Abstract
Background
Epidermal growth factor receptor (EGFR) expression is associated with hepatic fibrogenesis. Activated hepatic stellate cells (HSCs) release inflammatory cytokines and extracellular matrix (ECM). The aim of this in vitro study was to investigate HSCs, activated by lipopolysaccharide (LPS), and the role of EGFR using the small molecule EGFR inhibitor, AG1478, and using knockdown of the EGFR gene using small interfering RNA (siRNA) cell transfection.
Material/Methods
HSCs, isolated from male Sprague-Dawley rats, were cultured and treated with and without LPS (100 ng/mL), with and without AG1478 (2.5 μM and 5.0 μM) Cell survival and proliferation were studied using an MTT assay. Western blot was used to measure levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IκBα, cytoplasm and nuclear NFκB and EGFR in the cell lysates before and after small interfering RNA (siRNA) transfection. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to measure the mRNA levels of transforming growth factor (TGF)-β, Col-1, and α-smooth muscle actin (SMA). The Toll-like receptor 4 (TLR4) antagonist TAK-242 and the selective c-Src inhibitor, PP2 in LPS induced-EGFR phosphorylation was evaluated using Western blot.
Results
Inhibition of EGFR decreased LPS-induced HSC proliferation and inflammatory cytokines. The TLR4 antagonist TAK-242, and the c-Src inhibitor, PP2 reduced EGFR activation of HSCs, indicating a possible role for the TLR4/c-Src signaling cascade in LPS-induced HSC activation.
Conclusions
Activation of HSCs by LPS in vitro, including the expression of inflammatory cytokines and mediators of fibrogenesis, were shown to be dependent on the expression of EGFR.
MeSH Keywords: Fibrosis; Hepatic Stellate Cells; Inflammation; Lipopolysaccharides; Receptor, Epidermal Growth Factor
Background
Epidermal growth factor receptor (EGFR/ErbB1) is a tyrosine kinase receptor [1], which has been studied in both animal models of cancer and human cancer. Recently, EGFR signaling has been identified as having a key role in the progression of liver fibrosis [2]. In three different animal models of hepatic injury induced by bile duct ligation (BDL), carbon tetrachloride (CCl4), or diethylnitrosamine (DEN), the EGFR inhibitor, erlotinib, decreased the activation of hepatic stellate cells (HSCs), reduced liver fibrosis, and prevented the progression of cirrhosis [3]. Inhibition of EGFR has been shown to reduce extracellular matrix (ECM) deposition and inhibit HSC activation in a high lipid diet-induced model of nonalcoholic fatty liver disease (NAFLD) and promoted a pro-fibrogenic phenotype in HSCs [4]. These recent findings raise the possibility that inhibition of EGFR might be a future therapeutic approach in the prevention or control of hepatic fibrosis.
Activated HSCs are now recognized to have a role in the deposition of ECM, including collagen, in liver fibrosis [5]. The activation of HSCs can be driven by various stimuli, including lipopolysaccharide (LPS) [6]. Bacterial LPS, the classic ligand for toll-like receptor 4 (TLR4) [7], has been shown to be associated with hepatic fibrogenesis through direct interactions with HSCs [6]. LPS can also induce the activation of nuclear factor-κB (NF-κB) and the release of inflammatory cytokines that are required for the development of chronic inflammation and fibrosis, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, which are also involved in HSC activation [8]. However, the molecular mechanism underlying the effects of LPS on HSC activation remains poorly understood.
Therefore, the aim of this in vitro study was to investigate HSCs, activated by LPS, and the role of EGFR using the small molecule EGFR inhibitor, AG1478, and using knockdown of the EGFR gene using small interfering RNA (siRNA) cell transfection. The study included the investigation of the expression of inflammatory cytokines by HSCs, and the TLR4 antagonist TAK-242, and the c-Src inhibitor, PP2.
