Abstract
The black yeast genus Exophiala includes numerous potential opportunistic species that potentially cause systematic and disseminated infections in immunocompetent individuals. Species causing systemic disease have ability to grow at 37–40 °C, while others consistently lack thermotolerance and are involved in diseases of cold-blooded, waterborne vertebrates and occasionally invertebrates. We explain a fast and sensitive assay for recognition and identification of waterborne Exophiala species without sequencing. The ITS rDNA region of seven Exophiala species (E. equina, E. salmonis, E. opportunistica, E. pisciphila, E. aquamarina, E. angulospora and E. castellanii) along with the close relative Veronaea botryosa was sequenced and aligned for the design of specific padlock probes for the detection of characteristic single-nucleotide polymorphisms. The assay demonstrated to successfully amplify DNA of target fungi, allowing detection at the species level. Amplification products were visualized on 1% agarose gels to confirm specificity of probe–template binding. Amounts of reagents were reduced to prevent the generation of false positive results. The simplicity, tenderness, robustness and low expenses provide padlock probe assay (RCA) a definite place as a very practical method among isothermal approaches for DNA diagnostics.
Keywords: Waterborne Exophiala, Black yeasts, Identification, Rolling circle amplification
Introduction
Exophiala is a member of the ascomycete order Chaetothyriales (fungi), comprising the black yeasts and allies, which are frequently encountered as causative agents of disorders in humans and animals [1–7]. Human infections vary from commensalism or moderate cutaneous infection to fatal neurotropism with serious mutilation. Infections frequently involve patients without known immune disorder or underlying metabolic disease. Outside humans, especially cold-blooded waterborne vertebrates are susceptible to a variety of Exophiala species [2], some of these seem to be specific to certain host taxa [1]. As virulence factors, the capability to absorb alkylbenzenes, within sweat and nervous tissues of mammals and in the poisonous skin of amphibians, has been proposed [2, 8, 9]. Studies on epizootics from the older literature obviously show that black yeast infection is a relatively popular phenomenon in cold-blooded vertebrates [10–15]. Recent molecular reports demonstrate that various pathogenic species are concerned [1, 2, 16], which morphologically are extremely similar.
Some species can be classified by physiological characteristics, for example, temperature tolerance and nitrate assimilation, however, for many taxa molecular characterization are needed. [17]. Sequencing of the rRNA ITS location is generally adequate for routine species distinction in the genus Exophiala [18]. This method is relatively costly and time-consuming and less suitable for large numbers of strains in case of monitoring of epizootics.
Rolling circle amplification (RCA) is an isothermal DNA amplification technique applying so-called padlock probes. The method has been proven to be fast, cost-effective and specific for identification of human and plant pathogenic fungi [6, 19–25], including black yeasts and relatives [26–28]. The 3′- and 5′-end strands of the probes hybridize next to one another at the target strand, leading to circularization of the molecule upon ligation. The circular molecule is consequently amplified isothermally with a DNA polymerase that lacks exonuclease activity, and the resulting product subsequently can be utilized with a second primer causing a cascade of amplifications. Because of the necessary accurate base pairing, the padlock probes have the ability to identify single position mutations [29–31].
In the present paper, we developed eight padlock probes on the basis of the ITS location to identify the most relevant species of Exophiala in animal infection and epizootics, viz. E. equina, E. salmonis, E. opportunistica, E. aquamarina, E. angulospora and E. castellanii, together with Veronaea botryosa as out-group. The objective of the current study was to evaluate the practical applicability of the method and to assess its limitations.
Materials and Methods
Strains
The 62 isolates of Exophiala and Veronaea included 13 strains of E. equina, 3 of E. salmonis, 6 of E. opportunistica, 6 of E. pisciphila, 8 of E. aquamarina, 10 of E. angulospora, 7 of E. castellanii and 9 of V. botryosa (Table 1); together these strains formed the ‘salmonis-clade’ of waterborne mesophilic species [2]. Isolates originated from cold-blooded animals, from human infections and from the environment. Cultures are preserved on slants of 2% malt extract agar (MEA) and oatmeal agar (OA) (Difco, Brunschwig, Amsterdam, the Netherlands) at 24 °C in the reference collection of the Centraalbureau voor Schimmelcultures (housed at Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands). Affiliation to Exophiala was verified using a phylogenetic tree constructed with sequences of the partial SSU gene. Species identity was confirmed by sequencing rDNA internal transcribed spacer (ITS), partial β-tubulin (BT2), partial elongation factor 1-α (TEF1) and actin (ACT1) genes [2]. To evaluate the specificity of padlock probes, we tested four closely related species: Cladophialophora bantiana (CBS 678.79), Exophiala dermatitidis (CBS 525.76), E. bergeri (CBS 526.76) and Rhinocladiella mackenziei (CBS 650.93).
