Abstract
Breast cancer is the second leading cause of cancer death in women worldwide. Incurable metastatic breast disease presents a major clinical challenge and is the main cause of breast cancer-related death. The epithelial-mesenchymal transition (EMT) is a critical early promoter of metastasis. In the present study, we identified a novel role for the inhibitor of DNA binding 2 (Id2), a member of the basic helix–loop–helix protein family, during the EMT of breast cancer. Expression of Id2 was positively correlated with Notch3 in breast cancer cells. Low expression of Id2 and Notch3 was associated with worse distant metastasis-free survival in breast cancer patients. The present study revealed that Id2 activated Notch3 expression by blocking E2A binding to an E-box motif in the Notch3 promoter. The Id2-mediated up-regulation of Notch3 expression at both the mRNA and protein levels resulted in an attenuated EMT, which was associated with reduced motility and matrix invasion of ER-positive and -negative human breast cancer cells and the emergence of E-cadherin expression and reduction in the mesenchymal marker vimentin in triple-negative breast cancer cells. In summary, our findings identified Id2 as a suppressor of the EMT and positive transcriptional regulator of Notch3 in breast cancer. Id2 and Notch3 may serve as novel prognostic markers in a subpopulation of ER-positive breast cancer patients.
Introduction
Breast cancer is the most common malignant tumor in women and the second leading cause of cancer death of women worldwide [1], [2]. Different types of breast cancer cells, such as luminal A and B, Her-2, and triple-negative breast cancer (TNBC) display diverse histopathological features, genetic and genomic variability, and clinical outcomes. Metastatic breast cancer remains clinically challenging and very difficult to cure. Recent studies demonstrated that the epithelial-to-mesenchymal transition (EMT) plays a pivotal role in cancer metastasis [3], including breast cancer [4], [5]. The EMT is characterized by the loss of epithelial-like properties, including tight-junction proteins and E-cadherin, and the acquisition of mesenchymal properties, such as the intermediate filament protein, vimentin [6], [7]. These processes increase tumor aggressiveness and enhance the metastatic spread of breast cancer. Therefore, identifying key molecules in the EMT and elucidating the underlying mechanisms of the EMT will ultimately result in novel treatment options to suppress breast cancer metastasis.
Basic helix–loop–helix (bHLH) transcription factors are key regulators of cell cycle progression, proliferation [8], differentiation, and lineage commitment [9]. bHLH proteins are obligate dimers that dimerize through their HLH domains and bind DNA through the basic domains to regulate the transcription of target genes, which contain E-boxes (CANNTG) in their promoters [10]. The E2A gene encodes two class I bHLH members (E12 and E47), which are formed by means of variant splicing [11]. The E2A proteins (E12/E47) regulate the expression of target genes by homodimerization or heterodimerization with Class II bHLH proteins [12], [13]. Previous studies have shown that E12/E47 proteins are distributed broadly in most adult tissues [12]. Specifically, the expression of epithelial markers was repressed upon E12/E47 binding to the E-pal element in the E-cadherin promoter, which resulted in the suppression of the E-cadherin gene [14], [15].
E12 and E47 can be negatively regulated by the class V bHLH Inhibitor of DNA-binding protein family (Id) members, which lack the basic DNA binding element [16], [17]. Upon heterodimerization, Id proteins block the binding of E12 or E47 to DNA. Id1, 2, and 3 are ubiquitously expressed in mammalian tissues [18], [19]. Id proteins have diverse and complex biological effects depending on the cell lineage and differentiation state [20]. Both Id1 and Id2 are involved in the regulation of cell growth and differentiation during the normal development of the mammary gland [21]. Id2-deficient mice display impaired lobule-alveolar development during pregnancy and intrinsic defects in cell proliferation, survival, and lactogenic differentiation [22]. Previous studies demonstrated that overexpression of Id2 could antagonize the TGF-β-mediated induction of the EMT and Id2 has anti-oncogenic potential in normal mouse mammary epithelial cells (NMuMG), murine lens epithelium, and human renal proximal tubule epithelial cells [23], [24], [25]. Id2 regulates cell differentiation, proliferation, development, and tumorigenesis [18], [19], [26]. Signals from the extracellular microenvironment can promote the role of Id2 in tumorigenesis [27], [28], [29]. Overexpression of Id2 is linked to tumor progression in pancreatic cancer, neuroblastoma, and lung cancer [30], [31], [32]. In addition, elevated Id2 protein levels indicate poor clinical outcome in TNBC patients [33]. The cellular localization of Id2 has been recognized as an important factor in determining the outcome. In breast cancer, cytoplasmic, but not nuclear, localization of Id2 protein is associated with reduced invasive capacity and less aggressive tumor phenotypes [34], [35]. It is possible that the regulation, degradation, or localization of the Id2 mRNA and protein all have specific functions during breast cancer. However, this idea requires further clarification.
Notch signaling plays important fundamental roles in normal development. Notch transmembrane receptors (Notch 1–4) signal through ligand (i.e., Jagged and Delta-like family proteins) binding, and then undergo proteolytic cleavage, which results in the release of the Notch intracellular domain (NICD) that then translocates to the nucleus to promote the expression of downstream target genes, such as Hes1 and Hey-1 [36], [37]. Notch signaling has been implicated in many processes, including cell fate determination and oncogenesis. Notch signaling has a role in mammary gland development [38], [39], [40] and Notch3 marks clonogenic mammary luminal progenitor cells in transgenic mice [41]. A recent study connected Notch3 with Wnt signaling by showing that Notch3 can uniquely modulate the expression of the Wnt signaling receptor, Frizzled7 (FZD7) in a human luminal progenitor-enriched subpopulation. This report concluded that Notch3 was considered a key gene for luminal cell commitment [42].
TGF-β is a key cytokine promoting the EMT [43], and bone morphogenetic protein 7 (BMP7) can suppress the EMT by antagonizing TGF-β [44]. BMP7, Id2, and Id3 have also been found to delay the injury-induced EMT of lens epithelial cells [23]. BMP7 has also been reported to abolish TGF-β-mediated alpha-SMA expression by restoring Id2 levels [24]. Ectopic Id2 or Id3 expression renders human mammary carcinoma epithelial cells refractory to growth inhibition and EMT induced by TGF-β. Knockdown of endogenous Id2 or Id3 sensitizes epithelial cells to BMP, leading to a robust growth inhibition and induction of transdifferentiation [20]. Intriguingly, our group recently showed that Notch3 could repress the EMT in breast cancer [45], [46]. Although certain evidence points to a role for Id2 and Notch3 in the maintenance of the epithelial phenotype [47], the relationship between Id2 and Notch3 in human epithelial breast cancer cells remains largely unknown. Public database analysis of a large cohort of breast cancer patients revealed a significant association between Id2 and Notch3 and distant metastasis-free survival (DMFS; http://co.bmc.lu.se/gobo). In the current study, we describe two E-boxes in the Notch3 promoter. We identified E-box 1 as a docking site for the Id2/E2A heterodimer, which leads to the activation of Notch3 expression. Notch3 signaling antagonized the EMT and resulted in the epithelial phenoconversion of ER-/PR-/Her2- TNBC cells. We conclude that Id2 is a novel regulator of Notch3 expression and attenuates the mesenchymal dedifferentiation of human breast cancer cells.
