We have previously suggested that the very low levels of simian immunodeficiency virus (SIV) maternal-to-infant transmissions (MTIT) in African nonhuman primates that are natural hosts of SIVs are due to a low availability of target cells (CCR5+ CD4+ T cells) in the oral mucosa of the infants, rather than maternal and milk factors. To confirm this new MTIT paradigm, we performed a proof-of-concept study in which we therapeutically blocked CCR5 with maraviroc (MVC) and orally exposed MVC-treated and naive infant rhesus macaques to SIV. MVC had only a marginal effect on oral SIV transmission. However, the observation that the infant RMs that remained uninfected at the completion of the study, after 6 repeated viral challenges, had the lowest CCR5 expression on the CD4+ T cells prior to the MVC treatment appears to confirm our hypothesis, also suggesting that the partial effect of MVC is due to a limited efficacy of the drug. New, more effective CCR5 inhibitors may have a better effect in preventing SIV and HIV transmission.
KEYWORDS: simian immunodeficiency virus, rhesus macaques, oral transmission, real-time single-genome amplification, CCR5 coreceptor, maraviroc, target cells
ABSTRACT
Current approaches do not eliminate all human immunodeficiency virus type 1 (HIV-1) maternal-to-infant transmissions (MTIT); new prevention paradigms might help avert new infections. We administered maraviroc (MVC) to rhesus macaques (RMs) to block CCR5-mediated entry, followed by repeated oral exposure of a CCR5-dependent clone of simian immunodeficiency virus (SIV) mac251 (SIVmac766). MVC significantly blocked the CCR5 coreceptor in peripheral blood mononuclear cells and tissue cells. All control animals and 60% of MVC-treated infant RMs became infected by the 6th challenge, with no significant difference between the number of exposures (P = 0.15). At the time of viral exposures, MVC plasma and tissue (including tonsil) concentrations were within the range seen in humans receiving MVC as a therapeutic. Both treated and control RMs were infected with only a single transmitted/founder variant, consistent with the dose of virus typical of HIV-1 infection. The uninfected RMs expressed the lowest levels of CCR5 on the CD4+ T cells. Ramp-up viremia was significantly delayed (P = 0.05) in the MVC-treated RMs, yet peak and postpeak viral loads were similar in treated and control RMs. In conclusion, in spite of apparent effective CCR5 blockade in infant RMs, MVC had a marginal impact on acquisition and only a minimal impact on the postinfection delay of viremia following oral SIV infection. Newly developed, more effective CCR5 blockers may have a more dramatic impact on oral SIV transmission than MVC.
IMPORTANCE We have previously suggested that the very low levels of simian immunodeficiency virus (SIV) maternal-to-infant transmissions (MTIT) in African nonhuman primates that are natural hosts of SIVs are due to a low availability of target cells (CCR5+ CD4+ T cells) in the oral mucosa of the infants, rather than maternal and milk factors. To confirm this new MTIT paradigm, we performed a proof-of-concept study in which we therapeutically blocked CCR5 with maraviroc (MVC) and orally exposed MVC-treated and naive infant rhesus macaques to SIV. MVC had only a marginal effect on oral SIV transmission. However, the observation that the infant RMs that remained uninfected at the completion of the study, after 6 repeated viral challenges, had the lowest CCR5 expression on the CD4+ T cells prior to the MVC treatment appears to confirm our hypothesis, also suggesting that the partial effect of MVC is due to a limited efficacy of the drug. New, more effective CCR5 inhibitors may have a better effect in preventing SIV and HIV transmission.
INTRODUCTION
Despite enormous success in preventing mother-to-infant transmission (MTIT), recently, the World Health Organization (WHO) has intensified international efforts to significantly reduce or eliminate infection of infants. In 2013, UNAIDS reported that approximately 210,000 infants worldwide become human immunodeficiency virus (HIV) infected annually (1). More than 90% of these HIV-1 infections occur in sub-Saharan Africa. MTIT can occur in utero, directly by hematogenous transplacental spread or by infection of the amniotic membranes and fluid (2); during the delivery, by contact of the infant with maternal blood and cervicovaginal secretions (3, 4); or postnatally, through breast-feeding (5, 6). The last mode of transmission accounts for most MTIT cases and is difficult to prevent, because its mechanisms are not completely understood. Differently from HIV vaginal or rectal transmission, in which the virus-host interactions at the portal of entry have been intensively studied (7), little emphasis has been placed on the role of the infant mucosa in HIV breast-feeding transmission. This paucity of information is mainly due to the inherent limitations of sampling human infants. Further challenges to studying infant oral transmission include the long duration of exposure from breast milk and dramatic age-related changes in the infant mucosa during that time. In addition, most HIV-infected women are receiving some form of antiretroviral therapy (ART) or peripartum prophylaxis (8), which reduces MTIT but makes it more difficult to study breakthrough infections. As such, MTIT studies have focused almost exclusively on maternal virological and immunologic factors (9–11) and on immune effectors present in breast milk (12–16). High HIV-1 maternal plasma viral loads (VLs) and low CD4+ T cell counts in women that breast-feed are correlated with increased HIV breast-feeding transmission (17, 18), but these correlations are not always substantiated, as mothers with low VLs can also transmit HIV by milk (17, 18). Conversely, 63% of the infants breast-fed by mothers with <200 CD4+ T cells/μl and >105 viral RNA (vRNA) copies/ml remain uninfected (19). Furthermore, the correlation between milk viral shedding and the plasma VL is weak, and substantial discrepancies exist, with some women having low VLs in milk but high VLs in plasma, and vice versa (19). The rates of breast-feeding transmission are also correlated with the duration of lactation rather than the absolute CD4+ T cell count (20). These data highlight the complex and dynamic process of infant oral transmission.
Breast-feeding transmission studies in macaques have also focused only on maternal and milk factors (13, 16, 21, 22). Neither maternal plasma VLs nor CD4+ T cells clearly predict breast-feeding transmission in macaques, with only 20% of acutely infected dams successfully transmitting infection. Importantly, over 50% of simian immunodeficiency virus (SIV) breast-feeding transmissions occurred at 9 months post-dam infection, when the offspring were older, highlighting an age-related susceptibility to SIV infection, with higher doses of virus needed to infect younger RMs (23). Finally, it has been reported that an occult peripartum/postpartum simian-human immunodeficiency virus (SHIV) infection that may occur early may go undetected until later, suggesting that maturation of the immune system and generation of target cells in the infant are needed to support virus replication (21).
Our previous work in African nonhuman primates (NHPs) that are natural hosts of SIVs demonstrated that in these species MTIT of SIV is virtually nonexistent (<5%) (24–26) and below the level targeted by the WHO for virtual elimination of HIV-1 MTIT in humans (27). The low levels of MTIT in natural hosts contrast with the massive offspring exposure to SIV both in utero and through breast-feeding (25) due to the high SIV prevalence in the wild (>80%) and high levels of acute and chronic viral replication in dams (25, 26, 28). In African green monkeys (AGMs) and mandrills, resistance to SIV breast-feeding transmission is strongly associated with low levels of SIV target cells at the mucosal sites of the offspring (24). Furthermore, AGM susceptibility to experimental SIV mucosal transmission is proportional to the availability of CD4+ T cells expressing the SIV coreceptor CCR5+ at the mucosal sites (29, 30).
Based on these observations, we hypothesized that the levels of target cells (CCR5+ CD4+ T cells) at the oral mucosa of breast-fed infants may drive the efficacy of HIV/SIV transmission through breast-feeding and that the CCR5 blockade could represent a new potential therapeutic strategy to prevent HIV/SIV breast-feeding transmission. We tested this hypothesis in an infant rhesus macaque (RM) model of HIV breast-feeding transmission (16), in which we administered maraviroc (MVC) to block oral SIVmac transmission. MVC was shown to effectively block CCR5 expression in mucosal CD4+ T cells and prevent SIV transmission upon topic administration (31), but systemic CCR5 blockade to prevent oral HIV/SIV transmission has never been performed. MVC has low toxicity (32) and a high penetrability into the mucosal sites and is available for oral administration, thus being suitable for use in infants. As such, we reasoned that demonstrating MVC efficacy in blocking oral HIV transmission may lead to an efficient way to prevent HIV breast-feeding transmission. We report here that while systemic MVC administration to infant RMs was well tolerated and efficiently blocked CCR5 in peripheral blood and at mucosal sites, it had a minimal impact on viral acquisition and only marginally impacted the postinfection delay of viremia. The infant RMs that remained uninfected at the completion of the study had the lowest CCR5 expression on the CD4+ T cells prior to the MVC treatment, confirming our hypothesis that the availability of target cells may drive the efficacy of SIV/HIV breast-feeding transmission and also suggesting that the partial effect of MVC is due to a limited efficacy. New, more effective CCR5 inhibitors may have a better effect in preventing SIV and HIV transmission.
(This article was submitted to an online preprint archive [33].)
RESULTS
Study design.
To investigate whether or not blockade of the mucosal target cells can prevent/reduce HIV/SIV oral transmission, five infant RMs were administered MVC at a total daily dose of 300 mg/kg of body weight (150 mg/kg given twice daily [b.i.d.]) by mouth with food for up to 4 months. At 1 month after MVC initiation, the treated infants, together with four uninfected controls, received 10,000 IU of SIVmac766XII (a synthetic swarm of the transmitted/founder [T/F] SIVmac766 clone) (Fig. 1) (34) via oral, atraumatic administration. Viral challenges were repeated every 2 weeks until all the controls became SIV infected (after the 6th challenge).
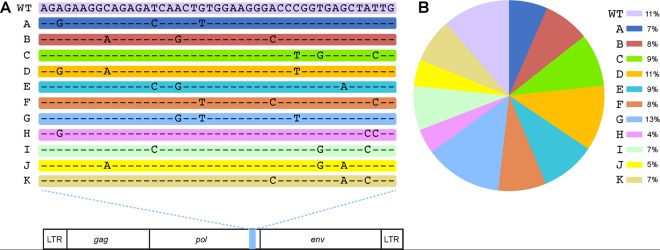
FIG 1.
Alterations in the SIVmac766 clone that allow for discriminating the number of unique T/F variants. (A) SIVmac766XII is an infection stock composed of 11 distinct viral clones differing from the wild-type virus by 3 synonymous mutations within the integrase gene. The entire remaining genome is identical between clones. (B) The proportion of each variant in the viral stock was determined by RT-SGA, with 334 sequences being examined. All mutations and the pie chart are color coded for each of the 12 clones within the synthetic swarm. WT, wild type; LTR, long terminal repeat.
At the time of the viral challenges, MVC was dosed in the circulation in all the MVC-treated infant RMs. Due to the nature of the study, which involved repeated oral challenges, we did not collect oral or tonsil biopsy specimens to dose the MVC at the site of virus exposure, to avoid increasing the risk of SIV transmission. However, we assessed the MVC concentration in tissues (including tonsils) in two additional MVC-treated SIV-unchallenged infant RMs, which were followed under the same conditions as the infants in the study group.
Blood (1.5 ml) was collected into EDTA-containing cell preparation tubes (CPTs) from all the infant RMs receiving MVC at the time of challenge, to monitor coreceptor occupancy (35) and measure the plasma concentrations of MVC. Blood was then collected every 3 days to detect the SIV infection. Once an animal was diagnosed as SIV infected, frequent blood samples were collected to monitor the acute and early chronic infection (10, 17, 24, 31, 38, 45, and 59 days postinfection [dpi]). Superficial lymph nodes (LNs), tonsils, and gut biopsy specimens were collected only from the RMs in the MVC-treated control group.
Orally administered MVC is well tolerated by infant RMs.
Throughout the MVC treatment (up to 101 days), all infant RMs receiving MVC were closely monitored for clinical or biological signs suggestive of side or adverse effects of the MVC. With no such signs being observed, we concluded that oral administration of MVC was safe and well tolerated by infant RMs.
PK of MVC in plasma and tissues.
