were selected with neomycin (Fisher Scientific) at 1.2 mg/ml.
Flow cytometry and sorting.
Cells were harvested with trypsin (Gibco) or phosphate-buffered saline (PBS) (Gibco) plus 2 μM EDTA (Fisher) when staining for trypsin-sensitive epitopes. Cells were blocked, stained, and washed in 1% bovine serum albumin (BSA; Fisher) in PBS. When applicable, cells were fixed in 4% paraformaldehyde (PFA; ThermoFisher Pierce) in PBS and permeabilized with 0.25% Triton X-100 (EM Science) in PBS. Live cells were stained with 4′,6-diamidino-2-phenylindole (DAPI; BioLegend), and fixed cells were stained with Ghost dye violet 510 (Tonbo Biosciences) for viability according to the manufacturers' instructions. Cells were analyzed using an LSR Fortessa or LSR Fortessa X-20 cell analyzer (BD Biosciences) and sorted using a BD Influx or BD FACSAria fusion cell sorter (BD Biosciences). Data were processed and visualized with FlowJo 10 (BD Biosciences).
Western blotting.
Cells were harvested by scraping in PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (150 mM sodium chloride, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, and a protease inhibitor cocktail [Roche]). The protein concentration in the lysate was quantified by a bicinchoninic acid (BCA) assay (ThermoFisher Pierce). Lysates were run on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. A buffer containing 3% BSA and 10% FBS in TBST (20 mM Tris, 150 mM sodium chloride, 0.1% Tween 20) was used for blocking and primary antibody incubation. Plain TBST was used for washing and secondary antibody incubation. Blots were visualized with IRDye 800CW and 680RD secondary antibodies from Li-Cor Biosciences using a Li-Cor Odyssey infrared scanner and analyzed in ImageStudio Lite 5.2 (Li-Cor Biosciences).
Statistical analysis.
The indicated data sets were compared using Student's t test in Prism 7 (GraphPad). A P value of <0.05 is denoted with an asterisk in the figures.
Supplementary Material
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We thank Hector Nolla and Alma Valeros from the University of California, Berkeley, Flow Cytometry Core Facility for technical assistance and advice and Ella Hartenian and Britt Glaunsinger for helpful data analysis, which helped guide the experiments. We additionally thank Ellen Robey, Eva Harris, Trever Greene, Andrew Birnberg, Kristina Geiger, and Valerie Vargas-Zapata for enlightening discussion about this project.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.00803-18.
REFERENCES
- 1.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol 72:5182–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackbourn DJ, Lennette E, Klencke B, Moses A, Chandran B, Weinstein M, Glogau RG, Witte MH, Way DL, Kutzkey T, Herndier B, Levy JA. 2000. The restricted cellular host range of human herpesvirus 8. AIDS 14:1123–1133. doi: 10.1097/00002030-200006160-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bechtel JT, Liang Y, Hvidding J, Ganem D. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J Virol 77:6474–6481. doi: 10.1128/JVI.77.11.6474-6481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar B, Chandran B. 2016. KSHV entry and trafficking in target cells—hijacking of cell signal pathways, actin and membrane dynamics. Viruses 8:305. doi: 10.3390/v8110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Tschachler E, Colombini S, Ensoli B, Stürzl M. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol 71:7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol 176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 7.Rappocciolo G, Jais M, Piazza PA, DeLucia DC, Jenkins FJ, Rinaldo CR. 2017. Human herpesvirus 8 infects and replicates in Langerhans cells and interstitial dermal dendritic cells and impairs their function. J Virol 91:e00909-. doi: 10.1128/JVI.00909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesri EA, Cesarman E, Arvanitakis L, Rafii S, Moore MA, Posnett DN, Knowles DM, Asch AS. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med 183:2385–2390. doi: 10.1084/jem.183.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackbourn DJ, Ambroziak J, Lennette E, Adams M, Ramachandran B, Levy JA. 1997. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 349:609–611. doi: 10.1016/S0140-6736(96)10004-0. [DOI] [PubMed] [Google Scholar]
