Abstract
Fish are good indicators of aquatic environment pollution because of their capability to uptake pollutants contained in water. Therefore, accumulation of pharmaceutical compounds in freshwater and marine fish and other aquatic organisms has been studied extensively in the last decade. In this context, the present study investigates the occurrence of pharmaceutical compounds in wild fish from 25 polluted river sites in the USA, downstream from wastewater treatment plants (WWTPs). Sample sites constitute a subset of urban rivers investigated in the U.S. EPA’s 2008–2009 National Rivers and Streams Assessment. Thirteen pharmaceuticals (out of the twenty compounds analyzed) were quantified in fish fillets at concentrations commonly below 10 ng g−1, in accordance with the findings from previous studies in the USA and Europe. The psychoactive drugs venlafaxine, carbamazepine and its metabolite 2-hydroxy carbamazepine were the most prevalent compounds (58%, 27% and 42%, respectively). This group of drugs is highly prescribed and rather resistant to degradation during conventional treatment in WWTPs as well as in natural aquatic environments. Salbutamol, a drug used to treat asthma, and the diuretic hydrochlorothiazide were also frequently detected (in >20% of the samples). Occurrence of six pharmaceutical families due to chronic exposure at environmental concentrations in water was detected in eight fish species.
Keywords: Pharmaceuticals, Fish, Accumulation, Trophic level
Graphical abstract
1. Introduction
Many contaminants have been detected in wastewater effluents and surface water in recent years, including pharmaceuticals. Some of these compounds are not completely removed in the wastewater treatment plants (WWTPs) (Kolpin et al., 2002; Kostich et al., 2014) and are released to the environment. Fish and other organisms downstream from WWTP effluent discharges are constantly exposed to mixtures of biologically active pharmaceuticals, which could lead to the alteration of important physiological processes, such as development, reproduction and nervous systemfunction (Liu et al., 2015). This has resulted in an increasing amount of literature exploring environmental occurrence, fate, effects, and risk assessment of these compounds. The impact of the contaminants can be evaluated by assessing selected toxicological effects or by measuring the bioaccumulation of the target compounds in the organisms. Bioaccumulation has indeed been proposed as a hazard criterion itself, since some effects may only be recognized in a later phase of life, are multigenerational effects, or manifest only in higher members of a food web (van der Oost et al., 2003). The accumulation of pharmaceutical compounds in freshwater and marine fish has been studied extensively in the last decade (Huerta et al., 2012; Rodríguez-Mozaz et al., 2017, Rodríguez-Mozaz et al., 2016).
Fish are good indicators of aquatic environment pollution because of their capability to uptake pollutants contained in water through their gills, as well as by ingestion of contaminated forage organisms. First studies were published by Brooks et al. (Brooks et al., 2005) in a single stream, and Ramirez et al. (Ramirez et al., 2009), in a series of streams heavily impacted by effluent discharges in the USA reporting concentrations of psychoactive drugs, antihistamines, and β-blockers in fish tissues. Simultaneously, the presence of psychoactive drugs in fish samples collected in Canada was reported (Chu and Metcalfe, 2007). In these studies, fish tissue concentrations were in the low ng g−1 range.
In Europe, initially most of the data on the presence of pharmaceuticals, mainly antibiotics, in aquatic organisms emerged from aquaculture and food control studies closely related to human consumption and regulation demands, where concentrations were found up to 150 ng g−1 (Berrada et al., 2008; Cháfer-Pericás et al., 2010). However, in the last few years, the interest in this subject has exponentially increased, and many reports have been published. For instance, anti-inflammatories, psychoactive drugs, and β-blockers were detected in Spain below 10 ng g−1 in fish homogenates (Huerta et al., 2013). Other countries, such as Argentina (Valdés et al., 2016), China (Liu et al., 2015; Zhao et al., 2015) or Japan (Tanoue et al., 2014), have also reported the presence of multi-class pharmaceuticals - including antibiotics, anti-inflammatories, β-blockers, synthetic hormones at low concentrations.
There are only limited data available on the effect of pharmaceuticals on aquatic organisms, although some studies have highlighted the link between the bioaccumulation and the possible biological effect of these compounds on aquatic organisms (Margiotta-Casaluci et al., 2014; Mimeault et al., 2005; Valenti et al., 2012). Logically, humans can also be exposed to the pollutants in water and food (Fedorova et al., 2014).
In this context, U.S. EPA conducted the first statistically based survey of persistent and bio-accumulative contaminants in fish designed to assess the condition of streams and rivers. Fish samples commonly consumed by humans were collected at 542 randomly selected river locations in 48 states in 2008–2009 (See website for more details https://www.epa.gov/fish-tech/studies-fish-tissue-contamination). Tissue analyses were conducted on these samples for mercury (Wathen et al., 2015), legacy organics (Batt et al., 2017), and perfluorinated compounds (Stahl et al., 2014). In addition, 182 water samples were collected from 164 targeted urban river sites and analyzed for 46 pharmaceuticals (Batt et al., 2016). The objectives of the present study were to investigate the occurrence of pharmaceutical compounds in 8 wild fish species collected from selected 26 sampling sites downstream from WWTPs (25 polluted and 1 reference site, where none of the target analytes were present).
2. Materials and methods
2.1. Standards and reagents
Chemical standards with a high purity grade (>95%) were purchased from Sigma–Aldrich in the case of atenolol carbamazepine, carazolol, citalopram, clopidogrel, codeine, diazepam, diclofenac, hydrochlorothiazide, levamisole, lorazepam, nadolol, propanolol, salbutamol, and sotalol. Sertraline and velafaxine were purchased from the European Pharmacopeia (EP), while metoprolol was acquired from the US Pharmacopeia (USP). Metabolites 2-hydroxycarbamazepine (2-HydroxyCBZ) and 10, 11-epoxycarbamazepine (10, 11-EpoxyCBZ) were purchased from Toronto Research Chemicals (TRC). Internal standards diazepam-d5, fluoxetine-d5, ibuprofen-d3, and ronidazole-d3 were purchased from Sigma–Aldrich, while atenolol-d7, carbamazepine-d10, citalopram-d4, and hydrochlorothiazide-d2 were acquired from CDN isotope, as well as antipyrine-d3 and cimetidine-d3, used as surrogate standards, and venlafaxine-d6 was acquired from TRC. All stock standards were prepared in methanol at a concentration of 1000 mg L−1 and stored at −20 °C. Fresh working standard solutions (20 and 1 mg L−1) of all compounds were prepared in methanol/water (10:90, v/v) before each analytical run.
