Abstract
Background
Cognitive training has been focused on the interventions of amnestic mild cognitive impairment (aMCI) in recent years, with poor understanding.
Material/Methods
The study participants with aMCI were screened in a previous intervention trial. Functional magnetic resonance imaging (fMRI) was adopted to investigate effects of single-domain cognitive training (SDCT) and multi-domain cognitive training (MDCT) on aMCI and to explore potential mechanisms.
Results
There were significant differences in the grey matter volume of the middle frontal gyrus, superior parietal lobule, inferior temporal gyrus, fusiform gyrus, and ventral V3 between the MDCT/SDCT group and the control group (P<0.05). Regional homogeneity (ReHo) increased significantly in the right and left inferior frontal gyrus as well as in the left and right precentral gyrus after intervention in the MDCT group and the SDCT group. ReHo increased significantly in the right and left lingual gyrus of the MDCT group and the control group. ReHo reduced significantly in the right middle temporal gyrus of the MDCT group but increased significantly in the left middle temporal gyrus in the SDCT group and the control group. The voxel of grey matter in the precuneus was positively related to the language scores on RBANS (Repeatable Battery for the Assessment of Neuropsychological Status), and amygdala, fusiform gyrus, and hippocampus also had a positive relationship with delayed memory scores in RBANS of the MDCT group. In the MDCT group, the attention and reasoning scores were also positively related to the ReHo of middle temporal gyrus.
Conclusions
Both MDCT and SDCT may improve the aMCI at brain functional and structural levels; however, the MDCT group exhibited higher ReHo values in middle temporal gyrus and superior occipital gyrus. Also, it was confirmed that MDCT leads to better results than SDCT, showing a significant correlation of cognitive functions such as attention, memory, reasoning, and visual-spatial ability.
MeSH Keywords: Cognitive Therapy, Magnetic Resonance Imaging, Mild Cognitive Impairment
Background
Mild cognitive impairment (MCI) is an intermediate state between normal aging and dementia [1,2]. Of note, patients with amnestic MCI (aMCI), which is characterized by memory impairment, are more likely to develop to Alzheimer disease (AD) [3]. Studies have revealed that auditory language, attention, working memory, and spatial memory are impaired to different extents in aMCI patients, but findings about executive function are still controversial in these patients. Zhang et al. [4] found the inhibition capability was preserved, but the planning capacity was significantly damaged. There is evidence showing that patients with aMCI had extensive damage to the executive function, and working memory, and response inhibition and task switching (3 main components of executive function) were damaged [5]. However, symptoms of executive dysfunction are usually not as prominent as those of memory impairment in complaints of these patients, while it is a major factor affecting the daily activity in aMCI patients. Thus, it is imperative to prevent the progression of aMCI into AD and improve the quality of life in the elderly of our aging population.
Currently, the efficacy of pharmacotherapy for aMCI is not satisfactory. With respect to pathogenic factors, cognitive reserve or activation may become a strategy in the prevention of occurrence and development of aMCI. In recent years, cognitive training has been one of the important methods in preventing and delay cognition dysfunction and AD [6–8]. Greenaway et al. found cognitive training could effectively improve the activities of daily living as well as the memory self-efficacy in MCI patients [9]. In a randomized, controlled trial focusing on the single-domain cognitive training (SDCT), elderly participants who were living independently in good functional and cognitive status received memory training, reasoning training, or speed-of-processing training [10]. After 12 months of training, the cognition function in the 3 groups was significantly improved, as demonstrated during the follow-up period. However, SDCT neglects the complicated interactions between multiple mental processes required to create and preserve a viable and healthy mental state capable of the flexible thinking necessary to interact appropriately with one’s world [11].
Most previous single-domain cognitive training (SDCT) methods were specified in some part of the cognitive ability testing, such as simple memory or reasoning, which would only improve the corresponding cognitive category. Thus, in recent years, some studies focused on the multi-domain cognitive training (MDCT). For example, reasoning, memory, speed of processing, and executive function trainings are integrated to improve different domains of cognitions. Studies have revealed that MDCT not only improves a single cognitive domain, but also affects multiple cognitive domains, or even leads to the generalization of the improvement as compared to SDCT [12]. In this study, MDCT and SDCT (reasoning training) were employed with aMCI patients, and efficacy of both methods was compared.
In recent years, functional magnetic resonance imaging (fMRI) has revealed that activation increased in the regions (frontal lobe, parietal lobe, and bilateral hippocampus) related to memory in healthy and aMCI individuals after cognitive training [13,14]. A recent resting state fMRI study indicated that cognitive training may induce plastic changes in neural functional connectivity of healthy older people, and these changes may underlie the positive effect of cognitive training [15]. In the resting state of fMRI studies, regional homogeneity (ReHo) is often employed to reflect the functional activation of the brain. ReHo is used to describe that the BOLD (blood-oxygen-level dependent) signal change of a given voxel over time is similar to that of its nearest neighbors in a specific functional region of the brain. To weigh the similarity of time series of different voxels in a specific functional region of the brain, ReHo method is used to determine the Kendairs coefficient of concordance of 3 or more voxels which is then endowed with the selected voxel. These procedures are repeated to achieve the ReHo of each region in the brain [16,17]. In the present study, ReHo was employed to evaluate the functional status of each region of the brain, and the effects of different cognitive trainings on the volume and function of grey matter were investigated in aMCI patients.
