Abstract
Background
Limited efficacy of immune checkpoint blockades was observed in clinical trials in colorectal (CRC) patients, especially in the microsatellite-stable patients. Interleukin-6 (IL-6) is critical in modeling immune responses in cancers. However, the effects of targeting IL-6 in combination with immune checkpoint blockades is unknown in CRC.
Material/Methods
In the present study, we investigated the profile of IL-6 expression in tumor tissues of CRC patient and we established CRC mouse models with various IL-6 expression levels using CT26 cells and MC38 cells. Effects of anti-IL-6 and anti-PD-L1 combination treatment were tested in these models.
Results
A total of 105 CRC patients were included in this study, with 41 (39%) females and 64 (61%) males. Sixty patients showed IL-6 high expression and 45 patients showed IL-6 low expression. The patients with IL-6 high expression tended to have shorter survival (median survival time of 25.5 months) than the patients with IL-6 low expression (median survival time of 46 months, P value=0.013). In the CRC mouse models, tumors with IL-6 overexpression tended to grow faster than the tumors with IL-6 knockout. The numbers of CD8+ T cells and CD4+ T cells were decreased in IL-6 overexpressed tumors. On the contrary, myeloid-derived suppressor cells and regulatory/suppressor T cells were more numerous in tumors with IL-6 overexpression. PD-L1 expression was upregulated in the tumors with IL-6 overexpression. Importantly, an IL-6 blockade reversed the anti-PD-L1 resistance and prolonged tumor-bearing mouse survival.
Conclusions
Our study indicates that IL-6 induces strong immunosuppression in the CRC microenvironment by recruiting immunosuppression cells and impairing T cell infiltration. Inhibition of IL-6 enhanced the efficacy of anti-PD-L1 in CRC, providing a novel strategy to overcome anti-PD-L1 resistance in CRC.
MeSH Keywords: Antigens, CD274; Colorectal Neoplasms, Hereditary Nonpolyposis; Immunity, Cellular; Interleukin-6
Background
Tumor-specific adaptive immunity, such as cytotoxic T lymphocyte (CTL) response, can result in promising anti-tumor effects in human malignancies [1,2]. Immune checkpoints are either inhibitory or stimulatory pathways hardwired into the immune system for maintaining self-tolerance and modulating the duration and amplitude of immune responses [3,4]. In cancers, the inhibitory immune checkpoints, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1) limit the anti-tumor immune response, thereby facilitating tumor progression [3,4]. Immune checkpoint blockades (ICBs) blocking the immune inhibitory pathways have shown promising effects in certain late-stage cancers [5,6]. However, in colorectal cancer (CRC), especially in the microsatellite-stable CRC subtype, their efficacy is limited [7,8].
IL-6 is a pleiotropic cytokine that is involved in tumor growth, invasion, and metastasis [9–11]. It is known that IL-6 is a pivotal modulator in the initiation of prostate tumorigenesis, tumor growth, metastasis, and resistance to chemotherapy [12]. In pancreatic cancer, IL-6 is necessary for pancreatic intraepithelial neoplasia (PanIN) maintenance and progression [13]. In CRC patients, the serum levels of IL-6 are elevated in CRC patients with advanced tumors and poor prognosis, suggesting that IL-6 facilitates CRC development [14,15]. However, the immunoregulatory roles of IL-6 and potential therapeutic effects of blocking IL-6 are unknown in CRC.
In the present study, we aimed to validate the prognostic value of IL-6 in a Chinese CRC patients cohort and to elucidate the immunomodulatory effects of IL-6 in CRC. More importantly, we sought determine if blocking IL-6 would sensitize CRC tumors to the existing ICBs, thereby reducing drug resistance in CRC patients.
Material and Methods
Cell culture
Mouse CRC cell lines CT26 (CRL-2638) and MC38 were obtained from ATCC (VA, USA) and the Cell Bank of Chinese Academy of Sciences (Shanghai, China). They were cultured with RPMI-1640 medium with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, MA, USA), 100 U/ml penicillin (Sigma-Aldrich, MO, USA), and 100 mg/ml streptomycin (Sigma-Aldrich, MO, USA) in a humidified incubator with 5% CO2 at 37°C.