- 10.Minhas V, Wood C. 2014. Epidemiology and transmission of Kaposi's sarcoma-associated herpesvirus. Viruses 6:4178–4194. doi: 10.3390/v6114178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerimele F, Curreli F, Ely S, Friedman-Kien AE, Cesarman E, Flore O. 2001. Kaposi's sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J Virol 75:2435–2443. doi: 10.1128/JVI.75.5.2435-2443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duus K, Lentchitsky V, Wagenaar T, Grose C, Webster-Cyriaque J. 2004. Wild-type Kaposi's sarcoma-associated herpesvirus isolated from the oropharynx of immune-competent individuals has tropism for cultured oral epithelial cells. J Virol 78:4074–4084. doi: 10.1128/JVI.78.8.4074-4084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AS, Maronian N, Vieira J. 2005. Activation of Kaposi's sarcoma-associated herpesvirus lytic gene expression during epithelial differentiation. J Virol 79:13769–13777. doi: 10.1128/JVI.79.21.13769-13777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifi A, Weaver EM, Whipple ME, Ikoma M, Farrenberg J, Huang ML, Vieira J. 2011. The lytic activation of KSHV during keratinocyte differentiation is dependent upon a suprabasal position, the loss of integrin engagement, and calcium, but not the interaction of cadherins. Virology 410:17–29. doi: 10.1016/j.virol.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Gong D, Wu NC, Xie Y, Feng J, Tong L, Brulois KF, Luan H, Du Y, Jung JU, Wang CY, Kang MK, Park NH, Sun R, Wu TT. 2014. Kaposi's sarcoma-associated herpesvirus ORF18 and ORF30 are essential for late gene expression during lytic replication. J Virol 88:11369–11382. doi: 10.1128/JVI.00793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagas CA, Endo LH, Sakano E, Pinto GA, Brousset P, Vassallo J. 2006. Detection of herpesvirus type 8 (HHV8) in children's tonsils and adenoids by immunohistochemistry and in situ hybridization. Int J Pediatr Otorhinolaryngol 70:65–72. doi: 10.1016/j.ijporl.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Akula SM, Wang FZ, Vieira J, Chandran B. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245–255. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- 18.Akula SM, Pramod NP, Wang FZ, Chandran B. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235–249. doi: 10.1006/viro.2001.0921. [DOI] [PubMed] [Google Scholar]
- 19.Wang FZ, Akula SM, Pramod NP, Zeng L, Chandran B. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J Virol 75:7517–7527. doi: 10.1128/JVI.75.16.7517-7527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birkmann A, Mahr K, Ensser A, Yağuboğlu S, Titgemeyer F, Fleckenstein B, Neipel F. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J Virol 75:11583–11593. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark L, Lee WH, Spiller OB, Villoutreix BO, Blom AM. 2006. The Kaposi's sarcoma-associated herpesvirus complement control protein (KCP) binds to heparin and cell surfaces via positively charged amino acids in CCP1-2. Mol Immunol 43:1665–1675. doi: 10.1016/j.molimm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Spiller OB, Mark L, Blue CE, Proctor DG, Aitken JA, Blom AM, Blackbourn DJ. 2006. Dissecting the regions of virion-associated Kaposi's sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding. J Virol 80:4068–4078. doi: 10.1128/JVI.80.8.4068-4078.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn A, Birkmann A, Wies E, Dorer D, Mahr K, Stürzl M, Titgemeyer F, Neipel F. 2009. Kaposi's sarcoma-associated herpesvirus gH/gL: glycoprotein export and interaction with cellular receptors. J Virol 83:396–407. doi: 10.1128/JVI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar B, Roy A, Veettil MV, Chandran B. 2018. Insight into the roles of E3 ubiquitin ligase c-Cbl, ESCRT machinery, and host cell signaling in Kaposi's sarcoma-associated herpesvirus entry and trafficking. J Virol 92:e01376-. doi: 10.1128/JVI.01376-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue N, Winter J, Lal RB, Offermann MK, Koyano S. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J Virol 77:8147–8152. doi: 10.1128/JVI.77.14.8147-8152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaleeba JA, Berger EA. 2006. Broad target cell selectivity of Kaposi's sarcoma-associated herpesvirus glycoprotein-mediated cell fusion and virion entry. Virology 354:7–14. doi: 10.1016/j.virol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Garrigues HJ, Rubinchikova YE, Dipersio CM, Rose TM. 2008. Integrin alphaVbeta3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J Virol 82:1570–1580. doi: 10.1128/JVI.01673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrigues HJ, DeMaster LK, Rubinchikova YE, Rose TM. 2014. KSHV attachment and entry are dependent on αVβ3 integrin localized to specific cell surface microdomains and do not correlate with the presence of heparan sulfate. Virology 464–465:118–133. doi: 10.1016/j.virol.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akula SM, Pramod NP, Wang FZ, Chandran B. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407–419. doi: 10.1016/S0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 30.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol 77:1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol 78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, Schmidt K, Holzer A, Schmidt M, Chen J, König S, Ensser A, Myoung J, Brockmeyer NH, Stürzl M, Fleckenstein B, Neipel F. 2012. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi's sarcoma-associated herpesvirus. Nat Med 18:961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty S, Veettil MV, Bottero V, Chandran B. 2012. Kaposi's sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection. Proc Natl Acad Sci U S A 109:E1163–E1172. doi: 10.1073/pnas.1119592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta D, Chakraborty S, Bandyopadhyay C, Valiya Veettil M, Ansari MA, Singh VV, Chandran B. 2013. EphrinA2 regulates clathrin mediated KSHV endocytosis in fibroblast cells by coordinating integrin-associated signaling and c-Cbl directed polyubiquitination. PLoS Pathog 9:e1003510. doi: 10.1371/journal.ppat.1003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandyopadhyay C, Valiya-Veettil M, Dutta D, Chakraborty S, Chandran B. 2014. CIB1 synergizes with EphrinA2 to regulate Kaposi's sarcoma-associated herpesvirus macropinocytic entry in human microvascular dermal endothelial cells. PLoS Pathog 10:e1003941. doi: 10.1371/journal.ppat.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandyopadhyay C, Veettil MV, Dutta S, Chandran B. 2014. p130Cas scaffolds the signalosome to direct adaptor-effector cross talk during Kaposi's sarcoma-associated herpesvirus trafficking in human microvascular dermal endothelial cells. J Virol 88:13858–13878. doi: 10.1128/JVI.01674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn AS, Desrosiers RC. 2014. Binding of the Kaposi's sarcoma-associated herpesvirus to the ephrin binding surface of the EphA2 receptor and its inhibition by a small molecule. J Virol 88:8724–8734. doi: 10.1128/JVI.01392-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veettil MV, Sadagopan S, Sharma-Walia N, Wang FZ, Raghu H, Varga L, Chandran B. 2008. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J Virol 82:12126–12144. doi: 10.1128/JVI.01146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari V, Darmani NA, Thrush GR, Shukla D. 2009. An unusual dependence of human herpesvirus-8 glycoproteins-induced cell-to-cell fusion on heparan sulfate. Biochem Biophys Res Commun 390:382–387. doi: 10.1016/j.bbrc.2009.08.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn AS, Desrosiers RC. 2013. Rhesus monkey rhadinovirus uses Eph family receptors for entry into B cells and endothelial cells but not fibroblasts. PLoS Pathog 9:e1003360. doi: 10.1371/journal.ppat.1003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zou Z, Deng Z, Liang D, Zhou X, Sun R, Lan K. 2017. Male hormones activate EphA2 to facilitate Kaposi's sarcoma-associated herpesvirus infection: implications for gender disparity in Kaposi's sarcoma. PLoS Pathog 13:e1006580. doi: 10.1371/journal.ppat.1006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Großkopf AK, Ensser A, Neipel F, Jungnickl D, Schlagowski S, Desrosiers RC, Hahn AS. 2018. A conserved Eph family receptor-binding motif on the gH/gL complex of Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus. PLoS Pathog 14:e1006912. doi: 10.1371/journal.ppat.1006912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker LR, Hussein HA, Akula SM. 2014. Disintegrin-like domain of glycoprotein B regulates Kaposi's sarcoma-associated herpesvirus infection of cells. J Gen Virol 95:1770–1782. doi: 10.1099/vir.0.066829-0. [DOI] [PubMed] [Google Scholar]
- 44.Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. 2006. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J Virol 80:11432–11446. doi: 10.1128/JVI.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrigues HJ, DeMaster LK, Rubinchikova YE, Rose TM. 2018. Corrigendum to: “KSHV attachment and entry are dependent on αVβ3 integrin localized to specific cell surface microdomains and do not correlate with the presence of heparan sulfate” [Virology 464–465 (2014) 118–133]. Virology 515:264–265. doi: 10.1016/j.virol.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stürzl M, Gaus D, Dirks WG, Ganem D, Jochmann R. 2013. Kaposi's sarcoma-derived cell line SLK is not of endothelial origin, but is a contaminant from a known renal carcinoma cell line. Int J Cancer 132:1954–1958. doi: 10.1002/ijc.27849. [DOI] [PubMed] [Google Scholar]
- 47.TerBush AA, Hafkamp F, Lee HJ, Coscoy L. 2018. A Kaposi's sarcoma-associated herpesvirus infection mechanism is independent of integrins α3β1, αVβ3, and αVβ5. bioRxiv doi: 10.1101/270108. [DOI] [PMC free article] [PubMed]
