Abstract
Background:
Diabetes mellitus is a metabolic disease leading to abnormal fat, carbohydrate and protein metabolism. Reduced salivary flow rate caused by hyperglycemia is characteristic mainly for periods of poor metabolic control of diabetes, thereby facilitating the growth of aciduric bacteria and caries-lesion development. The objective of our study was to evaluate the effects of diabetes mellitus on dental caries micro-organisms responsible for caries.
Materials and Methods:
This study was carried out on 60 subjects consisting of 2 groups. The Group A (study group) consisted of 30 subjects with diabetes mellitus and dental caries, and the Group B (control group) consisted of 30 subjects with dental caries but no systemic disease. DFS/dfs index in all subjects was evaluated and compared. Unstimulated salivary flow was collected and levels of Streptococcus mutans were analyzed.
Results:
It was found that the fasting blood sugar in Group A subjects because of which there was increased streptococcus mutans count and hence high caries index as compared to that of Group B.
Conclusion:
From our study, we could conclude that with increased age, blood sugar levels, DMFT values, dental caries increases in diabetics than in normal (control) subjects and therefore relationship does exist between diabetis mellitus, oral microbiota and dental caries.
Keywords: Biochemical tests, culture media, dental caries, diabetes mellitus, microbiological tests, oral microbiota, Robertson's cooked-meat media
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disease leading to abnormal fat, carbohydrate and protein metabolism. Two basic types of primary DM have been described as follows: insulin-dependent DM (IDDM; type 1) and non-IDDM type 2.[1] Several studies have demonstrated that selected caries indicators, such as reduced salivary flow and counts of mutans streptococci, can be related to the metabolic control of DM and therefore may influence the caries process.
Reduced salivary flow rate caused by hyperglycemia is characteristic mainly for periods of poor metabolic control of diabetes. During this period, glucose leakage into the oral cavity may occur, thereby facilitating the growth of aciduric and acidogenic bacteria and caries lesion development.[2] The main complications of DM affect the organs and tissues rich in capillary vessels, such as the kidney, retina and nerves. These complications are secondary to the development of microangiopathy.[3] Similar changes in small vessels can be found in the oral tissues.[4,5]
The World Health Organization (WHO) estimates that the proportions of annual health budgets spent on DM-related illnesses range from 2.5% to 15%. At least 171 million people worldwide have DM. This figure is likely to more than double to 366 million by 2030 (WHO, 2006a).[6] The WHO estimates that the number of diabetics in Iran will rise to 6,421,000 by the year 2030 (WHO, 2006b).[7]
India ranks second with 66.8 million people with diabetes in 2014 following China which ranks first with 96.2 million diabetics. International Diabetes Federation estimates that currently, more than 387 million people worldwide have diabetes and it is estimated to increase to 592 million by 2035.[8,9] Several studies have reported a higher prevalence of caries for participants with DM, particularly for those with poorly controlled DM.[10]
Our main purpose of the study was
To study and correlate the Streptococcus mutans (SM) count with dental caries and
To evaluate the effects of DM on dental caries in diabetic patients.
MATERIALS AND METHODS
Diabetic patients who reported to Rural Dental College, PIMS, Loni, Maharashtra, were included in this study. Case history of the patients was taken and decayed, missing and filled teeth (DMFT) were recorded. Informed consent was signed by the patient before sample collection. The study was approved by the Ethical Committee of Pravara Institute of Medical Sciences (Deemed University), Loni, Maharashtra, India.
Study group (Group A) consisted of 30 diagnosed cases of controlled DM and control group (Group B) consisted of 30 healthy nondiabetic participants with no specific age group. Type 2 diabetic patients with and without habits were included in this study. Patients with other systemic diseases were excluded from the study.
A participant was asked to rinse his/her mouth with water. Unstimulated saliva was collected by contacting the sterilized swab stick with the saliva of the oral cavity and was immediately passed into the Robertson's cooked-meat media and incubated at 37°C for 24 h. Next day, the turbidity appeared in the medium, which was then inoculated on sterile blood agar and MacConkey agar plates, using the sterile inoculating loop. The plates were again incubated at 37°C overnight. If growth occurred, it was studied in detail. Next day, colony characteristics were observed and Gram staining was done. Organisms were identified using standard biochemical tests.