Materials and Methods
Cell Culture and Establishment of Stable Cell Line
MCF-7, T47D, SKBR3, BT549, and MDA-MB-231 cell lines were obtained from the American Type Tissue Collection and cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) (Gibco, CA, USA) and antibiotics. MDA-MB-231-Id2 and the control cell line were generated by stable transfection with 2 ng pcDNA3-Id2 or pcDNA3, respectively, by Lipo2000 (Life Technology, NY, USA) and selection with G418 (1000 μg/ml) for 3 to 4 weeks.
Plasmids Construction and Small Interfering RNA
pCS2.E12, pcDNA3.E47, pCLE.N3ICD, and control vectors were purchased from Addgene (Cambridge, MA, USA). The pcDNA3.Id2 was obtained by cloning a PCR product of full-length human Id2 into the eukaryotic expression vector pcDNA3.1. The Notch3 promoter region (−900 to −650 upstream of exon 1) was subcloned into the KpnI/XhoI sites of the luciferase reporter gene pRL-SV40 (Promega, WI, USA), and named pGL3-Notch3-E1. The region +161 to +292 downstream of exon 1 was subcloned into the KpnI/XhoI sites and named pGL3-Notch3-E2. These Notch3 promoter regions were generated by PCR with the primers listed in Supplementary Table S1. Id2, E2A, Notch3, and control siRNAs were synthesized by the Genepharma Company (Suzhou, China). The oligonucleotides sequences of the siRNAs are listed in Supplementary Table S2.
RNA Purification and Real-Time PCR Analysis
Total RNA was isolated from cells using TRIzol (Life Technology, NY, USA) following the manufacturer's instructions and stored at −80 °C. Reverse transcription was performed using the PrimeScript RT reagent kit (Takara Bio Inc., Dalian, China) according to the manufacturer's instructions. qRT-PCR was performed using SYBR Premix Ex Taq (Takara Bio Inc., Dalian, China) on a CFX96 Real-Time PCR Detection System (Bio-Rad, CA, USA). The primer sequences for qRT-PCR are listed in Supplementary Table S1. PCR reactions were performed at 50 °C for 2 min, 95 °C for 2 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min, 10 min at 72 °C, and finally 4 °C. The relative expression levels for each gene were normalized to β-actin as an internal control.
Immunoblot Analysis
Protein extraction and western blot were performed as described previously [48]. Briefly, cells were lysed in RIPA buffer with 1 mM phenylmethylsulfonyl fluoride and phosphatase inhibitors (5 mM sodium orthovanadate). Protein lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted with primary antibodies at 4 °C overnight. A list of primary antibodies is given in Supplementary Table S3.
Immunofluorescence
Fix cells with 4% paraformaldehyde for 15 minutes at room temperature (RT), RT), wash cells 2 times with 1x phosphate-buffered saline (PBS) at RT, cover with 0.5% triton X-100 (Solarbio, Tongzhou Dist., Beijing, China) 5 to 8 minutes, wash cells 2 times with 1x PBS at RT, blocked with reagent 2.5% goat serum. Solute primary antibody 1:250 by 2.5% goat serum, incubate with primary antibody overnight at 4 °C, washed cells 3 times with PBS, incubate with secondary antibody(1:1000) for 1 hours at RT, wash cells 3 times with 1× PBS at RT. Mount in DAPI (Solarbio, Tongzhou Dist., Beijing, China) 3-5 minutes, wash cells 3 times with 1x PBS at RT. A list of primary antibodies is given in Supplementary Table S3.
Wound Healing Assay
Cells were pretreated with mitomycin C (25 mg/ml) for 30 min before an injury line was made with a 2-mm wide plastic tip on cells plated in culture dishes at 90% confluency. After rinsing in PBS, breast cancer cells were allowed to migrate in complete medium, and photographs were taken (x40) after 24 h (MDA-MB-231) or 48 h (MCF-7). Five randomly chosen widths of the injury line were selected for quantitation.
Transwell Migration and Invasion Assay
Cell culture inserts (8μM pore size; BD, CA, USA) and matrigel invasion chambers (BD, CA, USA) were used according to the manufacturer's instructions. A total of 2 × 105 MDA-MB-231 cells or 5 × 105 MCF-7 cells in serum-free medium were inoculated in the upper chamber after breast cancer cells had been serum-starved for 24 h. Complete medium was added to the bottom chamber. The cells that migrated to the underside of the filter were stained with 0.1% crystal violet after 24 h for MDA-MB-231 and 48 h for MCF-7. For the invasion assays, cells were stained and counted after 48 h for MDA-MB-231 and 72 h for MCF-7. The cell numbers in five different fields for each well were counted independently by two investigators and the average numbers are shown.
Chromatin Immunoprecipitation (ChIP) Assay
Breast cancer cells were fixed with 1% formaldehyde for 10 min at RT, lysed, and sonicated. Samples were precleared for 1 h with protein G beads, and ChIP was performed overnight using an anti-E2A antibody (Cell Signaling Technology, Inc. MA, USA) or control normal rabbit IgG (Santa Cruz Biotechnology, Inc.). After washing and elution of the beads, cross-linking was reversed, and protein was digested with proteinase K. ChIP DNA was isolated using the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany) and analyzed by quantitative PCR using Brilliant SYBR Green Master Mix. The PCR primer pairs were designed to amplify a 250 bp E-box1 fragment and a 132 bp E-box2 fragment (Supplementary Table S1).
Dual-Luciferase Reporter Assay
The luciferase assay was performed using the Dual Luciferase Reporter Assay System (Promega, Madison, USA) according to the manufacturer's instructions. The luciferase activity was measured 48 h after transfection. MCF-7 cells were transiently transfected with pGL3-Notch3-E1 or pGL3-Notch3-E2 in the presence of pCS2.E12, pcDNA3.E47, pcDNA3.Id2, or control vector in 24-well plates and pRL-SV40 (Promega, Madison, USA) was used as a control vector to normalize for the transfection efficiency.
Statistical Analysis
All experiments were performed in triplicate. Data are shown as the mean value ± S.E.M. unless otherwise stated and statistically analyzed by the two-sided Student's t-test. The levels of significance were set at *P < .05 and **P < .01. The survival curves were plotted using the Kaplan–Meier method, and statistical relevance between groups was calculated using the log-rank test.
