Abstract
Background
Pulmonary aspiration of the gastric contents is a serious perioperative complication. The aim of this study was to evaluate the efficacy of portable ultrasonography in the preoperative evaluation of the gastric contents of patients. The secondary aim was to examine the relationship between gastric antrum cross-sectional area and age and body mass index (BMI).
Material/Methods
This single-center, prospective, cross-sectional study included 120 patients who underwent surgery. Measurements the gastric antral cross-sectional areas and quantitative and qualitative measurements of the stomach were taken by ultrasonography guidance in all patients.
Results
With the patient in a supine position, the mean gastric antrum cross-sectional area was found to be 3.4±2.43 cm2 (range, 0.79–17.3 cm2). As the number of hours of fasting increased, the gastric antral cross-sectional area statistically significantly decreased (P<0.05). Increased age and BMI values were determined to increase the gastric antrum cross-sectional area in a linear correlation; r=0.209, P<0.05 and r=0.252, P=0.05, respectively. It was determined that 20.8% of the patients exceeded the high-risk stomach antral cutoff cross-sectional area that was defined as 340 mm2 in patients fasting for at least 8 hours.
Conclusions
It was determined that bedside ultrasonography is a useful, non-invasive tool in the determination of gastric content and volume. A significant proportion of surgical patients may not present with an empty stomach despite the recommended fasting protocols.
MeSH Keywords: Fasting, Pyloric Antrum, Respiratory Aspiration of Gastric Contents, Ultrasonography
Background
Although perioperative aspiration of the stomach contents is seen rarely in anesthesia practice, it is a complication that can lead to severe morbidity and mortality [1,2]. The risk of morbidity and mortality has been reported as 8%–10% in large case series studies that included long-term mechanical ventilation as a result of aspiration pneumonia [3,4]. However, different clinical conditions emerge depending on the type and amount of aspirated material, the frequency of aspiration, and the response of the patient. Solid particles, liquids with a low pH, and an excessive amount of content increase morbidity. Identification of patients at risk is a cornerstone of safe anesthesia practice regarding preoperative fasting and therapeutic drug aspiration. General anesthesia or sedation block the physiological mechanisms preventing aspiration (lower esophagus sphincter and upper airway reflexes) [5]. Therefore, the liquid and solid food intake of a patient before general anesthesia is important for patient safety. In the revised preoperative fasting guidelines, at least 2 hours without clear liquids is recommended, 6 hours of fasting for liquids containing solid particles and toasted sandwich type solid foods, and 8 hours of fasting for high calorie and fatty foods [6]. For patients at risk of aspiration, rapid series induction, tracheal intubation without mask ventilation, or face mask ventilation with cricoid pressure are recommended [7].
Despite the use of methods such as paracetamol absorption, polyethylene glycol dilution, and electric impedance tomography for visualization of the structure of the stomach content, volume, and time to emptying, stomach scintigraphy has been accepted as the gold standard imaging method [8,9]. However, it is not considered a practical measurement method as it is costly, causes wide spread radiation exposure, and requires special equipment. Gastric ultrasonography guidance (USG) has come into common use as it is a practical, inexpensive, and useful imaging method. Recent studies which have used bedside gastric USG have shown that the nature of the stomach content (liquid, solid, or empty) and the volume of the stomach content is correlated to the antral cross-sectional area [10–14]. Its accuracy and reproducibility have been shown in multiple studies [10–12]. Gastric USG may be useful in many clinical situations such as lack of patient adherence to fasting instructions (e.g., due to emergency/urgent procedure or miscommunication), unreliable fasting history (e.g., altered sensorium, language barrier, or cognitive dysfunction), potential delay in gastric emptying (e.g., pregnancy, diabetes mellitus, severe liver or kidney dysfunction, or neuromuscular disorders) in which aspiration risk is unclear or undetermined.
The primary aim of this prospective study was to identify patients at risk of perioperative aspiration of stomach contents, by calculating the gastric antrum cross-sectional area with preoperative stomach antrum USG of patients. The secondary aim was to examine the relationship between gastric antrum cross-sectional area and age and body mass index (BMI).
