Abstract
A serious challenge to antimicrobial therapies has emerged due to rapid increase in drug-resistant infections creating an urge for the development of alternative therapeutics. Antimicrobial peptides (AMPs) have gained importance because of their broad-spectrum antimicrobial activities and mediator-like functions linking innate and adaptive immune responses. The multidimensional properties of these peptides hold promising potentials as prophylactic and antimicrobial agents. This review discusses various AMPs and their role in combating microorganisms and infections along with its clinical implication.
Keywords: Antimicrobial, inflammation and innate immunity, periodontitis
To make the reader understand the concept of how antimicrobial peptides (AMPs) play a role in the host defense and thereby provide an outlook for the implications of these proteins in therapeutics.
INTRODUCTION
The oral cavity comprises approximately of 700 microorganisms, of which nearly 150–200 species are present in all the individuals.[1] Four hundred bacterial species can be found in periodontal pocket; however, only eight bacterial species have consistently been associated with the development of periodontitis including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia), Treponema denticola, Fusobacterium nucleatum, Eubacterium nodatum, Prevotella intermedia and Prevotella nigrescens.[2] The innate immune system rich in antimicrobial proteins and peptides initially controls this bacterial microflora.
AMPs are oligopeptides that are biologically active molecules produced by different sources including plants, animals, microorganisms and mammals. In humans, AMPs are widely distributed in saliva, epithelium and neutrophils having a broad range of antimicrobial activity and are effective in immune activation, wound healing and inflammation.[3] AMPs are less toxic and have antimicrobial specificity due to which they kill specific target cells without affecting the host cells; therefore, decreased resistance is developed by target cells against them.[4] They also serve as antitumor agents, contraceptive agents, drug delivery vectors, mitogenic agents and signaling molecules in signal transduction pathways. Approximately 106 human AMPs have been identified to date, of which at least 45 different AMPs are present in human saliva and gingival crevicular fluid (GCF).[5]
MECHANISM OF ACTION
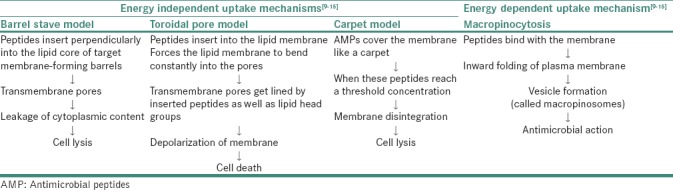
Interactions of AMPs with microbial cell membranes have led to dynamic interchange in their structure and topologies.[6] The primary mechanism for antimicrobial activity of AMPs is the electrostatic interaction of peptides with negatively charged molecules on the membrane. In target cells, AMPs can also exert antimicrobial activity by cell membrane translocation and inhibition of essential cellular processes including nucleic acid synthesis, cell wall synthesis, protein synthesis and enzymatic activities.[7] Based on the mechanism of action, they are broadly categorized into membrane acting peptides, for example, defensin, LL-37, melittin and nonmembrane acting peptides, for example, human neutrophil peptide (hNP)-1, buforin II, pleurocidin and dermaseptin.[8,9] In target cells, the damage of membrane is promoted by AMPs either by the formation of pores, by thinning of membrane or by disruption of lipid bilayer as explained by various models summarized in Table 1.[9,10,11,12,13,14]
Table 1.
Mechanism of action of antimicrobial peptides
CLASSIFICATION OF ANTIMICROBIAL PEPTIDES
Antimicrobial peptide database (APD) has proposed a three-dimensional structure classification approach.[15] According to the classification, AMP structures are classified into four families: α, β, αβ and non-αβ based on the types of secondary structures. AMPs in the α family consist of α-helical structure. Peptides in the β family are characterized by at least a pair of two β-strands in the structure. The αβ family contains both α and β structures; in contrast, the non-αβ family has neither α nor β structure.