The pharmacokinetic (PK) profile of MVC was evaluated in all the infant RMs from the study group by measuring the MVC plasma concentrations 4 h after the morning administration, when we expected drug levels to be maximal and when viral challenges were performed. Additional testing of the MVC plasma concentrations was performed at 2, 3, and 7 days post-viral challenge, just before the morning administration of the MVC, when we expected the plasma concentrations to be minimal (Fig. 2). The median plasma MVC concentrations at the time of each of the 6 viral challenges were 410 ng/ml (range, 77 to 1,040 ng/ml), 886 ng/ml (range, 29 to 1,910 ng/ml), 115 ng/ml (range, 23 to 267 ng/ml), 1,960 ng/ml (range, 815 to 3,720 ng/ml), 1,435 ng/ml (range, 5 to 5,340 ng/ml), and 64 ng/ml (range, 27 to 1,520 ng/ml), respectively (Fig. 2). In the unchallenged MVC-treated controls, plasma MVC concentrations were in the same range: 248 and 261 ng/ml (Fig. 3A). These levels are similar to the range seen in humans receiving a single 300-mg dose of MVC (618 to 888 ng/ml) (36, 37). The median MVC concentrations in plasma just prior to the morning dose (the minimal coverage concentration) were 59 ng/ml (range, 25 to 271 ng/ml), 46 ng/ml (range, 15 to 214 ng/ml), 28 ng/ml (range, 13 to 144 ng/ml), 11 ng/ml (range, 5 to 21 ng/ml), 33 ng/ml (range, 21 to 62 ng/ml), and 25 ng/ml (range, 19 to 44 ng/ml), respectively, at 3 days postchallenge, demonstrating steady and measurable MVC trough levels. In the MVC-treated controls, the minimal concentrations of MVC were 207 and 33 ng/ml (Fig. 3A). Overall, these levels were slightly lower than those measured at the same interval post-MVC administration in humans receiving a single 300-mg dose (34 to 43 ng/ml) (36, 37).
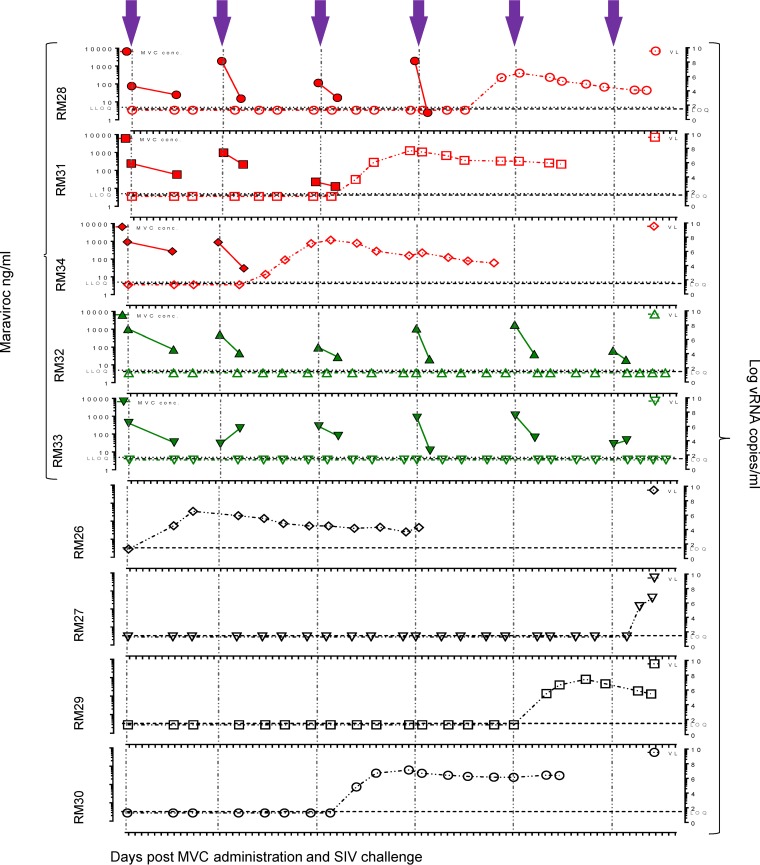
FIG 2.
Comparative assessment of MVC pharmacokinetics and plasma VLs at the time of and after the SIVmac766XII challenge. MVC concentrations in plasma at 4 h (maximum concentration) and 16 h (minimum concentration) after systemic administration of 150 mg/kg of MVC are shown; plasma VLs are shown at the corresponding time points of treatment and viral challenge for infant RMs in the MVC-treated group and untreated controls. Closed symbols, MVC concentration; open symbols, viral loads. The MVC concentration is expressed as nanograms per milliliter of plasma, and the viral load is expressed as the logarithm of the number of viral RNA copies per milliliter of plasma. Gray dotted lines, the lower limit of quantification (LLOQ; 5 ng/ml) of the bioanalytical LC-MS/MS method; short dashed lines, the limit of viral load quantification (LOQ; 30 copies per ml); violet arrows, time of viral challenge.
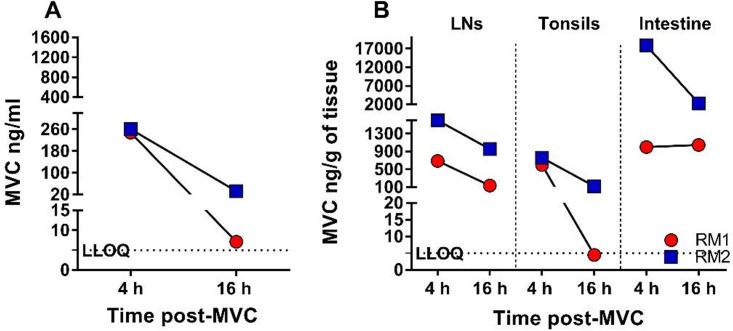
FIG 3.
Pharmacokinetic analysis of the MVC concentrations in the plasma and tissues of two infant RMs from the MVC-treated control group. (A) MVC concentrations in plasma at 4 h (maximum concentration) and 16 h (minimum concentration) after systemic administration of 150 mg/kg of MVC. The MVC concentration is expressed as nanograms per milliliter of plasma. (B) MVC concentrations in LNs, tonsils, and intestine at 4 h and 16 h after systemic administration. The MVC concentration is expressed as nanograms per gram of tissue. The black dotted lines show the lower limit of quantification (LLOQ; 5 ng/ml) of the bioanalytical LC-MS/MS method.
At 4 h after drug administration, the MVC concentrations in the tissues collected from the MVC-treated controls were 689 and 1,597 ng/g in the LNs, 597 and 759 ng/g in the tonsils, and 998 and 17,869 ng/g in the gut (Fig. 3B). The MVC concentrations in tissues immediately prior to the morning dose were 136 and 958 ng/g in the LNs, 5 and 122 ng/g in the tonsils, and 1,046 and 2,322 ng/ml in the gut (Fig. 3B). In only two of the collected samples (plasma from RM28 2 days postchallenge 4 (i.e., after the 4th SIV infection challenge) and tonsil from RM1), MVC concentrations were below the limit of quantification (BLQ) of 5 ng/ml of the method used (Fig. 2 and 3). We imputed a numerical value for these samples (5 ng/ml) because it was within 20% of the lower limit of quantification (LLOQ) (38). Interestingly, in RM28, the MVC concentration below the limit of quantification was followed by SIV infection (Fig. 2).
Taken together, these data demonstrate that an oral MVC dose of 150 mg/kg bid given to infant RMs 4 h prior to the viral challenge produced a plasma MVC concentration that approximated the plasma MVC concentration in humans, that the tissue concentrations of MVC were similar to those observed in humans (39) and high enough to block CCR5, and that the minimal concentrations of MVC were generally sufficient to compete with the virus for CCR5 coreceptor occupancy, albeit the concentrations of MVC decreased dramatically prior to the daily administration, in some instances to below 5 ng/ml.
Orally administered MVC effectively blocks CCR5 expression on the surface of CD4+ T cells.
To investigate whether or not CCR5 blockade with MVC impacts oral SIV transmission to infant RMs, we first determined the therapeutic impact of MVC by measuring the CCR5 receptor occupancy in blood, LNs, tonsil, and gut. This test monitors the levels of internalization of CCR5 receptors on the surface of CD4+ T cells following ex vivo macrophage inflammatory protein 1β (MIP-1β) exposure; these levels are indicative of the level of receptor occupancy. Complete prevention of CCR5 internalization indicates complete coreceptor occupancy.
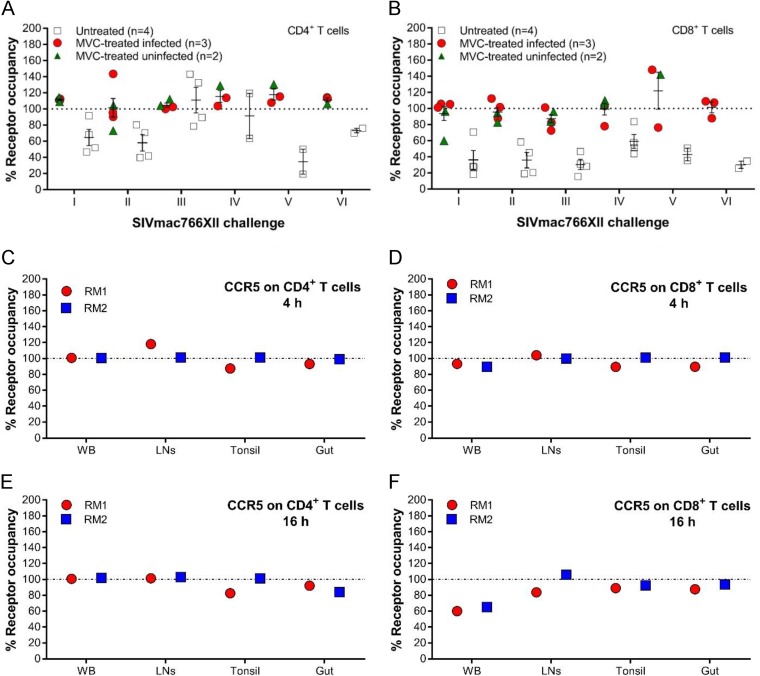
Close monitoring of CCR5 occupancy on the surface of circulating CD4+ and CD8+ T cells (Fig. 4) identified significant differences between the MVC-treated and untreated groups before the first viral challenge (CD4+ T cell CCR5 occupancy, P = 0.0159; CD8+ T cell CCR5 occupancy, P = 0.0317), before the second viral challenge (CD4+ T cell CCR5 occupancy, P = 0.0317; CD8+ T cell CCR5 occupancy, P = 0.0159), and before the third viral challenge (CD4+ T cell CCR5 occupancy, P = 0.0286; CD8+ T cell CCR5 occupancy, P = 0.0159) (Fig. 4A and B). For the remaining 3 challenges, statistical analyses could not be performed because the number of uninfected RMs was too low.
FIG 4.
CCR5 receptor occupancy on CD4+ and CD8+ T cells from blood from infant RMs. (A and B) Percentage of CCR5 receptor occupancy on circulating CD4+ T cells (A) and CD8+ T cells (B) from infant RMs included in the SIV challenge study at the time of SIVmac challenge. Data are presented as individual values with the group means (long solid lines) and standard errors of the means (short solid lines). The Mann-Whitney test was used to calculate the exact P value. (C to F) Percent coreceptor occupancy in the blood of the infant RMs from the MVC-treated unchallenged control group on CD4+ (C) and CD8+ (D) T cells 4 h after MVC administration (maximum concentration) and on CD4+ (E) and CD8+ (F) T cells 16 h after MVC administration (minimum concentration). WB, whole blood.
In the MVC-treated controls, MVC efficiently blocked CCR5 on CD4+ T cells in all tissue samples analyzed (Fig. 4C and D). In the gut, CCR5 blockade was not complete, even though the blocking efficiency was high, with average levels of 96% when the MVC concentration was expected to be high (Fig. 4C) and 88% when the MVC concentration was expected to be low (Fig. 4D). Similarly, MVC partially blocked CCR5 expression on CD8+ T cells (Fig. 4E and F), with an average CCR5 occupancy of 91% when the MVC concentration in whole blood was expected to be high and 63% when the MVC concentration was expected to be low; when the concentrations were expected to be high and low, the blockade of expression was 102% and 95%, respectively, in the LNs; 95% and 91%, respectively, in the tonsil; and 95% and 91%, respectively, in the gut (Fig. 4).
Systemic MVC administration only marginally impacted oral SIVmac transmission to infant RMs.