2.2. Site selection and sample collection
This national fish survey was conducted under the framework of EPA’s National Rivers and Streams Assessment (NRSA), a probability-based survey designed to assess the condition of the Nation’s streams and rivers (https://www.epa.gov/national-aquatic-resource-surveys/national-rivers-and-streams-assessment-2008-2009-report). Sampling sites were selected using a probability-based approach (Stevens and Olsen, 2004). Field teams applied consistent methods nationwide to collect samples of fish commonly consumed by humans at 542 randomly selected river locations in the lower 48 states during June through October 2008–2009 (see website for more detailed information https://www.epa.gov/fish-tech/studies-fish-tissue-contamination). Rivers were designated as fifth order or greater based on Strahler stream order (Strahler, 1957). The 542 nationwide sites were further stratified into 164 urban river sites where at least one water sample was collected for pharmaceutical analyses. The 164 urban sites were then ranked based on the highest number of target PhACs detected (among the total of 46 pharmaceuticals analyzed in water; Fig. 1).
Fig. 1.
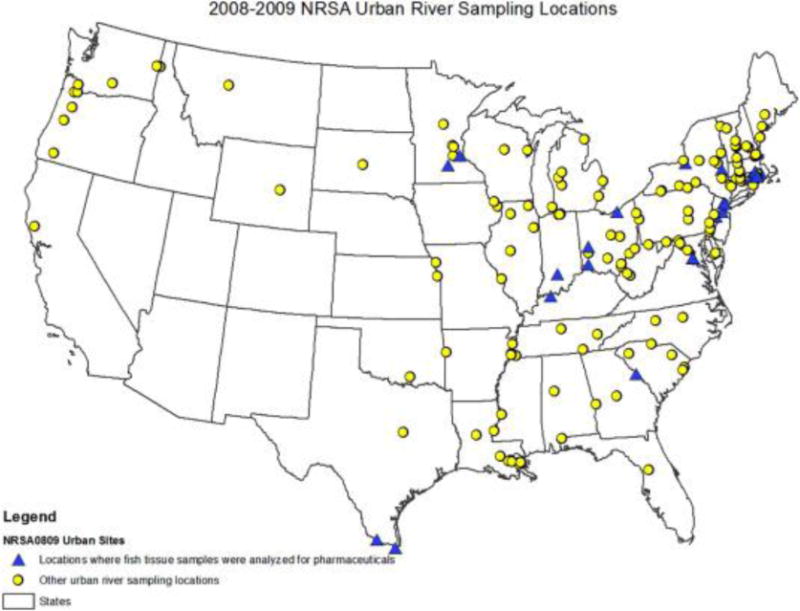
Map of sample collection locations.
Among those sites, only 25 sites were selected for analysis of fish tissue samples for 20 pharmaceuticals for this study (see Table S2 & Fig. S1 in Supporting Information). Criteria used for selecting sites for pharmaceutical analyses of fish fillets included:
The fish species collected for fish tissue analyses were selected based on a relatively broad continental U.S. distribution, and were also selected by their consumption by humans (see https://www.epa.gov/sites/production/files/2013-/documents/nrsa_field_manual_4_21_09.pdf for target species and size).
Forty-six pharmaceuticals, including 6 biologically active metabolites, were measured and reported in the water samples, but only 5 of those (sertraline, atenolol, metoprolol, carbamazepine and hydrochlorothiazide) were in common with the method applied to determine pharmaceutical presence in fish samples. The occurrence of those 5 pharmaceuticals that could be measured not only in water, but also in fish, was used to prioritize the sites for fish tissue analyses. Sites were prioritized based on the highest concentrations found for these 5 pharmaceuticals in water (see Fig. S1). One site (FW08TX038) was selected as a reference site, as only one pharmaceutical (the antibioticsulfamethoxazole) out of 45 was measured sa previous study (Batt et al., 2016).
The site had to be proximally 5 miles or less downstream from a WWTP (Wathen et al., 2015), as concentrations of pharmaceuticals are heavily linked to WWTP discharges.
Field teams used active methods, primarily electrofishing, to collect one fish composite sample that consisted of five similarly sized adult fish of the same species from each site. Selected fish species were ubiquitous, abundant, and easily identified. Largemouth and smallmouth bass were the primary species collected for the 2008–2009 National Sand Streams Assessment, accounting for 25% and 34% of all fish composites, respectively. Fish samples were shipped on dry ice to the designated sample preparation laboratory for storage until subsequent fillet tissue sample preparation and analysis. Other aspects of fish collection and handling methods are further described elsewhere (U.S. EPA, 2013).
Fish were filleted in the laboratory. Scales were removed, then lateral muscle fillets from both sides of each fish were prepared with skin on and the belly flap (ventral muscle and skin) attached (US-EPA, 2000). Fillet composites were homogenized using a tissue grinder (Batt et al., 2017; Stahl et al., 2014). Fillet tissue composite samples for this study were freeze-dried and stored at −20° C until analysis. A total of eight fish species were collected for the analysis of pharmaceuticals, including smallmouth bass (Micropterus dolomieu), largemouth bass (Micropterus salmoides), white sucker (Catostomus commersonii), yellow perch (Perca flavescens), channel catfish (Ictalurus punctatus), common snook (Centropomus undecimalis), freshwater drum (Aplodinotus grunniens), and spotted bass (Micropterus punctulatus).