Results
Id2 is Co-Expressed With Notch3 and Up-Regulates Notch3 Expression in Breast Cancer Cells
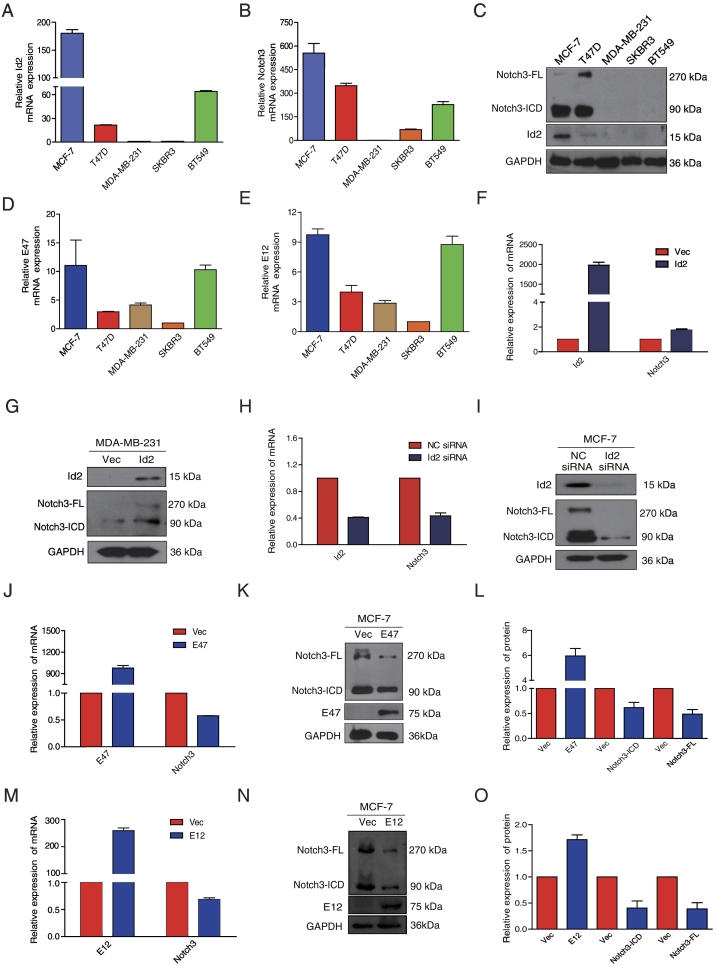
The Gene Set Analysis (GSA)-Cell line database revealed the Id2 mRNA expression levels in a panel of human breast cancer cell lines [49]. GSA-Cell lines clustered by genes selected by PAM50 analysis were identified as luminal (ERBB3- and ESR1-positive) or basal-like (ESR1-negative, CAV1-positive). The basal-like cell lines contained two major subdivisions: basal A (KRT5- and KRT14-positive) and basal B (VIM-positive). Elevated Id2 expression was observed in the luminal and basal A subgroups compared to the basal B subgroup (Supplementary Fig. S1A). Similar expression profiles were obtained for Notch3 in the different subgroups (Supplementary Fig. S1B). Consistent with the GSA-Cell line database, we confirmed higher expression of both Id2 and Notch3 mRNA and protein in ER+ breast cancer cells (MCF-7 and T47D) but lower expression in ER- (MDA-MB-231 and BT549) or HER2-positive (SKBR3) cell lines (Figure 1, A–C). More importantly, lower levels of Id2 had poor prognoses in the subgroup of luminal B, ER(+) and lymph node negative patients as evidenced by shorter DMFS (all P < .05, Supplementary Fig. S1,C-F). Intriguingly, a low expression level of Notch3 was also associated with shorter DMFS (database ID: 25379, n = 358, P = .02201, Supplementary Fig. S1,G). Thus, these data suggested there might be a potential link between Id2 and Notch3 in ER+ breast cancer.
Figure 1.
Id2, E12 and E47 dysregulate Notch3 expression in breast cancer cells. (A, B) The mRNA expression level of Id2 and Notch3 was analyzed by qRT-PCR in breast cancer cell lines. (C) Id2 and Notch3 protein levels were analyzed by western blot in breast cancer cell lines. (D, E) The mRNA expression level of E12 and E47 was analyzed by qRT-PCR in breast cancer cell lines. (F, G) Expression of Id2 and Notch3 mRNA and protein in Id2 overexpressing MDA-MB-231 cells were analyzed by qRT-PCR and western blot, respectively. (H, I) The expression of Id2 and Notch3 mRNA and protein in Id2 knock-down MCF-7 cells were analyzed by qRT-PCR and western blot, respectively. (J, K, L) The expression of E47 and Notch3 in MCF-7 cells was analyzed by qRT-PCR and western blot 48 h after transfection of pcDNA3-E47. (M, N, O) The expression of E12 and Notch3 in MCF-7 cells was analyzed by qRT-PCR and western blot 48 h after transfection of pCS2-E12.
We further investigated a potential role for Id2 in the regulation of Notch3 expression by either silencing or overexpressing Id2 in human breast cancer cells. Overexpression of Id2 in MDA-MB-231 increased Notch3 mRNA expression by 1.75 fold (Figure 1F), which resulted in an increase in full-length Notch3 (Notch3-FL) and Notch3-ICD protein levels (Figure 1G). In contrast, Id2 silencing resulted in a 57% decrease in Notch3 mRNA expression (Figure 1H). Notch3-FL and ICD proteins were also significantly down-regulated in MCF-7 cells (Figure 1I). These results demonstrated a consistent trend in Notch3 expression by either up-regulation or down-regulation of Id2 in breast cancer cells.
Id2 Up-Regulates Notch3 by Antagonizing E2A
To investigate whether Id2 regulated Notch3 expression by antagonizing E2A, a negative regulator of the DNA-binding protein family, we try to find the relation among Id2, Notch3 and E2A. In accordance with the expression of Id2 and Notch3, the expression of E2A (E12/E47) was higher in MCF-7 than MDA-MB-231 cells (Figure 1, D–E). Then we expressed either E47 or E12 in MCF-7 cells. We found that Notch3 mRNA was reduced by ectopic E47 expression (42%, Figure 1J) or E12 expression (31%, Figure 1M). Accordingly, the protein levels of Notch3-ICD and Notch3-FL were decreased by 38% and 51% in E47-expressing MCF-7 cells (Figure 1, K–L), respectively, or 60% and 61% in E12-expressing MCF-7 cells (Figure 1, N–O), respectively.
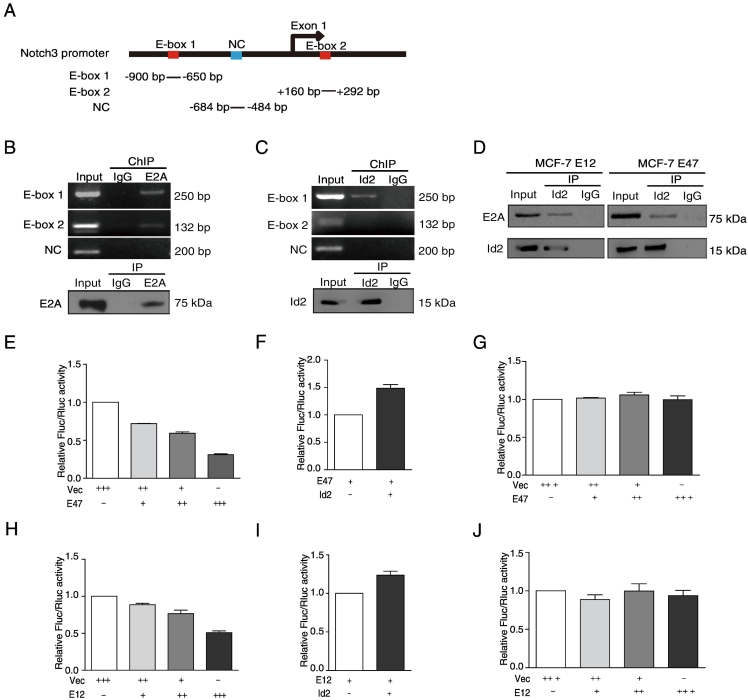
We identified two E-boxes (CANNTG) as potential binding sites for the Id2/E2A heterodimer [10] that were located at −799 to −783 bp (E-box 1) and+ 192 to +197 bp (E-box 2) in exon one of Notch3 (Figure 2A). First, western blot results confirmed that E2A and Id2 antibodies efficiently pull-down E2A and Id2 protein from the IP complex (Figure 2, B and C). Next, we performed ChIP assays with primer pairs spanning the two E-box binding sites to investigate whether Id2 and E2A bind to the Notch3 promoter. Our ChIP data revealed a strong binding of E2A to E-box 1 and E-box 2 (Figure 2B), but strong binding of Id2 to only E-box1 (Figure 2C). To investigate whether Id2 forms a complex with E2A, IP analyses were performed in E47 and E12 expressing MCF-7 cells. The data demonstrated that both E47 and E12 proteins bound to endogenous Id2 (Figure 2D).
