Abstract
The use of specialized centers has been the main alternative for an appropriate diagnosis, management and follow up of patients affected by inborn errors of metabolism (IEM). These centers facilitate the training of different professionals, as well as the research at basic, translational and clinical levels. Nevertheless, few reports have described the experience of these centers and their local and/or global impact in the study of IEM. In this paper, we describe the experience of a Colombian reference center for the research, diagnosis, training and education on IEM. During the last 20 years, important advances have been achieved in the clinical knowledge of these disorders, as well as in the local availability of several diagnosis tests. Organic acidurias have been the most frequently detected diseases, followed by aminoacidopathies and peroxisomal disorders. Research efforts have been focused in the production of recombinant proteins in microorganisms towards the development of new enzyme replacement therapies, the design of gene therapy vectors and the use of bioinformatics tools for the understanding of IEM. In addition, this center has participated in the education and training of a large number professionals at different levels, which has contributed to increase the knowledge and divulgation of these disorders along the country. Noteworthy, in close collaboration with patient advocacy groups, we have participated in the discussion and construction of initiatives for the inclusion of diagnosis tests and treatments in the health system.
Keywords: Inborn errors of metabolism, Colombia, Latin America, Research, Education, Diagnosis, Training, Rare diseases
Background
Inborn errors of metabolism (IEM) is a group of monogenic diseases with low frequency that constitute a challenge for health professionals due to their clinical, genetic and biochemical heterogeneity. In fact, to assure an appropriate diagnosis and management, it is ideal to have specialized centers that bring together technical and professional resources. The main work models for this kind of centers are those developed by the European Union where many countries articulate to optimize resources in benefit of the patient [1–3]. These centers facilitate patient’s care and follow up, as well as knowledge sharing among different professionals, which is particularly useful in the cases of very rare diseases and difficult clinical cases. In addition, this system facilitates clinical and basic research and allows training programs for professionals within and outside the network [1–3].
In Latin America, diagnosis and research in IEM, and other rare diseases, is widely affected by variant and complicated economic, politic, geographic and social context of this region. In fact, economic and technical resources are not equally distributed among countries. Furthermore, most of Latin American countries are considered developing countries, which implies that economical resources must be invested to overcome social and health challenges such as malnutrition, access and quality of public services, basic education, unemployment and international external debts, among others [4]. In addition, difficulties in geographical access to some areas and border policies limit communication and collaboration among specialized centers in some cases. Moreover, the tropical location of most countries establishes infectious diseases as a health priority [4]. All these circumstances greatly vary among countries within the region requiring individual national efforts that have proven successful in different areas, such as the establishment of full coverage newborn screening (NBS) for small molecule diseases in countries like Costa Rica, Cuba and Uruguay; and the development of clinical and research specialized centers in Brazil, Argentina and Mexico [5–7]. In 1996 it was created the Latin American Society for Inborn Errors and Neonatal Screening (SLEIMPN) allowing the integration of professionals working in NBS and IEM along Latin American countries, the interchange and cooperation between members, and the training and education of health and non-health professionals. In addition, this organization has promoted the development of reference centers and the necessity to implement quality control standards for diagnosis and NBS tests.
Colombia is a developing country of around 50 million inhabitants with a complex geography, located near the equator in the north west of South America. Health system coverage reaches more than 97% of the population, but quality and sustainability are challenges to be faced. In general, IEM and rare diseases were practically absent from health programs and policies until about 10 years ago. An ambitious law in favor of orphan diseases was issued in 2010 but its implementation is advancing slowly. The current situation is that Colombia is one of the countries in Latin America with the biggest number of patients being treated with enzyme replacement therapy and with a very rapid increase in diagnosis and treatment of organic acidemias, aminoacidopathies and neurological diseases. In terms of NBS only hypothyroidism is actively searched as part of a national funded program, while the IEM are mainly diagnosed after clinical onset. On the other hand, the research in IEM is led by academic institutions [6, 8, 9]. However, while the current trends and debates on diagnosis, treatment and research for IEM in developed countries are widely published (e.g. NBS, gene therapy, international collaboration, among others), limited information is found about these topics in developing countries. In this article, we describe 20 years of experience in diagnoses, research, training, education and social advocacy in IEM in Colombia from a reference center (Instituto de Errores Innatos del Metabolismo -IEIM-, Pontificia Universidad Javeriana, Bogotá D.C.). It is important to note that there are other centers in Colombia working in diagnosis and research of IEM such as Centro de Investigaciones en Bioquímica (Universidad de los Andes, Bogotá D.C.), Instituto de Genética Humana (Pontificia Universidad Javeriana, Bogotá D.C.), Instituto de Genética (Universidad Nacional de Colombia, Bogotá D.C.), Centro de Investigaciones en Anomalías Congénitas y Enfermedades Raras (Universidad Icesi, Cali), and Grupo de Medicina Genómica y Metabolismo (Fundación Cardiovascular de Colombia, Floridablanca, Santander).
We consider that the information presented in this review will contribute to the knowledge of a broad spectrum of the situation of IEM in the context of a country without NBS and where metabolic testing is mainly performed by private institutions. In addition, we consider that this kind of reports will encourage other laboratories to share their experiences, which might facilitate the identification of common strategies and challenges from closer scenarios and contribute to the development of public policies and the consolidation of new reference centers.
Biochemical diagnosis
Biochemical testing for diagnosis of IEM
Since the establishment of the IEIM a primary goal has been to provide biochemical tools to improve the diagnosis of IEM in Colombia. This center was among the first institutions in the country offering diagnostic tests for IEM. Twenty years ago, the diagnostic service began offering qualitative tests for diagnosis of aminoacidopathies and defects of monosaccharides metabolism, as well as enzymatic assays for some lysosomal storage diseases (LSD). Nowadays, the diagnostic service offers tests that include the biochemical confirmation of aminoacidopathies (amino acids quantitation by high performance liquid chromatography, HPLC), organic acidurias (gas chromatography–mass spectrometry, GC-MS), most common LSD and neurodegenerative diseases, among others [10–18]. Summed to the increase in the tests offered, it has been observed that the number of tests processed have increased from 1203 samples in 2007 to 5915 in 2017 (Fig. 1a). This behavior has been mainly influenced by the improvement in the clinician’s knowledge about these disorders and the local availability of confirmatory tests. It is also important to consider the availability of specific treatment for several disorders, which in most of the cases can change the natural course of the disease (e.g. phenylketonuria, propionic aciduria, isovaleric aciduria, among others).
Fig. 1.
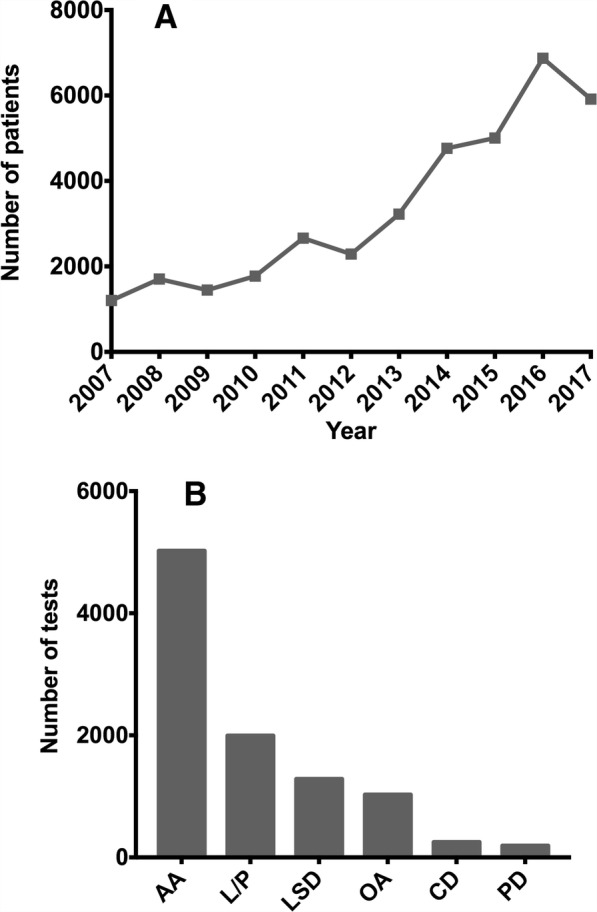
Diagnostic tests processed in the reference center. a Number of patient samples analyzed in the last 10 years. b Distribution of requested tests according to clinical suspicion in the last 10 years. AA: aminoacidopathies; OA: organic acidurias; LSD: lysosomal storage disorders; CD: carbohydrate disorders; PD: peroxisomal diseases (Total tests = 9772)
The above-mentioned circumstances constitute an important progress in the diagnosis of IEM in Colombia, since currently the diagnosis is carried out under clinical suspicion in high-risk population. In fact, in Colombia NBS is performed only for congenital hypothyroidism, while for other metabolic disorders it is available on demand and generally performed outside the country, limiting the accessibility to such biochemical tools. Recently, some private institutions are performing screening tests for diagnose amino acids and organic acidurias through tandem mass spectrometry. Nevertheless, these diagnostics analyzes are carried out selectively, which limits the generation of relevant epidemiological data [19, 20]. In the case of small molecule disorders (i.e. aminoacidophaties, organic acidurias and galactosemia, among others), this situation contrasts with that observed in developed countries where NBS has been stablished since 60’s [5]. In Latin America, different schemes of NBS are available depending on the country. For instance, Costa Rica has universal expanded NBS with high population coverage [5, 6, 21–23], while Uruguay, Cuba, Brazil, Chile and Mexico have selective NBS for common treatable IEM (i.e. phenylketonuria, biotinidase deficiency, galactosemia, maple syrup urine disease -MSUD-, and congenital adrenal hyperplasia, among others) and some of them have regional or private access to expanded NBS [6, 21, 24–29]. Screening of IEM in other Latin American countries is not universal and it is mainly provided by private institutions [6, 21, 30–33].
