Abstract
Objective
High dose IL-2 (HDIL-2) is associated with complete and durable responses in only 5–10% of patients with stage IV melanoma and the toxicity profile is significant. In vivo human models have recently demonstrated a stimulatory effect of exogenous IL-2 on both the Th17 and regulatory T cell (TREG) compartments. We investigated and compared the effect of HDIL-2 on the Th17 and TREG compartments in HDIL-2 responders versus non-responders.
Methods
High-dose IL-2 (HDIL-2) was administered at a dose of 720,000 IU/kg to patients with melanoma (n=6) and peripheral blood was collected at baseline and at 24, 48, 72, and 96 hours during treatment. PBMCs were isolated and underwent intracellular cytokine and extracellular receptor staining for flow cytometry.
Results
5 of 6 patients clinically progressed on HDIL-2 therapy, and these patients demonstrated an increase in the frequency of regulatory T cells on day 4 of treatment. A single patient responded to HDIL-2 therapy and demonstrated a decrease in the frequency of TREG cells on day 4 of treatment. We found that HDIL-2 resulted in a larger increase in the frequency of IFNγ+Th17 cells in the responder when compared to all non-responders. As such, all non-responders demonstrated a negative IFNγ+Th17:TREG ratio, whereas the complete responder demonstrated a positive IFNγ+Th17:TREG ratio.
Conclusion
Our results suggest a distinct immunophenotype may be associated with response to HDIL-2. The peripheral Th17:TREG ratio may serve as an early biomarker in the setting of HDIL-2 to help identify those patients who would benefit from subsequent cycles.
Keywords: HDIL-2, Th17:TREG ratio, IFNγ+Th17 cells, Th17 cells, melanoma
Introduction
Melanoma is a highly immunogenic tumor capable of inducing adaptive immunity[1]. As such, treatments have focused on immunomodulation as a means of inducing an anti-tumor response. High-dose IL-2 (HDIL-2) is the longest used immunotherapy for advanced stage melanoma. While HDIL-2 is associated with significant toxicity, it induces complete and durable responses in 5–10% of patients – a feature unique to HDIL-2 [2]. However, the mechanisms of HDIL-2 success and failure are poorly understood, and there is a paucity of data detailing the immunophenotypic and functional changes that occur during this treatment regimen. As such, reliable biomarkers predictive of response are lacking, and many patients experience the medication-associated toxicities without oncologic benefit.
Recent advancements in cancer immunotherapy have focused largely on the activation and function of CD8+ cytotoxic T cells (CTLs). However, CD4+ effector T cells are also able to directly induce tumor cell death [3]. While much emphasis has been placed on IFNγ-secreting Th1 cells, a more recently identified subset of effector CD4+ T cells, Th17 cells, have demonstrated potent antitumor effects in both in vitro and in vivo models [3–7]. It is important to note that the role of Th17 cells in tumorigenesis remains controversial, with studies demonstrating pro-tumorigenic qualities of both endogenous and exogenously administered IL-17, particularly in the setting of immunodeficiency [8–10]. It appears the effect of IL-17 on tumor growth is highly influenced by environmental factors, and the effect of Th17 cells in an in vivo human model of cancer warrants specific investigation. Prior studies have identified soluble IL-2 as an important regulator of both the Th17 and regulatory T cell (TREG) compartments; and we have recently shown that in vivo IL-2 increases Th17 cells in patients with advanced stage melanoma (Diller et al. unpublished data; manuscript under review). Here, we further interrogated the impact of HDIL-2 on the balance between Th17 and regulatory T cells in HDIL-2 non-responders vs. a patient exhibiting complete oncologic response.
Methods
Patient enrollment and sample collection
All patients undergoing HDIL-2 therapy at Emory University Hospital were enrolled in an IRB-approved protocol (IRB 46593) after informed consent was obtained (n=6). Of the 6 patients treated, 1 patient demonstrated a complete radiographic response to therapy, ongoing stable for 14 months. Patients were treatment naïve with regards to anti-PD-1 and anti-CTLA4 therapy with the average age of non-responders vs. responder 54 years-old vs. 68 years-old, respectively (Table 1). HDIL-2 (aldesleukin; Promethus, San Diego CA) was administered as an IV infusion over 15 minutes at a dose of 720,000 IU/kg every 8 hours and continued for a maximum of 5 days. Peripheral blood was collected in BD Vacutainer tubes containing 0.1 M sodium heparin. Samples were collected pretreatment and every 24 hours for the duration of HDIL-2 therapy. PBMCs were purified from peripheral blood samples via density gradient centrifugation (cell preparation tubes, BD Pharmingen) within 12 hours of sample collection and cryopreserved at −80 degrees C for future intracellular and extracellular staining and analysis via flow cytometry. All experiments performed for this study were completed within 30 days of PBMC purification.
