Abstract
Sponges are ubiquitous on coral reefs, mostly long lived and therefore adaptive to changing environmental conditions. They feed on organic matter withdrawn from the passing water and they may harbor microorganisms (endosymbionts), which contribute to their nutrition. Their diets and stable isotope (SI) fractionation determine the SI signature of the sponge holobiont. Little is known of spatio–temporal variations in SI signatures of δ13C and δ15N in tropical sponges and whether they reflect variations in the environment. We investigated the SI signatures of seven common sponge species with different functional traits and their potential food sources between 15 and 32 m depth along the S-SE and E-NE side of the Saba Bank, Eastern Caribbean, in October 2011 and October 2013. SI signatures differed significantly between most sponge species, both in mean values and in variation, indicating different food preferences and/or fractionation, inferring sponge species-specific isotopic niche spaces. In 2011, all sponge species at the S-SE side were enriched in d13C compared to the E-NE side. In 2013, SI signatures of sponges did not differ between the two sides and were overall lighter in δ13C and δ15N than in 2011. Observed spatio–temporal changes in SI in sponges could not be attributed to changes in the SI signatures of their potential food sources, which remained stable with different SI signatures of pelagic (particulate organic matter (POM): δ13C −24.9‰, δ15N +4.3‰) and benthic-derived food (macroalgae: δ13C −15.4‰, δ15N +0.8‰). Enriched δ13C signatures in sponges at the S-SE side in 2011 are proposed to be attributed to predominantly feeding on benthic-derived C. This interpretation was supported by significant differences in water mass constituents between sides in October 2011. Elevated NO3 and dissolved organic matter concentrations point toward a stronger reef signal in reef overlying water at the S-SE than N-NE side of the Bank in 2011. The depletions of δ13C and δ15N in sponges in October 2013 compared to October 2011 concurred with significantly elevated POM concentrations. The contemporaneous decrease in δ15N suggests that sponges obtain their N mostly from benthic-derived food with a lower δ15N than pelagic food. Average proportional feeding on available sources varied between sponge species and ranged from 20% to 50% for benthic and 50% to 80% for pelagic-derived food, assuming trophic enrichment factors of 0.5‰ ± sd 0.5 for δ13C and 3‰ ± sd 0.5 for δ15N for sponges. We suggest that observed variation of SI in sponges between sides and years were the result of shifts in the proportion of ingested benthic- and pelagic-derived organic matter driven by environmental changes. We show that sponge SI signatures reflect environmental variability in space and time on the Saba Bank and that SI of sponges irrespective of their species-specific traits move in a similar direction in response to these environmental changes.
Keywords: Sponge diet, Stable isotopes, Benthic–pelagic coupling, Saba Bank
Introduction
The stable isotope (SI) approach is a widely used method to study sources of food and trophic transfer in food web studies. Application to sponges revealed a wide range in bivariate δ13C and δ15N signatures, illustrating interspecific differences in diet and isotopic niche space of sponges (Van Duyl et al., 2011; Freeman, Easson & Baker, 2014). In shallow water habitats, such as coral reefs, diets of sponges mainly comprise of pico- and nanophytoplankton, bacterioplankton, small detrital particles, dissolved organic matter (DOM) and metabolites provided by endosymbionts, which are hosted by various sponges (Erwin & Thacker, 2007; Freeman & Thacker, 2011; Maldonado, Ribes & Van Duyl, 2012). Therefore, coral reef sponges are considered as primary consumers living mainly on products of primary production, besides bacteria. Like other consumers, sponges are considered to reflect the SI signature of the ingested food mixture within 0–1‰ enrichment for carbon (δ13C), and 1.5–3.5‰ for nitrogen (δ15N) (Fry et al., 1982; Vander Zanden & Rasmussen, 2001; Fry, 2006). Endosymbiont communities also influence the SI signatures of their sponge hosts. Both sponge host and associated bacteria jointly determine the typical δ13C and δ15N signature of the sponge holobiont (Thacker & Freeman, 2012; Freeman, Easson & Baker, 2014). Sponges hosting phototrophic endosymbionts, which fix CO2 tend to have lower δ13C values than sponges without phototrophic endosymbionts and sponges hosting N2 fixing endosymbionts tend to have lower δ15N values (Fiore et al., 2010). Many sponges feed on DOM apart from plankton (Yahel et al., 2003; De Goeij et al., 2008; Mueller et al., 2014a; McMurray, Pawlik & Finelli, 2017; Hoer et al., 2018). It is widely recognized that sponges draw food from the pelagos to the benthos (benthic–pelagic coupling), but that sponges also ingest food derived from the benthos is less well-known (Southwell, 2007; Van Duyl et al., 2011; Rix et al., 2016a, 2016b).
The current phase shift from scleractinian coral to macroalgal, turf algal and cyanobacterial mat dominated communities (McCook, Jompa & Diaz-Pulido, 2001; Jackson et al., 2014; De Bakker et al., 2017) is considered to lead to an increased supply of DOM and suspended detrital material from the benthos to the reef overlying water (Haas et al., 2016). These non-calcifying benthic primary producers produce more DOM than corals per unit surface (Naumann et al., 2010a; Haas et al., 2010a, 2010b, 2011; Mueller et al., 2014b, 2016; Mueller, Meesters & Van Duyl 2017; Brocke et al., 2015). Moreover, there is increasing evidence that besides pelagic-derived food (mainly consisting of microbial loop food, e.g., pico- and nano(phyto)plankton), benthic-derived DOM may be an important food source for sponges (Van Duyl et al., 2011; Rix et al., 2016a, 2016b; Pawlik, Burkepile & Thurber, 2016). Additionally, suspended detrital material either derived from benthic or pelagic producers has also been identified as sponge food (Maldonado, Ribes & Van Duyl, 2012; McMurray et al., 2018).
The stable isotopic ratios (δ13C and δ15N) of benthic- and pelagic-derived food differ substantially (Fry et al., 1982; Raven et al., 1995). Pelagic primary producers (i.e., phytoplankton) in oligotrophic reef waters have usually lower δ13C values than benthic primary producers (Faganeli et al., 1988; France, 1995; Duarte, 1992; Van Duyl et al., 2011). Contrary, δ15N tends to be lower in benthic than in pelagic primary producers (Naumann et al., 2010b; Van Duyl et al., 2011; Kolasinski et al., 2016), particularly when N2 fixing takes place by turf algae, cyanobacterial mats or nitrogen fixing bacteria associated with various reef organisms (e.g., corals and sponges) (Lesser et al., 2007; Cardini et al., 2014; Den Haan et al., 2014). DOM released by phytoplankton, macroalgae and corals is generally assumed to closely reflect the SI signature of its source with little isotopic fractionation during formation and degradation (Williams & Gordon, 1970; Fry et al., 1998; Benner et al., 1997). Depending on sponge species, food from these pelagic and/or benthic sources appears to be assimilated in different proportions leading to distinct SI signatures (Van Duyl et al., 2011). For sponge diet changes to be reflected in the SI signature a 1–2 months delay related to the integration time for new source information is required (Freeman & Thacker, 2011; Simister et al., 2013). Variations in hydrographic conditions may be drivers of such changes (current directions and velocities, upwelling) in the tropics. Besides variations in water mass constituents flowing toward coral reefs, water turbulence and vertical mixing affect the availability of benthic and pelagic food for sessile organisms (Lowe & Falter, 2015). Fine scale hydrodynamic conditions vary in geographic space and time around reefs, with reefs differently exposed to incoming waves and currents and different water mass properties. Whether such variations are reflected in sponge diets is still unknown.
The aim of this study was to explore and explain variations in δ13C and δ15N of sponges on fore reefs with different orientation toward incoming currents and waves (space) over time on the Saba Bank. It was hypothesized that sponges with different functional traits along these stretches (sides) of the Bank receive food from different sources over time. We sampled food reaching sponges from open water transported by currents, and local food derived from benthic sources on the Saba Bank to estimate the contribution of pelagic- and benthic-derived food to their diet under different hydrodynamic conditions. To identify different water masses and infer the source of its constituents, we measured concentrations of organic matter and inorganic nutrients in space and time.
Materials and Methods
Fieldwork was performed under the research permits #WSH/2011/1400 and RWS-2013/42681 issued by Rijkswaterstaat, Dutch Ministry of Infrastructure and Environment and Ministry of Economic Affairs.