Material and Methods
Reagents
Lipopolysaccharide (LPS), the small molecule EGFR inhibitor AG1478, the c-Src inhibitor, PP2, and the Toll-like receptor 4 (TLR4) antagonist, TAK-242 were purchased from Sigma-Aldrich (St. Louis, MO, USA). AG1478, PP2, and TAK-242 were dissolved in dimethyl sulfoxide (DMSO) for in vitro experiments. Primary antibodies to transforming growth factor (TGF)-β, collagen 1, α-smooth muscle actin (α-SMA), c-Src and phosphorylated c-Src (p-c-Src), Lamin B, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Technology (Santa Cruz, CA, USA). Primary antibodies to epidermal growth factor receptor (EGFR), phospho-EGFR (p-EGFR), Tyr835, TLR4, tumor necrosis factor (TNF)-α, interleukin (IL)-6, IκB-α (an inhibitor of NF-κB) and NFκB P65 were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell culture and treatment
Hepatic stellate cells (HSCs) were isolated from male Sprague-Dawley rats, as previously described [9]. Briefly, after in situ perfusion of the liver with a two-step pronase-collagenase digestion, HSCs were separated from other nonparenchymal cells by density-gradient centrifugation using OptiPrep (Axis-Shield density gradient media, 1114542). HSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 mg/ml of streptomycin in a humidified atmosphere of 5% CO2 at 37°C. After overnight incubation, the cells were incubated with or without 100 ng/mL LPS for the following indicated times: 15 min for Western blot analysis of p-EGFR, TLR4, and p-c-Src; 1 h for Western blot analysis of NF-κB; 6 h for quantitative reverse transcription polymerase chain reaction (RT-qPCR); and 24 h for the MTT assay, Western blot analysis of TGF-β, Col-1, α-SMA, TNF-α and IL-6, in the presence or absence of the small molecule EGFR inhibitor AG1478 (2.5 μM and 5.0 μM), which was incubated for 2 h. All experiments were performed in triplicate.
Measurement of cell viability using the MTT assay
Cell viability was assessed by the MTT assay. HSCs were plated in 96-well plates at 5,000 cells per well and then treated with or without LPS for 24 h. After incubation with MTT reagent for 3 h, the reduction of MTT to the purple formazan dye was detected by a microplate reader at 540 nm. Cell viability was calculated using as: cell viability=A treated/A control×100%.
Small interfering RNA (siRNA)-induced EGFR gene silencing
Gene silencing in cells was achieved using a specific siRNA sequence. EGFR siRNA was purchased from Gene Pharma Co. Ltd. (Shanghai, China). The specific siRNA sequence was 5′-CCGUGCCUGAAUAUAUAAATT-3′ for rat EGFR. Transfection of HSCs with siRNA was carried out using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instruction. EGFR gene knockdown was verified by Western blotting. Transfected cells were then treated with LPS for further experiments.
Western blot analysis
HSCs were lysed, and proteins were measured by the Bradford spectroscopic protein assay (Bio-Rad). Nuclear and cytoplasmic proteins were extracted from HSCs using a nuclear and cytoplasmic protein extraction kit (Beyotime Biotech, Nantong, China). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to a polyvinylidene difluoride (PVDF) membrane. Each membrane was blocked for 1.5 h with Tris-buffered saline containing 0.05% Tween 20 and 5% dried non-fat milk powder. The PVDF membranes were then incubated with specific primary antibodies. Immunoreactive bands were detected by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized using enhanced chemiluminescence (ECL) (Bio-Rad). The proteins levels were analyzed using Image J analysis software version 1.38e and normalized to their respective control.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HSCs using TRIZOL (Invitrogen). Reverse transcription and quantitative PCR were carried out using a two-step Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen). Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) was used for RT-qPCR analysis. Primers for genes including TNF-α, IL-6, Collagen1, TGF-β, α-SMA, and β-actin were obtained from Invitrogen (sequences are listed in the Supplementary Table 1). Target mRNA was normalized to β-actin.
Statistical analysis
Data were presented as the mean ±SEM. Differences between groups were determined by the Student’s t-test, where appropriate, using GraphPad Pro (GraphPad, San Diego, CA, USA). Values of p<0.05 were considered to be statistically significant.