Currently, our center performs the analyses for aminoacidopathies, organic acidurias (OA), carbohydrates disorders, lysosomal storage disorders, and peroxisomal diseases (Fig. 1b). Additionally, we performed other supporting tests like lactic-pyruvic ratio and glucose-6-phosphate dehydrogenase, which together correspond to 21% of the total processed samples. In the period of 2007 to 2017, 36,858 samples were processed, being those related with aminoacidopathies diagnosis the most frequently requested tests (Fig. 1b). It is difficult to compare this data with other experiences, due to limited available reports from countries with circumstances similar to those observed in Colombia. For instance, evidence from middle east countries and Brazil cannot be directly compared, since those countries perform diagnosis panels (including different biochemical tests for a group of diseases) to any patient fulfilling certain clinical criteria suggestive of an IEM [34–39]. In contrast, other experience, like Lebanese, Indian, Cuban and Brazilian ones, report findings of targeted screening in high-risk population [40–42]. To the best of our knowledge, the closest experience published so far was made by an Egyptian group that reported a similar pattern than that observed in our center [43].
Among the diagnosed cases, OA are the most frequently detected diseases with 81 cases confirmed, corresponding to 49% of total performed diagnosis, followed by aminoacidopathies (20%) (Fig. 2a). These results contrast with the experiences reported by Brazilian groups, where aminoacidopathies represent around 20% of the diagnosed cases and OA account for less than 10%. Moreover, for those laboratories, LSD are the most frequent diagnosis representing more than 45% of total cases [44, 45]. Comparisons with other reports from Latin America is difficult considering that those reports are focused only on the diagnosis of aminoacidopathies and OA. For instance, Cornejo et al. [29] reported 63% of confirmed OA versus 37% of aminoacidopathies. However, it is important to note that PKU was not included in this report since it is detected by NBS. Besides, Ibarra-González et al. [26] found in Mexican population a similar number of cases diagnosed with OA and AA, while Queiruga in 2015 reported that AA was the most frequent group detected in Uruguay, followed by congenital hypothyroidism, congenital adrenal hyperplasia and cystic fibrosis [24]. In addition, although most of the worldwide available literature is based on NBS scenarios, it is also observed a high heterogeneity in the detected IEM depending on the studied region [24, 46–50]. This data remarks the high variability in the IEM detection capability of each country, which might be influenced by circumstances such as: 1) particular interests and experience of each center, 2) technology available for screening and confirmatory tests, 3) accessibility to testing by national or private programs, 4) timeframe of each report, and 5) genetic variations among populations.
Fig. 2.
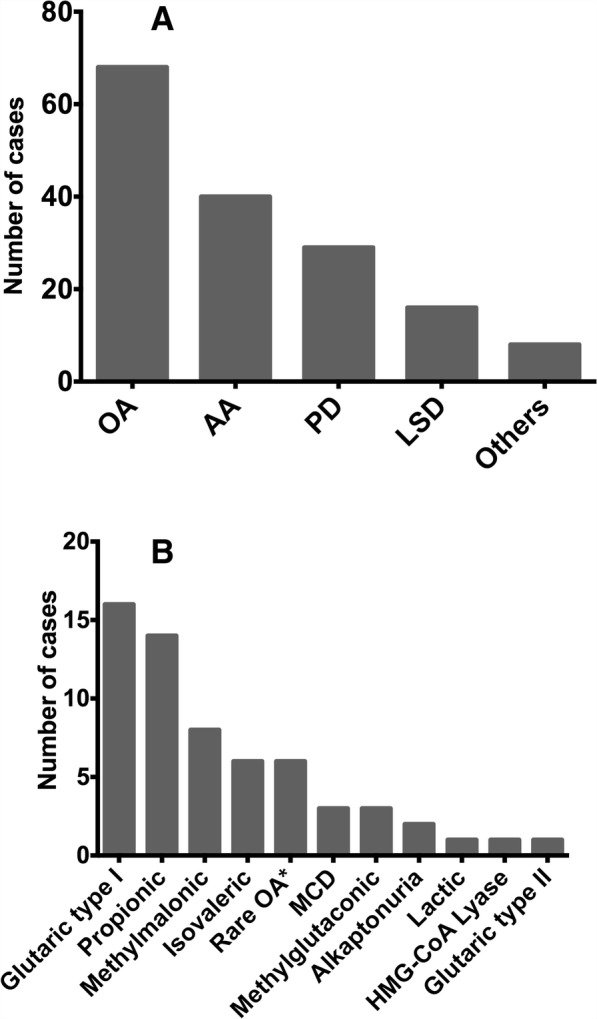
IEM detected in the reference center. a Diagnosis made according to biochemical-cellular classification of IEM. Data include diagnosis made in the period 2007–2017. b Organic acidurias diagnosed during the last 10 years. These data correspond to cases detected with typical biochemical profiles detected by GC-MS. AA: Aminoacidopathies; LSD: Lysosomal storage disorders; MCD: Multiple carboxylase deficiency; OA: Organic acidurias; PD: Peroxisomal diseases
The most frequent OA observed in our center are glutaric aciduria type I (26%) and propionic aciduria (23%) (Fig. 2b). On the other hand, methylmalonic acidurias, which have been reported as very frequent in Asian populations [40, 51], correspond only to 13% of the detected OA. In contrast, OA reported as very rare corresponded to about 8% of the identified cases (Fig. 2b), including 2-hydroxyglutaric aciduria, piroglutamic aciduria, and succinyl-CoA dehydrogenase deficiency [52]. As observed in Table 1, this behavior is similar to that reported for Chile; while reports from Brazil and Cuba show high differences in the frequency of glutaric, propionic, methylmalonic and isovaleric acidemias. Moreover, the high frequency observed for glutaric aciduria type I does not coincide with reports available from European and Asian countries [29]. These differences can be associated to the possibility that in the Colombia it is more feasible to detect chronic or progressive diseases, such as glutaric aciduria, than neonatal lethal conditions like the severe forms of propionic and isovaleric acidemias [53].
Table 1.
Organic acidemias detected in high risk population in Latin America
| Brazil [40] | Cuba [42] | Chile [29] | Colombia | |
|---|---|---|---|---|
| Time frame | 15 years | 5.5 years | 7.5 years | 9 years |
| OA diagnosed | 218 | 46 | 40 | 61 |
| Glutaric aciduria type I | 33 (14,1%) | 0 | 8 (20%) | 16 (26,2%) |
| Propionic aciduria | 18 (7,7%) | 3 (6,5%) | 11(27,5%) | 14 (22,9%) |
| Methylmalonic aciduria | 34 (14,5%) | 5 (10,8%) | 7 (17,5%) | 8 (13,1%) |
| Isovaleric acidemia | 7 (3%) | 0 | 5 (12,5%) | 6 (9,8%) |
During the period of 2007 to 2017, 40 patients with aminoacidopathies have been diagnosed, being non-ketotic hyperglycinemia (NKHG) the most frequently aminoacidopathy (Table 2). Data from aminoacidopathies detection in high risk population is scarce due to their inclusion in most of the NBS programs. Nevertheless, similar to the results observed for OA, our data differ from those reported for other countries, where PKU, MSUD and urea cycle disorders (UCD) are the most frequent entities [39, 48–50]. Such behavior may be explained by the mixed genetic background of Colombian population. In addition, it is important to highlight that since the diagnosis is done through a symptom-based approach, it is possible to miss those patients with severe phenotypes that lead to early patient death, as it is the case for severe phenotypes of MSUD and UCD [54, 55]. In addition, NBS studies usually do not actively look for some aminoacidopathies like NKHG [56–58].