Location and hydrography
The Saba bank (17°25′N; 63°18′W) is a 2,200 km2 submerged carbonate platform as measured to the 200 m isobath in the northeastern Caribbean Sea. It is raised about 700–1,000 m above the general depths of the surrounding sea floor and lies 10–100 m below sea level with shallowest depths (∼ 10 m) along the E-NE fringes. The nearest island is Saba (ca five km from the NE border of the Bank), which is separated from the Saba Bank by a 600 m deep channel. The Saba Bank is fringed by relatively undisturbed (negligible local human impact) coral reefs along its S-SE and E-NE side with an average stony coral and sponge cover of 7.1% and 7.8%, respectively, and a benthic algal cover of approximately 50% in the 15–32 m depth range (Van Beek & Meesters, 2014). The mainly wind-driven Caribbean Current flushes and envelopes the Saba Bank from the SSE to the WNW. The source water for the Caribbean Current is from the equatorial Atlantic Ocean (North Equatorial, North Brazil and Guiana Currents) and enters the Caribbean basin mainly through the southern part of the Antillean Arc. The Saba Bank also receives oligotrophic water from the Antillean Current flowing into the Caribbean Basin directly from the Atlantic (Van Duyl, 2016).
Fieldwork on the Saba Bank was conducted from the Caribbean Explorer II during two expeditions, the first from 22 to 28 October in 2011 and the second from 20 to 26 October in 2013. A total of 11 permanent coral reef stations, five stations along the S-SE side and six stations along the E-NE side of the bank were sampled, covering a distance of approximately 20 km along each side of the Bank (Fig. 1, see supplementary for coordinates and depth of stations). One site at the S-SE side (Dutch plains) was only sampled in 2013. Tidal amplitude is less than 10 cm per diurnal tidal cycle on the Bank, due to proximity of an amphidromic point (Kjerfve, 1981). Current speeds on the Bank in 2011 ranged from less than 0.25 up to 0.5 m/s (De Graaf, 2012). Water temperature varied between 29 and 30 °C in October 2011 and between 28 and 29 °C in October 2013.
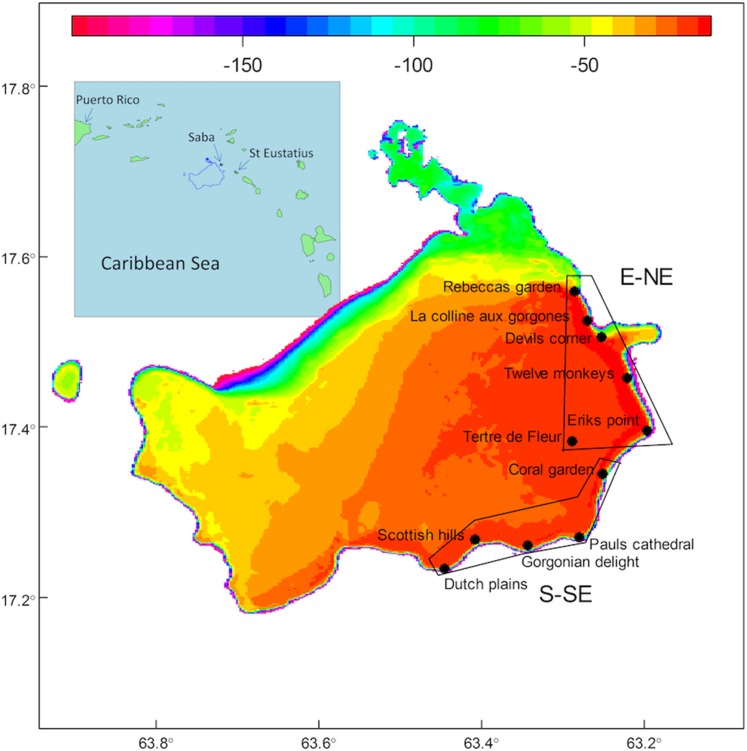
Figure 1. Map of the Saba Bank with inset.
Map of the Saba Bank with depth contours (color scale in meter) and stations which were visited in October 2011 and October 2013. Fore-reef stations were in the 17–32 m depth range and the patch reef on top of the Saba Bank, Tertre de Fleur, was at 15 m depth. Stations Dutch plains until Coral garden were exposed to the S-SE and the rest of the stations had an E-NE exposition. Inset shows the position of the Saba Bank in the Caribbean Sea, with nearest islands Saba (∼5 km NE of the Saba Bank) and St. Eustatius (∼20 km East of the Saba Bank).
Collection of samples
Sponges and benthic macroalgae were collected by SCUBA diving at stations varying in depth between 15 and 32 m on gently sloping fore reefs. At each station, up to seven commonly occurring sponge species (one to three specimen per species) were collected belonging to three different functional groups varying in microbial abundance and chlorophyll-a content (Table 1). Chlorophyll-a content in sponges is indicative of the presence and abundance of phototrophic endosymbionts (Wilkinson, 1983; Erwin & Thacker, 2007; Freeman & Thacker, 2011). As representatives of benthic food sources two common brown macroalgal species Dictyota spp. and Lobophora spp. were collected at each station (one to two samples per species). On a few stations six or less sponge species were found. Particularly at the shallowest site (15–17 m, Tertre de Fleur) only four of the sponge species and one macroalgal species (Dictyota spp.) were found. At this station some other benthic algae, Sargassum and filamentous cyanobacteria were sampled for SI values. Particulate organic matter (POM) and dissolved (in)organic nutrients were sampled with a six L niskin bottle between zero and two m depth from the research vessel at each station. In 2011, two water samples were taken per station and in 2013 one water sample. The suspended particulate matter obtained from the surface water was considered to represent pelagic food for sponges. Vertical temperature and salinity profiles over reefs down to 35 m depth showed that the water column was well mixed (temperature nor salinity changed with depth).
Table 1. Sponge species.
Collected sponge species with their functional traits, representing low microbial abundance sponges (LMA), high microbial abundance sponges with high chlorophyll-a (HMA-H) and low chlorophyll-a content (HMA-L). Selected sponge species were common on coral reefs of the Saba Bank (Thacker et al., 2010).
| Sponge species | Functional trait | Chlorophyll-a (μg/g sponge tissue) | Reference |
|---|---|---|---|
| Amphimedon compressa | LMA | Negligible | Erwin & Thacker (2007) and Gloeckner et al. (2014) |
| Callyspongia plicifera | LMA | Negligible | Erwin & Thacker (2007) and Gloeckner et al. (2014) |
| Aplysina cauliformis | HMA-H | >125 | Gloeckner et al. (2014) |
| Xestospongia muta | HMA-H | 50–125 | Erwin & Thacker (2007) and Gloeckner et al. (2014) |
| Plakortis spp. | HMA-H | 50–125? | Gloeckner et al. (2014) |
| Agelas conifera | HMA-L | <50 | Freeman, Easson & Baker (2014) |
| Aiolochroia crassa | HMA-L | <50 | Erwin & Thacker (2007) and Gloeckner et al. (2014) |
Sample processing
Water samples for inorganic nutrients were filtered over 0.2 μm acrodisc syringe filters (25 mm diameter). Filtrate was collected in pony vials, which were stored in a deep freezer (−20 °C) until analysis. Samples were processed on an autoanalyzer (TRAACS) for PO43−, NH4+, NOx, NO2−, (NOx − NO2− = NO3−). Water samples for DOM were also filtered over 0.2 μm acrodisc filters (25 mm diameter) and fixed with concentrated (38%) HCl (six drops for fixing 20 mL seawater) in combusted EPA vials (40 mL). Analysis of samples was done on a TOC-VCPN, Shimadzu analyzer yielding total and dissolved organic carbon (TOC, DOC) and total and dissolved nitrogen (TN, TDN). TDN minus dissolved inorganic nitrogen (DIN) (DIN = NH4 + NO2− + NO3−) renders dissolved organic nitrogen (DON).
Suspended matter was collected on combusted glass fiber filters (Whatman GF/F, 47 mm diameter and 10 mm diameter). The volume of water filtered was noted and filters were folded with tweezers, wrapped in aluminum foil, labelled and stored in a freezer at (−20 °C). Subsequently, filters were fumed with acid vapor (HCl) in a desiccator to remove possible carbonates, and lyophilized.
Sponges and macroalgae were individually labelled and packed in aluminum foil immediately after the dive and stored in a freezer (−20 °C) until processing. Sponge and algal samples were treated with 2M HCl overnight on a shaking table to remove possible carbonates. This procedure was repeated if necessary. Afterward samples were washed with UltraPure water until a pH between 5 and 6 was reached. Subsequently, samples were lyophilized.
Stable isotope analyses
For the SI analyses (δ13C and δ15N) homogenized organic matter portions of sponges and macroalgae (0.8–1 mg) and pieces of GF/F filters with suspended matter were transferred to tin cups, which were closed with tweezers. The C and N content of the dry weight and the isotope composition of the samples was determined on a Flash 2000 elemental analyzer coupled online with a Delta V Advantage-isotope monitoring mass spectrometer (Thermo Scientific, Waltham, Bremen, Germany) relative to standards (acetanilide, urea and casein). Values were normalized to acetanilide. C and N isotope ratios were expressed as δ13C to Vienna Pee Dee Belemnite standard and as δ15N to air. Standard error of measurements of standards was ∼0.15‰.