When material containing growth was cultured by plating, the different bacteria were seen to grow as separate colonies, each of which was usually a pure culture descended from a single inoculated cell. Isolation is generally done by careful subculture from a plate culture bearing well-separated colonies. A single colony, suspected from its appearance of being that of a significant or pathogenic organism, was picked with an inoculating wire loop and subcultured in a tube or a plate of fresh sterile culture medium. The morphological and cultural characteristics of the organism were then used to do definitive tests.
Streptococci produced either α-hemolysis or β-hemolysis. The cultured colonies of streptococci were then passed in mannitol sugar for fermentation and kept in the incubator for 24 h. If the sugar after fermentation changed the color from blue to yellow, then it is streptococcus.
RESULTS
Age- and sex-wise distribution of the cases under study showed that for Group A (study group), the average age in years for males was 59.47 and for females was 55.91. For Group B (control group), the average age in years for males was 50.79 and for females was 45.91, with a male: female ratio of 1.19:1 [Table 1].
Table 1.
Age and sex wise distribution of the cases under study in control and study group
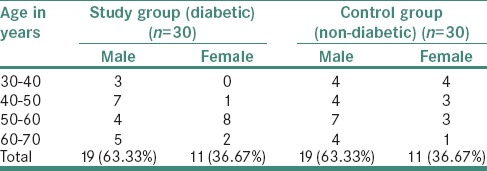
Distribution of organisms according to study and control groups showed that by applying Z-test for difference between two proportions, there was a highly significant difference between proportions of organisms SM, Pseudomonas aeruginosa (PA) and Staphylococcus aureus (SA) in study and control group (i.e., P < 0.01) and significant for organisms, namely, Klebsiella (K) and non-hemolytic streptococci (i.e., P < 0.05) and not significant for organism Enterobacter (E) (i.e., P > 0.05) [Table 2].
Table 2.
Distribution of Organisms according to study and control groups
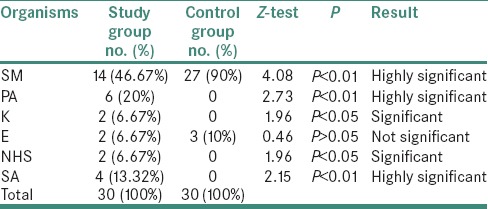
Comparison of mean values of DMFT index in study and control group showed that by applying Student's t-test, there was a highly significant difference between mean values of DMFT score in study and control group (i.e., P < 0.01). Thus, dental caries is more in study group as compared to control group [Table 3].
Table 3.
Comparison of mean values of DMFT index in study and control group

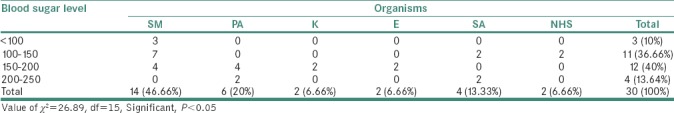
Association/correlation between blood sugar level and organisms in study group showed that by applying Chi-square test, it was found that there was a significant association between fasting blood sugar level and organisms in study group (i.e., P < 0.05). Thus, as fasting blood sugar level increases, organisms also increase [Table 4].
Table 4.
Association/correlation between Blood sugar level and Organisms in Study group. Average fasting Blood Sugar level=153.33
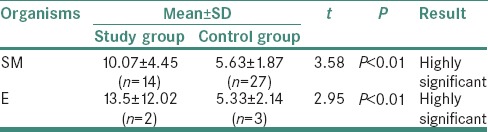
Comparison of mean values of DMFT in control and study group according to organisms showed that by applying Student's t-test, there was a highly significant difference between mean values of DMFT in organism type SM and E in study and control group (i.e., P < 0.01) [Table 5].
Table 5.
Comparison of mean values of DMFT in Control and Study group according to organisms
DISCUSSION
The term “diabetes mellitus” is used to identify a group of disorders characterized by elevated levels of glucose in the blood. This elevation is the result of a deficiency in insulin secretion or an increased cellular resistance to the actions of insulin, leading to a variety of metabolic abnormalities involving carbohydrates, fats and proteins.