The main goal of this study was to investigate whether or not CCR5 blockade with MVC impacts oral SIV transmission to infant RMs. MVC-treated and control infant RMs were repeatedly challenged with 10,000 IU of SIVmac766XII orally in an atraumatic fashion until all 4 RM controls became infected (6 challenges). At the end of the challenge experiments, 3/5 (60%) of the MVC-treated infant RMs were also SIV infected, while 2/5 infant RMs remained uninfected, in spite of being challenged 6 times under the same conditions (Fig. 2). However, the levels of protection in the MVC-treated RMs were not significant (P = 0.15). We conclude that systemic MVC administration does confer significant protection of the infant RMs against oral SIVmac challenge. This conclusion is also supported by the observation that the numbers of exposures necessary to infect the infant RMs in the two groups were similar, with the 4 control infant RMs becoming infected after 1, 3, 5, and 6 SIVmac766XII oral challenges, respectively, and the 3 SIV-infected MVC-treated infant RMs becoming infected after 2, 3, and 4 inoculations, respectively.
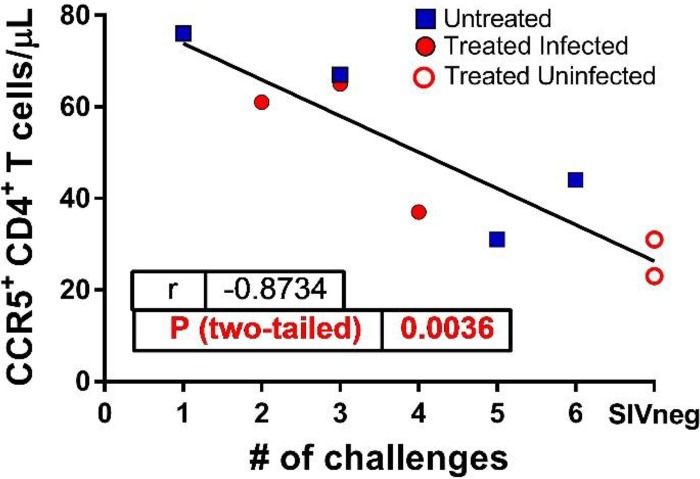
We next sought to correlate the efficacy of SIVmac766XII transmission (estimated on the basis of the number of viral challenges) with the availability of CCR5+ CD4+ T target cells. This analysis was prompted by our previous correlative studies in natural hosts of SIVs that found strong correlations between the target cell availability at mucosal sites and the efficacy of mucosal (intrarectal, intravaginal, and oral) transmission (24, 29). We assessed CCR5 expression on circulating CD4+ T cells of the infant RMs prior to the MVC treatment and correlated it to the number of viral exposures prior to infection. In a conservative approach, we listed the uninfected RMs as infected at the seventh challenge. These two variables were very strongly correlated (P = 0.0036) (Fig. 5), confirming our hypothesis and strongly supporting the paradigm that target cell availability determines susceptibility to infection in natural hosts of SIVs.
FIG 5.
Correlation between the levels of CCR5 expression on peripheral CD4+ T cells and the number of viral challenges required to infect MVC-treated and untreated RMs. Results for the two MVC-treated, SIV-uninfected RMs are also shown.
The SIVmac766XII stock consists of a swarm of 12 viral variants equally represented and phenotypically matched, allowing for variant enumeration (Fig. 1) (40); therefore, the numbers of transmitted viral variants in the MVC-treated group and the untreated controls were determined. The number of transmitted/founder lineages did not identify any difference in the number of transmitted variants between the two groups, with each animal being infected with only 1 of the 12 possible variants. This result suggests that the infant RMs were not overexposed to virus, which could have offset the protective effect of MVC.
SIVmac766XII uses CCR5 and GPR15 to enter transfected target cells.
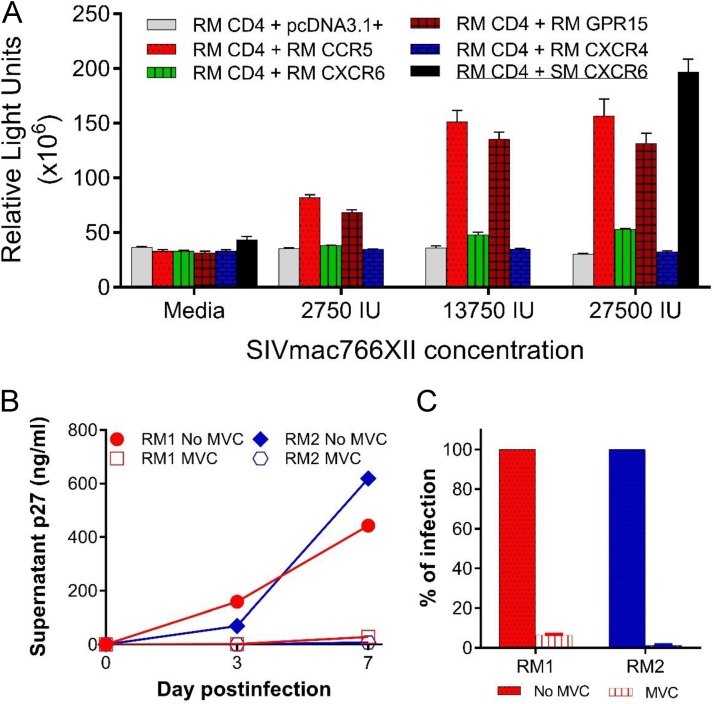
To understand why the MVC administration only marginally impacted oral SIV transmission in infant RMs, we first investigated the coreceptor usage of SIVmac766XII. Several SIVsmm strains from sooty mangabeys were reported to use CXCR6 (41, 42); if CXCR6 use were true for SIVmac, it could have resulted in a more promiscuous coreceptor use and the inefficacy of the CCR5 blockade. First, we assessed SIVmac766XII coreceptor usage in a CF2th-Luc reporter cell system and documented robust viral entry through both RM CCR5 and RM GPR15 (Fig. 6A) but only minimal entry through RM CXCR6 and no virus entry through RM CXCR4, in agreement with the findings of previous studies of coreceptor usage of the SIVmac strains (41). As controls, other SIVsmm strains showed a robust entry through sooty mangabey CXCR6 (Fig. 6A, SM CXCR6, black bar), as previously reported (42).
FIG 6.
SIVmac766XII use of RM coreceptors. (A) CF2th-Luc cells that contain a Tat-driven luciferase reporter were transfected with expression plasmids containing RM CD4 and coreceptor. Cells were infected 48 h later with SIVmac766XII (2,750 IU, 13,750 IU, and 27,500 IU), and entry was quantified 72 h later by measuring luciferase production as the number of relative light units (RLU). Infections were carried out in triplicate, and bars represent mean and standard error of the mean (SEM) values. Sooty mangabey CXCR6 (SM CXCR6), which is a functional coreceptor, was included at the highest inoculum for comparison. (B) SIVmac766XII infectivity on PBMCs. PBMCs from two RMs were stimulated for 3 days with ConA and IL-2 and then pretreated for 1 h with maraviroc (MVC; 15 μM) or with vehicle alone (no drug) and infected with SIVmac766XII (550 IU). Infection was measured by determination of the level of p27 production in the supernatant. Each line indicates one infection condition per animal, and the data represent the mean and standard error of the mean. (C) Day 7 p27 antigen level in the supernatant as a percentage of that in cells without blocking agent.
CCR5 is the main coreceptor used by SIVmac766XII to infect primary RM PBMCs.
We further assessed the SIVmac766XII coreceptor usage during infection of RM primary lymphocytes. Peripheral blood mononuclear cells (PBMCs) from two different RMs were infected with SIVmac766XII in the presence or absence of MVC, and virus replication was monitored by measuring the p27 Gag antigen level in the supernatant. As shown in Fig. 6B, MVC blocking of CCR5 dramatically inhibited infection of primary lymphocytes, reducing replication at 7 dpi by 94 to 99% (Fig. 6C). Although blocking was not 100% complete, indicating limited entry through non-CCR5 pathways, these data demonstrate that CCR5 is the overwhelmingly dominant pathway for SIVmac766XII in primary PBMCs, despite the efficient in vitro use of both RM CCR5 and RM GPR15 in transfected cells. This finding is concordant with previous results showing that SIVmac is highly dependent on CCR5 for primary lymphocyte infection (42–44). As such, our results showed that SIVmac766XII is an appropriate viral strain to model oral transmission of HIV-1.
Postinfection effects of the MVC treatment.
We further analyzed the impact of the MVC treatments on the natural history of SIV infection in infant RMs. In these studies, we included the three MVC-treated RMs and the four untreated infant RMs that became infected with SIVmac766XII.
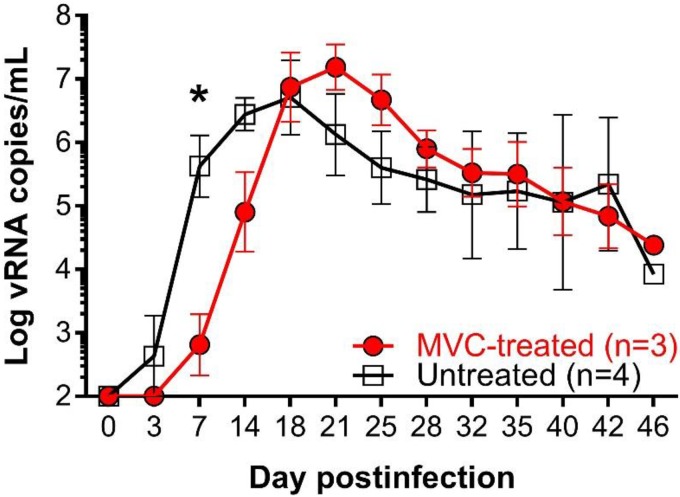
MVC treatment delayed ramp-up viremia.
A significant delay of the ramp-up VLs was observed in the MVC-treated infants (P = 0.05) (Fig. 7). In addition to the delay in ramp-up dynamics, the peak VL for MVC-treated animals was reached at 21 dpi, whereas the peak VL was reached at 18 dpi for the control RMs. However, the MVC effects on the timing and magnitude of the peak VL post-peak resolution and later in infection were not significantly different between the two groups (Fig. 7).
FIG 7.
Changes in the viral loads in the SIVmac-infected RMs treated with MVC from those in the untreated controls. The plasma vRNA loads (number of copies per milliliter expressed in the logarithmic format) in the MVC-treated and untreated groups are shown. Data are geometric means, with the bars representing the standard error of the mean. The Mann-Whitney test was used to calculate the exact P value (P = 0.05).
MVC treatment did not alter the dynamics of the peripheral CD4+ and CD8+ T cell populations or subsets in SIV-infected infant RMs.
In humans, MVC treatment does not significantly impact CD4+ and CD8+ T cell populations (45). The peripheral CD4+ (Fig. 8A) and CD8+ (Fig. 8B) T cell counts were compared throughout the follow-up between MVC-treated and untreated RMs, and no significant difference was observed between the two groups (Fig. 8). Peripheral CD4+ T cell depletion was transient, with the CD4+ T cell counts being partially restored by 24 dpi in both groups and declining slowly during the follow-up (Fig. 8A).
FIG 8.
Longitudinal analysis of absolute CD4+ and CD8+ T cell counts in blood from the SIV-infected infant RMs. (A) Changes in CD4+ T cells; (B) changes in CD8+ T cells. (Left) Results for individual animals. (Right) Average results. The vertical bars in the right panels are the standard errors of the means.
We next monitored the impact of MVC treatment on the memory subsets of CD4+ and CD8+ T cells, prompted by a recent report that CCR5 blockade in vivo might affect the trafficking of memory T cells expressing CCR5 to the site of the cognate antigen, preventing their proper stimulation and acquirement of effector functions and antiviral activity (46). However, comparison between MVC-treated and untreated infant RMs throughout the follow-up did not reveal any significant difference in the peripheral naive (CD28+ CD95neg), central memory (CD28+ CD95+), and effector memory (CD28neg CD95+) subsets of CD4+ or CD8+ T cells (data not shown). Our data indicate that MVC treatment had no discernible impact on the major T cell populations and subsets in the SIV-infected infant RMs.