Table 2.
Aminoacidopathies detected in the IEIM between 2007 and 2016
| No. Cases | Percentage | |
|---|---|---|
| NKHG | 13 | 32 |
| MSUD | 10 | 24 |
| UCD | 8 | 20 |
| HPA | 7 | 17 |
| Tyrosinemia | 2 | 5 |
| Homocistinuria | 1 | 2 |
In addition, our center offers some biochemical tools for other groups of IEM. In this context, in the last 10 years we have diagnosed 16 LSD, mainly mucopolysaccharidoses (MPS) and sphingolipidosis (gangliosidosis GM1 and GM2). In addition, by very long chain fatty acid (VLCFA) analyses we have been able to detect 29 patients with peroxisomal disorders, most of them (93%) corresponding to X-linked adrenoleukodystrophy (X-ALD).
Currently, the diagnostic area is in continuous development, with several challenges that has to be faced. On one hand, as a referral laboratory receiving samples from all over the country, it is difficult to keep track of the clinical histories of the patients and have personal contact with clinicians. Such contact is essential for a adequate following up of the patients, as well as for enhancement of the diagnostic process in complex cases. On the other hand, we are in a constant improvement of the technical and professional resources. Therefore, we look forward to improve the technical tools available for other group of disorders, such as LSD and mitochondrial disorders.
Interdisciplinary work
Complementary to the work performed in the biochemical diagnosis area, an important work has been done in the construction and consolidation of interinstitutional groups for the discussion and analysis of clinical cases of difficult diagnosis. Within these groups, it has been observed the valuable participation of biochemists, since they offer an important support for the interpretation and clinical correlation of the biochemical diagnostic tests. These types of alliances were established initially with one institution, which progress to at least once meeting per month in three different clinical centers. Finally, as a result of the continuous search to improve the diagnosis of IEM patients, the first inborn errors of metabolism clinic in Colombia was recently funded. This clinic was the first multidisciplinary approach developed in Colombia for the diagnosis and integral management of patients with IEM. During the last 3 years, this initiative has consolidated an important group of physicians from different medical specialties (i.e. child neurologist, geneticist, pediatrician, and child endocrinologist), nutritionists, psychologist and biochemists. This initiative is recognized as one of the reference groups for diagnosis and integral follow up for patients with diagnosis or suspicion of IEM. In addition, the model of attention is being used by other centers to organize similar groups along the country. Currently, the efforts are focused on increasing the time for patient attention and to improve the administrative and clinical resources offered for long term following up of patients, emergency care for critical patients, adult care and nationwide coverage.
In summary, during the last 20 years, we have worked in the consolidation of an integral center for the study of IEM. Specially, this institution has become in a reference center for the diagnosis of IEM of small molecule. Simultaneously, we have given to the medical community the possibility to improve the diagnosis, which allows an early and proper treatment, a follow-up of the patient, and the consolidation of an interdisciplinary work. These results contribute to establish the incidence of this type of entities and to extend the knowledge of the IEM in our population.
Education and training
One of the main purposes of our center has been to contribute to the clinical training and academic formation in basic, applied and clinical aspects of IEM at all education levels. Academic activities performed for undergraduate students are focused on topics related to biochemistry, IEM and biotechnology through courses mainly addressed to students of basic sciences and health related careers (Table 3). In addition, we offer the students the opportunity to get involved in research activities through internships and development of undergraduate research projects.
Table 3.
Training programs
| Program | Type | Frequency | Students Profile | Intensity (weeks) | Mean of students per year |
|---|---|---|---|---|---|
| Training in biochemical diagnosis of IEM | Internship | Permanent | Geneticists (2007) | 6/Full timea | 2 |
| Pediatric Neurologists (2005) | 8/Full timea | 3 | |||
| Pediatricians | 4/Part timeb | Occasional | |||
| Neonatologists | 4/Part timeb | 3 | |||
| Metabolic disease fundamentals | Theoretical Course | Permanent | Undergraduate students basic sciences | 4 h per week (17 weeks) | 30 |
| Health biotechnology | Theoretical Course | Permanent | Undergraduate students basic sciences | 4 h per week (17 weeks) | 100 |
| Trainig in biochemical and biotechnological tools | Internship | Permanent | Undergraduate students basic sciences | 17 weeks – Minimum 6 h per week depending on student schedule | 4 |
| Undergraduate research project | Internship | Permanent | Undergraduate students basic sciences | 17 – Part timeb | 2 |
| MSc and PhD | Academic program | Permanent | Graduate students with clinical or basic science background. | 2–4 years/Full timea | 1 |
| Young research program | Internship | On demand | Professionals from basic sciences. | 1 year /full time | 1 |
| Introduction to IEM for pediatricians. | Theoretical Course | On demand | Pediatricians | 8 h/w 3 w |
25c |
| Diploma in IEM of small molecule | Theoretical Course | On demand | Health professionals | 130 h | 23c |
aBetween 30 and 40 h per week
bAround 20 h per week
cMedia of student among the courses offered
At graduate level, academic activities on the clinical area involves the training in biochemical tools for diagnostic of IEM for healthcare professionals. Such training also involves different aspects of the biochemistry and physiopathology of these diseases. This kind of training is provided primarily to clinicians of different medical specialties including child neurologists (42%), geneticists (35%), neonatologists (21%) and pediatricians (2%). On the other hand, graduate training is offered to professionals in basic sciences that get involved in basic and applied research in different research lines that include development of new therapies, molecular biology and basic biology of IEM. In addition, we have published two handbooks about clinical aspects of inborn errors of metabolism [59, 60], as well several chapters in pediatrics textbooks [61–64].
Social advocacy in favor of the inborn errors of metabolism
Colombia is a developing country of 50 million inhabitants, with a health system comprised of a subsidized regime and a contributory regime. In this system, the contributory regime, through the mandatory payroll contributions along with the money from taxes, help to pay the health expenses of the subsidized regime. People belonging to either one system have had access to a specified package of benefits known as POS (obligatory plan of health) [65]. Although the Organization for Economic Co-operation and Development (OECD) recognized that Colombia has made great improvement’s in the health system [66]; rare diseases, and particularly IEM, were not part of the health programs neither private nor public, until very recently. These recent improvements have led to the progressive inclusion of some diagnostic test and treatments as part of the POS.
We led and participated in the organization of a The Colombian Association for Rare Diseases (ACER). The main objectives of ACER, among others, were to represent and work in favor of all rare diseases, but specially the more neglected ones. Later, ACER was included as part of a patient advocacy group (Colombian Federation of Rare Diseases – FECOER), who is currently working for the recognition of these diseases and their patients and families.
In cooperation with patient advocacy groups, we played an important role in the discussions that gave rise to the Orphan Diseases Act (Law 1392 of 2010), for which the orphan diseases are recognized of public interest and norms are adopted to guarantee the social inclusion of the patients and caregivers. This law covers important aspects for orphan diseases, such as: 1) the obligation of the government to establish a national registry of patients with rare diseases, 2) to create a system to import and distribute orphan drugs aiming to get fair access to all the patients, and 3) the establishment of specialized networks for diagnosis, treatment and orphan drugs distribution (specialized pharmacies). This Act also covers the education of human talent on these diseases in all the educational levels. This Act asks the government to stimulate research in prevention and treatment including psychological and psychiatric disorders associated with these diseases, social inclusion, and integration of patients into the society. The law is an advanced piece of legislation, unfortunately, most of the aspects have not been implemented and it has been through the tutela mechanism that Colombia has been able to advance in the diagnosis and treatment of those diseases. The tutela is a mechanism that protects the fundamental rights and speeds up the legal decisions; and that also protects persons who feels that his or her fundamental rights have been infringed.
The import of orphan drugs has been a problem still not fully solved despite of many advances in the last years. Most of orphan drugs must be imported and the process usually takes up to four to 6 months. The last few years, due to the growing number of diagnosed patients, several companies have started to commercialize these drugs in Colombia and there are now orphan products readily available in the country. In addition, we also participate in the discussions that gave rise to the Decree 481 of 2004, which regulates the processes, requirements and incentives for research, development, production, import and marketing of vital drugs not available in our country. Since many of these vital drugs were also orphan drugs, this norm represented an important contribution for the proper and timely treatment of inborn errors of metabolism.