Table 1.
Patient characteristics.
| Patient identification number | Age | Sex | BRAF V600E mutation | Prior treatment with IFN | LDH | M category | CNS metastases | ECOG PS | HDIL-2 administered | Response |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 63 | M | Positive | Yes | 158 | M1a | No | 1 | Two cycles | NR |
| 5 | 51 | M | Positive | No | 187 | M1c | Yes | 3 | One cycle | NR |
| 11 | 68 | M | Positive | No | 167 | M1a | No | 0 | Two cycles | CR |
| 14 | 50 | F | Positive | No | 183 | M1a | No | 0 | Two cycles | NR |
| 21 | 62 | F | Negative | Yes | 122 | M1c | No | 0 | One course + two cycles | NR |
| 22 | 43 | M | Negative | No | 180 | M1a | No | 0 | One course + two cycles | NR |
LDH, normal lactato dehydrogenase = 140–280 U/I; M category: M1a, metastasis to skin, subcutaneous tissue, or lymph nodes with a normal LDH level; M1c, metastasis to any other organs or distant spread to any site along with an elevated LDH level; Eastern Cooperative Oncology Group (ECOG) Performance Status (PS): PS 0, fully active; PS 1, restricted in physically strenuous activity; PS 2, ambulatory and capable of all self-care; PS 3, capable of only limited self-care; PS 4, completely disabled; PS 5, dead; one cycle of HDIL-2, 5 days of treatment; one course of HDIL-2, two cycles of treatment with 9 days of rest between cycles.
CNS, central nervous system; CR, complete responder; HDIL-2, high-dose IL-2; IFN, interferon; NR, nonresponder.
Ex vivo frequency and phenotype analysis of isolated PBMCs
Extracellular flow cytometry staining was performed on PBMCs using the following fluorophore-labeled antibodies: CD3-Alexa700 (Biolegend), CD14/CD20-PacOrange (Invitrogen), CD8-APC-Cy7 (BD pharmingen), CD4-Pac Blue (Biolegend), CD45RA-Qdot655 (Invitrogen), CCR7-PECy7 (Biolegend). For FoxP3 staining, cells were stained using the FoxP3 staining kit (eBioscience) according to manufacturer’s protocol. Frequencies of cell populations and co-signalling molecule expression were determined by flow cytometry. The flow cytometer was calibrated daily using Cytometer Setup and Tracking (CS&T) beads (BD pharmingen). Mid-range beads were utilized and photomultiplier tube (PMT) voltages adjusted prior to analyzing each experiment. Data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Ex vivo intracellular cytokine staining
For determination of ex vivo cytokine production, PBMCs were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine (200mM), 1% penicillin/streptomycin (100x), 1% hepes (1M), 1% 2-ME (14.3M) (R10). 1 × 106 PBMCs were placed in a 96 well plate and stimulated for 4 hours at 37 degrees C with a mixture of PMA (Sigma) and ionomycin (Sigma) at a concentration of 1 μg/mL each. Brefeldin A (GolgiPlug, BD Biosciences) was added to cells after 1 hour of stimulation at a concentration of 1 μg/mL. Intracellular staining was performed after fixation and permeabilization according to manufacturer’s instructions (BD Biosciences) utilizing fluorophore-labeled antibodies to IFNγ-APC, IL-2-PE, IL-17-FITC (all Biolegend). Cytokine production was determined via flow cytometry and analyzed using FlowJo software. Unstimulated (US) samples measured at the baseline timepoint were utilized as negative controls and were prepared and analyzed in the same manner listed above without the addition of PMA/ionomycin (average frequency of IL-17+CD4+ T cells =0.1% unstimulated vs. 0.8% stimulated; average frequency of IFNγ+CD4+ T cells=3% unstiumulated vs. 35% stimulated).
Statistics
Statistical analysis was performed using 2-tailed paired Student’s t tests and linear regression models with Prism 5.0 (GraphPad) software. P values of less than 0.05 were considered statistically significant.
