Despite the availability of vaccines, annual influenza virus epidemics cause 250,000 to 500,000 deaths worldwide. Currently licensed inactivated vaccines, which are standardized for the amount of the hemagglutinin (HA) antigen, primarily induce strain-specific antibodies, whereas the immune response to the neuraminidase (NA) antigen, which is also present on the viral surface, is usually low. Using NA-expressing single-cycle vesicular stomatitis virus replicons, we show that the NA antigen conferred protection of mice and ferrets against not only the matched influenza virus strains but also viruses carrying NA proteins from other strains of the same subtype. The extent of protection correlated with the level of cross-reactive NA-inhibiting antibodies. This highlights the potential of the NA antigen for the development of more broadly protective influenza vaccines. Such vaccines may also provide partial protection against newly emerging strains with the same NA but a different HA subtype.
KEYWORDS: correlates of protection, functional antibodies, influenza A virus, neuraminidase protein, VSV replicon vaccine platform
ABSTRACT
Immune responses induced by currently licensed inactivated influenza vaccines are mainly directed against the hemagglutinin (HA) glycoprotein, the immunodominant antigen of influenza viruses. The resulting antigenic drift of HA requires frequent updating of the vaccine composition and annual revaccination. On the other hand, the levels of antibodies directed against the neuraminidase (NA) glycoprotein, the second major influenza virus antigen, vary greatly. To investigate the potential of the more conserved NA protein for the induction of subtype-specific protection, vesicular stomatitis virus-based replicons expressing a panel of N1 proteins from prototypic seasonal and pandemic H1N1 strains and human H5N1 and H7N9 isolates were generated. Immunization of mice and ferrets with the replicon carrying the matched N1 protein resulted in robust humoral and cellular immune responses and protected against challenge with the homologous influenza virus with an efficacy similar to that of the matched HA protein, illustrating the potential of the NA protein as a vaccine antigen. The extent of protection after immunization with mismatched N1 proteins correlated with the level of cross-reactive neuraminidase-inhibiting antibody titers. Passive serum transfer experiments in mice confirmed that these functional antibodies determine subtype-specific cross-protection. Our findings illustrate the potential of NA-specific immunity for achieving broader protection against antigenic drift variants or newly emerging viruses carrying the same NA but a different HA subtype.
IMPORTANCE Despite the availability of vaccines, annual influenza virus epidemics cause 250,000 to 500,000 deaths worldwide. Currently licensed inactivated vaccines, which are standardized for the amount of the hemagglutinin (HA) antigen, primarily induce strain-specific antibodies, whereas the immune response to the neuraminidase (NA) antigen, which is also present on the viral surface, is usually low. Using NA-expressing single-cycle vesicular stomatitis virus replicons, we show that the NA antigen conferred protection of mice and ferrets against not only the matched influenza virus strains but also viruses carrying NA proteins from other strains of the same subtype. The extent of protection correlated with the level of cross-reactive NA-inhibiting antibodies. This highlights the potential of the NA antigen for the development of more broadly protective influenza vaccines. Such vaccines may also provide partial protection against newly emerging strains with the same NA but a different HA subtype.
INTRODUCTION
Influenza A viruses cause an acute respiratory infection that can affect up to 30% of the population during seasonal epidemics (1–4). The viruses undergo continuous antigenic drift to escape preexisting immune responses (5), necessitating the regular update of influenza vaccines. The hemagglutinin (HA) protein, which mediates viral attachment to the target cell and subsequent entry (6), is the main target of neutralizing antibodies and thus is subject to continuous antigenic drift. Consequently, the majority of antibodies directed against the HA protein are highly strain specific (5, 7). The neuraminidase (NA) protein, which facilitates viral egress, is more conserved (8). However, since the frequently used inactivated influenza vaccines are standardized only for HA protein content (9), the amount and quality of the NA protein are known to vary considerably among vaccine preparations and virus strains used (10, 11).
There is increasing evidence that NA-specific antibodies can provide protection from influenza viruses, including reports about broad binding and inhibition of NA activity (12–18). When administered separately, HA and NA proteins induced comparable total antibody titers (19). Immunization with NA alone decreased lung virus titers in mice and reduced severe clinical signs and viral shedding in patients (11, 19–22). The resulting antibody responses cross-reacted with other NA proteins of the same subtype, and NA-specific immunity protected mice from infection with antigenic drift variants but not from strains carrying a heterosubtypic NA protein (11, 23). The extent of protection from strains of the same NA subtype seemed to correlate with the phylogenetic distance between the vaccine and challenge virus NA proteins (12, 24). The level of cross-reacting neuraminidase-inhibiting antibodies has thus been proposed as an indicator for protection (25).
Vesicular stomatitis virus (VSV) is an attractive vector for in vivo antigen expression and vaccine development (26–28). Due to the affinity of the VSV glycoprotein (G) for the low-density lipoprotein (LDL) receptor, it is able to infect and replicate in a variety of tissues, thereby eliciting strong humoral and cellular immune responses (29). The lack of preexisting immunity and clinical disease associated with VSV infection in humans has led to the extensive exploration of this vaccine platform. A notable example is the VSV-Ebola virus (EBOV) vaccine candidate, for which efficacy has recently been reported (30–36). In contrast to propagation-competent VSV vectors, VSV replicons, which lack the G protein gene, have an improved biosafety profile. They can be amplified in G protein-expressing cells but perform only a single cycle of replication in all other cells (37). In chicken, immunization with influenza A virus NA-expressing single-cycle VSV replicons resulted in antibodies that efficiently inhibited the sialidase activity of the same subtype and prevented sustained viral shedding, highlighting the potential of NA-expressing VSV replicons as vaccine candidates (13).
To investigate the potential of the NA protein to confer protection in mammals against influenza virus strains carrying the same NA subtype, we generated single-cycle VSV replicon particles expressing different NA and HA proteins. After analysis of the immune response kinetics and the levels of cross-reactive antibodies in mice and ferrets, protective efficacy against challenge with H1N1 Puerto Rico/8/34 (PR8) and A/Mexico/InDRE4487/2009, respectively, was assessed. The contribution of antibodies to the observed protection was investigated with a passive transfer experiment in mice, and sialidase-inhibiting antibodies were identified as correlates of protection.
RESULTS
Generation of VSV replicon particles expressing the HA or NA proteins of various subtypes.