Material and Methods
Ethical approval
Approval for the study was granted by the Institutional Ethics Committee (Decision no: 82, Diyarbakir Training and Research Hospital Ethics Committee). Written informed consent was obtained from all patients.
Patients
This prospective study included 123 patients who were to undergo surgery. Inclusion criteria for the study were patient age 18–85 years old, height >145 cm, weight 40–110 kg, BMI <35 kg/m2, ASA classification (I, II, III), and elective or emergency surgery. Patients were excluded from the study if they had abnormal anatomy of the upper gastrointestinal system (previous esophagus or gastric surgery, including hiatus hernia), if they had active gastric or duodenal ulcer, upper gastrointestinal system bleeding, vagal nerve denervation, diabetes mellitus, subocclusive syndrome, or infiltrative diseases such as scleroderma or amyloidosis, medullar lesion above T10 level, or pregnancy. The presence of gastroesophageal reflux disease alone was not an exclusion criterion.
Preprocedural assessment
In the preoperative section of the operating theater all patients were questioned in detail about their fasting status, demographic data were recorded, and a medical examination was made. Before anesthesia induction, the aforementioned-described standardized screening protocol was applied to the patients for gastric examination and USG by an experienced USG operator (with at least 5 years USG experience and >50 gastric USG examinations). The doctor applying the USG had no influence on the anesthesia method or any other procedures applied to the patient.
Procedure
With the patient in a supine position, the USG examination was made with a 2–5 MHz curvilinear array low frequency transducer (Sonosite® M-Turbo Bothell WA, USA). The transducer was placed in the parasagittal plane of the epigastric region. To obtain general qualitative observations of the gastric antrum and body, the stomach cavity, and the stomach contents, the transducer was scanned curving from right to left. The antrum is generally seen in the parasagittal plane immediately to the right of the midline. Perlas et al. showed reference points to be the left anterior lobe or caudal lobe of the liver, the pancreas head, and the superior mesenteric vessels with the vena cava inferior or abdominal aorta [10–12]. Quantitative and qualitative evaluation of the gastric antrum was made by the USG operator. If the antrum had a smooth appearance when the anterior and posterior walls were next to each other, it was evaluated as empty. When the antrum had a hypoechoic content and the walls had a swollen endocavitary lumen, this was evaluated as evidence that it contained fluid. All the measurements were of the resting stomach at the moment when peristaltic contractions ceased.
The cross-sectional antral area was measured using the free-tracing method (FTM), using calculations of the manually drawn lines as determined by the ultrasound caliper section. The traditional two-diameter method (TDM) described by Bolondi et al. [15] was selected for this study as the calculation is simple and easy to apply according to the formula, CSA=AP×CC×π÷4. In a study using FTM and TDM, Kruisselbrink et al. showed that the results of the measurements of both methods were similar [14]. In this study, the cutoff value of antral cross-sectional area of 340 mm2 was accepted as the diagnosis of an at-risk stomach according to the study by Bouvet et al. [13].
Statistical analysis
A descriptive analysis of the demographic data (age, weight, height, and BMI), gender, and ASA classification was performed. The data were summarized using the mean and standard deviation. The assumption of normal distribution of continuous variables was checked using the Shapiro-Wilk test. If variables were normally distributed, central tendency was expressed as the mean (SD). Means were compared using Student’s t-test or one-way analysis of variance as appropriate. Categorical data are expressed as the count and percentages or ratios and analyzed with the Fisher’s exact test. Differences were considered significant if P<0.05. Statistical analysis was performed using SPSS 20 software (IBM, Armonk NY, USA).
Results
The study included a total of 123 patients. Three patients were excluded: 2 patients where detailed images could not be taken because of gas in the stomach, and 1 patient who had a BMI of >35 kg/m2. Therefore, the study evaluation included 120 patients of which 76 were male and 44 were female; the mean age was 40.4±16.54 years (range, 18–82 years). The demographic data of the patients are shown in Table 1. A period of more than 8 hours of fasting was determined in 91 patients (75.8%) and less than 8 hours of fasting in 29 patients (24.2%). The mean cross-sectional area of the stomach antrum was found to be 3.34±2.43 cm2 (range, 0.79–17.3 cm2).