Table 1.
Strains analyzed
| Name | Number | Source | Geography | GenBank (ITS) |
|---|---|---|---|---|
| E. equina | CBS 109789 | Human, dialysis | The Netherlands | JF747086 |
| E. equina | CBS 115143 | Bottled water | Australia | JF747080 |
| E. equina | CBS 120906 | Stool | USA | JF747093 |
| E. equina | CBS 120905 | Human, ulcer cornea | The Netherlands | JF747088 |
| E. equina | CBS 120904 | Water from water machine | The Netherlands | JF747081 |
| E. equina | CBS 121285 | Human, skin flakes | The Netherlands | JF747090 |
| E. equina | CBS 121282 | Human | USA | JF747091 |
| E. equina | CBS 121501 | Drinking water | The Netherlands | JF747077 |
| E. equina | CBS 121513 | Water system of packaging machine | The Netherlands | JF747082 |
| E. equina | CBS 116009 | Galapagos turtle | USA | JF747095 |
| E. equina | CBS 116922 | Silica gel | The Netherlands | JF747097 |
| E. equina | CBS 109913 | Drinking water | Germany | JF747145 |
| E. equina | CBS 150,93 | Washed Tilia root | Germany | JF747096 |
| E. salmonis | CBS 110371 | Frog | USA | … |
| E. salmonis | CBS 157,67 | Trout, brain | Canada | JF747137 |
| E. salmonis | CBS 120274 | Drinking water tap | The Netherlands | JF747138 |
| E. opportunistica | CBS 631,69 | Unknown | The Netherlands | JF747128 |
| E. opportunistica | CBS 122268 | Human, foot | Denmark | JF747125 |
| E. opportunistica | CBS 660,76 | Rhizosphere, Triticum aestivum | West Australia | JF747126 |
| E. opportunistica | CBS 122269 | Human, nail | Denmark | JF747124 |
| E. opportunistica | CBS 637,69 | Polyvinyl alcohol | Unknown | JF747127 |
| E. opportunistica | CBS 109811 | Drinking water | Germany | JF747123 |
| E. pisciphila | CBS 119913 | Potbelly seahorse | Unknown | JF747132 |
| E. pisciphila | CBS 119914 | Potbelly seahorse | Unknown | JF747133 |
| E. pisciphila | CBS 121500 | Human, nail | Germany | JF747134 |
| E. pisciphila | CBS 101610 | Water pipe | Germany | JF747130 |
| E. pisciphila | CBS 121505 | Swimming pool | Germany | JF747129 |
| E. pisciphila | CBS 537,73 | Catfish | USA | JF747131 |
| E. aquamarina | CBS 119915 | Little tunnyfish | USA | JF747061 |
| E. aquamarina | CBS 120417 | Leafy seadragon, bone | USA | JF747057 |
| E. aquamarina | CBS 119919 | Leafy seadragon, skull | USA | JF747056 |
| E. aquamarina | CBS 119912 | Winter flounder | USA | JF747060 |
| E. aquamarina | CBS 119921 | Weedy seadragon | USA | JF747059 |
| E. aquamarina | CBS 119916 | Leafy seadragon, necrotic tissue | USA | JF747055 |
| E. aquamarina | CBS 119917 | Leafy seadragon | USA | JF747058 |
| E. aquamarina | CBS 119918 | Leafy seadragon, skin | USA | JF747054 |
| V. botryosa | CBS 121506 | Human, wrist skin | Japan | JF747140 |
| V. botryosa | CBS 122826 | Railway tie | Brazil | … |
| V. botryosa | CBS 122236 | Railway tie | Brazil | … |
| V. botryosa | CBS 122823 | Railway tie | Brazil | … |
| V. botryosa | CBS 122824 | Railway tie | Brazil | … |
| V. botryosa | CBS 122825 | Railway tie | Brazil | … |
| V. botryosa | CBS 102593 | Human, disseminated in child | China | JF747142 |
| V. botryosa | CBS 101462 | Human, skin | Unknown | JF747141 |
| V. botryosa | CBS 254,57 | Sansa olive slag | Italy | JF747143 |