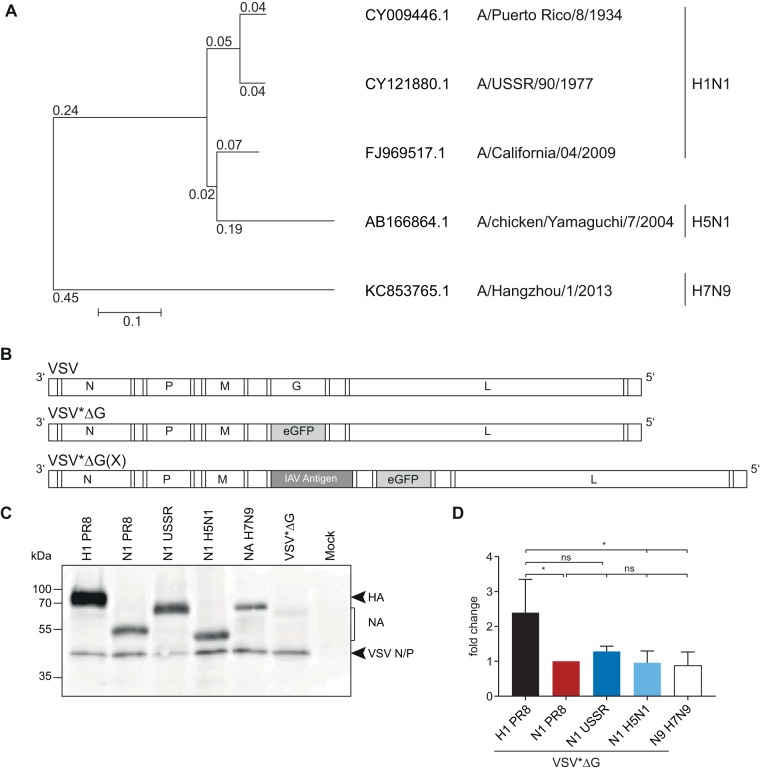
The majority of antibodies induced by inactivated influenza vaccines are directed against the HA protein and act in a strain-specific manner (5–7). To explore the protective potential of an immune response against the more conserved NA protein, we generated propagation-incompetent VSV by replacing the VSV G gene with either the HA or NA gene of PR8 (H1N1) or NA genes from prototype seasonal and pandemic H1N1 strains and a human H5N1 isolate with differing phylogenetic distances (Fig. 1A). An additional transcription cassette encoding the enhanced green fluorescence protein (eGFP) gene was added downstream of the influenza virus antigen (Fig. 1B) in order to ease the monitoring of infected cells. The resulting VSV replicon particles were successfully propagated on genetically modified BHK-21 cells that inducibly express the VSV G protein, yielding virus titers of up to 5 × 108 focus-forming units (FFU)/ml. Upon infection of parental baby hamster kidney (BHK) cells, no infectious particles were detected (data not shown), confirming that these vectors perform only a single round of replication (37). To assess the surface expression levels of the different NA proteins, Vero E6 cells were infected with the different replicons, followed by cell surface biotinylation and subsequent immunoprecipitation with the respective homologous mouse sera adjusted to the same antibody titer. Staining with peroxidase-labeled streptavidin revealed that the NAUSSR and NAH7N9 proteins migrated at a molecular mass of about 75 kDa, whereas the NAPR8 and NAH5N1 proteins were detected at around 55 kDa, reflecting the increased glycosylation of NAUSSR and NAH7N9. All NA proteins were expressed at comparable levels (Fig. 1C and D), suggesting similar antigen expression levels in vivo.
FIG 1.
Generation and characterization of influenza antigen-expressing VSV replicons. (A) Phylogenetic relationship of influenza A virus NA genes included in this study. Amino acid sequences were aligned using ClustalW and a phylogenetic tree constructed using the neighbor-joining method and 1,000 bootstrapped replications with observed amino acid differences as the distance on the aligned sequences using MEGA7. (B) Scheme of the genomes of the parental VSV and the recombinant VSV*ΔG replicons. VSV*ΔG lacks the gene encoding the VSV G protein and includes the eGFP gene in an additional transcription unit, depicted in gray. VSV*ΔG(X) encodes the respective heterologous influenza virus antigens, depicted in dark gray. (C) Vero E6 cells were surface biotin labeled with EZ-Link sulfo-NHS-biotin (Pierce) 12 h after infection with the respective VSV*ΔG replicons, followed by cell lysis and immunoprecipitation of 50 μg of cell extracts with homologous mouse antiserum. Proteins in the pellet fraction were separated on an SDS-PAGE gel, and surface biotin-labeled proteins were visualized using an avidin-HRP secondary antibody. (D) Protein bands from three independent replicates were quantified and normalized relative to NAPR8 for each blot and are shown as fold changes. Error bars represent the standard deviations of the means. Groups were compared using one-way ANOVA with a Tukey posttest, and statistical significance is indicated (*, P < 0.05; ns, not significant).
HA and NA induce robust antibody responses against the homologous strain.
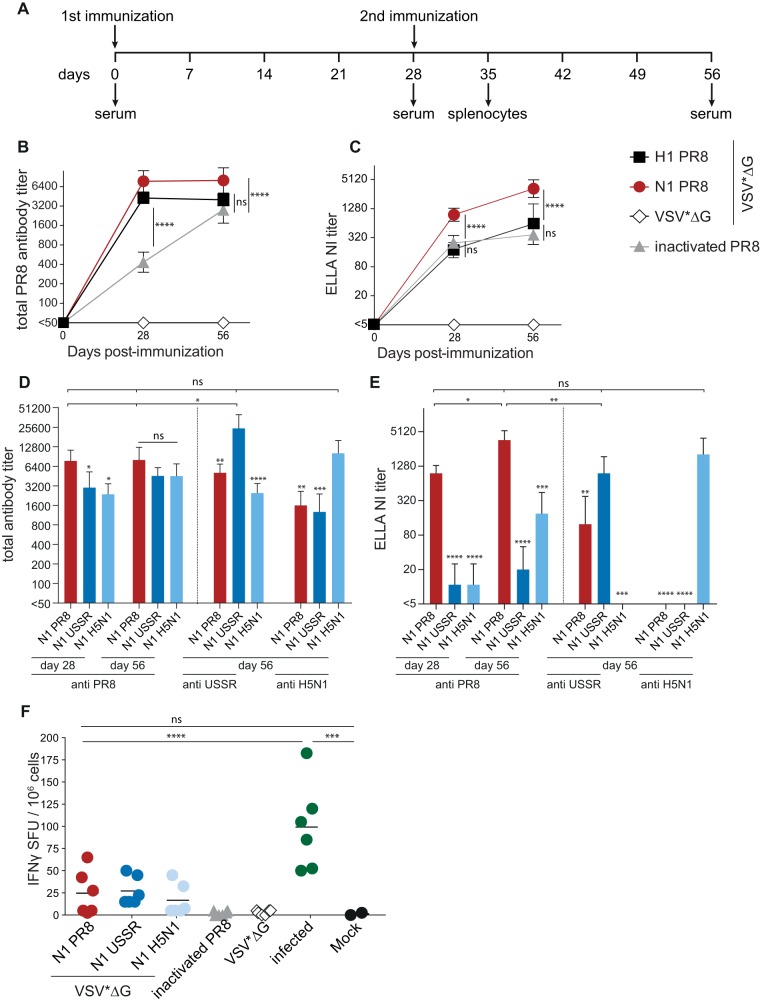
To evaluate the immunogenicity of NA-expressing VSV replicon particles, 6- to 8-week-old C57BL/6 mice were immunized intramuscularly with 106 FFU of VSV*ΔG(NAPR8) and boosted with the same dose 28 days later (Fig. 2A). VSV*ΔG, VSV*ΔG(HAPR8), and inactivated PR8 virus were included as controls. Intramuscular inoculation was chosen to reproduce the route of immunization of the seasonal inactivated influenza vaccine and to allow the side-by-side comparison of the elicited immune responses. Sera were collected at the indicated times, and total antibody titers directed against the homologous virus strain were quantified by an immunoperoxidase monolayer assay with PR8-infected Madin-Darby canine kidney (MDCK) cells (Fig. 2B). Mice immunized with the control replicon VSV*ΔG did not develop PR8-specific antibodies. Animals vaccinated with VSV*ΔG(HAPR8) or VSV*ΔG(NAPR8) produced comparable anti-influenza virus titers after the first immunization, which did not increase further after the boost. Compared to the replicon vaccines, antibody titers elicited by the inactivated vaccine were significantly low after the first immunization (P ≤ 0.0001) but increased after the second immunization, reaching levels similar to those for the VSV replicon vaccine groups.
FIG 2.