Statistical analyses
Bayesian techniques were adopted to analyze SI data (Zuur, Hilbe & Ieno, 2013). Bayesian approaches use statistical distributions to characterize the uncertainties in the data. Bayesian methods were solely applied for analysis of SI data of sponges and potential organic food sources for sponges. Means and credible intervals of the separate isotopes have been calculated using R (R Core Team, 2017) and R-INLA (Rue, Martino & Chopin, 2009). All models included a Gaussian error distribution that was allowed to differ per species because initial data explorations indicated heterogeneity between sponge species. R-INLA is used frequently for analyses that include spatial and temporal correlations (Zuur, Ieno & Saveliev, 2017), but can be used for any type of (Bayesian) analysis (see extensive model specification on www.r-inla.org). For model selection Watanabe–Akaike information criteria was used.
For bivariate isotopic niche space identification all sponge data collected on the Bank in 2011 and 2013 were pooled per sponge species. The isotope niche area of each sponge species was determined with Stable Isotope Bayesian Ellipses in R (Jackson et al., 2011). The method estimated the standard ellipse area (SEAc) of the bivariate niche space of different sponges, after a correction for small sample size. Standard ellipses enveloped approximately 40% of the bivariate δ13C and δ15N data of the different sponge species. Means of isotopes with 95% credible intervals of different sponge species were also determined by Bayesian statistics using R-INLA.
Stable isotope samples of sponges and potential food sources collected at the S-SE were compared with samples at the E-NE of the Bank for 2011 and for 2013. Data analyses of δ13C and δ15N in sponges and potential food sources were conducted for each isotope separately because C and N metabolism and fractionation are not necessarily coupled (Maldonado, Ribes & Van Duyl, 2012).
An isotope mixing model was applied using R package SIAR, SI analysis in R, R package version 4.2 (Parnell & Jackson, 2013) to calculate the proportional contribution of benthic- and pelagic-derived food to the sponge diet. The model used the bivariate isotope data of sponges and potential food sources and fitted a Bayesian model to determine the dietary proportions of the different sources (Parnell et al., 2013; Phillips et al., 2014). Suspended POM was used as pelagic-derived food and fleshy benthic macroalgae (SI data of Dictyota spp. and Lobophora spp. were combined) as representative for benthic-derived food. An average trophic enrichment factors (TEFs) of primary consumers (sponges) of 0.5 ± sd 0.5% for δ13C and 3 ± sd 0.5 for δ15N (Peterson & Fry, 1987) was assumed. The model was run for sponges at S-SE and the E-NE sides of the Bank and for the different years with vague priors using default settings.
A non-metric multidimensional scaling approach (nMDS) was applied to characterize water samples on the bank and to investigate whether there were differences between sides of the bank or between years. Concentrations of PO43−, NH4+, NO2−, NO3−, DIN, DIN/PO43− ratio, TOC, TON, DOC and DON and particulate organic carbon and nitrogen (POC, PON) and the ratio between POC and PON were used. The nMDS plot was generated after normalizing (scaling) values of environmental variables. Differences between samples were tested by Permanova (Anderson, 2001).
Results
Isotopic bivariate niche space of sponge species
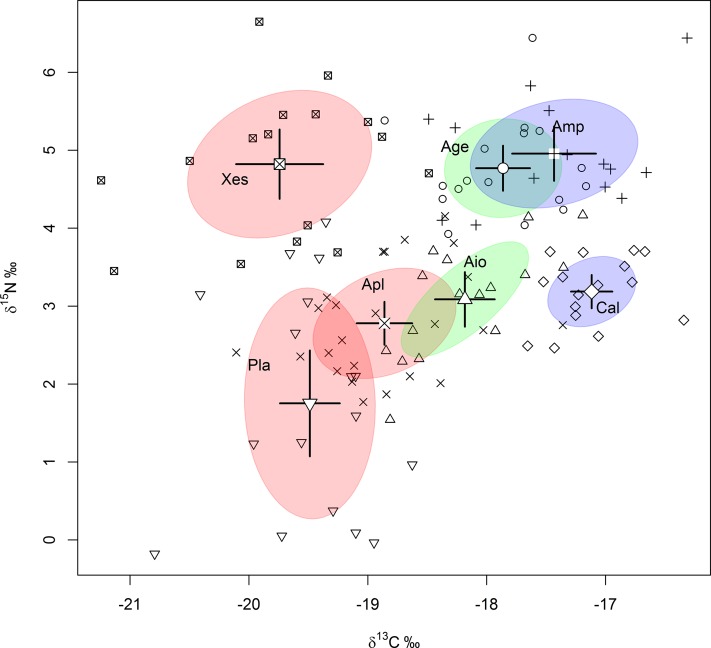
Combining data collected in this study over space and time, yielded ranges in δ13C and δ15N of sponges which showed considerable overlap between sponge species (Table 2). C/N ratios of sponges resembled those of bacteria with exception of the C/N ratio of Plakortis spp., which was surprisingly high for a High Microbial Abundance sponge (Table 2). Evident differences in the diet of sponges was best reflected in the isotopic bivariate niche spaces (Fig. 2). The placement, area and tilt of the SEAc around the different sponge species clearly varied (Fig. 2). HMA species were separated from Low Microbial Abundance species by differences in δ13C. Differences within functional groups (HMA-H, HMA-L and LMA) were mainly separated by differences in δ15N. The slight tilt of the ellipses of most sponge species indicated that SI ratios of C and N were mostly positively related.
Table 2. Sponge SI’s.
Ranges and (Bayesian) means of δ13C and δ15N of stable isotope signatures and averages of C:N ratio’s (mol weight, raw data) of sponges with standard deviations. Sponges were collected between 15 and 32 m depth.
| Sponges | n | Range ‰ δ13C | δ13C mean (sd) | Range ‰ δ15N | δ15N mean (sd) | Average C/N ratio (sd) |
|---|---|---|---|---|---|---|
| Aplysina cauliformis | 24 | −20.11 to −17.36 | −18.86 (0.58) | 1.77–4.16 | 2.78 (0.69) | 4.7 (0.39) |
| Plakortis sp. | 17 | −20.79 to −18.63 | −19.49 (0.52) | −0.18–4.08 | 1.75 (1.41) | 8.2 (1.80) |
| Xestospongia muta | 16 | −21.24 to −18.49 | −19.74 (0.72) | 3.45–6.65 | 4.82 (0.89) | 4.9 (0.49) |
| Agelas conifera | 17 | −18.86 to −17.16 | −17.86 (0.46) | 3.92–6.44 | 4.77 (0.60) | 4.3 (0.27) |
| Aiolochroia crassa | 16 | −18.85 to −17.19 | −18.18 (0.51) | 1.54–4.17 | 3.09 (0.70) | 4.9 (0.32) |
| Amphimedon compressa | 14 | −18.49 to −16.31 | −17.43 (0.67) | 4.04–6.44 | 4.96 (0.66) | 4.1 (0.52) |
| Callyspongia plicifera | 16 | −17.66 to −16.34 | −17.12 (0.34) | 2.46–3.71 | 3.19 (0.42) | 3.6 (0.05) |
Figure 2. Sponge niche spaces.
Isotopic bivariate niche space of seven sponge species on fore reefs of the Saba Bank, based on data collected in space and time. Standard ellipse areas of different sponge species were corrected for small sample size (SEAc). Bayesian means of different sponge species are shown within ellipses with the 95% credible intervals of the mean. HMA high chlorophyll-a sponges (HMA-H) are pink, HMA low chlorophyll-a sponges are green (HMA-L) and LMA sponges are purple. (+)Amp, A. compressa; (○)Age, A. conifera; (□)Xes, X. muta; (◊)Cal, C. plicifera; (▵)Aio, A. crassa; (x)Apl, A. cauliformis; (▽)Pla, Plakortis spp.
Spatial and temporal variation in SI signatures of sponges
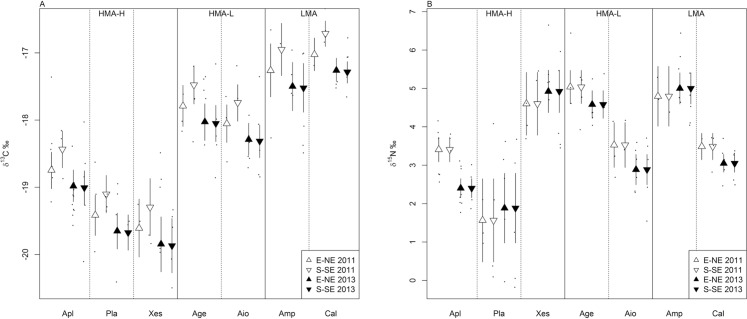
We found that δ13C of the different sponge species varied in space and time, and that δ15N only varied in time on the Saba Bank (Figs. 3A and 3B). The δ13C values of the sponges were best described by a model that included species, sampling year, side of the bank, as well as an interaction between these latter two variables:
Figure 3. Variation in δ13C (A) and δ15N (B) of different sponge species along the S-SE and E-NE side of the Saba Bank in 2011 and 2013.