| E. angulospora | CBS 119911 | Weedy seadragon | USA | JF747050 |
| E. angulospora | CBS 122237 | Hydrocarbon polluted soil | Brazil | … |
| E. angulospora | CBS 120272 | Drinking water tap | The Netherlands | JF747045 |
| E. angulospora | CBS 121503 | Fish | Russia | JF747049 |
| E. angulospora | CBS 122264 | Human, leg | Denmark | JF747052 |
| E. angulospora | CBS 146,93 | Tilia wood | Germany | JF747053 |
| E. angulospora | CBS 441,92 | Man, nail | Netherland | … |
| E. angulospora | CBS 617,96 | Wood | New Zealand | JF747040 |
| E. angulospora | CBS 109906 | Drinking water | Germany | JF747047 |
| E. angulospora | CBS 482,92 | Drinking water | Japan | JF747046 |
| E. castellanii | CBS 110025 | Drinking water | Germany | JF747072 |
| E. castellanii | CBS 122325 | Human, foot | Denmark | JF747068 |
| E. castellanii | CBS 121496 | Drinking water | Germany | JF747074 |
| E. castellanii | CBS 109812 | Drinking water | Germany | JF747075 |
| E. castellanii | CBS 109914 | drinking water | Germany | JF747076 |
| E. castellanii | CBS 109915 | Drinking water | Germany | JF747073 |
| E. castellanii | CBS 158,58 | Human, skin | Sri Lanka | JF747070 |
DNA Extraction and Amplification
DNA extraction and quality tests were executed using glass beads (Sigma G9143) based on the methods described formerly [32]. DNA concentration and quality were tested spectrophotometrically at 260 and 280 nm (Shimadzu, Kyoto, Japan). ITS amplicons were produced with primers V9G and LS266 as described earlier [33]. PCR conditions were as follows: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, with final extension at 72 °C for 10 min. Amplification products were recognized by electrophoresis on 1% agarose gels.
Padlock Probe Design
For the design of the RCA padlock probes, sequences of ITS regions of all tested Exophiala species, Veronaea botryosa, and closely related species from the CBS reference collection were aligned and adjusted manually using BioNumerics v. 4.61 (Applied Maths, St-Martens-Latem, Belgium) to identify informative nucleotide polymorphisms. Padlock probes targeting the ITS region were designed and were purchased from Invitrogen Inc. (Breda, the Netherlands). In order to optimize joining efficiency to target DNAs, the padlock probes were designed with minimal secondary structure and with Tm of the 5′ end probe binding arm near to or above ligation temperature (63 °C). To improve their discriminative specificity, the 3′-end binding arm was designed with a Tm 10–15 °C under ligation temperature. The linker part of each Exophiala species-specific probe obtained from [22] and the 5′- and the 3′-binding arms were designed in that article. Sequences of the both primers used for RCA and the oligonucleotide padlock probes are shown in Table 2. The oligonucleotide probes applied were c. 92–99 bp in length and contained two adjacent target complementary sequences (14–20 bp) with a spacer region (63 bp) to help loop formation and provide a template for RCA primer binding. Specificity of the probes was proved by BLAST examination in GenBank and in a validated database of filamentous fungi available at CBS for research purposes.
Table 2.