Research
Enzyme replacement therapy (ERT) and gene therapy are part of the main alternatives for the treatment of LSD [67]. Our group have worked in the design, development, production and evaluation of proteins and vectors for both type therapies. For ERT we have reported the production and characterization of human recombinant lysosomal iduronate-2-sulfate sulfatase (IDS), N-acetylgalactosamine-6-sulfate sulfatase (GALNS), and β-N-acetylhexosaminidases (Hex-A, Hex-B, and Hex-S) in the bacteria Escherichia coli and the yeast Pichia pastoris [68–74], as well as the phenylalanine hydroxylase in Lactobacillus plantarum [75] (Table 4). In the two first expression platforms, we have evaluated different strains, vectors, and culture conditions [73, 76–80]. All recombinant proteins have shown activity levels similar or higher that those reported for native or recombinant proteins produced in other expression systems, even IDS and GALNS produced in E. coli [73, 81]. Likewise, they have shown similar pH and temperature stability profiles compared to proteins produced in mammalian cells or native proteins. In addition, proteins obtained from P. pastoris are taken up by cultured cells and delivered to the lysosome in a dose dependent manner through an endocytic pathway, possibly mediated by mannose or mannose-6-phosphate receptors [68, 69, 74], showing the potential of this host to produce therapeutic enzymes for LSD. Recombinant proteins produced in E. coli were not uptake by cell lines, which demonstrated that the absence of N-glycosylations are necessary to mediate the cellular uptake of the enzymes but not to produce active or stable lysosomal enzymes [73]. Finally, the use of a genetically modified lactic acid bacteria, as an in situ (i.e. gut) expression system to produce a recombinant phenylalanine hydroxylase (PAH) for the treatment of PKU, showed promising results in the evaluation of a new strategy to facilitate the oral administration of recombinant enzymes for the treatment of IEM [75]. This approach could be used to avoid the intravenous administration of the purified enzyme in ERT [82], improving the patient quality of life, adherence to therapy, and reducing production costs [82].
Table 4.
Summary of recombinant lysosomal enzymes produced in microorganisms
| Enzyme | Disease | Expression system | Culture condition | Enzyme activity (nmol h− 1 mg− 1) |
|---|---|---|---|---|
| Iduronate-2-sulfate sulfatase IDS (E.C. 3.1.6.13) | Hunter (MPS II) | Escherichia coli K12 JM 109 | 100 mL | 1.2 to 2.8 |
| Escherichia coli DH5α | 100 mL | 25.9 to 34.2 | ||
| Pichia pastoris GS115 | 100 mL | 4.2 | ||
| 1.65 L | 29.5 | |||
| 1.65 L (optimized gene) | 49.7 | |||
| N-acetylgalactosamine 6 sulfate sulfatase GALNS (E.C.3.1.6.4) | Morquio IVA (MPS IVA) | Escherichia coli BL21 (DE3) | 3 L | 0.067 to 0.078 |
| 3 L (improved culture conditions) | 6.8 | |||
| Purified enzyme | 2.9 | |||
| Pichia pastoris GS115 | 1.65 L | 0.02 to 0.09 | ||
| 1.65 L (coexpression of SUMF1) | 16.69 | |||
| β-N-acetylhexosaminidases Hex-A Hex-B, and Hex-S (E.C.3.2.1.52) | GM2 Gangliosidoses (Tay Sachs and Sandhoff diseases) | Pichia pastoris GS115 | 1.65 L ( AOX promoter) | Hex-A: 13,124 Hex-B: 12,779 Hex-S: 14,606 |
| Purified enzymes | Hex-A: 1.35 × 106 Hex-B: 1.27 × 106 Hex-S: 1.39 × 106 |
|||
| 1.65 L (GAP promoter) | Hex-A: 32,666 | |||
| Phenylalanine hydroxylase PAH (EC 1.14.16.1) | Phenylketonuria (PKU) | Lactobacillus plantarum CM_PUJ411 | 100 mL | Active |
In terms gene therapy, we have focused on the design and evaluation of vectors for Morquio A disease. Overall, the use of AAV-derived vectors, eukaryotic promoters (elongation factor 1α or α1-antitrypsin) and co-expression of GALNS and SUMF1 genes resulted in a significant increase in enzyme activity and secretion in patient fibroblasts or Morquio A mouse chondrocytes [83–86]. In vivo assays showed that a single intravenous administration allowed GALNS activity up to 20% of wild-type levels plasma and between 3 and 36% of wild-type levels in tissues (Table 5) [87, 88]. Additionally, the AAV vector was modified by insertion of a short acidic amino acid peptide within the viral capsid, to confer affinity of the virus for hydroxyapatite (HA), the major constituent of bone matrix [89]. This engineered vector allowed higher vector genome copies in bone, which led to enzyme activity levels in bone of 42% of those observed in wild-type animals [89].
Table 5.
Summary of gene therapy in-vivo results
| Tissue | AAV-GALNS | AAV-GALNS AAV-SUMF1 |
Bone-tagged AAV-GALNS |
|---|---|---|---|
| Plasma | 8.5 | 19.4 | NA |
| Liver | 21.9 | 36.6 | 24.3 |
| Spleen | 4.5 | 5.4 | 3.2 |
| Kidney | 3.2 | 3.1 | 1.8 |
| Lung | 4.2 | 4.1 | 3.0 |
| Heart | 6.5 | 30.6 | 61.8 |
| Brain | 4.0 | 9.1 | 35.8 |
| Bone marrow | 2.0 | 10.4 | 13.8 |
| Bone (leg) | 0.2 | 33.3 | 41.9 |
Results are expressed as percentage of wild-type levels. NA not assayed
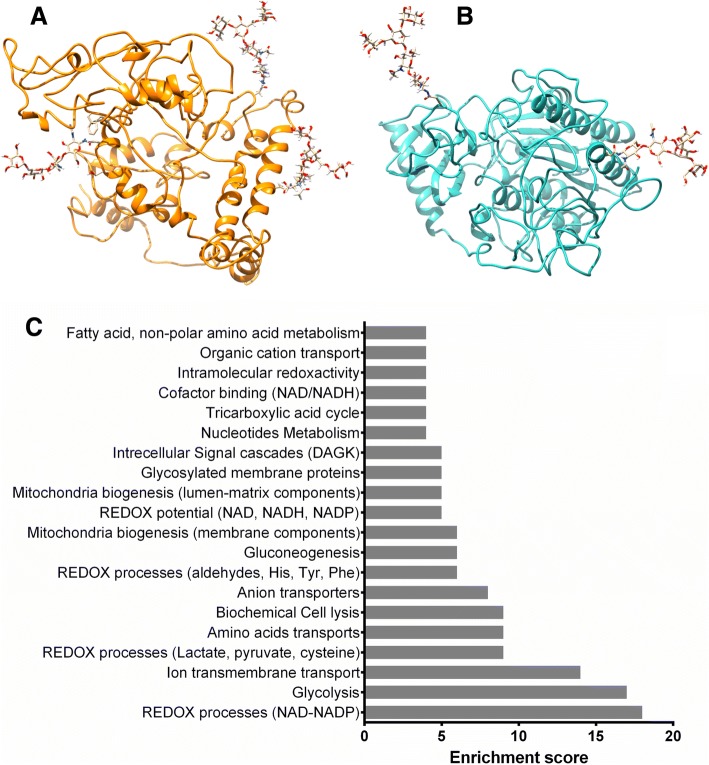
Bioinformatics studies have involved the study of phenotype-genotype correlations, evolution, modelling of metabolic alterations using an in-silico systems biology approach, and mechanical and mechanobiological mathematical models of growth plate. IDS and GALNS were modeled (Fig. 3) allowing the understanding, correlation and prediction of phenotype-genotype correlations, as well as docking and molecular dynamic modeling against natural and artificial substrates [90–92]. Structural modelling of IDS allowed the identification and design of peptides to produce chicken immunoglobulin Y (IgY) anti-IDS antibodies, which were used for the development of an ELISA test [93].
Fig. 3.
Use of bioinformatics tools for the study of IEM. Tertiary structure of human IDS (a) and GALNS (b) enzymes were modeled by protein threading based on the reported structure of other lysosomal enzymes. IDS and GALNS 3D models have been used in phenotype-genotype correlation studies as well as for the design of epitope-specific antibodies. N-glycosylations were modeled by using GlyProt at GLYCOSCIENCES.de server. c Gene enrichment analysis for impaired cellular process in MPS models, identified by a computational systems biology approach
To understand the global metabolic consequences of protein mutation, an in-silico systems biology approach was used to identify metabolic pathways impaired in each MPS [94]. The results predicted several commonly affected pathways, including oxidative stress, activation of β-oxidation, synthesis of ROS by NADH dehydrogenase, and cytochrome C oxidase, among others (Fig. 3c) [85]. A similar strategy was used to model the biochemical consequences of Arylsulfatase A (ARSA) deficiency, showing that that mitochondrial metabolism and amino acid transport c the main reactions affected in a glia cell model [95]. These findings allowed us to hypothesize that ARSA deficiency might lead to metabolic consequences that not only compromise the myelin band or the glycosphingolipids metabolism but also the overall metabolic function of the nervous system.