More recently, the APD3 proposed a classification according to the covalent bonding pattern of polypeptide chains.[15] In this, AMPs were divided into four classes. All linear peptides where chemical modifications occur within the same amino acid belonged to the first class (UCLL). In the second class (UCSS), peptides in which one chemical bond is present between the side chains of different amino acids of the polypeptide were included in the study. The peptides in which a chemical bond occurred between the side chain of one residue and backbone of other residue were grouped in Class 3 (UCSB). The fourth class (UCBB) comprised of all peptides with a circular backbone wherein a covalent bond is formed between the N- and C-termini of the polypeptide.[15]
TYPES OF ORAL ANTIMICROBIAL PEPTIDES AND THEIR ROLE IN DISEASE
Defensins
These are innate defense molecules due to their capability of killing all kinds of Gram-positive and negative bacteria, fungi as well as viruses such as herpes simplex.[5] These peptides are short, cationic with low molecular weight and unique, characteristic beta-sheet fold structure which consists of three disulfide bonds among six cysteines. On the basis of their length, location, position of cysteine and folding of peptide chains, human defensins are classified as α-defensins (hNP) and β-defensins.[16]
Alpha-defensins
They are further subclassified into six types, such as hNP-1, hNP-2, hNP-3, hNP-4 and hNP-5 and hNP-6 (paneth cells of intestinal mucosa). In amino acid sequences, the neutrophils secreting them are almost identical except at the N-terminus resulting a change in antimicrobial spectrum of defensins. The hNP-1 or hNP-2 actively destroy Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli as compared to hNP-3 and hNP-4[17] which are active against Candida albicans, E. coli and Streptococcus faecalis.[18] hNP-5 and hNP-6 are not expressed in the oral cavity as they are present in the enteric system.[19]
Polymorphonuclear neutrophils present in the junctional epithelium consist of hNP in the periodontium, hence can be detected in GCF. The most abundant peptide present in saliva is hNP-1-3 (99%).[19] In patients with lichen planus, leukoplakia and squamous cell carcinoma, a higher concentration of salivary hNP-1 is seen.[20,21] Patients having dental caries have low salivary levels of α-defensins (hNP-1, -2 and-3) and are used as caries risk assessment.[22]
Beta-defensins
They are small, cationic peptides having antimicrobial activity that are principally expressed in epithelial cells of various tissues and organs such as gingiva, skin, gastrointestinal tract, respiratory tract and kidney.[1] In the oral cavity, only human beta-defensins (hBD-1, hBD-2 and hBD-3) are expressed.[23] Out of 28 hBD genes, only 4 (hBD 1-4) have been detected in the gingival epithelium.[24] Within the suprabasal layer of normal gingiva, hBD-1 and-2 are localized, and within the basal layer in undifferentiated epithelial cells, hBD-3 peptide is expressed.[25]
During gingivitis, chronic periodontitis and aggressive periodontitis, different patterns of expression for hBD have been suggested. hBD-1 obstructs normal flora from becoming opportunistic and is expressed continuously; on the other hand, hBD-2 and-3 are more effective against almost all pathogens and are induced in response to bacterial lipopolysaccharides (LPS), tumor necrosis factors (TNF-α), pro-inflammatory mediators (interleukins [IL-1 β] and interferons).[26,27,28] hBD-1 and-2 were detected less frequently in tissue samples from patients with gingivitis as compared to healthy subjects; however, expression of hBD-3 was at similar levels in both. The expression of hBD-2 gene was higher in gingival tissue samples when compared with expression of hBD-1 and-3 and detected more strongly in aggressive periodontitis patients as compared to gingivitis and chronic periodontitis subjects.[29,30] Furthermore, in samples from patients with peri-implantitis, hBD-1 was expressed more strongly than hBD-2.[31]
Histatins
These are cationic peptides with low molecular weight, synthesized by the parotid and submandibular salivary ducts cells in healthy adults at a concentration of 50–425 μg/ml.[32] They are named as histidine-rich proteins, comprising 7–38 amino acid residues in length and have at least 12 histidine residues. These are predominantly antifungal and the three main members are His-1, His-3 and His-5. However, by means of proteolytic cleavage of these members, the other members are generated.[33] Certain functions of histatins are inhibition of growth of Candida species, bonding of metal ions in saliva, regulation of oral hemostasis[34] and formation of acquired enamel pellicle due to high affinity for enamel surfaces.[35]
Histatins at physiological concentration (15–30 lM), especially Hst-5, inhibit Candida species. The Candidacidal activity of Hst M (middle portion of Hst-3) is similar to the full-length molecule indicating the potential future use of short length antifungal peptides for oral ointments.[36] In patients with human immunodeficiency virus (HIV), histatin 5 12-mer P113 (Demegen) appears to be a promising AMP and works as a mouth rinse for oral candidiasis.[1]
Cathelicidins (LL-37)
These belong to the α-helical peptides family, do not have cysteine and are located at the carboxyl terminus of a 15–18 kDa highly conserved cathepsin-L-inhibitor (cathelin)-like domain.[37] Human cationic antimicrobial peptide (hCAP18) is the only cathelicidin that has been found in humans in the oral cavity and respiratory tract.[38] It was demonstrated that saliva, sweat, neutrophils, monocytes and epithelial cells of tongue, buccal and lingual gingival epithelium express LL-37/CAP18.[39]
Functions[40,41,42,43,44]
It acts as a chemotactic factor for monocytes, neutrophils, mast cells and T-cells
Potent antimicrobial and antiviral activity against many Gram-negative and positive bacteria, fungi, viruses and parasites
Neutralizes the activity of LPS by binding with it
Suppresses the reverse transcriptase activity of HIV-1.