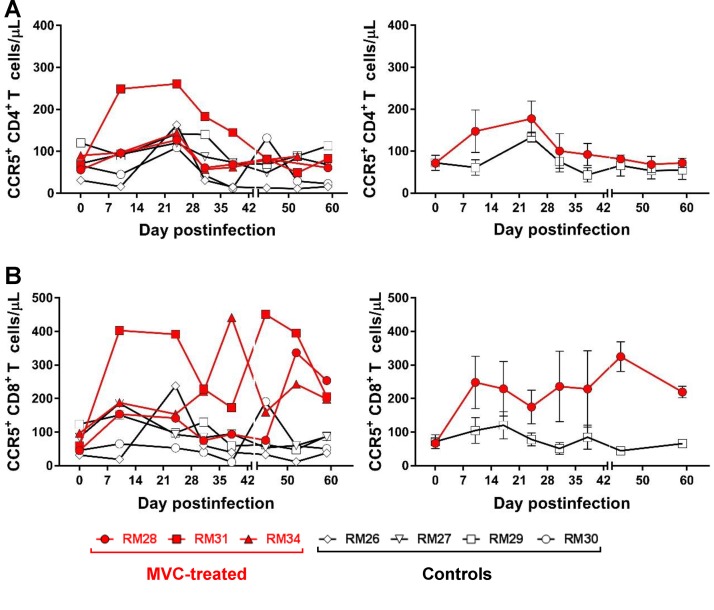
MVC administration did not impact the levels of circulating CD4+ and CD8+ cells expressing CCR5 in SIV-infected infant RMs.
CCR5 expression on the surface of CD4+ T cells is highly variable, depending on CCR5 polymorphisms and expression of its chemokine ligand (47, 48), leading to variations in HIV target cell availability that impact virus entry, susceptibility to infection (49), and the therapeutic efficacy of CCR5 inhibitors (50). We therefore monitored CCR5 expression on both CD4+ and CD8+ T cells throughout the follow-up (Fig. 9) and report that they were similar between the two groups, being increased during the first weeks of treatment (Fig. 9) and returning to preinfection levels by 28 dpi. The CD4+ T cells expressing CCR5 gradually declined during the follow-up (Fig. 9A and B), likely as a result of the direct virus targeting of the CD4+ T cells expressing CCR5.
FIG 9.
Longitudinal analysis of absolute counts of CD4+ and CD8+ T cells expressing CCR5 in blood from the SIV-infected infant RMs. (A, B) Changes in CCR5+ CD4+ T cells; (C, D) changes in CCR5+ CD8+ T cells. (Left) Results for individual animals; (right) average results. The vertical bars in the right panels are the standard errors of the means.
MVC treatment had no discernible impact on the levels of T cell activation and proliferation in SIV-infected infant RMs.
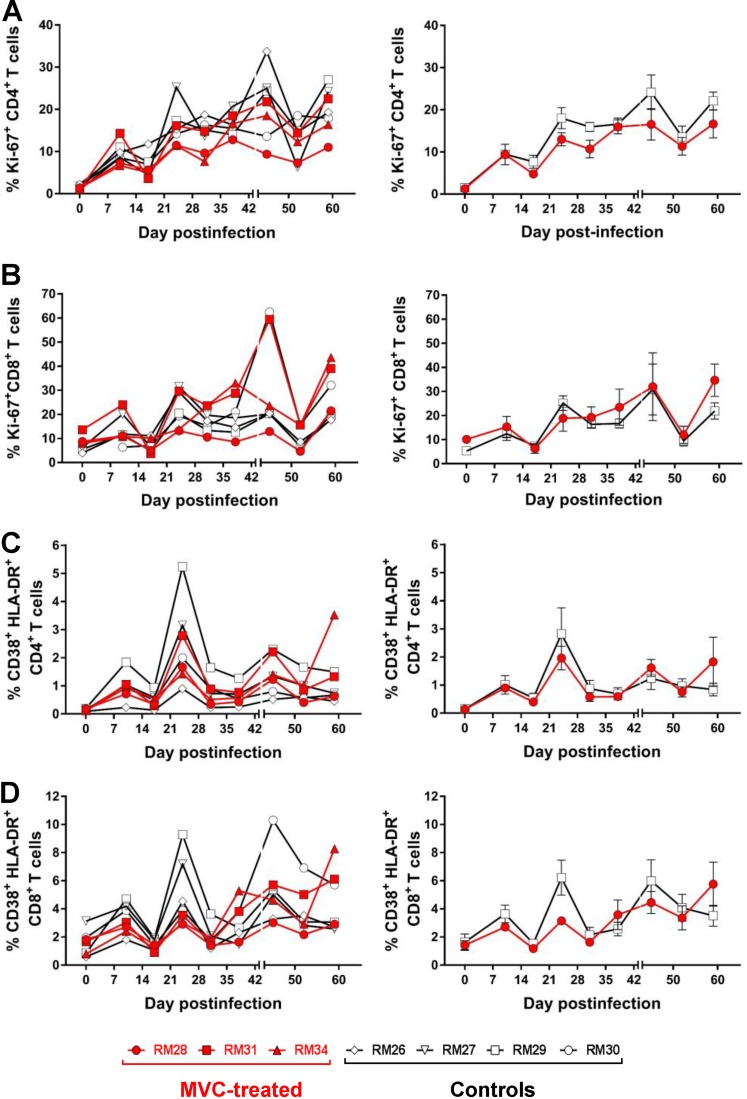
These analyses were prompted by studies reporting either that MVC treatment results in a resolution of chronic immune activation that goes beyond the levels of viral control (51) or, conversely, that MVC administration increases the levels of T cell activation (52). While SIV infection was associated in both MVC-treated and untreated RMs with increased levels of CD4+ and CD8+ T cell proliferation (Fig. 10A and B) and immune activation (Fig. 10C and D), no significant difference was observed throughout the follow-up between the two groups. We conclude that MVC administration did not significantly influence the levels of CD4+ and CD8+ T cell immune activation and proliferation in SIV-infected infant RMs.
FIG 10.
Changes in the frequency of CD4+ and CD8+ T cells expressing proliferation and immune activation markers in blood from the SIV-infected infant RMs. (A) Frequency of CD4+ T cells expressing the proliferation marker Ki-67; (B) frequency of CD8+ T cells expressing the proliferation marker Ki-67; (C) frequency of CD4+ T cells expressing immune activation markers CD38 and HLA-DR; (D) frequency of CD8+ T cells expressing immune activation markers CD38 and HLA-DR. (Left) Results for individual animals. (Right) Average results. The vertical bars in the right panels are the standard errors of the means. The Mann-Whitney test was used to calculate the exact P value.
DISCUSSION
While breast-feeding is the healthiest practice for feeding infants, breast milk can also transmit SIV/HIV infection when mothers are infected (53). Without prevention, 13 to 48% of babies born to HIV-1-infected mothers become HIV infected (4). Perinatal administration of short-term ART to HIV-infected mothers dramatically decreases the rates of HIV-1 MTIT (54), yet even with ART prophylaxis, breast-feeding transmission accounts for half of the MTIT cases (55), with overall HIV breast-feeding transmission rates being approximately 13% higher in the mothers that seroconvert postpartum (29%) (55) or are in the AIDS stage (37%) (56). Administration of ART to breast-feeding mothers and prolonged ART prophylaxis to infants significantly impact HIV breast-feeding transmission (57), but this strategy has yet to assess the rates of residual transmission, the degree of drug toxicity for the infant, and the risk for transmission/selection of drug-resistant viruses. Also, to be effective, this strategy still has to circumvent multiple barriers related to implementation (27).
HIV breast-feeding transmission is devastating in developing countries, where 95% of babies are breast-fed for up to 2 years (58) and where the benefits of breast-feeding outweigh the risks of breast-feeding transmission (58), as the use of replacement feeding is hindered by access to clean water, the cost, the availability of formula, and cultural background (54). Strategies allowing HIV-infected mothers to breast-feed while completely controlling breast-feeding transmission are badly needed.
The rates of SIV MTIT are negligible in African NHP species (AGMs, mandrills, or sooty mangabeys) that are natural hosts of SIVs (24–26, 59), in spite of the fact that milk VLs are comparable to those observed in pathogenic infections (60). In experimental studies, we failed to document any SIV breast-feeding transmission in mandrills, even during the acute infection of lactating dams (24). Meanwhile, these low rates of SIV breast-feeding transmission are associated with low levels of mucosal target cells (24, 26), and we documented that, under experimental conditions, susceptibility to mucosal SIV infection of natural hosts is age related and correlates with the availability of target cells at the mucosal sites (29). These features led us to hypothesize that the mucosal milieu of breast-fed infants represents an effective barrier to HIV breast-feeding transmission and that the experimental blockade of mucosal target cell availability may represent an effective new strategy to prevent HIV breast-feeding transmission.
There are multiple rationales for blocking CCR5 to prevent HIV transmission, the most important of which is that CCR5 is the main coreceptor for HIV-1 (61, 62) and is thus relevant for the first steps of infection; furthermore, transmitted/founder viruses always use CCR5 for virus entry (63). CCR5 antagonists inhibit the replication of R5-tropic HIV variants by blocking viral entry into the target cells (64). MVC is the only FDA-approved CCR5 antagonist (65) and blocks HIV-1 entry by binding CCR5 and suppressing viral infection (66). In addition to modulating CCR5 expression and function (67), MVC may have immunomodulatory effects by blocking binding of the natural ligands of CCR5 (MIP-1α, MIP-1β, and RANTES) (68). As a result, CCR5 blockade in HIV-infected subjects reduces immune activation and improves CD4+ T cell restoration (69, 70). For the CCR5 blockade, we used MVC, which is FDA approved, reasoning that, if proven effective, our strategy could be readily implemented to prevent HIV breast-feeding transmission.
Similar to previous studies on MVC safety and tolerance (71, 72), orally administered MVC was safe and well tolerated in all infant RMs, without any obvious side effects, adverse reactions, or impact on the development of the immune system of the infants due to the blockade of a chemokine that may decisively contribute to immune system maturation (73). As such, we concluded that prolonged CCR5 blockade did not have any deleterious effects on the immune effectors.
Surprisingly, systemic MVC administration only marginally impacted oral SIVmac transmission to infant RMs. At the end of the SIV challenge experiments, when all the RMs in the untreated control group were infected with SIVmac, 60% (3 out of 5 RMs) of the MVC-treated infant RMs were also infected. Furthermore, MVC treatment had no effect on the number of viral challenges needed to transmit SIV or the outcome of SIV infection in infant RMs. The only discernible difference observed between the SIV-infected MVC-treated and untreated infant RMs was a significant delay of ramp-up viremia in the MVC-treated infants.
This lack of efficacy of MVC in preventing oral SIV transmission to infant RMs and its limited impact on the key parameters of SIV infection in SIV-infected infant RMs might be due to multiple causes, such as (i) underdosing of MVC, which could limit its therapeutic efficacy; (ii) overexposure to the virus during the challenge experiments, which might have offset the effects of MVC; and (iii) the biological promiscuity of SIVs, which may use coreceptors other than CCR5 to infect their target cells (74–76). To examine our MVC dosing strategy, we performed two sets of experiments: first, as the MVC interactions with CCR5 might be different between macaques and humans, we sought to demonstrate that MVC successfully blocks CCR5 in infant RMs, and to this end, we performed an occupancy test (72). In this test, the binding of MVC to CCR5 coreceptors prevents internalization of CCR5 by MIP-1β, and thus, the degree of CCR5 internalization by MIP-1β provides an indirect measurement of MVC binding to CCR5. The occupancy test demonstrated that, at the time of viral challenge, 4 h after oral administration of MVC, CCR5 internalization was robustly blocked. Similarly, testing of the samples collected just prior to drug administration showed that the minimal levels of MVC were generally sufficient to compete with the virus for CCR5 coreceptor occupancy. Note, however, that the lowest coreceptor occupancy was observed in tonsils, a potential site of virus entry upon oral transmission (77).
Next, we measured the MVC plasma concentrations at the time of viral challenge, and we documented that the steady-state exposure to MVC was similar to the therapeutic concentrations in HIV-infected patients. In a different group of infant RMs, we performed an MVC dosage determination in tissues and showed that the drug reaches steady levels both in tonsils and at the mucosal sites, suggesting that the dose of MVC employed here was sufficient to realize a clinical effect. We noted, however, that the minimal levels of MVC, measured just prior to the morning administration of the drug, were low and, at least in two instances, below the limit of detection of the assay. Interestingly, the infant RMs which remained uninfected at the completion of the study also had very low minimal levels of MVC, suggesting that the clinical dose used here is probably sufficient for prevention. Nevertheless, drug monitoring revealing a relatively abrupt decline in the MVC levels in infant RMs may also point to a different metabolism of the drug in RMs compared to humans, thus calling for a more detailed evaluation. This aspect is particularly important, as the MVC effect observed here was marginal, and in one case (RM28), a documented undetectable plasma level of MVC was followed by SIV infection.