2.3. Analysis of pharmaceuticals in water
Surface water samples were extracted and analyzed by Batt et al. (Batt et al., 2016) using a previously reported method (Batt et al., 2008). Briefly, 500 mL of each sample was extracted with 150 mg Oasis HLB MCX (Waters) cartridges at an unadjusted pH. Acidic and neutral analytes were eluted by acetonitrile, and basic analytes were eluted by 95% acetonitrile and 5% ammonium hydroxide into separate silanized glass tubes. Immediately before analysis, extracts were concentrated to dryness under a constant flow of nitrogen prior to reconstitution in either 20% acetonitrile (acidic and neutral analytes) or 20% methanol (basic analytes). Reconstituted extracts were analyzed by LC-MS/MS in a Waters Acquity ultra performance liquid chromatograph coupled to a Micromass Quattro Micro triple quadrupole mass spectrometer with an electrospray ionization source operated using multiple reaction monitoring. Analytes were separated on a BEH C18 column (1.0 × 100 mm, 1.7 μm).
2.4. Analysis of pharmaceuticals in fish
Fillet composite samples were treated according to the method developed by Huerta et al. (Huerta et al., 2013). Briefly, 1 g of freeze-dried sample was extracted with methanol in an automated solvent extractor system (ASE® 350, Dionex). Extracts were concentrated to 1 ml and then 250 μl was passed through a preparative column (EnviroPrep, 300 mm × 21.2 mm, 10 μm pore size) in a gel permeation chromatography system (Agilent 1260 Infinity) in tandem with a diode array detector (HPLC-DAD) for lipid removal. Fractions were collected between minutes 13.5 to 26.5 and evaporated to dryness. All fractions from each sample were collected and combined in a final purified extract in methanol/water (10:90) and analyzed by ultra-performance liquid chromatography (UPLC Acquity, Waters, Mildford, USA) coupled to a QTRAP® 5500 (AB SCIEX, Framingham, USA) with electrospray ionization (ESI), according to the method developed by Gros et al. (Gros et al., 2012). An Acquity HSS T3 with 10 mM formic acid/ammonium formate (pH 3.2) and methanol as the mobile phase at a flow rate of 0.5 mL min−1 was applied for the compounds analyzed in the positive mode, whereas an Acquity BEH C18 with acetonitrile and 5 mM ammonium acetate/ammonia (pH = 8) as mobile phase at a flow rate of 0.6 mL min−1 was used for the compounds analyzed in negative mode. Quantification was performed with a matrix-matched calibration curve together with the internal standard approach (9 labeled compounds) in the concentration range from 0.1 to 50 ng g−1. Methodological blanks were extracted and purified following the same method. One blank was extracted each 10 samples. Analytical blanks were injected together with the samples each 5 injections. Method detection limits (MDL) were in the range of 0.03–0.50 ng g−1.
2.5. Calculation of bioaccumulation factors
Bioaccumulation factors (BAFs, in L·kg−1) of pharmaceuticals in fish samples were estimated as the ratio between the concentration of the pharmaceutical in the fish fillet composite (μg kg−1 wet weight) divided by the concentration of the same pharmaceutical in water (μg L−1), assuming stationary state. Predicted BAFs were obtained from the linear regression proposed by Arnot and Gobas (Arnot and Gobas, 2006), using the octanol–water distribution coefficient (Dow) instead of the octanol–water partition coefficient (Kow): log BAF = 0.86 log Dow + 0.12.
3. Results & discussion
3.1. Analysis of pharmaceuticals in fish
A summary of the results are shown in Table 1 (see also Table S3 in Supporting Information). Thirteen pharmaceuticals (out of 20 analyzed) were detected in fish fillets collected from the 25 selected urban sites. In the majority of cases, measured concentrations (all in dry weight in this study) were below 10 ng g−1, as seen in previous studies (Huerta et al., 2013).
Table 1.
Concentrations (ng g−1, dry weight) of pharmaceuticals in fish fillet composites.
| Therapeutic Family | Compound | MQL | Mean Conc. # | Max. Conc. | # DetectedN | Fish Species |
|---|---|---|---|---|---|---|
| Anti-inflammatories | Diclofenac | 0.2 | 0.7 | 0.7 | 1 | White sucker |
| Codeine | 0.2 | – | – | 0 | – | |
| Antihelmintics | Levamisole | 0.1 | – | – | 0 | – |
| Antiplatelet agent | Clopidogrel | 0.1 | 7.7 | 8 | 2 | Channel catfish White sucker |
| β-Blockers | Atenolol | 0.2 | – | – | 0 | – |
| Carazolol | 0.1 | – | – | 0 | – | |
| Metoprolol | 0.7 | – | – | 0 | – | |
| Nadolol | 0.1 | 2.1 | 2.3 | 2 | Channel catfish White sucker |
|
| Propanolol | 0.3 | 0.4 | 0.4 | 1 | Smallmouth bass | |
| Sotalol | 0.9 | 21.4 | 37.5 | 2 | Smallmouth bass Yellow perch |
|
| Diuretics | Hydrochlorothiazide | 0.2 | 0.8 | 1.1 | 5 | Channel catfish Common snook Largemouth bass |
| Psychoactive drug | Carbamazepine | 0.1 | 3 | 8.2 | 4 | Smallmouth bass Largemouth bass Yellow perch White sucker |
| Citalopram | 0.4 | 1.2 | 2.4 | 3 | Smallmouth bass Yellow perch White sucker |
|
| Diazepam | 0.2 | 1.9 | 1.9 | 1 | Common snook | |
| 10,11-epoxyCBZ | 0.3 | – | – | 0 | – | |
| 2-hydroxyCBZ | 0.1 | 0.7 | 2.5 | 5 | Smallmouth bass Largemouth bass Channel catfish Freshwater drum |
|
| Lorazepam | 1.6 | – | – | 0 | – | |
| Sertraline | 1.1 | 17.1 | 17.1 | 1 | White sucker | |
| Venlafaxine | 0.1 | 4.6 | 22.9 | 13 | Smallmouth bass Largemouth bass Channel catfish Freshwater drum Common snook Spotted brass |
|
| Antiasthmatic | Salbutamol | 0.2 | 3.4 | 6.5 | 7 | White sucker Channel catfish |
Detected out of 25 samples.
Not detected.
calculated only with positive samples.