In patients with aggressive and chronic periodontitis, high levels of LL-37 were found.[45] An inherited bone marrow disorder with a severe congenital neutropenia is seen in patients with Kostmann syndrome, as there is a lack of LL-37 in saliva and plasma along with severe periodontal destruction.[46] Treating patients with recombinant granulocyte colony-stimulating factor restores the neutrophil levels, but this result is not similar in higher concentrations of LL-37 and is also associated with recurrence of periodontal infection. On the contrary, restored neutrophil levels with normal plasma concentration of LL-37 are seen in patients who have received bone marrow transplants.[47]
Adrenomedullin
Adrenomedullin is produced from cells of adrenal medulla, kidney, lung as well as epithelial lining of skin, gut and oral cavity[48] when microbes come in contact with epithelial cells. The expression of adrenomedullin gene is upregulated by pro-inflammatory cytokines such as IL-1 and TNF-α.[49]
AMP is present mostly in the GCF and saliva with larger concentrations in whole saliva approximately 55–65 pg/mL. It is effective against both Gram-positive and Gram-negative bacteria of the oral cavity.[50,51] Adrenomedullin is increased in periodontally affected sites as compared to healthy sites.[52]
Statherin
It is a 5.4 kDa basic histadine-rich peptide present in saliva and GCF with antimicrobial properties. In C-terminal peptide, growth of statherin inhibits anaerobic bacteria and prevents plaque formation as crystallization of calcium phosphate is restrained by them.[53] It is used as a biomarker in the proteomic analysis of saliva.[54]
Azurocidin
It is a 37 kDa cationic antimicrobial protein identified by salivary proteomics and is expressed in azurophil granules of neutrophils. Azurocidin is a 251-amino acid protein and consists of two cysteine residues in positions 52 and 68. They have a strong affinity for LPS and therefore exhibit strong antibacterial properties towards Gram-negative bacteria.[55]
C–C motif chemokine 28
It is present mostly in saliva and exhibits both broad-spectral antimicrobial activity and chemotactic activity. This is a 128-amino acid peptide secreted by epithelial cells and salivary glands.[54] C–C motif chemokine 28 is a salt-sensitive peptide and causes an increase in permeability of cell membrane.[56]
Neuropeptides
The neuropeptides, calcitonin gene-related peptide and substance P are expressed in GCF,[57] salivary fluids contains neuropeptide Y and vasoactive intestinal peptide.[58] Since the minimum inhibitory concentrations (MIC) required to be effective against C. Albicans and bacteria are higher than their concentrations which varies from 2 to 45 pg/ml; therefore, their antimicrobial role is extremely limited.[59]
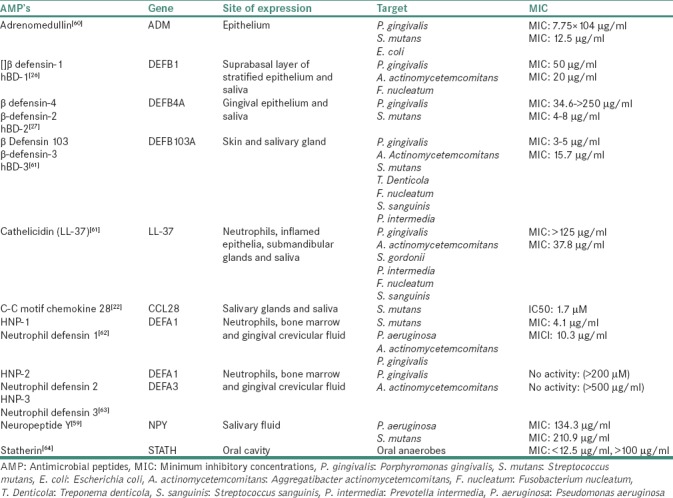
The site of expression of the explained AMPs along with their MIC for targeted microorganisms is summarized in Table 2.[26,27,28,59,60,61,62,63,64]
Table 2.