We demonstrated that the MVC dose resulted in 100% receptor occupancy of circulating CD4+ T cells at the time of SIV challenge (4 h postdose) for all but 1 of the 6 challenges. Additionally, Massud et al. have demonstrated that MVC concentrations of >10 ng/ml are sufficient to saturate CCR5 receptor binding in RM PBMCs (39). Given that only 1 of 21 trough plasma concentrations (Ctrough) was less than 10 ng/ml (Fig. 2), we believe that there is sufficient evidence that our dosing strategy recapitulated what would be deemed an effective plasma exposure. Furthermore, the human plasma PK of MVC among pediatric patients (2 to 6 years of age), which were not available at the time of submission of this article, demonstrate that the concentrations achieved in our infant RMs were in the range of or higher than those achieved by the use of effective dosing strategies in human children (average maximum concentration in plasma [Cmax] = 581 ng/ml at 2 h postdose, average concentration at 4 h = ∼375 ng/ml, and average Ctrough = ∼50 ng/ml [78]). Intensive PK studies to characterize MVC absorption and clearance are not feasible in infant RMs because the required blood volume exceeds safety thresholds. Thus, this PK characterization would require our use of an adult RM model, from which distribution and clearance may not extrapolate well to infant RMs. Consistent with the human pediatric data, intensive PK studies in adult macaques following a single oral 44-mg/kg dose demonstrate that maximal concentrations are achieved by 2 h postdose in the plasma. Thus, it is possible that our 4-h collection time does not capture the Cmax in our infant RMs. Even so, the concentrations demonstrated herein would represent a worst-case scenario that still saturates CCR5 receptor binding at 4 h postdose, which (on the basis of previous exposure-response data) should have been maintained until our next dose was administered at 12 h. Thus, there is sufficient evidence to suggest that the dosing strategy used in this model of pediatric transmission recapitulates effective and physiologic plasma exposure.
To rule out the possibility of virus overdosing, we performed single-genome amplification of the molecular tag (34, 79) and showed that none of the MVC-treated or control infant RMs were infected with more than one viral variant, thus discarding the possibility of an eventual SIVmac overexposure that could have offset the protective effect of MVC.
Finally, to discard the hypothesis of a more promiscuous receptor usage by SIVmac than by HIV-1, we assessed the coreceptor usage of SIVmac766XII. Differently from HIV-1, which uses CCR5 as the main coreceptor and which may expand to use CXCR4 in advanced stages of infection, the majority of SIV strains are more promiscuous, being able to use, in addition to CCR5, BOB/GPR15 (80, 81) and Bonzo/STRL33 (82, 83) for efficient entry into the target cells. More recently, multiple SIV strains were reported to use alternative coreceptors for viral entry, most notably, CXCR6 (74–76). This coreceptor usage pathway was reported for the SIVs isolated from both AGMs and sooty mangabeys (74–76). However, testing of our viral stock for coreceptor usage clearly demonstrated that CCR5 is the only coreceptor used by SIVmac766XII in vivo, which is similar to the receptor usage of transmitted/founder HIV-1 strains and which validates our choice of challenge strain. While SHIV env strains might have been preferable to SIVmac in this study, fully functional transmitted/founder SHIVs became available only after the completion of this study (84, 85).
As such, our study indicates that MVC was relatively efficient in blocking CCR5 and well tolerated in infant RMs but exerted only a marginal effect on SIV oral transmission. Failure to block infection was not due to underdosing of MVC, overexposure to the virus during the challenge phase, or alternative coreceptor usage. While a more rapid MVC metabolism in RMs than in humans might have impacted MVC efficacy to prevent infection, additional studies would be needed to explore the prophylactic efficacy of target cell blockade for preventing oral HIV transmission through breast-feeding.
Finally, note that the systemic administration of MVC did not prevent the intrarectal transmission of SHIV (39). Furthermore, CCR5 blockade with MVC was reported to be ineffective in preventing rectal HIV transmission in humans in an ex vivo challenge model (86), in stark contrast to the results obtained by the systemic administration of pre- and postexposure prophylaxis, which is extremely effective at preventing HIV transmission (87, 88). Furthermore, subcutaneous administration of tenofovir effectively prevented oral infection with SIV (89). Interestingly, this lack of efficacy of systemic MVC in preventing SIV/HIV mucosal transmission is in contrast to the results observed after topic administration, which indicated the efficacy of MVC in preventing both vaginal (31) and rectal (90) SHIV infection in RMs. As such, it is possible that systemic CCR5 blockade by MVC is not sufficiently effective in blocking CCR5 and preventing HIV transmission and that the use of new, more potent CCR5 inhibitors will have a better effect in preventing oral SIV transmission, as recently suggested (91).
MATERIALS AND METHODS
Ethics statement.
Eleven RMs aged 6 months were included in this study. They were all housed and maintained at the Plum Borough animal facility of the University of Pittsburgh in agreement with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The RMs were fed and housed according to regulations set forth by the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (92). The RM infants were socially housed indoors in stainless steel cages, had a 12-h light/12-h dark cycle, were fed twice daily, and were provided water ad libitum. They were also given various toys and feeding enrichments. The animals were observed twice daily, and any signs of disease or discomfort were reported to the veterinary staff for evaluation. For sample collection, animals were anesthetized with 10 mg/kg ketamine hydrochloride (Parke-Davis, Morris Plains, NJ, USA) or 0.7 mg/kg tiletamine hydrochloride and zolazepam (Telazol; Fort Dodge Animal Health, Fort Dodge, IA), injected intramuscularly. After viral challenge, all the infant RMs that became infected were followed for 120 days and sacrificed by intravenous administration of barbiturates prior to the onset of any clinical signs of disease. The animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) (protocol number 13112685).
Virus stock.
The SIVmac766XII stock (Fig. 1) is composed of parental SIVmac766, previously identified to be a transmitted/founder virus and infectious molecular clone (40), and 11 other viral variants differing from the parental clone by 3 synonymous mutations in integrase, similar to the SIVmac239X swarm previously described (34). The virus stock was generated by transfecting 293T cells with equal amounts of each of the 12 molecularly modified plasmids for 24 h using the TransIT HEK-293 transfection reagent (Mirus Bio) according to the manufacturer's instructions. The culture medium was changed at 24 h posttransfection and again at 48 h posttransfection. At 72 h posttransfection, virus-containing supernatant was clarified by centrifugation, sterile filtered through a 0.45-μm-pore-size filter, aliquoted, and stored at −80°C. A series of small-scale cotransfections with subsequent sequencing analyses to determine the relative proportion of each tagged variant in the virus pool was used to identify the relative input proportion of each plasmid in the DNA cotransfection pool that would yield roughly equal proportions of tagged viruses in the SIVmac766XII stock. Virus titers were determined using TZM-bl reporter cells (NIH AIDS Reagent Program), which contain a Tat-inducible luciferase and a β-galactosidase gene expression cassette. Infectious titers were measured by counting the individual β-galactosidase-expressing cells per well in cultures infected with serial 3-fold dilutions of virus. The results for wells containing dilution-corrected blue cell counts within the linear range of the virus dilution series were averaged to generate an infectious titer in infectious units (IU) per milliliter. The SIVmac766XII stock contained 2.75 × 105 IU/ml. This newly derived infectious stock has been used to successfully infect rhesus macaques intrarectally (n = 3) at 3 × 105 IU/ml with 6 to 8 detectable T/F variants and intravaginally (n = 2) at 3 × 105 IU/ml with 3 to 4 T/F variants and one additional animal intravaginally at 6 × 105 IU/ml with 10 variants (B. Keele, unpublished results).
MVC treatment and animal infection.
Five RMs received a total daily dose of 300 mg/kg administered as two divided doses (150 mg/kg) per os with food. The MVC dose was allometrically scaled to twice the dose of humans (300 mg). One month after MVC initiation, all the MVC-treated infants, together with 4 untreated infant RMs, were orally administered 10,000 IU of SIVmac766XII. Viral challenge occurred 4 h after the morning MVC administration, when the concentrations of MVC were demonstrated to be maximal. For infant RM infections, the animals were placed on their backs prior to inoculation. The virus stock (2.75 × 105 IU/ml) was placed in 1-ml syringes, the needle was removed, and 0.5 ml of the viral stock was placed inside each pouch. After each exposure on the two mouth sides, we waited at least 5 min for the stock to disperse around the pouch. Viral challenge was repeated every 2 weeks up to 6 times. At the time of the viral challenges, CCR5 coreceptor occupancy (35) was also closely monitored. To evaluate the concentrations and pharmacokinetics of MVC in the tissues, we enclosed in our experimental design two RMs treated with MVC similarly to the infants in the study group but not challenged.
Sampling.
At the time of viral challenge, blood (1.5 ml) was collected into EDTA-containing CPTs to monitor coreceptor occupancy and MVC plasma levels and then every 3 days to monitor SIV infection. Once an animal was demonstrated to be SIV infected, sampling was scheduled to monitor the acute and early chronic infection (10, 17, 24, 31, 38, 45, 59 dpi). Superficial LNs, tonsils, and intestine were collected from just the two MVC-treated SIV-unexposed infant RM controls. To prevent changes in CCR5 expression due to storage and shipping of unprocessed peripheral blood mononuclear cells, all blood and tissue samples were processed within 60 min from the time of collection.
Analysis of plasma MVC concentrations.
MVC concentrations in plasma samples collected 4 h after administration of one oral dose of 150 mg/kg were measured by a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method using a Shimadzu high-performance liquid chromatography system for separation and an AB Sciex API 5000 mass spectrometer (AB Sciex, Foster City, CA, USA) equipped with a turbo spray interface for detection. The calibrated linear range was 5 to 5,000 ng/ml in plasma. All samples were extracted by protein precipitation, with a stable, isotopically labeled internal standard (MVC-d6) added for quantification. All calibration standards and quality controls (QCs) were prepared in drug-free NHP plasma. Calibration standards and QC samples met the 15% acceptance criterion for precision and accuracy. Plasma MVC concentrations were expressed as the number of nanograms of MVC per milliliter.
Analysis of tissue MVC concentration.
MVC concentrations in LNs, tonsils, and intestine were measured on samples collected either 4 h after administration of an oral dose of 150 mg/kg MVC or immediately before MVC administration. Tissue MVC concentrations were measured with the same methodology used to measure plasma MVC concentrations and were expressed as the number of nanograms of MVC per gram of tissue.
MIP-1β internalization assay.
The coreceptor occupancy test was performed to assess MVC binding to CCR5 in blood and in LNs, tonsils, and intestine (35, 72, 93). The principle of this test is that the binding of MVC prevents internalization of CCR5 by MIP-1β and, thus, that the degree of CCR5 internalization by MIP-1β provides an indirect measurement of MVC binding to CCR5.
PBMC-rich plasma samples were isolated by centrifugation of the CPT at 2,200 rpm for 25 min. The cell pellets were resuspended in the plasma at 5 × 106 cells/ml, obtaining cell-enriched plasma samples. For the assay in blood, a 5 × 105-cell-enriched plasma sample (100 μl) was aliquoted into three separately labeled 5-ml fluorescent-activated cell sorting (FACS) tubes containing the isotype control (tube 1), MVC-stabilized CCR5 (tube 2), and the test sample (tube 3). Cells from LNs, tonsils, and intestinal biopsy specimens were collected as described previously (94), and 5 × 106 cells were resuspended in the plasma and aliquoted (100 μl) in three tubes as described above for blood. Fifty microliters of 1 μM MVC (CCR5 stabilizing solution) was added to tube 2; the same volume of 50 μl of 1% (wt/vol) phosphate-buffered saline (PBS)–bovine serum albumin was added to tubes 1 and 3. Tubes 1 to 3 were briefly vortexed and incubated at 37°C for 30 min, followed by centrifugation at 1,500 rpm for 5 min. The supernatant was discarded, and 15 μl of MIP-1β (100 nM) was added to all tubes. The mixture was then vortexed and incubated uncapped for 45 min at 37°C to enable CCR5 internalization. Then, 1 ml of 0.5% paraformaldehyde in PBS was added to each tube, and the tubes were then vortexed and incubated in the dark at room temperature (RT) for 10 min. The cells were then washed with PBS by centrifugation at 1,500 rpm for 5 min and stained with a combination of antibodies (Table 1) appropriate for the identification of CD4+ and CD8+ T cells expressing CCR5 and a combination of isotype and fluorescence-minus-one controls. Labeled cells were washed once with 1% fetal bovine serum (FBS)–PBS, fixed in 2% formaldehyde PBS (Affymetrix, Santa Clara, CA), and then acquired on the same day on a custom four-laser BD LSRII instrument (BD Bioscience). Only singlet events were gated, and a minimum of 250,000 live CD3 cells were acquired. Populations were analyzed using FlowJo software (version 7.6.5; TreeStar Inc., Ashland, OR), and graphs were generated with GraphPad Prism (version 6.04) software. The percentage of receptor occupancy was calculated using CCR5 expression data obtained for peripheral blood lymphocyte aliquots incubated with the chemokine in the presence of 1 μM MVC (tube 2) and in the absence of additional MVC (tube 3), as follows: percent receptor occupancy = [(percent CCR5 expression in tube 3)/(percent CCR5 expression in tube 2)] × 100.