We hypothesized that both MDCT and SDCT may alter the function or structure of the aMCI brain as compared to a control group but features in the improvement would be different after MDCT and SDCT.
Material and Methods
Ethics
This study was approved by the Ethics Committee of Shanghai Tongji Hospital, and written informed consent was obtained before study (LL[H]-09-04).
Participants
Elderly individuals from 3 communities of Shanghai were recruited and divided into 3 groups: the MDCT group, the SDCT group, and the control (CON) group; and participants were screened for aMCI [18]. Patients with aMCI further received MRI examination.
The inclusion criteria were as follows: 1) patients aged 65–75 years; 2) patients received education for ≥1 year; 3) patients complained of memory impairment of at least 3 months or the insiders speculated that the memory impairment last for more than 3 months; 4) the score of memory in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was ≤ mean −1.0 standard deviation (the mean is calculated according to the norm on the basis of age and education level); 5) the score of Clinical Dementia Rating Scale (CDRS) was 0.5, while there was no evident cognition impairment (score of Mini Mental State Examination [Chinese edition] ≥17 [19]); 6) patients had no severe impairment to vision and hearing; no physical disability, no severe physical diseases, and no severe mental disorders, and were living independently.
A total of 25 patients with aMCI were recruited into the present study. The demographics of these patients are shown in Table 1. All these patients received resting state MRI (MDCT: SDCT: CON=8: 8: 9); brain structural analysis was performed in 23 patients due to concern on data matching (MDCT: SDCT: CON=7: 8: 8).
Table 1.
Characteristics of participants in this study.
| Characteristics | MDCT | SDCT | CON | F/χ2 value | P |
|---|---|---|---|---|---|
| Female/male | 4/4 | 4/4 | 5/4 | 0.071 | 0.965 |
| Age (years) | 69.63±3.54 | 72.13±3.56 | 68.13±2.80 | 1.92 | 0.17 |
| Education (years) | 10.75±3.54 | 10.35±4.33 | 11.00±2.78 | 1.83 | 0.63 |
| RBANS | 85.75±8.45 | 79.88±9.01 | 86.56±11.79 | 1.10 | 0.35 |
| CWST-word | 36.38±6.57 | 35.00±8.90 | 40.44±13.34 | 0.67 | 0.52 |
| Reasoning | 5.00±1.07 | 4.13±2.10 | 4.67±2.50 | 0.39 | 0.68 |
RBANS – the Repeatable Battery for the Assessment of Neuropsychological Status; CWST-word – word-interference time of the Color Word Stroop Test; Reasoning – the visual reasoning test of the World Health Organization Neuropsychological Battery of Cognitive Assessment Instruments for the elderly (WHO-BCAI).
Study design
This was a single blind, prospective study aiming to investigate the effects of different cognitive training methods on the structure and function of the brain in aMCI. The cognitive function was evaluated, and MRI examination was conducted before the study intervention and 1 year after the intervention.
Cognitive training
The cognitive training used into the present study included 4 components: repeated exercises, training focusing on the problem-solving ability, standardized training, and training targeting one or more cognitive domains.
MDCT focuses on the memory, reasoning, problem-solving ability, and visual-spatial reading skills. In these dimensions, the validity of each dimension has been confirmed by previous studies [19,20]. Moreover, studies have also confirmed the effectiveness of interventions with each dimension. SDCT focuses on reasoning, including picture arrangement, digital reasoning, and the Raven reasoning test [15,19,21,22].
Cognitive training was performed in a face-to-face manner for 1 hour twice weekly for a total of 12 weeks. The in-home practice was administered once weekly, and the completion of homework was checked in a timely manner. In the control group, cognitive training was not administered.
Methods for evaluation
In the present study, the comprehensive cognitive assessment tool and the Elderly Community Health Assessment Questionnaire of Shanghai were employed to evaluate the cognitive function and health status. Evaluations were performed in a blinded manner to assure the accuracy of results. All the evaluators received consistency test with Kappa value of >0.8 and intergroup correlation coefficient was >0.75.
The RBANS [23], the Color Word Stroop test (CWST) [24], the visual reasoning test [25] and trail making test [26] of the World Health Organization Neuropsychological Battery of Cognitive Assessment Instruments for the elderly were employed for the evaluation.
Data acquisition of MRI
MRI was performed with Siemens Magnetom Trio 3.0T in the Shanghai East China Normal University. Patients were in a supine position and wore noise-canceling headphones, and their head was fixed with a sponge mat. The patients were asked to keep calm and minimize the movement of the head. Resting state MRI was performed with Echo Planner Imaging (EPI) sequence; scanning was done at the plane parallel to the posterior-anterior line with the following parameters: time of repetition=2000 ms; time of echo=25 ms; flip angle=90°; FOV=240 mm; matrix=64×64; slice thickness=5 mm; slice number=32.