Results
IL-2 induced expansion of TREG during HDIL-2 therapy has been implicated as a potential mechanism of treatment failure [11]. We interrogated changes within the CD25+FoxP3+CD4+ T cell compartment over the course of HDIL-2 therapy in two patient populations: those who responded to treatment (CR, n=1) and those who did not (NR, n=5). Flow cytometric analyses were performed on days 0 and 4 of HDIL-2 therapy. 5 of 6 patients failed HDIL-2 therapy and demonstrated an increase in TREG frequency following treatment (4% on day 0 +/− 1% to 14 +/− 6% on day 4, p=0.06; Fig. 1B–C). Interestingly, only the complete responder demonstrated a decrease in TREG frequency (9% on day 0 to 7% on day 4; Figure 1B–C).
Figure 1. Complete response is associated with a decrease in the frequency of CD25+FoxP3+CD4+ T cells (TREG) and an increase in IFNγ–secreting Th17 cells on day 4 of HDIL-2 therapy.
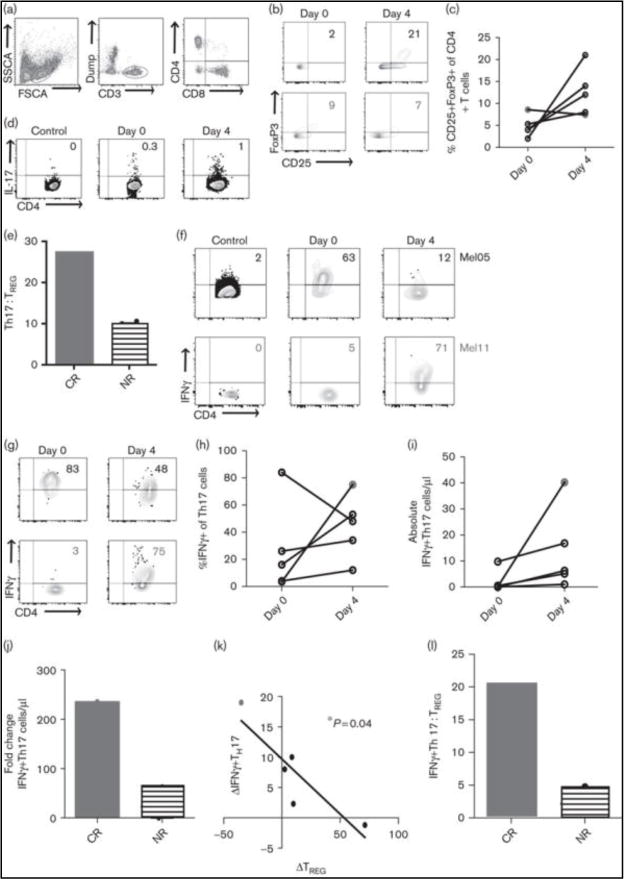
A. Representative flow plots demonstrate gating strategy for lymphocytes (left), CD3+ T cells (middle), and CD4+ T cells (right). B. Representative flow plots demonstrate changes in TREG frequency in a complete responder (CR, red) and a non-responder (NR, black). C. Summary data for all patients demonstrates change in frequency of TREG cells (ns). D. Representative flow plot demonstrates gating strategy for IL-17+CD4+ (Th17) cells. The control plots (left) represent unstimulated staining and served as the negative control for the patient shown. E. HDIL-2 resulted in a positive Th17:TREG ratio on day 4 of therapy in a complete responder and a negative Th17:TREG ratio in all non-responders. F. (bottom) demonstrate gating strategy for IFNγ-secreting T cells. The control plots (left) represent unstimulated staining and served as the negative control for the patients shown. G. Representative flow plots demonstrate changes in IFNγ expression on Th17 cells. H. Summary data for all patients demonstrates change in the frequency of IFNγ+Th17 cells (ns). I. The complete responder demonstrates a larger increase in the frequency of IFNγ+Th17 cells on day 4 of HDIL-2. J. Change in frequency of TREG cells inversely correlates with frequency of IFNγ+Th17 cells (p = 0.04). K. HDIL-2 resulted in a positive IFNγ+Th17:TREG ratio on day 4 of therapy in a complete responder and a negative IFNγ+Th17:TREG ratio in non-responders.
Seminal studies have demonstrated potent yet opposing effects of IL-2 on the regulatory and Th17 cell compartments, whereby IL-2 stimulates TREG growth and development and inhibits Th17 expansion[12]. We previously reported that in vivo IL-2 increased Th17 cells in patients with melanoma and that this was closely associated with changes in the TREG compartment (Diller et al. unpublished data; manuscript under review). As Th17 cells have been shown to induce an anti-tumor response [3, 4], we hypothesized that the ratio between Th17 and regulatory T cells may be associated with clinical response to HDIL-2. To investigate this further, we divided the change in frequency of Th17 cells by TREG cells. While all non-responders demonstrated a negative Th17:TREG ratio, the complete responder (CR) uniquely demonstrated a positive Th17:TREG ratio (Figure 1E).