TABLE 1.
Anti-human monoclonal antibodies used for flow cytometry and FACSa
| Marker | Clone | Fluorochrome | Company |
|---|---|---|---|
| CD3 | SP34-2 | V450 | BD Pharmingen |
| CD4 | L200 | APC | BD Pharmingen |
| CD8 | 3B5 | PE-Texas Red | Invitrogen |
| CD28 | CD28.2 | PE-Cy7 | BD Pharmingen |
| CD95 | DX2 | FITC | BD Pharmingen |
| CCR5 (CD195) | 3A9 | PE | BD Pharmingen |
| CD38 | AT-1 | FITC | BD Pharmingen |
| Ki-67 | B56 | PE | BD Pharmingen |
| HAL-DR | L243 | PE-Cy7 | BD Pharmingen |
| Viability dye | NA | Blue fluorescent reactive dye | Life Technologies |
APC, allophycocyanin; PE, phycoerythrin; FITC, fluorescein isothiocyanate; NA, not applicable.
Alternative coreceptor usage by SIVmac76XII in CF2th-Luc cells.
CF2th-Luc cells, which contain a Tat-driven luciferase reporter, were transfected, using the Fugene 6 reagent (Promega), with two expression plasmids: one containing RM CD4 and one containing the coreceptor (or the pcDNA3.1 empty vector). Cells were infected 48 h later with SIVmac766XII (using 2,750, 13,750, or 27,500 IU) by spinoculation for 2 h at 1,200 × g. Cells were incubated for 48 h at 37°C, and then they were lysed and the luciferase content was quantified as the number of relative light units (RLU), as previously described (74).
Alternative coreceptor usage by SIVmac76XII in PBMCs.
Cryopreserved PBMCs from two RMs (RM1 and RM2) were thawed in complete medium (RPMI 1640 with 10% FBS, 1% l-glutamine, and 1% penicillin-streptomycin) and stimulated for 3 days with 5 μg/ml concanavalin A (ConA) and 100 U/ml interleuikin-2 (IL-2). Activated PBMCs were plated at 2 × 105 per well in 96-well plates, pretreated for 1 h with 15 μM MVC (NIH AIDS Reagent Program) or with vehicle alone (dimethyl sulfoxide), and then infected with SIVmac766XII (550 IU) by spinoculation for 1.5 h at 1,200 × g. The cells were then washed and incubated at 37°C, the supernatants were collected, and 50% medium was replaced every 3 to 4 days. Replication was measured by SIV p27 Gag antigen production in the supernatant by enzyme-linked immunosorbent assay (Perkin-Elmer).
Assessment of the T/F virus variants.
The number of T/F variants was determined using a real-time single-genome amplification (RT-SGA) approach described previously (34). Briefly, a 300-bp portion of the integrase gene surrounding the mutated site was amplified and sequenced using a limiting dilution PCR where only a single genome is amplified (SGA) per reaction. Viral RNA was extracted using a QIAamp viral RNA minikit (Qiagen) and then reverse transcribed into cDNA using SuperScript III reverse transcription according to the manufacturer's recommendations (Invitrogen) and the antisense primer SIVmacIntR1 (5′-AAG CAA GGG AAA TAA GTG CTA TGC AGT AA-3′). PCR was then performed with 1× PCR buffer, 2 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 0.2 μM each primer, and 0.025 U/μl Platinum Taq polymerase (Invitrogen) in a 10-μl reaction mixture with sense primer SIVmacIntF1 (5′-GAA GGG GAG GAA TAG GGG ATA TG-3′) and antisense primer SIVmacIntR3 (5′-CAC CTC TCT AGC CTC TCC GGT ATC C-3′) under the following conditions: 1 cycle of 94°C for 2 min and 40 cycles of 94°C for 15 s, 55°C for 30 s, 60°C for 1.5 min, and 72°C for 30 s. Template-positive reactions were determined by real-time PCR using gene-specific probe SIVIntP (5′-TCC CTA CCT TTA AGA TGA CTG CTC CTT CCC CT-3′) with 6-carboxyfluorescein and ZEN/Iowa black hole quencher (Integrated DNA Technologies) and directly sequenced with SIVmacIntR3 using the BigDye Terminator technology (Life Technologies). To confirm PCR amplification from a single template, chromatograms were manually examined for multiple peaks, indicative of the presence of amplicons resulting from PCR-generated recombination events, Taq polymerase errors, or multiple variant templates.
Flow cytometry.
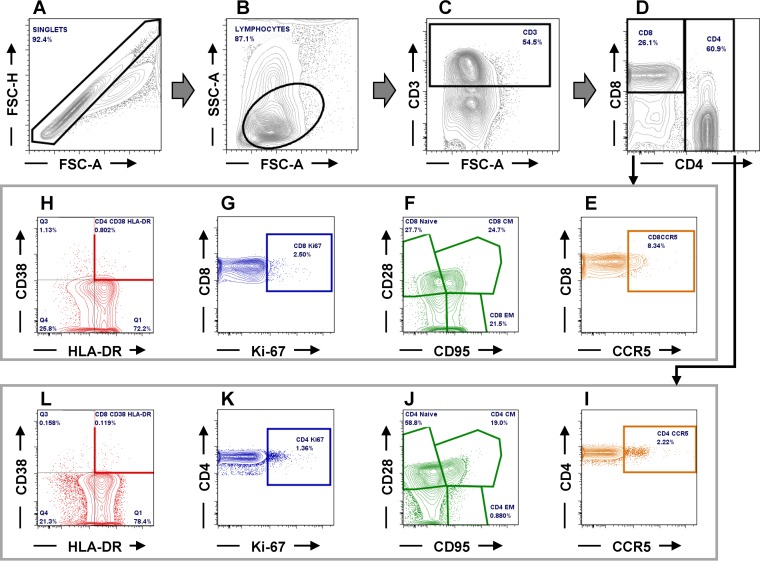
Flow cytometry was used to assess changes in CD4+ and CD8+ T cell populations, the frequency of CD4+ and CD8+ cells expressing CCR5, as well as their proliferation and immune activation status, as described previously (95–97). Briefly, 2 × 106 cells were stained with viability dye (blue dye; Life Technologies) and incubated for 15 min in the dark at RT. The cells were then washed with 1× PBS and stained for 30 min at RT in the dark with combinations of antibodies and combinations of isotype and fluorescence-minus-one controls (Table 1) appropriate for the identification of different T cell populations (Fig. 11). Stained cells were washed in 1× PBS, fixed with 2% paraformaldehyde (PFA) solution, and stored at 4°C prior to acquisition. For intracellular staining, viable cells stained as described above were washed with 1× PBS, permeabilized with a solution containing 0.1% of saponin, and incubated for 30 min at room temperature in the dark. Cells were then stained with an anti-Ki-67 antibody (Table 1). Cells were then washed with 1× PBS, fixed with 2% PFA, and stored at 4°C prior to acquisition. A minimum of 250,000 CD3 live cells were acquired with FACSDiva software (version 8.0). Acquired cells were analyzed using FlowJo (version 7.6.5) software.
FIG 11.
Gating strategy employed to characterize the CD4+ and CD8+ T cells and their levels of expression of CCR5, as well as the frequency of activated and proliferating T cells (illustrative plots from RM34). (A to D) CD4+ and CD8+ T cells were gated on singlets followed by lymphocytes and CD3+; (E and I) CD4+ (E) and CD8+ (I) T cells expressing CCR5; (F and J) CD4+ (F) and CD8+ (J) T cell naive and memory subsets (CM, central memory; EM, effector memory); (G and K) CD4+ (G) and CD8+ (K) T cells expressing Ki-67; (H and L) activated CD4+ (H) and CD8+ (L) T cells expressing CD38 and HLA-DR. FSC-A, forward scatter area, FSC-H, forward scatter height; SSC-A, side scatter area.
Statistical analyses.
All statistical analyses were performed with GraphPad Prism software (version 6.02; GraphPad Software Inc., San Diego, CA, USA). Data were expressed as averages ± standard errors of the means (SEM). An unpaired nonparametric Mann-Whitney t test was used for determination of significant differences between the experimental groups with regard to the absolute cell counts and the frequency of cells expressing CCR5, immune activation, and cell proliferation markers. The Wilcoxon paired nonparametric test was used to determine significant differences between the baselines of the mean cell frequencies and absolute counts at a single time point of MVC treatment for each experimental group. The chi-square test was used to establish significant differences between animals that became infected in both groups. Differences were considered statistically significant at a P value of ≤0.05.
ACKNOWLEDGMENTS
We thank Guido Silvestri and Isaac Rodriguez-Chavez for helpful discussions.
This work was supported by grant R56 DE023508 (to C.A.) from the National Institute of Dental and Craniofacial Research (NIDCR). Parts of this study were supported with funds from grants RO1 HL117715 (to I.P.), R01 AI119346 (to C.A.), and R01 HL123096 (to I.P.). K.D.R. and B.B.P. were supported by T32 AI065380 (University of Pittsburgh AIDS Research Training [PART]). B.B.P. was supported by RO1 AI104373. This work was also partially supported by the UNC Center for AIDS Research (CFAR) (P30 AI50410) and received assistance from the University of Pennsylvania Center for AIDS Research (P30 AI045008). Finally, this project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
We declare that no conflict of interest exists.
E.B.-C., C.A., and I.P. designed experiments; E.B.-C., C.A., I.P., A.D.K., R.G.C., and B.F.K., analyzed data; B.F.K. and K.M. provided virus stock and sequence analysis; E.B.-C., C.X., K.S.W., M.L.C., B.B.P., K.D.R., T.D., G.S.H.-R., D.M., and K.M. performed experiments; E.B.-C., C.A., I.P. B.F.K., M.L.C., A.D.K., K.S.W., and R.G.C. wrote the manuscript.