T1 weighed scanning was performed with spin echo sequence at the sagittal plane with the following parameters: time of repetition=1900 ms; time of echo=3.43 ms; flip angle=90°; FOV=256 mm; matrix=256×256; slice thickness=1 mm; slice number=160.
Data processing
Data processing of brain structure
SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) software was used for data processing, and DARTEL for the Voxel based morphometry (VBM) analysis of brain structure as follows: 1) T1 weighed images were standardized into a 3-dimensional space; 2) the standardized brain images were divided into grey matter part, white matter part, and cerebral spinal fluid part; 3) DARTEL was used for space standardization of 3 parts; (4) data smoothening was done for 6-mm 3-dimensional Gaussian kernel with full width half maximum (FWMH).
Data processing of resting state fMRI
Convert software was used to transform the data of DICOM format into those of NIFTI format, followed by pre-treatment as follows: the first 4 images were removed considering the adaptation of participants, followed by adjustment for head movement, space standardization, removal of linear drift and wave filtering in which the data were sampled into 3×3×3 mm3 area. Rest software (REST Version: 1.8) [27] was used to calculate ReHo which was then smoothened. The 4-mm FWHM Gaussian kernel was used to space smoothening, aiming to reduce spatial noise and anatomical difference between individuals. After smoothening, data in different groups were tested with analysis of variance and paired t-test. In addition, correlation of these parameters with the behaviors was also evaluated.
Results
Characteristics of participants
As shown in Table 1, there were no significant differences in the age, gender, education level, and scores of 3 scales among the 3 groups.
Data analysis of brain structure
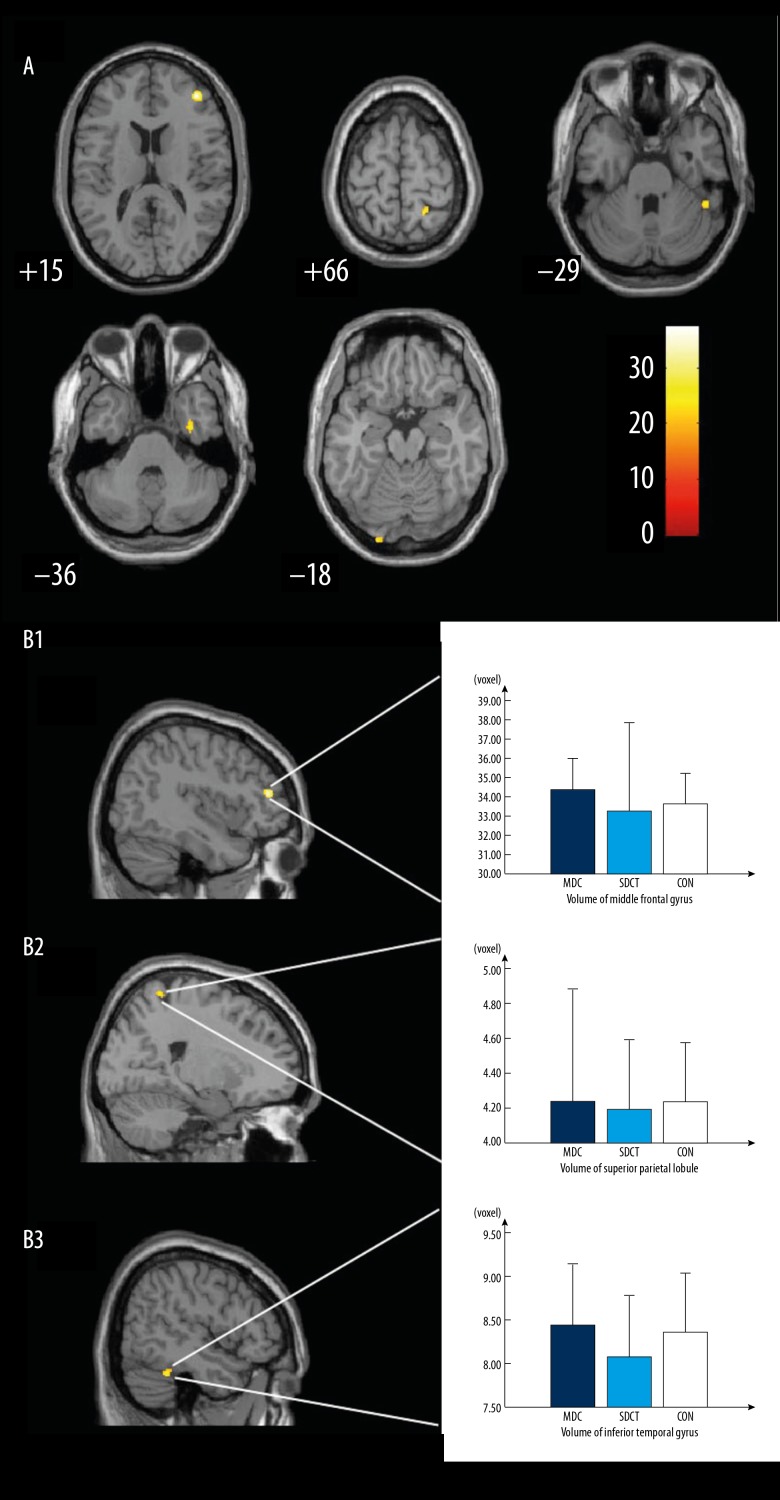
Age and gender served as covariates and 2-way-ANOVA was employed for statistical analysis. Results showed significant differences among 3 groups in the middle frontal gyrus, superior parietal lobule, inferior temporal gyrus, fusiform gyrus, and ventral V3 (Table 2, Figure 1A), which were more obvious in the right hemisphere.