Humoral and cellular immune responses in mice induced by the different VSV*ΔG replicons. (A) Schematic overview of the immunization strategy and blood and splenocyte sampling time points. Six- to eight-week-old naive C57BL/6 mice were immunized intramuscularly twice with 106 FFU of the VSV*ΔG replicons or with 2.5 μg of BPL-inactivated PR8 virus. (B and C) Kinetics of total antibody titers (B) and 50% neuraminidase-inhibiting (NI) antibody titers (C) against PR8 in animals immunized with matched antigens. Symbols represent the means for each group (n = 6), and error bars indicate the standard deviations of the means. (D and E) Total antibody titers (D) and NI titers (E) against all PR8, USSR, and H5N1 viruses. Bars represent the means for each group (n = 6), and error bars indicate the standard deviations of the means. Log2-transformed groups were compared to the NAPR8 group using one-way ANOVA with a Tukey posttest. (F) IFN-γ ELISpot analysis results after restimulation with purified PR8 virus. H1N1 PR8-infected mice were used as positive controls, and noninfected mice were used as negative controls. Symbols indicate spot-forming units (SFU) for individual animals, and black bars represent the respective means. Groups were compared using one-way ANOVA with a Tukey posttest, and statistical significance is indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
To assess the functionality of NA-specific antibodies, their neuraminidase-inhibiting activity was quantified using an enzyme-linked lectin assay (ELLA) with whole PR8 virus as the sialidase source (Fig. 2C). Both VSV replicons and the inactivated vaccine induced robust NA-inhibiting (NI) antibody titers after the first immunization, and titers increased in all groups after the second immunization. However, titers of functional antibodies were significantly higher in the VSV*ΔG(NAPR8) group (P ≤ 0.0001), demonstrating that the NA-expressing VSV replicon vaccine is more effective in inducing neuraminidase-inhibiting antibodies. The NI antibody titers of the group immunized with the HA-expressing VSV replicon were similar to those for the inactivated virus, illustrating the extent of sterical interference of HA-specific antibodies with NA access to sialic acids and suggesting that HA-specific antibodies also contribute to the NI titer detected in the latter group.
VSV*ΔG(N1)s elicit functional subtype-specific antibodies.
Data from several studies suggest an inverse correlation between high NI antibody titers and reduced morbidity (25, 52). To systematically assess the breadth of NA-specific immune responses, mice were immunized twice with VSV replicons expressing the NA proteins of PR8, A/USSR/90/1977 (H1N1) (USSR) as a prototypic seasonal H1N1 strain, and A/chicken/Yamaguchi/7/2004 (H5N1) and A/Hangzhou/1/2013 (H7N9) as avian influenza viruses that cause severe disease in humans. For all N1-carrying replicons, total as well as NI antibody titers against the homologous virus were in the same range (Fig. 2D and E), indicating similar NA expression levels in vivo. Immunization with NAUSSR and NAH5N1 resulted in NA-specific antibodies that reacted with PR8-infected MDCK cells as efficiently as antibodies that were obtained following immunization with NAPR8 (Fig. 2D). In contrast, the matched antisera resulted in 5- to 10-fold-higher total antibody titers for USSR and H5N1, respectively (Fig. 2D). The NAUSSR- and NAH5N1-specific antisera also inhibited PR8 sialidase activity (Fig. 2E) but with lower efficacy than NAPR8-specific antibodies (P ≤ 0.0001). While the NAPR8-specific antisera displayed NI activity against USSR, no cross-reactivity was observed for the NAH5N1 group (anti-USSR) (Fig. 2E), and none of the heterologous antisera were able to inhibit the NAH5N1 sialidase activity (anti-H5N1) (Fig. 2E). Interestingly, NI antibody titers were detected in groups with total antibody titers above 3,200, suggesting that a correlation between total and NI antibodies could be established. No cross-reactive antibodies were detected after immunization with the heterologous N9 subtype (data not shown), suggesting that NA-specific antibodies cross-react only with NA proteins of the same subtype.
NA-expressing VSV replicons also induce influenza virus-specific cellular immune responses.
In contrast to other vaccine platforms, VSV replicons elicit not only humoral but also cellular immune responses (38). To evaluate the extent of cellular immune responses after immunization with NA-specific VSV*ΔGs, splenocytes of animals vaccinated with the different N1 vaccine candidates were isolated 7 days after the second immunization and analyzed for antigen-specific gamma interferon (IFN-γ) secretion by an enzyme-linked immunosorbent spot (ELISpot) assay using purified PR8 virus for restimulation (Fig. 2F). In samples from mice sacrificed 7 days after intranasal infection with 1 × 103 50% tissue culture infectious doses (TCID50) of PR8, 50 to 185 IFN-γ-secreting cells per 1 × 106 splenocytes were observed, while a background response below 3 IFN-γ-secreting cells was seen in naive control animals. PR8 stimulation of splenocytes from groups immunized with the inactivated vaccine or the VSV*ΔG control replicon resulted in IFN-γ-secreting cell numbers close to the background (0 to 5 positive cells). Following vaccination with the homologous VSV*ΔG(NAPR8), up to 65 spots per 1 × 106 splenocytes were observed, while the heterologous N1 antigens were associated with lower but still positive responses ranging between 10 and 80 spots per 106 splenocytes. However, these responses were not statistically different from the background, indicating that the protective effect associated with the NA antigen in this context is primarily antibody mediated.
N1-expressing VSV replicon particles partially protect mice from heterologous but not heterosubtypic virus challenge.
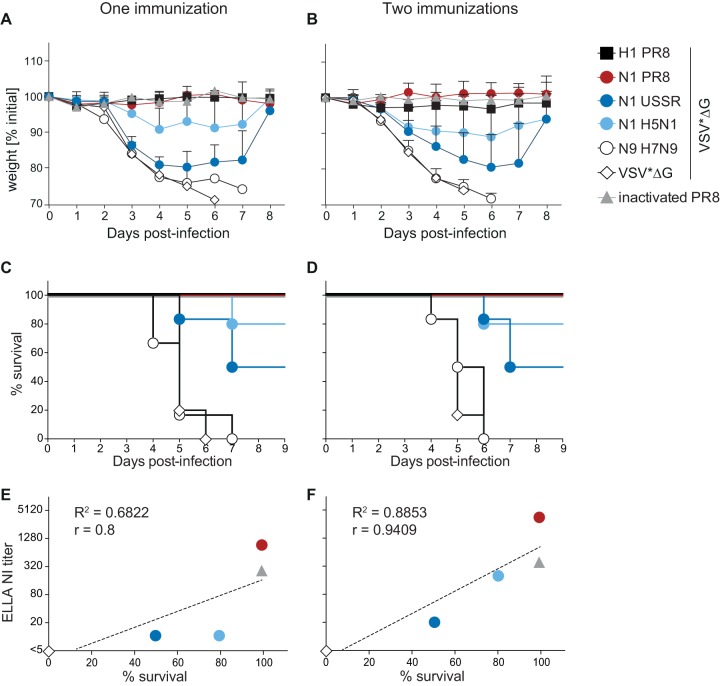
NA-specific immune responses have previously been shown to protect from homologous as well as heterologous challenge with a strain carrying an NA protein of the same subtype (11, 13, 23, 39). To determine the extent of protection and survival conferred by VSV replicon-based vaccines from challenge with heterologous virus strains, we infected C57BL/6 mice intranasally with 1 × 104 TCID50 PR8 4 weeks after the last immunization. Animals that received the homologous HA- or NA-expressing VSV*ΔG replicons or a matched inactivated vaccine were already fully protected from weight loss and mortality after a single immunization (Fig. 3A to D), while mice immunized with the VSV*ΔG control replicon or with VSV*ΔG(NAH7N9) reached humane endpoints within 6 to 7 days (Fig. 3A to D). A single immunization with the mismatched N1-expressing VSV*ΔG replicons resulted in partial protection from weight loss and mortality, specifically 50% for VSV*ΔG(NAUSSR) and 80% for VSV*ΔG(NAH5N1) (Fig. 3A and C), and a second immunization had no added beneficial effect (Fig. 3B and D). There was a clear correlation between mean NI antibody titers and percent survival for each group (Fig. 3E), and the correlation coefficient further improved after two immunizations (Fig. 3F), strengthening the link between sialidase-inhibiting antibodies and protection.
FIG 3.
Protective efficacy of the different vaccine candidates. Mice were vaccinated once or twice in 4-week intervals and then challenged with 1 × 104 TCID50 of H1N1 PR8 4 weeks later. Upon challenge, animals were monitored daily for clinical signs and body weight and euthanized as soon as the weight loss reached 25%. (A to D) Percent weight changes after challenge following one (A) or two (B) immunizations and the respective percent survivals (C and D) (n = 6; N1 H5N1, n = 5). (E and F) Correlation between mean NI titer and percent survival 4 weeks after the first (E) or the second (F) immunization.