Results
The small-molecule epidermal growth factor receptor (EGFR) inhibitor, AG1478, inhibited lipopolysaccharide (LPS)-induced hepatic stellate cell (HSC) activation
The tyrosine kinase activity of epidermal growth factor receptor (EGFR) can be detected in hepatic stellate cells (HSCs) and activated HSCs by the expression of cell surface EGFR [3,4,10]. The small-molecule inhibitor of EGFR, AG1478, was used in this study (Figure 1A) [11]. Freshly isolated HSCs were treated with LPS (100 ng/mL for 15 min), and the effects on EGFR activation were determined. The results showed that LPS induced a significant increase in EGFR phosphorylation, which was inhibited in a dose-dependent way with AG1478 treatment (2.5 μM and 5.0 μM) with pretreatment for 2 h (Figure 1B). To evaluate the effect of AG1478 on the activation in HSCs, HSC viability was then determined. As supported by a previous study [12], LPS significantly stimulated HSC proliferation (Figure 1C), indicating LPS could increase HSCs activation. Treatment with AG1478 reduced LPS related-cell viability (Figure 1C).
Figure 1.
The epidermal growth factor receptor (EGFR) inhibitor, AG1478, reduced lipopolysaccharide (LPS)-induced hepatic stellate cell (HSC) activation. (A) Chemical structure and epidermal growth factor receptor (EGFR)-inhibitory IC50 values of AG1478. Hepatic stellate cells (HSCs) were pretreated with AG1478 (2.5 μM and 5.0 μM) for 2 hours, and then exposed to lipopolysaccharide (LPS) (100 ng/mL) for the indicated time. (B) HSCs incubated with LPS for 15 min. The p-EGFR level was detected using Western blot. (C) HSCs incubated with LPS for 24 h. The cell viability of HSCs was detected by the MTT assay. (D–F) HSCs incubated with LPS for 24 h. The levels of transforming growth factor (TGF)-β (D), Col-1 (E), and α-smooth muscle actin (SMA) (F) in the cell lysates were detected by Western blot. (G) HSCs incubated with LPS for 6 h. The mRNA levels of TGF-β, Col-1, and α-SMA were detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR) and normalized against β-actin. * P<0.05, ** P<0.01, *** P<0.001, vs. Ctrl group; # P<0.05, ## P<0.01, ### P<0.001, vs. the LPS-treated group
Increased production of transforming growth factor-β (TGF-β) is critical for sustaining HSC activation [13,14]. In this study, with sustained LPS treatment for 24 h, the expression of TGF-β was stimulated (Figure 1D). Pretreatment with AG1478 decreased TGF-β protein expression in a dose-dependent way (Figure 1D). LPS also stimulated the expression of extracellular matrix (ECM) components, including collagen I (Col-1) (Figure 1E), and α-smooth muscle actin (α-SMA) (Figure 1F), which were reduced by AG1478 pretreatment in a dose-dependent manner (Figure 1E, 1F). AG1478 also reversed the effects of LPS on the expression of genes associated with fibrosis, such as TGF-β, Col-1, and α-SMA, and in a dose-dependent way (Figure 1G). These findings indicated that the EGFR small-molecule inhibitor, AG1478, inhibited LPS-related fibrosis in HSCs, and the inhibition of expression of proteins associated with liver fibrosis by AG1478 may also have been associated with reduced viability of HSCs.