Diabetes represents an extreme disturbance in glucose metabolism with severe hyperglycemia and insulin deficiency. A number of oral disorders have been associated with DM such as dental caries, gingivitis, periodontitis, salivary dysfunction, altered taste, oral mucosal diseases and infections such as lichen planus, recurrent aphthous stomatitis and candidiasis.[11,12,13]
The prevention of periodontal breakdown in diabetic patients is mostly based on the education of the individual. Thus, patients should be informed about the importance of oral health for diabetics, and they should be taught that the main symptom of periodontal disease is gingival bleeding.[14] Candidiasis is a manifestation of an immunocompromised state, and a reduction in salivary flow is another risk factor for oral candidiasis.[11]
The relationship between dental caries and DM is complex. Children with type 1 diabetes often are given diets that restrict their intake of carbohydrate-rich, cariogenic foods, whereas children and adults with type 2 diabetes – which often is associated with obesity and intake of high-calorie and carbohydrate-rich food – can be expected to have a greater exposure to cariogenic foods. Furthermore, a reduction in salivary flow has been reported in people with diabetes who have neuropathy.[15] To know the effect of diabetes on dental caries in diabetic and control group, we used the DMFT index and found that the average score was more in diabetics (10.66) than in the control (5.6) group and the results were also statistically significant (P < 0.01). The literature presents no consistent pattern regarding the relationship between dental caries and diabetes.[10,16,17,18,19] However, Jones reported an elevated risk of caries due to DM[19] which was in accordance with our study as we found that there was a highly significant difference between mean values of DMFT score in study and control group (i.e., P < 0.01). Thus, dental caries was more in study group as compared to control group. We also found that in the study group, increased blood sugar levels caused increased SM count and were statistically significant (P < 0.05). Therefore, higher the SM count more is the caries risk.
Siudikiene et al. found that high caries levels in diabetics were significantly associated with age, plaque score and decreased unstimulated salivary flow rate.[2] Reduced salivary secretion increases the likelihood of caries, but good metabolic control prevents the most dangerous salivary changes such as high glucose content and lower pH, while a good diabetic diet, rich in fiber and low in simple carbohydrates, can slow down the production of plaque and the proliferation of acidogenic bacterial microflora.[15,20,21,22,23,24]
Sheridan et al. found that the presence of plaque, calculus, pocket formation, increased tooth mobility and tooth loss occurred with greater frequency in patients with decreased glucose tolerance.[1,25,26,27,28,29]
Twetman et al. demonstrated that diabetes with poor metabolic control developed three times more lesions during the study period than those with better metabolic control which was in accordance with our study, where increased blood sugar levels caused increased SM counts and were statistically significant (P < 0.05).[30]
Orbak et al. suggest that type 1 DM plays a significant role for dentition and oral health in children and adolescents. The children with type 1 DM are more likely to experience infections in connective tissues than children without type 1 DM. This is due to the fact that for children with type 1 DM, infection leads to loss of teeth.[1]
Miko et al. found from their study that poor glycaemic control and the early onset of DM may increase the risk of dental caries, but appropriate oral hygiene together with satisfactory metabolic control may prevent the development of dental caries in adolescents with type 1 DM. According to their study, studied individuals had fewer decayed and more filled teeth.[31]
Sri Kenneth et al. found from their study that decreased salivary pH and an increased incidence of dental caries was observed in participants with uncontrolled diabetes as compared to control group.[32]
Satish et al. found from their study that glycosylated hemoglobin A1c was also determined in both type 2 diabetic patients and control group and a significant correlation was found between HbA1c and serum glucose concentrations in diabetic and control group, respectively.[33]