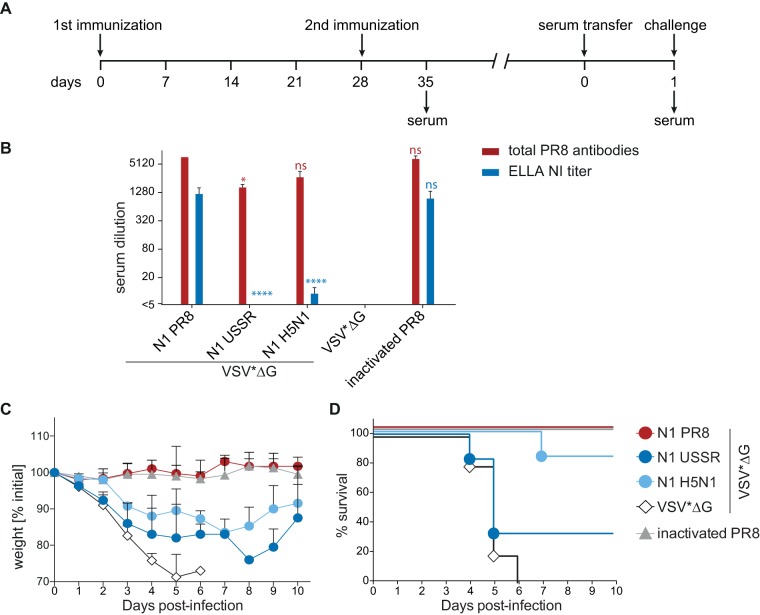
Functional NA-specific antibodies are sufficient for cross-protection.
To confirm that the observed cross-protection is due to functional NA-specific antibodies, sera from animals immunized twice with the respective VSV*ΔG replicons or inactivated PR8 were transferred into naive 6- to 8-week-old C57BL/6 mice (Fig. 4A). Total and NI antibody titers were measured in donor serum before transfer and 24 h after transfer in serum collected from recipient animals (Fig. 4B). The levels of PR8-binding and NI antibodies were similar to those in the above-described experiment (compare Fig. 2 and 4B). However, the NI antibody titers were generally lower than those observed previously, leading to titers below detection levels for VSV*ΔG(NAUSSR). The weight loss and survival rates of the recipient animals mirrored those of the originally vaccinated groups, with antisera directed against NAH5N1 being more protective than those against NAUSSR (compare Fig. 3B and D and 4C and D), demonstrating that subtype-specific cross-protection is largely antibody mediated.
FIG 4.
Efficacy of passive serum transfer. (A) Schematic overview of the experimental design. Mice were vaccinated on days 0 and 28 with the respective VSV*ΔG replicons, and serum was isolated 7 days after the boost immunization. Sera were subsequently transferred into naive mice. Blood samples were taken 24 h later, and animals were challenged with 1 × 104 TCID50 of H1N1 PR8. (B) Total antibody responses against PR8 and NI titers of donor mice. Bars represent the means for three repeated measurements of pooled serum, and error bars indicate the standard deviations of the means. Log2-transformed groups were compared to the NAPR8 group using one-way ANOVA with a Tukey posttest. Statistical significance is indicated (*, P < 0.05; ****, P < 0.0001). (C and D) Percent weight change (C) and percent survival (D) in recipient mice. Upon challenge, body weight was monitored as a measure of morbidity. Animals were euthanized as soon as the weight loss reached 25%.
Influenza virus HA- or NA-expressing VSV replicon particles reduce spread to the lower respiratory tract in ferrets.
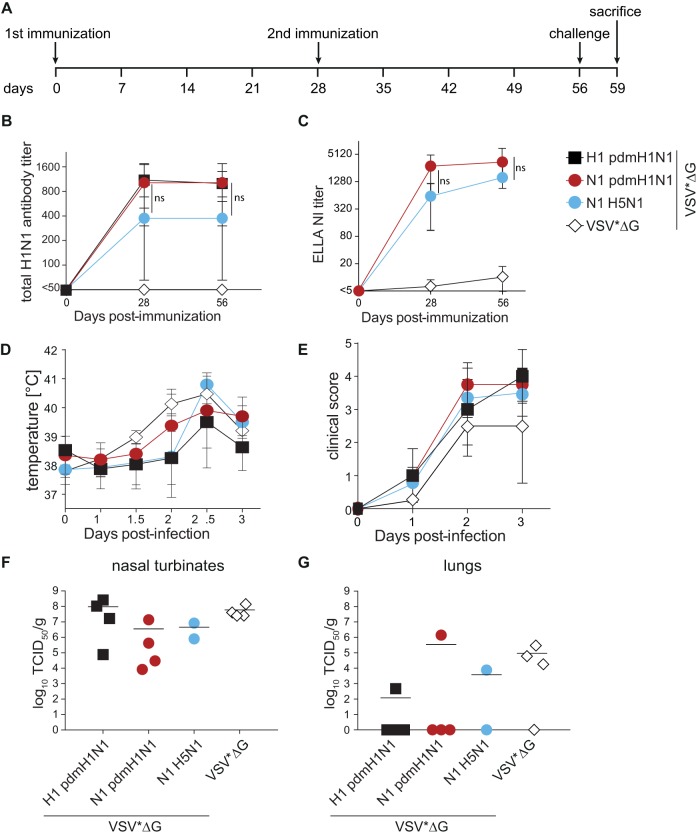
Ferrets are frequently used to assess the immunogenicity and efficacy of influenza vaccines since they reproduce many of the clinical signs and much of the overall course of disease seen in patients (40). Following the same experimental design as the one described above (Fig. 5A), immunization with VSV*ΔG replicons expressing the matched HA or NA proteins of pdmH1N1 A/California/04/2009 or VSV*ΔG(NAH5N1) elicited robust total antibody titers after the first immunization, which remained stable after a second immunization (Fig. 5B). While matched HA and NA proteins induced similar responses, levels in the NAH5N1 group were slightly but not statistically significantly lower (Fig. 5B). In contrast, both groups immunized with NA-expressing replicons induced similar NI titers, with a slight increase after the second immunization (Fig. 5C), thereby reproducing the antibody response kinetics seen in mice. Intranasal challenge of the animals with 105 TCID50 pdmH1N1 A/Mexico/InDRE4487/2009 resulted in an increase in body temperature in all groups, starting on day 2, and mild to moderate clinical disease characterized by reduced activity, congestion, runny nose, and sneezing (Fig. 5D and E). Unfortunately, two animals in the VSV*ΔG(NAH5N1) group succumbed to anesthesia immediately after challenge. All remaining animals were sacrificed on day 3 after infection, and viral loads in nasal turbinates and lungs as representatives of the upper and lower respiratory tracts were quantified. Nasal turbinate titers were slightly lower in NA-immunized groups albeit not statistically different (Fig. 5F), and vaccination reduced spreading to the lung irrespective of the antigen used (Fig. 5G). It is of note that the two NA-immunized animals with detectable virus in the lung had the lowest NA-specific antibody titers in their respective groups. One of the two remaining animals in the VSV*ΔG(NAH5N1) group was completely protected from lower respiratory tract infection, and the lung titer of the other one was around 10-fold lower than the control group mean, suggesting an efficacy similar to those of the matched vaccines and providing further evidence for the correlation between functional NA-specific antibody responses and protection.
FIG 5.
Humoral immune responses in ferrets induced by the different VSV*ΔG replicons and viral load after infection. (A) Schematic overview of the immunization-and-infection strategy and blood sampling time points. Naive ferrets were immunized twice with 108 FFU VSV*ΔG replicons intramuscularly. All animals were challenged intranasally with 105 TCID50 A/Mexico/InDRE4487/2009 and sacrificed for virus titration on day 3 after infection. (B and C) Kinetics of total antibody titers (B) and NI titers (C) against pdmH1N1 in animals immunized with matched or mismatched antigens. Log2-transformed groups were compared using two-way ANOVA with a Tukey posttest. (D and E) Temperature changes (E) and clinical scores (F) measured over 3 days after infection. Symbols represent the means for each group (n = 4; postinfection N1 H5N1, n = 2), and error bars indicate the standard deviations of the means. (F and G) Virus titers in the nasal turbinates (F) and lungs (G) in terms of TCID50 per gram on MDCK cells. Symbols indicate data for individual animals, and black bars represent the respective means.