Patient samples
We collected a total of 105 formalin-fixed and paraffin-embedded (FFPE) CRC tissue samples. All patients were diagnosed from April 2011 to June 2012 at the Cancer Hospital of China Medical University. The samples were collected during the surgery. The follow-up started from the date of the surgery and ended in June 2016. Survival time was defined as the interval between the date of diagnosis and the date of death or the end of follow-up. This study was approved by the local Ethics Committee of Cancer Hospital of China Medical University. All patients signed the informed consent before surgery.
Immunohistochemistry
Expression of IL-6 in the FFPE tumor tissue sections was evaluated by standard immunohistochemistry (IHC). Briefly, the slides were deparaffinized with xylene and rehydrated with ethanol. Then, they were submerged in the 10 Mm citric acid buffer (Sigma-Aldrich, MO, USA) and heated in a microwave oven for 20 min for antigen retrieval. After cooling to room temperature, the slides were incubated with 3% hydrogen peroxide for 20 min and then incubated with 5% bovine serum albumin buffer (these 2 steps were both performed at room temperature). The primary antibody of IL-6 (Santa Cruz, CA, USA) was diluted by 1: 100 and added to the slides for incubation overnight at 4°C. Horseradish-peroxidase-conjugated secondary antibody (Santa Cruz, CA, USA) was diluted to 1: 1000 and added to the slides for incubation for 1 h at room temperature. The stain was developed using DAB (Abcam, MA, USA). Counterstaining was performed using hematoxylin. PBS replaced the primary antibody in the negative control. All the slides were observed under a microscope by 2 pathologists independently and without knowing the aim of this study and the clinical features of the patients. The slides with more than 30% IL-6 positive cells were defined as having IL-6 high expression.
Overexpression and knockout
Expression of IL-6 was regulated in CT26 cells and MC38 cells. Lentivirus IL-6 overexpression vector (Il6 Mouse ORF Clone Lenti Vector, Origene) and CRISPR vector (Il6 - mouse gene knockout kit via CRISPR, Origene) were used. We used the corresponding empty vector as a transfection control. HEK293T cells were transfected with vectors for amplification of the lentivirus. Then, CT26 and MC38 cells were infected with the harvested lentivirus. A standard lentivirus packaging and infection protocol was followed (from Origene). IL-6 expression was confirmed by flow cytometry.
Animal model
BALB/c mice and C57BL/6J mice (6-week-old, 20–22 g, male) were used to establish CT26 and MC38 syngeneic mouse models, respectively. All the mice were obtained from Shanghai SLAC Laboratory Animal Center of the Chinese Academy of Sciences, China. Before inoculation, cells were harvest from cell culture flasks with medium removed and counted after trypan blue staining. Then, the cells were washed with PBS and resuspended by Matrigel to a concentration of 1×106 cells/100 μl. Afterwards, a 100-μl cell suspension was slowly injected into the right flank of each mouse. Treatment started 1 week after the inoculation. We administered 10 mg/kg anti-PD-1 ligand 1 (PD-L1) and 5 mg/kg anti-IL-6 intraperitoneally twice per week. Tumor volume and survival status were measured and recorded regularly. Tumor length and width were measured weekly using calipers. Tumor volume was calculated as: tumor volume=length×width2×ϖ/6. Survival time was defined as the interval between the date of tumor cell inoculation and the date of death or the observation endpoint. Mice with extreme volume (more than 2000 mm3), obvious weight loss (more than 30%), excessive ascites, or any other distress were sacrificed and considered as deaths. All mice were raised in specific-pathogen-free conditions with free access to standard food and clean water.