Table 1.
Demographic data of the patients.
| Characteristic | |
|---|---|
| Age (years) | 40.4±16.54 (18–82) |
| Gender (M/F) | 76/44 |
| Height (cm) | 169.01±9.11 (148–187) |
| Weight (kg) | 72.97±12.27 (44–105) |
| Area (cm2) | 3.34±2.43 (0.79–17.3) |
| BMI (kg/m2) | 25.56±4.02 (17.72–37.78) |
| ASA | |
| ASA I | 84 |
| ASA II | 29 |
| ASA III | 7 |
| Preoperative fasting time (h) | 9.41±4.08 (1.0–18.50) |
| >8 hours fasting | 91 |
| <8 hours fasting | 29 |
| Type of surgery | |
| General surgery | 44 |
| Orthopedia | 24 |
| Urology | 24 |
| Otolaryngology | 8 |
| Ophtalmology | 13 |
| Neurosurgery | 4 |
| Plastic and Reconstructive surgery | 3 |
| Planned Anesthesia | |
| General | 59 |
| Spinal | 35 |
| Regional blok | 18 |
| Sedation | 4 |
| Local | 4 |
| Total | 120 |
ASA – American Society of Anesthesiologists; mean ± standard deviation; n – patient number.
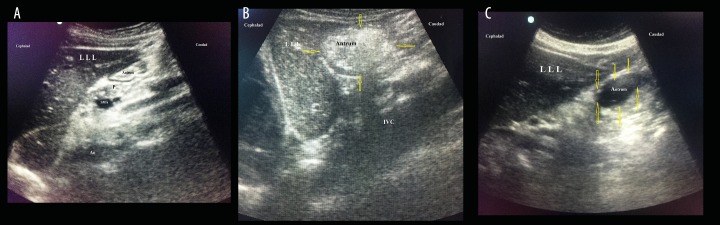
USG images of the patients are shown in Figure 1. Patients with an empty stomach were seen to have oval or round images in the sagittal plane; this appearance is described as “bullseye” (Figure 1A). The walls of an empty stomach appear relatively thick. In a stomach with liquid content, the antrum appears stretched and round, and the stomach walls are seen to be thin and hypoechoic (Figure 1B). When there is solid content, the antrum appearance is described as frosted glass (Figure 1C).
Figure 1.
(A) Gastric sonographic image of the antrum in the epigastric area obtained in a sagittal plane using a curved-array low-frequency probe after 8 hours of fasting. Note a hypoechoic layer within the gastric wall that corresponds to the muscularis propria. Ao – aorta; LLL – left lobe of the liver; P – pancreas; SMA – superior mesenteric artery. (B) Gastric ultrasound images of the antrum 2 hour after ingestion of a solid meal. (C) Gastric ultrasound images of the antrum 1.5 hour after ingestion of clear fluid. The clear fluid in the stomach seen as hypoechoic-anechoic content.
The mean, standard deviation, and the minimum and maximum values of the antral cross-sectional area of patients with at least 8 hours of fasting and less than 8 hours of fasting are shown in Table 2. The antral cross-sectional area of the group with less than 8 hours of fasting was determined to be statistically significantly higher (P<0.05). No statistically significant difference was determined between the mean antrum cross-sectional areas of emergency and elective cases (P>0.05). No difference was seen between the fasting periods of elective and emergency cases (P>0.05). According to the cutoff value of 340 mm2 determined for the diagnosis of stomach fullness risk, an increased rate was determined in 20.8% of patients with at least 8 hours of fasting and the mean cross-sectional area was found to be 461.65±135.4 mm2 (Table 2).
Table 2.
Characteristics of antral cross-sectional area.
| Characteristic | n (%) | Anral area (mm2) | p |
|---|---|---|---|
| Surgery | 0.17 | ||
| Emergency | 24 | 394.21±21.2 | |
| Elective | 96 | 319.25±24.9 | |
| Emergency (fasting time) | |||
| At least 8 hours fasting | |||
| Antral area | |||
| >340 mm2 | 20 (% 20.8) | 461.65±135.4 | |
| <340 mm2 | 72 (%79.2) | 217.7±68.9 | |
| Total | 25 | ||
A linear correlation curve was seen such that as the fasting period increased, the antral cross-sectional area decreased (Figure 2). The correlation coefficient was determined as r=−0.499 (P<0.01).