DISCUSSION
Currently licensed influenza vaccines are highly strain specific and have to be updated as soon as HA antigenic drift variants emerge (41, 42). Even though inactivated influenza vaccines contain NA protein, its content and quality are not standardized. The contribution of NA-specific immunity to protection has only recently become a focus of investigation (11, 12, 21, 43). While there is increasing evidence that NA proteins can induce subtype-specific protection, its extent as well as possible correlates of this protection remained to be characterized. Using VSV*ΔG replicons encoding N1 proteins of the mouse-adapted PR8, prototypic seasonal and pandemic H1N1, and human H5N1 strains, we observed that in the absence of HA, NA induced robust humoral immune responses that protected mice and ferrets from challenge with a matched strain as efficiently as HA-specific immunity. For heterologous N1 proteins, the level of cross-reactive NI antibodies correlated with protection, and antibodies alone were sufficient to confer this protection. These findings highlight the potential of NA as a promising antigen to induce broader protection and demonstrate the potential of single-cycle VSV replicons as a vector platform for vaccine development.
VSV-based single-round replicons are a promising vaccine platform.
The success of the propagation-competent VSV-based vaccine candidate against Ebola virus demonstrates the potential of VSV as a vaccine platform (35, 36). However, the benefit-risk profile of a propagation-competent viral vector may not be appropriate for all target pathogens. VSV replicons, which are limited to a single round of replication due to the lack of an essential viral gene in their genome, constitute an attractive alternative, as they maintain the high-level protein expression capacity of the VSV replication machinery but are unable to produce progeny virus (37, 44). Consistent with this de novo protein synthesis, we were able to detect IFN-γ secretion after stimulation of splenocytes from VSV*ΔG(N1)-immunized mice with influenza virus. However, the responses were low compared to those of previously influenza virus-infected animals, which may be due to the lack of strong T cell epitopes in the NA protein (45). In contrast, we were able to demonstrate that NA proteins expressed with this system elicit robust functional antibody responses that exceed the titers induced by an inactivated vaccine. Repeated immunization resulted in an increase of these responses, and this effect might be improved further by including viral proteins with known conserved T cell epitopes, such as the NP or M1 protein in the VSV replicon (46), and by taking advantage of G proteins from heterologous, antigenically different VSV strains (47).
Similar efficacies of NA- and HA-specific immune responses against matched virus strains.
While a recent study found HA- and NA-reactive B cells at similar frequencies after influenza infection (48), an immunodominance of the HA over the NA protein has been postulated based on the analysis of antibody prevalence in sera from individuals immunized with inactivated vaccines (49). However, the variability in NA content makes it difficult to assess the contribution of NA-specific antibodies to vaccine efficacy (11, 50). Nevertheless, after separate administration of purified HA and NA proteins, comparable antibody levels have been observed in study participants (19). Moreover, NA-based vaccines reduced clinical disease and viral shedding in patients (20, 39, 51), and immunization of mice with purified NA protein resulted in lower lung titers and protection from lethal challenge with the matched as well as heterologous strains carrying the same subtype (11, 52). Here we show that in the context of de novo protein expression, the protection conferred by NA-specific immune responses is similar to that of HA-specific responses against the matched strain, further strengthening the potential of the NA protein as a vaccine antigen. Standardizing the NA protein content and quality in existing vaccines might thus be a straightforward approach for inducing more broadly protective immune responses.
Sialidase-inhibiting antibody titers predict the extent of protection against heterologous strains.
While HA-mediated protection is primarily strain specific, the immune response elicited by NA proteins protects at least partially against heterologous strains carrying the same NA subtype (11, 23, 25, 51). This aspect makes NA a promising candidate for the development of more broadly protective influenza vaccines. However, such development programs require robust correlates of protection to measure vaccine efficacy. Our study demonstrates that phylogenetic distance, which has been postulated to correlate with the extent of protection (21, 24, 53), may be only partially relevant. Instead, we observed a strong link between NI antibody titers and protection, irrespective of amino acid homology, phylogenetic distance, or the HA subtype present. This is in agreement with previous reports showing that N1-specific NI antibody titers correlated with cross-protection against infection with low-pathogenic avian influenza virus (LPAIV) H5N1 infection (25, 54).
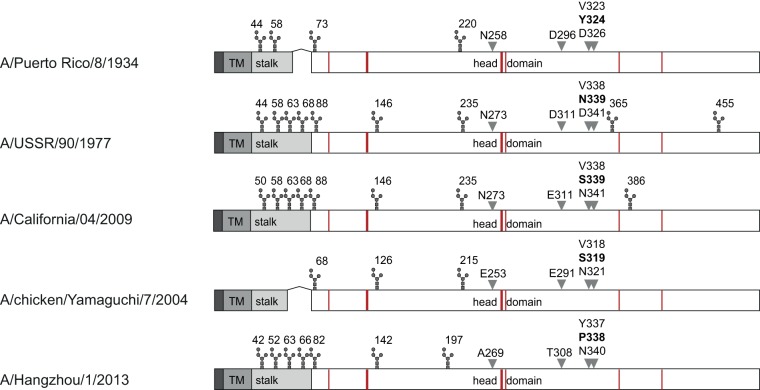
The extent of these cross-reactive antibodies and the cross-protection observed here is in line with previous publications identifying critical residues for the cross-reactivity of NA-specific antibodies (55, 56). Particularly, amino acid 339, located in a region associated with sialic acid binding and catalytic activity, has been identified as being essential for epitope recognition by antigenic mapping with mouse monoclonal antibodies (56). This residue differs in NAPR8, NAUSSR, and NAH5N1 but turns out to be conserved between NApdmH1N1 and NAH5N1, which may explain the high cross-reactivity observed in our ferret study (Fig. 6). Of note, the two NA proteins with stalk deletions characteristic of avian-origin viruses and the lowest number of potential N-glycosylation sites, NAPR8 and NAH5N1, elicited the highest sialidase-inhibiting antibody titers in our study, suggesting that stalk length and glycosylation pattern may also influence the extent of functional antibodies (Fig. 6). However, a causal connection between either characteristic and antigenicity would require validation involving a larger selection of proteins, similar to an influenza B virus neuraminidase study (14). Together with a recent report that natural influenza infection, in contrast to immunization with inactivated vaccines, induces broadly cross-reactive NA-specific antibodies (48), our study underlines the potential of NA-based vaccines and demonstrates that neuraminidase-inhibiting antibodies may serve as promising functional correlates of protection.
FIG 6.
Linear comparison of the NA proteins. The cytoplasmic domain, transmembrane region (TM), stalk region, and head domain are depicted in dark, medium and light gray, and white, respectively. Putative glycosylation sites are shown above, active sites are in red, and previously identified critical residues for inhibiting antibody binding are indicated by gray arrowheads and the respective amino acid numbering.
MATERIALS AND METHODS
Cells.
BHK-21 cells were obtained from the German Cell Culture Collection (DSZM, Braunschweig, Germany) and grown in Earle's minimal essential medium (MEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Life Technologies) and 200 mM l-glutamine (Sigma-Aldrich). BHK-G43 cells, which inducibly express the VSV G protein, were maintained as described previously (54). Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) and Vero E6 cells (ATCC CRL-1586) were maintained in Dulbecco's modified Eagle medium (DMEM; Sigma-Aldrich) supplemented with 5% FBS and 200 mM l-glutamine (Sigma-Aldrich).
Virus production and quantification.