Flow cytometry
Flow cytometry was performed to classify the tumor-infiltrating immune cells in the CRC mouse models: CD4+ T cell (CD3+CD19−CD4+), CD8+ T cell (CD3+CD19−CD8+), myeloid-derived suppressor cells (MDSCs, CD11b+Gr-1+), and regulatory/suppressor T cells (Tregs, CD3+CD19−CD4+CD25+Foxp3+). All the fluorescence-labeled antibodies were purchased from Biolegend. Standard flow cytometry procedures were followed. Briefly, fresh tumor tissues harvested from mouse models were chopped into small pieces and digested into single cells with collagenases type I and type IV. Cells were first stained for cell membrane markers and then processed by fixation and permeabilization buffer for cytoplasm staining. Samples were analyzed using an FACSCanto II machine (Becton Dickinson, NJ, USA). The data were visualized using Flow Jo software.
Statistical analysis
GraphPad (CA, USA) and SPSS 17.0 software (Chicago, IL, USA) were used to conduct all the statistical analyses in this study. One-way ANOVA and the t test were performed to evaluate the difference between different experimental groups. Bonferroni’s pairwise comparisons were used to analyze the difference between 2 individual groups after one-way ANOVA. Kaplan-Meier survival analysis and the log-rank test were used to evaluate the difference in survival between colon cancer patients with different IL-6 expressions. Two-tailed P<0.05 was considered as statistically significant.
Result
IL-6 high expression was related to poor survival of CRC patients
To evaluate the role of IL-6 in CRC development, we collected tumor tissues from 105 CRC patients, of whom 41 (39%) were females and 64 (61%) were males. Sixty patients with obvious IL-6 expression were considered as IL-6 high. The average age of the patients with IL-6 high expression was 56.4 years and that of the patients with IL-6 low expression was 58.3 years. When they were diagnosed as having CRC, 57.8% of the high-IL-6 patients were at an advanced stage vs. 51.7% of the low-IL-6 patients, but the difference was not significant. Other clinicopathological parameters such as tumor size and lymph node metastasis were not correlated with IL-6 expression. The survival analysis indicated that patients with IL-6 high expression tended to have poorer survival than the patients with IL-6 low expression (log-rank test, P value=0.013, Figure 1). The patients with IL-6 high expression had a median survival time of 25.5 months, while the patients with IL-6 low expression had a median survival time of 46 months (P value=0.013). This data suggested that IL-6 high expression in tumor tissue may promote tumor development of CRC.
Figure 1.
Prognostic value of IL-6 in CRC patients. (A) IL-6 expression in CRC tissues. The major staining was seen in cytoplasm. (B) The survival analysis indicated that the patients with IL-6 high expression (n=60) tended to have shorter survival time than the patients with IL-6 low expression (n=45, P value=0.013). IL-6 low, IL-6 low expression; IL-6 high, IL-6 high expression.
IL-6 overexpression promoted PD-L1 expression in the CRC mouse model
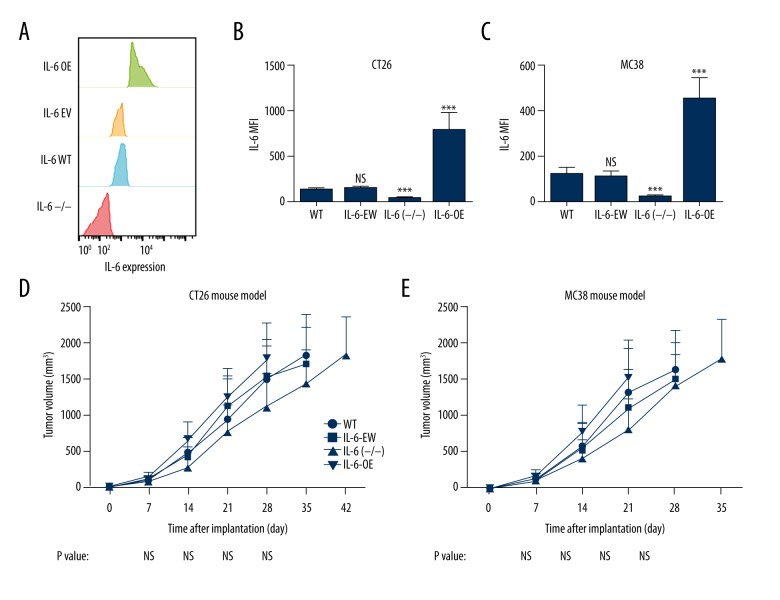
To examine the role of IL-6 in CRC development, we established CRC mouse models using CT26 and MC38 cell lines with altered IL-6 expression: wild-type cell lines (WT), cells transfected with empty vector (IL-6-EV), cells transfected with IL-6 CRISPR (IL-6−/−), and cells transfected with IL-6 overexpression vector (IL-6-OE) (Figure 2A–2C). Tumor growth of these mouse models was recorded (Figure 2D, 2E). The data indicated that the tumors with IL-6 overexpression tended to grow faster than the tumors with IL-6 knockout, but the difference was not significant (Figure 2D, 2E).