Means and 95% credible intervals are based on Bayesian statistics. Black dots represent the raw data of δ13C (A) and of δ15N (B), respectively, of the different sponge species. Sponges are grouped according to their functional traits (HMA-H, HMA-L and LMA). For sponge species abbreviations see Fig. 2.
The best model for values of δ15N included only the main effects, “species” and “year of sampling”:
“Species” refers to sponge species, “Side” to the E-NE facing or S-SE facing side of the Saba Bank and “Year” to October 2011 or October 2013. Raw data for Figs. 3A and 3B are presented in Supplementary Information.
Sponge species was the main driver of variations in δ13C and δ15N. In space and time, however, the δ13C of sponge species varied in concert irrespective of their different functional traits (Fig. 3A). Mean δ13C was higher at the S-SE than the E-NE side in 2011 and decreased from 2011 to 2013 in all sponge species. An evident interaction effect between Side and Year for variations in δ13C was observed, implying that spatial differences in δ13C in sponges between sides with different orientation toward incoming currents and waves occurred, but varied in time. In 2011, the δ13C signatures in sponges was on average 0.31‰ lower at the E-NE side than at the S-SE side (mean 0.31, 95% credible interval 0.04–0.59). From 2011 to 2013 δ13C signatures decreased in all sponges species on average with 0.23‰, but sponges at the S-SE side decreased even further than at the E-NE side with 0.34‰ (median 0.34, 95% credible interval −0.67 to −0.01). This decrease of δ13C in sponges at the S-SE side compensated the difference that existed in 2011 between sides, making sides again comparable in 2013.
Overall the δ15N in sponges (Fig. 3B) showed an evident temporal effect with a mean decrease of 0.51‰ (95% credible interval 0.27–0.75) from 2011 to 2013. Spatial variations were not found and variations in δ15N of sponges in time were not consistent for species within HMA-H and LMA groups. Only A. cauliformis, A. conifera (Age), A. crassa and C. plicifera showed a clear decrease in δ15N from 2011 to 2013 with little or no overlap of 95% credible intervals. The mean δ15N of other sponge species (Plakortis spp., X. muta and A. compressa) did not clearly change in time. No relations were found between sponge SI signatures and depth.
Stable isotope signatures of potential food sources for sponges
Stable isotope signatures of benthic genera of fleshy brown algae and cyanobacterial mats largely overlapped and were evidently higher in δ13C and lower in δ15N than the pelagic-derived food, POM (Table 3). Sargassum spp. and cyanobacteria were only collected at up to three stations. Therefore, only SI data of Dictyota spp. and Lobophora spp. were used and pooled as benthic-derived food representative, and compared with the pelagic food source (POM). SI signatures of suspended POM (δ13C: −24.91‰ [95% credible interval −24.20‰ to −25.63‰]; δ15N: 4.33‰ [95% credible interval 3.66–4.99‰]) clearly differed from the benthic source (δ13C: 15.38‰ [95% credible interval −14.69‰ to−16.07‰] and δ15N: 0.80‰ [95% credible interval 0.47–1.14‰]), with lower mean δ13C (9.5‰ lower) and higher δ15N values (3.5‰ higher). The C/N ratio of pelagic POM was lower compared to that of benthic macroalgae, but overlapped with benthic cyanobacteria (Table 3). No differences in geographic space or time were observed for δ13C and δ15N SI signatures of benthic food sources. The same was found for the δ13C in pelagic food (POM). For δ15N in POM insufficient data were available to test its variation in space and time (only one observation in 2011 and seven in 2013). Values obtained in 2013, however, did not show spatial variation and covered the single value available for 2011 assuming that also temporal variation was negligible.
Table 3. Benthic algae and POM.
Ranges and (Bayesian) means of δ13C and δ15N of stable isotope signatures of benthic algae, benthic cyanobacterial mats and particulate organic matter (POM) and averages of C:N ratio’s (mol weight, raw data) with standard deviations. Benthic algae were collected between 15 and 32 m depth. POM was obtained from surface water (0 to 2 m).
| Benthic algae and POM | n | Range in ‰ δ13C | δ13C mean (sd) | Range in ‰ δ15N | δ15N mean (sd) | Average C/N ratio (sd) |
|---|---|---|---|---|---|---|
| Dictyota spp. | 15 | −18.44 to −15.04 | −16.60 (1.17) | −0.26–1.19 | 0.47 (0.60) | 18.7 (4.08) |
| Lobophora spp. | 16 | −18.97 to −11.21 | −14.23 (2.10) | −0.19–3.38 | 1.12 (0.93) | 33.6 (6.00) |
| Sargassum spp. | 3 | −19.50 to −14.62 | −16.31 (2.36) | −0.41–2.52 | 1.31 (1.32) | 35.4 (3.80) |
| Filamentous benthic cyanobacteria | 1 | −15.07 | 0.00 | 8.1 | ||
| POM in surface water | 19.8 | −27.67 to −17.78 | −24.91 (1.77) | 2.92–6.25 | 4.33 (1.24) | 12.9 (5.52) |
Source partitioning
The proportional contribution of pelagic- and benthic-derived food to the diet of the seven different sponge species revealed that based on the assumed TEFs of C (0.5‰ ± sd 0.5) and N (3.0‰ ± sd 0.5), diets varied depending on sponge species from 51%/49% (LMA sponge C. plicifera) to 21%/79% (HMA-H sponge X. muta) with regard to benthic/pelagic feeding. LMA sponges appeared to rely more on benthic-derived food than HMA sponges. Five (X. muta, A. conifera, A. crassa, A. compressa and C. plicifera) of the seven sponge species were estimated to consume relatively more benthic food at the S-SE side than at the E-NE side in 2011 based on the means of the 95% credibility internals (Table 4). In 2013, X. muta and Plakortis spp. were estimated to consume on average relatively less benthic food and thereby more pelagic-derived food than their conspecifics in 2011. Results showed that average shifts in benthic–pelagic feeding of ∼1 (A. crassa) to ∼13% (A. compressa) would be sufficient to cover the recorded differences in bivariate isotope signatures of sponges in space in 2011. To cover changes in time in sponge SI’s along the S-SE and E-NE side, shifts of up to 19% (Plakortis spp.) in benthic–pelagic feeding were estimated. It should be noted here that the 95% credibility internals were large (Table 4), which made it impossible to assess evident differences in proportional feeding of sponges between different sides of the Bank in 2011 and between years.
Table 4. Contribution of benthic food to sponge diet.
Bayesian means with 95% credibility internals (between brackets) of benthic food contribution (%) to the diet of different sponge species in space and time according to the Stable Isotope mixing model (Parnell et al., 2013). Complementary % to reach 100% is ascribed to pelagic food (not shown).
