Abstract
Cardiovascular diseases are leading cause of mortality and morbidity, and are responsible for 1 out of every 6 deaths in the United States.1 Cardiac remodeling is an important determinant of long term outcomes and occurrence of heart failure (HF). Many times one requires additional tools like biomarkers to identify high risk individuals, to diagnose disease conditions accurately and to effectively prognosticate the patients. ST2 is a member of the interleukin 1 receptor family and exists in two forms, a trans-membrane receptor (ST2L) as well as a soluble decoy receptor (sST2).2 The ligand of ST2 is Interleukin-33 (IL-33), which is involved in reducing fibrosis and hypertrophy in mechanically strained tissues.3
In heart failure (HF), the change in ST2 levels is an independent predictor of subsequent mortality or need for transplantation.4 ST2 has also been found to be an independent predictor of HF and adverse outcomes in patients with ST elevation myocardial infarction.5
1. Introduction
1.1. Definition & description of biomarker
The term ‘Biomarker’ (biological marker) was introduced in 1989 as a Medical Subject Heading (MeSH) term: 6
“Measurable and identifiable biological parameters, which serve as indices for health and physiology related assessments, such as disease risk, psychiatric disorders, environmental exposure and its effects, disease diagnosis, metabolic processes, substance abuse, pregnancy, cell line development, epidemiological studies etc.”
In 2001, National Institute of Health (NIH) working group standardized the definition of a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”7
A simplistic way to think of biomarkers is as indicators of disease trait (risk factor or risk marker), disease state (preclinical or clinical), or disease rate (progression).8
1.2. Biomarkers in cardiovascular disease (CVD)
There are several biomarkers which have been identified to aid diagnosis, management and prognosticate patients with CVD. Gibbons et al9 published the potential and progress of genetic markers. Cohn et al10 published about the functional surrogate markers in cardiovascular diseases while Macini et al11 published about the structural surrogate markers.
1.3. ST2 as a biomarker (Fig. 1)
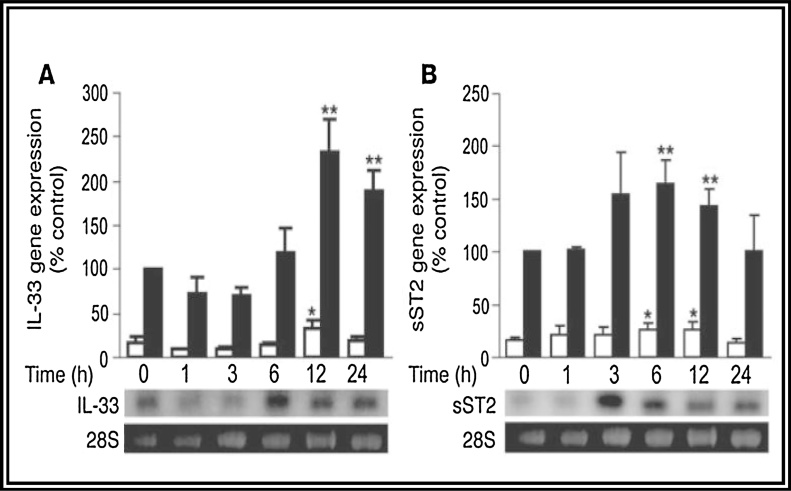
Fig. 1.
Interleukin-33 (IL-33) is induced by mechanical strain in cardiac fibroblasts. (A and B) Quantitative analyses of gene expression of IL-33 by quantitative PCR (A) and sST2 by Northern analysis (B) in rat neonatal cardiomyocytes (white bars) and fibroblasts (black bars) are shown above with representative images from Northern analyses of cardiac fibroblast RNA. Cells were subjected to cyclic strain (8%, 1 Hz) for the indicated periods.13
Interleukin-1 receptor-like-1 (IL1RL1) is the approved symbol for ST2 from the Human Gene Nomenclature Database. The gene is located on Chromosome 2.12 The protein product of ST2 gene encodes a membrane receptor of the interleukin-1 receptor family and a truncated soluble receptor that can be detected in human serum.2 Originally identified in 1989 as an orphan receptor, the ST2 ligand was discovered to be IL-33 in 2005.3 Due to alternative splicing and 3′ processing at the RNA level, ST2 is expressed in a trans-membrane form (ST2 ligand, or ST2L) as well in a soluble, circulating form (sST2) .
