Abstract
An acute bout of physical activity and exercise can increase pain in individuals with chronic pain, but regular exercise is an effective treatment. This review will discuss these two dichotomous findings by summarizing studies in human and animal subjects. We will provide the data that supports the role of physical activity in modulating central nervous system excitability and inhibition, immune system function, and psychological constructs associated with pain. We show evidence that the sedentary condition is associated with greater excitability and less inhibition in both the central nervous system (brainstem inhibitory/facilitatory sites) and the immune system. We further show that exercise and regular physical activity decreases excitability and improves inhibition in both the central nervous system (brainstem inhibitory/facilitatory sites) and the immune system. We will then discuss the clinical implications of these findings, make recommendations for clinical application of exercise, and suggest future research directions.
Keywords: pain, exercise, analgesia, physical activity, immune system, central sensitization, opioid, central inhibition, macrophage
Introduction
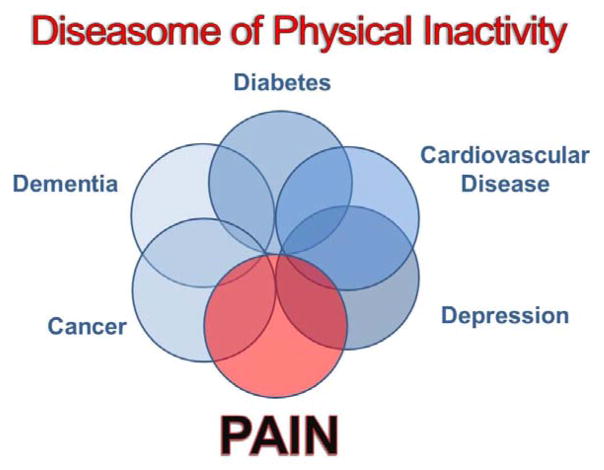
Physical inactivity or a sedentary lifestyle is a significant health concern world-wide. The Centers for Disease Control recommends 150 minutes per week of moderate to vigorous activity for health benefits [1]. World-wide the great majority of the population does not meet these physical activity guidelines. Furthermore, physical inactivity is a recognized risk factor for many conditions including cardiovascular disease, diabetes, cancer, dementia, and depression [70](Figure 1). In fact, this has been referred to as the “diseasome of physical inactivity” [70]. Physical inactivity is also a risk factor for development of pain [50–52,90]. The HUNT study performed a population based analysis of 4219 subjects and showed that those with moderate levels physical activity report less musculoskeletal pain [50,51]. Similarly, higher leisure time physical activity is associated with a lower risk of chronic pelvic pain in men [90], and those with a greater number of years of leisure physical activity decreased the risk of low back pain during pregnancy [64]. Thus, physical inactivity may be a risk factor for development of chronic pain, while physical activity reduces this risk.
Figure 1.
Diagram representing the diseasome of physical inactivity. Physical inactivity is a risk factor for development of a number of diseases including pain. Modified from [70].
Regular physical activity can be achieved through regular lifestyle activity or by structured exercise. In chronic pain, prescribed exercise is an effective treatment for most pain conditions, and use of exercise and physical therapy has long been recognized for its effectiveness in reducing disability and health care costs [40,42,85]. Despite this, an acute bout of exercise can exacerbate pain, in those with chronic pain. As an example we have shown that an upper body fatiguing exercise increases pain by 3 points on a 10 point scale in those with fibromyalgia (Figure 2A)[23], and isometric contractions in individuals with fibromyalgia show no increase in pain thresholds that normally occurs in healthy controls [41,53]. Furthermore, people with chronic pain are generally less active than age-matched healthy controls (Figure 2B,C) [20,29,56,60].
Figure 2.
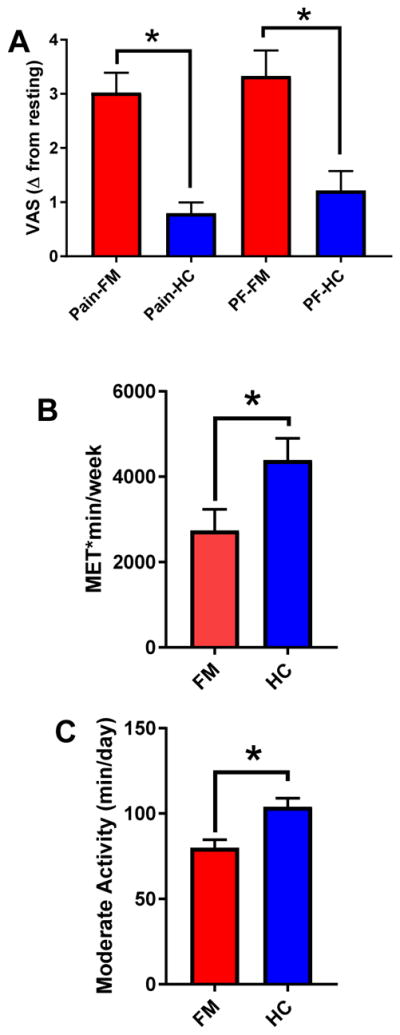
A. Graphs showing the increase in pain and physical fatigue in people with fibromyalgia compared to healthy controls after a whole body fatiguing exercise task. *, p<0.05. Data are mean ± S.E.M. Data are regraphed from [23]. FM=fibromyalgia; HC=healthy controls; PF=physical fatigue. B. Graph showing self-reported activity levels in METS*min/week for those with fibromyalgia and healthy controls. *, p<0.05. Data are mean ± S.E.M. Data are graphic representations from tables in [60]. C. Graph showing moderate physical activity levels measured by accelerometry in fibromyalgia compared to healthy controls. *, p<0.05. Data are mean ± S.E.M. Data are graphic representations from tables in [60].
Effects of exercise on the central nervous system
We propose that regular physical activity changes the state of central pain inhibitory pathways and the immune system to result in a protective effect against a peripheral insult. This normal protective state that occurs with regular physical activity is not found in physically inactive individuals and results in a greater risk for development of chronic long-lasting pain.
Figure 3 depicts two states of the nervous system for cells in the brainstem that modulate pain. Brainstem sites, like the rostral ventromedial medulla (RVM), both facilitate and inhibit nociceptive signals [37,71]. We suggest that in the sedentary condition that muscle insult results in increased phosphorylation of the NR1 subunit of the NMDA receptor, which would result in increased facilitation. There is substantial research suggesting that NMDA receptors in the RVM facilitate pain, and phosphorylation of the NMDA receptor enhances channel conductance and increases insertion of NMDA receptors into the synapse [18,21,22,27,82,87,88]. Simultaneously, we propose there is an increased expression of the serotonin transporter (SERT), which would result in reduced inhibition. Classical studies show that injection of serotonin or a SERT inhibitor into the RVM is analgesic, blockade of serotonin receptors prevents analgesia by stimulation of the PAG, and systemic morphine increases serotonin in the RVM [47,48,57,58,79]. Further, in the sedentary condition there is less opioid tone in the nervous system to prevent these excitatory effects upon peripheral nerve damage. In the physically active state we propose that activation of opioid receptors modulates neuron activity so that there is less phosphorylation of the NDMA receptor and less expression of the serotonin transporter. Basic research studies support this hypothesis. We show, in sedentary animals, that there is increased expression of the serotonin transporter and increased phosphorylation of the NR1 subunit of the NMDA receptor in the RVM in animals with nerve injury or chronic muscle pain [3,9,55]. These increases do not occur in physical active animals with nerve injury or chronic muscle pain [3,9,55]. Further blockade of opioid receptors systemically, in the PAG or the RVM prevents the protective effects of regular physical activity, and mu-opioid receptor knockouts do not develop analgesia to regular physical activity [55]. Further we show that naloxone-treated or mu-opioid receptor knockout physically active animals do not show the increases in SERT in the RVM supporting an interaction between endogenous opioids and serotonin [55]. In human studies, greater exercise-induced analgesia was associated with a gene for stronger opioid signaling (OPRM1 G) in combination with weak 5-HT tone (5-HTT low/5-HT1a G), suggesting interactions between opioid and serotonergic mechanisms for exercise-induced analgesia [83]. Thus, regular physical activity prevents hyperalgesia through activation of opioids and serotonin to produce analgesia.
Figure 3.
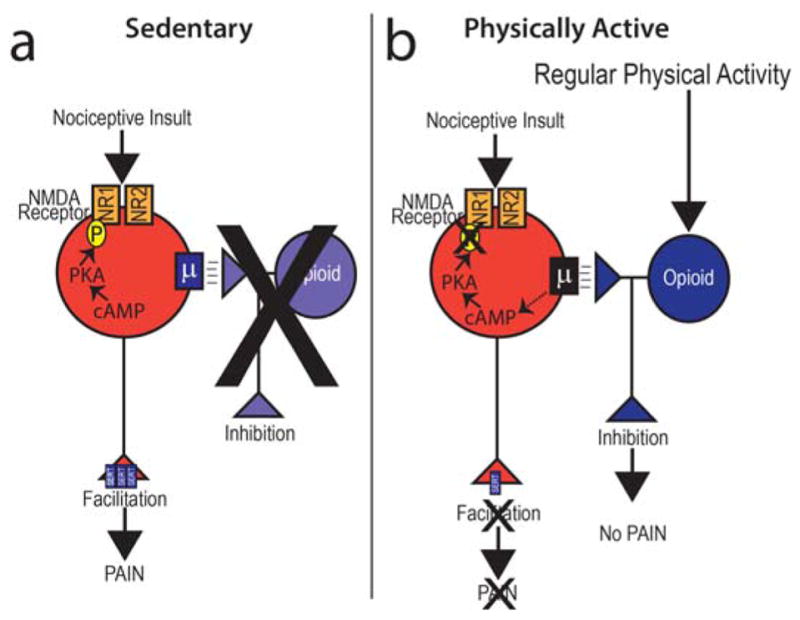
A schematic diagram representing the neurons in the brainstem, rostral ventromedial medulla, that facilitate and those that inhibit pain and how sedentary lifestyle or physical activity could modulate their activity. Based on data outlined in the text, we propose that in sedentary conditions there is less opioid tone in the brainstem and overall less inhibition. This results in the neurons showing more facilitation after nociceptive input with increases in phosphorylation of the NR1 subunit of the NMDA receptor and increased expression of the serotonin transporter (SERT). We further propose that regular physical activity increases release of endogenous opioids in the brainstem that inhibit facilitatory neurons to reduce facilitation. This would be associated with less phosphorylation of the NR1 subunit of the NMDA receptor and reduced expression of SERT. Overall, in the physically active condition there would be more inhibition from opioids and serotonin, and less excitation.
In humans, several studies have emerged suggesting greater physical activity is associated with equal or reduced pain sensitivity across a wide range of assessments. Quantitative sensory testing (QST) is increasingly used as an indirect measure of centrally mediated pain processing. Healthy individuals routinely participating in vigorous activity demonstrate enhanced conditioned pain modulation, a measure of central pain inhibition, compared to less active individuals [32,67]. In people with osteoarthritis, a 12-week exercise program increased pain thresholds and decreased temporal summation [38]. However, in one study temporal summation, a measure of central pain facilitation, to cold pain was unchanged [32] whereas temporal summation to heat pain was reduced in the other [67]. Similarly, a meta-analysis of athletes versus normally active adults indicates reduced pain sensitivity overall in athletes [80]. These studies suggest that engagement in regular physical activity is related to decreased pain sensitivity in healthy adults. However, few studies have examined associations between daily lifestyle physical activity and pain sensitivity in FM or other chronic pain populations.
Epidemiological investigations also support the protective nature of physical activity on the development of chronic pain, which may be due to peripheral or central mechanisms. A population-based study from Norway showed chronic musculoskeletal pain incidence was 10 – 38% less in individuals participating in moderate leisure-time activity one to three times per week compared to those with no leisure-time activity [50,51]. However, in patient populations the relationships between regular physical activity and central pain processing is less clear. Increasing physical activity and exercise reduces symptomology in a variety of patient population, and is a first line treatment in a number of chronic pain populations, from fibromyalgia to low back pain [59,72]. Further, non-pharmacological therapies, in general, are considered first-line treatments, with exercise having strong support [59,72]. However, QST and physical activity levels have not been routinely investigated in patient populations. In a small study of 18 women with fibromyalgia, fitness levels, as assessed by cycle ergometry or the six-minute walk test (6MWT), were not associated with pain thresholds or temporal summation assessments [25]. However, this area of study remains sparse and may be limited by the reduced range of lifestyle physical activity levels observed in many chronic pain populations.
Effects of exercise on the immune system
We also propose that regular physical activity modulates the immune system locally at the site of insult, systemically, and in the central nervous system. In the physically inactive condition there are more inflammatory cytokines and less anti-inflammatory cytokines. After regular physical activity this balance shifts to more anti-inflammatory cytokines and less inflammatory cytokines. Inflammatory cytokines activate receptors on nociceptors to produce pain while anti-inflammatory cytokines reduce activity of nociceptors to prevent pain [26,33,46,91]. Figure 4 shows our theory that physical activity levels modulate phenotype of macrophages in muscle. Macrophages are located in muscle and release inflammatory or anti-inflammatory cytokines depending on two relevant phenotypes: classically-activated (M1) macrophages release inflammatory cytokines and regulatory (M2) macrophages release anti-inflammatory cytokines [66]. In support, we show that in uninjured animals physically active animals show an increased proportion of M2 macrophages [54]. Similarly, in animals with nerve injury, sedentary animals show an increased proportion of M1 and less M2 macrophages at the site of injury, while physically active animals show increases in M2 and less M1 macrophages [4]. The analgesia produced by regular physical activity and exercise is prevented by blockade of either IL-10 (muscle insult) or IL-4 (nerve injury) [4,54]. Thus, at the peripheral site of insult there are alterations in macrophage phenotype that underlie the analgesia produced by regular exercise.
Figure 4.
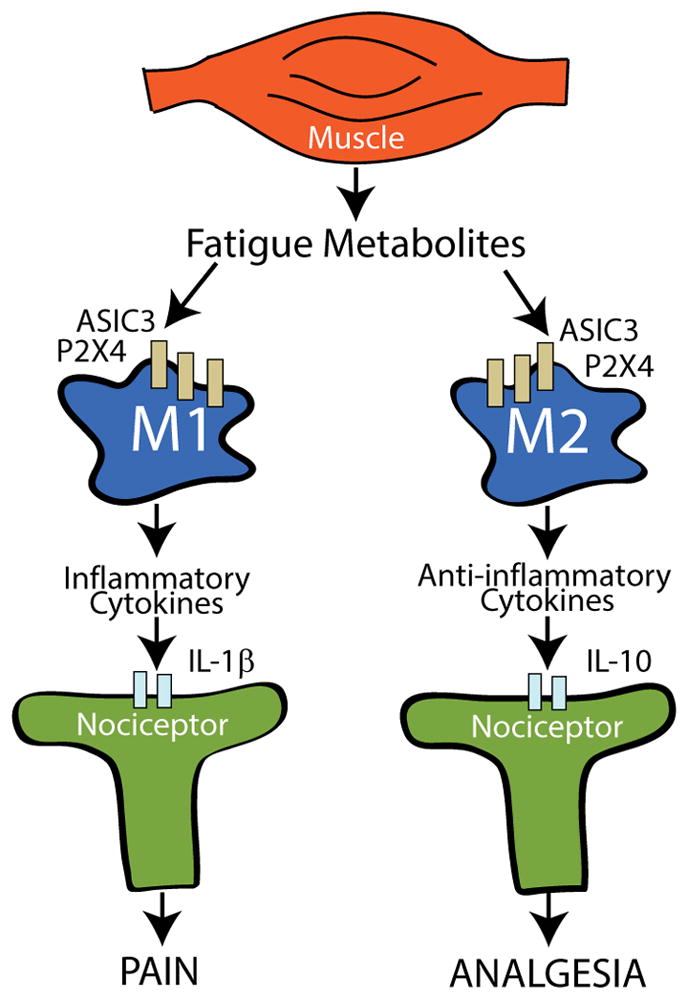
A schematic diagram representing the interaction between muscle, macrophages, and nociceptors in the peripheral nervous system. Macrophages, found in local tissue, can be polarized to and M1 phenotype that releases pro-inflammatory cytokines which activate nociceptors, or an M2 phenotype that releases anti-inflammatory cytokines which inhibit nociceptors. Our data support that there are greater M1 macrophages at the site of insult or injury in the sedentary state and that regular physical activity increases the proportion of M2 macrophages. Our data further support the notion that regular physical activity changes the state of the immune system so that there is a greater proportion of M2 macrophages and greater anti-inflammatory cytokine that mediate the analgesia of regular physical activity.
In chronic pain conditions, systemic inflammation is suggested as an underlying pathology [61,76,77]. Systemically, immune cells, i.e., peripheral blood mononuclear cells (PBMCs), are highly plastic, can alter levels of cytokines systemically or locally in tissue, and secrete inflammatory or anti-inflammatory cytokines based on their phenotype. In support, people with FM show enhanced circulating inflammatory cytokines and enhanced evoked-release of inflammatory cytokines from circulating monocytes [6,7,30,68,69]. In contrast, a 4- or 8-month aquatic exercise program for individuals with FM decreases circulating and stimulated release from monocytes of inflammatory cytokines of IL-8, IL-1β, and TNF [6,68,69]. In healthy controls, exercise-training also reduces the percentage of inflammatory monocytes in healthy men and women [81]. However, it should be noted that the number of subjects in the majority of these studies was low, and there are mixed results in the literature, likely a result of low sample size, use of mixed populations of immune cells, use of different stimuli to evoke and cytokine release from immune cells [62,77,84]. Thus, preliminary studies show that exercise can alter systemic cytokines, and reduce systemic inflammation, a proposed mechanism of chronic pain.
In the central nervous system, glia cells modulate inflammatory and anti-inflammatory cytokines, and play a significant role in a variety of pain conditions [63]. In animals with nerve injury, there is activation of glial cells, increases in inflammatory cytokines, and decreases in anti-inflammatory cytokines [4,34,63,86]. Regular physical activity and exercise reduce glial cell activation, reduce inflammatory cytokines and increase anti-inflammatory cytokines in the spinal cord dorsal horn [4,34]. Specifically, the enhanced astrocyte (GFAP) and microglial (Iba-1) immunoreactivity produced by nerve injury was significantly reduced by treadmill running [4]. In parallel decreases in the anti-inflammatory cytokines -IL-4, Il-1ra, and IL-5-induced by nerve injury are reversed by treadmill running [4]. On the other hand the increase in inflammatory cytokine IL-1beta is reduced by regular physical activity [34]. Further, there are increases in transcription factors that regulate IL-1β, NFκB and NLRP3 inflammasome, that are also reduced by regular physical activity [34]. Thus, regular exercise normalizes neuroimmune signaling in the central nervous system to prevent and reverse the development of hyperalgesia.
Effects of exercise on psychological co-morbidities
In addition to the beneficial effects of exercise on immune health, people who participate in regular physical activity typically have enhanced mental health and psychological well-being whereas individuals that are physically inactive are more likely to experience depression and anxiety. Specific to chronic pain, individuals that report low levels of physical activity are more likely to report higher kinesiophobia, fear avoidance beliefs, and pain catastrophizing compared with those that report higher physical activity levels [28,49]. However, in a cohort of patients with nonspecific LBP, fear of movement was not associated with subjective and objective (i.e., questionnaire and accelerometry, respectively) measures of physical activity [17]. Therefore, the relation between physical activity and psychological health is less clear for individuals with chronic pain.
Despite the frequent recommendation of exercise in the treatment of depression and anxiety [16,19], the prescription of exercise on improving psychological functioning for individuals with chronic pain is equivocal. In an overview of Cochrane Reviews to determine the effectiveness of physical activity and exercise interventions for adults with chronic pain [31], only five of the twenty-one reviews included psychological well-being (i.e., mental health, anxiety, and depression). Variable effects were reported that included positive and no effects of exercise on psychological health.
The variability in the response may be related to how exercise is incorporated with other interventions in promoting psychological well-being. For example, in a systematic review and meta-analysis, the strongest effects for reducing pain catastrophizing in adults with chronic non-cancer pain was with multimodal treatment that included cognitive behavioral therapy and exercise [75]. The authors propose several explanations such that participating in exercise produces positive benefits that subsequently promotes cognitive restructuring; increases self-efficacy by encouraging self-management; attenuates rumination through increased attentional demands of exercise and decreases pain via activation of descending inhibitory systems. Similarly, in patients with chronic low back pain, a multimodal program that included cognitive behavioral training and exercise produced better effects than exercise alone in improving quality of life and reducing disability and fear avoidance beliefs [65]. It is important to note that improvements occur with exercise alone; participating in regular physical activity that included both aerobic and strength training reduced pain catastrophizing in patients with chronic low back pain which mediated the improvements in disability and depression [78]. Thus, exercise prescription that incorporates a biopsychosocial approach that addresses the multitude of factors that occur with prolonged pain is important to maximize the overall positive effects [5,40].
Clinical Implications
Pain with activity is a significant barrier to activity participation11–13. We routinely show that in sedentary animals there is an increase in hyperalgesia with a single bout of fatiguing exercise [12,35,36,89]. We further show that in human subjects there is a significant increase in pain with fatiguing exercise in people with fibromyalgia [23]. Treatments designed to reduce pain with activity have the potential to improve participation in regular activity. We recently show that application of transcutaneous electrical nerve stimulation (TENS) to the spine in people with fibromyalgia reduces movement-evoked pain, but has no effect on resting pain [24]. Similarly, in people with postoperative pain, Rakel and Frantz applied TENS and showed a reduction in movement-evoked pain but not in resting pain [73]. Thus, TENS may be an effective treatment to reduce movement-evoked pain to encourage activity participation in individuals with chronic pain.
The type of exercise may be less important than the act of doing exercise. Several studies have compared different types of exercise for different types of pain and show no difference between active exercise interventions [15,39,45,74]. For example, in individuals with low back pain, comparison of spinal stabilization exercises to conventional physical therapy which included general exercise showed no differences between groups [14]. For those with neck pain, comparison of proprioceptive training to craniocervical flexion showed no differences in outcomes between groups [45]. Similarly, comparison of graded exercise and graded exposure for those with chronic low back pain showed similar effects [15]. Further, significant effects of strengthening and aerobic exercise are shown in low back pain, osteoarthritis, and fibromyalgia, and are both part of recommended guidelines for these conditions [2,10,11,13,59,72]. In fact, a recent Cochrane review comparing motor control exercise to other forms of exercise for those with chronic low back pain concluded “Given the evidence that MCE [motor control exercise] is not superior to other forms of exercise, the choice of exercise for LBP [low back pain] should probably depend on patient and therapist preferences, therapist training, costs and safety [74].” We suggest this lack of specificity of exercise may be related to the multiple and widespread mechanisms by which exercise works to reduce pain.
Future research directions
Basic science studies have only just begun to examine the underlying mechanisms of exercise. A better understanding of the molecular and cellular mechanisms of exercise can lead increase pain or decrease pain will help to develop novel strategies to address chronic pain and improve implementation and adherence for this important intervention for chronic pain. It is abundantly clear that regular exercise and physical activity are effective for reduction in pain. It has also become increasingly clear that the type of exercise for reduction in pain is less important than doing the exercise. While the Centers for Disease Control (CDC) recommends 150 minutes of moderate physical activity per week and 2 days of strengthening per week for health benefits [1], it is unclear if this dose is needed for pain relief [5]. Indeed multiple clinical trials use less time and lower intensities and still produce clinical effects in those with chronic pain [40]. However, we do not know the most effective dose, or the minimal effective dose. Further, like all interventions, adherence and compliance with the program is extremely important to producing an effect. Barriers to exercise adherence include pain with exercise, low levels of physical activity, low self-efficacy and psychological dysfunction, and poor social support [43]. Supervised exercise, individualized therapy, and self-management techniques may improve adherence; however, the quality of trial assessing these interventions is low [44]. Thus, future clinical studies will need to determine the most effective and minimally effective doses of exercise, if physical activity is equally beneficial to regular prescribed exercise, and develop methods and programs to improve adherence. Lastly, while accepted that exercise is an important intervention for chronic pain, it is often not used as a first-line treatment, relying rather on medication prescriptions. The CDC opioid prescribing guidelines recommend the use of non-pharmacological approaches as the preferred approach to chronic pain (CDC guidelines). As much as 50% of all visits to primary care practitioners is for chronic pain [8], yet non-pharmacological treatments are underutilized. Therefore future studies should develop innovative methods to improve utilization of non-pharmacological treatments by health care practitioners for both acute and chronic pain.
Footnotes
Conflict of Interest: All authors have no conflicts of interest.
References
- 1.Centers for Diesase Control and Prevention. How much physical activity do adults need? 2016 https://www.cdc.gov/cancer/dcpc/prevention/policies_practices/physical_activity/guidelines.htm.
- 2.Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Goes SM, Boden C, Foulds HJ. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;6:CD012700. doi: 10.1002/14651858.CD012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobinski F, Ferreira TA, Cordova MM, Dombrowski PA, da CC, Santo CC, Poli A, Pires RG, Martins-Silva C, Sluka KA, Santos AR. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156(12):2595–2606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobinski F, Teixeira JM, Sluka KA, Santos ARS. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain. 2017 doi: 10.1097/j.pain.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth J, Moseley GL, Schiltenwolf M, Cashin A, Davies M, Hubscher M. Exercise for chronic musculoskeletal pain: A biopsychosocial approach. Musculoskeletal Care. 2017;15(4):413–421. doi: 10.1002/msc.1191. [DOI] [PubMed] [Google Scholar]
- 6.Bote ME, Garcia JJ, Hinchado MD, Ortega E. An exploratory study of the effect of regular aquatic exercise on the function of neutrophils from women with fibromyalgia: Role of IL-8 and noradrenaline. Brain Behav Immun. 2013;39:107–112. doi: 10.1016/j.bbi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Bote ME, Garcia JJ, Hinchado MD, Ortega E. Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS One. 2013;8(9):e74524. doi: 10.1371/journal.pone.0074524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Brito RRL, SLuka KA. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. PainReports. 2017;2(5):e618. doi: 10.1097/PR9.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Mizusaki Imoto A, Toupin-April K, Westby M, Alvarez Gallardo IC, Gifford W, Laferriere L, Rahman P, Loew L, De Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clin Rehabil. 2017;31(5):612–624. doi: 10.1177/0269215517691085. [DOI] [PubMed] [Google Scholar]
- 11.Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Mizusaki Imoto A, Toupin-April K, Westby M, Alvarez Gallardo IC, Gifford W, Laferriere L, Rahman P, Loew L, De Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31(5):596–611. doi: 10.1177/0269215517691084. [DOI] [PubMed] [Google Scholar]
- 12.Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1347–R1355. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, Danyliw A, Sawant A, Dal Bello-Haas V, Rader T, Overend TJ. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;12:CD010884. doi: 10.1002/14651858.CD010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, rendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006;169(4):467–472. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- 15.Calley DQ, Jackson S, Collins H, George SZ. Identifying patient fear-avoidance beliefs by physical therapists managing patients with low back pain. J Orthop Sports Phys Ther. 2010;40(12):774–783. doi: 10.2519/jospt.2010.3381. [DOI] [PubMed] [Google Scholar]
- 16.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41(1):15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho FA, Maher CG, Franco MR, Morelhao PK, Oliveira CB, Silva FG, Pinto RZ. Fear of Movement Is Not Associated With Objective and Subjective Physical Activity Levels in Chronic Nonspecific Low Back Pain. Arch Phys Med Rehabil. 2017;98(1):96–104. doi: 10.1016/j.apmr.2016.09.115. [DOI] [PubMed] [Google Scholar]
- 18.Chen BS, Braud S, Badger JD, Isaac JT, Roche KW. Regulation of NR1/NR2C N-methyl-D-aspartate (NMDA) receptors by phosphorylation. J Biol Chem. 2006;281(24):16583–16590. doi: 10.1074/jbc.M513029200. [DOI] [PubMed] [Google Scholar]
- 19.Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper NA, Rakel BA, Zimmerman B, Tonelli SM, Herr KA, Clark CR, Noiseux NO, Callaghan JJ, Sluka KA. Predictors of multidimensional functional outcomes after total knee arthroplasty. J Orthop Res. 2017;35(12):2790–2798. doi: 10.1002/jor.23596. [DOI] [PubMed] [Google Scholar]
- 21.Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78(1):59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 22.da Silva LFS, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. Pain. 2010;11(4):378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dailey DL, Keffala VJ, Sluka KA. Cognitive and physical fatigue tasks enhance pain, cognitive fatigue and physical fatigue in people with fibromyalgia. Arthritis Care Res (Hoboken ) 2015;67:288–296. doi: 10.1002/acr.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, Lee KS, Lee JE, Sluka KA. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554–2562. doi: 10.1016/j.pain.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bruijn ST, van Wijck AJ, Geenen R, Snijders TJ, van der Meulen WJ, Jacobs JW, Veldhuijzen DS. Relevance of physical fitness levels and exercise-related beliefs for self-reported and experimental pain in fibromyalgia: an explorative study. J Clin Rheumatol. 2011;17(6):295–301. doi: 10.1097/RHU.0b013e31822c5196. [DOI] [PubMed] [Google Scholar]
- 26.Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15(8):796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlers MD, Tingley WG, Huganir RL. Regulated subcellular distribution of the NR1 subunit of the NMDA receptor. Science. 1995;269(5231):1734–1737. doi: 10.1126/science.7569904. [DOI] [PubMed] [Google Scholar]
- 28.Elfving B, Andersson T, Grooten WJ. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int. 2007;12(1):14–24. doi: 10.1002/pri.355. [DOI] [PubMed] [Google Scholar]
- 29.Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13(2):195–206. doi: 10.1016/j.jpain.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia JJ, Cidoncha A, Bote ME, Hinchado MD, Ortega E. Altered profile of chemokines in fibromyalgia patients. Ann Clin Biochem. 2013;51:576–581. doi: 10.1177/0004563213506413. [DOI] [PubMed] [Google Scholar]
- 31.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279. doi: 10.1002/14651858.CD011279.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geva N, Defrin R. Enhanced pain modulation among triathletes: a possible explanation for their exceptional capabilities. Pain. 2013;154(11):2317–2323. doi: 10.1016/j.pain.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident macrophages in muscle contribute to development of hyperalgesia in a mouse model of non-inflammatory muscle pain. J Pain. 2016;17:1081–1094. doi: 10.1016/j.jpain.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN, Maier SF. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–2023. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregory NS, Brito R, Fusaro MCGO, Sluka KA. ASIC3 is required for development of fatigue-induced hyperalgesia. Molecular Neurobiology. 2016;53:1020–1030. doi: 10.1007/s12035-014-9055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain. 2013;154:2668–2676. doi: 10.1016/j.pain.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinricher MM, Fields HL. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Melzack and Wall’s Textbook of Pain. Philadelphia: Elsevier; 2013. pp. 129–142. [Google Scholar]
- 38.Henriksen M, Klokker L, Graven-Nielsen T, Bartholdy C, Schjodt JT, Bandak E, Danneskiold-Samsoe B, Christensen R, Bliddal H. Association of exercise therapy and reduction of pain sensitivity in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res (Hoboken ) 2014;66(12):1836–1843. doi: 10.1002/acr.22375. [DOI] [PubMed] [Google Scholar]
- 39.Henry SM, Van Dillen LR, Ouellette-Morton RH, Hitt JR, Lomond KV, DeSarno MJ, Bunn JY. Outcomes are not different for patient-matched versus nonmatched treatment in subjects with chronic recurrent low back pain: a randomized clinical trial. Spine J. 2014;14(12):2799–2810. doi: 10.1016/j.spinee.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeger Bement MK, Sluka KA. Exercise-induced hypoalgesia: An Evidence-based review. In: Sluka KA, editor. Pain Mechanisms and Management for the Physical Therapist. Philadelphia: Wolters Kluwer; 2016. pp. 177–202. [Google Scholar]
- 41.Hoeger Bement MK, Weyer A, Hartley S, Drewek B, Harkins AL, Hunter SK. Pain perception after isometric exercise in women with fibromyalgia. Arch Phys Med Rehabil. 2011;92(1):89–95. doi: 10.1016/j.apmr.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Hurley MV, Walsh NE, Mitchell H, Nicholas J, Patel A. Long-term outcomes and costs of an integrated rehabilitation program for chronic knee pain: a pragmatic, cluster randomized, controlled trial. Arthritis Care Res (Hoboken) 2012;64(2):238–247. doi: 10.1002/acr.20642. [DOI] [PubMed] [Google Scholar]
- 43.Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther. 2010;15(3):220–228. doi: 10.1016/j.math.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan JL, Holden MA, Mason EE, Foster NE. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956. doi: 10.1002/14651858.CD005956.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jull G, Falla D, Treleaven J, Hodges P, Vicenzino B. Retraining cervical joint position sense: the effect of two exercise regimes. J Orthop Res. 2007;25(3):404–412. doi: 10.1002/jor.20220. [DOI] [PubMed] [Google Scholar]
- 46.Kanaan SA, Poole S, Saade NE, Jabbur S, Safieh-Garabedian B. Interleukin-10 reduces the endotoxin-induced hyperalgesia in mice. J Neuroimmunol. 1998;86(2):142–150. doi: 10.1016/s0165-5728(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 47.Kiefel JM, Cooper ML, Bodnar RJ. Inhibition of mesencephalic morphine analgesia by methysergide in the medial ventral medulla of rats. Physiol Behav. 1992;51(1):201–205. doi: 10.1016/0031-9384(92)90224-p. [DOI] [PubMed] [Google Scholar]
- 48.Kiefel JM, Cooper ML, Bodnar RJ. Serotonin receptor subtype antagonists in the medial ventral medulla inhibit mesencephalic opiate analgesia. Brain Res. 1992;597(2):331–338. doi: 10.1016/0006-8993(92)91490-6. [DOI] [PubMed] [Google Scholar]
- 49.Koho P, Orenius T, Kautiainen H, Haanpaa M, Pohjolainen T, Hurri H. Association of fear of movement and leisure-time physical activity among patients with chronic pain. J Rehabil Med. 2011;43(9):794–799. doi: 10.2340/16501977-0850. [DOI] [PubMed] [Google Scholar]
- 50.Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241–2247. doi: 10.1016/j.pain.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 51.Landmark T, Romundstad PR, Borchgrevink PC, Kaasa S, Dale O. Longitudinal associations between exercise and pain in the general population--the HUNT pain study. PLoS One. 2013;8(6):e65279. doi: 10.1371/journal.pone.0065279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landmark T, Romundstad PR, Borchgrevink PC, Kaasa S, Dale O. Longitudinal Associations between Exercise and Pain in the General Population-The HUNT Pain Study. PloS one. 2013;8(6):e65279. doi: 10.1371/journal.pone.0065279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain. 2010;151(1):77–86. doi: 10.1016/j.pain.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 54.Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by alatering resident muscle macrophage phenotype and increasing IL-10 in mice. Pain. 2016;157:79–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lima LV, DeSantana JM, Rasmussen LA, Sluka KA. Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms. Pain. 2017;158(9):1697–1710. doi: 10.1097/j.pain.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin CW, McAuley JH, Macedo L, Barnett DC, Smeets RJ, Verbunt JA. Relationship between physical activity and disability in low back pain: a systematic review and meta-analysis. Pain. 2011;152(3):607–613. doi: 10.1016/j.pain.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Llewelyn M, Azami J, Roberts M. The effect of modification of 5-hydroxytryptamine function in nucleus raphe magnus on nociceptive threshold. Brain research. 1984;306(1–2):165–170. doi: 10.1016/0006-8993(84)90365-2. [DOI] [PubMed] [Google Scholar]
- 58.Llewelyn MB, Azami J, Roberts MHT. Effects of 5-hydroxytryptamine applied into the nucleus raphe magnus on nociceptive thresholds and neuronal firing rate. Brain Res. 1983;258:59–68. doi: 10.1016/0006-8993(83)91226-x. [DOI] [PubMed] [Google Scholar]
- 59.Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, Choy E, Kosek E, Amris K, Branco J, Dincer F, Leino-Arjas P, Longley K, McCarthy GM, Makri S, Perrot S, Sarzi-Puttini P, Taylor A, Jones GT. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–328. doi: 10.1136/annrheumdis-2016-209724. [DOI] [PubMed] [Google Scholar]
- 60.McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc. 2011;43(5):905–912. doi: 10.1249/MSS.0b013e3181fca1ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendieta D, la Cruz-Aguilera DL, Barrera-Villalpando MI, Becerril-Villanueva E, Arreola R, Hernandez-Ferreira E, Perez-Tapia SM, Perez-Sanchez G, Garces-Alvarez ME, Aguirre-Cruz L, Velasco-Velazquez MA, Pavon L. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol. 2016;290:22–25. doi: 10.1016/j.jneuroim.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Menzies V, Lyon DE. Integrated review of the association of cytokines with fibromyalgia and fibromyalgia core symptoms. Biol Res Nurs. 2010;11(4):387–394. doi: 10.1177/1099800409348328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mogren IM. Previous physical activity decreases the risk of low back pain and pelvic pain during pregnancy. Scand J Public Health. 2005;33(4):300–306. doi: 10.1177/140349480503300410. [DOI] [PubMed] [Google Scholar]
- 65.Monticone M, Ferrante S, Rocca B, Baiardi P, Farra FD, Foti C. Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain: results of a randomized controlled trial. Clin J Pain. 2013;29(11):929–938. doi: 10.1097/AJP.0b013e31827fef7e. [DOI] [PubMed] [Google Scholar]
- 66.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naugle KM, Riley JL., III Self-reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc. 2014;46(3):622–629. doi: 10.1249/MSS.0b013e3182a69cf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ortega E, Bote ME, Giraldo E, Garcia JJ. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand J Med Sci Sports. 2012;22(1):104–112. doi: 10.1111/j.1600-0838.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 69.Ortega E, Garcia JJ, Bote ME, Martin-Cordero L, Escalante Y, Saavedra JM, Northoff H, Giraldo E. Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exerc Immunol Rev. 2009;15:42–65. [PubMed] [Google Scholar]
- 70.Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587(Pt 23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porreca F, Ossipov MH, Gebhart G. Chronic pain and medullary descending facilitation. Trends in neurosciences. 2002;25(6):319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 72.Qaseem A, Wilt TJ, McLean RM, Forciea MA Clinical Guidelines Committee of the American College of P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 73.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. Journal of Pain. 2003;4(8):455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 74.Saragiotto BT, Maher CG, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016;(1):CD012004. doi: 10.1002/14651858.CD012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schutze R, Rees C, Smith A, Slater H, Campbell JM, O’Sullivan P. How Can We Best Reduce Pain Catastrophizing in Adults With Chronic Noncancer Pain? A Systematic Review and Meta-Analysis. J Pain. 2018;19(3):233–256. doi: 10.1016/j.jpain.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152(12):2802–2812. doi: 10.1016/j.pain.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7(4):261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Taylor BK, Basbaum AI. Systemic morphine-induced release of serotonin in the rostroventral medulla is not mimicked by morphine microinjection into the periaqueductal gray. Journal of neurochemistry. 2003;86(5):1129–1141. doi: 10.1046/j.1471-4159.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- 80.Tesarz J, Schuster AK, Hartmann M, Gerhardt A, Eich W. Pain perception in athletes compared to normally active controls: a systematic review with meta-analysis. Pain. 2012;153(6):1253–1262. doi: 10.1016/j.pain.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84(5):1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 82.Tingley WG, Roche KW, Thompson AK, Huganir RL. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993;364(6432):70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- 83.Tour J, Lofgren M, Mannerkorpi K, Gerdle B, Larsson A, Palstam A, Bileviciute-Ljungar I, Bjersing J, Martin I, Ernberg M, Schalling M, Kosek E. Gene-to-gene interactions regulate endogenous pain modulation in fibromyalgia patients and healthy controls-antagonistic effects between opioid and serotonin-related genes. Pain. 2017;158(7):1194–1203. doi: 10.1097/j.pain.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011;12:245. doi: 10.1186/1471-2474-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wainwright JM. The Influence of Physical Therapy in Reducing Disability Time in Fractures of the Long Bones. Ann Surg. 1921;74(3):304–305. doi: 10.1097/00000658-192109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001;93(3):201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 87.Wei H, Pertovaara A. MK-801, an NMDA receptor antagonist, in the rostroventromedial medulla attenuates development of neuropathic symptoms in the rat. Neuroreport. 1999;10(14):2933–2937. doi: 10.1097/00001756-199909290-00011. [DOI] [PubMed] [Google Scholar]
- 88.Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127(3):253–262. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R, Chomistek AK, Dimitrakoff JD, Giovannucci EL, Willett WC, Rosner BA, Wu K. Physical Activity and Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Med Sci Sports Exerc. 2015;47:757–764. doi: 10.1249/MSS.0000000000000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng W, Huang W, Liu S, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with ddC in rats. Mol Pain. 2014;10:49. doi: 10.1186/1744-8069-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]