Abstract
Aim
To identify factors influencing toxicity in patients affected by localized prostate cancer treated with conformal image-guided radiotherapy.
Background
Image guidance in combination with conformal techniques is the standard of care in localized prostate cancer, but factors affecting toxicity are still under investigation.
Materials and methods
294 patients were analyzed. Median age at diagnosis was 71 year. 76 Gy (38 × 2 Gy) were delivered to the target volume. We used the χ2 test to analyse associations between toxicity and dosimetric and clinical parameters. Multivariate analysis was performed using binary logistic regression. Kaplan–Meier method was used for survival analysis.
Results
Median follow-up was 62.9 months. Acute grade ≥2 gastro-intestinal toxicity (GI) was 12.1%. Acute genito-urinary (GU) toxicity of grade ≥2 was 33.9%. Actuarial 4 and 5 years late grade ≥2 GI was 3% and 4%, respectively. Four and 5-year late grade ≥2 GU toxicity was 6% and 10%. At multivariate analysis for acute toxicity rectal V70 was correlated with GI toxicity (p = 0.01, HR 2.73 CI 1.19–6.26), and smoking habit with GU toxicity (p < 0.01, HR 2.50 CI 1.51–4.14). For late toxicity, rectal V70 was correlated with gastro-intestinal toxicity (p = 0.04, HR 4.76 CI 1.07–21.13), and pre-radiotherapy urinary symptoms with genito-urinary toxicity (p = 0.01, HR 2.84 CI 1.29–6.22).
Discussion
Conformal image-guided radiotherapy shows low rates of toxicity. Smoking should be avoided during radiotherapy. Besides the evaluation of high doses received by the organs at risk, individual factors, such as co-morbidities and lifestyle choices, have an impact on normal-tissue complication risk.
Keywords: Cone-beam CT, Conformal radiotherapy, Prostate cancer, Toxicity, Volumetric image-guidance
1. Background
Three-dimensional conformal radiotherapy (3DCRT) is a therapeutic option in the treatment of localized prostate disease, and favorable results have been reported in dose escalation-studies,[1], [2], [3] despite the delivery of high doses to the tumor is associated with an increased risk of acute and late toxicity.[4], [5] In order to decrease the irradiation of organs at risk (OARs) allowing dose escalation to the target volume, new technologies such as intensity-modulated radiation therapy (IMRT) and image guidance have been introduced in the clinical practice.
Regarding the treatment planning and delivery, micro-multi-leaf collimators (micro-MLCs) which are characterized by small leaf width (3–5 mm) improve target dose distribution and normal tissues sparing both in 3DCRT and in IMRT,[6], [7], [8] and volumetric image guidance allows to check the daily treatment reproducibility. More specifically, by using an on-board cone-beam computed tomography (CBCT) system it is possible to match online the pelvic anatomy of the computed tomography (CT) with that of the cone-beam CT, comparing prostate position and rectal and bladder filling.[9], [10] Together with the clinical implementation of new technologies, which will improve the dosimetric features of treatment plans and the radiotherapy delivery, the process of understanding those clinical factors that affect treatment tolerance and toxicity will be crucial for the selection of patients for a more personalized therapy.
2. Aim
In this retrospective study, we report acute and late toxicity in 294 patients affected by localized prostate cancer who underwent 3D conformal image-guided radiotherapy using a micro-MLC (4 mm leaf width at the isocenter) and a linac-integrate kV-cone-beam CT. Furthermore, we sought to identify dosimetric and clinical factors influencing toxicity.