Antimicrobial peptide genes and their dosage for targeted microorganisms
Advantages of antimicrobial peptides[22]
Broad-spectrum activity (anti-inflammatory, antibacterial, antiviral and antifungal)
Rapid onset of killing with potentially low levels of induced resistance.
Disadvantages of Antimicrobial peptides[22]
Systemic and local toxicity with susceptibility to proteolysis
Reduced activity based on salt, serum and pH sensitivity
Sensitization and allergy after repeated application with natural resistance
Confounding biological functions (e.g., angiogenesis)
High manufacturing costs.
Future implications of antimicrobial peptides
AMPs have multiple functions including antimicrobial activity, innate immune response and play a key role in cancer biology.[65] Peptides from plants exhibiting antimicrobial activity may also be applied to prevent or treat infectious disease, as an alternative to human AMPs.[66] Potential use of hydroxychavicol, a piper betel leaf extract, as an oral health-care agent has been suggested, due to its inhibitory activity against oral microorganisms. Synthetically generated AMPs may also have a great potential for clinical application in addition to natural peptides.[67] At present, determination whether AMPs should be applied for prevention or treatment of periodontal infections is in primitive stage.[68]
CONCLUSION
AMPs have diverse structural and antimicrobial properties and are one of the most promising future drug candidates for reduction of infections and resistance of microbial drugs. They are potentially applied as drug delivery vectors, signaling molecules, immune modulators, antitumor agents and may also possess other biological activities. Therefore, for clinical development of peptide-based therapeutics, understanding the versatile biological properties of AMPs can be of extreme importance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gorr SU, Abdolhosseini M. Antimicrobial peptides and periodontal disease. J Clin Periodontol. 2011;38(Suppl 11):126–41. doi: 10.1111/j.1600-051X.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 2.Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000. 2006;42:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 3.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 4.Hancock RE, Patrzykat A. Clinical development of cationic antimicrobial peptides: From natural to novel antibiotics. Curr Drug Targets Infect Disord. 2002;2:79–83. doi: 10.2174/1568005024605855. [DOI] [PubMed] [Google Scholar]
- 5.Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel) 2014;7:545–94. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sansom M. Peptides and lipid bilayers: Dynamic interactions. Curr Opin Colloid Interface Sci. 1998;3:518–24. [Google Scholar]
- 7.Brogden KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 8.Henzler Wildman KA, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–58. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–85. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Rozek A, Hancock RE. Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem. 2001;276:35714–22. doi: 10.1074/jbc.M104925200. [DOI] [PubMed] [Google Scholar]
- 11.Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47:451–63. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta D, Leontiadou H, Mark AE, Marrink SJ. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta. 2008;1778:2308–17. doi: 10.1016/j.bbamem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Madani F, Lindberg S, Langel U, Futaki S, Gräslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011. 2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G. Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol Biol. 2015;1268:43–66. doi: 10.1007/978-1-4939-2285-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greer A, Zenobia C, Darveau RP. Defensins and LL-37: A review of function in the gingival epithelium. Periodontol 2000. 2013;63:67–79. doi: 10.1111/prd.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilde CG, Griffith JE, Marra MN, Snable JL, Scott RW. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem. 1989;264:11200–3. [PubMed] [Google Scholar]
- 19.Gomes Pde S, Fernandes MH. Defensins in the oral cavity: Distribution and biological role. J Oral Pathol Med. 2010;39:1–9. doi: 10.1111/j.1600-0714.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 20.Dunsche A, Açil Y, Siebert R, Harder J, Schröder JM, Jepsen S, et al. Expression profile of human defensins and antimicrobial proteins in oral tissues. J Oral Pathol Med. 2001;30:154–8. doi: 10.1034/j.1600-0714.2001.300305.x. [DOI] [PubMed] [Google Scholar]