Figure 2.
E2A inhibits Notch3 expression by antagonizing Id2. (A) Schematic representation of the E-box binding sites in the Notch3 promoter. (B, C) The ChIP assays for MCF-7 cells were performed with anti-E2A and anti-Id2 antibodies. The pull-down of E2A or Id2 protein from the IP complex was analyzed by western blot using E2A or Id2 antibodies. Rabbit IgG was used as a control for the ChIP assays. (D) The Co-IP assay for E12- or E47-expressing MCF-7 cells was performed using an anti-Id2 antibody to pull-down the protein complex, and then E2A and Id2 antibodies were used for western-blot. Rabbit IgG was used as a control for the IP assay. (E, H) After co-transfection of different amounts of pcDNA3-E47 or pCS2-E12 2/5 in the presence of reporter plasmids pGL3-Notch3-E1 in MCF-7 cells, dual-luciferase reporter assays were performed to detect the luciferase activity in the cell lysates. (F, I) Dual-luciferase reporter assays were also performed on MCF-7 cells after cotransfection of pcDNA3-E47 or pCS2-E12 2/5 with pcDNA3.1.Id2 and reporter plasmid pGL3-Notch3-E1. (G, J) Dual-luciferase reporter assays were also performed on MCF-7 cells after cotransfection of pcDNA3-E47 or pCS2-E12 2/5 with pGL3-Notch3-E2.
To address whether Id2 antagonizes E2A to the E-box in the Notch3 promoter, pGL3-Notch3-E1 and pGL3-Notch3-E2 luciferase reporter systems were employed. Both ectopic E47 and E12 expression decreased the luciferase activity of pGL3-Notch3-E1 by 1.4 to 3.22 fold in the E47-transfected MCF-7 cells (Figure 2E) and 1.14 to 2.0 fold in the E12-transfected MCF-7 cells (Figure 2H) in a dose-dependent manner. The inhibitory effect of E47 and E12 on the luciferase activity of pGL3-Notch3-E1 was partially rescued by Id2 (Figure 2, F and I). However, both ectopic E47 and E12 expression had no effects on the luciferase activity in the MCF-7 cells containing the pGL3-Notch3-E2 luciferase construct (Figure 2, G and J). Taken together, these data suggest that Id2 up-regulates the expression of Notch3 at the transcriptional level through an interaction with E2A at the binding site of E-box 1 in the Notch 3 promoter.
Concordance of Id2 and Notch3 Expression in TGF-β-Induced EMT or BMP-Induced MET Models
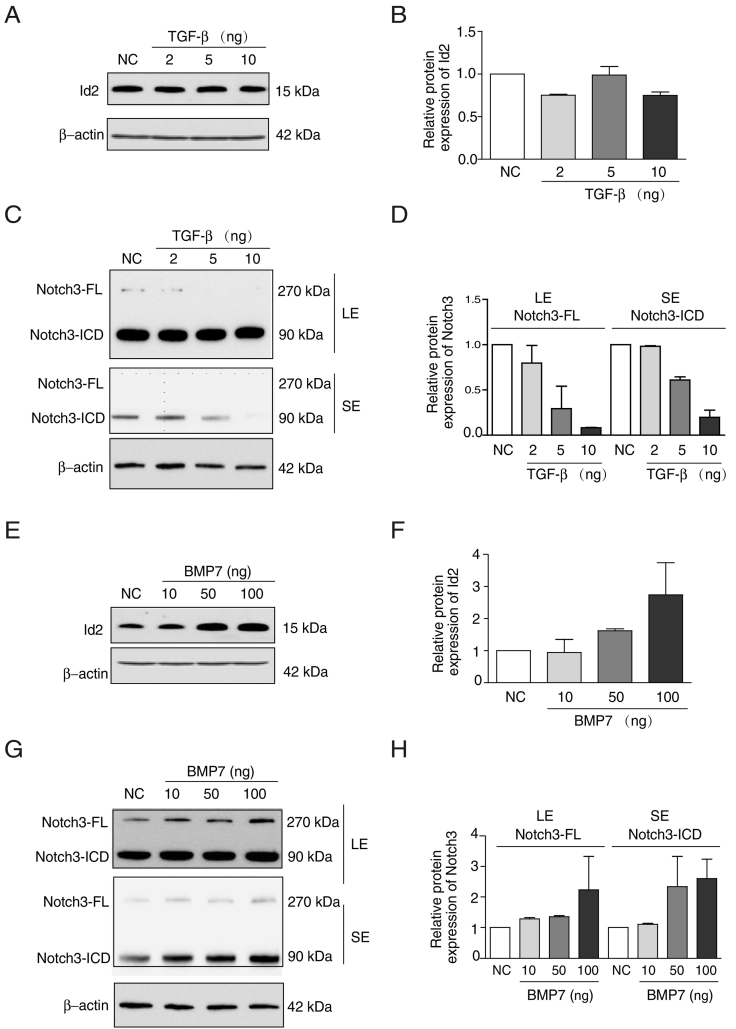
To investigate the roles of Id2 and Notch3 in the progression of the EMT, we treated MCF-7 cells with TGF-β and quantified the expression of Id2 and Notch3. TGF-β treatment decreased the Id2 protein level by more than 30% (Figure 3, A and B), and Notch3-FL and Notch3-ICD protein levels by over 80% in a dose-dependent manner (Figure 3, C and D). Treatment of MCF-7 cells with BMP7 caused a dose-dependent increase of Id2 (up to 2.7 fold) (Figure 3, E and F) and Notch3 (Notch3-FL and Notch3-ICD, 2.3 fold; Figure 3, G and H) protein levels.
Figure 3.
Alteration of Id2 and Notch3 expression in EMT or MET models. (A, B, C, D) The expression of Id2 and Notch3 proteins were analyzed by western blot following the treatment of MCF-7 with TGF-β (2, 5, 10 ng/ml) for 72 h. LE: Long Exposure; SE: Short Exposure. (E, F, G, H) Id2 and Notch3 proteins were analyzed by western blot following treatment of MCF-7 cells with BMP7 (10, 50, 100 ng/ml) for 72 h. LE: Long Exposure; SE: Short Exposure.