3. Materials and methods
Between December 2006 and April 2016, 294 patients diagnosed with localized prostate cancer were treated with conformal (3DCRT) image guided radiotherapy (IGRT) in our Department. All patients provided informed consent. The clinical details of patients’ cohort are shown in Table 1. Median age at diagnosis was 71 years (interquartile [IQR], 67–74). Median PSA level was 7.7 ng/ml (IQR, 5.3–11.2). All patients had pathologically confirmed prostate cancer and were stratified according to the National Comprehensive Cancer Network Criteria (NCCN): 121 patients were classified in the low-risk group, 69 in the favorable intermediate, 31 in the unfavorable intermediate-risk group and 73 in the high-risk group or locally advanced disease (67 and 6 patients, respectively). Androgen deprivation therapy (ADT) was prescribed in 132 patients at the time of radiotherapy (LH–RH analogues in 75 patients, antiandrogen in 18 patients and total androgen blockade in 39). We collected data about pre-treatment urinary symptoms, co-morbidities (diabetes, colitis, previous abdominal/pelvic surgery), the use of antihypertensive medication and anticoagulants and smoking habitude during radiotherapy.
Table 1.
Patient characteristics (a), and co-morbidities status (b) (294 patients).
| Mean | Median | IQR | No. of patients | ||
|---|---|---|---|---|---|
| (a) Patient characteristics | |||||
| Age (years) | 70 | 71 | 67–74 | ||
| PSA (ng/ml) at diagnosis | 12.7 | 7.7 | 5.3–11.2 | ||
| Clinical stage | T1c | 190 | |||
| T2a | 55 | ||||
| T2b | 19 | ||||
| T2c | 19 | ||||
| T3a | 5 | ||||
| T3b | 6 | ||||
| Gleason score | 6 | 148 | |||
| 7 | 92 | ||||
| 8 | 36 | ||||
| 9 | 16 | ||||
| 10 | 2 | ||||
| Risk class | Low | 121 | |||
| Intermediate | 100 | ||||
| High | 73 | ||||
| Pre-EBRT urinary symptomsa | Yes | 125 | |||
| No | 169 | ||||
| Androgen deprivation therapy | Yes | 132 | |||
| No | 162 | ||||
| (b) Co-morbidities status | |||||
| Diabetes | Yes | 35 | |||
| No | 259 | ||||
| Colitis | Yes | 12 | |||
| No | 282 | ||||
| Smoking abitude | Yes | 112 | |||
| No | 182 | ||||
| Abdominal surgery | Yes | 135 | |||
| No | 159 | ||||
| Antihypertensive medication | Yes | 187 | |||
| No | 107 | ||||
| Anticoagulants | Yes | 104 | |||
| No | 190 | ||||
BRT = external beam radiation therapy.
Stress incontinence, frequency, dysuria.
All patients underwent planning CT with empty rectum and comfortably full bladder, and planning MRI was obtained in 128 patients within 20 min after CT scanning. Treatment protocol was described elsewhere.[11], [12] Briefly, patients underwent planning CT in the supine position using ankle stocks for immobilization. Conformal treatment plans were obtained on the Pinnacle treatment planning system (Philips Medical System, Andover, MA), and were delivered using an Elekta Synergy S linear accelerator equipped with a micro-MLC (Beam Modulator™) and with an on-board kV-cone-beam CT used for volumetric image guidance.
In Table 2, for the whole cohort we reported the median values of the clinical target volume (CTV), the planning target volume (PTV) and those of the bladder and rectum. The CTV included the prostate in the low-risk group, and the prostate plus 2/3 of the seminal vesicles in the intermediate and high-risk groups. PTV was obtained by anisotropic expansion of CTV (5 mm in the posterior direction, 6 mm in all the others). The rectum and the bladder were contoured as solid organs.12 A total dose of 76 Gy (38 × 2 Gy) was delivered to the prostate in low-risk patients, whereas intermediate and high-risk patients received 66 Gy (33 × 2 Gy) to the prostate and 2/3 of the seminal vesicles plus a sequential boost of 10 Gy (5 × 2 Gy) to the prostate only.
Table 2.