Table 2.
Grey matter volume of the brain after interventions in different groups.
| Region | Hemisphere | F Value | P | Cluster size | Coordinates (mm) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Middle frontal gyrus | R | 37.43 | 0.001 | 123 | 40.5 | 43.5 | 15.0 |
| Superior parietal lobule | R | 26.17 | 0.011 | 25 | 21.0 | −49.5 | 66.0 |
| Inferior temporal gyrus | R | 25.42 | 0.003 | 59 | 48.0 | −43.5 | −28.5 |
| Fusiform gyrus | R | 25.35 | 0.002 | 74 | 34.5 | −7.5 | −36.0 |
| Ventral V3 | L | 24.36 | 0.000 | 178 | −25.5 | −99.0 | −18.0 |
P<0.05 (FWE-corrected). Cluster size at least 18 voxels.
Figure 1.
Brain morphology differences based on volume pixel in the MDCT group and the SDCT group. MDCT – multi-domain cognitive training; SDCT – single-domain cognitive training.
Comparisons between 2 groups showed the grey matter volume of the middle frontal gyrus, superior parietal lobule, and inferior temporal gyrus in the MDCT group was significantly higher than in the SDCT group (P<0.05; Figure 1, B1–B3 respectively). This suggests that the effect on the MDCT and the SDCT group was different in grey matter volume, especially in the right cerebellum (Table 3).
Table 3.
ReHo in MDCT group and SDCT group before and after intervention.
| Region | Hemisphere | Cluster size | Coordinates (mm) | t Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Putamen | R | 154 | 30 | −6 | −3 | 3.96 |
| Calcarine | R | 232 | 27 | −60 | 12 | 4.44 |
| Postcentral gyrus | R | 111 | 57 | −21 | 18 | −3.77 |
| Superior parietal lobule | R | 125 | 24 | −51 | 57 | −3.69 |
| Inferior temporal gyrus | L | 119 | −48 | −21 | −18 | 4.41 |
| Superior parietal lobule | L | 235 | −33 | −48 | 60 | −4.22 |
P<0.05 (Alphasim corrected). Cluster size at least 85 voxels.
Resting state fMRI
Intergroup comparison of ReHo before and after intervention
The ReHo value was compared before and after the intervention among the groups. Results showed significantly differences in the ReHo of cerebellum (peak voxel of MNI: x=−36, y=−42, z=−30, F score11.3417, corrected P=0.05), right fusiform (peak voxel of MNI: x=36, y=−36, z=18, F score=14.0737, corrected P=0.05), left pars triangularis inferior frontal gyrus (peak voxel of MNI: x=−51, y=−30, z=3, F score=7.5399, corrected P=0.05), left postcentral gyrus (peak voxel of MNI: x=−18, y=−33, z=75, F score=6.9578, corrected P=0.05) among the 3 groups. Paired comparison showed the ReHo of putamen, calcarine and inferior temporal gyrus in the MDCT group was significantly higher than in the SDCT group, while that of superior parietal lobule and postcentral gyrus was markedly lower than in the SDCT group. This suggests that MDCT and SDCT may differentially affect the brain function of several regions.
Intragroup comparison of ReHo before and after intervention
As shown in Table 4, the ReHo of the precentral gyrus, superior temporal gyrus, inferior frontal gyrus, and lingual gyrus increased significantly in the MDCT group, while ReHo of the superior temporal gyrus and lingual gyrus increased in the control group. The ReHo of superior frontal gyrus remained unchanged in the MDCT group but reduced in the control group. The ReHo of the middle temporal gyrus and superior occipital gyrus reduced significantly in the MDCT group but increased in the control group.
Table 4.