Overexpression of Id2 Increases Epithelial Marker and Knock-Down of Id2 Induces Mesenchymal Markers
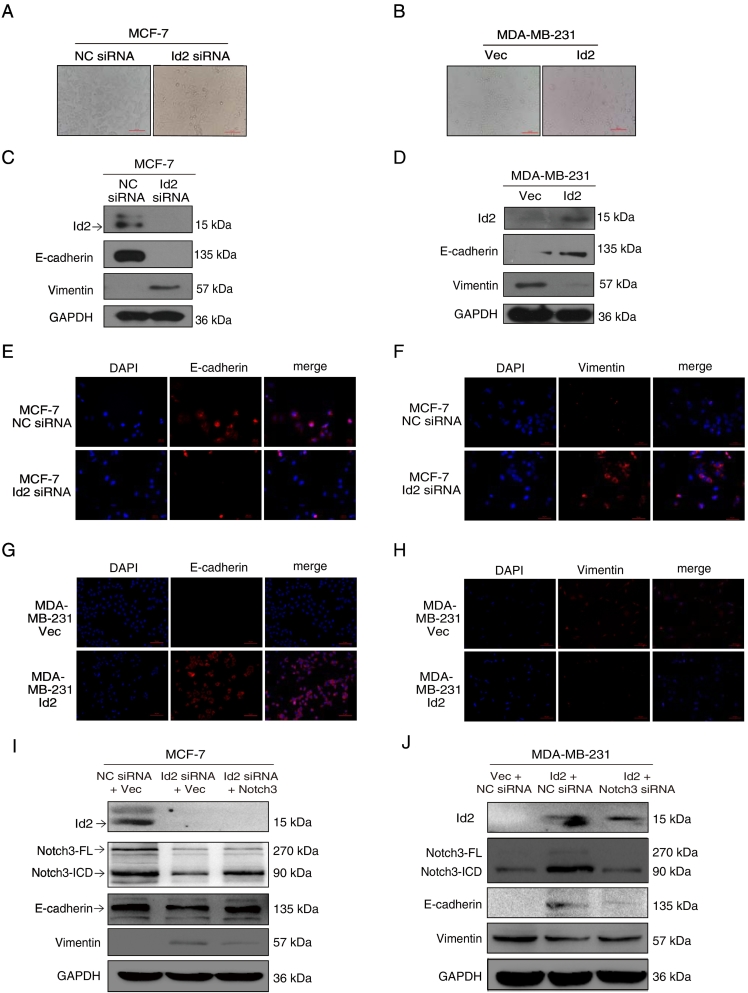
To investigate the effect of Id2 on the epithelial-mesenchymal phenoconversion of breast cancer cells, we knocked down Id2 with siRNA in MCF-7 cells or overexpressed Id2 in MDA-MB-231 cells. We observed morphological changes associated with the EMT in MCF-7 cells following Id2 knockdown. Compared to MCF-7 cells, the morphology of Id2 siRNA-expressing cells was changed from an epithelial-like phenotype to a fibroblast-like morphology (Figure 4A). In addition, these cells had decreased E-cadherin and increased vimentin as demonstrated by western blot and immunofluorescence (Figure 3, C-F). In contrast, MDA-MB-231 cells, which normally have a spindle-shaped, mesenchymal morphology, exhibited a cobblestone-like, epithelial phenotype when expressing Id2 (Figure 4B). These Id2-expressing cells had increased E-cadherin and decreased vimentin as shown by western-blot and immunofluorescence (Figure 3, D–H). Furthermore, when Notch3 was over-expressed in the silincing-Id2 MCF-7 cell, the expression of E-cadherin was partially rescued and vimentin was re-inhibited (Figure 4I), while opposite change of E-cadherin and vimentin were observed when Notch3 was silenced in the overexpressing-Id2 MDA-MB-231 cell (Figure 4J), suggesting that Id2 inhibits EMT through the activation of Notch3 in breast cancer.
Figure 4.
Id2/Notch3 axis attenuated EMT. (A, B, C, D) After the silencing of Id2 by siRNA or transfecting of the Id2 expression vector in MCF-7 for 48 h, the representative micrographs (×200) were taken, and E-cadherin and vimentin expression were analyzed by western blot. (E, F, G, H) Immunofluorescence was also performed to analyze the expression of E-cadherin and vimentin in Id2 siRNA-expressing MCF-7 cells or Id2-expressing MDA-MB-231 cells. (I) Following transfection with Id2 siRNA or cotransfection of Id2 siRNA and pCLE.Notch3-ICD in MCF-7 cells, Id2, Notch3, E-cadherin and vimentin expression were analyzed by western blot. (J) Following cotransfection of the Id2 expression vector and Notch3 siRNA in MDA-MB-231 cells, Id2, Notch3, E-cadherin and vimentin expression were analyzed by western blot.
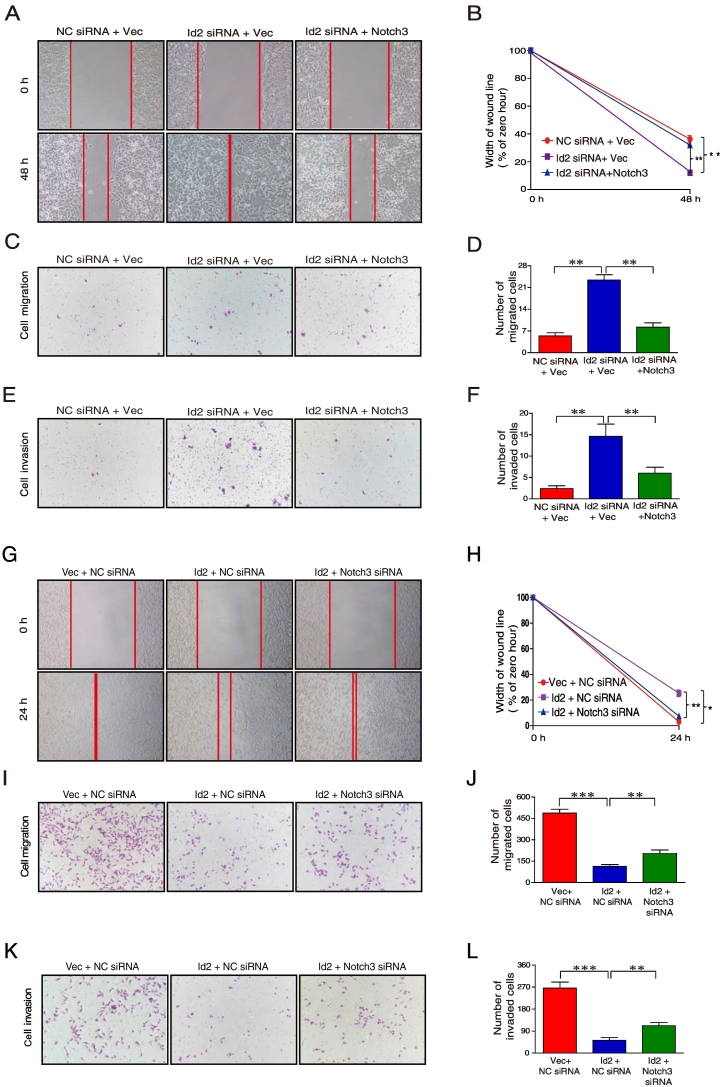
Id2/Notch3 Axis Inhibited Cell Motility and Cell Invasion
Wound healing and matrix invasion assays were performed to investigate the effect of the Id2/Notch3 axis on the motility and invasiveness of MCF-7 cells. Wound healing assays showed that Id2 knockdown increased the cellular mobility by 22.3% whereas overexpression of Notch3 attenuated this increase (Figure 5, A and B). Id2 silencing caused a 4.3-fold increase in migration (Figure 5, C and D) and a 3.2-fold enhancement of invasion (Figure 5, E and F) by MCF-7 cells compared to scrambled siRNA. Ectopic Notch3-ICD expression was sufficient to attenuate the increase the migration and invasion of MCF-7 (Figure 5, C–F). Conversely, wound healing assays revealed a 22% decrease in cell motility upon ectopic Id2 expression in MDA-MB-231, which was partially rescued by specific knockdown of Notch3 (Figure 5, G and H). Upon overexpression of Id2 in MDA-MB-231 cells, transwell migration was reduced by 78% (Figure 5, I and J) and invasion by 89% (Figure 5, K and L). Intriguingly, specific silencing of Notch3 by siRNA blocked the inhibitory effects of Id2 overexpression on the migration and invasion of MDA-MB-231 cells (Figure 5, I–L). Thus, these data suggest that the inhibitory effect of Id2 on EMT is partially mediated by Notch3.