Psychoactive drugs (antiepileptics and antidepressants) were the most prevalent therapeutic family in this study, as at least one compound of this group was detected in 15 samples out of 25, and in all fish species (see table S4). Six out of eight psychoactive compounds were detected at least once. This group of drugs is highly prescribed and the consumption of these drugs increased considerably in western countries over the last decade (OECD, 2013). In fact, these compounds have been consistently detected in surface water at relatively high concentrations (Silva et al., 2015). Consequently, they have been frequently measured in tissues (brain, liver, plasma) of fish and mollusks, normally at concentrations in the low ng g−1 range (Brooks et al., 2005; Du et al., 2015; Grabicova et al., 2015; Huerta et al., 2013; Silva et al., 2015). This may be cause for concern, as many studies have reported biological alterations (e.g. reproduction, physiology, and behavior alterations in fish) induced by these compounds in aquatic organisms (Silva et al., 2015).
Many of these compounds are relatively hydrophobic (Log Kow > 3), therefore they may have the ability to accumulate (Du et al., 2015; Ramírez et al., 2007; Silva et al., 2015). Many of these drugs are weakly alkaline compounds. In waters with a high pH they appear in a neutral form, while in a low pH environment in an ionic form.
Venlafaxine was the most detected compound, in concentrations from below quantification limit to 23 ng g−1 (detection frequency: 52%). Venlafaxine is an antidepressant belonging to the class of the serotonin-norepinephrine reuptake inhibitors. This compound has been measured in wastewater effluent and in wastewater-dominated streams at very high concentrations (>1000 ng L−1) in several studies carried out in the USA (Schultz et al., 2010; Schultz and Furlong, 2008). Venlafaxine values found in this study were higher than those found in wild fish from Iberian rivers (Huerta et al., 2013) and from streams in the USA (Schultz et al., 2010) and similar to those detected in caged fishes exposed to effluent from a WWTP (21 ng g−1) (Grabicova et al., 2014). This compound has also been detected previously in marine mollusks at similar (Álvarez-Muñoz et al., 2015) or lower concentrations (up to 3 ng g−1) in samples collected from the Mediterranean (Martínez Bueno et al., 2014, Martínez Bueno et al., 2013; Moreno-González et al., 2016).
The second most detected psychoactive drug was carbamazepine, which was detected at concentrations up to 8 ng g−1, together with its metabolite 2-hydroxy-carbamazepine, which was detected at lower concentrations (up to 2.5 ng g−1) (detection frequencies were 16% and 20%, respectively). Several studies have previously detected carbamazepine and its metabolites 2-hydroxy-carbamazepine and EPO-Carbamazepine in wild fish (Huerta et al., 2013; Moreno-González et al., 2016; Valdés et al., 2014) at concentrations (in dry weight) similar to this study. Carbamazepine, primarily used for the treatment of epilepsy, is excreted by humans in its unaltered form along with several metabolites, including the metabolite 2-OH-CBZ (Zhang et al., 2008). Both compounds have been found in wastewaters at 219 and 370 ng L−1 for carbamazepine and 2-OH-CBZ, respectively (Snip et al., 2016), as well as in surface waters at 32 and 59 ng L−1 respectively (Aymerich et al., 2016). Therefore, the presence of 2-OH-CBZ in the fish from the current study could be attributed, on one hand, to the direct uptake of the metabolite from the aquatic environment, and on the other hand, to the metabolization of carbamazepine by the organism itself as other studies suggest. Bioaccumulation and metabolization of carbamazepine in fish were studied in mesocosms experiments by Valdés et al., who detected 2-OH-CBZ and EPO-Carbamazepine in different tissues of J. multidentata(Cyprinodontiformes, Anablepidae), although at much lower concentrations than the parent compound (Valdés et al., 2014). The CBZ:2-OH-CBZ ratio obtained ranged between 14:1 and 6:1 for all the organs of J. multidentata. However, metabolization of carbamazepine seems to be species dependent, since 2-OH-CBZ was not detected in biota samples from the carbamazepine exposure studies carried out with mussels (Mytilus galloprovincialis) (Boillot et al., 2015) or zebra mussel (Daniele et al., 2017) nor in vitro studies using rainbow trout liver fractions (Connors et al., 2013).
Sotalol and nadolol were also detected in fish from a few sampling sites. These compounds belong to the β-blocker therapeutic family, a group of cardiovascular drugs, which are highly prescribed and will be even more in the future due to the great prevalence of hypertension and rapid aging of western populations (Puckowski et al., 2016). Sotalol has been detected at very low concentrations in freshwater fish and mollusks in previous studies (Álvarez-Muñoz et al., 2015; Huerta et al., 2013). Salbutamol and hydrochlorothiazide, both extremely polar and therefore, not likely to be accumulated, were detected also with relatively high prevalence. Salbutamol is widely used in the treatment of asthma, and up to 80% of it is excreted as unchanged compound (Depaolini et al., 2016). Thus, this compound has been detected in wastewater effluents at 48 ng L−1 and in surface water below 5 ng L−1 (Gros et al., 2012; Santos et al., 2013). Hydrochlorothiazide was detected at levels up to 600 ng L−1 in surface waters and 3 μg L−1 in effluent waters by Batt et al. (Batt et al., 2016). This is probably due in part to high prescription amounts for hydrochlorothiazide, but also due to its reduced metabolism (for instance, excretion rate as unchanged form is in the range of 80–99%) (Stankiewicz et al., 2015). Both, salbutamol and hydrochlorothiazide, were also previously detected in fish samples at very low concentrations (<20 ng g−1, dry weight) (Huerta et al., 2013; Moreno-González et al., 2016; Valdés et al., 2016).