ReHo in MDCT group and control group before and after intervention.
| Brain Regions | Hemisphere | Group | Cluster size | Coordinates | t Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Precentral gyrus | L | MDCT | 162 | −9 | −30 | 69 | −7.74 |
| CON | – | – | – | – | – | ||
| Superior frontal gyrus | L | MDCT | – | – | – | – | – |
| CON | 7091 | −18 | 24 | 51 | 10.53 | ||
| Inferior frontal gyrus | R | MDCT | 993 | 51 | 27 | 0 | −8.26 |
| CON | – | – | – | – | |||
| Lingual gyrus | R | MDCT | 149 | 3 | −78 | −3 | −9.05 |
| L | CON | 123 | −21 | −57 | −9 | −4.21 | |
| Superior temporal gyrus | R | MDCT | 115 | 33 | 15 | −27 | −4.71 |
| R | CON | 2322 | 60 | 3 | −6 | −11.21 | |
| Middle temporal gyrus | L | MDCT | 1159 | −45 | −48 | 9 | 13.76 |
| R | CON | 262 | 51 | −63 | 3 | −10.93 | |
| Superior occipital gyrus | R | MDCT | 380 | 27 | −69 | 21 | 9.97 |
| R | CON | 899 | 15 | −54 | 6 | −6.28 | |
x, y, z – coordinates of primary peak locations in the space of MNI; t – statistical value of peak voxel showing ReHo before and after intervention (positive t value means decreased ReHo). P<0.05 (Alphasim corrected). Cluster size at least 85 voxels.
As shown in Table 5, the ReHo of the inferior frontal gyrus and precentral gyrus increased significantly in the SDCT group but remained unchanged in the control group. The ReHo of the lingual gyrus remained stable in the SDCT group but increased dramatically in the control group. The ReHo of middle temporal gyrus, superior temporal gyrus, superior occipital gyrus, and superior frontal gyrus showed similar trend in both the SDCT group and the control group, but it was more obvious in the control group. These findings suggest that SDCT and MDCT have differential effects on the brain function, which are more evident after MDCT.
Table 5.
ReHo in SDCT group and control group before and after intervention.
| Brain Regions | Hemisphere | Group | Cluster size | Coordinates | t Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Inferior frontal gyrus | L | SDCT | 87 | −54 | 39 | −3 | −4.07 |
| CON | – | – | – | – | – | ||
| Precentral gyrus | R | SDCT | 114 | 54 | 12 | 42 | −5.39 |
| CON | – | – | – | – | – | ||
| Lingual gyrus | SDCT | – | – | – | – | – | |
| L | CON | 123 | −21 | −57 | −9 | −4.21 | |
| Middle temporal gyrus | R | SDCT | 139 | 54 | −72 | 15 | −4.08 |
| R | CON | 262 | 51 | −63 | 3 | −10.93 | |
| Superior temporal gyrus | R | SDCT | 997 | 63 | 6 | 0 | −11.03 |
| R | CON | 2322 | 60 | 3 | −6 | −11.21 | |
| Superior occipital gyrus | R | SDCT | 89 | 21 | −72 | 48 | −4.86 |
| R | 993 | 18 | −81 | 18 | −6.07 | ||
| R | CON | 899 | 15 | −54 | 6 | −6.28 | |
| Superior frontal gyrus | L | SDCT | 4435 | −21 | −3 | 72 | 8.32 |
| L | CON | 7091 | −18 | 24 | 51 | 10.53 | |
P<0.05 (Alphasim corrected). Cluster size at least 85 voxels.
Correlation between MRI findings and behaviors
Correlation between grey matter volume and behaviors
The correlation between grey matter volume and the behavioral score was evaluated in the MDCT group. Results showed the voxel of the precuneus was positively related to language section in RBANS, but that of the amygdala, fusiform gyrus, and hippocampus had a positive relationship with delayed memory section in RBANS in the MDCT group (Table 6). However, there was no significant correlation between grey matter volume and behavioral score in the SDCT group.
Table 6.
Correlation between grey matter volume and behavioral score after intervention in MDCT group.
| Behavior score | Region | Hemisphere | t Value | P | Cluster size | Coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Delayed Memory | Amygdala | R | 23.98 | 3.45×10−7 | 948 | 24.0 | −4.5 | −25.5 |
| R | 22.72 | 4.76×10−7 | 28.5 | −7.5 | −19.5 | |||
| R | 13.44 | 1.05×10−5 | 30.0 | −9.0 | 30.0 | |||
| Fusiform gyrus | L | 9.55 | 7.53×10−5 | 907 | −36.0 | −4.5 | −39.0 | |
| L | 8.38 | 1.57×10−4 | −31.5 | −4.5 | −30.0 | |||
| Hippocampus | L | 8.27 | 1.69×10−4 | −25.5 | −9.0 | −22.5 | ||
| Language | Precuneus | L | 14.70 | 6.23×10−6 | 169 | 0.0 | −66.0 | 27.0 |
Delayed Memory: P<0.001, FWE-corrected. Cluster size at least 23 voxels. Language: FWE-corrected. Cluster size at least 27 voxels.