Seethalakshmi et al. found that the mean DMFT index was higher in the study group when compared to that of the control group, whose findings are similar to our study.[8] This is due to loss of protective mechanism of the saliva in diabetics. The cleansing and buffering action of saliva is also impaired. Low salivary pH promotes the growth of aciduric bacteria which then allows the acidogenic bacteria to proliferate creating an inhospitable environment for the protective oral bacteria. This allows for a shift in the oral environmental balance to favor cariogenic bacteria, which further lowers the salivary pH and the cycle continues.[34]
Studies by Jawed et al. and Akpata et al. have reported increased incidence of dental caries among DM patients.[35,36]
Singh et al. reported that patients with type 2 DM have high rate of dental caries and are at high risk of caries development.[37]
CONCLUSION
We conclude from our study that with increased age, blood sugar levels, DMFT values, dental caries increases in diabetes group than in control group
Thus, a diabetic patient should always see that he/she maintains his/her oral hygiene by following proper toothbrushing habits
Diabetics should take care to see that they get their teeth restored as early as possible, if decayed
They should follow the instructions given by the physician or the dietician for the intake of noncariogenic diet.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Orbak R, Simsek S, Orbak Z, Kavrut F, Colak M. The influence of type-1 diabetes mellitus on dentition and oral health in children and adolescents. Yonsei Med J. 2008;49:357–65. doi: 10.3349/ymj.2008.49.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siudikiene J, Machiulskiene V, Nyvad B, Tenovuo J, Nedzelskiene I. Dental caries and salivary status in children with type 1 diabetes mellitus, related to the metabolic control of the disease. Eur J Oral Sci. 2006;114:8–14. doi: 10.1111/j.1600-0722.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 3.Hanssen KF. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes. 1997;46(Suppl 2):S101–3. doi: 10.2337/diab.46.2.s101. [DOI] [PubMed] [Google Scholar]
- 4.Listgarten MA, Ricker FH, Jr, Laster L, Shapiro J, Cohen DW. Vascular basement lamina thickness in the normal and inflamed gingiva of diabetics and non-diabetics. J Periodontol. 1974;45:676–84. doi: 10.1902/jop.1974.45.9.676. [DOI] [PubMed] [Google Scholar]
- 5.Frantzis TG, Reeve CM, Brown AL., Jr The ultrastructure of capillary basement membranes in the attached gingiva of diabetic and nondiabetic patients with periodontal disease. J Periodontol. 1971;42:406–11. doi: 10.1902/jop.1971.42.7.406. [DOI] [PubMed] [Google Scholar]
- 6.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 7.Regensteiner J, Reush J, Stewart K, Veves A. Dibetes and Exercise. Humana Press, LLC. 2009;5:6. [Google Scholar]
- 8.Seethalakshmi C, Reddy RC, Asifa N, Prabhu S. Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: A cross-sectional study. J Clin Diagn Res. 2016;10:ZC12–4. doi: 10.7860/JCDR/2016/16310.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Diabetes Federation. IDF Diabetes Atlas Update poster. 6th ed. Vol. 10. Brussels, Belgium: International Diabetes Federation; J Clin Diagn Res; 2016. pp. ZC12–4. [Google Scholar]
- 10.Syrjälä AM, Niskanen MC, Ylöstalo P, Knuuttila ML. Metabolic control as a modifier of the association between salivary factors and dental caries among diabetic patients. Caries Res. 2003;37:142–7. doi: 10.1159/000069020. [DOI] [PubMed] [Google Scholar]
- 11.Lamster IB, Lalla E, Borgnakke WS, Taylor GW. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139(10 Suppl):19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 12.Siudikiene J, Maciulskiene V, Nedzelskiene I. Dietary and oral hygiene habits in children with type I diabetes mellitus related to dental caries. Stomatologija. 2005;7:58–62. [PubMed] [Google Scholar]
- 13.Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: II. Prevalence and characteristics of Candida and Candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:570–6. doi: 10.1067/moe.2000.104477. [DOI] [PubMed] [Google Scholar]
- 14.Iughetti L, Marino R, Bertolani MF, Bernasconi S. Oral health in children and adolescents with IDDM – A review. J Pediatr Endocrinol Metab. 1999;12:603–10. [PubMed] [Google Scholar]
- 15.Moore PA, Guggenheimer J, Etzel KR, Weyant RJ, Orchard T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:281–91. doi: 10.1067/moe.2001.117815. [DOI] [PubMed] [Google Scholar]