3.2. Bioaccumulation factors
The calculation of bioaccumulation factors for each particular compound can help to assess its distribution in environmental compartments (biota and water). Bioaccumulation of two pharmaceuticals (hydrochlorothiazide and carbamazepine), among the five compounds analyzed both in water and fish analysis, was assessed. Only these two compounds were present in more than one fish sample, as sertraline was measured in fish only in one sample. Bioaccumulation factors (BAFs) were calculated as the ratio between the concentration of the pharmaceutical in the fish sample divided by the concentration of the same pharmaceutical in water (water concentrations were obtained from the study previously published by Batt et al. (Batt et al., 2016)). Results are presented in Table 2. BAFs were calculated collating data from 4 sampling sites where both water and fish data were available. In both cases, the observed mean BAF was lower (carbamazepine) or higher (hydrochlorothiazide) than the predicted BAF. This is often the case, as changes in pharmaceutical concentrations in river water over time may control effective exposure of fish during their lifespan. Location and time may affect the concentration of a pollutant in water, as well as the mobility of organisms. Thus, a relative disparity between predicted and observed BAFs is expected, and particularly in this case where only a few data points were viable for BAFs calculation due to the limitations of this study (only 5 compounds measured both in water and fish). Other known factors explaining variation in BAFs are, for instance, differences in lipid content among collected fish (higher lipid contents imply a higher capacity to store hydrophobic compounds), size (larger-bodied animals have slower elimination rates) or life stage (age reflects organism effective exposure under pseudo-persistent conditions) (Ruhi et al., 2015). However, lipid normalization has been proved to be ineffective to explain bioaccumulation of pharmaceuticals, in contrast with other organic contaminants (e.g. polychlorinated biphenyls (PCBs), dioxins, furans) (Du et al., 2014; Ramirez et al., 2009).
Table 2.
Calculation of BAFs of two selected compounds.
| Carbamazepine | Fish Species | Hydrochlorothiazide | Fish Species | |
|---|---|---|---|---|
| log P | 2.8 | – | −0.6 | – |
| log Dow (pH 7) | 2.8 | – | −0.01 | – |
| Max fish conc. (ng g−1) | 7.4 | – | 8.4 | – |
| Mean water conc. (ng L−1)a | 97.6 | – | 55.9 | – |
| Mean fish conc. (ng g−1) | 3.0 | – | 0.8 | – |
| Mean BAF (L kg−1) | 49 | – | 20 | – |
| Predicted BAFb | 318 | – | 0.4 | – |
| Measured BAFs (L kg−1)c | 4.3 ± 0.2 | Largemouth bass | 4.5 ± 0.5 | Largemouth bass |
| 5.1 ± 0.2 | White sucker | 9.7 ± 1.2 | Channel catfish | |
| 11.3 ± 0.4 | Yellow perch | 12.5 ± 1.5 | Channel catfish | |
| 90.9 ± 3.6 | Smallmouth bass | 16.7 ± 2.0 | Common snook |
As published by Batt et al. (Batt et al., 2016).
Log BAF = 0.86 log Dow + 0.12.
Concentration ± analytical standard deviation.
The bioaccumulation potential is often based on the log Kow (or log P), as it was in the case of the predictive model applied (Arnot and Gobas, 2006). However, when this approach is used with pharmaceuticals, which are ionizable, it forgoes the interactions of charged compounds with the heterogeneous membrane system, which contain polar and charged phospholipids and membrane proteins (Ismail et al., 2014; Puckowski et al., 2016). In this study, however, carbamazepine and hydrochlorothiazide were not ionized, so this approach was considered accurate for this particular case.
In summary, relatively low (when compared with other organic compounds) BAFs values for carbamazepine were calculated in this study, in agreement with previous reports in snails (BAF: 3.2) (Du et al., 2015), crustaceans (BCF: 12.6) (Vernouillet et al., 2010) and fish (BCF: 0.5–9) (Garcia et al., 2012; Tanoue et al., 2015; Valdés et al., 2016) both in field and laboratory studies.
3.3. Trophic niche and accumulation
A total of eight fish species were collected for the analysis of pharmaceuticals, including four carnivores (largemouth bass, smallmouth bass, common snook, spotted bass), two omnivores (white sucker and channel catfish), and two invertivores (yellow perch and freshwater drum). Fig. 2 represents the average accumulation of the measured pharmaceutical groups according to their feeding habits. Among them, channel catfish, white sucker, and smallmouth bass were the ones with a higher number of compounds detected (≥5). Smallmouth bass, channel catfish and white sucker also presented the highest average concentrations among all the species: between 4.9 and 6.3 ng g−1 (see Table S4).
Fig. 2.
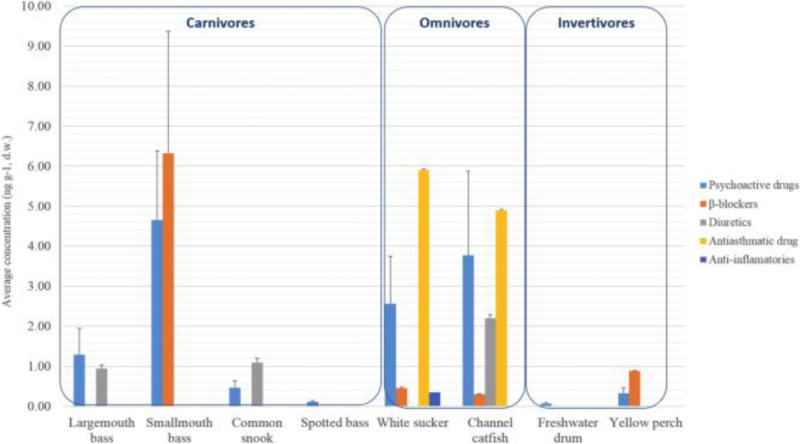
Pharmaceutical average concentration according to the feeding strategies.
There is little information about the degree of influence of the trophic niche and feeding strategy, if any, in the accumulation of pharmaceuticals in fish. In the studies published in the literature, no systematic approach to study species with similar feeding strategies or trophic niche has been found. Although previous studies have assessed concentrations of these contaminants in a variety of species, some of them can be considered comparable to the ones in the present studies. For instance, Brooks et al. (Brooks et al., 2005) detected antidepressants in channel catfish at concentrations ≤10 ng g−1 (wet weight). Silurus glanis, the European catfish, has also been found to accumulate β-blockers at low concentrations (Huerta et al., 2013). Antibiotics were detected in wild leather catfish (Clarias fuscus), with maximum concentrations up to tens of μg kg−1 (wet weight) (Zhao et al., 2015). Catfish feeding strategy is considered omnivorous and, in natural conditions, they feed on a large variety of items, including insects, plant seeds, and fish. However, most of the catfishes are carnivorous in the early beginning of their life. Afterwards, their feeding regime switches progressively to an omnivorous one (Bailey and Harrison, 1948).