Correlation between ReHo and behavioral score
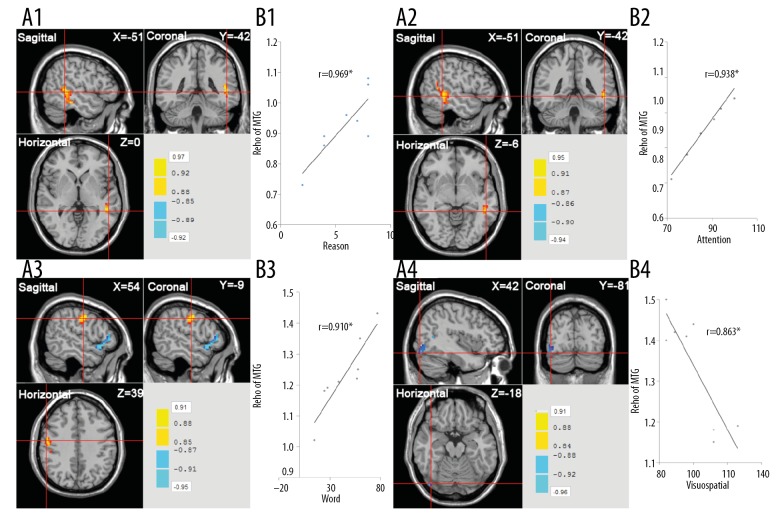
The correlation between ReHo and behavioral score was further evaluated in the brain regions with significant difference in ReHo of MDCT. Results showed the attention score of RBANS and reasoning score had positive relationship with the ReHo of middle temporal gyrus; word interfere score of the CWST was positively related to the ReHo of the precentral gyrus; visuospatial score of RBANS was negatively associated with the ReHo of the lingual gyrus. These findings suggest that the ReHo of some brain regions was related to the behavioral scores (such as reasoning and attention) in aMCI patients after MDCT (Figure 2).
Figure 2.
Correlation between behavioral score and ReHo of different brain regions in the MDCT group. * r: P<0.05. (A1) Correlation between reasoning score and ReHo of middle temporal gyrus (r=0.969); (A2) correlation between attention score of RBANS and ReHo of middle temporal gyrus (r=0.938); (A3) correlation between word interfere score of the CWST and ReHo of precentral gyrus (r=0.910); (A4) correlation between visuospatial of RBANS score and ReHo of lingual gyrus (r=−0.863). MDCT – multi-domain cognitive training; SDCT – single-domain cognitive training; ReHo – regional homogeneity; RBANS – Repeatable Battery for the Assessment of Neuropsychological Status; CWST – Color Word Stroop Test.
Discussion
In this study, the grey matter volume and resting state of specific brain regions were investigated. After the intervention, the grey matter volume was significantly different in the right middle frontal gyrus, right inferior temporal gyrus, and temporal fusiform gyrus among the 3 groups, and the grey matter volume in the MDCT group was significantly higher than in the SDCT group and the control group. In addition, from the discussion of the resting state results and the correlation with behavioral data described, it can be assumed that the MDCT group and the SDCT group leads to better results than in the control group before and after the intervention. This indicates that SDCT and MDCT may affect aMCI at both structural and functional levels of the brain. This study, for the first time, elucidates the beneficial effects of SDCT and MDCT on aMCI from the structural and functional levels of the brain and identifies the difference in the effectiveness on aMCI between SDCT and MDCT, which provides reference for the prevention and therapy of aMCI.
There were similarities between the findings of our study and previous studies. 1) the change of grey matter volume of the right middle frontal gyrus, right inferior temporal gyrus, and temporal fusiform gyrus was significantly different among the 3 groups (Table 2). The grey matter volume of these regions in the MDCT group was significantly higher than in the SDCT group and the control group. Available findings suggest that memory training is able to increase the cortical thickness of some brain regions, especially those related to cognitive impairment and AD, such as temporal lobe, supramarginal gyrus, and entorhinal gyrus, as well as cortex of the frontal and prefrontal lobes [28–30], which also explains the elevated grey matter volume of the inferior temporal gyrus and temporal fusiform gyrus after intervention in the MDCT group. 3) Brain resting state analysis showed ReHo of the inferior frontal gyrus and precentral gyrus increased significantly in both the SDCT group and the MDCT group after intervention, but it remained unchanged in the control group. When compared with healthy individuals, ReHo was reduced significantly in the inferior frontal gyrus and precentral gyrus of aMCI. This indicates that both MDCT and SDCT may improve aMCI to a certain extent. For the middle temporal gyrus and superior occipital gyrus, 2 regions with significant difference in ReHo, the ReHo in the SDCT group and the control group consistently was increased, but that in the MDCT group it was reduced. This suggests that intervention may reduce ReHo, which was also observed in the studies of Machulda et al. and Yetkin et al. several brain regions (such as middle temporal gyrus and fusiform gyrus) were activated in executing an assignment in aMCI patients and thus they speculated that aMCI was characterized by memory impairment in early stages when the executive function is relatively intact and there might be compensatory mechanism [31–35].