Rolling circle amplification (RCA) padlock probes and padlock probe-specific primers used in this study
| Probe or primer | Target species | Sequences and locations of the two binding arms in comparison with relevant reference |
|---|---|---|
| RCA1 | 5′-ATGGGCACCGAAGAAGCA-3′ | |
| RCA2 | 5′-CGCGCAGACACGATA-3′ | |
| Equi | E. equina | 5′ P GGTTGGGCTACCGACGAGCG |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| TRGTTAAAGATTTTAAT 3 | ||
| Esal | E. Salmonis | 5′ p AGGGGCCTCCACCAAACCGTC |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| GGGGCAGATGCCCGCA 3′ | ||
| Eopp | E. opportunistica | 5′ p RAAGACCCCCCGGCGGTCCG |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| GCGGGCCAAGGGGTRC 3′ | ||
| Epis | E. pisciphila | 5′ p AGACGGGCTCGCCGAAGCAAC |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| CCCGGCGGTCCATTAC 3 | ||
| Eagu | E. aquamarina | 5′ p GGGGCGTCCACCAAGCCGTCCAA |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| TGGACGCCCCGTGC 3′ | ||
| Vbot | V. botryosa | 5′ p CTGTTAGGGGTCCCCCGGCG |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| GCGGGCCAGGAGACT 3′ | ||
| Eang | E. angulospora | 5′ p GACGGGCCCGCCGAAGCAAC |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| CTCCGGCGGTCACGAA 3′ | ||
| Ecas | E. castellanii | 5′ p ACACCAAACCGTCCAACACCAA |
| GatcaTGCTTCTTCGGTGCCCATtacgaggtgcggatagctacCGCGCAGACACGATAgtcta | ||
| GGGGTGACGTTGCCG 3′ |
P: 5′-phosphorylation. Underlined: the binding arms of the padlock probes, which are joined by the backbone of the probe including the non-specific linker region. Bold: the binding region of the RCA1 and RCA2
Ligation of Padlock Probe
One microliter of ITS amplicon was mixed with 2 U pfu DNA ligase (Epicentre Biotechnologies, Madison, WI, U.S.A.) and 0.1 μmol l−1 padlock probe in 20 mmol l−1 Tris–HCl (pH 7.5), 20 mmol l−1 Cl, 10 mmol l−1 MgCl2, 0.1% Igepal, 0.01 mmol l−1 rATP and 1 mmol l−1 DTT, with an overall total reaction volume of 10 μl. Padlock probe ligation reaction was performed as described by [34] by one denaturation cycle for 5 min at 94 °C, followed by five cycles of 30 s. at 94 °C and 4 min ligation at 63 °C.
Exonucleolysis
Exonucleolysis is needed to eliminate unligated padlock probe and template PCR product and therefore minimize subsequent ligation-independent amplification events. It had been done in a 20-μl vol by addition of 10 U every one of exonuclease I and III (New England Biolabs, Hitchin, UK) to the ligation mixture and incubation at 37 °C for 30 min, followed by 94 °C for 3 min to inactive the exonuclease reaction.
Rolling Circle Amplification (RCA) Reaction
Rolling circle amplification was executed in 50 µl reaction mixture containing 8 U Bst DNA polymerase (New England Biolabs), 400 μmol l−1 deoxynucleoside triphosphate mix and 10 pmol of every RCA primer in distilled water with 2 µl ligation product as template. Probe signals were amplified by incubation at 65 °C for 60 min, and accumulation of double stranded DNA products was visualized on a 1% agarose gel to validate the specificity of probe–template binding. Positive responses revealed a ladder-like structure, while negative responses showed a clean background. When the exonuclease step is omitted, some weak signal might be visible in gel electrophoresis [24].
Results
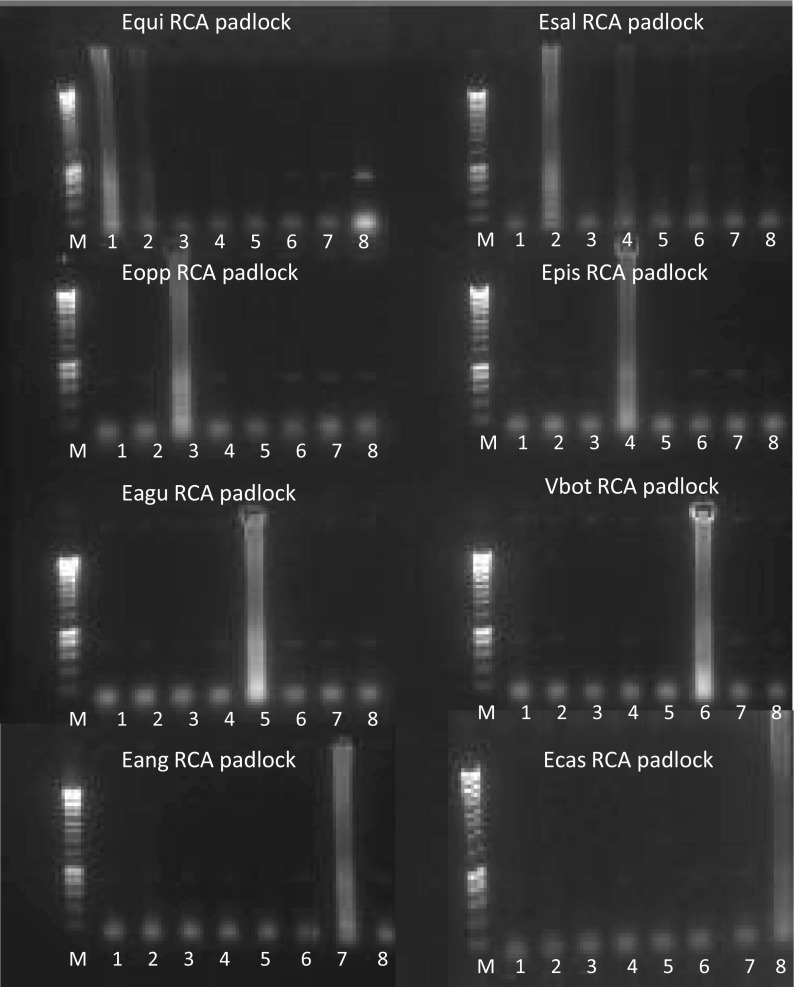