Figure 2.
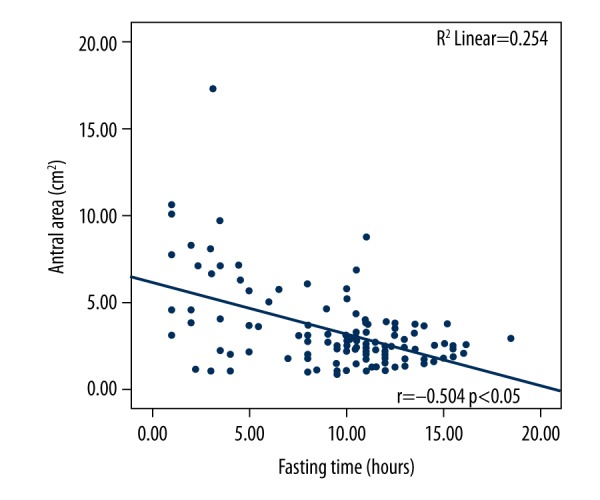
Correlation of the antral cross-sectional area with fasting time (hours).
The correlation of BMI and the antral cross-sectional area of patients with at least 8 hours of fasting is shown in Figure 3. A weak positive linear correlation was determined (r=0.252, P=0.05). Similar results were determined in the relationship between age and antral cross-sectional area (r=0.209, P<0.05) (Figure 4).
Figure 3.
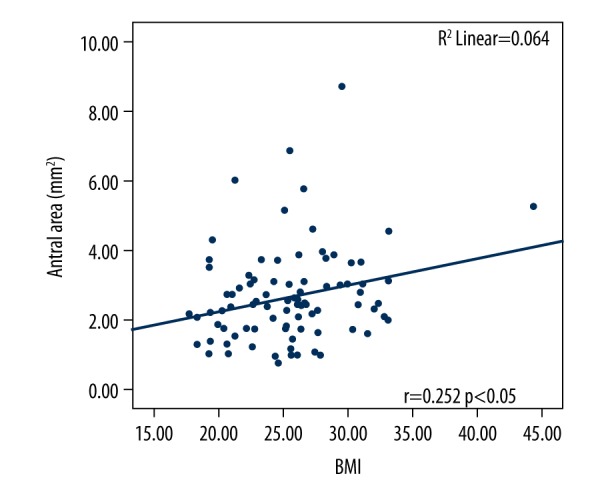
Correlation of the antral cross-sectional area with body mass index, at least 8 hours of fasting.
Figure 4.
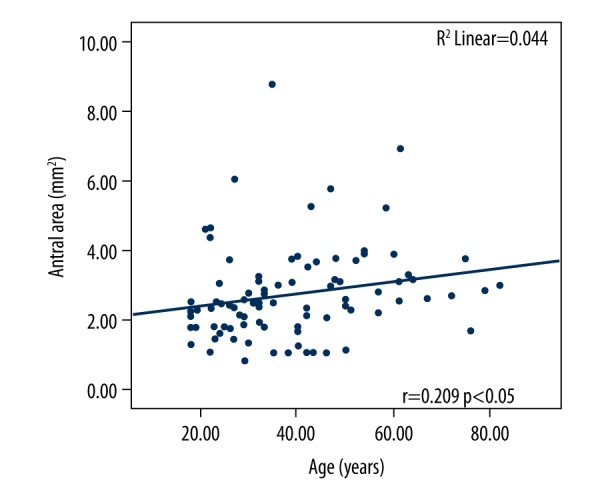
Correlation of the antral cross-sectional area with age (years), at least 8 hours of fasting.
Discussion
The results of this study showed that the use of USG on patients for preoperative gastric antrum measurements was simple and effective for the examination of stomach contents. In the different periods of fasting, it was seen that as the period of fasting increased, the stomach antrum area decreased; in patients with at least 8 hours of fasting, a statistically significant change was determined in the stomach antrum area, independent of factors such as age and BMI.