Dose–volume histogram parameters (294 conformal plans).
| Median/mean value of organ volume (cc) | Median/mean value of volume (%) | Median/mean value of maximum dose (Gy) | Median/mean value of mean dose (Gy) | |
|---|---|---|---|---|
| (IQR) | (IQR) | (IQR) | ||
| Rectum | 41.3/43.2 (29.6–48.9) | 76.3/76 (75.4–77.3) | 43.5/42.4 (38.6–47.2) | |
| V75 | 3/4.2 (1–7) | |||
| V70 | 13/14 (10–19) | |||
| V60 | 27/26.6 (20–32) | |||
| V50 | 37/35.8 (29–43) | |||
| Bladder | 131.7/165.6 (72.1–177.3) | 78/78 (77.2–79.6) | 37/37.8 (26.7–48) | |
| V75 | 8/10 (4–15) | |||
| V70 | 17/18.8 (10–27) | |||
| V60 | 27/29.3 (17–40) | |||
| V50 | 34/36.3 (22–49) | |||
| CTV | 43.2/45.19 (32–57.2) | |||
| PTV | 132.4/139.7 (110–163.8) |
Toxicity was registered according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Acute toxicity (within 90 days from the start of radiotherapy) and late toxicity (>90 days from the start of radiotherapy) were analyzed, and grade ≥2 toxicity was correlated with clinical and dosimetric parameters. Dose–volume-histograms (DVHs) were used to provide a quantitative analysis. The maximum dose, the mean dose, and a set of appropriate V× (percent of OAR volume receiving the × dose) were evaluated for the rectum and bladder. For statistical analysis, dosimetric parameters were dichotomized by the median value. Concerning clinical variables, the assumption of antihypertensive medication and/or anticoagulants, the smoking habit during radiotherapy, a positive history for diabetes, colitis and previous abdominal surgery were analyzed.
Statistical Package for the Social Sciences version 22.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis. The χ2 test was used to analyse associations between grade ≥2 toxicities and dosimetric and clinical parameters. Multivariate analysis to predict the risk of grade ≥2 toxicity development was performed using binary logistic regression. Statistical significance was assumed at p < 0.05. The survival analysis was performed with the Kaplan–Meier method.
4. Results
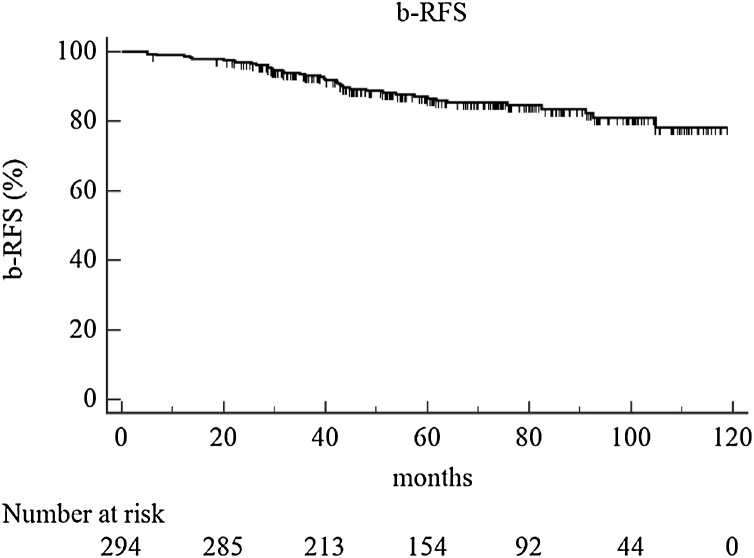
The median follow-up for the whole population was 62.9 months (IQR, 43.2–86.9 months), calculated from the end date of radiotherapy. At 4 and 5-year overall survival (OS) was 94% and 89%, respectively, and cancer specific survival (CSS), 99% and 96%. Biochemical relapse-free survival (b-RFS) at 4 and 5 years was 89% and 87% (Fig. 1). Regarding dose–volume histograms parameters, the median volumes of the CTV, rectum and bladder were 43.2 cc, 41.3 cc and 131.7 cc, respectively (Table 2). Median values of rectum V75, V70, V60 and V50 were 3%, 13%, 27% and 37%, respectively. Median values of bladder V75, V70, V60 and V50 were 8%, 17%, 27% and 34%, respectively.