The EGFR inhibitor AG1478 inhibited the expression of LPS-induced inflammatory cytokines in HSCs
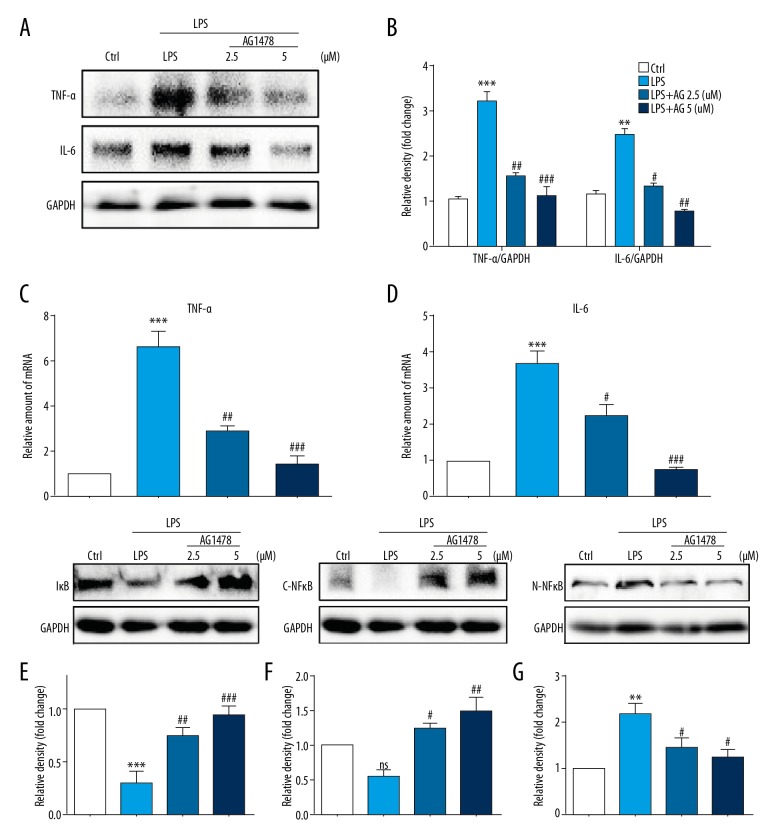
LPS has previously been reported to increase inflammatory responses in the liver, to activate HSCs, chronic inflammation, and fibrosis [6,8,15]. As part of this study, treatment of HSCs with AG1478 was found to alter the expression levels of pro-inflammatory cytokines. Immunoblotting showed increased expression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in HSCs (Figure 2A, 2B), which was also associated with increased mRNA levels of TNF-α and IL-6 (Figure 2C, 2D). AG1478 treatment reduced both protein and mRNA levels TNF-α and IL-6 (Figure 2A–2D).
Figure 2.
Treatment with AG1478 reduced levels of lipopolysaccharide (LPS)-induced hepatic stellate cell (HSC) inflammatory cytokines. Hepatic stellate cells (HSCs) were pretreated with AG1478 (2.5 μM and 5 μM) for 2 h, and then exposed to lipopolysaccharide (LPS) (100 ng/mL) for the indicated times. (A, B) HSCs incubated with LPS for 24 h. Levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6, in the cell lysates were detected by Western blot (A). The figures in the columns show the normalized optical density (OD) for the data from three independent experiments (B). (C, D) HSCs incubated with LPS for 6 h. The mRNA levels of TNF-α (C) and IL-6 (D) were detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR) and normalized against β-actin. (E–G) Western blot analysis of IκBα (E), cytoplasm NFκB P65 (C-NFκB) (F) and nuclear NFκB P65(N-NFκB) (G) levels in HSCs incubated with LPS for 1h. GAPDH was used as a loading control for IκBα/C-NFκB and laminin B as loading control for N-NFκB. ns – not significant vs. Ctrl group; ** P<0.01, *** P<0.001, vs. Ctrl group; # P<0.05, ## P<0.01, ### P<0.001, vs. the LPS-treated group.
To determine the possible underlying signaling mechanism of the anti-inflammatory activity of AG1478, the NF-κB pathway was examined, as this signaling pathway has previously been implicated in the expression of pro-inflammatory cytokines following LPS simulation of HSCs [8]. In the present study, HSCs stimulated with LPS and then treated with AG1478 showed reduced cytosolic IκB-α (Figure 2E), cytoplasm NF-κB p65 subunit (Figure 2F) and increased nuclear NF-κB p65 subunit (Figure 2G) levels. AG1478 treatment of HSCs prevented LPS-induced reduction in cytosolic IκB-α, p65 and increased nuclear p65 levels (Figure 2E–2G). These results showed that LPS induced a pro-inflammatory phenotype in HSCs and that these effects could be inhibited with the use of the specific EGFR inhibitor, AG1478.