Weinberg et al. identified a novel stress-activated signalling pathway: induction of ST2, an interleukin-1 receptor family member, after myocardial stress or injury, by studying neonatal rat myocytes exposed to mechanical strain. The potential importance of this is supported by the finding of increased levels of circulating ST2 in patients after myocardial infarction (MI).13
IL-33 is mechanically induced in cardiac fibroblasts and antagonizes hypertrophic stimuli. Analysis of rat neonatal cardiomyocytes and cardiac fibroblasts revealed that gene expression of IL-33 as well as sST2 was more than 5-fold greater in cardiac fibroblasts than in cardiomyocytes.14
Expression of ST2 is markedly upregulated as early as 1 h following mechanical strain in cultured myocytes and in patients with acute MI.13
Based on these findings they suggest that ST2 participates in the cardiovascular response to injury and that serum ST2 may be a useful biomarker in pre dieting prognosis.
1.4. ST2 and vascular inflammation
In 2005, the discovery of interleukin-33 (IL-33) as an ST2 ligand provided new insights into ST2 signalling10. IL-33 is clearly a potential mediator of diverse inflammatory diseases.15 However, despite its heritage in the investigation of classical inflammatory diseases such as asthma and urticaria, IL-33 has now also been shown to participate in cardiovascular pathophysiology. As will be discussed in this review, myocardial production of IL-33 can protect cardiac function in response to pressure overload.16 Furthermore, the IL-33/ST2 system may play a part in the progression of atherosclerotic vascular disease.17
1.5. Measurement of ST2
Recently, a highly sensitive ELISA for sST2 has been developed (Presage ST2), which has low imprecision (coefficient of variation <5%) even at very low analyte concentrations.18
While ST2 is associated with allergic and immunologic diseases such as asthma, among normal subjects, sST2 was not found to be higher in them. Unlike natriuretic peptides, sST2 values were not significantly affected by body-mass index or renal insufficiency, a major weak spot for BNP or NT-pro BNP.
An upper reference limit of 35 ng/mL has been set for this assay; 95% of normal subjects are below this threshold value. The Presage ST2 assay was recently approved by regulatory agencies in Europe and the United States for prognostication in HF.18
1.6. Post myocardial infarction ventricular remodeling
Cardiac remodeling can be described as a physiologic and pathologic response that may follow MI, pressure overload (aortic stenosis, hypertension), inflammatory heart muscle disease (myocarditis), idiopathic dilated cardiomyopathy or volume overload conditions (valvular regurgitation).
It refers to alteration of ventricular architecture. Inspite of different etiologies the pathophysiological changes at cellular and biochemical levels are common. After acute myocardial infarction, which involves myocardial necrosis and loss of myocardium, there is triggering of numerous biochemical and intracellular mechanisms, as an adaptive and reparative response of the ventricle to changed loading conditions. This involves dilatation, hypertrophy, and the formation of a discrete collagen scar.