The general fungal primers ITS1 and ITS4 amplified the ITS location of all studied isolates. The ITS alignment revealed appropriate positions for the development of padlock probes that were able to differentiate between seven Exophiala species and the Veronaea out-group tested in this study. Each of the eight infectious species had several distinctive nucleotide positions. The duration of the RCA assay was 2 h. The tested species were unambiguously distinguished from each other and from different black yeast and relatives in the order Chaetothyriales compared by ITS sequence analysis and included as negative controls: the four closely related, clinically relevant species used for comparison, i.e., Cladophialophora bantiana (CBS 678.79), Exophiala dermatitidis (CBS 525.76), E. bergeri (CBS 526.76) and Rhinocladiella mackenziei (CBS 650.93) yielded negative results with the species-specific padlock probes (data not shown). Positive responses proved to be consisted and highly specific in all strains; all individual strains responded with respective probes being and were correctly identified as Exophiala equina, E. salmonis, E. opportunistica, E. aquamarina, E. angulospora and E. castellanii, as well as Veronaea botryosa, the nearest neighbor of the salmonis-clade [2]. No cross reaction was observed between any of the Exophiala species (Fig. 1). Products of the RCA responses were visualized by electrophoresis on 1% agarose gels. Positive reaction showed ladder-like patterns after RCA, while with negative results the background stayed clean. When the exonucleolysis step was deleted, a single poor band was apparent on the gel representing a non-specific band that did not interfere with the RCA reaction (data not shown). The concordance of RCA results and identification by multilocus sequencing was 100%.
Fig. 1.
Proof of species specificity of RCA padlock probes and intraspecific variation of RCA response. Amplification and subsequent fluorescent banding were seen only with appropriate template–probe mixtures (Empty lanes denote the absence of signals with unmatched template–probe mixtures.) The species-specific probes are labeled as listed in Table 1 (Equi, E. equina; Esal, E. Salmonis; Eopp, E. opportunistica; Epis, E. pisciphila; Eagu, E. aquamarina; Vbot, V. botryosa; Eang, E. angulospora; Ecas, E. castellanii) lanes: M is 200-bp DNA MW marker (Eurogentec, the Netherlands); 1 to 8, RCA reaction with DNA of E. equina (CBS 109879) (lane 1), E. Salmonis (CBS 110371) (lane2), E. opportunistica (CBS 631.69) (lane 3), E. pisciphila (CBS 119913) (lane 4), E. aquamarina (CBS 119915) (lane 5), V. botryosa (CBS 121506) (lane 6), E. angulospora (CBS 119911) (lane 7) and E. castellanii (CBS 110025) (lane 8)
Discussion
Thermotolerance is generally considered as a prime condition for vertebrate pathogenicity. During the last decades, several black yeasts have been described in Exophiala that constantly lacked thermotolerance, but still were associated with animal disease, indicating that these fungi have other, intrinsic, temperature-independent infectious abilities [2]. Infections were especially within fish and amphibians, but sometimes also in invertebrates [14]. Such infections seem to be relatively regular, at least in captive and farmed fish and amphibians. Outbreaks in farmed and aquarium animals could cause serious losses to aquaculture and fishery industries [34], but because of the spread nature of reports it is hard to estimate the magnitude of the problem.