| Sponges | S-SE 2011 (%) | E-NE 2011 (%) | S-SE 2013 (%) | E-NE 2013 (%) |
|---|---|---|---|---|
| Aplysina cauliformis | 38.7 (20–57) | 40.7 (29–53) | 45.2 (26–64) | 40.5 (28–53) |
| Plakortis spp. | 36.4 (14–58) | 53.0 (15–91) | 30.3 (11–50) | 33.9 (15–53) |
| Xestospongia muta | 29.1 (0–59) | 25.7 (4–47) | 20.7 (7–34) | 22.9 (5–41) |
| Agelas conifera | 37.6 (17–58) | 35.5 (10–61) | 34.2 (18–51) | 39.0 (24–54) |
| Aiolochroia crassa | 43.3 (15–71) | 42.7 (24–61) | 44.3 (30–58) | 44.2 (32–56) |
| Amphimedon compressa | 48.8 (5–93) | 36.5 (2–71) | 37.4 (21–45) | 45.2 (30–61) |
| Callyspongia plicifera | 50.9 (37–65) | 49.1 (4–94) | 51.2 (40–62) | 49.1 (37–61) |
Water mass nutrient concentrations
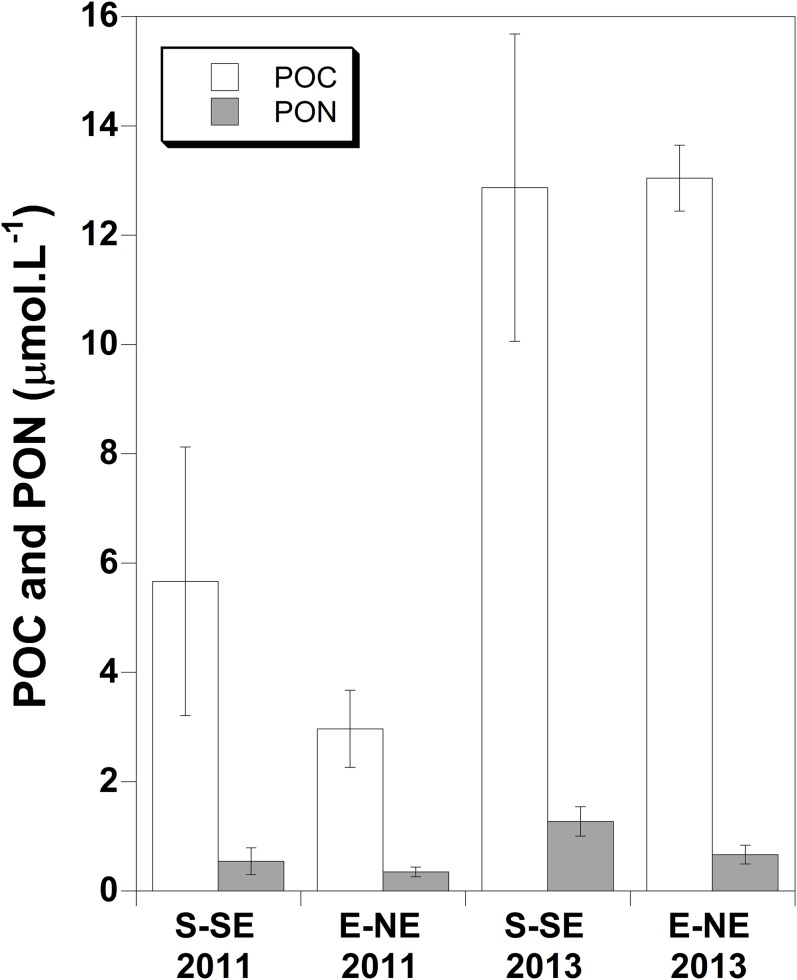
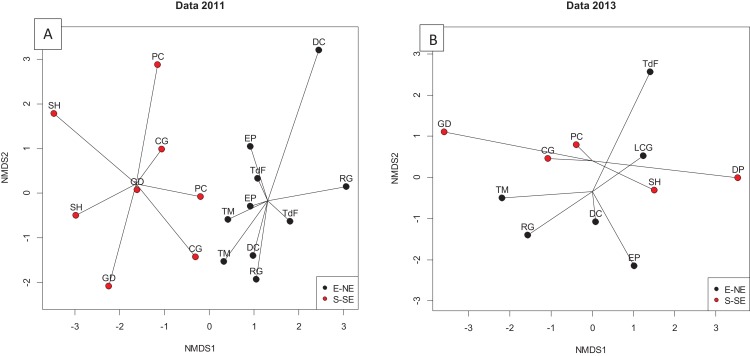
In October 2013, POM concentrations in surface waters were significantly higher along both sides of the Bank than in 2011 (Fig. 4). POC/PON ratios differed between sides in 2013 with a ratio of 20.5 ± sd 4.8 at the E-NE and 10.2 ± sd 2.0 at the S-SE side. The latter ratio was comparable to the ratios found in 2011 along both sides (10.1 ± sd 0.8 and 9.2 ± sd 0.7 at the S-SE and E-NE side, respectively). Concentrations of all other organic and inorganic nutrient variables were not significantly different between sides in 2011 or in 2013. However, taking all nutrient variables in surface water into account (except the ones showing high autocorrelation, e.g., NO3−, TN, TOC), a clear difference in water mass constituent proportions (NH4+, NO2−, PO43−, DOC, DON, POC concentrations and DIN/PO43− and POC/PON ratio’s) between the S-SE and E-NE side was found in 2011 (Fig. 5A). Testing the distance matrix ∼ side of the nMDS results, yielded a p-value of <0.001 (Permanova test). The difference in water masses between sides in 2011 was characterized by higher average DIN (notably NO3−), DOC, POC and PON concentrations and DIN/PO43− ratio along the S-SE than E-NE side of the Bank, and lowest average PO43− values at the S-SE side in 2011. Particularly the higher concentrations of NO3− of 0.197 ± sd 0.15 at the S-SE vs 0.111 ± sd 0.04 μmol/L at the E-NE (Fig. S1) and lower concentrations of PO43− (∼0.013 μmol/L, Fig. S2) at S-SE side in combination with higher DOC concentrations (Fig. S3) and DIN/PO43− ratios (Fig. S4) than the E-NE side pointed to a reef signal in the water mass in 2011. In 2013, the water mass at the S-SE was not significantly different from the water mass at the E-NE side (Fig. 5B, Permanova test, p = 0.596). See Supplemental Information for concentrations of all nutrients.
Figure 4. POC and PON concentrations on the Saba Bank.
Variations in particulate organic carbon (POC) and nitrogen (PON) concentrations in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
Figure 5. Water mass characterization.
Numerical dimensional scaling plots with water mass differences on basis of the following variables: average PO43−, NH4+, NO2−, DIN, DIN/PO43−, ratio, DOC, DON, TOC, TON, POC and POC/PON ratio at different sides of the Saba Bank (S-SE and E-NE) in (A) October 2011 and (B) October 2013. Samples along the S-SE side were collected at stations DP, SH, GD, PC, CG and along the E-NE side at EP, TdF, DC, TM, LCG and RG (i.e., abbreviations of station names, see Fig. 1 or Supplemental Data for full names of locations).
Discussion
We show that carbon and nitrogen SI compositions of sponges on the Saba Bank vary in geographic space and through time. Trajectories of change in SI signatures were comparable between most sponge species irrespective of different functional traits. Variations in sponge δ13C signature in geographic space concurred with differences in water mass constituents. Differences in the composition of these constituents suggest different exposure times to reef waters due to differences in hydrodynamic conditions between the E-NE and S-SE side of the Bank in 2011. Observed lower δ13C and δ15N values of sponges in 2013 compared to 2011 was suggested to be induced by an increase in pelagic food, which might have influenced the proportion of C and N absorbed from pelagic- and benthic-derived food by sponges differently.
Variation in bivariate isotopic niche space in sponges
Sponge species in this study showed ranges in SI signatures, which agree well with ranges for similar sponge species in the Caribbean (Southwell, 2007; Weisz et al., 2007; Fiore, Baker & Lesser, 2013; Freeman, Easson & Baker, 2014). This might imply that bivariate isotope niche space of these sponge species persisted in space and time and that sponges maintained their trophic position relative to other sponge species. The placement of the niche spaces (SEAsc) in bivariate plots showed clear differences between sponge species indicating distinct diets. Larger niche spaces suggest that species have a broader access to food sources and might be more apt to maintain themselves under varying trophic conditions than species with relatively small niche spaces (Freeman, Easson & Baker, 2014, 2016). The observation that the ellipses of most sponges slightly tilted to the right indicate that the δ13C and δ15N tended to follow comparable trajectories of change irrespective functional traits (HMA vs LMA sponges). This suggested that the basic sponge metabolism overruled the contribution of its endosymbionts.
Interestingly, the dichotomy of HMA and LMA sponges (Gloeckner et al., 2014) was mainly covered by differences in δ13C. HMA-H sponges had lower δ13C values than LMA sponges. This difference might be attributed to the presence of phototrophic endosymbionts, which preferably fix inorganic 12C over 13C (Freeman, Easson & Baker, 2014) and supply HMA-H sponges with photosynthates. Niche spaces of HMA-L sponges were found to be in between HMA-H and LMA sponges, which suggest that they rely more on external food sources and to a lesser part on food supplied by their endosymbionts Patterns for δ15N were unresolved with respect to HMA–LMA dichotomy, suggesting that N might be obtained from a different source and/or that fractionation varies between sponge species. The division we found between the low <3.5 and high >4‰ δ15N values in HMA sponges has also been reported by others, although at a slightly different separation of the low δ15N group (Weisz et al., 2007; Mohamed et al., 2010). The LMA group mainly resides in the high δ15N group (Freeman, Easson & Baker, 2014), although in our study the LMA sponge C. plicifera was part of the low group. Complex biochemical N cycling pathways involving different N fractionation have been reported for both HMA (Weisz et al., 2007; Southwell et al., 2008; Fiore et al., 2010; Fiore, Baker & Lesser, 2013) and LMA sponges (Schläppy et al., 2010; Cardoso et al., 2013; Diaz & Ward, 1997; Jiménez & Ribes, 2007; Southwell, Popp & Martens, 2008; Southwell et al., 2008; Van Duyl et al., 2008). Nitrification and other microbial metabolic pathways (e.g., denitrification, anammox, N2 fixation) probably influenced the δ15N signature of the sponge holobiont (Weisz et al., 2007; Southwell et al., 2008; Mohamed et al., 2010). Nitrification and denitrification increased the δ15N values in sponges (Southwell et al., 2008), while sponges harboring N2 fixing microbes tended to decrease in δ15N (Wilkinson & Fay, 1979; Southwell, Popp & Martens, 2008; Southwell et al., 2008; Taylor et al., 2007; Weisz et al., 2007; Mohamed et al., 2008; Ribes et al., 2015). To what extent N2 fixation contributed to the differences in δ15N of the sponge holobiont is still questionable (Southwell et al., 2008) and might depend on ambient nutrient conditions. Overall these and our results suggest that the carbon and nitrogen incorporated in the sponge holobiont were not necessarily retrieved from the same source.