Fig. 1.
Biochemical relapse-free survival (b-RFS).
Table 3 shows the frequency of acute and late toxicity. More specifically, acute G2 gastro-intestinal toxicity was observed in 11.5% of cases and acute G3 toxicity in 0.6%. Acute genito-urinary toxicity of grade 2 and 3 was reported in 31.9% and 2% of cases, respectively.
Table 3.
Common terminology criteria for adverse events (CTCAE v4.0) toxicity scale (294 patients).
| G1 | G2 | G3 | |
|---|---|---|---|
| Gastro-intestinal | |||
| Acute | 13.6% (40/294) | 11.5% (34/294) | 0.6% (2/294) |
| Late | 1.3% (4/294) | 2.7% (8/294) | 2.3% (7/294) |
| Genito-urinary | |||
| Acute | 30.2% (89/294) | 31.9% (94/294) | 2% (6/294) |
| Late | 7.1% (21/294) | 7.5% (22/294) | 3.4% (10/294) |
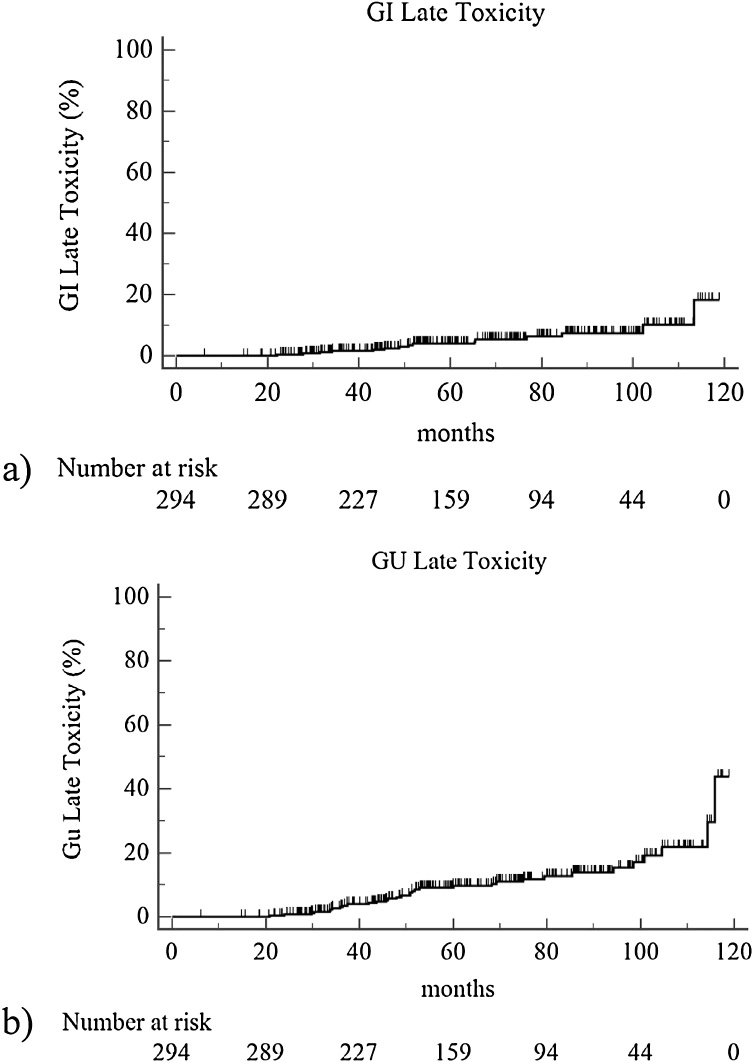
About late toxicity, 2.7% and 2.3% of patients developed a late gastro-intestinal G2 and G3 toxicity, respectively. Late genito-urinary G2 and G3 toxicity was observed in 7.5% and 3.4% of cases. Actuarial 4 and 5 years late grade ≥2 GI toxicity was 3% and 4%, respectively (Fig. 2a), while 4 and 5-year late grade ≥2 GU toxicity was 6% and 10% (Fig. 2b).