In the present study, the correlation between fMRI findings and behaviors was further evaluated. Results showed the language score and delayed memory section of RBANS had positively relationships with the grey matter volume of the precuneus and hippocampus in the MDCT group; the attention score of RBANS and reasoning score were positively related to the ReHo of the middle temporal gyrus; after intervention, the ReHo reduced significantly at this region in the MDCT group. Thus, we speculate that the attention and reasoning were significantly improved after intervention, which was consistent with the improvement of corresponding scores. The CWST-word score was positively related to the ReHo of the precentral gyrus. This suggests that both MDCT and SDCT can improve the executive function in aMCI patients. Visuospatial score of RBANS was negatively associated with the ReHo of the lingual gyrus, but ReHo of this region increased significantly in the MDCT group and remained unchanged in the SDCT group. The ReHo values in lingual gyrus increased, based on the brain-behavior relationships between MRI and behavior data, and revealed a significant negative correlation with the visuospatial score and ReHo value in lingual gyrus. We hypothesized that the visuospatial score of the MDCT group would decrease. Excellent agreements were found with our results, i.e., the visuospatial score of the aMCI participants in the MDCT group was decreased, and visual-spatial ability was improved. We speculated that MDCT may confer more beneficial effects on the visuospatial capability of aMCI patients compared to SDCT.
One of major findings in this study was that changes in both grey matter volume and ReHo after intervention in the MDCT group and SDCT group were mainly found in the right hemisphere. That is, grey matter atrophy and brain function deterioration were different between left and right hemispheres at the speed and extent with the conduction of cognitive training, and the degeneration of the right hemisphere was generally slower than that of left hemisphere. Cabeza et al. and Allali et al. [36,37] found that brain activity was reduced in MCI patients, which could result in asymmetrical reduction in 2 hemispheres. The lateral development of 2 hemispheres indicated that the more advanced the hemisphere, the more evident the lateral development was. Thus, there was lateral processing in the cognitive function, a component of advanced function of the brain. Based on our study findings, the change of ReHo value after intervention was compared between the MDCT and the SDCT group. Significant differences were observed in right brain, indicating that the change in the right brain activity after intervention was more significant in the MDCT group than in the SDCT group. Thus, MDCT could attenuate the cognition degradation of right brain, keeping the asymmetry between the left and right brain. MDCT and SDCT may attenuate the degradation of the brain to a certain extent, but MDCT is more likely to preserve the laterality of the brain (Table 3). In addition, the laterality of the brain function is considered a marker of specialization of cognitive function in humans. In the elderly, studies on the influence of network laterality of the brain on cognitive training have indicated that the network laterality, including advanced cognition, is sensitive to MDCT [38]. Taken together, cognitive training, especially MDCT, has its own advantages in preventing cognitive impairment of the elderly.
There were several limitations to this study: 1) there might be systemic error (difference in the quality of brain images) in the fMRI examination before and after intervention; 2) the follow-up was conducted for 1 year, causing some patients to be lost to follow-up, and thus the small sample size was an important limitation of this study. In our future studies, more participants will be recruited to confirm our findings. As compared to previous studies, we compared the effects of MDCT and SDCT on aMCI, and fMRI was employed to evaluate them at the structural and functional levels. Thus, our findings or conclusions may be theoretically important for the clinical prevention and treatment of cognitive impairment.
Conclusions
Both MDCT and SDCT may improve the aMCI at the brain functional and structural levels, and MDCT is more likely to block the reduction of laterality. In the MDCT group, results suggest not only that memory impairment is attenuated by MDCT, but also the compensatory activation of several brain regions (such as middle temporal gyrus and fusiform gyrus) may conceal the deterioration of executive dysfunction in aMCI patients.
Acknowledgments
We thank the investigators involved in the MRI scanning, data collection, and data analysis. We thank Dr. Wu WY and Luo M for providing helpful suggestions on this study.