REFERENCES
- 1.UNAIDS. 2013. 2013 Progress report on the global plan. UNAIDS, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/20130625_progress_global_plan_en_0.pdf. [Google Scholar]
- 2.Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. 1992. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med 327:1246–1247. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn L, Abrams EJ, Chinchilla M, Tsai WY, Thea DM. 1996. Sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period. AIDS 10:1181–1182. [PubMed] [Google Scholar]
- 4.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, Overbaugh J, Bwayo J, Ndinya-Achola JO, Kreiss JK. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis 183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 5.Kourtis AP, Butera S, Ibegbu C, Belec L, Duerr A. 2003. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis 3:786–793. doi: 10.1016/S1473-3099(03)00832-6. [DOI] [PubMed] [Google Scholar]
- 6.Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, Van der Hoeven L, Chiphangwi JD, Liomba G, Biggar RJ. 1999. HIV transmission through breastfeeding: a study in Malawi. JAMA 282:744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, Shang L, Wietgrefe S, Southern PJ, Reilly CS, Skinner PJ, Zupancic ML, Carlis JV, Piatak M Jr, Waterman D, Reeves RK, Masek-Hammerman K, Derdeyn CA, Alpert MD, Evans DT, Kohler H, Muller S, Robinson J, Lifson JD, Burton DR, Johnson RP, Haase AT. 2014. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol 193:3113–3125. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, McIntyre J, Gnanashanmugam D, Siberry GK, Coletti AS, Taha TE, Klingman KL, Martinson FE, Owor M, Violari A, Moodley D, Theron GB, Bhosale R, Bobat R, Chi BH, Strehlau R, Mlay P, Loftis AJ, Browning R, Fenton T, Purdue L, Basar M, Shapiro DE, Mofenson LM, IMPAACT 1077BF/1077FF PROMISE Study Team. 2016. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 375:1726–1737. doi: 10.1056/NEJMoa1511691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll O, Hernandez M, Boucher CA, Fortuny C, de Tejada BM, Canet Y, Caragol I, Tijnagel J, Bertran JM, Espanol T. 1997. Vertical HIV-1 transmission correlates with a high maternal viral load at delivery. J Acquir Immune Defic Syndr Hum Retrovirol 14:26–30. doi: 10.1097/00042560-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kumar SB, Rice CE, Milner DA Jr, Ramirez NC, Ackerman WE IV, Mwapasa V, Turner AN, Kwiek JJ. 2012. Elevated cytokine and chemokine levels in the placenta are associated with in-utero HIV-1 mother-to-child transmission. AIDS 26:685–694. doi: 10.1097/QAD.0b013e3283519b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Louis ME, Kamenga M, Brown C, Nelson AM, Manzila T, Batter V, Behets F, Kabagabo U, Ryder RW, Oxtoby M, Quinn TC, Heyward WL. 1993. Risk for perinatal HIV-1 transmission according to maternal immunologic, virologic, and placental factors. JAMA 269:2853–2859. [PubMed] [Google Scholar]
- 12.Lohman-Payne B, Slyker JA, Moore S, Maleche-Obimbo E, Wamalwa DC, Richardson BA, Rowland-Jones S, Mbori-Ngacha D, Farquhar C, Overbaugh J, John-Stewart G. 2012. Breast milk cellular HIV-specific interferon gamma responses are associated with protection from peripartum HIV transmission. AIDS 26:2007–2016. doi: 10.1097/QAD.0b013e328359b7e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, Seaton KE, Deal A, Edwards RW, Tegha G, Kamwendo D, Kumwenda J, Nelson JA, Liao HX, Brinkley C, Denny TN, Ochsenbauer C, Ellington S, King CC, Jamieson DJ, van der Horst C, Kourtis AP, Tomaras GD, Ferrari G, Permar SR. 2015. Association of HIV-1 envelope-specific breast milk IgA responses with reduced risk of postnatal mother-to-child transmission of HIV-1. J Virol 89:9952–9961. doi: 10.1128/JVI.01560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahl A, Swanson MD, Nochi T, Olesen R, Denton PW, Chateau M, Garcia JV. 2012. Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog 8:e1002732. doi: 10.1371/journal.ppat.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter J, Ghosh MK, Kuhn L, Semrau K, Sinkala M, Kankasa C, Thea DM, Aldrovandi GM. 2009. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J Infect Dis 200:1498–1502. doi: 10.1086/644603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amedee AM, Rychert J, Lacour N, Fresh L, Ratterree M. 2004. Viral and immunological factors associated with breast milk transmission of SIV in rhesus macaques. Retrovirology 1:17. doi: 10.1186/1742-4690-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn DT, Newell ML, Ades AE, Peckham CS. 1992. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 340:585–588. doi: 10.1016/0140-6736(92)92115-V. [DOI] [PubMed] [Google Scholar]
- 18.Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew JF. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med 341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 19.Aldrovandi GM, Kuhn L. 2010. What infants and breasts can teach us about natural protection from HIV infection. J Infect Dis 202(Suppl 3):S366–S370. doi: 10.1086/655972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, Hu CC, Tsai WY, Thea DM, Aldrovandi GM. 2010. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis 50:437–444. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaraman P, Haigwood NL. 2006. Animal models for perinatal transmission of HIV-1. Front Biosci 11:2828–2844. doi: 10.2741/2012. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman P, Zhu T, Misher L, Mohan D, Kuller L, Polacino P, Richardson BA, Bielefeldt-Ohmann H, Anderson D, Hu SL, Haigwood NL. 2007. Evidence for persistent, occult infection in neonatal macaques following perinatal transmission of simian-human immunodeficiency virus SF162P3. J Virol 81:822–834. doi: 10.1128/JVI.01759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chenine AL, Ferrantelli F, Hofmann-Lehmann R, Vangel MG, McClure HM, Ruprecht RM. 2005. Older rhesus macaque infants are more susceptible to oral infection with simian-human immunodeficiency virus 89.6P than neonates. J Virol 79:1333–1336. doi: 10.1128/JVI.79.2.1333-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandrea I, Onanga R, Souquiere S, Mouinga-Ondeme A, Bourry O, Makuwa M, Rouquet P, Silvestri G, Simon F, Roques P, Apetrei C. 2008. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol 82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, Schmitt C, Raehtz K, Feyertag F, Martinez Sosa N, Wijewardana V, Burke DS, Robertson DL, Tracy R, Pandrea I, Freimer N, Apetrei C, International Vervet Research Consortium. 2013. SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations. PLoS Pathog 9:e1003011. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma D, Jasinska AJ, Feyertag F, Wijewardana V, Kristoff J, He T, Raehtz K, Schmitt CA, Jung Y, Cramer JD, Dione M, Antonio M, Tracy R, Turner T, Robertson DL, Pandrea I, Freimer N, Apetrei C, International Vervet Research Consortium. 2014. Factors associated with simian immunodeficiency virus transmission in a natural African nonhuman primate host in the wild. J Virol 88:5687–5705. doi: 10.1128/JVI.03606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciaranello AL, Perez F, Keatinge J, Park JE, Engelsmann B, Maruva M, Walensky RP, Dabis F, Chu J, Rusibamayila A, Mushavi A, Freedberg KA. 2012. What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis. PLoS Med 9:e1001156. doi: 10.1371/journal.pmed.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch V, Apetrei C. 2006. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J Med Primatol 35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 29.Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, Kristoff J, Gautam R, Zhong F, Haret-Richter GS, Trichel A, Shaw GM, Hahn BH, Apetrei C. 2012. Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites. J Virol 86:4158–4168. doi: 10.1128/JVI.07141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raehtz K, Pandrea I, Apetrei C. 2016. The well-tempered SIV infection: pathogenesis of SIV infection in natural hosts in the wild, with emphasis on virus transmission and early events post-infection that may contribute to protection from disease progression. Infect Genet Evol 46:308–323. doi: 10.1016/j.meegid.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. 2010. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis 202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasmuth JC, Rockstroh JK, Hardy WD. 2012. Drug safety evaluation of maraviroc for the treatment of HIV infection. Expert Opin Drug Saf 11:161–174. doi: 10.1517/14740338.2012.640670. [DOI] [PubMed] [Google Scholar]
- 33.Brocca-Cofano E, Xu C, Wetzel K, Cottrell M, Policicchio B, Raehtz K, Ma D, Dunsmore T, Haret-Richter G, Musaitif K, Keele BF, Kashuba ADM, Collman R, Pandrea I, Apetrei C. 2018. Marginal effects of systemic CCR5 blockade with maraviroc on oral simian immunodeficiency virus transmission to infant macaques. bioRxiv doi: 10.1101/299206. [DOI] [PMC free article] [PubMed]
- 34.Del Prete GQ, Park H, Fennessey CM, Reid C, Lipkey L, Newman L, Oswald K, Kahl C, Piatak M Jr, Quinones OA, Alvord WG, Smedley J, Estes JD, Lifson JD, Picker LJ, Keele BF. 2014. Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J Virol 88:8077–8090. doi: 10.1128/JVI.01026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosario MC, Jacqmin P, Dorr P, James I, Jenkins TM, Abel S, van der Ryst E. 2008. Population pharmacokinetic/pharmacodynamic analysis of CCR5 receptor occupancy by maraviroc in healthy subjects and HIV-positive patients. Br J Clin Pharmacol 65(Suppl 1):S86–S94. doi: 10.1111/j.1365-2125.2008.03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown KC, Patterson KB, Malone SA, Shaheen NJ, Prince HM, Dumond JB, Spacek MB, Heidt PE, Cohen MS, Kashuba AD. 2011. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis 203:1484–1490. doi: 10.1093/infdis/jir059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, Tressler R, Worsley J, Kashuba AD. 2009. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr 51:546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.FDA. 2001. Guidance for industry: bioanalytical method validation. FDA, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. [Google Scholar]
- 39.Massud I, Aung W, Martin A, Bachman S, Mitchell J, Aubert R, Solomon Tsegaye T, Kersh E, Pau CP, Heneine W, Garcia-Lerma JG. 2013. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol 87:8952–8961. doi: 10.1128/JVI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopker MJ, Del Prete GQ, Estes JD, Li H, Reid C, Newman L, Lipkey L, Camus C, Easlick JL, Wang S, Decker JM, Bar KJ, Learn G, Pal R, Weiss DE, Hahn BH, Lifson JD, Shaw GM, Keele BF. 2016. Derivation and characterization of pathogenic transmitted/founder molecular clones from simian immunodeficiency virus SIVsmE660 and SIVmac251 following mucosal infection. J Virol 90:8435–8453. doi: 10.1128/JVI.00718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pohlmann S, Lee B, Meister S, Krumbiegel M, Leslie G, Doms RW, Kirchhoff F. 2000. Simian immunodeficiency virus utilizes human and sooty mangabey but not rhesus macaque STRL33 for efficient entry. J Virol 74:5075–5082. doi: 10.1128/JVI.74.11.5075-5082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott ST, Wetzel KS, Francella N, Bryan S, Romero DC, Riddick NE, Shaheen F, Vanderford T, Derdeyn CA, Silvestri G, Paiardini M, Collman RG. 2015. Dualtropic CXCR6/CCR5 simian immunodeficiency virus (SIV) infection of sooty mangabey primary lymphocytes: distinct coreceptor use in natural versus pathogenic hosts of SIV. J Virol 89:9252–9261. doi: 10.1128/JVI.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiene M, Marzi A, Urbanczyk A, Bertram S, Fisch T, Nehlmeier I, Gnirss K, Karsten CB, Palesch D, Munch J, Chiodi F, Pohlmann S, Steffen I. 2012. The role of the alternative coreceptor GPR15 in SIV tropism for human cells. Virology 433:73–84. doi: 10.1016/j.virol.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Riddick NE, Wu F, Matsuda K, Whitted S, Ourmanov I, Goldstein S, Goeken RM, Plishka RJ, Buckler-White A, Brenchley JM, Hirsch VM. 2015. Simian immunodeficiency virus SIVagm efficiently utilizes non-CCR5 entry pathways in African green monkey lymphocytes: potential role for GPR15 and CXCR6 as viral coreceptors. J Virol 90:2316–2331. doi: 10.1128/JVI.02529-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puertas MC, Massanella M, Llibre JM, Ballestero M, Buzon MJ, Ouchi D, Esteve A, Boix J, Manzardo C, Miro JM, Gatell JM, Clotet B, Blanco J, Martinez-Picado J, MaraviBoost Collaborative Group. 2014. Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS 28:325–334. doi: 10.1097/QAD.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 46.Mencarelli A, Cipriani S, Francisci D, Santucci L, Baldelli F, Distrutti E, Fiorucci S. 2016. Highly specific blockade of CCR5 inhibits leukocyte trafficking and reduces mucosal inflammation in murine colitis. Sci Rep 6:30802. doi: 10.1038/srep30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin MP, Dean M, Smith MW, Winkler C, Gerrard B, Michael NL, Lee B, Doms RW, Margolick J, Buchbinder S, Goedert JJ, O'Brien TR, Hilgartner MW, Vlahov D, O'Brien SJ, Carrington M. 1998. Genetic acceleration of AIDS progression by a promoter variant of CCR5. Science 282:1907–1911. doi: 10.1126/science.282.5395.1907. [DOI] [PubMed] [Google Scholar]