Finally, mechanical and mechanobiological mathematical models were formulated to develop theoretical approximations to understand the growth plate physiology and the pathological changes observed in MPS [96, 97]. The results predicted that the main factors involved in growth plate pathology are the altered cell differentiation and the changes in structure organization [98].
Conclusions and future remarks
We have described the different contributions from a reference center to the advance in the diagnosis, research, education, training and divulgation of the IEM in Colombia. Noteworthy, the continuous growth of this reference center has had a significant impact in the inclusion and recognition of this group of disorders within the Colombia’s health system. The strategy to achieve this goal has involved the continuous work in the knowledge and divulgation of the biochemical and clinical characteristics of these disorders and their treatment alternatives. The education and training of health and non-health professionals has also played an important role on the recognition of this group of disorders. One important aspect has been the collaborative work and communication between the clinical laboratory and the clinicians, which has been essential for the timely diagnosis of patients, as well as for the enhancement of the diagnostic of complex cases and the follow up of patients. Although significant improvements have been done during the last years in the diagnosis, treatment and follow up of these disorders in Colombia, future efforts should be focus in the decentralization and consolidation of specialized centers, as well as in the construction of knowledge networks, since until now the work in the field has focused on consolidation of individualized centers. In addition, we need to work in the empowerment of different patient advocacy groups, working together to achieve important goals such as the extended newborn screening program [32, 99], the transition to new omics technologies [100], and the establishment of research programs sponsored by the government.
Acknowledgments
We thank to Eugenia Espinosa, Ines Stella Morales, Leonardo Lareo, Raúl Poutou, Homero Sáenz, Patricia Landázuri, Henry Córdoba, Mónica Gutierrez, Jenny Granados, Shunji Tomatsu and Adriana Montaño, for all they collaboration and support during different stages of the Institute growth. We also thank to all our past and current students, since none of the research could been possible without them.
Funding
CJAD was supported by Pontificia Universidad Javeriana (Proposal ID 7204 and 7520) and COLCIENCIAS (Grant ID 05174, contract No. 120356933205; and Grant ID 5170, contract No. 120356933427). OYEP was supported by Pontificia Universidad Javeriana (Grant ID 4197). JMGM was supported by Pontificia Universidad Javeriana (Grant ID 5633).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AA
Aminoacidopathies
- AAV
Adeno-associated virus
- ACER
Colombian Association for Rare Diseases
- ARSA
Arylsulfatase A
- CMV
Cytomegalovirus
- ERT
Enzyme replacement therapy
- FECOER
Colombian Federation of Rare Diseases
- FGE
Formylglycine-generating enzyme
- GALC
Galactocerebrosidase
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
- GBA
Glucocerebrosidase
- GC-MS
Gas chromatography–mass spectrometry
- HA
Hydroxyapatite
- Hex
β-N-acetylhexosaminidases
- HPLC
High performance liquid chromatography
- IDS
Iduronate-2-sulfate sulfatase
- IEM
Inborn errors of metabolism
- IgY
Immunoglobulin Y
- LAB
Lactic acid bacteria
- LSD
Lysosomal storage diseases
- MPS
Mucopolysaccharidoses
- MS/MS
Tandem mass spectrometry
- MSUD
Maple syrup urine disease
- NBS
Newborn screening
- NKHG
Non-ketotic hyperglycinemia
- OA
Organic acidurias
- OECD
Organization for Economic Co-operation and Development
- PAH
Phenylalanine hydroxylase
- PKU
Phenylketonuria
- POS
Obligatory plan of health
- SLEIMPN
Latin American Society for Inborn Errors and Neonatal Screening
- SNPs
Single nucleotide polymorphisms
- SUMF1
Sulfatase modifying factor 1
- UCD
Urea cycle disorders
- VLCFA
Very long chain fatty acid
- X-ALD
X-linked adrenoleukodystrophy
Authors’ contributions
OYE, JMG, AA, NP, MR and LAB wrote the Biochemical Diagnosis and Education sections. OYE, JMG, AJEM, ARL and CJAD wrote the Research section. LAB wrote the Social Advocacy in Favor of the Inborn Errors of Metabolism section. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olga Y. Echeverri, Email: oyecheve@javeriana.edu.co
Johana M. Guevara, Email: johana.guevara@javeriana.edu.co
Ángela J. Espejo-Mojica, Email: aespejo@javeriana.edu.co
Andrea Ardila, Email: yardila@husi.org.co.
Ninna Pulido, Email: nfpulido@husi.org.co.
Magda Reyes, Email: mrreyes@husi.org.co.
Alexander Rodriguez-Lopez, Email: rodriguez.edwin@javeriana.edu.co.
Carlos J. Alméciga-Díaz, Phone: +57-1-3208320, Email: cjalmeciga@javeriana.edu.co
Luis A. Barrera, Email: abarrera@javeriana.edu.co
References
- 1.Evangelista T, Hedley V, Atalaia A, Johnson M, Lynn S, Le Cam Y, Bushby K. The context for the thematic grouping of rare diseases to facilitate the establishment of European Reference Networks. Orphanet J Rare Dis. 2016;11:17. doi: 10.1186/s13023-016-0398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayme S, Rodwell C. The European Union Committee of Experts on Rare Diseases: three productive years at the service of the rare disease community. Orphanet J Rare Dis. 2014;9:30. doi: 10.1186/1750-1172-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centres of Reference for rare diseases in Europe: State-of-the-art in 2006 and recommendations of the Rare Diseases Task Force. 2006. http://www.eucerd.eu/?post_type=document&p=1334.
- 4.Giugliani R. Inborn errors of metabolism in Latin America: challenges and opportunities. J Inherit Metab Dis. 2010;33:S315–S320. doi: 10.1007/s10545-010-9112-8. [DOI] [PubMed] [Google Scholar]
- 5.Therrell BL, Padilla CD, Loeber JG, Kneisser I, Saadallah A, Borrajo GJ, Adams J. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39:171–187. doi: 10.1053/j.semperi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Borrajo GJ. Newborn screening in Latin America at the beginning of the 21st century. J Inherit Metab Dis. 2007;30:466–481. doi: 10.1007/s10545-007-0669-9. [DOI] [PubMed] [Google Scholar]
- 7.Giugliani R, Vairo FP, Riegel M, de Souza CF, Schwartz IV, Pena SD. Rare disease landscape in Brazil: report of a successful experience in inborn errors of metabolism. Orphanet J Rare Dis. 2016;11:76. doi: 10.1186/s13023-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrera Avellaneda LA. Los errores innatos del metabolismo, fuente inagotable de conocimiento médico por más de un siglo, siguen sin diagnosticarse y tratarse en Colombia. Rev NOVA. 2005;3:13–17. [Google Scholar]
- 9.Barrera Avellaneda LA. Estudios Bioquímicos de los Errores Innatos del Metabolismo en Colombia durante dos décadas. Rev Acad Colombiana Cienc. 2009;XXXIII:377–394. [Google Scholar]
- 10.Ardila S, Echeverri OY, Guevara J, Espinosa E, Barrera LA. Tyrosinemia type I success and errors. Pediatria (Santiago) 2014;47:55–59. [Google Scholar]
- 11.Echeverri P, Espinosa E, Moser W, Peña S, Barrera A. Adrenoleucodistrofia ligada al X en ocho casos colombianos. Acta Neurol Colomb. 2005;21:299–305. [Google Scholar]
- 12.Espinosa E, Montaña M, Mera P, Echeverri O, Guevara J, Barrera L. Methylmalonic aciduria: case presentation and literature review. Rev Med. 2014;22:62–67. doi: 10.18359/rmed.1031. [DOI] [Google Scholar]
- 13.Espinosa E, Perez-poveda JC, Echeverri OY, Barrera A. Acute neuropathic (type 2) variant of Gaucher disease with mutation K198E. Rev Neurol. 2005;41:443–444. [PubMed] [Google Scholar]