Rolling circle amplification is a powerful and easy, isothermal in vitro DNA amplification method emerging as a tool for quick detection of specific nucleic-acid sequences in DNA samples [35]. The use of a padlock probe to circularize oligonucleotides was produced by Nilsson [36]. The technique is on the basis of the replication of a short, single-stranded DNA circle by Bst DNA polymerases at constant temperature. Sequencing of the internal transcribed spacer (ITS) is the gold standard for species recognition of black yeast and relatives, as it provides sufficient resolution between species [18]. For analysis of large numbers of isolates in case of outbreaks and epidemiological monitoring, sequencing is nevertheless costly, time-consuming and impractical [22]. Furthermore, validated databases for comparison are needed, as GenBank information is polluted with wrongly identified sequences; we used a research database on black fungi housed at the Westerdijk Fungal Biodiversity Institute and of which a selection has been deposited in the ISHAM ITS Database (www.its.mycologylab.org). The RCA reaction is relatively free of requirement for high priced laboratory equipment and could be done within 2 h isothermally at 65 °C in a water bath, thermocycler, heat block or microwave. Nevertheless, positive signals are often visible 15 min after commencement of the RCA reaction when recognized by real-time PCR [19, 22]. The amplification product can be visualized by agarose gel electrophoresis, but can also be visualized in gel-free methods applying fluorescence staining of amplified product by SYBR Green in combination with a UV transilluminator. The progress of RCA probes to distinguish single species or groups of species depends on the presence of adequate sequence information and useful species-specific polymorphisms in genes of precisely identified species.
The objective of the current study was to begin a screening technique based on RCA for highly specific and rapid detection of waterborne Exophiala species which repeatedly occur in the form of outbreaks in farmed fish, enabling their unambiguous differentiation from related melanized fungi. The RCA method performed well elsewhere in the fungal Kingdom, e.g., in Candida, Aspergillus, Scedosporium [22], Cryptococcus [37], Trichophyton [19], Fusarium [21], human-pathogenic Exophiala [28], Talaromyces marneffei [24], Scedosporium [20], Rhizopus [23] and Fonsecaea [27]. A low-cost alternative to RCA might be loop-mediated isothermal amplification (LAMP). This technique uses a set of six oligonucleotide primers with eight joining sites hybridizing particularly to various parts of a target gene [38]. Najafzadeh et al. [27] compared RCA and LAMP detection for human-pathogenic Fonsecaea species and discovered that LAMP was extremely sensitive, but RCA become more specific.
In conclusion, RCA is a very fast (less than 1 working day), specific (down to the single-nucleotide level) and economical (no additional equipment required) method for specific and rapid identification of fungal pathogens where large numbers of strains need to be processed. Our results show a considerable potential of the method in the future in laboratories for fungal outbreak control, e.g., in farmed fish. The establishment of the test is relatively expensive, but with high throughput applications, the final result per strain will be rapid and cost-effective.
Acknowledgements
M. J. Najafzadeh was supported by the Deputy of Research, Mashhad University of Medical Sciences, Mashhad, Iran (Grant No. 922320).
Compliance with Ethical Standards
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
M. J. Najafzadeh, Email: najafzadehmj@mums.ac.ir
G. S. de Hoog, Email: s.hoog@westerdijkinstitute.nl
References
- 1.Nyaoke A, Weber ES, Innis C, et al. Disseminated phaeohyphomycosis in weedy seadragons (Phyllopteryx taeniolatus) and leafy seadragons (Phycodurus eques) caused by species of Exophiala, including a novel species. J Vet Diagn Invest. 2009;21(1):69–79. doi: 10.1177/104063870902100111. [DOI] [PubMed] [Google Scholar]