Figure 2.
Role of IL-6 in tumor growth in CRC mouse models. (A) Expression of IL-6 was tested by flow cytometry in modified cells. (B, C) IL-6 expression values in modified cells. (D, E) Syngeneic mouse models were established using CT26 and MC38 cells with various levels of IL-6 expression. Tumors with IL-6 deficiency (IL-6 (−/−)) grew slightly slower than the tumors with IL-6 overexpression (IL-6-OE) and control groups, but no significant difference was observed. *** P value less than 0.001; WT – wild-type; EV – empty vector; OE – overexpression; MFI – mean fluorescence intensity; sample size of every experimental group was 10.
IL-6 expression suppressed immune response in the CRC mouse model
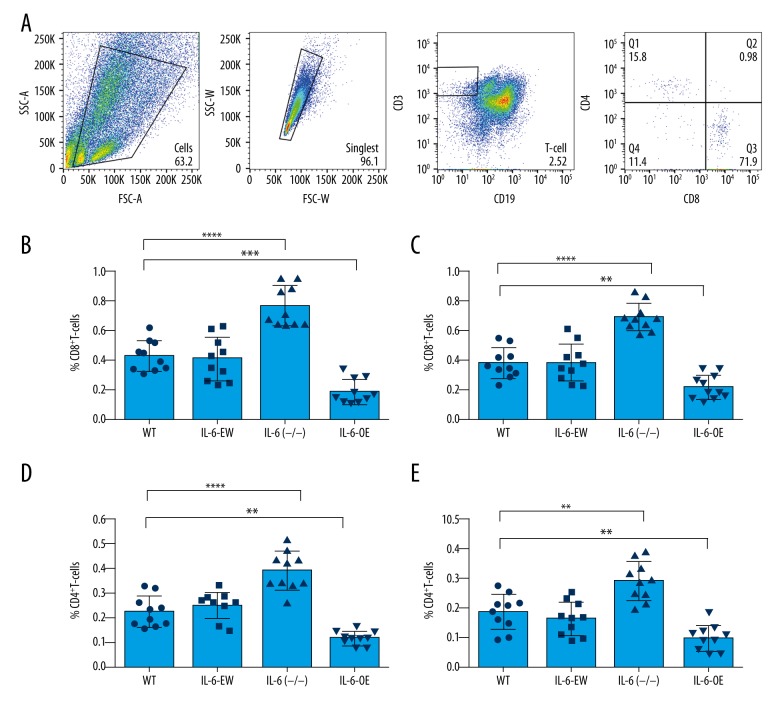
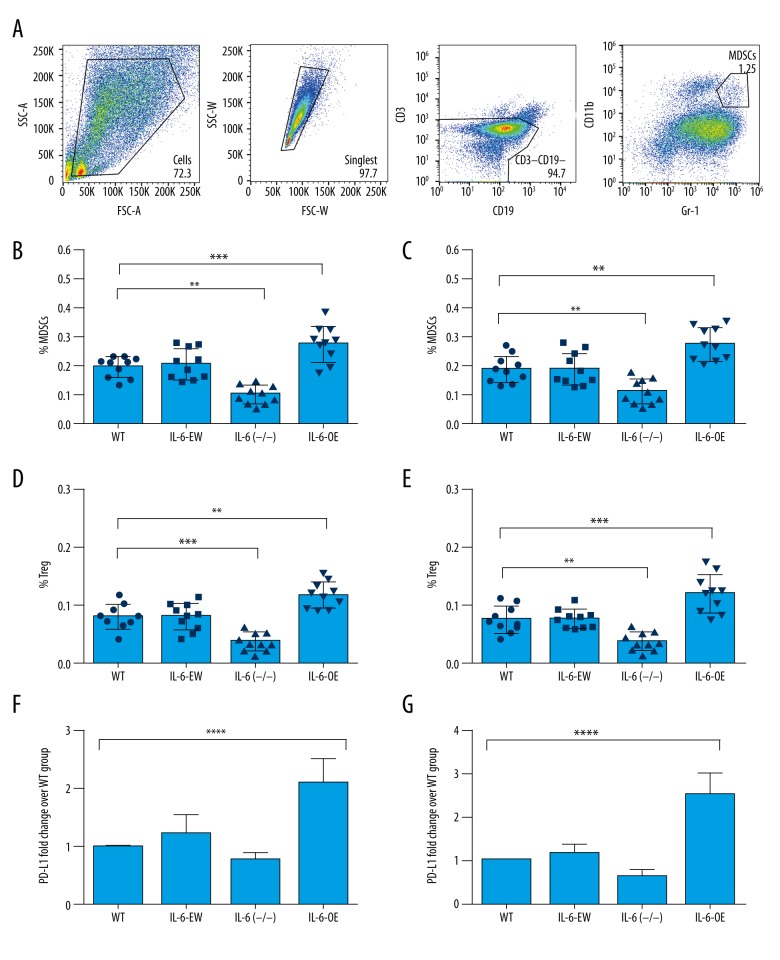
We evaluated the numbers of tumor-infiltrating immune cells in CT26 and MC38 tumors, aiming to characterize the immune signature in CRC models with different IL-6 expression levels. Interestingly, we found that the numbers of CD8+ T cells and CD4+ T cells were decreased in IL-6-overexpressed tumors but were increased in the IL-6-deficient tumors (Figure 3). However, the numbers of MDSCs and Tregs were increased in the tumors with IL-6 overexpression and decreased in the tumors with IL-6 deficiency (Figure 4). PD-L1 is a potent inhibitory regulator of anti-tumor immunity. We found that PD-L1 expression was significantly increased in the tumors with IL-6 overexpression (Figure 4F, 4G). Taken together, our results show that IL-6 expression causes a compromised anti-tumor immunity in CRC.
Figure 3.
Effects of IL-6 on immune cells tumor infiltration in CRC mouse models. (A) The tumor-infiltrating immune cells were measured by flow cytometry. Gating plots for T cells are shown. (B, C) The ratio of CD8+ T cells was significantly increased in the tumor tissues with IL-6 deficiency but was decreased in the tumors with IL-6 overexpression. (D, E) The number of CD4+ T cells in tumors with IL-6 deficiency was significantly enhanced but was decreased in tumors with IL-6 overexpression. ** P value less than 0.01; *** P value less than 0.001; **** P value less than 0.0001; WT – wild-type; EV – empty vector; OE – overexpression; sample size of every experimental group was 10 mice.
Figure 4.