In 1946, Mendelson first identified pneumonia associated with the aspiration of gastric contents during general anesthesia in obstetric patients, and this complication has since been emphasized as a serious problem in anesthesia practice [16]. In recent prospective and retrospective large case series studies in the UK and France, aspiration of the stomach contents has been shown to be the leading cause of anesthesia-related mortality [4,17]. Lung damage related to aspiration of stomach acid has been reported to be the result of 2 mechanisms. The first is rapid tissue damage which is a direct effect, and the second is the indirect development of widespread inflammatory response. Severe complications, such as pneumonia, pulmonary edema, acute respiratory distress syndrome, sepsis, and hypoxic brain injury, are associated with aspiration [18].
Until recently, USG was used in anesthesiology for venous and arterial punctures, peripheral and central nerve blocks, cardiac interventions, and at increasing rates in orotracheal intubation interventions. Bedside determination of gastric fullness with USG has become a part of the daily practice of anesthesia used in intensive care units and emergency care units for the evaluation of the risk of pulmonary aspiration. Koenig et al. determined the stomach contents of intensive care patients who were to be intubated in the supine position using USG, then aspirated the stomach contents and applied the procedures [19]. Gastric USG has also been used by gastroenterologists to determine gastric motility and emptying, or lesions in the gastric wall. In the evaluation of gastric motility, the results of USG have been seen to be close to those obtained with scintigraphy, which is considered the gold standard [20]. After efficacy is proven in the calculations of gastric volume of bedside ultrasound [12], the efficacy is further determined by showing the nature of the stomach contents (solid, liquid, or empty). Just as in the current study, previous studies have calculated that the gastric content volume increases in with a linear correlation (Pearson correlation coefficient 0.6–0.91) with increasing antral area [10,11,21–23].
The volume of gastric content is defined with different mathematical models after the calculation of the gastric antrum cross-sectional area with USG imaging [10–13]. The Perla’s and Bouvet mathematical models are acceptable with respect to reliability and applicability. In the Bouvet model, gastric volumes up to 250 mL can be calculated from stomach serosa-to-serosa in non-pregnant adults with BMI of 14–31 kg/m2 (correlation coefficient 0.72) [13]. In the Perlas model, gastric volumes up to 500 mL can be calculated from stomach serosa-to-serosa in non-pregnant adults with BMI of up to 40 kg/m2 (correlation coefficient 0.86). As the current study was conducted with patients in the supine position, the Perlas et al. mathematical formula was determined to be appropriate for the gastric volume calculation in this position [12]. However, because the variable values in this formula (19 years < age <58 years, 45 kg < weight <105 kg, 150 cm < height <192 cm, 2.3cm2 < CSA-supine <16.27 cm2) were not suitable for the current study group, correct results could not be obtained.
There is ongoing debate related to the cutoff values of gastric volume which would lead to aspiration of stomach contents. Previous studies have shown basal values of gastric content volume varying between 100 mL and 130 mL in individuals with an average fasting period, which has been shown to be equivalent to 0.6 mL/kg [24,25]. In animal studies adapted to humans, while the stomach content of 25–50 mL volume (0.4–0.8 mL/kg) has been accepted as the cutoff value [26], Perlas et al. stated that 1.5 mL/kg could be appropriate [10]. In risk evaluation according to gastric antrum area measurements, while there are studies that have taken 320 mm2 as a threshold value [27,28], in another study by Bouvet et al. which evaluated aspiration of stomach contents in the supine position after gastric antrum measurements, used the risk of solid food or liquid content of 0.8 mL/kg with antral cross-sectional area of 340 mm2 with 91% sensitivity, 71% specificity, and 94% negative predictive value [13]. According to the results of the current study, in 20.8% of the patients with a fasting period of at least 8 hours, the antral cross-sectional area was higher than 340 mm2. In a study by Bisinoti et al., the stomach was determined not to be empty by gastric measurements of 26.4% of patients [29]. It has been suggested that there could be patient incompatibility or known or unknown gastric motility impairment because of increased gastric content in elective patients [30].