Figure 5.
Id2 expression inhibited EMT via Notch3 and triggered phenoconversion. (A, B, C, D, E, F) Following transfection with Id2 siRNA or cotransfection of Id2 siRNA and pCLE.Notch3-ICD in MCF-7 cells, representative micrographs (×200) were taken to document cell motility in wound healing assays and transwell assays for migration and invasion. (G, H, I, J, K, L) Following cotransfection of the Id2 expression vector and Notch3 siRNA in MDA-MB-231 cells, representative micrographs (×200) were taken to document cell motility in wound healing assays and transwell assays for migration and invasion. All experiments were performed at least three times, and data were statistically analyzed by the two-sided t test. *, P < .05; **, P < .01; ***, P < .001. Error bars indicate S.E.M.
Discussion
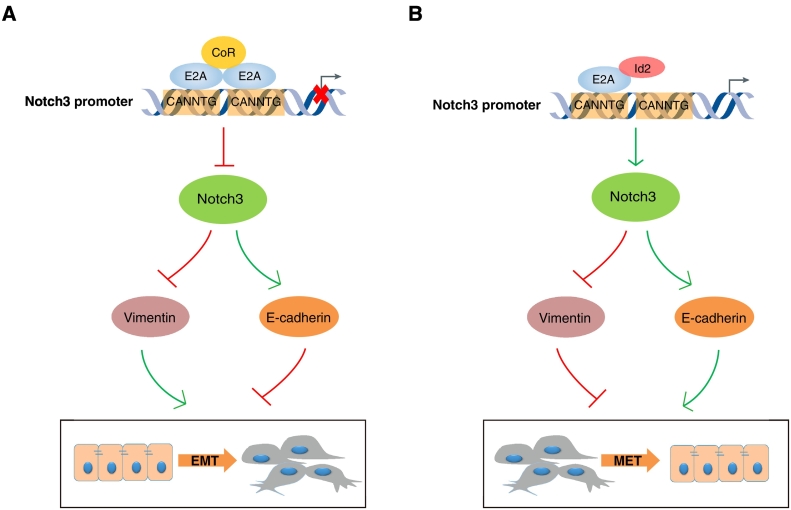
The identification of molecules and mechanisms that regulate the invasive phenotype of breast cancer cells is important for understanding tumor metastasis and to identify novel therapeutic targets. Both Notch3 [41], [50] and Id2 [34] are indispensable during the development of the mammary gland. In this study, we showed coexpression of Id2 and Notch3 in various human breast cancer lines with higher expression in the luminal type compared to basal-like breast cancers. As shown in Figure 6, we found that Id2 blocked the binding of the bHLH transcriptional factor E2A to E-box 1 of the Notch3 promoter and increased Notch3 gene activity. Concomitant up-regulation of Id2 and Notch3 diminished the migration and invasion of breast cancer cells, which was associated with a mesenchymal-to-epithelial (MET) phenoconversion. A public GSA-Patient database search (http://co.bmc.lu.se/gobo) of a cohort of breast cancer patients revealed that patients in the Id2+ (database: 26582 and 26,740) and Notch3+ (database: 25379) luminal B primary breast cancer subgroup had longer distant metastasis-free survival (DMFS; all P < .05, Supplementary Fig. S1, C-E). This association suggested that Id2 and Notch3 may be potential prognostic biomarkers for a subgroup of ER+ breast cancer patients.
Figure 6.
Proposed model of how Id2 and Notch3 attenuate EMT in breast cancer. (A) the E2A-E2A homodimerization with the co-repressors (CoR) binds to E-box sequences of Notch3 promoter and inactivates Notch3 transcription, permitting the repression of E-cadherin and up-regulation of vimentin to ultimately induce EMT in breast cancer. (B) the Id2-E2A heterodimerization E2A complex releases the binding with E-box of Notch3 promoter and activates Notch3 transcription, permitting the repression of vimentin and up-regulation of E-cadherin to attenuate EMT in breast cancer.
This study is the first report to find two E-box motifs (−788 to −793 and+ 192 to 197) within the Notch3 promoter that serve as binding sites for the bHLH factor E2A, a negative transcriptional regulator of Notch3. The E-box 1 motif (−788 to −793) might serve as a docking site for Id2, which competes with E2A. Id2 binding effectively antagonized E2A transcriptional repression and allowed the activation of Notch3 transcription (Supplementary Fig. S1G). This finding is consistent with previous reports that Id2 is a negative regulator of E2A for the activation of downstream target genes [23], [24], [25].
Intriguingly, we found that the Id2/Notch3 axis plays a novel role in the epithelial–mesenchymal and mesenchymal-epithelial transition. Several TGF-β or BMP epithelial phenoconversion models showed that Id2 plays a key role in maintaining a MET-like epithelial phenoconversion [20], [24], [34], [47]. Moreover, Id2 is part of a network of interacting molecular switches that governs mammary epithelial cell phenotypes [21] (i.e., prevents down-regulation of E-cadherin and β4 integrin [47], [51]). Additionally, Ohashi et al. reported that Notch3 is a key factor that limits the overexpression of ZEBs, which are transcription factors that are essential for TGF-β-mediated EMT [52]. Notch3 could be involved in luminal cell differentiation by modulating Wnt signaling via FZD7 [42]. Thus, addressing the interplay between Id2 and Notch3 may be a significant mechanism for EMT. Indeed, our study identified a novel Id2-Notch3-EMT axis whereby the inhibitory function of Id2 on E2A facilitates up-regulation of Notch3 expression, and this opposes functional EMT.
The Id2-Notch3-EMT axis revealed in the present study together with our recent publication on Notch3 [45], [46] and previous report on Id2 [34] indicate that Id2 and Notch3 signaling might be important in the progression of breast cancer. However, previous reports for the role of Id2 or Notch3 in the migration or invasion of breast cancer cells remain controversial. Differentiated breast cancer cells, such as T47D and MCF-7, strongly express Id2 and Notch3. In contrast, the mesenchymal and invasive MDA-MB-231 has very low Id2 and Notch3 protein levels. Ectopic expression of Id2 in MDA-MB-231 and MDA-MB-436 markedly reduced the invasiveness of these TNBC cells [34], [35]. We confirmed that overexpression of Id2 in MDA-MB-231 cells coincided with a reduced migratory and invasive phenotype and up-regulation of Notch3. Ectopic Id2 expression appeared to initiate a complex epithelial-like phenoconversion with a reduced migratory behavior of MDA-MB-231 cells. These results differed markedly from the aggressive phenotype associated with the up-regulation of Notch3. Human mammary epithelial cells with ectopic Wnt-1 expression showed tumorigenic transformation accompanied by Notch activation and elevated expression of Notch members, including Notch3 [53]. In MCF-7 cells, autocrine IL-6 triggered Notch3-dependent mammosphere formation, resistance to hypoxia, and invasive behavior [54]. Notch3 signaling is associated with an invasive/metastatic phenotype and poor survival in patients with hepatocellular carcinoma [55]. In contrast, high Notch3 transcript levels correlated with better overall survival only in ER+ breast cancer patients [56]. These controversial reports did not investigate the involvement of Id2 and suggest a potential Id2-independent regulation of the Notch3 gene by other factors (e.g., Wnt, IL6). In this study, the enhanced cell migration/invasion upon Id2 silencing is critically rescued by the presence of Notch3, which supports a role for Notch3 in the prevention of the aggressive breast cancer behavior.