1.7. Remodeling is divided into two phase (Fig. 2)19
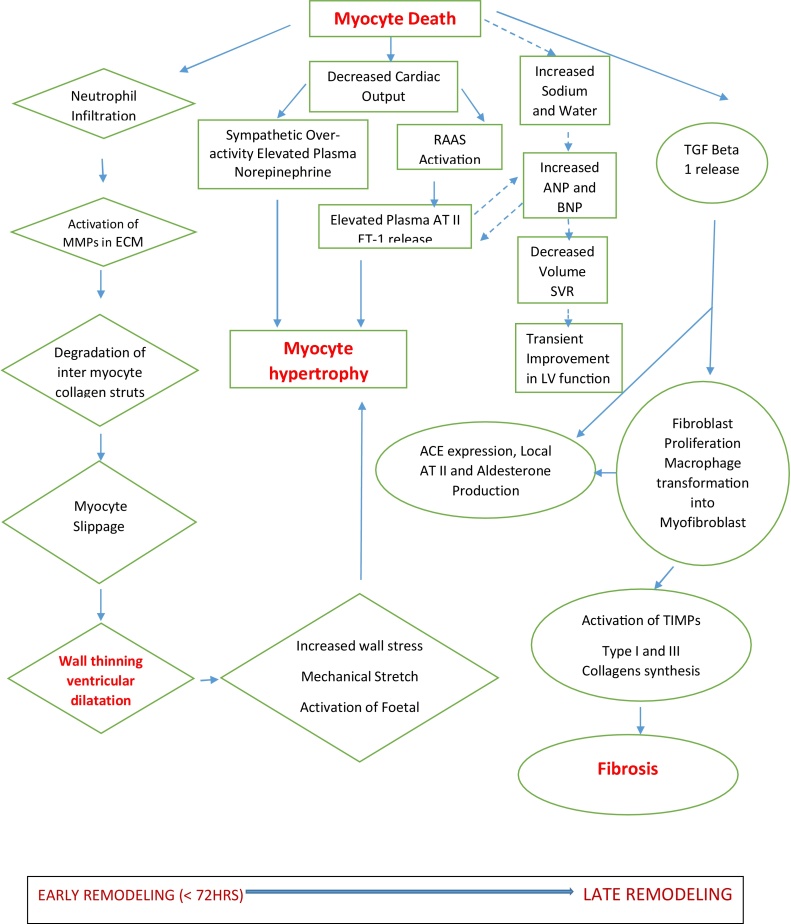
Fig. 2.
Diagrammatic representation of the many factors involved in the pathophysiology of ventricular remodeling. ECM indicates extracellular matrix; RAAS, renin-angiotensin-aldosterone system; CO, cardiac output; SVR, systemic vascular resistance; LV, left ventricular; TGF-beta 1, Transforming growth factor beta-1;ET-1, Endothelin-1 and ATII, angiotensin II.16
Early phase (within 72 h) – Expansion of the infarct zone occurs within hours of myocyte injury, which leads to wall thinning and ventricular dilatation. This in turn triggers adaptive responses like stimulation of renin-angiotensin-aldosterone system and increased synthesis of catecholamine.
Late phase (beyond 72 h) – Remodeling involves myocyte hypertrophy and alterations in ventricular architecture to distribute the increased wall stresses more evenly as the extracellular matrix forms a collagen scar to stabilize the distending forces and prevent further deformation.
In human and animal models, fibroblast stimulation increases collagen synthesis and causes fibrosis of both the infarcted and non-infarcted regions of the ventricle, thus contributing to remodeling.20
1.8. Non ischemic ventricular remodeling
Hypertension is the most important risk factor for HF and 75% of HF patients are known hypertensives.21 Terminally differentiated cardiac myocytes respond to pressure-overload stress by enlarging. Increases in wall thickness tend to diminish wall stress and to decrease oxygen demand; hence, they are adaptive. This response, called hypertrophy, ultimately leads to ventricular wall thickening and stiffening. When the pressure stress is persistent, however, the myocardium slowly transits to a state of decompensation and clinical HF.
1.9. Ventricular remodeling and heart failure
As the heart remodels, its geometry changes; it becomes less elliptical and more spherical. There are also changes in ventricular mass, composition and volume, all of which may adversely affect cardiac function. Progressive remodeling is considered deleterious and is associated with poor prognosis. If cardiac dilation persists without hypertrophy, myocardial wall stress is increased. A number of mechanisms may be stimulated by increased wall stress, and this may lead to further dilation of the heart. Without therapy to reduce ventricular dilation, decrease wall stress the process progresses towards overt chronic HF.22
In 1987, White et al observed that left ventricular (LV) ejection fraction and LV end systolic volume index (ESVi) at 1- 2months post MI were powerful predictors of prognosis.23
Migrino et al demonstrated a continuous relationship between ESVi and mortality as well as development of HF symptoms.24
2. Role of ST2 in cardiovascular diseases
2.1. ST2 in heart failure
The first large-scale analysis of sST2 in patients with heart failure was from the Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. In this analysis of 593 patients admitted to the emergency department with acute dyspnea with and without acute destabilized HF were evaluated with the measurement of ST2 concentrations. Independent predictors of death at 1 year were identified. Concentration of ST2 was higher in those with dyspnea due to HF than those without HF. Although NT-pro BNP was superior in the diagnosis of HF, ST2 values were directly associated with worse NYHA class and symptom severity.