EGFR silencing reduced LPS-induced inflammatory cytokines and activation of HSCs
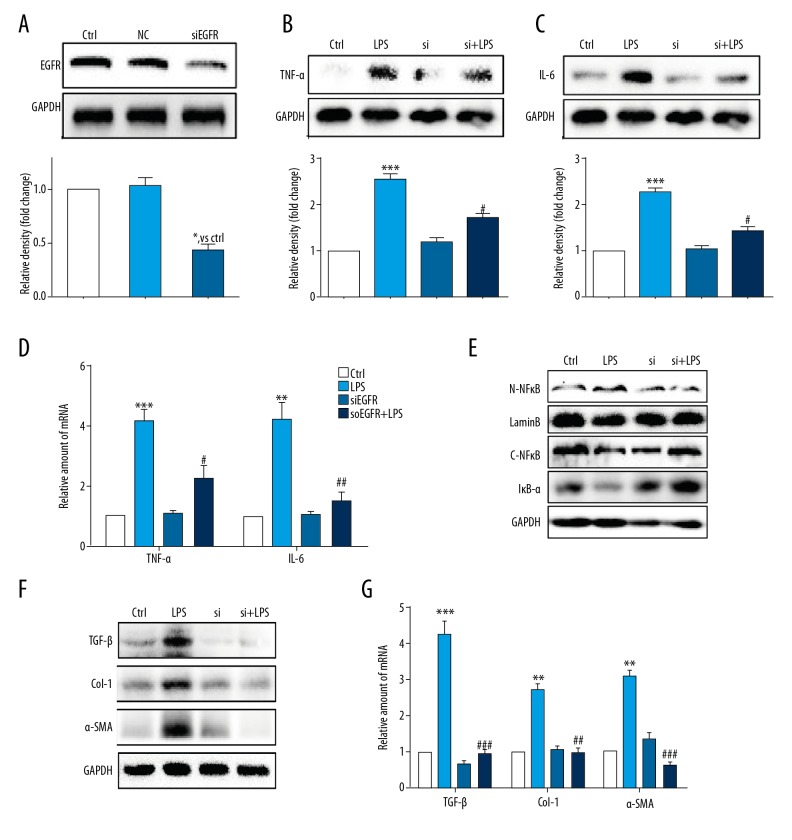
As an additional method to overcome the possibility of non-specific inhibition by the EGFR inhibitor, AG1478 and to provide further support for the role of EGFR, the EGFR gene was silenced by using small interfering RNA (siRNA) cell transfection. Transfection of si-EGFR led to decreased levels of EGFR 1 protein expression in HSCs (Figure 3A), and reduced EGFR protein levels (Figure 3B, 3C) and gene expression levels (Figure 3D) and expression levels of TNF-α and IL-6 in LPS-stimulated HSCs. Also, LPS-induced NF-κB activity was not evident following silencing EGFR expression in HSCs (Figure 3E). Also, si-EGFR decreased LPS-treated activation of HSCs, as reduced levels of the liver fibrosis markers, including of TGF-β, Col-1, and α-SMA at both the protein level (Figure 3F) and the mRNA level (Figure 3G). These findings, together with the results of the anti-inflammation and anti-fibrosis effect of AG1478, confirmed that EGFR has a potential role in regulating LPS-related HSCs activation.
Figure 3.
Knockdown of the EGFR gene following small interfering RNA (siRNA) transfection reduced lipopolysaccharide (LPS)-induced hepatic stellate cell (HSC) injury. (A) Western blot analysis of epidermal growth factor receptor (EGFR) following small interfering RNA (siRNA) transfection in HSCs (NC – negative control for transfection). After incubation of the transfected hepatic stellate cells (HSCs) for 24 h, EGFR gene knockdown HSCs were stimulated with lipopolysaccharide (LPS) (100 ng/mL) for the indicated times (Si – EGFR siRNA). (B, C) HSCs incubated with LPS for 24 h. Tumor necrosis factor (TNF)-α (B) and interleukin (IL)-6 (C) in cell lysates were detected using Western blot. (D) HSCs incubated with LPS for 6 h. The mRNA levels of TNF-α and IL-6 were detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR) and normalized by β-actin. (E) HSCs incubated with LPS for 1 h. IκB-α, cytoplasm NFκB (C-NFκB), and nuclear NFκB (N-NFκB) protein levels were detected using Western blot. (F) HSCs incubated with LPS for 24 h. The levels of transforming growth factor (TGF)-β, Col-1, and α-smooth muscle actin (SMA) were detected using Western blot. (G) HSCs incubated with LPS for 6 h. The mRNA levels of TGF-β, Col-1, and α-SMA were detected by RT-qPCR and normalized against β-actin. * P<0.05, ** P<0.01, *** P<0.001, vs. Ctrl group; # P<0.05, ## P<0.01, ### P<0.001, vs. the LPS-treated group.