The association of Id2 expression with disease severity and poor prognosis is contradictory and differs in different human tumor types. Although the reasons are largely unknown, recent reports suggest that not only the overall Id2 levels but the specific intracellular localization of Id2 in tumor cells may be critical in assessing Id2 function. A negative correlation was reported for Id2 protein expression and histological grade in breast cancer [51] whereas tissue immunodetection studies identified Id2 as a predictor of poor prognosis in TNBC [33]. The intensity of the cytoplasmic, but not nuclear, staining of Id2 protein was positively correlated with patient survival [35]. Recently, qRT-PCR analysis of 2688 genes in breast cancer tissue (n = 80) and histologically normal epithelium samples (n = 16) revealed that the expression of Id2 was reduced in luminal type A and B breast cancer patients [57]. For Notch3, high transcript levels correlated with better overall survival only in ER+ breast cancer patients (n = 3554) [56]. We also demonstrated that breast cancer patients with moderate to high Id2 and Notch3 mRNA expression had significantly longer distant metastasis-free survival (DMFS) when using the GSA-Patient database. Cumulatively, these data suggest that Id2 and Notch3 may qualify as predictors of better prognosis in ER+ breast cancer patients.
In summary, the results of this study investigated whether Id2 was a novel positive regulator of Notch3 by blocking E2A binding to an E-box motif in the Notch3 promoter. The Id2/Notch3 axis promotes a differentiated non-invasive phenotype in breast cancer cells, and both Id2 and Notch3 may serve as novel biomarkers of good prognosis for breast cancer subtypes.
The following are the supplementary data related to this article.
Supplementary Table S1. Primers Sequences.
Supplementary Table S2. RNAi Sequences.
Supplementary Table S3. Antibodies.
Expression of Id2 and Notch3 in breast cancer cell lines and its prognostic value in a breast cancer patient cohort.
Conflict of Interest
None of the authors have any potential conflicts to disclose.
Acknowledgements
The study was supported by the Guangdong Provincial Key Laboratory on Breast Cancer Diagnosis and Treatment Research, Canadian Cancer Society Research Institute (CCSRI), and the Natural Sciences and Engineering Council of Canada (NSERC). This study was funded in part by grants from Major International Collaborative Research Project of NSFC (81320108015), research team project of Natural Science Foundation of Guangdong Province (2016A030312008), special fund for public welfare research and capacity building in Guangdong Province (2014A020210026), and the Canadian Breast Cancer Foundation (CBCF).
Contributor Information
Thomas Klonisch, Email: Thomas.Klonisch@umanitoba.ca.
Guo-Jun Zhang, Email: gjzhang@xah.xmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, Boyle P, Levi F. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol. 2010;21(6):1323–1360. doi: 10.1093/annonc/mdp530. [DOI] [PubMed] [Google Scholar]
- 3.Serrano MJ, Ortega FG, Alvarez-Cubero MJ, Nadal R, Sanchez-Rovira P, Salido M, Rodriguez M, Garcia-Puche JL, Delgado-Rodriguez M, Sole F. EMT and EGFR in CTCs cytokeratin negative non-metastatic breast cancer. Oncotarget. 2014;5(17):7486–7497. doi: 10.18632/oncotarget.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamiya T, Goto A, Kurokawa E, Hara H, Adachi T. Cross talk mechanism among EMT, ROS, and histone acetylation in phorbol ester-treated human breast cancer MCF-7 cells. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/1284372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/beta-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iavarone A, Garg P, Lasorella A, Hsu J, Israel MA. The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev. 1994;8(11):1270–1284. doi: 10.1101/gad.8.11.1270. [DOI] [PubMed] [Google Scholar]
- 9.Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13(8):4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty T, Brennan TJ, Li L, Edmondson D, Olson EN. Inefficient homooligomerization contributes to the dependence of myogenin on E2A products for efficient DNA binding. Mol Cell Biol. 1991;11(7):3633–3641. doi: 10.1128/mcb.11.7.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Pooter RF, Kee BL. E proteins and the regulation of early lymphocyte development. Immunol Rev. 2010;238(1):93–109. doi: 10.1111/j.1600-065X.2010.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9(3):175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 14.Beck K, Peak MM, Ota T, Nemazee D, Murre C. Distinct roles for E12 and E47 in B cell specification and the sequential rearrangement of immunoglobulin light chain loci. J Exp Med. 2009;206(10):2271–2284. doi: 10.1084/jem.20090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66(19):9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 16.Biggs J, Murphy EV, Israel MA. A human Id-like helix-loop-helix protein expressed during early development. Proc Natl Acad Sci U S A. 1992;89(4):1512–1516. doi: 10.1073/pnas.89.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellmeier W, Aguzzi A, Kleiner E, Kurzbauer R, Weith A. Mutually exclusive expression of a helix-loop-helix gene and N-myc in human neuroblastomas and in normal development. EMBO J. 1992;11(7):2563–2571. doi: 10.1002/j.1460-2075.1992.tb05321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 19.Ling F, Kang B, Sun XH. Id proteins: small molecules, mighty regulators. Curr Top Dev Biol. 2014;110:189–216. doi: 10.1016/B978-0-12-405943-6.00005-1. [DOI] [PubMed] [Google Scholar]
- 20.Kowanetz M, Valcourt U, Bergstrom R, Heldin CH, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004;24(10):4241–4254. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrinello S, Lin CQ, Murata K, Itahana Y, Singh J, Krtolica A, Campisi J, Desprez PY. Id-1, ITF-2, and Id-2 comprise a network of helix-loop-helix proteins that regulate mammary epithelial cell proliferation, differentiation, and apoptosis. J Biol Chem. 2001;276(42):39213–39219. doi: 10.1074/jbc.M104473200. [DOI] [PubMed] [Google Scholar]
- 22.Kim NS, Kim HT, Kwon MC, Choi SW, Kim YY, Yoon KJ, Koo BK, Kong MP, Shin J, Cho Y. Survival and differentiation of mammary epithelial cells in mammary gland development require nuclear retention of Id2 due to RANK signaling. Mol Cell Biol. 2011;31(23):4775–4788. doi: 10.1128/MCB.05646-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saika S, Ikeda K, Yamanaka O, Flanders KC, Ohnishi Y, Nakajima Y, Muragaki Y, Ooshima A. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am J Physiol Cell Physiol. 2006;290(1):C282–C289. doi: 10.1152/ajpcell.00306.2005. [DOI] [PubMed] [Google Scholar]