The mouse-adapted H1N1 influenza virus strain A/Puerto Rico/8/34 (PR8) and pdmH1N1 A/Mexico/InDRE4487/2009 were grown on MDCK cells in serum-free DMEM supplemented with 0.75 μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin (TPCK-trypsin; Sigma-Aldrich). When cytopathic effect was widespread, the supernatant was harvested, clarified by centrifugation at 3,000 × g for 15 min at 4°C, aliquoted, and stored at −80°C. To quantify virus titers, MDCK cells were seeded into 96-well plates at 90% confluence, and quadruplicate wells were inoculated with 10-fold serial dilutions of the supernatant. After 2 days of incubation at 37°C and 5% CO2, the plates were washed once with phosphate-buffered saline (PBS) diluted 1:3 in double-distilled H2O, air dried for 15 min, and fixed at 65°C for 8 h. Infected cells were stained by adding ferret anti-H1N1 serum at a dilution of 1:500, followed by incubation for 2 h at room temperature. The plates were then washed with PBS and incubated with a 1:750 dilution of a horseradish peroxidase (HRP)-coupled anti-ferret secondary antibody (Biomol GmbH) for 1 h at room temperature. Following a final washing step, positive cells were visualized with 3-amino-9-ethyl-carbazole staining (Sigma-Aldrich). Virus titers were expressed as the 50% tissue culture infectious dose (TCID50) per milliliter.
For egg-grown PR8, 11-day-old embryonated eggs were inoculated by the allantoic route and incubated at 37°C and 60% humidity in an egg incubator. Infected allantoic fluid was harvested under sterile conditions 48 h after inoculation and purified through a 20% (wt/vol) sucrose cushion at 135,000 × g for 2 h at 4°C. Pellets were resuspended in PBS with 10% FBS. The hemagglutination titer of each virus was determined by mixing 2-fold dilutions in U-bottomed microtiter plates with an equal volume of 0.5% guinea pig red blood cells (RBCs) according to standard protocols (57).
Generation of recombinant VSV replicon particles.
The plasmid pVSV*ΔG(NAH5N1) was recently described (13). It encodes a G protein gene-deleted VSV genome encoding NA of A/chicken/Yamaguchi/7/2004 (H5N1) and a GFP reporter. For the generation of influenza HA- or NA-expressing recombinant VSV replicons, the respective HA or NA genes of A/Puerto Rico/8/1934 (H1N1) (HAPR8 or NAPR8 [GenBank accession number CY009444.1 or CY009446.1, respectively]), A/California/04/2009 (H1N1) (HApdmH1N1 or NApdmH1N1 [GenBank accession number GQ117044.1 or FJ969517.1, respectively]), A/USSR/90/1977 (H1N1) (NAUSSR [GenBank accession number CY121880.1]), A/chicken/Yamaguchi/7/2004 (H5N1) (NAH5N1 [GenBank accession number AB166864.1]), or A/Hangzhou/1/2013 (H7N9) (NAH7N9 [GenBank accession number KC853765.1]) were used to replace the NAH5N1 gene in the fourth transcription unit of pVSV*ΔG(NAH5N1), taking advantage of the flanking MluI and BstEII endonuclease restriction sites. The sequences of all clones used for further experiments were confirmed by Sanger sequencing.
To obtain VSV replicon particles, BHK-G43 cells were infected with recombinant modified vaccinia virus Ankara (MVA) virus expressing the T7 RNA polymerase (58). Prior to infection, the expression of the VSV G protein was induced by adding mifepristone at a concentration of 10−9 M (Sigma-Aldrich) to the cell culture medium. Cells were cotransfected with the respective genomic plasmids along with three helper plasmids encoding the VSV N, P, and L proteins under the control of the T7 promoter, as described above. After 24 h of incubation, the cells were trypsinized, seeded along with an equal number of fresh BHK-G43 cells, and incubated for an additional 24 h at 37°C in the presence of mifepristone (59). The supernatant was clarified by low-speed centrifugation at 1,000 × g for 15 min at 4°C and stored at −80°C. For further passaging of the replicons, BHK-G43 cells were induced to express the VSV G protein by adding mifepristone and infected with the respective replicons. When cytopathic effect was widespread, the supernatant was harvested, clarified by centrifugation at 1,000 × g for 15 min at 4°C followed by purification through a 20% (wt/vol) sucrose cushion at 100,000 × g for 1 h at 4°C, and resuspended in PBS. Replicon stocks were stored at −80°C. Virus stocks were titrated on BHK-21 cells, taking advantage of the GFP reporter for detection of infected cells. Infectious virus titers were expressed as focus-forming units (FFU) per milliliter.
Western blot analysis.
To analyze cell surface protein expression, Vero E6 cells were infected with the respective VSV replicons at a multiplicity of infection (MOI) of 10, and cell surface proteins were biotinylated with 2 mM EZ-Link sulfo-NHS-LC-LC-biotin (Pierce) in PBS at 12 h postinfection. Noninfected BHK-21 cells were used as a control. Cells were subsequently lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 8], 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100) supplemented with Halt protease inhibitors (Thermo). Cell lysates were centrifuged at 14,000 × g for 10 min at 4°C to remove cell debris, and the supernatants were stored at −20°C until further use. Protein concentration was determined by using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific). For immunoprecipitation, 50 μg of total protein was coincubated with 5 μl of equal amounts of homologous mouse antibody and incubated under rotation at 4°C for 3 h with the addition of protein G plus agarose beads (Santa Cruz) during the final 1 h. Beads were then precipitated by centrifugation, washed 4 times with lysis buffer, and separated by 10% SDS-polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore) and blocked overnight at 4°C in 5% milk powder prepared in Tris-buffered saline containing 1% Tween (TBS-T). The membranes were then incubated with Pierce high-sensitivity streptavidin-HRP (ThermoFisher). After three wash steps with TBS-T, bands were visualized using the Amersham ECL Prime Western blotting detection system (GE Healthcare) with a MicroChemi 4.2 chemiluminescent imaging system (DNR Bio Imaging Systems Ltd.), and the resulting bands were quantified from underexposed TIF images using the ImageJ analysis software package (NIH).
Animal experiments.
All animal experiments were carried out in compliance with the regulations of German animal protection laws and were authorized by the responsible state authority. Six- to eight-week-old female C57BL/6N mice (Janvier Labs) were immunized twice intramuscularly 4 weeks apart with 1 × 106 FFU of the respective VSV replicon particles diluted in 50 μl PBS or beta-propiolactone (BPL)-inactivated PR8 (catalog number NR-19325; BEI Resources, NIAID, NIH) as a control. On days 28, 35, and 56 after the initial immunization, 6 animals of each group were anesthetized with isoflurane and exsanguinated by cardiac puncture. Serum samples were stored at −20°C for further analysis. For virus challenge, mice were anesthetized by intraperitoneal injection with ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg) and inoculated intranasally with 104 TCID50 of PR8 in 30 μl, corresponding to 3× the 100% lethal dose (LD100). Animals were monitored daily for clinical signs and body weight and euthanized as soon as weight loss reached 25%. For T cell response analysis, spleens were harvested 7 days after the second immunization.
To investigate the role of the antibodies in protection from infection, naive animals were injected intraperitoneally with 500 μl of pooled sera collected on day 35 after initial immunization. The next day, mice were anesthetized, bled via the retrobulbar route, and infected intranasally with PR8 as outlined above. Recipient animals with total anti-PR8 antibody titers below 50% of the initial donor titer were considered a transfer failure and subsequently excluded from the study (NAPR8, n = 2; inactivated PR8, n = 2).
Male and female ferrets (Mustela putorius furo) 16 weeks of age or older and seronegative for influenza viruses were used for the study. Groups of 4 animals were immunized twice intramuscularly 4 weeks apart with 1 × 108 FFU of the respective VSV replicon particles, and blood samples were collected before the first and second immunizations and the challenge. Two months after the initial immunization, animals were challenged by intranasal inoculation with 105 TCID50 of pdmH1N1 A/Mexico/InDRE4487/2009. Three days after infection, all animals were sacrificed, and tissues were harvested for titration.
Total influenza virus antibody assay.
Total influenza virus-specific antibody responses were quantified using an immunoperoxidase monolayer assay with PR8- or pdmH1N1-infected MDCK cells as described previously (60). Briefly, MDCK cells were seeded into 96-well plates at 90% confluence and infected with PR8 at an MOI of 0.01. After 48 h, cells were washed once with PBS diluted 1:3 in double-distilled H2O, air dried, and fixed at 65°C for 8 h. Serial 2-fold dilutions of mouse sera were added to the fixed MDCK cells, and antibody titers were detected by immunostaining as outlined above and expressed as the reciprocal of the last serum dilution at which antibody-positive cells were detected.