Footnotes
Source of support: This work was supported by the National Nature Science Foundation of China (81371505, 81200831, 30770769); the Nature Science Foundation of Shanghai (17ZR1426400); the Fundamental Research Funds for the Central Universities (22120170037); the Top Priority of Clinical Center and Key Discipline Construction in Shanghai(2017ZZ02020); the Science and Technology Commission of Shanghai Municipality (134119a2501, 13dz2260500); Humanities and Social Sciences Interdisciplinary Project of Tongji University (1430219044, 1430219042) and Shanghai Municipal Commission of Health and Family Planning (2015ZB0502)
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Berg AI, Wallin A, Nordlund A, Johansson B. Living with stable MCI: experiences among 17 individuals evaluated at a memory clinic. Aging Ment Health. 2013;17:293–99. doi: 10.1080/13607863.2012.751582. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Han B, Verhaeghen P, Nilsson LG. Executive functioning in older adults with mild cognitive impairment: MCI has effects on planning, but not on inhibition. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:557–70. doi: 10.1080/13825580600788118. [DOI] [PubMed] [Google Scholar]
- 5.Zheng DM, Dong XY, Sun HZ, et al. Executive function deficits in patients with amnestic mild cognitive impairment. Chin J Nervous Mental Dis. 2012;38:266–70. [Google Scholar]
- 6.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 8.Pieramico V, Esposito R, Cesinaro S, et al. Effects of non-pharmacological or pharmacological interventions on cognition and brain plasticity of aging individuals. Front Syst Neurosci. 2014;8:153. doi: 10.3389/fnsys.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenaway MC, Duncan NL, Smith GE. The memory support system for mild cognitive impairment: Randomized trial of a cognitive rehabilitation intervention. Int J Geriatr Psychiatry. 2013;28:402–9. doi: 10.1002/gps.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–81. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckroth-Bucher M, Siberski J. Preserving cognition through an integrated cognitive stimulation and training program. Am J Alzheimers Dis Other Demen. 2009;24:234–45. doi: 10.1177/1533317509332624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozzini L, Costardi D, Chilovi BV, et al. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriatr Psychiatry. 2007;22:356–60. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- 13.Belleville S, Clement F, Mellah S, et al. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134:1623–34. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff BA, Anderson BA, Barch DM, Jacoby LL. Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb Cortex. 2012;22:788–99. doi: 10.1093/cercor/bhr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T, Yao Y, Cheng Y, et al. Cognitive training can reduce the rate of cognitive aging: a neuroimaging cohort study. BMC Geriatr. 2016;16:12. doi: 10.1186/s12877-016-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Liu Y, Li W, et al. Increased regional homogeneity of blood oxygen level-dependent signals in occipital cortex of early blind individuals. Neuroreport. 2011;22:190–94. doi: 10.1097/WNR.0b013e3283447c09. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Wu W, Feng W, et al. The effects of multi-domain versus single-domain cognitive training in non-demented older people: A randomized controlled trial. BMC Med. 2012;10:30. doi: 10.1186/1741-7015-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Wu W, Jin H, et al. Successful aging in Shanghai, China: Definition, distribution and related factors. Int Psychogeriatr. 2006;18:551–63. doi: 10.1017/S1041610205002966. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Kawas C, Morrison JH, et al. Session II: Mechanisms of age-related cognitive change and targets for intervention: Neural circuits, networks, and plasticity. J Gerontol A Biol Sci Med Sci. 2012;67:747–53. doi: 10.1093/gerona/gls111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng W, Yokoyama JS, Yu S, et al. APOE genotype affects cognitive training response in healthy Shanghai community-dwelling elderly individuals. J Alzheimers Dis. 2015;47:1035–46. doi: 10.3233/JAD-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng W, Li C-b, Chen Y, et al. Integrative cognitive training for healthy elderly Chinese in community: A controlled study. Biomed Res. 2013;24:223–29. [Google Scholar]
- 23.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–19. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 24.van Boxtel MP, ten Tusscher MP, Metsemakers JF, et al. Visual determinants of reduced performance on the Stroop color-word test in normal aging individuals. J Clin Exp Neuropsychol. 2001;23:620–27. doi: 10.1076/jcen.23.5.620.1245. [DOI] [PubMed] [Google Scholar]
- 25.Xiao S, Yao P, Li X, Zhang M. Neuropsychological testing profiles of patients with Alzheimer’s Disease and mild cognitive impairment: A case-control study. Hong Kong J Psych. 2002;12:2–5. [Google Scholar]
- 26.Ashendorf L, Jefferson AL, O’Connor MK, et al. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol. 2008;23:129–37. doi: 10.1016/j.acn.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song XW, Dong ZY, Long XY, et al. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyke J, Driemeyer J, Gaser C, et al. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–35. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engvig A, Fjell AM, Westlye LT, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–76. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Engvig A, Fjell AM, Westlye LT, et al. Effects of cognitive training on gray matter volumes in memory clinic patients with subjective memory impairment. J Alzheimers Dis. 2014;41:779–91. doi: 10.3233/JAD-131889. [DOI] [PubMed] [Google Scholar]
- 31.Bai F, Zhang Z, Yu H, et al. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: A combined structural and resting-state functional MRI study. Neurosci Lett. 2008;438:111–15. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Balardin JB, Batistuzzo MC, Martin Mda G, et al. Differences in prefrontal cortex activation and deactivation during strategic episodic verbal memory encoding in mild cognitive impairment. Front Aging Neurosci. 2015;7:147. doi: 10.3389/fnagi.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao Y, Miao D, Huan Y, et al. Altered regional homogeneity with short-term simulated microgravity and its relationship with changed performance in mental transformation. PLoS One. 2014;8:e64931. doi: 10.1371/journal.pone.0064931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–6. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yetkin FZ, Rosenberg RN, Weiner MF, et al. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur Radiol. 2006;16:193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- 36.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 37.Allali G, Annweiler C, Predovan D, et al. Brain volume changes in gait control in patients with mild cognitive impairment compared to cognitively healthy individuals; GAIT study results. Exp Gerontol. 2016;76:72–79. doi: 10.1016/j.exger.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Luo C, Zhang X, Cao X, et al. The Lateralization of intrinsic networks in the aging brain implicates the effects of cognitive training. Front Aging Neurosci. 2016;8:32. doi: 10.3389/fnagi.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]