The patient’s age is not expected to have an effect on the calculated gastric volume and antral area. In a study by Perlas et al., the antral cross-sectional area of elderly patients was determined to be greater than that of young patients estimated to have the same gastric volume [10]. In other words, a lower gastric volume was found in the right lateral antral cross-sectional area measurements in elderly patients compared to young patients. In the current study, it was determined that the antral cross-sectional area of patients fasting for at least 8 hours increased with a low linear correlation with advancing age. The reason for this may be that the stomach walls of elderly patients are more compliant than those of young patients, and it could be that there is reduced gastric motility at an advanced age.
In the current study, no statistically significant difference was determined in the antral cross-section measurements of those undergoing elective or emergency surgery. Due to the low number of patients, and because the majority of those in the emergency patient group were appendectomy cases who had delayed time to admittance for surgery for diagnosis from fasting periods, the period of fasting exceeded the mean 8 hours.
There were some limitations to this study. It was not applied to specific patient groups such as children, the elderly, and those with certain comorbidities. The USG examinations were made by a single practitioner and it was not possible for the results to be confirmed by another researcher. As the study was conducted with the patients in a supine position, there was no clear mathematical formula related to the stomach content volume. Therefore, high-risk stomach classification could not be applied according to the estimated gastric content volume.
Conclusions
In conclusion, because of the preoperative gastric antrum measurements, it was determined that the use of bedside USG was simple and effective in the examination of stomach content volume and the evaluation of the related risk of aspiration. It should be taken into consideration that patients have a risk of gastric filling despite recommended fasting protocols, increased age, and BMI.
Footnotes
Source of support: Departmental resources
Conflict of interest
None.
References
- 1.Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg. 2001;93(2):494–513. doi: 10.1097/00000539-200108000-00050. [DOI] [PubMed] [Google Scholar]
- 2.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Shime N, Ono A, Chihara E, Tanaka Y. Current status of pulmonary aspiration associated with general anesthesia: A nationwide survey in Japan. Japanese J Anesthesiol. 2005;54(10 PG-1177–1185):1177–85. [PubMed] [Google Scholar]
- 4.Lienhart A, Auroy Y, Pequignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105(6):1087–97. doi: 10.1097/00000542-200612000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Vanner RG, Pryle BJ, O’dwyer JP, Reynolds F. Upper oesophageal sphincter pressure and the intravenous induction of anaesthesia. Anaesthesia. 1992;47(5):371–75. doi: 10.1111/j.1365-2044.1992.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures. Anesthesiology. 2011;114(3):495–511. doi: 10.1097/ALN.0b013e3181fcbfd9. [DOI] [PubMed] [Google Scholar]
- 7.El-Orbany M, Connolly LA. Rapid sequence induction and intubation: Current controversy. Anesth Analg. 2010;110:1318–25. doi: 10.1213/ANE.0b013e3181d5ae47. [DOI] [PubMed] [Google Scholar]
- 8.Seok JW. How to interpret gastric emptying scintigraphy. J Neurogastroenterol Motil. 2011;17(2):189–91. doi: 10.5056/jnm.2011.17.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Näslund E, Bogefors J, Grybäck P, et al. Gastric emptying: comparison of scintigraphic, polyethylene glycol dilution, and paracetamol tracer assessment techniques. Scand J Gastroenterol. 2000;35(4):375–79. doi: 10.1080/003655200750023930. [DOI] [PubMed] [Google Scholar]