- 14.Forero Sánchez E, Echeverri Peña OY, Espinosa García E, Guevara Morales JM, Barrera Avellaneda LA. Acidemia glutárica tipo 1: presentación de un caso y revisión de la literatura. Iatreia. 2015;28:193–197. doi: 10.17533/udea.iatreia.v28n2a09. [DOI] [Google Scholar]
- 15.Gomez JF, Espinosa E, Barrera L, Echeverri OY. Maple Syrup Urinaty Disease: Clincal improvement associated with earlu detection and management. Case report and literature review. Rev Med. 2008;16:99–105. [Google Scholar]
- 16.Londoño JP, Espinosa E, Echeverri Peña OY, Ardila A, Barrera A, Alejandro L. Gangliosidosis GM1: About a clinical case. Univ Med. 2015;56:366–377. [Google Scholar]
- 17.Ortiz Á, Cabarcas L, Espinosa E, Echeverri O, Guevara J, Ruiz E, Cifuentes Z, Barrera L. Glutaric Aciduria type 1. Acta Neurol Colomb. 2012;28:157–165. [Google Scholar]
- 18.Ortiz B, González C, Espinosa E, Guevara J, Echeverri OY, Barrera L. Juvenile GM1 gangliosidosis as a cause of regression in neurodevelopment: a case report. Acta Neurol Colomb. 2012;28:37–41. [Google Scholar]
- 19.Cespedes N, Valencia A, Echeverry CA, Arce-Plata MI, Colon C, Castineiras DE, Hurtado PM, Cocho JA, Herrera S, Arevalo-Herrera M. Reference values of amino acids, acylcarnitines and succinylacetone by tandem mass spectrometry for use in newborn screening in Southwest Colombia. Colomb Med (Cali) 2017;48:113–119. doi: 10.25100/cm.v48i3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermúdez FAJ, Robayo GDB. Vigilancia por laboratorio de las enfermedades crónicas: una estrategia para las enfermedades metabólicas. Nova. 2016;14:85–93. doi: 10.22490/24629448.1754. [DOI] [Google Scholar]
- 21.Gerner de Garcia B, Gaffney C, Chacon S, Gaffney M. Overview of newborn hearing screening activities in Latin America. Rev Panam Salud Publica. 2011;29:145-52. [PubMed]
- 22.de Céspedes C, Saborío M, Trejos R, Casco T. Prevención de retardo mental y otras discapacidades por tamizaje neonatal masivo en Costa Rica. 2003. [Google Scholar]
- 23.Borrajo GJC. Newborn screening for phenylketonuria:Latin American consensus guidelines. J Inborn Errors Metab Screen. 2016;4:2326409816682764. doi: 10.1177/2326409816682764. [DOI] [Google Scholar]
- 24.Queiruga G. Neonatal screening: its importance and impact in Latin America. J Int Fed Clin Chem Lab Med. 2015;26:326–331. [Google Scholar]
- 25.Larrandaburu M, Matte U, Noble A, Olivera Z, Sanseverino MT, Nacul L, Schuler-Faccini L. Ethics, genetics and public policies in Uruguay: newborn and infant screening as a paradigm. J Commun Genet. 2015;6:241–249. doi: 10.1007/s12687-015-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarra-González I, Fernández-Lainez C, Belmont-Martínez L, Guillén-López S, Monroy-Santoyo S, Vela-Amieva M. Caracterización de errores innatos del metabolismo intermediario en pacientes mexicanos. An Pediatr. 2014;80:310–316. doi: 10.1016/j.anpedi.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Aparicio-Rodriguez JM, Hurtado-Hernandez MDL. Five cases report with maple syrup disease over a period of 16 years. Metabolic screening, detection of inborn errors of metabolism at the Hospital Para El Nino Poblano, Mexico. J Asian Sci Res. 2012;2:884–892. [Google Scholar]
- 28.Torres-Sepúlveda MR, Martínez-de Villarreal LE, Esmer C, González-Alanís R, Ruiz-Herrera C, Sánchez-Peña A, Mendoza-Cruz JA, Villarreal-Pérez JZ. Tamiz metabólico neonatal por espectrometría de masas en tándem: dos años de experiencia en Nuevo León, México. Salud Pública Méx. 2008;50:200–206. doi: 10.1590/S0036-36342008000300003. [DOI] [PubMed] [Google Scholar]
- 29.Cornejo V, Raimann E, Cabello JF, Valiente A, Becerra C, Opazo M, Colombo M. Past, present and future of newborn screening in Chile. J Inherit Metab Dis. 2010;33(Suppl 3):S301–S306. doi: 10.1007/s10545-010-9165-8. [DOI] [PubMed] [Google Scholar]
- 30.Ascurra M, Caballero EG, Samudio M. Detección neonatal en el Paraguay. Brechas para su cobertura universal. Mem Inst Invest Cienc Salud. 2015;13:107-13.
- 31.De Gouveia Roche YD, Márquez Herrera CE, Carniato Pérez LA. Detección temprana de Hipotiroidismo congénito y Fenilcetonuria a través del cribado neonatal en el estado Cojedes. Arch Venez Pueric Pediatr. 2016;79:003–007. [Google Scholar]
- 32.Céspedes N, Valencia A, Echeverry CA, Arce-Plata MI, Colón C, Castiñeiras DE, Hurtado PM, Cocho JA, Herrera S, Arévalo-Herrera M. Reference values of amino acids, acylcarnitines and succinylacetone by tandem mass spectrometry for use in newborn screening in Southwest Colombia. Colomb Méd. 2017;48:113–119. doi: 10.25100/cm.v48i3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvear CC, Barboza M, Viola M, Moneriz C, Araque LM. Pilot study of hemoglobinopathies in newborns of the Rafael Calvo maternity clinic of Cartagena, Colombia. 2012. [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X, Yang L, Tong F, Yang R, Zhao Z. Screening for inborn errors of metabolism in high-risk children: a 3-year pilot study in Zhejiang Province, China. BMC Pediatr. 2012;12:18. doi: 10.1186/1471-2431-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karam PE, Habbal MZ, Mikati MA, Zaatari GE, Cortas NK, Daher RT. Diagnostic challenges of aminoacidopathies and organic acidemias in a developing country: a twelve-year experience. Clin Biochem. 2013;46:1787–1792. doi: 10.1016/j.clinbiochem.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Arif AH, Thejeal RF, Farhan A. Inborn Errors of Metabolism Status in Iraq. IOSR J Pharm Biol Sci. 2016;11:58–62. doi: 10.4103/0975-7406.171685. [DOI] [Google Scholar]
- 37.AlObaidy H. Patterns of inborn errors of metabolism: a 12 year single-center hospital-based study in Libya. Qatar Med J. 2013;2013:57–65. doi: 10.5339/qmj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Riyami S, Al Maney M, Joshi SN, Bayoumi R. Detection of inborn errors of metabolism using tandem mass spectrometry among high-risk Omani patients. Oman Med J. 2012;27:482–485. doi: 10.5001/omj.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfadhel M, Benmeakel M, Hossain MA, Al Mutairi F, Al Othaim A, Alfares AA, Al Balwi M, Alzaben A, Eyaid W. Thirteen year retrospective review of the spectrum of inborn errors of metabolism presenting in a tertiary center in Saudi Arabia. Orphanet J Rare Dis. 2016;11:126. doi: 10.1186/s13023-016-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wajner M, Coelho Dde M, Ingrassia R, de Oliveira AB, Busanello EN, Raymond K, Flores Pires R, de Souza CF, Giugliani R, Vargas CR. Selective screening for organic acidemias by urine organic acid GC-MS analysis in Brazil: fifteen-year experience. Clin Chim Acta. 2009;400:77–81. doi: 10.1016/j.cca.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Narayanan MP, Kannan V, Vinayan KP, Vasudevan DM. Diagnosis of major organic acidurias in children: two years experience at a tertiary care Centre. Indian J Clin Biochem. 2011;26:347–353. doi: 10.1007/s12291-011-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camayd Viera I, Nuevas Paz L, Alvarez AC. Diagnóstico bioquímico de acidurias orgánicas en Cuba: periodo 2008–2013. Acta Bioquím Clín Latinoam. 2015;49:209–214. [Google Scholar]
- 43.Fateen EM, Gouda AS, Ibrahim MM, Abdallah ZY. Fifteen years experience: Egyptian metabolic lab. Egypt J Med Hum Genet. 2014;15:379–385. doi: 10.1016/j.ejmhg.2014.07.002. [DOI] [Google Scholar]
- 44.Coelho JC, Wajner M, Burin MG, Vargas CR, Giugliani R. Selective screening of 10,000 high-risk Brazilian patients for the detection of inborn errors of metabolism. Eur J Pediatr. 1997;156:650–654. doi: 10.1007/s004310050685. [DOI] [PubMed] [Google Scholar]