Fig. 2.
Actuarial late grade ≥2 toxicity: (a) gastro-intestinal; (b) genito-urinary.
We performed the univariate analysis (χ2 test) for the correlations between toxicity of grade ≥2 and clinical and dosimetric variables (Table 4). Acute GI toxicity is strongly correlated with diabetes (p = 0.043), with the use of anticoagulants (p = 0.024) and with a rectal V70 higher than 13% (p = 0.01). Acute GU toxicity is strongly associated with smoking habitude (p < 0.001), and with a PTV volume higher than 43.2 cc (p = 0.009).
Table 4.
Univariate analysis for CTCAE grade ≥2 toxicity.
| Acute toxicity |
Late toxicity |
|||
|---|---|---|---|---|
| GI | GU | GI | GU | |
| p-value | p-value | |||
| Clinical variables | ||||
| Diabetes (yes vs no) | 0.043* | 0.677 | 0.520 | 0.541 |
| Colitis (yes vs no) | 0.641 | – | 0.063 | – |
| Smoking habitude (yes vs no) | 0.422 | 0.001* | 0.876 | 0.550 |
| Abdominal surgery (yes vs no) | 0.352 | – | 0.952 | – |
| Antihypertensive medication (yes vs no) | 0.730 | 0.385 | 0.421 | 0.698 |
| Anticoagulants (yes vs no) | 0.024* | 0.394 | 0.323 | 0.903 |
| Urinary symptoms pre-EBRT (yes vs no) | – | 0.172 | – | 0.009* |
| ADT (yes vs no) | 0.269 | 0.811 | 0.941 | 0.682 |
| Acute grade ≥2 GI toxicity | – | – | 0.903 | – |
| Acute grade ≥2 GU toxicity | – | – | – | 0.930 |
| Dosimetric variables | ||||
| CTV volume (cc) (<43.2 vs ≥43.2) | 0.965 | 0.009* | 0.194 | 0.211 |
| Rectum | ||||
| Dmax (Gy] (<76.3 vs ≥76.3) | 0.685 | 0.255 | ||
| Dmean (Gy] (<43.5 vs ≥43.5) | 0.306 | 0.064 | ||
| V50 (%) (<37 vs ≥37) | 0.361 | 0.078 | ||
| V60 (%) (<27 vs ≥27) | 0.381 | 0.128 | ||
| V70 (%) (<13 vs ≥13) | 0.001* | 0.004* | ||
| V75 (%) (<3 vs ≥3) | 0.726 | 0.227 | ||
| Bladder | ||||
| Dmax (Gy) (<78 vs ≥78) | 0.463 | 0.242 | ||
| Dmean (Gy) (<37 vs ≥37) | 0.101 | 0.182 | ||
| V50 (%) (<34 vs ≥34) | 0.093 | 0.150 | ||
| V60 (%) (<27 vs ≥27) | 0.111 | 0.138 | ||
| V70 (%) (<17 vs ≥17) | 0.110 | 0.175 | ||
| V75 (%) (<8 vs ≥8) | 0.754 | 0.147 | ||
p < 0.05.
The rectal V70 higher than 13% is also statistically significant in the correlation with late GI toxicity (p = 0.004), whereas late GU toxicity is associated with the presence of urinary symptoms (stress incontinence) before the start of radiotherapy (p = 0.009).
Multivariate analysis for acute toxicity confirmed the importance of rectal V70 correlating with GI toxicity (p = 0.01, HR 2.73 CI 1.19–6.26), and smoking habit correlating with GU toxicity (p < 0.01, HR 2.50 CI 1.51–4.14). Multivariate analysis for late toxicity showed that a high rectal V70 significantly influences the onset of late GI side effects (p = 0.04, HR 4.76 CI 1.07–21.13), and that patients with pre-EBRT urinary symptoms have a high probability to develop late GU toxicity (p = 0.01, HR 2.84 CI 1.29–6.22).