LPS triggered EGFR activation in HSCs through Toll-like receptor 4 (TLR4)/c-Src signaling cascade
Included in this study was an initial attempt to determine the possible pathway for the activation of EGFR expression by LPS stimulation of HSCs. LPS has been shown by previous studies to bind directly to Toll-like receptor 4 (TLR4) [7]. TLR4 signaling activation promotes c-Src phosphorylation [16]. It has also previously been reported that c-Src mediates atopic airway inflammation via EGFR activation [17]. Supported by these previous findings, this study included a preliminary evaluation of whether LPS activated EGFR in HSCs through the TLR4/c-Src signaling cascade. Either EGFR gene knockdown (Figure 4A–4C) or pharmacological inhibition (Figure 4D, 4E) of EGFR did not inhibit the expression of TLR4 and phosphorylation of c-Src induced by LPS treatment, suggesting that EGFR may not be the upstream regulator of TLR4/c-Src. We then evaluated the role of the TLR4 antagonist, TAK-242 and the c-Src inhibitor, PP2 on LPS induced-EGFR phosphorylation. As shown in Figure 4F, both TAK-242 and PP2 reduced EGFR activation in HSCs. These findings show a novel mechanism of LPS-induced EGFR activation, one which involves the TLR4/c-Src signaling cascade.
Figure 4.
Lipopolysaccharide (LPS) triggered epidermal growth factor receptor (EGFR) phosphorylation in hepatic stellate cells (HSCs) through the TLR4/c-Src signaling cascade. (A–C) Hepatic stellate cells (HSCs) were pretreated with AG1478 (2.5 μM and 5 μM) for 2 h. After LPS treatment for 15 min, the phosphorylation levels of EGFR (A), the expression of TLR4 (B), and phosphorylation level of c-Src (C) were detected using Western blot. (D, E) Small interfering RNA (siRNA) transfection did not reduce LPS (15 min)-induced increased expression of TLR4 and c-Src activation. (F) HSCs were pretreated with TAK-242 (a TLR4 inhibitor), or PP2 (a c-Src inhibitor) for 1 h, followed by LPS treatment for 15 min. Phosphorylation of EGFR was detected using Western blot. * P<0.05, ** P<0.01, *** P<0.001, vs. Ctrl group; ns – not significant vs. the LPS-treated group; ## P<0.01, vs. the LPS-treated group.
Discussion
Hepatic fibrosis represents healing that follows chronic inflammation and is a response to frequent and repeated chronic liver injury, which can eventually lead to cirrhosis and even liver cancer [18]. During hepatic injury, quiescent hepatic stellate cells (HSCs), which are located between hepatocytes and endothelial cells in the space of Diss, can convert to an activated phenotype that produce both inflammatory cytokines and extracellular matrix (ECM) components [5]. Therefore, reduction of both HSC proliferation and activation might be a potential therapeutic approach to prevent or inhibit the progression of hepatic fibrosis [5]. HSCs can also directly interact with and be activated by lipopolysaccharide (LPS) [19], the plasma concentrations of which are elevated during chronic liver injury [6,8]. Previously published studies have shown that epidermal growth factor receptor (EGFR) expression is associated with hepatic fibrogenesis [3,4]. Feng et al. reported that in dimethylnitrosamine (DMN)-induced hepatic fibrosis in rats, a thio derivative of matrine inhibited fibrogenesis, and improved hepatic function via inhibiting the phosphorylation of EGFR [10]. The findings of the present study showed that the use of small-molecule antagonists of EGFR, or knockdown of the EGFR gene using small interfering RNA (siRNA) cell transfection significantly reduced the expression of ECM-associated cytokines, including transforming growth factor (TGF)-β, α-smooth muscle actin (α-SMA) and collagen 1, and reduced cell viability in the HSCs treated with LPS.