- 16.Ship JA. Diabetes and oral health: An overview. J Am Dent Assoc. 2003;134:4S–10S. doi: 10.14219/jada.archive.2003.0367. [DOI] [PubMed] [Google Scholar]
- 17.Sampaio N, Mello S, Alves C. Dental caries-associated risk factors and type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metab. 2011;17:152–7. [PubMed] [Google Scholar]
- 18.Collin HL, Uusitupa M, Niskanen L, Koivisto AM, Markkanen H, Meurman JH, et al. Caries in patients with non-insulin-dependent diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:680–5. doi: 10.1016/s1079-2104(98)90035-x. [DOI] [PubMed] [Google Scholar]
- 19.Jones RB, McCallum RM, Kay EJ, Kirkin V, McDonald P. Oral health and oral health behaviour in a population of diabetic outpatient clinic attenders. Community Dent Oral Epidemiol. 1992;20:204–7. doi: 10.1111/j.1600-0528.1992.tb01717.x. [DOI] [PubMed] [Google Scholar]
- 20.Karjalainen KM, Knuuttila ML, Käär ML. Relationship between caries and level of metabolic balance in children and adolescents with insulin-dependent diabetes mellitus. Caries Res. 1997;31:13–8. doi: 10.1159/000262367. [DOI] [PubMed] [Google Scholar]
- 21.Karjalainen KM, Knuuttila ML, Käär ML. Salivary factors in children and adolescents with insulin-dependent diabetes mellitus. Pediatr Dent. 1996;18:306–11. [PubMed] [Google Scholar]
- 22.Got I, Fontaine A. Teeth and diabetes. Diabete Metab. 1993;19:467–71. [PubMed] [Google Scholar]
- 23.Ciglar I, Sutalo J, Sjaljac-Staudt G, Bozikov J. Saliva as a risk factor for caries in diabetic patients. Acta Stomatol Croat. 1991;25:143–9. [PubMed] [Google Scholar]
- 24.Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: A review of the literature. Compend Contin Educ Dent. 2004;25:179. [PubMed] [Google Scholar]
- 25.Goteiner D, Vogel R, Deasy M, Goteiner C. Periodontal and caries experience in children with insulin-dependent diabetes mellitus. J Am Dent Assoc. 1986;113:277–9. doi: 10.14219/jada.archive.1986.0166. [DOI] [PubMed] [Google Scholar]
- 26.Hatun S, Tezic T. Insulin-dependent diabetes mellitus prevalence in ankaradaki school children. Child Sagligiv Journal of Diseases. 1996;39:465–71. [Google Scholar]
- 27.Swanljung O, Meurman JH, Torkko H, Sandholm L, Kaprio E, Mäenpää J, et al. Caries and saliva in 12-18-year-old diabetics and controls. Scand J Dent Res. 1992;100:310–3. doi: 10.1111/j.1600-0722.1992.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 28.Wegner H. Dental caries in young diabetics. Caries Res. 1971;5:188–92. doi: 10.1159/000259746. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan RC, Jr, Cheraskin E, Flyn AC. Epidemiology of diabetes mellitus II 100 dental patients. J Periodontol. 1959;30:298–323. [Google Scholar]
- 30.Twetman S, Petersson GH, Bratthall D. Caries risk assessment as a predictor of metabolic control in young type 1 diabetics. Diabet Med. 2005;22:312–5. doi: 10.1111/j.1464-5491.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 31.Miko S, Ambrus SJ, Sahafian S, Dinya E, Tamas G, Albrecht MG, et al. Dental caries and adolescents with type 1 diabetes. Br Dent J. 2010;208:E12. doi: 10.1038/sj.bdj.2010.290. [DOI] [PubMed] [Google Scholar]
- 32.Sri Kenneth JA, Sanjay R, Peramachi P. Evaluation of correlation between salivary pH and prevalence of dental caries in subjects with and without diabetes mellitus. Res J Recent Sci. 2014;3:224–6. [Google Scholar]
- 33.Satish BN, Srikala P, Maharudrappa B, Awanti SM, Kumar P, Hugar D, et al. Saliva: A tool in assessing glucose levels in diabetes mellitus. J Int Oral Health. 2014;6:114–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Deepak G, Harshaminder K, Manveen KJ, Sonika V, Swati P. Salivary pH and dental caries in diabetes mellitus. Int J Oral Maxillofac Pathol. 2012;3:13–6. [Google Scholar]
- 35.Jawed M, Shahid SM, Qader SA, Azhar A. Dental caries in diabetes mellitus: Role of salivary flow rate and minerals. J Diabetes Complications. 2011;25:183–6. doi: 10.1016/j.jdiacomp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Akpata ES, Alomari Q, Mojiminiyi OA, Al-Sanae H. Caries experience among children with type 1 diabetes in Kuwait. Pediatr Dent. 2012;34:468–72. [PubMed] [Google Scholar]
- 37.Singh I, Singh P, Singh A, Singh T, Kour R. Diabetes an inducing factor for dental caries: A case control analysis in Jammu. J Int Soc Prev Community Dent. 2016;6:125–9. doi: 10.4103/2231-0762.178748. [DOI] [PMC free article] [PubMed] [Google Scholar]