5. Discussion
The strengths of this study are the long follow-up, the homogeneity of patients population and treatment modality, which is characterized by daily rectal and bladder filling procedures, the use of highly conformal 3D plans and online volumetric check of pelvic anatomy and rectal and bladder filling. Limitations are the retrospective analysis and the absence of patient self-assessed toxicity. According to data in literature and taking into account our previous dosimetric analysis on hypofractionated prostate cancer treatment using Elekta Beam Modulator™,8 from May 2016 we used an IMRT technique with daily CBCT image-guidance to deliver a hypofractionated regimen (60 Gy, 20 × 3 Gy) to patients affected by localized prostate cancer.
Data on long-term follow-up are lacking, but several published studies demonstrated the positive impact of IGRT on localized prostate cancer radiotherapy decreasing GI and GU toxicity and improving biochemical tumor control at 2–3 years.[13], [14]
With a median follow-up of 63 months, we had a very low 5 years rectal and bladder toxicity corresponding to 5% and 10%, respectively. We found that current smokers are at a higher risk of developing acute GU toxicity (p < 0.01, HR 2.50 CI 1.51–4.14) compared with non-smokers. Healthy elderly smokers have a significantly higher risk to develop urgency, over-activity and urinary incontinence compared with never smokers.15 This could be explained from the pathophysiological point of view by the chronic damage to the bladder epithelium and microvasculature induced by cigarette smoke. Chronic ischemia significantly increases the production of cytokines, which seems to be responsible for noninfectious cystitis and increased smooth muscle contraction, altering the neuronal regulation of bladder contractility. Eventually, the contractility dysfunction may lead to local functional changes in the contractile component of bladder smooth muscle.
The association between radiotherapy and smoking-related hypoxia may lead to poor wound healing and may contribute to the onset of acute and late GU toxicity. Current smoking has a significant negative impact on both general and disease specific QoL after radiotherapy for prostate cancer.16 Previous reports found a correlation between smoking and genito-urinary symptoms in patients who underwent radiotherapy for pelvic tumors.[17], [18], [19] Thomas et al. showed a statistically significant association between smoking and urinary symptoms after radical radiotherapy for prostate cancer.18 In the study by Solanki et al.,20 smokers had a higher risk of grade 3 late GU toxicity compared with non-smokers. Steinberger et al. found a correlation between current smokers and former smokers and long-term genito-urinary toxicity after external beam radiation therapy (EBRT).19
About acute GU toxicity, there are few studies demonstrating a correlation with smoking habitude during radiotherapy for localized prostate cancer.[21], [22] Cozzarini et al. demonstrate that smoking habitude is a predictor of acute genito-urinary toxicity worsening emptying, frequency, intermittency, urgency and straining.21 In the study by Stankovich et al., smoking status was a predictive factor of acute grade ≥2 GU toxicity.22
Pre-treatment prostate volume may influence the onset of acute GU toxicity in localized prostate cancer radiotherapy.[23], [24] At univariate analysis, we found that a prostate volume >43.2 cc is associated with the risk of developing grade ≥2 acute GU toxicity. In the study by Pinkawa et al., patients with a large prostate volume (between 44 and 151 cc) had an increased risk of developing acute GU toxicity compared with patients with a small prostate volume (between 11 and 43 cc).23 Aizer et al. analyzed acute GU toxicity in 214 patients treated with IMRT for localized prostate cancer. Patients with a prostate volume >50 cc had a higher risk of developing acute G3 genito-urinary toxicity.24
In our series, at multivariate analysis patients with pre-EBRT urinary symptoms (stress incontinence and/or dysuria and/or frequency) had a higher risk for grade ≥2 late GU toxicity (p > 0.01, HR 2.84, CI 1.29–6.22). In the RADAR trial, baseline symptoms were the most predictive parameters of late genito-urinary toxicity.25 Other authors reported a correlation between pre-EBRT urinary symptoms and late incontinence and frequency.[26], [27]