- 48.Jarousse N, Chandran B, Coscoy L. 2008. Lack of heparan sulfate expression in B-cell lines: implications for Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 infections. J Virol 82:12591–12597. doi: 10.1128/JVI.01167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brulois KF, Chang H, Lee AS, Ensser A, Wong LY, Toth Z, Lee SH, Lee HR, Myoung J, Ganem D, Oh TK, Kim JF, Gao SJ, Jung JU. 2012. Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone. J Virol 86:9708–9720. doi: 10.1128/JVI.01019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol 77:7978–7990. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerur N, Veettil MV, Sharma-Walia N, Sadagopan S, Bottero V, Paul AG, Chandran B. 2010. Characterization of entry and infection of monocytic THP-1 cells by Kaposi's sarcoma associated herpesvirus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology 406:103–116. doi: 10.1016/j.virol.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang FZ, Akula SM, Sharma-Walia N, Zeng L, Chandran B. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J Virol 77:3131–3147. doi: 10.1128/JVI.77.5.3131-3147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitulescu ME, Adams RH. 2010. Eph/ephrin molecules—a hub for signaling and endocytosis. Genes Dev 24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bocharov EV, Mayzel ML, Volynsky PE, Goncharuk MV, Ermolyuk YS, Schulga AA, Artemenko EO, Efremov RG, Arseniev AS. 2008. Spatial structure and pH-dependent conformational diversity of dimeric transmembrane domain of the receptor tyrosine kinase EphA1. J Biol Chem 283:29385–29395. doi: 10.1074/jbc.M803089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garrigues HJ, Rubinchikova YE, Rose TM. 2014. KSHV cell attachment sites revealed by ultra sensitive tyramide signal amplification (TSA) localize to membrane microdomains that are up-regulated on mitotic cells. Virology 452–453:75–85. doi: 10.1016/j.virol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY. 2010. An extracellular steric seeding mechanism for Eph-ephrin signalling platform assembly. Nat Struct Mol Biol 17:398–402. doi: 10.1038/nsmb.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. 2010. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A 107:10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seiradake E, Schaupp A, del Toro Ruiz D, Kaufmann R, Mitakidis N, Harlos K, Aricescu AR, Klein R, Jones EY. 2013. Structurally encoded intraclass differences in EphA clusters drive distinct cell responses. Nat Struct Mol Biol 20:958–964. doi: 10.1038/nsmb.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu K, Tzvetkova-Robev D, Xu Y, Goldgur Y, Chan YP, Himanen JP, Nikolov DB. 2013. Insights into Eph receptor tyrosine kinase activation from crystal structures of the EphA4 ectodomain and its complex with ephrin-A5. Proc Natl Acad Sci U S A 110:14634–14639. doi: 10.1073/pnas.1311000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PIH, Lackmann M. 2004. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol 164:661–666. doi: 10.1083/jcb.200312001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janes PW, Griesshaber B, Atapattu L, Nievergall E, Hii LL, Mensinga A, Chheang C, Day BW, Boyd AW, Bastiaens PI, Jørgensen C, Pawson T, Lackmann M. 2011. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J Cell Biol 195:1033–1145. doi: 10.1083/jcb.201104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holmberg J, Clarke DL, Frisén J. 2000. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- 63.Greene AC, Lord SJ, Tian A, Rhodes C, Kai H, Groves JT. 2014. Spatial organization of EphA2 at the cell-cell interface modulates trans-endocytosis of ephrinA1. Biophys J 106:2196–2205. doi: 10.1016/j.bpj.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaupp A, Sabet O, Dudanova I, Ponserre M, Bastiaens P, Klein R. 2014. The composition of EphB2 clusters determines the strength in the cellular repulsion response. J Cell Biol 204:409–422. doi: 10.1083/jcb.201305037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beauchamp A, Debinski W. 2012. Ephs and ephrins in cancer: ephrin-A1 signalling. Semin Cell Dev Biol 23:109–115. doi: 10.1016/j.semcdb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wykosky J, Debinski W. 2008. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res 6:1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS, Longnecker R. 2018. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat Microbiol 3:172–180. doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, Dong XD, Li SB, Du Y, Xiong D, He JY, Li MZ, Liu YM, Zhou AJ, Zhong Q, Zeng YX, Kieff E, Zhang Z, Gewurz BE, Zhao B, Zeng MS. 2018. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat Microbiol 3:164–171. doi: 10.1038/s41564-017-0080-8. [DOI] [PubMed] [Google Scholar]
- 69.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. 2009. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci U S A 106:20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chesnokova LS, Hutt-Fletcher LM. 2011. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J Virol 85:13214–13223. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myoung J, Ganem D. 2011. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J Virol Methods 174:12–21. doi: 10.1016/j.jviromet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hynes RO. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.