- 45.Scalco FB, Oliveira MLC, Simonia RE, Aquino Neto FR. Inborn errors of metabolism, an important group of orphan neglected diseases: investigation of 8,000 patients in Rio de Janeiro, Brazil. J Braz Chem Soc. 2014;25:1914–1917. [Google Scholar]
- 46.Guo K, Zhou X, Chen X, Wu Y, Liu C, Kong Q. Expanded newborn screening for inborn errors of metabolism and genetic characteristics in a Chinese population. Front Genet. 2018;9:122. doi: 10.3389/fgene.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatr. 2000;105:e10. [DOI] [PubMed]
- 48.Shi XT, Cai J, Wang YY, Tu WJ, Wang WP, Gong LM, Wang DW, Ye YT, Fang SG, Jing PW. Newborn screening for inborn errors of metabolism in mainland China: 30 years of experience. JIMD Rep. 2012;6:79–83. doi: 10.1007/8904_2011_119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niu DM, Chien YH, Chiang CC, Ho HC, Hwu WL, Kao SM, Chiang SH, Kao CH, Liu TT, Chiang H, Hsiao KJ. Nationwide survey of extended newborn screening by tandem mass spectrometry in Taiwan. J Inherit Metab Dis. 2010;33:S295–S305. doi: 10.1007/s10545-010-9129-z. [DOI] [PubMed] [Google Scholar]
- 50.Dionisi-Vici C, Rizzo C, Burlina AB, Caruso U, Sabetta G, Uziel G, Abeni D. Inborn errors of metabolism in the Italian pediatric population: a national retrospective survey. J Pediatr. 2002;140:321–329. doi: 10.1067/mpd.2002.122394. [DOI] [PubMed] [Google Scholar]
- 51.Vockley J, Zschocke J, Knerr I, Vockley CW, Michael Gibson K. Branched chain organic acidurias. In: Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G, editors. The Online Metabolic and Molecular Bases of Inherited Disease. New York: The McGraw-Hill Companies, Inc.; 2014. [Google Scholar]
- 52.Echeverri OY, Guevara JM, Espinosa E, Pulido NF, Ardila A, Cabarcas L, Barrera LA. ICEIM 2013 XII International congress of inborn errors of metabolism. Barcelona: Journal of Inherited Metabolic Disease; 2013. Infrequent organic acidurias in Colombia, South America; p. S185. [Google Scholar]
- 53.Goodman SI, Frerman FE. Organic acidemias due to defects in lysine oxidation: 2-ketoadipic acidemia and glutaric acidemia. In: Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G, editors. The online metabolic and molecular bases of inherited disease. New York: The McGraw-Hill Companies, Inc.; 2014. [Google Scholar]
- 54.Gropman AL, Summar M, Leonard JV. Neurological implications of urea cycle disorders. J Inherit Metab Dis. 2007;30:865–879. doi: 10.1007/s10545-007-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogier de Baulny H, Saudubray JM. Branched-chain organic acidurias. Semin Neonatol. 2002;7:65–74. doi: 10.1053/siny.2001.0087. [DOI] [PubMed] [Google Scholar]
- 56.Tan ES, Wiley V, Carpenter K, Wilcken B. Non-ketotic hyperglycinemia is usually not detectable by tandem mass spectrometry newborn screening. Mol Genet Metab. 2007;90:446–448. doi: 10.1016/j.ymgme.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Van Hove JCCI, Scharer G. Glycine Encephalopathy In: Pagon RAAM, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington; 1993–2017. 2002 Nov 14 [Updated 2013 Jul 11].
- 58.Pitt JJ. Newborn screening. Clin Biochem Rev. 2010;31:57–68. [PMC free article] [PubMed] [Google Scholar]
- 59.Barrera Avellaneda LAEM, Angela J, Espinosa Garcia E, Echeverri Peña OY. Errores Innatos del Metabolismo. Un abordaje integral del diagnóstico al tratamiento. Bogota: Editorial Pontificia Universidad Javeriana; 2014. [Google Scholar]
- 60.Barrera LA, Saenz H, Cuellar JM, Ospina S, Garzón K, Cabrera-Salazar MA, Marquez W, Torres AL. Manual de Enfermedades Metabólicas. Bogotá: La Piragua; 2004. [Google Scholar]
- 61.Barrera L, Echeverri O, Almeciga C, Malaver L. Fundamentos de las acidemias orgánicas y desordenes del ciclo de la urea: diagnóstico y tratamiento. Parte I. Temas Pediatr. 2006;23:1-37.
- 62.Barrera L, Echeverri O, Almeciga C, Malaver L. Fundamentos de las acidemias orgánicas y desordenes del ciclo de la urea: diagnóstico y tratamiento. Parte II. Temas Pediatr. 2007;24:1-25.
- 63.Barrera L, Espinosa E, Echeverri OY. Errores Innatos del Metabolismo. In: Correa VJ, Gomez RJ, Posada SR, editors. Fundamentos de Pediatría: Genética, inmunología, alergología, reumatología, hematología, cardiología y oncología. 3. Medellin: Corporación para investigaciones biomédicas; 2006. [Google Scholar]
- 64.Barrera L, Espinosa E, Echeverri OY. Errores Innatos del Metabolismo. In: Correa VJ, Gomez RJ, Posada SR, editors. Fundamentos de Pediatría: Genética, inmunología, alergología, reumatología, hematología, cardiología y oncología, vol. 2. 4th ed. Medellín: Corporación para investigaciones biomédicas; 2012.
- 65.Gianella-Malca C. The health system of Colombia. Salud Publica Mex. 2011;53:369. doi: 10.1590/S0036-36342011000500003. [DOI] [PubMed] [Google Scholar]
- 66.Organisation for Economic Co-operation and Development (OECD) OECD Reviews of Health Systems: Colombia 2016. Paris: OECD Publishing; 2015. [Google Scholar]
- 67.Demain AL, Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv. 2009;27:297–306. doi: 10.1016/j.biotechadv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Espejo-Mojica AJ, Mosquera A, Rodríguez-López A, Díaz D, Beltrán L, Hernandez FL, Alméciga-Díaz CJ, Barrera LA. Characterization of recombinant human lysosomal beta-hexosaminidases produced in the methylotrophic yeast Pichia pastoris. Univ Sci. 2016;21:195–217. doi: 10.11144/Javeriana.SC21-3.corh. [DOI] [Google Scholar]
- 69.Rodríguez-López A, Alméciga-Díaz CJ, Sánchez J, Moreno J, Beltran L, Díaz D, Pardo A, Ramírez AM, Espejo-Mojica AJ, Pimentel L, Barrera LA. Recombinant human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) produced in the methylotrophic yeast Pichia pastoris. Sci Rep. 2016;6:29329. doi: 10.1038/srep29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poutou-Piñales RA, Córdoba-Ruíz H, Quevedo-Hidalgo BE, Landázuri P, Echeverri Peña OY, Sáenz H, Vanegas A, Acero J, González A, Herrera J, et al. Expresión de Iduronato 2-sulfato sulfatasa humana recombinante (IDShr) en Pichia pastoris. Univ Sci. 2005;10:75–96. [Google Scholar]
- 71.Landázuri P, Poutou-Piñales RA, Acero-Godoy J, Córdoba-Ruiz H, Echeverri-Peña OY, Sáenz H, Delgado J, Barrera-Avellaneda LA. Cloning and shake flask expression of hrIDS-Like in Pichia pastoris. Afr J Biotechnol. 2009;8:2871–2877. [Google Scholar]
- 72.Córdoba-Ruiz HA, Poutou-Piñales RA, Echeverri-Peña OY, Algecira-Enciso NA, Landázuri P, Sáenz H, Barrera-Avellaneda LA. Laboratory scale production of the human recombinant iduronate 2-sulfate sulfatase-Like from Pichia pastoris. Afr J Biotechnol. 2009;8:1786–1792. [Google Scholar]
- 73.Mosquera A, Rodríguez A, Soto C, Leonardi F, Espejo A, Sánchez OF, Alméciga-Díaz CJ, Barrera LA. Characterization of a recombinant N-acetylgalactosamine-6-sulfate sulfatase produced in E. coli for enzyme replacement therapy of Morquio A disease. Process Biochem. 2012;47:2097–2102. doi: 10.1016/j.procbio.2012.07.028. [DOI] [Google Scholar]
- 74.Pimentel N, Rodríguez-López A, Díaz S, Losada J, Díaz-Rincón D, Cardona C, Espejo-Mojica A, Ramírez A, Ruiz F, Landázuri P, et al. Production and characterization of a human lysosomal recombinant iduronate-2-sulfatase produced in Pichia pastoris. Biotechnol Appl Biochem. 2018; In press [DOI] [PubMed]
- 75.Ramírez AM, Rodriguez-López A, Ardila A, Beltran L, Patarroyo CA, Del Pilar Melendez A, Sánchez OF, Alméciga-Díaz CJ. Production of human recombinant phenylalanine hydroxylase in Lactobacillus plantarum for gastrointestinal delivery. Eur J Pharm Sci. 2017;109:48-55. [DOI] [PubMed]
- 76.Poutou-Piñales RA, Vanegas A, Landázuri P, Sáenz H, Lareo L, Echeverri O, Barrera LA. Human sulfatase transiently and functionally active expressed in E. coli K12. Electron J Biotechnol. 2010;13:1-10.