Our data about the correlation between clinical factors and gastro-intestinal toxicity are in agreement with several studies in literature.[28], [4], [29], [30] In fact, we found out that acute GI toxicity is strongly correlated with diabetes and use of anticoagulant therapy. In a recent review,28 diabetes has been reported as a risk factor for rectal toxicity. In the study by Fiorino et al., gastro-intestinal toxicity was associated with diabetes, use of anticoagulants and presence of haemorrhoids.4
Regarding dosimetric parameters, the volume of the rectum receiving high doses is associated with late complications.29 In our series, at multivariate analysis, V70 >13% is associated with acute (p = 0.01, HR 2.73 CI 1.19–6.26) and late (p = 0.04, HR 4.76, CI 1.07–21.13] grade ≥2 GI toxicity. This finding is consistent with the RTOG 0126 trial, in which patients with a planned V70 >15% were at a higher risk of developing late grade ≥2 rectal toxicity.30
6. Conclusions
In summary, patients treated in empty rectum and full bladder conditions with 3D conformal radiotherapy using a micro-MLC and with volumetric image-guidance show low rates of acute and late toxicity. The retrospective nature of this study is hypothesis generating. It seems that current smokers have a higher risk of developing acute genito-urinary toxicity compared with non-smokers. There could be an additive effect of tobacco with EBRT and, therefore, smoking should be avoided during a radiotherapy treatment course. Baseline urinary function should be carefully evaluated with the aim of minimizing the risk of late genito-urinary toxicity. About gastro-intestinal toxicity, besides high doses received by the rectum, individual factors, such as co-morbidities and lifestyle choices, have an impact on normal-tissue complication risk.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Beckendorf V., Guerif S., Le Prisé E. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 2.Soete G., Verellen D., Michielsen D. Image-guided conformal arc therapy for prostate cancer: early side-effects. Int J Radiat Oncol Biol Phys. 2006;66:S141–144. [Google Scholar]
- 3.Moon D.H., Efstathiou J.A., Chen R.C. What is the best way to radiate the prostate in 2016. Urol Oncol. 2016;35:59–68. doi: 10.1016/j.urolonc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Fiorino C., Valdagni R., Rancati T. Dose volume effects for normal tissues in external radiotherapy: pelvis. Radiother Oncol. 2009;93:153–167. doi: 10.1016/j.radonc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Maund I.F., Benson R.J., Fairfoul J. Image-guided radiotherapy of the prostate using daily CBCT: the feasibility and likely benefit of implementing a margin reduction. Br J Radiol. 2014;87:20140459. doi: 10.1259/bjr.20140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carosi A., Ingrosso G., Ponti E. Dosimetric effect of Elekta Beam modulator micromultileaf in three-dimensional conformal radiotherapy, intensity-modulated radiotherapy for prostate cancer. Med Dosim. 2014;39:180–184. doi: 10.1016/j.meddos.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Hoban P., Paskalev K. Dosimetric advantage and clinical implication of a micro-multileaf collimator in the treatment of prostate with intensity-modulated radiotherapy. Med Dosim. 2005;30:97–103. doi: 10.1016/j.meddos.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Carosi A., Ingrosso G., Ponti E. Intensity-modulated and 3D-conformal radiotherapy in hypofractionated prostate cancer treatment using Elekta Beam Modulator™ micro-MLC: a dosimetric analysis. Acta Oncol. 2016;55(1):116–121. doi: 10.3109/0284186X.2015.1046559. [DOI] [PubMed] [Google Scholar]