The pro-inflammatory cytokines, interleukin (IL)-6 and tumor necrosis factor (TNF)-α are involved in liver fibrosis [19]. The NF-κB pathway, a conserved mediator of inflammatory responses, also plays a central role in regulating the progression of liver fibrosis [20]. Inhibition of IκB kinases stimulates apoptosis of HSCs, indicating that NF-κB signaling plays a central role in the activation of HSC [8]. The findings of the present study showed that the same EGFR/NF-κB activation pathway enhanced inflammatory response in HSCs, which were reversed by EGFR inhibitors or by EGFR gene knockdown with si-EGFR.
There remains a need to understand how LPS activates EGFR signaling. Koff et al. found that the LPS/Toll-like receptor 4 (TLR4) axis activated EGFR to produce inflammation in the airway epithelium [21]. Also, use of the EGFR inhibitor, erlotinib, or EGFR gene silencing, have previously been shown to inhibit LPS-induced TLR4/NF-κB activation [22]. Therefore, in the present study, TLR4, a classical receptor for LPS, was identified as a potential activator of EGFR in HSCs. It has been reported in previously published studies that c-Src activation plays an important role in EGFR kinase activation [23,24]. The findings of the present study showed, for the first time, that TLR4 interacts with LPS to facilitate c-Src/EGFR interactions, thereby activating the downstream NF-κB signaling pathway and inflammatory response.
Conclusions
The findings of this study showed that the activation of hepatic stellate cells (HSCs) by lipopolysaccharide (LPS) in vitro, including the expression of inflammatory cytokines and mediators of fibrogenesis, was shown to be dependent on the expression of epidermal growth factor receptor (EGFR). Using EGFR gene knockdown and specific small-molecule antagonists and inhibitors, the findings showed that LPS mediated EGFR activation in HSCs, cell proliferation, and the secretion of cytokines involved in inflammation and fibrogenesis. LPS-induced EGFR activation in HSCs was associated with Toll-like receptor 4 (TLR4)/c-Src-related mechanisms. These findings suggest that EGFR antagonism may be a feasible approach for treating LPS-related activation of HSCs activation, preventing or limiting the progression of liver fibrosis.
Supplementary Table
Supplementary Table 1.
Sequences of primers for real-time qPCR assay used in the study.
| Gene | Species | FW | RW |
|---|---|---|---|
| TNF-α | Rat | TACTCCCAGGTTCTCTTCAAGG | GGAGGCTGACTTTCTCCTGGTA |
| IL-6 | Rat | GAGTTGTGCAATGGCAATTC | ACTCCAGAAGACCAGAGCAG |
| Collagen1 | Rat | CGAGTATGGAAGCGAAGGTT | ACGCTGTTCTTGCAGTGATA |
| TGF-β | Rat | AGGAGGAATTTGGCCAGGTG | GCTCACGAGGAGGCTAATCC |
| α-SMA | Rat | TGACCCAGATTATGTTTGAG | AGATAGGCACGTTGTGAGTC |
| β-actin | Rat | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
Acknowledgments
Thanks are given to Jibo Han and Liqin Jiang who supervised this study and who had full access to the data with responsibility for the accuracy of the data collection and interpretation.
Footnotes
Source of support: The project supported by research grants from the Zhejiang Provincial Program of Chinese Medical and Health Science Funding (20172A141), the Zhejiang Provincial Program of Medical and Health Science Funding (2017KY679), the Zhuji City Natural Science Funding and the Zhejiang Pharmaceutical Association Science Funding (2016ZYY30)
Conflict of interest
None.