The concentrations of the marker were higher in patients who were dead at 1 year as compared with survivors, with a dose-dependent relationship between ST2 concentrations and risk of death at 1 year.4, 25
In a larger pooled analysis of 346 patients with acutely decompensated HF, using the Presage ST2 assay, Rehman et al26 further examined the association between sST2 concentrations and clinical characteristics and prognosis. sST2 levels were higher in patients with acute HF who died at 1 year, and in adjusted models, an elevated sST2 was associated with a twofold risk of mortality independent of other clinical and biochemical parameters of risk (including BNP levels).
3. ST2 level and cardiovascular imaging
Filippi et al. reviewed both the associations with cross-sectional findings and longitudinal changes in cardiac structure and function measured by echocardiography and cardiac magnetic resonance imaging with sST2 levels in a variety of patient populations with or at-risk for cardiovascular disease.27 In a PRIDE substudy in patients with acute dyspnea, sST2 levels were found associated with left ventricular ejection fraction (LVEF), estimated right ventricular (RV) systolic pressure and RV hypokinesis. However, in the Framingham Heart Study, sST2 was not associated with either echocardiographic finding. In the Cardiovascular Health Study, sST2 appeared strongly associated with the presence of diastolic dysfunction. A substudy of Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) evaluated the association among LV remodeling (defined as an increase in LV end-systolic and −diastolic volumes), sST2, and the benefit of eplerenone and found that sST2 levels were good surrogates of LV remodeling. Similarly, the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) study found that more time spent with an sST2 level less than the cutoff of 35 ng/L identified patients with a greater probability of a decrease in LV diastolic index over 1 year.
3.1. ST2 in acute coronary syndromes
Acute MI is a condition which causes sudden mechanical stress to the myocardium. As soluble form of ST2 is secreted by biomechanically overloaded myocytes, in patients with STEMI, an elevated level of ST2 is a strong predictor of cardiovascular death or HF within 30 days.5
3.1.1. ST2 in STEMI
Shimpo et al. investigated sST2 levels in 810 patients in the Thrombolysis In Myocardial Infarction (TIMI) 14 and Enoxaparin and TNK-tPA With or Without GPIIb/IIIa Inhibitor as Reperfusion Strategy in STEMI (ENTIRE)-TIMI 23clinical trials. They found that elevated ST2 levels at admission were associated with death or new onset heart failure at 1 month. This prediction was independent of traditional risk factors.5
Also elevated sST2 levels were associated with unstable hemodynamic profile on admission. It also showed a positive association with heart rate, cardiac troponin I level, C-reactive protein, BNP, and serum creatinine.
Sabatine et al. extended these results in a study of 1239 patients with STEMI in the Clopidogrel as Adjunctive Reperfusion Therapy– Thrombolysis in Myocardial Infarction 28 (CLARITY-TIMI 28) trial and found a positive association between elevated ST2 level and adverse outcome at 1 month.28
Patients in the highest quartile of sST2 concentration had a nearly 3.5-fold risk of cardiovascular death or HF at 30 days as compared to patients in the lowest quartile.
Apart from predicting risk for onset of HF due to MI, measurement of ST2 helps in decision making regarding therapy, by identifying patients at risk and starting them on medications (like aldosterone antagonists, ACE-I or ARB) to prevent remodeling.