Variations in sponge SI signature in space and time
To date it is still poorly understood to what extent hydrodynamic conditions in geographic space and/or time affect variations in SI signature of different sponge species. Variations in space of δ13C and δ15N of corals, macroalgae, suspended matter, (in)organic nutrients and sponges on coral reefs have mostly been described in relation to onshore–offshore distances and depth (Risk, Sammarco & Schwarcz, 1994; Risk et al., 2009; Southwell, 2007; Lapointe et al., 2010; Wyatt et al., 2013). On the Saba Bank the distance from shore did not play a role and the depth gradient from 15 to 32 m, in which sponges were collected, did not influence the spatial variation in SI signatures of sponges on the Saba Bank. Nevertheless, in 2011 clear differences in SI signatures were found between the E-NE and the S-SE sides of the Bank. All sponges irrespective of species or functional traits had higher δ13C values at the S-SE side than at the E-NE side in 2011. Each species was on average (raw data) 0.01–0.64‰ higher in δ13C than its conspecifics at the other side. With Bayesian statistics the best estimate for all sponges yielded a difference of 0.31 (95% credible interval of 0.04–0.59) in δ13C between sides. This was comparable to ranges found for δ13C signatures of X. muta between different sites in the NE and W Caribbean (Fiore, Baker & Lesser, 2013; Freeman, Easson & Baker, 2014), but smaller than differences found for onshore–offshore gradients of up to 5‰ (Southwell, 2007). The fact that all seven sponge species had higher δ13C values at the S-SE-side than the E-NE side implied that this phenomenon was likely to be caused by general differences in organic matter supply of pelagic and/or benthic sources, rather than by species-specific functional traits, such as the presence and abundance of (phototrophic) endosymbionts. As sponges only differed in δ13C, but not in δ15N signatures, it further appeared that N-metabolism of sponges, irrespective of sponge species, was not affected by space on the Saba Bank. The effect of spatial differences in hydrodynamic conditions concerning pelagic food supply were apparently more important for the δ13C than for the δ15N in sponges, which is in accordance with other studies (Southwell, 2007; Freeman, Easson & Baker, 2014).
Spatial differences in δ13C of sponge species were only found in 2011, but not in 2013. This indicated that the difference was not determined by the fixed position of the Saba Bank toward incoming currents and waves. The environmental conditions were apparently different between sides (S-SE and E-NE side) in 2011 (see below), which lasted over a long enough period for sponges to obtain a distinct δ13C signal. Integration time for a distinct signal has been estimated on 1–2 months (Freeman & Thacker, 2011; Simister et al., 2013). Thus, it is likely that divergent environmental conditions led to the increased δ13C signature in sponges at the S-SE side and not at the E-NE side, where variations in δ13C in time were smaller than at the S-SE side.
Time affected both the δ13C and the δ15N values of sponges on the Saba Bank. The sponge SI signatures decreased between 2011 and 2013 with 0.23‰ for δ13C and with 0.51‰ for δ15N. That decreases in δ13C in time at the S-SE side (0.34‰) were larger than at the E-NE side suggested that the uptake of pelagic-derived food was higher, purportedly due to an increased supply rate at the S-SE than E-NE side preceding our sampling in 2013. The variability in hydrodynamics in time might have been larger at the S-SE than E-NE side of the Bank supporting the higher fluctuation in δ13C at the S-SE side. Comparable trajectories of excursions of bulk δ13C and δ15N in sponges in time were also observed by Simister et al. (2013). They (ibid) reported several significant excursions from the mean δ13C and δ15N values in the LMA sponges Ancorina alata and Tethya stolonifera without a significant change over a 2-year period. Interestingly the trajectories of the excursions were comparable between the sponge species irrespective species-specific differences in SI signatures, which is in accordance with our SI changes for most sponge species in space and time. Comparable patterns of change in the δ13C and δ15N of most sponges appeared particularly driven by temporal variation in dietary food sources.
Food sources contributing to sponge SI signatures
Contrasting to the spatio–temporal variations in sponge SI signatures, SI signatures of pelagic POM and the benthic macroalgae, as well as their difference remained stable in both space and time. Pelagic-derived food was ∼9.5‰ lower in δ13C and ∼3.5‰ higher in δ15N compared to these values in benthic-derived food. Comparable differences in SI signatures between pelagic and benthic primary producers in coral reef ecosystems have been reported by others (Granek, Compton & Phillips, 2009; Van Duyl et al., 2011; Wyatt et al., 2013; Kürten et al., 2014; Kolasinski et al., 2016). We therefore, propose that shifts in benthic vs pelagic feeding occurred to explain variations in SI signatures in space and time. On average 25–50% of the sponge diets on the Saba Bank consisted of benthic-derived food. The HMA-H sponges X. muta relied the least on benthic food (25% ± sd 4%) and the LMA sponge C. plicifera the most (50% ± sd 1%). Shifts of less than 10% in the diet of sponges (Table 4) would be sufficient to explain the recorded spatio–temporal patterns in most sponge bivariate SI signatures. Such shifts were likely triggered by local environmental (hydrodynamic) conditions influencing the availability of benthic- and pelagic-derived food.
Effect of environmental conditions on stable isotopic signatures in sponges
The spatial enrichment of δ13C in sponges in 2011 along the S-SE side of the Bank concurred with a small, but significant difference in water mass constituent proportion between sides (Fig. 5A). Although upwelling and different water mass intrusions occur along the Saba Bank and Saba (Van Duyl, 2016; De Nooijer & Van Heuven, 2016; Van Heuven et al., submitted), no clear indications were found to support that such events occurred during the sampling in October 2011 and October 2013. Therefore, water masses at the different sides of the bank might have come from different sources or the retention time of the water on the fore reefs along the S-SE and E-NE side of the Bank differed. The general trend is that the main surface flow approaching the Saba Bank comes from the SSE and slightly bends toward more westerly directions over and along the Bank to continue to the NW (Gordon, 1967). However, source water reaching the Bank and flow patterns along and over the Bank can be highly variable (Andrade & Barton, 2000). Model simulations of source, track and speed of water parcels reaching the Saba Bank in October 2011 and October 2013 were quite different (A. Candy, 2017, unpublished data). This might also have affected water mass constituents and SI signatures of sponges found between sides and years at the Saba Bank. Increased average NO3− concentrations, decreased PO43− and slightly enhanced DOM concentrations pointed to an elevated reef benthic influence on water mass constituents at the S-SE compared to the E-NE side in 2011. This implied that the retention time of water might have been longer on the reefs along the S-SE than E-NE side of the Bank. Sponge and coral holobionts are known for their potential to nitrify leading to increased nitrate concentrations above reefs (Webb et al., 1975; Corredor et al., 1988; Wafar, Wafar & David, 1990; Diaz & Ward, 1997; Gast et al., 1999; Southwell et al., 2008). Also increased DOC concentrations have been reported for reef overlying waters (Van Duyl & Gast, 2001; Dinsdale et al., 2008; Mueller et al., 2014b; Mueller, Meesters & Van Duyl, 2017). Sponges obtaining food from this water mass might therefore, have been higher in δ13C than sponges at the E-NE side in 2011. The fact that the δ15N signatures did not differ spatially in 2011 nor in 2013 might indicate that sponges obtain N for a large part from benthic-derived organic matter. Organic N requirements of sponges are high considering the low C:N ratio of most sponges in this study and the generally high inorganic N exudation rates (Maldonado, Ribes & Van Duyl, 2012).