In contrast, smallmouth bass (Micropterus dolomieu) is carnivorous through its lifespan, and its diet consists of crayfish, insects, and smaller fish (Poe et al., 1991). Catostomus commersonii is a catastomid (omnivore), which is probably the most broadly studied group in terms of pharmaceutical accumulation (Brooks et al., 2005; Huerta et al., 2013; Ramirez et al., 2009; Tanoue et al., 2015; Zhao et al., 2015). Catastomids feed on organisms located in bottom sediments, most commonly small invertebrates, algae and plant matter. It is still unclear why these three different species, with such diverse feeding strategies, showed more propensity to accumulate pharmaceuticals than others.
4. Conclusions
Evidence of occurrence of pharmaceuticals in fish tissues due to chronic exposure at environmental concentrations in water was established in this study. Thirteen pharmaceuticals were detected in fish fillets from urban systems, although they were measured at concentrations as low as 10 ng g−1, which probably does not represent an immediate risk for the exposed organisms. Psychoactive drugs were the most frequently detected family, with venlafaxine and carbamazepine (and its metabolites) as the most representative compounds.
Supplementary Material
Acknowledgments
The tissue analyses conducted in the present study were funded by the European Union through the European Regional Development Fund. The present study was partly supported by the Economy and Knowledge Department of the Catalan Government (Consolidated Research Group 2014 SGR 291-ICRA). S. Rodriguez-Mozaz acknowledges Ramon y Cajal research fellowship RYC-2014-16707 from the Spanish Ministry of Economy and Competitiveness. Collection of fish samples for this study was made possible by the collaborative efforts of U.S. EPA’s Office of Water and a national network of state, tribal, and federal agency partners. Disclaimer: The views expressed in this perspective are those of the authors and do not necessarily represent the views or policies of the US Environmental Protection Agency. Use of trade names does not imply endorsement of a particular product.
References
- Álvarez-Muñoz D, Rodríguez-Mozaz S, Maulvault AL, Tediosi A, Fernández-Tejedor M, Van den Heuvel F, Kotterman M, Marques A, Barceló D. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ Res. 2015;143:56–64. doi: 10.1016/j.envres.2015.09.018. Part. [DOI] [PubMed] [Google Scholar]
- Arnot JA, Gobas FA. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev. 2006;14:257–297. [Google Scholar]
- Aymerich I, Acuña V, Barceló D, García MJ, Petrovic M, Poch M, Rodriguez-Mozaz S, Rodríguez-Roda I, Sabater S, von Schiller D, Corominas L. Attenuation of pharmaceuticals and their transformation products in a waste water treatment plant and its receiving river ecosystem. Water Res. 2016;100:126–136. doi: 10.1016/j.watres.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Bailey RM, Harrison HM. Food habits of the southern channel catfish (Ictaluruslacustris punctatus) in the Des Moines River, Iowa. Trans Am Fish Soc. 1948;75:110–138. [Google Scholar]
- Batt AL, Kostich MS, Lazorchak JM. Analysis of ecologically relevant pharmaceuticals in wastewater and surface water using selective solid-phase extraction and UPLC−MS/MS. Anal Chem. 2008;80:5021–5030. doi: 10.1021/ac800066n. [DOI] [PubMed] [Google Scholar]
- Batt AL, Kincaid TM, Kostich MS, Lazorchak JM, Olsen AR. Evaluating the extent of pharmaceuticals in surface waters of the United States using a national-scale rivers and streams assessment survey. Environ Toxicol Chem. 2016;35:874–881. doi: 10.1002/etc.3161. [DOI] [PubMed] [Google Scholar]
- Batt AL, Wathen JB, Lazorchak JM, Olsen AR, Kincaid TM. Statistical survey of persistent organic pollutants: risk estimations to humans and wildlife though consumption of fish from U.S. rivers. En viron Sci Technol. 2017;51(5):3021–3031. doi: 10.1021/acs.est.6b05162. (acs.est.6b05162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrada H, Borrull F, Font G, Marcé RM. Determination of macrolide antibiotics in meat and fish using pressurized liquid extraction and liquid chromatography-mass spectrometry. J Chromatogr A. 2008;1208:83–89. doi: 10.1016/j.chroma.2008.08.107. [DOI] [PubMed] [Google Scholar]
- Boillot C, Bueno MJM, Munaron D, Le Drea UM, Mathieu O, David A, Fenet H, Casellas C, Gomez E. Science of the total environment in vivo exposure of marine mussels to carbamazepine and and met abolizati on. Sci Total En viron. 2015;532:564–570. doi: 10.1016/j.scitotenv.2015.05.067. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Kevin Chambliss C, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem. 2005;24:464. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- Cháfer-Pericás C, Maquieira Á, Puchades R, Company B, Miralles J, Moreno A. Multiresidue determination of antibiotics in aquaculture fish samples by HPLC-MS/MS. Aquac Res. 2010;41:217–225. [Google Scholar]
- Chu S, Metcalfe CD. Analysis of paroxetine, fluoxetine and norfluoxetine in fish tissues using pressurized liquid extraction, mixed mode solid phase extraction cleanup and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2007;1163:112–118. doi: 10.1016/j.chroma.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Connors K, Bowen D, Fitzsimmons P, Hoffman A, Chambliss K, Nichols J, Brooks B. Comparative pharmac eutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ Toxicol Chem. 2013;32:1810–1818. doi: 10.1002/etc.2240. [DOI] [PubMed] [Google Scholar]
- Daniele G, Fieu M, Joachim S, Bado-Nilles A, Beaudouin R, Baudoin P, James-Casas A, Andres S, Bonnard M, Bonnard I, Geffard A, Vulliet E. Determination of carbamazepine and 12 degradation products in various compartments of an out-door aquatic mesocosm by reli able analytical methods based on liquid chromatography-tandem mass spectrometry. Environ Sci Pollut Res. 2017;24:16893–16904. doi: 10.1007/s11356-017-9297-6. [DOI] [PubMed] [Google Scholar]