References
- 1.Tomas A, Futter CE, Edenet ER, et al. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014;24(1):26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berasain C, Avila MA. The EGFR signalling system in the liver: From hepatoprotection to hepatocarcinogenesis. J Gastroenterol. 2014;49(1):9–23. doi: 10.1007/s00535-013-0907-x. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–90. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang D, Chen H, Zhao L, et al. Inhibition of EGFR attenuates fibrosis and stellate cell activation in diet-induced model of nonalcoholic fatty liver disease. Biochim Biophys Acta. 2018;1864(1):133–42. doi: 10.1016/j.bbadis.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Yin C, Evason KJ, Asahina K, et al. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123(5):1902–10. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouts DE, Torralba M, Nelson KE, et al. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56(6):1283–92. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedayat M, Netea MG, Rezaei N, et al. Targeting of Toll-like receptors: A decade of progress in combating infectious diseases. Lancet Infectious Dis. 2011;11(9):702–12. doi: 10.1016/S1473-3099(11)70099-8. [DOI] [PubMed] [Google Scholar]
- 8.Oakley F, Meso M, Iredale JP, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128(1):108–20. doi: 10.1053/j.gastro.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Mederacke I, Dapito DH, Affo S, et al. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protocol. 2015;10(2):305–15. doi: 10.1038/nprot.2015.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Ying HY, Qu Y, et al. Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell. 2016;7(9):662–72. doi: 10.1007/s13238-016-0285-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollee G, Flamant M, Schordan S, et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med. 2011;17(10):1242–50. doi: 10.1038/nm.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Xu Y, Han X, et al. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-kappaB signaling pathway. Sci Rep. 2015;5:18038. doi: 10.1038/srep18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21(3):397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 14.Sui G, Cheng G, Yuan J, et al. Interleukin (IL)-13, prostaglandin E2 (PGE2), and prostacyclin 2 (PGI2) activate hepatic stellate cells via protein kinase C (PKC) pathway in hepatic fibrosis. Med Sci Monit. 2018;24:2134–41. doi: 10.12659/MSM.906442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceccarelli S, Panera N, Mina M, et al. LPS-induced TNF-alpha factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget. 2015;6(39):41434–52. doi: 10.18632/oncotarget.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan X, Zhang Y, Chen H, et al. Inhibition of epidermal growth factor receptor attenuates LPS-induced inflammation and acute lung injury in rats. Oncotarget. 2017;8(16):26648–61. doi: 10.18632/oncotarget.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Hashim AZ, Khajah MA, Renno WM, et al. Src-dependent EGFR transactivation regulates lung inflammation via downstream signaling involving ERK1/2, PI3Kdelta/Akt and NFkappaB induction in a murine asthma model. Sci Rep. 2017;7(1):9919. doi: 10.1038/s41598-017-09349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127(1):55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paik YH, Schwabe RF, Bataller R, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37(5):1043–55. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 20.Xiao C, Ghosh S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol. 2005;560:41–45. doi: 10.1007/0-387-24180-9_5. [DOI] [PubMed] [Google Scholar]
- 21.Koff JL, Shao MX, Ueki IF, et al. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):1068–75. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 22.De S, Zhou H, DeSantis D, et al. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Natl Acad Sci USA. 2015;112(31):9680–85. doi: 10.1073/pnas.1511794112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W, Graves LM, Gill GN, et al. Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced Ras activation. J Biol Chem. 2002;277(27):24252–57. doi: 10.1074/jbc.M200437200. [DOI] [PubMed] [Google Scholar]
- 24.Sutton P, Borgia JA, Bonomi P, et al. Lyn, a Src family kinase, regulates activation of epidermal growth factor receptors in lung adenocarcinoma cells. Mol Cancer. 2013;12:76. doi: 10.1186/1476-4598-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Sequences of primers for real-time qPCR assay used in the study.
| Gene | Species | FW | RW |
|---|---|---|---|
| TNF-α | Rat | TACTCCCAGGTTCTCTTCAAGG | GGAGGCTGACTTTCTCCTGGTA |
| IL-6 | Rat | GAGTTGTGCAATGGCAATTC | ACTCCAGAAGACCAGAGCAG |
| Collagen1 | Rat | CGAGTATGGAAGCGAAGGTT | ACGCTGTTCTTGCAGTGATA |
| TGF-β | Rat | AGGAGGAATTTGGCCAGGTG | GCTCACGAGGAGGCTAATCC |
| α-SMA | Rat | TGACCCAGATTATGTTTGAG | AGATAGGCACGTTGTGAGTC |
| β-actin | Rat | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |