Abstract
Poor graft function (PGF) is a life-threatening complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Current treatment strategies include the use of growth factors, CD34+-selected stem cell boost, mesenchymal stem cell transfusion, and second allo-HSCT, but these treatments are not effective in all patients. Eltrombopag, an oral thrombopoietin receptor agonist, which showed promising results in severe aplasia anemia, may be an alternative choice for PGF patients. Therefore, we treated 12 patients who responded poorly to standard treatments for secondary PGF after allo-HSCT with eltrombopag. The median duration was 116 (35–1000) days from transplantation to PGF diagnosis and 59 (30–180) days from PGF diagnosis to eltrombopag treatment. Eltrombopag was started at a dose of 25 mg/d for 3 days and then increased to 50 or 75 mg/d. Median treatment duration was 8 (2–23) weeks. Ten patients (83.3%) responded to the treatment: 8 achieved complete response (CR), and the remaining 2 achieved partial response. In the 10 responding subjects, median platelet count was 18 (5–27) × 109/L vs 74 (30–117) × 109/L prior to and after treatment. Neutrophil count was 0.51 (0.28–0.69) × 109/L vs 1.84 (0.78–4.90) × 109/L. Hemoglobin was 88 (63–123) vs 101 (78–134) g/L. In the 8 patients who achieved CR, the time from eltrombopag initiation to achieving CR was 29 (10–49) days; the response lasted until the last follow-up in all 8 CR subjects (10–18 months). The 12-month overall survival rate was 83.3%. There was no treatment-related mortality and no evidence of cataract, thrombosis, or any other grade 3/4 toxicities.
Keywords: Eltrombopag, Secondary poor graft function, Allogeneic hematopoietic stem cell transplantation
Poor graft function (PGF) is a life-threatening complication that occurs in 5–27% of the patients following allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1, 2]. Management strategies, including the use of growth factors [3], CD34+-selected stem cell boost [4], mesenchymal stem cell (MSC) transfusion [5], and second allo-HSCT [6], are not effective for all patients.
Eltrombopag, a c-mpl receptor agonist, is an effective treatment for immune thrombocytopenic purpura (ITP) and thrombocytopenia after transplantation [7, 8]. In a recent phase I/II study of 92 patients with severe aplastic anemia (SAA) [9], eltrombopag plus standard immunosuppression resulted in 94% hematological response rate. Considering the similarity between PGF and SAA, we speculated that eltrombopag is also effective against PGF. This retrospective analysis included 12 consecutive patients receiving eltrombopag for secondary PGF after allo-HSCT during a period from February 2016 to October 2017. Secondary PGF (sPGF) was defined as: cytopenia in at least two lineages (platelet < 20 × 109/L, neutrophil < 0.5 × 109/L, hemoglobin < 70 g/L), and/or with transfusion requirements beyond day + 28, with full donor chimerism, without relapse or severe graft versus host disease, and lasting at least for 14 consecutive days [5, 10].
Clinical characteristics of the subjects were summarized in Table 1. All 12 patients responded poorly to previous treatments, including growth factors (n = 12), MSCs (n = 2), and decitabine (n = 2). All but one patient were transfusion-dependent. The median duration was 116 (35–1000) days from transplantation to sPGF diagnosis and 59 (30–180) days from sPGF diagnosis to eltrombopag treatment. Eltrombopag was started at a dose of 25 mg/d for 3 days and then increased to 50 or 75 mg/d. Median duration of eltrombopag treatment was 8 (2–23) weeks. Total dosage was 2487.5 (700–10,500) mg.
Table 1.
Clinical Characteristics of the 12 sPGF patients
| No. | Age | Sex | Underlying disease | Cytopenia | Failed previous treatments (duration) | Eltrom duration, weeks | Total dose of eltrom, mg | Time to CR, days | Best response | Last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | ALL | N, PLT | G-CSF, EPO, TPO, IL-11 PLT transfusion-dependent for 12 months, MSC infusion for 4 times | 13 | 6475 | 43 | CR | Alive |
| 2 | 25 | F | ALL | N, HB, PLT | G-CSF, EPO, TPO, PLT transfusion-dependent for 2 months | 2 | 700 | 10 | CR | Alive |
| 3 | 35 | F | ALL | N, HB, PLT | G-CSF, EPO, TPO, RBC and PLT transfusion-dependent for 2 months | 2 | 700 | NA | PR | Dead |
| 4 | 22 | M | ALL | N, HB, PLT | G-CSF, PLT transfusion-dependent for 1 month | 8 | 4200 | NA | NR | Dead |
| 5 | 52 | M | AML | N, PLT | G-CSF, IL-11, PLT transfusion-dependent for 1 month | 4 | 700 | 36 | CR | Alive |
| 6 | 27 | F | AML | N, HB, PLT | G-CSF, EPO, TPO, RBC and PLT transfusion-dependent for 3 months | 7 | 1725 | NA | PR | Dead |
| 7 | 53 | M | AML | N, PLT | G-CSF, EPO, TPO, PLT transfusion-dependent for 1 month | 6 | 2175 | 25 | CR | Alive |
| 8 | 42 | M | MPAL | N, HB, PLT | G-CSF, EPO, TPO, RBC and PLT transfusion-dependent for 3 months; DAC for 1 course | 4 | 1400 | NA | NR | Alive |
| 9 | 42 | F | SAA | N, HB, PLT | G-CSF, PLT transfusion-dependent for 1 month; DAC for 1 course | 8 | 4200 | 30 | CR | Alive |
| 10 | 29 | F | SAA | N, PLT | G-CSF, TPO-dependent for 1 month | 23 | 10,500 | 28 | CR | Alive |
| 11 | 33 | M | SAA | N, PLT | G-CSF, EPO, TPO, PLT transfusion-dependent for 2 months, MSC infusion for 3 times | 8 | 4025 | 49 | CR | Alive |
| 12 | 47 | M | MF | N, PLT | G-CSF, PLT transfusion-dependent for 1 month | 8 | 2800 | 20 | CR | Alive |
M male, F female, N neutrophil, HB hemoglobin, PLT platelet, sPGF secondary poor graft function, AML acute myeloid leukemia, ALL acute lymphocytic leukemia, MPAL mixed phenotype acute leukemia, SAA severe aplasia anemia, MF myelofibrosis, CR complete response, PR partial response, NR no response, G-CSF granulocyte colony-stimulating factor, EPO erythropoietin, TPO thrombopoietin, MSC mesenchymal stem cell, DAC decitabine, NA not available
The overall response rate (ORR) was 83.3% (10/12). Eight patients achieved complete response (CR), as defined by platelet ≥ 50 × 109/L, neutrophil ≥ 1.0 × 109/L, and hemoglobin ≥ 90 g/L, without blood cell transfusion or granulocyte colony stimulating factor for ≥ 7 consecutive days [5]; the time from eltrombopag initiation to achieving CR was 29 (10–49) days. Two patients achieved partial response, as defined by hematopoietic engraftment of at least two lineages (platelet ≥20 × 109/L, neutrophil ≥0.5 × 109/L and hemoglobin ≥70 g/L) but not fulfilling CR criteria.
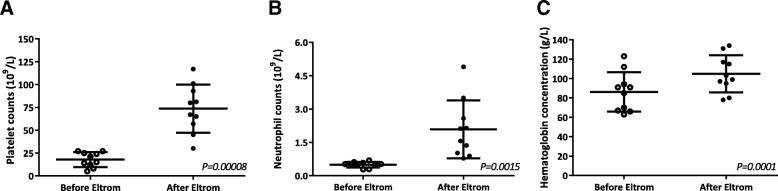
The follow-up was 18.5 (3–37) months post transplantation. Among the 10 responding patients, median platelet count was 18 (5–27) × 109/L vs 74 (30–117) × 109/L prior to and after treatment (P = 0.00008; Fig. 1a). Median neutrophil count was 0.51 (0.28–0.69) × 109/L vs 1.84 (0.78–4.90) × 109/L (P = 0.0015; Fig. 1b). Median hemoglobin was 88 (63–123) vs 101 (78–134) g/L (P = 0.0001; Fig. 1c). The response lasted to the last follow-up (10–18 months) in all 8 subjects who achieved CR.
Fig. 1.
Blood cell counts prior to and after eltrombopag treatment. The analysis included only 10 responding patients. a Median platelet count was 18 (5–27) × 109/L vs 74 (30–117) × 109/L before and after the treatment (P = 0.00008). b Median neutrophil count was 0.51 (0.28–0.69) × 109/L vs 1.84 (0.78–4.90) × 109/L (P = 0.0015). c Median hemoglobin level was 88 (63–123) vs 101 (78–134) g/L (P = 0.0001)
Eltrombopag was well tolerated by all 12 patients. There were no treatment-related mortality and no evidence of cataract, thrombosis, or any other grade 3/4 toxicities. Upon the last follow-up, 9 subjects were PGF-free; 9 had normal blood cell counts. The 12-month overall survival rate after transplantation was 83.3% (95% CI: 62–100%).
With increasing application of alternative donor in transplantation, especially haploidentical HSCT and cord blood transplantation, PGF has become a major obstacle contributing to higher non-relapse mortality. Eltrombopag, as a stimulator of platelet production, promotes the proliferation of megakaryocytes by binding with thrombopoietin receptor (c-mpl) [7], also can promote hematopoiesis along all three lineages. Indeed, clinical trials have establishedefficacy of eltrombopag against ITP, thrombocytopenia after transplantation, as well as SAA [7–9]. Considering the fact that all patients in the current study failed previous treatments for sPGF, the ORR (83.3%) and CR (66.7%) are encouraging. Another important finding is the relatively long duration of the response after eltrombopag withdrawal. The current study represents the first case series of using eltrombopag for secondary PGF after allo-HSCT. Due to the retrospective nature of the study and the small sample size, the results must be considered preliminary and should be verified by randomized controlled trials in the future.
In summary, we showed that eltrombopag could produce a rapid and sustaining response in patients with sPGF after allo-HSCT who failed treatment with conventional treatments. This finding is particularly interesting considering the increasing use of alternative donor HSCT and high rate of non-relapse mortality due to PGF.
Acknowledgments
Funding
This work was supported, in part by research grants from the National Key R&D Program of China (2016YFC0902800), National Natural Science Foundation of China (81270645, 81770154, 8187010379), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Frontier Clinical Technical Project of the Science and Technology Department of Jiangsu Province (BE2017655), and the Jiangsu Provincial Medical Talent (ZDRCA2016045).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- CR
Complete response
- ITP
Immune thrombocytopenic purpura
- MSC
Mesenchymal stem cell
- ORR
Overall response rate
- PGF
Poor graft function
- SAA
Severe aplastic anemia
- sPGF
Secondary PGF
Authors’ contributions
XT and DW designed the study. CT, FC, DK, QM, HD, ZL, JY, JC, and XZ participated in the collection and analysis the data. CT and FC wrote the manuscript. XM and XT were responsible of the critical review and revision of this manuscript. All authors provided the approval of the final manuscript for submission.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinliang Mao, Email: xinliangmao@suda.edu.cn.
Depei Wu, Phone: 86 13951102021, Email: drwudepei@163.com.
Xiaowen Tang, Phone: 86 13913538266, Email: xwtang1020@163.com.
References
- 1.Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation - frequency and outcomes. Bone Marrow Transplant. 2004;33(7):729–734. doi: 10.1038/sj.bmt.1704428. [DOI] [PubMed] [Google Scholar]
- 2.Woolfrey A, Anasetti C. Allogeneic hematopoietic stem-cell engraftment and graft failure. Pediatr Transplant. 1999;3(Suppl 1):35–40. doi: 10.1034/j.1399-3046.1999.00068.x. [DOI] [PubMed] [Google Scholar]
- 3.Bittencourt H, Rocha V, Filion A, Ionescu I, Herr AL, Garnier F, et al. Granulocyte colony-stimulating factor for poor graft function after allogeneic stem cell transplantation: 3 days of G-CSF identifies long-term responders. Bone Marrow Transplant. 2005;36(5):431–435. doi: 10.1038/sj.bmt.1705072. [DOI] [PubMed] [Google Scholar]
- 4.Ghobadi A, Fiala MA, Ramsingh G, Gao F, Abboud CN, Stockerl-Goldstein K, et al. Fresh or cryopreserved CD34(+)-selected mobilized peripheral blood stem and progenitor cells for the treatment of poor graft function after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(7):1072–1077. doi: 10.1016/j.bbmt.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014;23(9):1087–1098. doi: 10.3727/096368912X661319. [DOI] [PubMed] [Google Scholar]
- 6.Guardiola P, Kuentz M, Garban F, Blaise D, Reiffers J, Attal M, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Society of Bone Marrow Transplantation. Br J Haematol. 2000;111(1):292–302. doi: 10.1046/j.1365-2141.2000.02306.x. [DOI] [PubMed] [Google Scholar]
- 7.Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. doi: 10.1182/blood-2017-04-748707. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(5):919–924. doi: 10.1016/j.bbmt.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, et al. Association of an impaired bone marrow microenvironment with secondary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(10):1465–1473. doi: 10.1016/j.bbmt.2013.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.