3.1.2. Non – ST elevation MI
Kohli et al showed that among patients in the MERLIN TIMI 36 trial, concentrations of sST2 were only weakly correlated with troponin or BNP in 4426 subjects with unstable angina pectoris and non-ST segment elevation MI. However an sST2 > 35 ng/mL was associated with an increased risk of heart failure or adverse cardiac event at 30 days (6.6% versus 1.6% below; p < 0.001) and at one year (12.2% versus 5.2%; p < 0.001).29
3.2. ST2 in management of myocardial infarction and heart failure
Studies have demonstrated the role of ST2 biomarker in management of MI and HF. Although baseline ST2 values at admission have been proved to predict outcomes, serial measurements may be of even greater value. The biological variation and the low index of variation of ST2 make it a good marker for monitoring and possibly guiding therapy in acute HF.30, 31
One of the first studies to assess serial measurements of sST2 was reported by Boisot et al. 32 In this study sST2 was measured on a daily basis in patients admitted with acute decompensated HF and demonstrated that this biomarker quickly changes in response to treatment. Patients whose values decreased rapidly after admission had a good short-term outcome. They found that percentage change of ST2 level during treatment, is predictive of 90 days mortality. Thus ST2 values may be used in optimizing therapy.
In a small randomized trial of Eplerenone (a mineralocorticoid receptor antagonist with anti-remodeling effects), an elevated sST2 appeared to identify those patients likely to benefit from treatment with this drug: those treated with Eplerenone did not have significant ventricular dilation in follow-up, however placebo-treated subjects developed significant ventricular remodeling as predicted by the elevated sST2. 33
4. ST2 in comparison to other biomarkers
In a study by Bayes-Genis et al, where they compared ST2 and Galectin-3 in chronic HF, it was found that incorporation of ST2 into a full-adjusted model for all-cause mortality (including clinical variables and N-terminal pro–B-type natriuretic peptide) improved discrimination and calibration, and reclassified significantly. Incorporation of Gal-3 showed no significant increase in discrimination or reclassification and worse calibration metrics. On direct model comparison, ST2 was superior to Gal-3. Thus ST2 is superior to Galectin-3 in risk stratification.34
Also Dupuy et al, found that ST2 in combination with CRP is a more valuable tool for identifying patients at risk of death, than CRP alone.35
ST2 in comparison to Natriuretic peptides and Galectin 3, has other advantages in that its levels are not affected by confounding factors (age, body weight or serum creatinine); its levels rapidly change with patient’s changing disease state, hence easier to optimize drug therapy; the single cutpoint to assess treatment makes it easier to use and less confusing.
5. Conclusions
In the setting of STEMI, high levels of ST2 can be used for “Risk stratification” to identify patients at high risk of developing adverse cardiovascular events like progressive HF or death.
The ST2 level gives information about wall stress, inflammation, macrophage activation (fibrosis), hence a single sST2 measurement, should allow titration of therapy and monitoring of the patient.
The current ACC/AHA guideline for HF management, recommends monitoring of ST2 level in heart failure patients for additive risk stratification, as a class II, level of evidence B.36
Time only will tell us whether ST2 estimation continues to be in the diagnostic armamentarium in STEMI and HF or if it will wither away like many other biomarkers.
Contributor Information
Aditi Dattagupta, Email: aditi.dattagupta@sakraworldhospital.com.
Sathyamurthy Immaneni, Email: drsathyamurthy_i@apollohospitals.com.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwahana H., Yanagisawa K., Ito-Kosaka A., Kuroiwa K. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264(2):397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz J. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg E.O., Shimpo M., Hurwitz S., Tominaga S., Rouleau J.L. Identification of Serum Soluble ST2 Receptor as a Novel Heart Failure Biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 5.Shimpo M., Morrow D.A., Weinberg E.O., Sabatine M.S., Murphy S.A. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 6.Vasan Ramachandran S. Biomarkers of cardiovascular disease molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 7.Biomarkers Definitions Working Group Biomarkers and surrogate end- points: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 8.Fox N., Growdon J.H. Biomarkers and surrogates. Neuro Rx. 2004;1:181. [Google Scholar]
- 9.Gibbons G.H., Liew C.C., Goodarzi M.O. Genetic markers: progress and potential for cardiovascular disease. Circulation. 2004;109:IV–47. doi: 10.1161/01.CIR.0000133440.86427.26. [DOI] [PubMed] [Google Scholar]