Overexpression of IL-6 increased immunosuppression in CRC mouse models. (A) Gating plots for MDSCs are shown. (B, C) In IL-6 overexpressed tumors, the frequency of MDSCs were significantly increased. However, in the IL-6-deficient tumors, their ratios were significantly decreased. (D, E) The number of Tregs was increased in IL-6-overexpressed tumors. (F, G): Tumors with IL-6 overexpression showed increased PD-L1 expression but tumors with IL-6 deficiency showed decreased PD-L1 expression. ** P value less than 0.01; *** P value less than 0.001; **** P value less than 0.0001; WT – wild-type; EV – empty vector; OE – overexpression; MDSCs – myeloid-derived suppressor cells; Tregs – regulatory/suppressor T cells PD-L1, PD-1 ligand 1; sample size of every experimental group was 10 mice.
Anti-IL-6 treatment acted synergistically with anti-PD-L1 treatment in the CRC mouse model
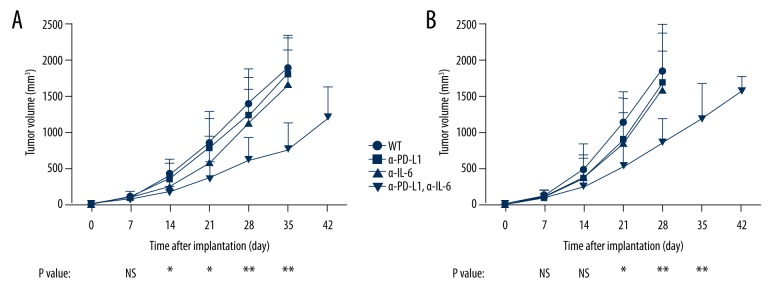
Because IL-6 overexpression promoted PD-L1 expression and inhibited anti-tumor immune activity in CRC, we explored the effect of anti-IL-6 treatment in combination anti-PD-L1 treatment in both the CT26 mouse model and the MC38 mouse model. Importantly, the result showed that anti-IL-6 treatment or anti-PD-L1 treatment alone did not have an obvious effect on these mouse models (Figure 5). However, anti-IL-6 and anti-PD-L1 combined treatment significantly suppressed tumor growth (Figure 5). Therefore, we believe that anti-IL-6 and anti-PD-L1 acts synergistically in inhibiting colon cancer growth.
Figure 5.
Effects of combined treatment of anti-PD-L1 and anti-IL-6 in CRC mouse models. CT26 (A) and MC38 (B) mouse models were treated by anti-PD-L1 or anti-IL-6 at 1 week after tumor implantation. Tumor growth of these models was significantly suppressed by the combined treatment with anti-PD-L1 and anti-IL-6 antibodies. * P value less than 0.05; ** P value less than 0.01; WT – wild-type; EV – empty vector; OE – overexpression; PD-L1 – PD-1 ligand 1; sample size of every experimental group was 10 mice.
Discussion
Accumulating evidence from preclinical and clinical studies suggests that the inflammatory cytokines and chemokines have critical roles in cancer immunotherapy [16]. In normal condition, IL-6 is secreted by T-helper cells and macrophages to stimulate immune response and maintain chronic inflammation [17]. IL-6 is one of the major cytokines in the tumor microenvironment and is an important factor involved in multiple processes of tumor development, such as apoptosis, survival, proliferation, angiogenesis, invasiveness, and metastasis, and, most importantly, the immune response [18]. In this study, we focused on the effects of IL-6 expression on immune regulation of CRC.
To understand the roles of IL-6 in CRC, we first correlated IL-6 expression with CRC patients’ survival. Patients with IL-6 high expressions had an obvious shorter survival time than the patients with IL-6 low expression. This data agrees with previous reports [15] and suggests that IL-6 high expression has an adverse effects on CRC patients. We further investigated the mechanisms by which IL-6 overexpression can promote CRC progression. Interestingly, overexpression of IL-6 in mouse CRC cell lines did not increase tumor growth dramatically, which made us think that IL-6 produced by CRC tumor cells may function in the tumor microenvironment to promote tumor progression.