Mouse models of B16 melanoma have shown that Th17-mediated tumor destruction is critically dependent on IFNγ rather than IL-17 secretion [4]. We therefore sought to investigate IFNγ production by Th17 cells in the setting of HDIL-2 in a complete responder and all non-responders. We found that HDIL-2 treatment increased the frequency of IFNγ-producers within the Th17 compartment on day 4 of therapy in 4 of 5 patients (Fig. 1G–H). Importantly, the complete responder demonstrated a much larger fold increase in the frequency of IFNγ+Th17 cells when compared to all non-responders (16% complete responder vs. 1.6% ± 1% non-responders; Figure 1I). It is important to note that fold change in the frequency of IFNγ+Th17 cells is reported relative to fold change in the frequency of CD4+ T cells. Fold change within the CD4+ T cell compartment was minimal (1% complete responder vs. 1.4% ± 0.1% non-responders; data not shown); therefore changes seen within the IFNγ+Th17 cell population is unlikely secondary to changes within the gross CD4+ T cell compartment. Changes in frequency of IFNγ+Th17 cells inversely correlated with changes in TREG frequency (p = 0.04, Figure 1J). Similar to the ratio of Th17:TREG cells, all non-responders demonstrated a negative IFNγ+Th17:TREG ratio, whereas the complete responder demonstrated a positive IFNγ+Th17:TREG ratio (Figure 1K).
Discussion
The mechanisms behind HDIL-2 success and failure are poorly understood: however, the stimulatory effect of HDIL-2 on the TREG compartment has served as a potential explanation of HDIL-2 failure [11]. While our cohort of patients was small, one patient demonstrated a complete and durable response to HDIL-2, and this response was associated with a distinct immunophenotype detected early in the course of therapy. Our findings showed that response to treatment was associated with a high Th17:TREG ratio and more specifically a positive IFNγ+Th17:TREG ratio. Emerging evidence now identifies CD4+ T cells as active participants during tumor rejection. CD4+ T cells are capable of eliminating tumors that are resistant to CD8-mediated rejection [3], and Th17 cells specifically demonstrate a superior anti-tumor response when compared to Th1 cells [4].
As the number of oncologic therapies available continues to increase, it becomes imperative that biomarkers to help categorize patients into specific treatment arms be identified. Early lymphocytosis, vitiligo, and metastases limited to subcutaneous or cutaneous sites have been associated with an antitumor response secondary to HDIL-2, however these parameters fail to accurately predict response in a large proportion of patients [13]. Additionally, while tissue immunohistochemistry (IHC) is a validated method for characterizing patterns of protein expression, proteins identified as biomarkers predictive of response to treatment (i.e. tumor PDL-1 expression as predictive of response to PD-1 blockade) [14, 15], do not function as clear cut inclusionary or exclusionary predictors [16]. Furthermore, these assays require tissue samples for analysis. Monitoring the ratios between T cell compartments within the peripheral blood has the benefit of easy sample collection and makes use of simple laboratory procedures, potentially serving as a valuable adjunct to currently utilized prognostic models.
Here, we show that a positive IFNγ+Th17:TREG ratio may serve as a biomarker in the setting of HDIL-2. As mentioned above, IFNγ-secreting Th17 cells have received recent notoriety for their anti-tumor effects [4]. Interestingly, IFNγ itself has both anti- and pro-tumorigenic functions, and its ultimate action depends on a multitude of host factors including tumor profile, microenvironment, and signal strength [17]. In vitro studies have demonstrated that IFNγ combined with IL-2 favors cytotoxic T cell proliferation and promotes a pro-inflammatory phenotype [18, 19]. These data further support the idea that measurement of peripheral IFNγ+Th17:TREG ratios could be used as a meaningful biomarker in the setting of HDIL-2.
Because all patients increased Th17 cells with HDIL-2 therapy, the high Th17:TREG ratio observed on day 4 of treatment in the complete responder was likely intensified by this patient’s accompanying decrease in TREG frequency. As stated above, the stimulatory effect of IL-2 on the TREG compartment is well-documented [11, 20]; therefore it is curious that any patient would demonstrate a decrease in either the frequency or total number of regulatory T cells. It is interesting to note that the complete responder demonstrated a decrease in both IL-2+CD4+ and IL-2+CD8+ T cells during the course of HDIL-2 therapy, and this decrease was larger than similar trends observed in other patients (data not shown). Because TREG development relies heavily on paracrine IL-2 signaling [21], the decrease in the number of neighboring IL-2-secreting T cells observed in the complete responder may have disrupted efficient TREG development.