- 2.de Hoog GS, Vicente VA, Najafzadeh MJ, et al. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia. 2011;27:46–72. doi: 10.3767/003158511X614258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kano R, Kusuda M, Nakamura Y, et al. First isolation of Exophiala dermatitidis from a dog: identification by molecular analysis. Vet Microbiol. 2000;76(2):201–205. doi: 10.1016/S0378-1135(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 4.Badali H, Najafzadeh MJ, van Esbroeck M, et al. The clinical spectrum of Exophiala jeanselmei, with a case report and in vitro antifungal susceptibility of the species. Med Mycol. 2010;48(2):318–327. doi: 10.3109/13693780903148353. [DOI] [PubMed] [Google Scholar]
- 5.Najafzadeh MJ, Suh MK, Lee MH, et al. Subcutaneous phaeohyphomycosis caused by Exophiala equina, with susceptibility to eight antifungal drugs. J Med Microbiol. 2013;62(5):797–800. doi: 10.1099/jmm.0.057406-0. [DOI] [PubMed] [Google Scholar]
- 6.Tsui CK, Woodhall J, Chen W, et al. Molecular techniques for pathogen identification and fungus detection in the environment. IMA Fungus. 2011;2(2):177–189. doi: 10.5598/imafungus.2011.02.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libert X, Chasseur C, Packeu A, et al. A molecular approach for the rapid, selective and sensitive detection of Exophiala jeanselmei in environmental samples: development and performance assessment of a real-time PCR assay. Appl Microbiol Biotechnol. 2016;100(3):1377–1392. doi: 10.1007/s00253-015-7175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prenafeta-Boldú FX, Summerbell R, de Hoog GS. Fungi growing on aromatic hydrocarbons: biotechnology’s unexpected encounter with biohazard? FEMS Microbiol Rev. 2006;30(1):109–130. doi: 10.1111/j.1574-6976.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- 9.do Nascimento MM, de Hoog GS, Gomes RR, et al. Shared physiological traits of Exophiala species in cold-blooded vertebrates, as opportunistic black yeasts. Mycopathologia. 2016;181(5–6):353–362. doi: 10.1007/s11046-016-0001-z. [DOI] [PubMed] [Google Scholar]
- 10.Fijan N. Systemic mycosis in channel catfish. Wildl Dis. 1969;5(2):109–110. doi: 10.7589/0090-3558-5.2.109. [DOI] [PubMed] [Google Scholar]
- 11.Otis EJ, Wolke RE, Blazer VS. Infection of Exophiala salmonis in Atlantic salmon (Salmo salar L.) J Wildl Dis. 1985;21(1):61–64. doi: 10.7589/0090-3558-21.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Reuter RE, Hutchinson W, Ham J, Davis S. Exophiala sp. infection in captured King George whiting (Sillaginodes punctata) Bull Eur Assoc Fish Pathol. 2003;23:128–134. [Google Scholar]
- 13.Richards RH, Holliman A, Helgason S. Exophiala salmonis infection in Atlantic salmon Salmo salar L. J Fish Dis. 1978;1:357–368. doi: 10.1111/j.1365-2761.1978.tb00040.x. [DOI] [Google Scholar]
- 14.Vicente VA, Orelis-Ribeiro R, Najafzadeh MJ, et al. Black yeast-like fungi associated with Lethargic Crab Disease (LCD) in the mangrove-land crab, Ucides cordatus (Ocypodidae) Vet Microbiol. 2012;158(1–2):109–122. doi: 10.1016/j.vetmic.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Guerra RS, do Nascimento MM, Miesch S, et al. Black yeast biota in the mangrove, in search of the origin of the lethargic crab disease (LCD) Mycopathologia. 2013;175(5–6):421–430. doi: 10.1007/s11046-013-9636-1. [DOI] [PubMed] [Google Scholar]
- 16.Manharth A, Lemberge K, Mylniczenko N, et al. Disseminated phaeohyphomycosis due to Exophiala species in a Galapagos tortoise, Geochelone nigra. J Herpetol Med Surg. 2005;15:20–26. doi: 10.5818/1529-9651.15.2.20. [DOI] [Google Scholar]
- 17.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23(4):884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng JS, de Hoog GS. Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Med Mycol. 2008;46(3):193–208. doi: 10.1080/13693780701799217. [DOI] [PubMed] [Google Scholar]
- 19.Kong F, Tong Z, Chen X, et al. Rapid identification and differentiation of Trichophyton species, based on sequence polymorphisms of the ribosomal internal transcribed spacer regions, by rolling-circle amplification. J Clin Microbiol. 2008;46(4):1192–1199. doi: 10.1128/JCM.02235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lackner M, Najafzadeh MJ, Sun J, et al. Rapid identification of Pseudallescheria and Scedosporium strains by using rolling circle amplification. Appl Environ Microbiol. 2012;78(1):126–133. doi: 10.1128/AEM.05280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davari M, van Diepeningen AD, Babai-Ahari A, et al. Rapid identification of Fusarium graminearum species complex using Rolling Circle Amplification (RCA) J Microbiol Meth. 2012;89(1):63–70. doi: 10.1016/j.mimet.