- 24.Veerasamy M, Phanish M, Dockrell ME. Smad mediated regulation of inhibitor of DNA binding 2 and its role in phenotypic maintenance of human renal proximal tubule epithelial cells. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0051842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gervasi M, Bianchi-Smiraglia A, Cummings M, Zheng Q, Wang D, Liu S, Bakin AV. JunB contributes to Id2 repression and the epithelial-mesenchymal transition in response to transforming growth factor-beta. J Cell Biol. 2012;196(5):589–603. doi: 10.1083/jcb.201109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zebedee Z, Hara E. Id proteins in cell cycle control and cellular senescence. Oncogene. 2001;20(58):8317–8325. doi: 10.1038/sj.onc.1205092. [DOI] [PubMed] [Google Scholar]
- 27.Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14(2):77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 28.Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3(6):525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 29.Sun XH, Copeland NG, Jenkins NA, Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991;11(11):5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rollin J, Blechet C, Regina S, Tenenhaus A, Guyetant S, Gidrol X. The intracellular localization of ID2 expression has a predictive value in non small cell lung cancer. PLoS One. 2009;4(1) doi: 10.1371/journal.pone.0004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen XS, Zhang YH, Cai QY, Yao ZX. ID2: A negative transcription factor regulating oligodendroglia differentiation. J Neurosci Res. 2012;90(5):925–932. doi: 10.1002/jnr.22826. [DOI] [PubMed] [Google Scholar]
- 32.Kleeff J, Ishiwata T, Friess H, Buchler MW, Israel MA, Korc M. The helix-loop-helix protein Id2 is overexpressed in human pancreatic cancer. Cancer Res. 1998;58(17):3769–3772. [PubMed] [Google Scholar]
- 33.Li K, Yao L, Chen L, Cao ZG, Yu SJ, Kuang XY, Hu X, Shao ZM. ID2 predicts poor prognosis in breast cancer, especially in triple-negative breast cancer, and inhibits E-cadherin expression. Onco Targets Ther. 2014;7:1083–1094. doi: 10.2147/OTT.S64759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itahana Y, Singh J, Sumida T, Coppe JP, Parrinello S, Bennington JL, Desprez PY. Role of Id-2 in the maintenance of a differentiated and noninvasive phenotype in breast cancer cells. Cancer Res. 2003;63(21):7098–7105. [PubMed] [Google Scholar]
- 35.Stighall M, Manetopoulos C, Axelson H, Landberg G. High ID2 protein expression correlates with a favourable prognosis in patients with primary breast cancer and reduces cellular invasiveness of breast cancer cells. Int J Cancer. 2005;115(3):403–411. doi: 10.1002/ijc.20875. [DOI] [PubMed] [Google Scholar]
- 36.McElhinny AS, Li JL, Wu L. Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways. Oncogene. 2008;27(38):5138–5147. doi: 10.1038/onc.2008.228. [DOI] [PubMed] [Google Scholar]
- 37.Acar A, Simoes BM, Clarke RB, Brennan K. A role for notch signalling in breast cancer and endocrine resistance. Stem Cells Int. 2016;2016 doi: 10.1155/2016/2498764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3(4):429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Rangel MC, Bertolette D, Castro NP, Klauzinska M, Cuttitta F, Salomon DS. Developmental signaling pathways regulating mammary stem cells and contributing to the etiology of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156(2):211–226. doi: 10.1007/s10549-016-3746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raafat A, Goldhar AS, Klauzinska M, Xu K, Amirjazil I, McCurdy D, Lashin K, Salomon D, Vonderhaar BK, Egan S. Expression of Notch receptors, ligands, and target genes during development of the mouse mammary gland. J Cell Physiol. 2011;226(7):1940–1952. doi: 10.1002/jcp.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafkas D, Rodilla V, Huyghe M, Mourao L, Kiaris H, Fre S. Notch3 marks clonogenic mammary luminal progenitor cells in vivo. J Cell Biol. 2013;203(1):47–56. doi: 10.1083/jcb.201307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhat V, Sun YJ, Weger S, Raouf A. Notch-induced expression of FZD7 requires noncanonical NOTCH3 signaling in human breast epithelial cells. Stem Cells Dev. 2016;25(7):522–529. doi: 10.1089/scd.2015.0315. [DOI] [PubMed] [Google Scholar]
- 43.Fuxe J, Karlsson MC. TGF-beta-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol. 2012;22(5–6):455–461. doi: 10.1016/j.semcancer.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Hirschberg R. Bone morphogenetic protein-7 signals opposing transforming growth factor beta in mesangial cells. J Biol Chem. 2004;279(22):23200–23206. doi: 10.1074/jbc.M311998200. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Liu X, Luo J, Xiao W, Ye X, Chen M, Li Y, Zhang GJ. Notch3 inhibits epithelial-mesenchymal transition by activating Kibra-mediated Hippo/YAP signaling in breast cancer epithelial cells. Oncogene. 2016;5(11) doi: 10.1038/oncsis.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dou XW, Liang YK, Lin HY, Wei XL, Zhang YQ, Bai JW, Chen CF, Chen M, Du CW, Li YC. Notch3 maintains luminal phenotype and suppresses tumorigenesis and metastasis of breast cancer via trans-activating estrogen receptor-alpha. Theranostics. 2017;7(16):4041–4056. doi: 10.7150/thno.19989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kondo M, Cubillo E, Tobiume K, Shirakihara T, Fukuda N, Suzuki H, Shimizu K, Takehara K, Cano A, Saitoh M. A role for Id in the regulation of TGF-beta-induced epithelial-mesenchymal transdifferentiation. Cell Death Differ. 2004;11(10):1092–1101. doi: 10.1038/sj.cdd.4401467. [DOI] [PubMed] [Google Scholar]
- 48.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 49.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu C, Dievart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol. 2006;168(3):973–990. doi: 10.2353/ajpath.2006.050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang C, Yang X, Pursell B, Mercurio AM. Id2 complexes with the SNAG domain of Snai1 inhibiting Snai1-mediated repression of integrin beta4. Mol Cell Biol. 2013;33(19):3795–3804. doi: 10.1128/MCB.00434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohashi S, Natsuizaka M, Naganuma S, Kagawa S, Kimura S, Itoh H, Kalman RA, Nakagawa M, Darling DS, Basu D. A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res. 2011;71(21):6836–6847. doi: 10.1158/0008-5472.CAN-11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103(10):3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou L, Zhang N, Song W, You N, Li Q, Sun W, Zhang Y, Wang D, Dou K. The significance of Notch1 compared with Notch3 in high metastasis and poor overall survival in hepatocellular carcinoma. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Xu J, Song F, Jin T, Qin J, Wu J, Wang M, Wang Y, Liu J. Prognostic values of Notch receptors in breast cancer. Tumour Biol. 2016;37(2):1871–1877. doi: 10.1007/s13277-015-3961-6. [DOI] [PubMed] [Google Scholar]
- 57.Zubor P, Hatok J, Moricova P, Kajo K, Kapustova I, Mendelova A, Racay P, Danko J. Gene expression abnormalities in histologically normal breast epithelium from patients with luminal type of breast cancer. Mol Biol Rep. 2015;42(5):977–988. doi: 10.1007/s11033-014-3834-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Primers Sequences.
Supplementary Table S2. RNAi Sequences.
Supplementary Table S3. Antibodies.
Expression of Id2 and Notch3 in breast cancer cell lines and its prognostic value in a breast cancer patient cohort.