While flow cytometry allows for rapid, multi-parameter phenotypic and functional analyses of individual cells [22], data is interpreted via hierarchical gating analysis and the boundaries between cell populations are not always easy to define. This allows for an element of subjectivity in gating strategies and in the subsequent interpretation of results. Use of controls such as an isotype control and/or fluorescence minus one (FMO) aid in identification of positive cell staining and minimize interpretive subjectivity. Unfortunately, due to patient lymphopenia, there were insufficient cell numbers to perform an FMO. Therefore, each patient served as their own control with an unstimulated ICCS performed as a negative control. Furthermore, in this study a single individual specially trained to operate a flow cytometer performed all experiments and analyzed all data, helping to minimize intra-operator variability.
An additional limitation worthy of discussion is the small sample size. While HDIL-2 continues to be used in oncologic practice today, there are only a few centers in the country equipped to manage this treatment regimen. Furthermore, few patients are candidates for HDIL-2 given its significant toxicity profile. Identification of those patients who are most likely to benefit from HDIL-2 may therefore afford its focused application in this patient population.
In summary, we show that complete response to HDIL-2 treatment was associated with a large increase in the frequency of IFNγ+Th17 cells and a decrease in TREG frequency. An early shift towards an inflammatory CD4+ T cell phenotype may help predict response to HDIL-2. Measurement of peripheral IFNγ+Th17:TREG ratios early in the course of treatment may identify those patients who would benefit from additional cycles of HDIL-2.
Acknowledgments
We would like to thank the clinical coordinators at Emory University Winship Cancer Institute, Cabell E. Eysmans and Susan Maio, for facilitating patient enrollment and sample collection. We also thank the Emory Transplant Center Biorepository for managing sample processing and storage. We give special thanks to the many anonymous donors for their contributions to the Winship Cancer Institute melanoma research fund.
Funding: This work was supported by the Kennedy Seed Grant, the Winship Skin Cancer and Melanoma Fund, and NIH GM104323. Additional support was provided by an anonymous donor to establish the Surgical Oncology/Medical Research Fellow Fund within the Division of Surgical Oncology in the Emory School of Medicine Department of Surgery.
Footnotes
Conflicts of Interest: The authors declare no competing interests, financial or otherwise.
Authorship
Contribution: M.L.D. and M.L.F. contributed to study concept, design, independent review of all studies, data collection and interpretation, drafting of manuscript and manuscript revision. K.A.D., R.R.K., and D.H.L. contributed to study concept, protocol development, data interpretation, and manuscript revision. All authors reviewed and approved the manuscript.
References
- 1.Blankenstein T, et al. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12(4):307–313. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. Jama. 1994;271(12):907–13. [PubMed] [Google Scholar]
- 3.Perez-Diez A, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryczek I, et al. Endogenous IL-17 contributes to reduced tumour growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei S, et al. Endogenous IL-17, tumour growth and metastasis. Blood. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartour E. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumours in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 9.Numasaki M. Interleukin-17 promotes angiogenesis and tumour growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 10.Wang L. IL-17 can promote tumour growth through an IL-6-Stat3 signalling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 13.Phan GQ, et al. Factors Associated With Response to High-Dose Interleukin-2 in Patients With Metastatic Melanoma. Journal of Clinical Oncology. 2001;19(15):3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 14.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taube JM, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clinical Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi MR, Merlino G. The Two Faces of Interferon-γ in Cancer. Clinical Cancer Research. 2011;17(19):6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maraskovsky E, Chen WF, Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. The Journal of Immunology. 1989;143(4):1210–4. [PubMed] [Google Scholar]
- 19.Gajewski TF, Joyce J, Fitch FW. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. The Journal of Immunology. 1989;143(1):15–22. [PubMed] [Google Scholar]
- 20.Kryczek I, et al. Cutting Edge: Th17 and Regulatory T Cell Dynamics and the Regulation by IL-2 in the Tumor Microenvironment. The Journal of Immunology. 2007;178(11):6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 21.Long M, Adler AJ. Cutting Edge: Paracrine, but Not Autocrine, IL-2 Signaling Is Sustained during Early Antiviral CD4 T Cell Response. The Journal of Immunology. 2006;177(7):4257–4261. doi: 10.4049/jimmunol.177.7.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muirhead KA, Horan PK, Poste G. Flow Cytometry: Present and Future. Nat Biotech. 1985;3(4):337–356. [Google Scholar]