- 21.Fanali C, Inzitari R, Cabras T, Pisano E, Castagnola M, Celletti R, et al. Alpha-defensin levels in whole saliva of totally edentulous subjects. Int J Immunopathol Pharmacol. 2008;21:845–9. doi: 10.1177/039463200802100409. [DOI] [PubMed] [Google Scholar]
- 22.Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–80. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 23.Dale BA, Krisanaprakornkit S. Defensin antimicrobial peptides in the oral cavity. J Oral Pathol Med. 2001;30:321–7. doi: 10.1034/j.1600-0714.2001.300601.x. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Duan D, Wang P, Han B, Xu Y. New finding of the expression of human beta defensin-4 in healthy gingiva. Hua Xi Kou Qiang Yi Xue Za Zhi. 2013;31:165–8. [PubMed] [Google Scholar]
- 25.Pisano E, Cabras T, Montaldo C, Piras V, Inzitari R, Olmi C, et al. Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur J Oral Sci. 2005;113:462–8. doi: 10.1111/j.1600-0722.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 26.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–96. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 27.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–9. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–8. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dommisch H, Açil Y, Dunsche A, Winter J, Jepsen S. Differential gene expression of human beta-defensins (hBD-1, -2, -3) in inflammatory gingival diseases. Oral Microbiol Immunol. 2005;20:186–90. doi: 10.1111/j.1399-302X.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- 30.Vardar-Sengul S, Demirci T, Sen BH, Erkizan V, Kurulgan E, Baylas H, et al. Human beta defensin-1 and -2 expression in the gingiva of patients with specific periodontal diseases. J Periodontal Res. 2007;42:429–37. doi: 10.1111/j.1600-0765.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 31.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 32.de Sousa-Pereira P, Amado F, Abrantes J, Ferreira R, Esteves PJ, Vitorino R, et al. An evolutionary perspective of mammal salivary peptide families: Cystatins, histatins, statherin and PRPs. Arch Oral Biol. 2013;58:451–8. doi: 10.1016/j.archoralbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Troxler RF, Offner GD, Xu T, Vanderspek JC, Oppenheim FG. Structural relationship between human salivary histatins. J Dent Res. 1990;69:2–6. doi: 10.1177/00220345900690010101. [DOI] [PubMed] [Google Scholar]
- 34.Oudhoff MJ, van den Keijbus PA, Kroeze KL, Nazmi K, Gibbs S, Bolscher JG, et al. Histatins enhance wound closure with oral and non-oral cells. J Dent Res. 2009;88:846–50. doi: 10.1177/0022034509342951. [DOI] [PubMed] [Google Scholar]
- 35.Richardson CF, Johnsson M, Raj PA, Levine MJ, Nancollas GH. The influence of histatin-5 fragments on the mineralization of hydroxyapatite. Arch Oral Biol. 1993;38:997–1002. doi: 10.1016/0003-9969(93)90113-z. [DOI] [PubMed] [Google Scholar]
- 36.Raj PA, Edgerton M, Levine MJ. Salivary histatin 5: Dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–905. [PubMed] [Google Scholar]
- 37.Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, et al. Cathelicidins: Family of antimicrobial peptides. A review. Mol Biol Rep. 2012;39:10957–70. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tecle T, Tripathi S, Hartshorn KL. Review: Defensins and cathelicidins in lung immunity. Innate Immun. 2010;16:151–9. doi: 10.1177/1753425910365734. [DOI] [PubMed] [Google Scholar]
- 39.Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81:845–50. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- 40.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 41.López-García B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 42.Zanetti M, Gennaro R, Scocchi M, Skerlavaj B. The Biology and Pathology of Innate Immunity Mechanisms. Boston, MA: Springer; 2002. Structure and biology of cathelicidins; pp. 203–18. [DOI] [PubMed] [Google Scholar]
- 43.Barlow PG, Findlay EG, Currie SM, Davidson DJ. Antiviral potential of cathelicidins. Future Microbiol. 2014;9:55–73. doi: 10.2217/fmb.13.135. [DOI] [PubMed] [Google Scholar]
- 44.Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Söderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–5. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 45.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008;23:328–35. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostman R. Infantile genetic agranulocytosis. A review with presentation of ten new cases. Acta Paediatr Scand. 1975;64:362–8. doi: 10.1111/j.1651-2227.1975.tb03847.x. [DOI] [PubMed] [Google Scholar]