Enzyme-linked lectin assay.
To determine the amount of neuraminidase-inhibiting antibodies, an enzyme-linked lectin assay (ELLA) was performed as described previously (61). Briefly, flat-bottom MaxiSorp polystyrene 96-well plates (Nunc) were coated with fetuin (Sigma-Aldrich). Serum samples were heat treated at 56°C for 45 min prior to serial 2-fold dilutions in PBS and subsequent coincubation with a predetermined 90% NA activity of PR8 at 37°C for 16 to 18 h. After three wash steps with PBS containing 0.05% Tween 20 (PBS-T), peroxidase-labeled lectin from Arachis hypogaea (Sigma-Aldrich) was added, and the mixture was incubated for 2 h at room temperature. Plates were washed again before the addition of o-phenylenediamine dihydrochloride (OPD) substrate (Sigma-Aldrich). The reaction was stopped with 1 N sulfuric acid before the absorbance at 490 nm was read. The sialidase-inhibiting antibody titer was expressed as the reciprocal of the highest dilution that exhibited ≥50% inhibition of NA activity.
ELISpot assay.
For the quantification of gamma interferon (IFN-γ)-expressing T cells, a commercial murine IFN-γ ELISpot assay (eBioscience) was used, according to the manufacturer's instructions. Splenocytes were isolated on day 35, 7 days after the second immunization, and 2 × 105 cells were coincubated with 320 hemagglutinating units (HAU) of egg-grown PR8 or Newcastle disease virus at 37°C in 200 μl RPMI (10% mouse serum, 2 nM l-Gln, 1% penicillin-streptomycin; Sigma-Aldrich) in MultiScreen ELISpot polyvinylidene difluoride 96-well plates (Millipore). C57BL/6 mice were infected with 1 × 103 TCID50 H1N1 PR8 virus 7 days before splenocyte isolation as a positive control, while uninfected mice were used as a negative control. Medium alone was used as a negative control, and concanavalin A (ConA; Sigma-Aldrich) at 6 μg/ml was added to positive-control wells to assess splenocyte reactivity. After 36 h, cells were removed from the wells, and the plates were washed before incubation with biotin-conjugated anti-IFN-γ antibodies and avidin-HRP according to the manufacturer's instructions. A 3-amino-9-ethyl-carbazole (Sigma-Aldrich) substrate solution was used for visualization of spots, and analysis was performed using the AID EL R04 ELISpot reader.
Statistical and phylogenetic analyses.
Statistical analyses were performed using GraphPad Prism 6 on log2-transformed data using one-way or two-way analysis of variance (ANOVA) with a Tukey posttest. All groups were compared, and P values of ≤0.05 were considered significant. Amino acid sequences were aligned using ClustalW, and a phylogenetic tree was constructed using the neighbor-joining method and 1,000 bootstrapped replications with observed amino acid differences as the distance on the aligned sequences using MEGA7.
ACKNOWLEDGMENTS
We thank all laboratory members for continuing support and lively discussions, Hanna Sediri for help with the enzyme-linked lectin assay, and Yvonne Krebs for excellent technical support.
This work was supported by funding from the German Center for Infection Research (DZIF) and the German Ministry of Health to V.v.M. and by a Swiss National Science Foundation grant (no. 166265) to G.Z.
We declare no conflict of interest.
REFERENCES
- 1.Balkovic ES, Goodman RA, Rose FB, Borel CO. 1980. Nosocomial influenza A (H1N1) infection. Am J Med Technol 46:318–320. [PubMed] [Google Scholar]
- 2.Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. 1999. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 281:908–913. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 3.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. 1997. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 15:1114–1122. doi: 10.1016/S0264-410X(97)00003-0. [DOI] [PubMed] [Google Scholar]
- 4.Horcajada JP, Pumarola T, Martínez JA, Tapias G, Bayas JM, de la Prada M, García F, Codina C, Gatell JM, de Anta MTJ. 2003. A nosocomial outbreak of influenza during a period without influenza epidemic activity. Eur Respir J 21:303–307. doi: 10.1183/09031936.03.00040503. [DOI] [PubMed] [Google Scholar]
- 5.Air GM, Laver WG, Webster RG. 1987. Antigenic variation in influenza viruses. Contrib Microbiol Immunol 8:20–59. [PubMed] [Google Scholar]
- 6.Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 7.Hensley SE, Das SR, Bailey AL, Schmidt LM, Hickman HD, Jayaraman A, Viswanathan K, Raman R, Sasisekharan R, Bennink JR, Yewdell JW. 2009. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science 326:734–736. doi: 10.1126/science.1178258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sylte MJ, Suarez DL. 2009. Influenza neuraminidase as a vaccine antigen. Curr Top Microbiol Immunol 333:227–241. doi: 10.1007/978-3-540-92165-3_12. [DOI] [PubMed] [Google Scholar]
- 9.Dobbelaer R, Levandowsky R, Wood JA. 2005. WHO technical report series, 2005. WHO, Geneva, Switzerland. [Google Scholar]
- 10.Creskey MC, Li C, Wang J, Girard M, Lorbetskie B. 2012. Simultaneous quantification of the viral antigens hemagglutinin and neuraminidase in influenza vaccines by LC-MSE. Vaccine 30:4762–4770. doi: 10.1016/j.vaccine.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F. 2015. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 6:e02556-14. doi: 10.1128/mBio.02556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlbold TJ, Krammer F. 2014. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6:2465–2494. doi: 10.3390/v6062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halbherr SJ, Ludersdorfer TH, Ricklin M, Locher S, Rentsch MB, Summerfield A, Zimmer G. 2014. Biological and protective properties of immune sera directed to the influenza virus neuraminidase. J Virol 89:1550–1563. doi: 10.1128/JVI.02949-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohlbold TJ, Podolsky KA, Chromikova V, Kirkpatrick E, Falconieri V, Meade P, Amanat F, Tan J, ten Oever BR, Tan GS, Subramaniam S, Palese P, Krammer F. 2017. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat Microbiol 2:1415–1424. doi: 10.1038/s41564-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson BE, Moran TM, Bona CA, Popple SW, Kilbourne ED. 1987. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. II. Sequential infection of mice simulates human experience. Virus Res 139:2010–2014. [PubMed] [Google Scholar]
- 16.Wan H, Yang H, Shore DA, Garten RJ, Couzens L, Gao J, Jiang L, Carney PJ, Villanueva J, Stevens J, Eichelberger MC. 2015. Structural characterization of a protective epitope spanning A(H1N1)pdm09 influenza virus neuraminidase monomers. Nat Commun 6:6114. doi: 10.1038/ncomms7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, Hirsh A, Krammer F. 2015. Hemagglutinin stalk- and neuraminidase-specific monoclonal antibodies protect against lethal H10N8 influenza virus infection in mice. J Virol 90:851–861. doi: 10.1128/JVI.02275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JR, Guo Z, Reber A, Kamal RP, Music N, Gansebom S, Bai Y, Levine M, Carney P, Tzeng W-P, Stevens J, York IA. 2016. An influenza A virus (H7N9) anti-neuraminidase monoclonal antibody with prophylactic and therapeutic activity in vivo. Antiviral Res 135:48–55. doi: 10.1016/j.antiviral.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson BE, Bucher DJ, Kilbourne ED. 1989. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol 63:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED. 1974. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis 129:411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- 21.Schulman JL. 1969. The role of antineuraminidase antibody in immunity to influenza virus infection. Bull World Health Organ 41:647–650. [PMC free article] [PubMed] [Google Scholar]