Anti-tumor immune cells are major components of the tumor microenvironment and control the most vulnerable point of cancer [19]. Intensive infiltration of immune cells, such as T cells and NK cells, is correlated with better survival of CRC patients [20,21]. Using flow cytometry, we found that CD4 and CD8 T cell populations were suppressed by IL-6 overproduction in the tumor tissue. However, the immunosuppressive cells, such as MDSCs and Tregs, were more numerous in IL-6-overexpressed tumors. PD-L1 is a transmembrane protein speculated to play a major role in suppressing the immune system during particular events, such as cancer. High expression of PD-L1 in tumor tissue has potent effects on inhibiting anti-tumor immunity [22]. In our animal model, we showed that IL-6 overexpression upregulated PD-L1 levels in tumor tissues. These data clearly indicate that IL-6 overexpression in CRC promotes tumor progression by inducing immunosuppression.
Immunotherapy targeting the immune checkpoints has been approved for the treatment of an expanding list of cancers, including microsatellite instable (MSI) CRC [23]. Clinical trials of PD-L1/PD-1 antibodies in MSI CRC patients have shown favorable results, but only about 15% of CRC patients are MSI subtype [24]. The rest of the microsatellite-stable (MSS) CRC patients showed a very low responsive rate [23]. These clinical observations underscore the compelling need to reverse the non-responsive CRC tumors for better therapeutic responses to immune checkpoint blockades. Previous studies have clearly shown that immune-suppressive factors in the tumor microenvironment are one of the major factors causing immunotherapy failure [25]. Each treatment can only reduce some of these suppressive factors. Thus, releasing more immunosuppressive factors by combining different treatments that target different immunosuppressive mechanisms will be a promising strategy to achieve better responses in immunotherapies. Since we showed that high IL-6 expression could induce a suppressive immune phenotype, we further investigated the feasibility of combining anti-IL-6 with anti-PD-L1 in CRC. Importantly, we noticed that anti-IL-6 treatment was able to reverse anti-PD-L1 resistance in CRC mouse models. Our results and those of previous studies [25] may help develop novel treatments for immune checkpoint-insensitive CRC patients.
Conclusions
We found that IL-6 has important roles in suppressing immune response in CRC and can serve as a target for sensitizing anti-PD-L1 treatment.
Footnotes
Source of support: This work was supported by the National Natural Science Fund from the National Natural Science Foundation of China (Grant No. 81672427)
Conflict of interest
None.
References
- 1.Koi M, Carethers JM. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncol. 2017;13(18):1633–47. doi: 10.2217/fon-2017-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813–20. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 9.He G, Dhar D, Nakagawa H, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155(2):384–96. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldner MJ, Neurath MF. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol. 2014;26(1):75–79. doi: 10.1016/j.smim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 11.IL-6 secreted by cancer-associated fibroblasts induces tamoxifen resistance in luminal breast cancer. Oncogene. 2014;33(35):4450. doi: 10.1038/onc.2014.224. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: The role of interleukin 6 (IL-6) BJU Int. 2014;113(6):986–92. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Yan W, Collins MA, et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73(20):6359–74. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83(4):222–26. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 15.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients – a summary of published results. Int J Colorectal Dis. 2010;25(2):135–40. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 16.McMillan DC. Cancer and systemic inflammation: Stage the tumour and stage the host. Br J Cancer. 2013;109(3):529. doi: 10.1038/bjc.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141(2):125–39. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Becht E, Giraldo NA, Dieu-Nosjean MC, et al. Cancer immune contexture and immunotherapy. Curr Opin Immunol. 2016;39:7–13. doi: 10.1016/j.coi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Galon J, Pagès F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–28. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–45. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 23.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smaglo BG, Marshall JL. Microsatellite instability in colorectal cancer. Clin Adv Hematol Oncol. 2013;11(10):659–61. [PubMed] [Google Scholar]
- 25.Zhao X, Subramanian S. Oncogenic pathways that affect antitumor immune response and immune checkpoint blockade therapy. Pharmacol Ther. 2018;181:76–84. doi: 10.1016/j.pharmthera.2017.07.004. [DOI] [PubMed] [Google Scholar]