- Depaolini AR, Fattore E, Cappelli F, Pellegrino R, Castiglioni S, Zuccato E, Fanelli R, Davoli E. Source discrimination of drug residues in wastewater: the case of salbutamol. J Chromatogr B. 2016;1023–1024:62–67. doi: 10.1016/j.jchromb.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Du B, Haddad SP, Luek A, Scott WC, Saari GN, Kristofco LA, Connors KA, Rash C, Rasmussen JB, Chambliss CK, Brooks BW. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369:1–10. doi: 10.1098/rstb.2014.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Haddad SP, Scott WC, Chambliss CK, Brooks BW. Pharmaceutical bioaccumulation by periphyton and snails in an effluent-dependent stream during an extreme drought. Chemosphere. 2015;119:927–934. doi: 10.1016/j.chemosphere.2014.08.044. [DOI] [PubMed] [Google Scholar]
- Fedorova G, Golovko O, Randak T, Grabic R. Storage effect on the analysis of pharmaceuticals and personal care products in wastewa ter. Chemosphere. 2014;111:55–60. doi: 10.1016/j.chemosphere.2014.02.067. [DOI] [PubMed] [Google Scholar]
- Garcia SN, Foster M, Constantine LA, Huggett DB. Field and laboratory fish tissue accumulation of the anti-convulsant drug carbamazepine. Ecotoxicol Environ Saf. 2012;84:207–211. doi: 10.1016/j.ecoenv.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Grabicova K, Lindberg RH, Östman M, Grabic R, Randak T, Joakim Larsson DG, Fick J. Tissue-specific bioconcentration of antidepressants in fish exposed to effluent from a municipal sewage treatment plant. Sci Total Environ. 2014;488–489:46–50. doi: 10.1016/j.scitotenv.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Grabicova K, Grabic R, Blaha M, Kumar V, Cerveny D, Fedorova G, Randak T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015;72:145–153. doi: 10.1016/j.watres.2014.09.018. 2015. [DOI] [PubMed] [Google Scholar]
- Gros M, Rodríguez-Mozaz S, Barceló D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem. J Chromatogr A. 2012;1248:104–121. doi: 10.1016/j.chroma.2012.05.084. [DOI] [PubMed] [Google Scholar]
- Huerta B, Rodríguez-Mozaz S, Barceló D. Pharmaceuticals in biota in the aquatic environment: analytical methods and environmental implications. Anal Bioanal Chem. 2012;404:2611–2624. doi: 10.1007/s00216-012-6144-y. [DOI] [PubMed] [Google Scholar]
- Huerta B, Jakimska A, Gros M, Rodríguez-Mozaz S, Barceló D. Analysis of multi-class pharmaceuticals in fish tissues by ultra-high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2013;1288:63–72. doi: 10.1016/j.chroma.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Ismail NS, Müller CE, Morgan RR, Luthy RG. Uptake of contaminants of emerging concern by the bivalves Anodonta californiensis and Corbicula fluminea. Environ Sci Technol. 2014;48:9211–9219. doi: 10.1021/es5011576. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Batt AL, Lazorchak JM. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ Pollut. 2014;184:354–359. doi: 10.1016/j.envpol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu G, Xie Z, Zhang Z, Li S, Yan Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci Total Environ. 2015;511:54–62. doi: 10.1016/j.scitotenv.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Margiotta-Casaluci L, Owen SF, Cumming RI, De Polo A, Winter MJ, Panter GH, Rand-Weaver M, Sumpter JP. Quantitative cross-species extrapolation between humans and fish: the case of the anti-depressant fluoxetine. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Bueno MJ, Boillot C, Fenet H, Chiron S, Casellas C, Gómez E. Fast and easy extraction combined with high resolution-mass spectrometry for residue analysis of two anticonvulsants and their transformation product s in marine mussels. J Chromatogr A. 2013;1305:27–34. doi: 10.1016/j.chroma.2013.06.071. [DOI] [PubMed] [Google Scholar]
- Martínez Bueno MJ, Boillot C, Munaron D, Fenet H, Casellas C, Gómez E. Occurrence of venlafaxine residues and its metabolites in marine mussels at trace levels: development of analytical method and a monitoring program. Anal Bioanal Chem. 2014;406:601–610. doi: 10.1007/s00216-013-7477-x. [DOI] [PubMed] [Google Scholar]
- Mimeault C, Woodhouse AJ, Miao XS, Metcalfe CD, Moon TW, Trudeau VL. The human lipid regulator, gemfibrozil bioconcentrates and reduces testosterone in the goldfish, Carassius auratus. Aquat Toxicol. 2005;73:44–54. doi: 10.1016/j.aquatox.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Moreno-González R, Rodríguez-Mozaz S, Huerta B, Barceló D, León VM. Do pharmaceuticals bioaccumulate in marine molluscs and fish from a coastal lagoon? Environ Res. 2016;146:282–298. doi: 10.1016/j.envres.2016.01.001. [DOI] [PubMed] [Google Scholar]
- OECD. Health at a Glance 2013: OECD Indicators. 2013 doi: 10.1787/health_glance-2013-en. [DOI]
- van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Poe TP, Hansel HC, Vigg S, Palmer DE, Prendergast LA. Feeding of predaceous. Trans Am Fish Soc. 1991;120:405–420. [Google Scholar]
- Puckowski A, Mioduszewska K, Łukaszewicz P, Borecka M, Caban M, Maszkowska J, Stepnowski P. Bioaccumulation and analytics of pharmaceutical residues in the environment: a review. J Pharm Biomed Anal. 2016;127:232–255. doi: 10.1016/j.jpba.2016.02.049. [DOI] [PubMed] [Google Scholar]
- Ramírez AJ, Mottaleb MA, Brooks BW, Chambliss CK. Analysis of pharmaceuticals in fish using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:3155–3163. doi: 10.1021/ac062215i. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Brain R, Usenko S, Mottaleb M, O’Donnell J, Stahl L, Wathen J, Snyder B, Pitt J, Perez-Hurtado P, Dobbins L, Brooks B, Chambliss C. Occurence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ Toxicol Chem. 2009;28:2587–2597. doi: 10.1897/08-561.1. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Mozaz S, Huerta B, Barceló D. Bioaccumulation of Emerging Contaminants in Aquatic Biota: Patterns of Pharmac euticals in Mediterranean River Networks. In: Petrovic M, Sabater S, Elosegi A, Barceló D, editors. Emerging Contaminants in River Ecosystems: Occu rrence and Effects Under Multiple Stress Conditions. Springer International Publishing; Cham: 2016. pp. 121–141. [Google Scholar]