- 48.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 49.Reynes J, Portales P, Segondy M, Baillat V, Andre P, Reant B, Avinens O, Couderc G, Benkirane M, Clot J, Eliaou JF, Corbeau P. 2000. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis 181:927–932. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 50.Heredia A, Gilliam B, DeVico A, Le N, Bamba D, Flinko R, Lewis G, Gallo RC, Redfield RR. 2007. CCR5 density levels on primary CD4 T cells impact the replication and enfuvirtide susceptibility of R5 HIV-1. AIDS 21:1317–1322. doi: 10.1097/QAD.0b013e32815278ea. [DOI] [PubMed] [Google Scholar]
- 51.Wilkin TJ, Lalama CM, McKinnon J, Gandhi RT, Lin N, Landay A, Ribaudo H, Fox L, Currier JS, Mellors JW, Gulick R, Tenorio AR. 2012. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4+ T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis 206:534–542. doi: 10.1093/infdis/jis376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, McLaughlin B, Landay AL, Adeyemi O, Gilman LE, Clagett B, Rodriguez B, Martin JN, Schacker TW, Shacklett BL, Palmer S, Lederman MM, Deeks SG. 2013. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 121:4635–4646. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, Alnwick DJ, Rogers M, Shaffer N. 2000. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 54.Rollins N, Meda N, Becquet R, Coutsoudis A, Humphrey J, Jeffrey B, Kanshana S, Kuhn L, Leroy V, Mbori-Ngacha D, McIntyre J, Newell ML. 2004. Preventing postnatal transmission of HIV-1 through breast-feeding: modifying infant feeding practices. J Acquir Immune Defic Syndr 35:188–195. doi: 10.1097/00126334-200402010-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, Kreiss J. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn L, Aldrovandi G. 2010. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol 37:843–862. doi: 10.1016/j.clp.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taha TE, Kumwenda J, Cole SR, Hoover DR, Kafulafula G, Fowler MG, Thigpen MC, Li Q, Kumwenda NI, Mofenson L. 2009. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis 200:1490–1497. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 58.Nagelkerke NJ, Moses S, Embree JE, Jenniskens F, Plummer FA. 1995. The duration of breastfeeding by HIV-1-infected mothers in developing countries: balancing benefits and risks. J Acquir Immune Defic Syndr Hum Retrovirol 8:176–181. doi: 10.1097/00042560-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Chahroudi A, Meeker T, Lawson B, Ratcliffe S, Else J, Silvestri G. 2011. Mother-to-infant transmission of simian immunodeficiency virus is rare in sooty mangabeys and is associated with low viremia. J Virol 85:5757–5763. doi: 10.1128/JVI.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilks AB, Perry JR, Ehlinger EP, Zahn RC, White R, Gauduin MC, Carville A, Seaman MS, Schmitz JE, Permar SR. 2011. High cell-free virus load and robust autologous humoral immune responses in breast milk of simian immunodeficiency virus-infected African green monkeys. J Virol 85:9517–9526. doi: 10.1128/JVI.00796-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clapham PR, McKnight A. 2001. HIV-1 receptors and cell tropism. Br Med Bull 58:43–59. doi: 10.1093/bmb/58.1.43. [DOI] [PubMed] [Google Scholar]
- 62.Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol 74:57–64. doi: 10.1128/JVI.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. 2012. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog 8:e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burke BP, Boyd MP, Impey H, Breton LR, Bartlett JS, Symonds GP, Hutter G. 2013. CCR5 as a natural and modulated target for inhibition of HIV. Viruses 6:54–68. doi: 10.3390/v6010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacArthur RD, Novak RM. 2008. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis 47:236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- 66.Latinovic O, Kuruppu J, Davis C, Le N, Heredia A. 2009. Pharmacotherapy of HIV-1 infection: focus on CCR5 antagonist maraviroc. Clin Med Ther 1:1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawana-Tachikawa A, Llibre JM, Bravo I, Escrig R, Mothe B, Puig J, Puertas MC, Martinez-Picado J, Blanco J, Manzardo C, Miro JM, Iwamoto A, Pozniak AL, Gatell JM, Clotet B, Brander C, MARAVIBOOST Investigators. 2014. Effect of maraviroc intensification on HIV-1-specific T cell immunity in recently HIV-1-infected individuals. PLoS One 9:e87334. doi: 10.1371/journal.pone.0087334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelly KM, Beck SE, Metcalf Pate KA, Queen SE, Dorsey JL, Adams RJ, Avery LB, Hubbard W, Tarwater PM, Mankowski JL. 2013. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS 27:F21–F28. doi: 10.1097/QAD.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Funderburg N, Kalinowska M, Eason J, Goodrich J, Heera J, Mayer H, Rajicic N, Valdez H, Lederman MM. 2010. Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLoS One 5:e13188. doi: 10.1371/journal.pone.0013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schroder C, Pierson RN III, Nguyen BN, Kawka DW, Peterson LB, Wu G, Zhang T, Springer MS, Siciliano SJ, Iliff S, Ayala JM, Lu M, Mudgett JS, Lyons K, Mills SG, Miller GG, Singer II, Azimzadeh AM, DeMartino JA. 2007. CCR5 blockade modulates inflammation and alloimmunity in primates. J Immunol 179:2289–2299. doi: 10.4049/jimmunol.179.4.2289. [DOI] [PubMed] [Google Scholar]
- 71.Walker DK, Abel S, Comby P, Muirhead GJ, Nedderman AN, Smith DA. 2005. Species differences in the disposition of the CCR5 antagonist, UK-427,857, a new potential treatment for HIV. Drug Metab Dispos 33:587–595. doi: 10.1124/dmd.104.002626. [DOI] [PubMed] [Google Scholar]
- 72.Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, Jenkins TM, Medhurst C, Sullivan JF, Ridgway C, Abel S, James IT, Youle M, van der Ryst E. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med 11:1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 73.Farquhar C, John-Stewart G. 2003. The role of infant immune responses and genetic factors in preventing HIV-1 acquisition and disease progression. Clin Exp Immunol 134:367–377. doi: 10.1111/j.1365-2249.2003.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elliott ST, Riddick NE, Francella N, Paiardini M, Vanderford TH, Li B, Apetrei C, Sodora DL, Derdeyn CA, Silvestri G, Collman RG. 2012. Cloning and analysis of sooty mangabey alternative coreceptors that support simian immunodeficiency virus SIVsmm entry independently of CCR5. J Virol 86:898–908. doi: 10.1128/JVI.06415-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wetzel KS, Yi Y, Elliott ST, Romero D, Jacquelin B, Hahn BH, Muller-Trutwin M, Apetrei C, Pandrea I, Collman RG. 2017. CXCR6-mediated simian immunodeficiency virus SIVagmSab entry into Sabaeus African green monkey lymphocytes implicates widespread use of non-CCR5 pathways in natural host infections. J Virol 91:e01626-. doi: 10.1128/JVI.01626-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, Li Y, Hahn BH, Derdeyn CA, Sodora DL, Apetrei C, Paiardini M, Silvestri G, Collman RG. 2010. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog 6:e1001064. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wood LF, Chahroudi A, Chen HL, Jaspan HB, Sodora DL. 2013. The oral mucosa immune environment and oral transmission of HIV/SIV. Immunol Rev 254:34–53. doi: 10.1111/imr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giaquinto C, Mawela MP, Chokephaibulkit K, Negra MD, Mitha IH, Fourie J, Fang A, van der Ryst E, Valluri SR, Vourvahis M, Zhang-Roper RY, Craig C, McFadyen L, Clark A, Heera J. 2018. Pharmacokinetics, safety and efficacy of maraviroc in treatment-experienced pediatric patients infected with CCR5-tropic HIV-1. Pediatr Infect Dis J 37:459–465. doi: 10.1097/INF.0000000000001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fennessey CM, Keele BF. 2013. Using nonhuman primates to model HIV transmission. Curr Opin HIV AIDS 8:280–287. doi: 10.1097/COH.0b013e328361cfff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pohlmann S, Stolte N, Munch J, Ten Haaft P, Heeney JL, Stahl-Hennig C, Kirchhoff F. 1999. Co-receptor usage of BOB/GPR15 in addition to CCR5 has no significant effect on replication of simian immunodeficiency virus in vivo. J Infect Dis 180:1494–1502. doi: 10.1086/315097. [DOI] [PubMed] [Google Scholar]
- 81.Gautam R, Carter AC, Katz N, Butler IF, Barnes M, Hasegawa A, Ratterree M, Silvestri G, Marx PA, Hirsch VM, Pandrea I, Apetrei C. 2007. In vitro characterization of primary SIVsmm isolates belonging to different lineages. In vitro growth on rhesus macaque cells is not predictive for in vivo replication in rhesus macaques. Virology 362:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang YJ, Zhang L, Ketas T, Korber BT, Moore JP. 2001. HIV type 1 molecular clones able to use the Bonzo/STRL-33 coreceptor for virus entry. AIDS Res Hum Retroviruses 17:217–227. doi: 10.1089/088922201750063133. [DOI] [PubMed] [Google Scholar]
- 83.Edinger AL, Hoffman TL, Sharron M, Lee B, O'Dowd B, Doms RW. 1998. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology 249:367–378. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, Kim E, Learn GH, Hahn P, Policicchio B, Brocca-Cofano E, Deleage C, Hao X, Chuang GY, Gorman J, Gardner M, Lewis MG, Hatziioannou T, Santra S, Apetrei C, Pandrea I, Alam SM, Liao HX, Shen X, Tomaras GD, Farzan M, Chertova E, Keele BF, Estes JD, Lifson JD, Doms RW, Montefiori DC, Haynes BF, Sodroski JG, Kwong PD, Hahn BH, Shaw GM. 2016. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A 113:E3413–E3422. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Del Prete GQ, Ailers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, Chertova E, Smedley J, LaBranche CC, Montefiori DC, Burton DR, Shaw GM, Markowitz M, Piatak M Jr, KewalRamani VN, Bieniasz PD, Lifson JD, Hatziioannou T. 2014. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe 16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coll J, Molto J, Boix J, Gomez-Mora E, Else L, Garcia E, Paredes R, Ouchi D, Carrillo A, Escrig R, Back D, Clotet B, Cabrera C. 2015. Single oral dose of maraviroc does not prevent ex-vivo HIV infection of rectal mucosa in HIV-1 negative human volunteers. AIDS 29:2149–2154. doi: 10.1097/QAD.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 87.Riddell Jt, Amico KR, Mayer KH. 2018. HIV preexposure prophylaxis: a review. JAMA 319:1261–1268. doi: 10.1001/jama.2018.1917. [DOI] [PubMed] [Google Scholar]
- 88.Irvine C, Egan KJ, Shubber Z, Van Rompay KK, Beanland RL, Ford N. 2015. Efficacy of HIV postexposure prophylaxis: systematic review and meta-analysis of nonhuman primate studies. Clin Infect Dis 60(Suppl 3):S165–S169. doi: 10.1093/cid/civ069. [DOI] [PubMed] [Google Scholar]
- 89.Van Rompay KK, Berardi CJ, Aguirre NL, Bischofberger N, Lietman PS, Pedersen NC, Marthas ML. 1998. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS 12:F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Dobard CW, Taylor A, Sharma S, Anderson PL, Bushman LR, Chuong D, Pau CP, Hanson D, Wang L, Garcia-Lerma JG, McGowan I, Rohan L, Heneine W. 2015. Protection against rectal chimeric simian/human immunodeficiency virus transmission in macaques by rectal-specific gel formulations of maraviroc and tenofovir. J Infect Dis 212:1988–1995. doi: 10.1093/infdis/jiv334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng Y, Han GW, Abagyan R, Wu B, Stevens RC, Cherezov V, Kufareva I, Handel TM. 2017. Structure of CC chemokine receptor 5 with a potent chemokine antagonist reveals mechanisms of chemokine recognition and molecular mimicry by HIV. Immunity 46:1005–1017.e1005. doi: 10.1016/j.immuni.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.National Research Council, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 93.Winters MA, Van Rompay KK, Kashuba AD, Shulman NS, Holodniy M. 2010. Maternal-fetal pharmacokinetics and dynamics of a single intrapartum dose of maraviroc in rhesus macaques. Antimicrob Agents Chemother 54:4059–4063. doi: 10.1128/AAC.00747-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol 179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gautam R, Gaufin T, Butler I, Gautam A, Barnes M, Mandell D, Pattison M, Tatum C, Macfarland J, Monjure C, Marx PA, Pandrea I, Apetrei C. 2009. Simian immunodeficiency virus SIVrcm, a unique CCR2-tropic virus, selectively depletes memory CD4+ T cells in pigtailed macaques through expanded coreceptor usage in vivo. J Virol 83:7894–7908. doi: 10.1128/JVI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mandell DT, Kristoff J, Gaufin T, Gautam R, Ma D, Sandler N, Haret-Richter G, Xu C, Aamer H, Dufour J, Trichel A, Douek DC, Keele BF, Apetrei C, Pandrea I. 2014. Pathogenic features associated with increased virulence upon simian immunodeficiency virus cross-species transmission from natural hosts. J Virol 88:6778–6792. doi: 10.1128/JVI.03785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, Keele BF, Ribeiro RM, Veazey RS, Apetrei C. 2011. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog 7:e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]