- 22.Rott R, Becht H, Orlich M. 1974. The significance of influenza virus neuraminidase in immunity. J Gen Virol 22:35–41. doi: 10.1099/0022-1317-22-1-35. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Kadowaki S, Hagiwara Y, Yoshikawa T, Matsuo K, Kurata T, Tamura S. 2000. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 18:3214–3222. doi: 10.1016/S0264-410X(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 24.Marcelin G, Bland HM, Negovetich NJ, Sandbulte MR, Ellebedy AH, Webb AD, Griffin YS, DeBeauchamp JL, McElhaney JE, Webby RJ. 2010. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1N1) 2009 virus in an age-dependent manner. J Infect Dis 202:1634–1638. doi: 10.1086/657084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockman S, Brown LE, Barr IG, Gilbertson B, Lowther S, Kachurin A, Kachurina O, Klippel J, Bodle J, Pearse M, Middleton D. 2013. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J Virol 87:3053–3061. doi: 10.1128/JVI.02434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol 72:4704–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regules JA, Beigel JH, Paolino KM. 2017. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 376:330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs JD, Frank I, Elizaga ML, Allen M. 2015. First-in-human evaluation of the safety and immunogenicity of a recombinant vesicular stomatitis virus human immunodeficiency virus-1 gag vaccine (HVTN 090). Open Forum Infect Dis 2:ofv082. doi: 10.1093/ofid/ofv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelshtein D, Werman A, Novick D. 2013. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomczyk T, Orzechowska B. 2013. Vesicular stomatitis virus (VSV) as a vaccine vector for immunization against viral infections. Postepy Hig Med Dosw 67:1345–1358. (In Polish.) doi: 10.5604/17322693.1083016. [DOI] [PubMed] [Google Scholar]
- 31.Kapadia SU, Simon ID, Rose JK. 2008. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology 376:165–172. doi: 10.1016/j.virol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalhoro NH, Veits J, Rautenschlein S, Zimmer G. 2009. A recombinant vesicular stomatitis virus replicon vaccine protects chickens from highly pathogenic avian influenza virus (H7N1). Vaccine 27:1174–1183. doi: 10.1016/j.vaccine.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Halbherr SJ, Brostoff T, Tippenhauer M, Locher S, Rentsch MB, Zimmer G. 2013. Vaccination with recombinant RNA replicon particles protects chickens from H5N1 highly pathogenic avian influenza virus. PLoS One 8:e66059. doi: 10.1371/journal.pone.0066059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer AV, Pahar B, Boudreaux MJ, Wakamatsu N. 2009. Recombinant vesicular stomatitis virus-based West Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent West Nile virus strain LSU-AR01. Vaccine 27:893–903. doi: 10.1016/j.vaccine.2008.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H. 2015. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banadyga L, Marzi A. 2017. Closer than ever to an Ebola virus vaccine. Expert Rev Vaccines 16:401–402. doi: 10.1080/14760584.2017.1309977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmer G, Locher S, Rentsch MB, Halbherr SJ. 2014. Pseudotyping of vesicular stomatitis virus with the envelope glycoproteins of highly pathogenic avian influenza viruses. J Gen Virol 95:1634–1639. doi: 10.1099/vir.0.065201-0. [DOI] [PubMed] [Google Scholar]
- 38.Schulman JL, Khakpour M, Kilbourne ED. 1968. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol 2:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sylte MJ, Hubby B, Suarez DL. 2007. Influenza neuraminidase antibodies provide partial protection for chickens against high pathogenic avian influenza infection. Vaccine 25:3763–3772. doi: 10.1016/j.vaccine.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Enkirch T, Von Messling V. 2015. Ferret models of viral pathogenesis. Virology 479–480:259–270. doi: 10.1016/j.virol.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hensley SE, Das SR, Bailey AL, Schmidt LM.. 2009. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science 326:734–736. doi: 10.1126/science.1178258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleury D, Wharton SA, Skehel JJ, Knossow M. 1998. Antigen distortion allows influenza virus to escape neutralization. Nat Struct Biol 5:119–123. doi: 10.1038/nsb0298-119. [DOI] [PubMed] [Google Scholar]
- 43.Marcelin G, Sandbulte MR. 2012. Contribution of antibody production against neuraminidase to the protection afforded by influenza vaccines. Rev Med Virol 22:267–279. doi: 10.1002/rmv.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Publicover J, Ramsburg E, Rose JK. 2005. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J Virol 79:13231–13238. doi: 10.1128/JVI.79.21.13231-13238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutiérrez AH, Loving C, Moise L, Terry FE, Brockmeier SL, Hughes HR, Martin WD, De Groot AS. 2016. In vivo validation of predicted and conserved T cell epitopes in a swine influenza model. PLoS One 11:e0159237. doi: 10.1371/journal.pone.0159237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreijtz JHCM, Fouchier RAM, Rimmelzwaan GF. 2011. Immune responses to influenza virus infection. Virus Res 162:19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Roberts A, Buonocore L, Price R, Forman J, Rose JK. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol 73:3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YQ, Wohlbold TJ, Zheng NY, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, Henry C, Palm AKE, Stamper CT, Lan LYL, Topham DJ, Treanor J, Wrammert J, Ahmed R, Eichelberger MC, Georgiou G, Krammer F, Wilson PC. 2018. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 173:417.e10–429.e10. doi: 10.1016/j.cell.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brett IC, Johansson BE. 2005. Immunization against influenza A virus: comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology 339:273–280. doi: 10.1016/j.virol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Kalbfuss B, Knöchlein A, Kröber T, Reichl U. 2008. Monitoring influenza virus content in vaccine production: precise assays for the quantitation of hemagglutination and neuraminidase activity. Biologicals 36:145–161. doi: 10.1016/j.biologicals.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Johansson BE, Matthews JT, Kilbourne ED. 1998. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine 16:1009–1015. doi: 10.1016/S0264-410X(97)00279-X. [DOI] [PubMed] [Google Scholar]
- 52.Liu WC, Lin CY, Tsou YT, Jan JT, Wu SC. 2015. Cross-reactive neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5N1 and pandemic H1N1 influenza A viruses. J Virol 89:7224–7234. doi: 10.1128/JVI.00585-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcelin G, DuBois R, Rubrum A, Russell CJ, McElhaney JE, Webby RJ. 2011. A contributing role for anti-neuraminidase antibodies on immunity to pandemic H1N1 2009 influenza A virus. PLoS One 6:e26335. doi: 10.1371/journal.pone.0026335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE. 2015. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis 212:1191–1199. doi: 10.1093/infdis/jiv195. [DOI] [PubMed] [Google Scholar]
- 55.Khurana S, Suguitan AL, Rivera Y, Simmons CP, Lanzavecchia A, Sallusto F, Manischewitz J, King LR, Subbarao K, Golding H. 2009. Antigenic fingerprinting of H5N1 avian influenza using convalescent sera and monoclonal antibodies reveals potential vaccine and diagnostic targets. PLoS Med 6:e1000049. doi: 10.1371/journal.pmed.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wan H, Gao J, Xu K, Chen H, Couzens LK, Rivers KH, Easterbrook JD, Yang K, Zhong L, Rajabi M, Ye J, Sultana I, Wan XF, Liu X, Perez DR, Taubenberger JK, Eichelberger MC. 2013. Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J Virol 87:9290–9300. doi: 10.1128/JVI.01203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.WHO. 2002. WHO manual on animal influenza diagnosis and surveillance. WHO, Geneva, Switzerland. [Google Scholar]
- 58.Sutter G, Ohlmann M, Erfle V. 1995. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett 371:9–12. doi: 10.1016/0014-5793(95)00843-X. [DOI] [PubMed] [Google Scholar]
- 59.Rentsch MB, Zimmer G. 2011. A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS One 6:e25858. doi: 10.1371/journal.pone.0025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Direksin K, Joo H, Goyal SM. 2002. An immunoperoxidase monolayer assay for the detection of antibodies against swine influenza virus. J Vet Diagn Invest 14:169–171. doi: 10.1177/104063870201400215. [DOI] [PubMed] [Google Scholar]
- 61.Couzens L, Gao J, Westgeest K, Sandbulte M. 2014. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods 210:7–14. doi: 10.1016/j.jviromet.2014.09.003. [DOI] [PubMed] [Google Scholar]