- 77.Morales-Álvarez ED, Rivera-Hoyos CM, Baena-Moncada AM, Landázuri P, Poutou-Piñales RA, Sáenz-Suárez H, Barrera LA, Echeverri-Peña OY. Low-scale expression and purification of an active putative iduronate 2-sulfate sulfatase-Like enzyme from Escherichia coli K12. J Microbiol. 2013;51:213–221. doi: 10.1007/s12275-013-2416-2. [DOI] [PubMed] [Google Scholar]
- 78.Rodriguez A, Espejo AJ, Hernandez A, Velasquez OL, Lizaraso LM, Cordoba HA, Sanchez OF, Almeciga-Diaz CJ, Barrera LA. Enzyme replacement therapy for Morquio A: an active recombinant N-acetylgalactosamine-6-sulfate sulfatase produced in Escherichia coli BL21. J Ind Microbiol Biotechnol. 2010;37:1193–1201. doi: 10.1007/s10295-010-0766-x. [DOI] [PubMed] [Google Scholar]
- 79.Hernandez A, Velasquez O, Leonardi F, Soto C, Rodriguez A, Lizaraso L, Mosquera A, Bohorquez J, Coronado A, Espejo A, et al. Effect of culture conditions and signal peptide on production of human recombinant N-acetylgalactosamine-6-sulfate sulfatase in Escherichia coli BL21. J Microbiol Biotechnol. 2013;23:689–698. doi: 10.4014/jmb.1211.11044. [DOI] [PubMed] [Google Scholar]
- 80.Reyes LH, Cardona C, Pimentel L, Rodriguez-Lopez A, Almeciga-Diaz CJ. Improvement in the production of the human recombinant enzyme N-acetylgalactosamine-6-sulfatase (rhGALNS) in Escherichia coli using synthetic biology approaches. Sci Rep. 2017;7:5844. doi: 10.1038/s41598-017-06367-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodríguez-López A, Díaz D, Beltrán L, Sánchez J, Moreno J, Díaz S, Pimentel N, Mosquera A, Soto C, Pardo A, et al. Production of active recombinant human sulfatases Iduronate-2-sulfate and N-acetylgalactosamine-6-sulfate in microorganisms. J Inborn Errors Metab Screen. 2014;2:76. [Google Scholar]
- 82.Kwon KC, Daniell H. Oral delivery of protein drugs bioencapsulated in plant cells. Mol Ther. 2016;24:1342–1350. doi: 10.1038/mt.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almeciga-Diaz CJ, Montano AM, Tomatsu S, Barrera LA. Adeno-associated virus gene transfer in Morquio A disease - effect of promoters and sulfatase-modifying factor 1. FEBS J. 2010;277:3608–3619. doi: 10.1111/j.1742-4658.2010.07769.x. [DOI] [PubMed] [Google Scholar]
- 84.Almeciga-Diaz CJ, Rueda-Paramo MA, Espejo AJ, Echeverri OY, Montano A, Tomatsu S, Barrera LA. Effect of elongation factor 1alpha promoter and SUMF1 over in vitro expression of N-acetylgalactosamine-6-sulfate sulfatase. Mol Biol Rep. 2009;36:1863–1870. doi: 10.1007/s11033-008-9392-3. [DOI] [PubMed] [Google Scholar]
- 85.Gutierrez MA, Garcia-Vallejo F, Tomatsu S, Ceron F, Almeciga-Diaz CJ, Dominguez MC, Barrera LA. Construction of an adenoassociated, viral derived, expression vector to correct the genetic defect in Morquio A disease. Biomedica. 2008;28:448–459. doi: 10.7705/biomedica.v28i3.85. [DOI] [PubMed] [Google Scholar]
- 86.Handley CJ, Samiric T, Ilic MZ. Structure, metabolism, and tissue roles of chondroitin sulfate proteoglycans. Adv Pharmacol. 2006;53:219–232. doi: 10.1016/S1054-3589(05)53010-2. [DOI] [PubMed] [Google Scholar]
- 87.Alméciga-Díaz C, Montaño A, Tomatsu S, Barrera LA. Contribución colombiana al conocimiento de la enfermedad de Morquio. Medicina (Mex) 2012;34:221–241. [Google Scholar]
- 88.Tomatsu S, Alméciga-Díaz C, Montaño A, Yabe H, Tanaka A, Dung VC, Giugliani R, Kubaski F, Mason RW, Yasuda E, et al. Therapies for the bone in mucopolysaccharidoses. Mol Genet Metab. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alméciga Díaz C, Montaño A, Barrera L, Tomatsu S. Tailoring the AAV2 capsid vector for bone-targeting. Pediatr Res. 2018; In press [DOI] [PMC free article] [PubMed]
- 90.Saenz H, Lareo L, Poutou RA, Sosa AC, Barrera LA. Computational prediction of the tertiary structure of the human iduronate 2-sulfate sulfatase. Biomedica. 2007;27:7–20. doi: 10.7705/biomedica.v27i1.229. [DOI] [PubMed] [Google Scholar]
- 91.Olarte-Avellaneda S, Rodríguez-López A, Alméciga-Díaz CJ, Barrera LA. Computational analysis of human N-acetylgalactosamine-6-sulfate sulfatase enzyme: an update in genotype-phenotype correlation for Morquio A. Mol Biol Rep. 2014;41:7073–7088. doi: 10.1007/s11033-014-3383-3. [DOI] [PubMed] [Google Scholar]
- 92.Olarte-Avellaneda S, Rodríguez-López A, Alméciga-Díaz CJ. In-silico Analysis of the active cavity of N-acetylgalactosamine-6-sulfate sulfatase in eight species. In: Castillo LF, Cristancho M, Isaza G, Pinzón A, Corchado JM, editors. Advances in Computational Biology. Switzerland: Springer International Publishing; 2014. p. 141–6. [Proceedings of the 2nd Colombian Congress on Computational Biology and Bioinformatics (CCBCOL)].
- 93.Sosa AC, Espejo AJ, Rodriguez EA, Lizaraso LM, Rojas A, Guevara J, Echeverri OY, Barrera LA. Development of a sandwich enzyme linked immunosorbent assay (ELISA) for the quantification of iduronate-2-sulfate sulfatase. J Immunol Methods. 2011;368:64–70. doi: 10.1016/j.jim.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 94.Salazar DA, Rodriguez-Lopez A, Herreno A, Barbosa H, Herrera J, Ardila A, Barreto GE, Gonzalez J, Almeciga-Diaz CJ. Systems biology study of mucopolysaccharidosis using a human metabolic reconstruction network. Mol Genet Metab. 2016;117:129–139. doi: 10.1016/j.ymgme.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Echeverri Olga Y, Salazar Diego A, Rodriguez-Lopez A, Janneth G, Almeciga-Diaz Carlos J, Barrera Luis A. Understanding the metabolic consequences of human arylsulfatase a deficiency through a computational systems biology study. Cent Nerv Syst Agents Med Chem. 2017;17:72-77. [PubMed]
- 96.Guevara JM, Moncayo MA, Vaca-Gonzalez JJ, Gutierrez ML, Barrera LA, Garzon-Alvarado DA. Growth plate stress distribution implications during bone development: a simple framework computational approach. Comput Methods Prog Biomed. 2015;118:59–68. doi: 10.1016/j.cmpb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Guevara JM, Moncayo MA, Vaca-Gonzalez JJ, Gutierrez ML, Barrera LA, Garzon-Alvarado DA. Exploratory analysis of growth plate mechanical environment during bone development. A computational model. In: 3th International Symposium on Mucopolysaccharidoses and Related Diseases; Bahia, Brazil: Journal of Inborn Errors of Metabolism and Screening; 2014. p. 18–19.
- 98.Guevara JM. Analysis of the biological implications of the mechanical environment within the growth plate during bone development in physiological and pathological scenarios. Pontificia Universidad Javeriana, Bogotá D.C., Colombia; 2016.
- 99.Rosselli D, Rueda JD, Ruiz-Patiño A. Análisis de costos de la tamización neonatal universal mediante espectrometría de masas en tándem para errores innatos del metabolismo en Colombia. Pediatria (Santiago) 2014;47:68–73. [Google Scholar]
- 100.Tebani A, Afonso C, Marret S, Bekri S. Omics-based strategies in precision medicine: toward a paradigm shift in inborn errors of metabolism investigations. Int J Mol Sci. 2016;17:1-27 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.