- 10.Cohn J.N., Quyyumi A.A., Hollenberg N.K., Jamerson K.A. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004;109:IV–31. doi: 10.1161/01.CIR.0000133442.99186.39. [DOI] [PubMed] [Google Scholar]
- 11.Mancini G.B.J., Dahlof B., Diez J. Surrogate markers for cardiovascular disease: structural markers. Circulation. 2004;109:IV–22. doi: 10.1161/01.CIR.0000133443.77237.2f. [DOI] [PubMed] [Google Scholar]
- 12.Dale M., Nicklin M.J.H. Interleukin-1 receptor cluster: gene organization of IL1R2, IL1R1, IL1RL2 (IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1 (IL-1Rrp) on human chromosome 2q. Genomics. 1999;57:177–179. doi: 10.1006/geno.1999.5767. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg E.O., Shimpo M., De Keulenaer G.W. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106(23):2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanada S., Hakuno D., Higgins L.J., Schreiter E.R., McKenzie A.N., Lee R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117(6):1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello C.A. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–462. doi: 10.1016/j.immuni.2005.10.004. [PubMed: 16286013] [DOI] [PubMed] [Google Scholar]
- 16.Sanada S., Hakuno D., Higgins L.J., Schreiter E.R., McKenzie A.N., Lee R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller A.M., Xu D., Asquith D.L. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieplinger B., Januzzi J.L., Jr., Steinmair M. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma–the Presage ST2 assay. Clin Chim Acta. 2009;409(1–2):33–40. doi: 10.1016/j.cca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 20.Cohn J.N., Ferrari R., Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 21.Go A.S., Mozaffarian D., Roger V.L. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman W., Jones D., McLaurin L.D. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White H.D., Norris R.M., Brown M.A., Brandt P.W.T., Whitlock R.M.L., Wild C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 24.Migrino R.Q., Young J.B., Ellis S.G. End–systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)-I Angiographic Investigators. Circulation. 1997;96:116–121. doi: 10.1161/01.cir.96.1.116. [DOI] [PubMed] [Google Scholar]
- 25.Januzzi J.L., Peacock W.F., Maisel A.S. Measurement of the interleukin family member ST2 in patients with acute dyspnea. Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) JACC. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Rehman S.U., Mueller T., Januzzi J.L. Characteristics of the novel Interleukin family biomarker ST2 in patients with acute heart failure. JACC. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 27.deFilippi C.L., Daniels L.B.2. Bayes-Genis A3. Structural heart disease and ST2: cross-sectional and longitudinal associations with echocardiography. Am J Cardiol. 2015;115(April 7 Suppl):59B–63B. doi: 10.1016/j.amjcard.2015.01.042. [Epub 2015 Jan 26] [DOI] [PubMed] [Google Scholar]
- 28.Sabatine M.S., Morrow D.A., Higgins L.J. Cannon CP, Gerszten RE, Lee RT. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptidein patients with ST-elevation myocardial infarction. Circulation. 2008;117(15):1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohli P.L., Bonaca M.P., Kakkar R. Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clin Chem. 2012;58(1):257–266. doi: 10.1373/clinchem.2011.173369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshikawa K., Kuroiw K., Tago K. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164(2):277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 31.Wu A.H., Wians F., Jaffe A. Biological variation of galectin-3 and soluble ST2 for chronic health failure: implication on interpretation of test results. Am Heart J. 2013;165(6):995–999. doi: 10.1016/j.ahj.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Boisot S., Beed J., Isakson S. Serial sampling of ST2 predicts 90-day mortality following destabilized Heart failure. J Card Fail. 2008;14(9):732–738. doi: 10.1016/j.cardfail.2008.06.415. [DOI] [PubMed] [Google Scholar]
- 33.Pitt B., White H., Nicolau J. EPHESUS Investigators. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46(3):425–431. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 34.Bayes-Genis A., De Antonio M., Vila J., Peñafiel J. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification ST2 versus galectin-3. JACC. 2014;63(2):158–166. doi: 10.1016/j.jacc.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 35.Dupuy A.M., Curinier C., Kuster N., Huet F. Multi-marker strategy in heart failure: combination of ST2 and CRP predicts poor outcome. PLoS One. 2016 doi: 10.1371/journal.pone.0157159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.2013 ACCF/AHA guideline for the management of heart failure a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]