- Rodríguez-Mozaz S, Alvarez-Muñoz D, Barceló D. Pharmaceuticals in the Marine Environment: Analytical Techniques and Applications, in: Environmental Problems in Marine Biology: Methodological Aspects and Applications. Taylor & Francis; Publisher: CRC Press; Boca Raton, FL: 2017. pp. 268–316. [Google Scholar]
- Ruhi A, Acuña V, Barcelò D, Huerta B, Mor JR, Rodriguez-Mozaz S, Sabater S. Bioaccumulation and trophic ma gnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web. Sci Total Environ. 2015;540:250–259. doi: 10.1016/j.scitotenv.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Santos LHMLM, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barceló D, Montenegro MCBSM. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ. 2013;461–462:302–316. doi: 10.1016/j.scitotenv.2013.04.077. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET. Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal Chem. 2008;80:1756–1762. doi: 10.1021/ac702154e. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM. Antidepressant Pharmaceuticals in two U.S. effluent-impacted streams: occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ Sci Technol. 2010;44:1918–1925. doi: 10.1021/es9022706. [DOI] [PubMed] [Google Scholar]
- Silva LJG, Pereira AMPT, Meisel LM, Lino CM, Pena A. Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: uptake, bioaccumulation and ecotoxicology. Environ Pollut. 2015;197:127–143. doi: 10.1016/j.envpol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Snip LJP, Flores-Alsina X, Aymerich I, Rodríguez-Mozaz S, Barceló D, Plósz BG, Corominas L, Rodriguez-Roda I, Jeppsson U, Gernaey KV. Generation of synthetic influent data to perform (micro)pollutant wastewater treatment modelling studies. Sci Total Environ. 2016;569–570:278–290. doi: 10.1016/j.scitotenv.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Stahl LL, Snyder BD, Olsen AR, Kincaid TM, Wathen JB, McCarty HB. Perfluorinated compounds in fish from U.S. urban rivers and the Great Lakes. Sci Total Environ. 2014;499:185–195. doi: 10.1016/j.scitotenv.2014.07.126. [DOI] [PubMed] [Google Scholar]
- Stankiewicz A, Giebułtowicz J, Stankiewicz U, Wroczyński P, Nałęcz-Jawecki G. Determination of selected cardiovascular active compounds in environmental aquatic samples – methods and results, a review of global publications from the last 10 years. Chemosphere. 2015;138:642–656. doi: 10.1016/j.chemosphere.2015.07.056. [DOI] [PubMed] [Google Scholar]
- Stevens DL, Olsen AR. Spatially balanced sampling of natural resources. J Am Stat Assoc. 2004;99:262–278. [Google Scholar]
- Strahler AN. Quantitativ e analysis of watershed geomorphology. Trans Am Geophys Union. 1957;38:913. [Google Scholar]
- Tanoue R, Nomiyama K, Nakamura H, Hayashi T, Kim JW, Isobe T, Shinohara R, Tanabe S. Simultaneous determination of polar pharmaceuticals and personal care products in biological organs and tissues. J Chromatogr A. 2014;1355:193–205. doi: 10.1016/j.chroma.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Tanoue R, Nomiyama K, Nakamura H, Kim JW, Isobe T, Shinohara R, Kunisue T, Tanabe S. Uptake and tissue distribution of pharmaceuticals and personal care products in wild fish from treated-wastewater-impacted streams. Environ Sci Technol. 2015;49:11649–11658. doi: 10.1021/acs.est.5b02478. [DOI] [PubMed] [Google Scholar]
- US-EPA. National Rivers and Streams Assessment Field Operations Manual 2013 [Google Scholar]
- US-EPA. Fish sampling and analysis. Guid Assess Chem Contam Data Use Fish Advis. 2000;1:1–485. [Google Scholar]
- Valdés ME, Amé MV, Bistoni M, de Los A, Wunderlin DA. Occurrence and bio-accumulation of pharmaceuticals in a fish species inhabiting the Suquia River basin (Cordoba, Argentina) Sci Total Environ. 2014;472:389–396. doi: 10.1016/j.scitotenv.2013.10.124. [DOI] [PubMed] [Google Scholar]
- Valdés ME, Huerta B, Wunderlin DA, Bistoni MA, Barcelò D, Rodriguez-Mozaz S. Bioaccumulation and bioconcentration of carbamazepine and other pharmaceuticals in fish under field and controlled laboratory experiments. Evidences of carbamazepine metabolization by fish. Sci Total Environ. 2016;557–558:58–67. doi: 10.1016/j.scitotenv.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fat head minnows. Environ Sci Technol. 2012;46:2427–2435. doi: 10.1021/es204164b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernouillet G, Eullaffroy P, Lajeunesse A, Blaise C, Gagné F, Juneau P. Toxic effects and bioaccumulation of carbamazepine evaluated by biomarkers measured in organisms of different trophic levels. Chemosphere. 2010;80:1062–1068. doi: 10.1016/j.chemosphere.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Wathen JB, Lazorchak JM, Olsen AR, Batt A. A national statistical survey assessment of mercury concentrations in fillets of fish collected in the U.S. EPA national rivers and streams assessment of the continental USA. Chemosphere. 2015;122:52–61. doi: 10.1016/j.chemosphere.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Geißen SU, Gal C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere. 2008;73:1151–1161. doi: 10.1016/j.chemosphere.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Liu YS, Liu WR, Jiang YX, Su HC, Zhang QQ, Chen XW, Yang YY, Chen J, Liu SS, Pan CG, Huang GY, Ying GG. Tissue-specific bioaccumulation of human and veterinary antibiotics in bile, plasma, liver and muscle tissues of wild fish from a highly urbanized region. Environ Pollut. 2015;198:15–24. doi: 10.1016/j.envpol.2014.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