- 9.Schallenkamp J.M., Herman M.G., Kruse J.J. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys. 2005;63:800–811. doi: 10.1016/j.ijrobp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 10.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Falco M.D., D'Andrea M., Fedele D. Preliminary experience of a predictive model to define rectal volume and rectal dose during the treatment of prostate cancer. Br J Radiol. 2011;84:819–825. doi: 10.1259/bjr/25741415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingrosso G., Carosi A., Ponti E. Acute and late toxicity after three-dimensional conformal image-guided radiotherapy for localized prostate cancer. Cancer Invest. 2014;32:526–532. doi: 10.3109/07357907.2014.970193. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky M.J., Kollmeier M., Cox B. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Guckenberger M., Ok S., Polat B. Toxicity after intensity modulated, image-guided radiotherapy for prostate cancer. Strahlenther Onkol. 2010;186:535–543. doi: 10.1007/s00066-010-2144-z. [DOI] [PubMed] [Google Scholar]
- 15.Nuotio M., Jylhä M., Koivisto A.M. Association of smoking with urgency in older people. Eur Urol. 2001;40:206–212. doi: 10.1159/000049774. [DOI] [PubMed] [Google Scholar]
- 16.Dieperink K.B., Hansen S., Wagner L. Living alone, obesity and smoking: important factors for quality of life after radiotherapy and androgen deprivation therapy for prostate cancer. Acta Oncol. 2012;51:722–729. doi: 10.3109/0284186X.2012.682627. [DOI] [PubMed] [Google Scholar]
- 17.Eifel P.J., Jhingran A., Bodurka D.C. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 18.Thomas R.J., Holm M., Williams M. Lifestyle factors correlate with the risk of late pelvic symptoms after prostatic radiotherapy. Clin Oncol. 2013;25:246–251. doi: 10.1016/j.clon.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Steinberger E., Kollmeier M., McBride S. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU Int. 2015;116:596–603. doi: 10.1111/bju.12969. [DOI] [PubMed] [Google Scholar]
- 20.Solanki A.A., Liauw S.L. Tobacco use and external beam radiation therapy for prostate cancer: influence on biochemical control and late toxicity. Cancer. 2013;119:2807–2814. doi: 10.1002/cncr.28128. [DOI] [PubMed] [Google Scholar]
- 21.Cozzarini C., Rancati T., Carillo V. Multi-variable models predicting specific patient-reported acute urinary symptoms after radiotherapy for prostate cancer: results of a cohort study. Radiother Oncol. 2015;116:185–191. doi: 10.1016/j.radonc.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Stankovic V., Džamic Z., Pekmezovic T. Acute and late genitourinary toxicity after 72 Gy of conventionally fractionated conformal radiotherapy for localised prostate cancer: impact of individual and clinical parameters. Clin Oncol. 2016;28:577–586. doi: 10.1016/j.clon.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Pinkawa M., Fischedick K., Asadpour B. Toxicity profile with a large prostate volume after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:83–89. doi: 10.1016/j.ijrobp.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 24.Aizer A.A., Anderson N.S., Oh S.C. The impact of pretreatment prostate volume on severe acute genitourinary toxicity in prostate cancer patients treated with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;79:379–384. doi: 10.1016/j.ijrobp.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Yahya N., Ebert M.A., Bulsara M. Dosimetry, clinical factors and medication intake influencing urinary symptoms after prostate radiotherapy: an analysis of data from the RADAR prostate radiotherapy trial. Radiother Oncol. 2015;116:112–118. doi: 10.1016/j.radonc.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Peeters S.T., Heemsbergen W.D., van Putten W.L. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005;61:1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 27.Barnett G.C., De Meerleer G., Gulliford S.L. The impact of clinical factors on the development of late radiation toxicity: results from the Medical Research Council RT01 trial (ISRCTN47772397) Clin Oncol. 2011;23:613–624. doi: 10.1016/j.clon.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Budäus L., Bolla M., Bossi A. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Michalski J.M., Gay H., Jackson A. Radiation dose–volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michalski J.M., Yan Y., Watkins-Bruner D. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]