The decrease of δ13C in sponges from 2011 to 2013 is most likely driven by higher POC concentrations in October 2013 than in October 2011 which might have affected the proportional feeding of benthic- and pelagic-derived food in favor of pelagic-derived food. The δ13C in phyto-, bacterioplankton comprising most of the POM is much lower than in benthic-derived organic matter, which might have been reflected in the SI signature of sponges at the S-SE as well as the E-NE side in 2013. That several sponge species had lower δ15N values (A. cauliformis, A. conifera and C. plicifera) in 2013 than 2011 is more difficult to explain considering the fact that plankton derived PON was higher in δ15N (mean δ15N is 4.3‰) compared to benthic-derived food (0.8‰). It suggests that also feeding on benthic-derived nitrogen was intensified by increasing the proportion of benthic N in the diet of these sponges. Additionally, the lower C/N ratio of POM along the E-NE side in 2013 than in 2011 potentially contributed to this proportional shift. Furthermore, it is well-known that cyanobacterial mats and turf algae on coral reefs fix N2 and that δ15N signatures of cyanobacterial mats are lower than of other benthic primary producers (Table 3; Kayanne et al., 2005; Kolasinski et al., 2016). Released products from these mats contain N compounds lower in δ15N than N released by macroalgae or pelagic primary producers (Kayanne et al., 2005; Thibodeau et al., 2013; Brocke et al., 2015). In the Caribbean significant increases in cyanobacterial mat cover on coral reefs were reported from less than 10 to more than 20% since ∼1990 (De Bakker et al., 2017). Such shifts in cover might have occurred on the Saba Bank as well, considering the fact that in October 2013 ca 30% of the cover of benthic primary producers and 20% of total cover on reefs of the Saba Bank was occupied by cyanobacterial mats (Wiltink, 2016). In case sponges feed on this cyanobacterial mat derived nitrogen, it is likely that δ15N in their bulk tissue further decreased in time. It is evident that various nitrogen sources contributed to the observed patterns of δ15N in sponges in time.
Conclusion
Bivariate SI signatures of sponges (δ13C, δ15N) on coral reefs of the Saba Bank differed between species with different functional traits. Orientation of reefs toward incoming currents and waves (space) influenced the variation of δ13C in sponges but not the δ15N. Bulk δ13C and δ15N in sponges moved in a comparable direction in time, while the δ13C appeared more responsive to changes in pelagic food concentrations than the δ15N. The variable supply of pelagic-derived organic carbon to sponges and the accumulation of benthic-derived food in reef overlying waters was ascribed to variations in hydrodynamic conditions in time and space (currents and retention time of water over reefs). Variations in SI signatures of sponges were most likely realized by mainly unselective shifts in proportional feeding on pelagic- and benthic-derived food sources, which differed significantly in SI composition. Shifts in diet were likely influenced by spatial differences in retention time of water over reefs and concentration of pelagic food relative to benthic-derived food.
Supplemental Information
Variations in inorganic nitrate (NO3−) in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
Variations in soluble reactive phosphate (PO43−) in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
Variations in dissolved organic carbon (DOC) in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
(Sheet Stations) Names and positions of stations sampled on the Saba Bank in October 2011 and October 2013.(Sheet Sponges SI) Stable isotope signatures of sponges collected on the Saba Bank in October 2011 and October 2013. Position of station numbers are presented on sheet Stations.(Sheet Macroalgae SI) Stable isotope signatures of benthic primary producers collected on the Saba Bank in October 2011 and October 2013. Position of station numbers are presented on sheet Stations. (Sheet POM SI) Stable isotope signatures of particulate organic matter (POM) collected in surface water on the Saba Bank in October 2011 andOctober 2013. Position of station numbers are presented on sheet Stations.(sheet Nutriënt concentrations) Organic and inorganic nutrient concentrations in surface water at stations sampled in October 2011 and 2013 on the Saba Bank. In 2011 replicate water samples were analysed. PO43− is soluble reactive phosphate; Sum of ammonium (NH4+) nitrite (NO2−) and nitrate (NO3−) represents dissolved inorganic nitrogen (DIN); DOC is dissolved organic carbon; DON is dissolved organic nitrogen obtained by distracting DIN from total dissolved nitrogen (TDN); TOC is total organic carbon; TON is total organic nitrogen obtained by distracting DIN from total nitrogen (TN); POC is particulate organic carbon; PON is particulate organic nitrogen. Position of station numbers are presented on sheet Stations.
Acknowledgments
We are grateful for the support of fellow divers and crew of the Caribbean Explorer II during the expeditions to the Saba Bank in 2011 and 2013. We thank our NIOZ-colleagues Karel Bakker, Richard Doggen, Santiago Gonzales, Kirsten Kooijman and Jort Ossebaar for analyses of stable isotopes, organic and inorganic nutrients.
Funding Statement
This work was supported by the Dutch Ministry of Economic Affairs (No. HD4302 BO-11-011.05-002 of Wageningen Marine Research (WMR)), World Wildlife Fund for Nature (WNF.nl) and the European Union Seventh Framework Program (FP 7/2007-2013, grant agreement No. 244161). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Fleur C. Van Duyl conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Benjamin Mueller performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Erik H. Meesters analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, statistics.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Research permits for conducting research on the Saba Bank in 2011 and 2013 were issued by Rijkswaterstaat, Dutch Ministry of Infrastructure and Environment and Ministry of Economic Affairs (No. WSH/2011/1400 and No. RWS-2013/42681).
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Anderson (2001).Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26(1):32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- Andrade & Barton (2000).Andrade CA, Barton ED. Eddy development and motion in the Caribbean Sea. Journal of Geophysical Research: Oceans. 2000;105(C11):26191–26201. doi: 10.1029/2000jc000300. [DOI] [Google Scholar]
- Benner et al. (1997).Benner R, Biddanda B, Black B, McCarthy M. Abundance, size distribution, and stable carbon and nitrogen isotopic compositions of marine organic matter isolated by tangential-flow ultrafiltration. Marine Chemistry. 1997;57(3–4):243–263. doi: 10.1016/s0304-4203(97)00013-3. [DOI] [Google Scholar]
- Brocke et al. (2015).Brocke HJ, Wenzhoefer F, De Beer D, Mueller B, Van Duyl FC, Nugues MM. High dissolved organic carbon release by benthic cyanobacterial mats in a Caribbean reef ecosystem. Scientific Reports. 2015;5(1):8852. doi: 10.1038/srep08852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardini et al. (2014).Cardini U, Bednarz VN, Foster RA, Wild C. Benthic N2 fixation in coral reefs and the potential effects of human induced environmental change. Ecology and Evolution. 2014;4(9):1706–1727. doi: 10.1002/ece3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso et al. (2013).Cardoso JFMF, Van Bleijswijk JDL, Witte H, Van Duyl FC. Diversity and abundance of ammonia oxidizing Archaea and Bacteria in tropical and cold-water coral reef sponges. Aquatic Microbial Ecology. 2013;68(3):215–230. doi: 10.3354/ame01610. [DOI] [Google Scholar]
- Corredor et al. (1988).Corredor JE, Wilkinson CR, Vicente VP, Morell JM, Otero E. Nitrate release by Caribbean reef sponges. Limnology and Oceanography. 1988;33(1):114–120. doi: 10.4319/lo.1988.33.1.0114. [DOI] [Google Scholar]
- De Bakker et al. (2017).De Bakker DM, Van Duyl FC, Bak RPM, Nugues MM, Nieuwland G, Meesters EH. 40 years of benthic community change on the Caribbean reefs of Curacao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs. 2017;36(2):355–367. doi: 10.1007/s00338-016-1534-9. [DOI] [Google Scholar]
- De Goeij et al. (2008).De Goeij JM, Van den Berg H, Van Oostveen MM, Epping EHG, Van Duyl FC. Major Bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Marine Ecology Progress Series. 2008;357:139–151. doi: 10.3354/meps07403. [DOI] [Google Scholar]
- De Graaf (2012).De Graaf N. Wind waves and tidal controls over flow processes in the Saba Bank. 2012. MSc thesis research, Hydrology and Quantitative Water Management, Wageningen University.