- 10.Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116(2):357–63. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 11.Perlas A, Davis L, Khan M, et al. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113(1):93–97. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 12.Perlas A, Vincent WSC, Catalin ML, et al. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–89. doi: 10.1097/ALN.0b013e3181a97250. [DOI] [PubMed] [Google Scholar]
- 13.Bouvet L, Mazoit J-X, Chassard D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114(5):1086–92. doi: 10.1097/ALN.0b013e31820dee48. [DOI] [PubMed] [Google Scholar]
- 14.Kruisselbrink R, Arzola C, Endersby R, et al. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;(1):1–6. doi: 10.1097/ALN.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 15.Bolondi L, Bortolotti M, Santi V, et al. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89(4):752–59. doi: 10.1016/0016-5085(85)90569-4. [DOI] [PubMed] [Google Scholar]
- 16.Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obs Gynecol. 1946;52:191–205. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 17.Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth. 2011;106(5):617–31. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 18.Sakai T, Planinsic RM, Quinlan JJ, et al. The incidence and outcome of perioperative pulmonary aspiration in a university hospital: A 4-year retrospective analysis. Anesth Analg. 2006;103(4):941–47. doi: 10.1213/01.ane.0000237296.57941.e7. [DOI] [PubMed] [Google Scholar]
- 19.Koenig SJ, Lakticova V, Mayo PH. Utility of ultrasonography for detection of gastric fluid during urgent endotracheal intubation. Intensive Care Med. 2011;37(4):627–31. doi: 10.1007/s00134-010-2125-9. [DOI] [PubMed] [Google Scholar]
- 20.Darwiche G, Thorsson O. Correlation between simultaneous scintigraphic and ultrasonographic measurement of gastric emptying in patients with type 1 diabetes mellitus. J Ultrasound Med. 2003;22:459–66. doi: 10.7863/jum.2003.22.5.459. [DOI] [PubMed] [Google Scholar]
- 21.Fujigaki T, Fukusaki M, Nakamura H, et al. Quantitative evaluation of gastric contents using ultrasound. J Clin Anesth [Internet] 1993;5(6):451–55. doi: 10.1016/0952-8180(93)90059-n. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz A, Thomas S, Melanie F, et al. Ultrasonographic gastric antral area and gastric contents volume in children. Paediatr Anaesth. 2012;22(2):144–49. doi: 10.1111/j.1460-9592.2011.03718.x. [DOI] [PubMed] [Google Scholar]
- 23.Hveem K, Hausken T, Berstad A. Ultrasonographic assessment of fasting liquid content in the human stomach. Scand J Gastroenterol. 1994;29(9):786–89. doi: 10.3109/00365529409092511. [DOI] [PubMed] [Google Scholar]
- 24.Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344–50. doi: 10.1097/00000539-200111000-00063. [DOI] [PubMed] [Google Scholar]
- 25.Maltby JR, Lewis P, Martin A, Sutherland LR. Gastric fluid volume and pH in elective patients following unrestricted oral fluid until three hours before surgery. Can J Anaesth. 1991;38(4):425–29. doi: 10.1007/BF03007577. [DOI] [PubMed] [Google Scholar]
- 26.Raidoo DM, Rocke DA, Brock-Utne JG, et al. Critical volume for pulmonary acid aspiration: Reappraisal in a primate model. Br J Anaesth. 1990;65(2):248–50. doi: 10.1093/bja/65.2.248. [DOI] [PubMed] [Google Scholar]
- 27.Benini L, Sembenini C, Heading RC, et al. Simultaneous measurement of gastric emptying of a solid meal by ultrasound and by scintigraphy. Am J Gastroenterol. 1999;94(10):2861–65. doi: 10.1111/j.1572-0241.1999.01429.x. [DOI] [PubMed] [Google Scholar]
- 28.Bouvet L, Miquel A, Chassard D, et al. Could a single standardized ultrasonographic measurement of antral area be of interest for assessing gastric contents? A preliminary report. Eur J Anaesthesiol. 2009;26(12):1015–19. doi: 10.1097/EJA.0b013e32833161fd. [DOI] [PubMed] [Google Scholar]
- 29.Bisinotto FMB, Patricia LP, Silveira LAM, et al. Qualitative and quantitative ultrasound assessment of gastric content. Rev Assoc MEd Bras. 2017;63(2):134–41. doi: 10.1590/1806-9282.63.02.134. [DOI] [PubMed] [Google Scholar]
- 30.Søreide E, Eriksson LI, Hirlekar G, et al. Pre-operative fasting guidelines: An update. Acta Anaesthesiol Scand. 2005;49:1041–47. doi: 10.1111/j.1399-6576.2005.00781.x. [DOI] [PubMed] [Google Scholar]