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Kong F, Sorrell TC, et al. Practical method for detection and identification of Candida, Aspergillus, and Scedosporium spp. by use of rolling-circle amplification. J Clin Microbiol. 2008;46(7):2423–2427. doi: 10.1128/JCM.00420-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolatabadi S, Najafzadeh MJ, de Hoog GS. Rapid screening for human-pathogenic Mucorales using rolling circle amplification. Mycoses. 2014;57(Suppl 3):67–72. doi: 10.1111/myc.12245. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Najafzadeh MJ, Zhang J, et al. Molecular identification of Penicillium marneffei using rolling circle amplification. Mycoses. 2011;54(6):e751–e759. doi: 10.1111/j.1439-0507.2011.02017.x. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri Tehrani S, Aliabadian M, Fata A, Najafzadeh MJ. Rolling Circle Amplification (RCA): an approach for quick detection and identification of fungal species. J Mycol Res. 2014;1(1):55–62. [Google Scholar]
- 26.Feng P, Klaassen CH, Meis JF, et al. Identification and typing of isolates of Cyphellophora and relatives by use of amplified fragment length polymorphism and rolling circle amplification. J Clin Microbiol. 2013;51(3):931–937. doi: 10.1128/JCM.02898-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najafzadeh MJ, Sun J, Vicente VA, de Hoog GS. Rapid identification of fungal pathogens by rolling circle amplification using Fonsecaea as a model. Mycoses. 2011;54(5):e577–e582. doi: 10.1111/j.1439-0507.2010.01995.x. [DOI] [PubMed] [Google Scholar]
- 28.Najafzadeh MJ, Dolatabadi S, Saradeghi Keisari M, et al. Detection and identification of opportunistic Exophiala species using the rolling circle amplification of ribosomal internal transcribed spacers. J Microbiol Methods. 2013;94(3):338–342. doi: 10.1016/j.mimet.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson M, Malmgren H, Samiotaki M, et al. Padlock probes: circularizing oligonucleotides for localized DNA detection. Science. 1994;265(5181):2085–2088. doi: 10.1126/science.7522346. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson M, Krejci K, Koch J, et al. Padlock probes reveal single-nucleotide differences, parent of origin and in situ distribution of centromeric sequences in human chromosomes 13 and 21. Nat Genet. 1997;16(3):252–255. doi: 10.1038/ng0797-252. [DOI] [PubMed] [Google Scholar]
- 31.Baner J, Nilsson M, Mendel-Hartvig M, Landegren U. Signal amplification of padlock probes by rolling circle replication. Nucleic Acids Res. 1998;26(22):5073–5078. doi: 10.1093/nar/26.22.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najafzadeh MJ, Sun J, Vicente VA, et al. Molecular epidemiology of Fonsecaea species. Emerg Infect Dis. 2011;17(3):464–469. doi: 10.3201/eid1703.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Najafzadeh MJ, Vicente VA, Sun J, et al. Fonsecaea multimorphosa sp. nov, a new species of Chaetothyriales isolated from a feline cerebral abscess. Fungal Biol. 2011;115(10):1066–1076. doi: 10.1016/j.funbio.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Potter SJ, Lin Y, et al. Rapid and sensitive detection of severe acute respiratory syndrome coronavirus by rolling circle amplification. J Clin Microbiol. 2005;43(5):2339–2344. doi: 10.1128/JCM.43.5.2339-2344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demidov VV. Rolling-circle amplification in DNA diagnostics: the power of simplicity. Expert Rev Mol Diagn. 2002;2(6):542–548. doi: 10.1586/14737159.2.6.542. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson M. Lock and roll: single-molecule genotyping in situ using padlock probes and rolling-circle amplification. Histochem Cell Biol. 2006;126(2):159–164. doi: 10.1007/s00418-006-0213-2. [DOI] [PubMed] [Google Scholar]
- 37.Trilles L, Wang B, Firacative C, et al. Identification of the major molecular types of Cryptococcus neoformans and C. gattii by hyperbranched rolling circle amplification. PLoS ONE. 2014;9(4):e94648. doi: 10.1371/journal.pone.0094648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J, Najafzadeh MJ, Vicente V, et al. Rapid detection of pathogenic fungi using loop-mediated isothermal amplification, exemplified by Fonsecaea agents of chromoblastomycosis. J Microbiol Methods. 2010;80(1):19–24. doi: 10.1016/j.mimet.2009.10.002. [DOI] [PubMed] [Google Scholar]