- 47.Pütsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus kostmann: An observation study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, Eto T, et al. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993;194:720–5. doi: 10.1006/bbrc.1993.1881. [DOI] [PubMed] [Google Scholar]
- 49.Kapas S, Bansal A, Bhargava V, Maher R, Malli D, Hagi-Pavli E, et al. Adrenomedullin expression in pathogen-challenged oral epithelial cells. Peptides. 2001;22:1485–9. doi: 10.1016/s0196-9781(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 50.Kapas S, Pahal K, Cruchley AT, Hagi-Pavli E, Hinson JP. Expression of adrenomedullin and its receptors in human salivary tissue. J Dent Res. 2004;83:333–7. doi: 10.1177/154405910408300412. [DOI] [PubMed] [Google Scholar]
- 51.Allaker RP, Grosvenor PW, McAnerney DC, Sheehan BE, Srikanta BH, Pell K, et al. Mechanisms of adrenomedullin antimicrobial action. Peptides. 2006;27:661–6. doi: 10.1016/j.peptides.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Lundy FT, O'Hare MM, McKibben BM, Fulton CR, Briggs JE, Linden GJ, et al. Radioimmunoassay quantification of adrenomedullin in human gingival crevicular fluid. Arch Oral Biol. 2006;51:334–8. doi: 10.1016/j.archoralbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7:1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudney JD, Staikov RK, Johnson JD. Potential biomarkers of human salivary function: A modified proteomic approach. Arch Oral Biol. 2009;54:91–100. doi: 10.1016/j.archoralbio.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhaifalah I, Andrys C, Drahosova M, Musilova I, Adamik Z, Kacerovsky M, et al. Azurocidin levels in maternal serum in the first trimester can predict preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014;27:511–5. doi: 10.3109/14767058.2013.820698. [DOI] [PubMed] [Google Scholar]
- 56.Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–61. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- 57.Awawdeh L, Lundy FT, Shaw C, Lamey PJ, Linden GJ, Kennedy JG, et al. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002;35:30–6. doi: 10.1046/j.1365-2591.2002.00451.x. [DOI] [PubMed] [Google Scholar]
- 58.Dawidson I, Blom M, Lundeberg T, Theodorsson E, Angmar-Månsson B. Neuropeptides in the saliva of healthy subjects. Life Sci. 1997;60:269–78. doi: 10.1016/s0024-3205(96)00627-3. [DOI] [PubMed] [Google Scholar]
- 59.El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200:11–6. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Allaker RP, Zihni C, Kapas S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol. 1999;23:289–93. doi: 10.1111/j.1574-695X.1999.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 61.Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y, et al. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007;42:410–9. doi: 10.1111/j.1600-0765.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 62.Lundy FT, Nelson J, Lockhart D, Greer B, Harriott P, Marley JJ, et al. Antimicrobial activity of truncated alpha-defensin (human neutrophil peptide (HNP)-1) analogues without disulphide bridges. Mol Immunol. 2008;45:190–3. doi: 10.1016/j.molimm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Raj PA, Antonyraj KJ, Karunakaran T. Large-scale synthesis and functional elements for the antimicrobial activity of defensins. Biochem J. 2000;347(Pt 3):633–41. [PMC free article] [PubMed] [Google Scholar]
- 64.Kochańska B, Kedzia A, Kamysz W, Maćkiewicz Z, Kupryszewski G. The effect of statherin and its shortened analogues on anaerobic bacteria isolated from the oral cavity. Acta Microbiol Pol. 2000;49:243–51. [PubMed] [Google Scholar]
- 65.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–38. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 66.Stotz HU, Thomson JG, Wang Y. Plant defensins: Defense, development and application. Plant Signal Behav. 2009;4:1010–2. doi: 10.4161/psb.4.11.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faccone D, Veliz O, Corso A, Noguera M, Martínez M, Payes C, et al. Antimicrobial activity of de novo designed cationic peptides against multi-resistant clinical isolates. Eur J Med Chem. 2014;71:31–5. doi: 10.1016/j.ejmech.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 68.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]