- Den Haan et al. (2014).Den Haan J, Visser PM, Ganase AE, Gooren EE, Stal LJ, Van Duyl FC, Vermeij MJA, Huisman J. Nitrogen fixation rates in algal turf communities of a degraded versus less degraded coral reef. Coral Reefs. 2014;33(4):1003–1015. doi: 10.1007/s00338-014-1207-5. [DOI] [Google Scholar]
- De Nooijer & Van Heuven (2016).De Nooijer LJ, Van Heuven SMAC. NIOZ Cruise report RV Pelagia 64PE414 Saba Bank: 19th of August—8th of September 2016 Guadeloupe—Curaçao. 2016. http://www.dcbd.nl/document/cruise-report-rv-pelagia-64pe414-saba-bank http://www.dcbd.nl/document/cruise-report-rv-pelagia-64pe414-saba-bank
- Diaz & Ward (1997).Diaz MC, Ward BB. Sponge-mediated nitrification in tropical benthic communities. Marine Ecology Progress Series. 1997;156:97–107. doi: 10.3354/meps156097. [DOI] [Google Scholar]
- Dinsdale et al. (2008).Dinsdale EA, Pantos O, Smriga S, Edwards RA, Angly F, Wegley L, Hatay M, Hall D, Brown E, Haynes M, Krause L, Sala E, Sandin SA, Thurber RV, Willis BL, Azam F, Knowlton N, Rohwer F. Microbial ecology of four coral atolls in the northern line Islands. PLOS ONE. 2008;3(2):e1584. doi: 10.1371/journal.pone.0001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte (1992).Duarte CM. Nutrient concentration of aquatic plants: patterns across species. Limnology and Oceanography. 1992;37(4):882–889. doi: 10.4319/lo.1992.37.4.0882. [DOI] [Google Scholar]
- Erwin & Thacker (2007).Erwin PM, Thacker RW. Incidence and identity of photosynthetic symbionts in Caribbean coral reef sponge assemblages. Journal of the Marine Biological Association of the United Kingdom. 2007;87(6):1683–1692. doi: 10.1017/s0025315407058213. [DOI] [Google Scholar]
- Faganeli et al. (1988).Faganeli J, Malej A, Pezdic J, Malacic V. C:N:P ratios and stable C isotope ratios as indicators of sources of organic matter in the Gulf of Trieste (Northern Adriatic) Oceanologica Acta. 1988;11:377–382. [Google Scholar]
- Fiore, Baker & Lesser (2013).Fiore CL, Baker DM, Lesser MP. Nitrogen biogeochemistry in the Caribbean sponge, Xestospongia muta: a source or sink of dissolved inorganic nitrogen? PLOS ONE. 2013;8(8):e72961. doi: 10.1371/journal.pone.0072961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore et al. (2010).Fiore CL, Jarett JK, Olson ND, Lesser MP. Nitrogen fixation and nitrogen transformations in marine symbioses. Trends in Microbiology. 2010;18(10):455–463. doi: 10.1016/j.tim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- France (1995).France RL. Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Marine Ecology Progress Series. 1995;124:307–312. doi: 10.3354/meps124307. [DOI] [Google Scholar]
- Freeman, Easson & Baker (2014).Freeman CJ, Easson CG, Baker DM. Metabolic diversity and niche structure in sponges from the Miskito Cays, Honduras. PeerJ. 2014;2:e695. doi: 10.7717/peerj.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, Easson & Baker (2016).Freeman CJ, Easson CG, Baker DM. Niche structure of marine sponges from temperate hard-bottom habitats within Gray’s Reef National Marine Sanctuary. Journal of the Marine Biological Association of the United Kingdom. 2016;96(2):559–565. doi: 10.1017/s0025315415000363. [DOI] [Google Scholar]
- Freeman & Thacker (2011).Freeman CJ, Thacker RW. Complex interactions between marine sponges and their symbiotic microbial communities. Limnology and Oceanography. 2011;56(5):1577–1586. doi: 10.4319/lo.2011.56.5.1577. [DOI] [Google Scholar]
- Fry (2006).Fry B. Stable Isotope Ecology. New York: Springer; 2006. p. 297. [Google Scholar]
- Fry et al. (1998).Fry B, Hopkinson CS, Jr, Nolin A, Wainright SC. 13C/12C composition of marine dissolved organic carbon. Chemical Geology. 1998;152(1–2):113–118. doi: 10.1016/s0009-2541(98)00100-4. [DOI] [Google Scholar]
- Fry et al. (1982).Fry B, Lutes R, Northam M, Parker PL, Ogden JC. A 13C/12C comparison of food webs in Caribbean seagrass meadows and coral reefs. Aquatic Botany. 1982;14:389–398. doi: 10.1016/0304-3770(82)90112-7. [DOI] [Google Scholar]
- Gast et al. (1999).Gast GJ, Jonkers PJ, Van Duyl FC, Bak RPM. Bacteria, flagellates and nutrients in island fringing coral reef waters: influence of the ocean, the reef and eutrophication. Bulletin of Marine Science. 1999;65:523–538. [Google Scholar]
- Gloeckner et al. (2014).Gloeckner V, Wehrl M, Moitinho-Silva L, Gernert C, Schupp P, Pawlik JR, Lindquist N, Erpenbeck D, Worheide G, Hentschel U. The HMA-LMA dichotomy revisited: and electron microscopical survey of 56 sponge species. Biological Bulletin. 2014;227(1):78–88. doi: 10.1086/bblv227n1p78. [DOI] [PubMed] [Google Scholar]
- Gordon (1967).Gordon AL. Circulation of the Caribbean Sea. Journal of Geophysical Research. 1967;72(24):6207–6223. doi: 10.1029/jz072i024p06207. [DOI] [Google Scholar]
- Granek, Compton & Phillips (2009).Granek EF, Compton JE, Phillips DL. Mangrove-exported nutrient incorporation by sessile coral reef invertebrates. Ecosystems. 2009;12(3):462–472. doi: 10.1007/s10021-009-9235-7. [DOI] [Google Scholar]
- Haas et al. (2016).Haas AF, Fairoz MF, Kelly LW, Nelson CE, Dinsdale EA, Edwards RA, Giles S, Hatay M, Hisakawa N, Knowles B, Lim YW, Maughan H, Pantos O, Roach TNF, Sanchez SE, Silveira CB, Sandin S, Smith JE, Rohwer F. Global microbialization of coral reefs. Nature Microbiology. 2016;1(6):16042. doi: 10.1038/nmicrobiol.2016.42. [DOI] [PubMed] [Google Scholar]
- Haas et al. (2010a).Haas AF, Jantzen C, Naumann MS, Iglesias-Prieto R, Wild C. Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ O2 availability. Marine Ecology Progress Series. 2010a;409:27–39. doi: 10.3354/meps08631. [DOI] [Google Scholar]
- Haas et al. (2010b).Haas AF, Naumann MS, Struck U, Mayr C, El-Zibdah M, Wild C. Organic matter release by coral reef associated benthic algae in the northern Red Sea. Journal of Experimental Marine Biology and Ecology. 2010b;389(1–2):53–60. doi: 10.1016/j.jembe.2010.03.018. [DOI] [Google Scholar]
- Haas et al. (2011).Haas AF, Nelson CE, Wegley KL, Carlson CA, Rohwer F, Leichter JJ, Wyatt A, Smith JE. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLOS ONE. 2011;6(11):e27973. doi: 10.1371/journal.pone.0027973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoer et al. (2018).Hoer DR, Gibson PJ, Tommerdahl JP, Lindquist NL, Martens CS. Consumption of dissolved organic carbon by Caribbean reef sponges. Limnology and Oceanography. 2018;63(1):337–351. doi: 10.1002/lno.10634. [DOI] [Google Scholar]
- Jackson et al. (2014).Jackson J, Donavan M, Cramer K, Lam V. Status and Trends of Caribbean Coral Reefs: 1970–2012. CEP Technical Report. 2014;71:330. [Google Scholar]
- Jackson et al. (2011).Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology. 2011;80(3):595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- Jiménez & Ribes (2007).Jiménez E, Ribes M. Sponges as a source of dissolved inorganic nitrogen: nitrification mediated by temperate sponges. Limnology and Oceanography. 2007;52(3):948–958. doi: 10.4319/lo.2007.52.3.0948. [DOI] [Google Scholar]
- Kayanne et al. (2005).Kayanne H, Hirota M, Yamamuro M, Koike I. Nitrogen fixation of filamentous cyanobacteria in a coral reef measured using three different methods. Coral Reefs. 2005;24(2):197–200. doi: 10.1007/s00338-004-0465-z. [DOI] [Google Scholar]
- Kjerfve (1981).Kjerfve B. Tides of the Caribbean Sea. Journal Geophysical Research. 1981;86(C5):4243–4247. doi: 10.1029/jc086ic05p04243. [DOI] [Google Scholar]
- Kolasinski et al. (2016).Kolasinski J, Nahon S, Rogers K, Chauvin A, Bigot L, Frouin P. Stable isotopes reveal spatial variability in the trophic structure of a macro-benthic invertebrate community in a tropical coral reef. Rapid Communications in Mass Spectrometry. 2016;30(3):433–446. doi: 10.1002/rcm.7443. [DOI] [PubMed] [Google Scholar]
- Kürten et al. (2014).Kürten B, Al-Aidaroos AM, Struck U, Khomayis HS, Gharbawi WY, Sommer U. Influence of environmental gradients on C and N stable isotope ratios in coral reef biota of the Red Sea, Saudi Arabia. Journal of Sea Research. 2014;85:379–394. doi: 10.1016/j.seares.2013.07.008. [DOI] [Google Scholar]
- Lapointe et al. (2010).Lapointe BE, Langton R, Bedford BJ, Potts AC, Day O, Hu C. Land-based nutrient enrichment of the Buccoo Reef Complex and fringing coral reefs of Tobago, West Indies. Marine Pollution Bulletin. 2010;60(3):334–343. doi: 10.1016/j.marpolbul.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Lesser et al. (2007).Lesser MP, Falcon LI, Rodriguez-Roman A, Enriquez S, Hoegh-Guldberg O, Iglesias-Prieto R. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastrea cavernosa. Marine Ecology Progress Series. 2007;346:143–152. doi: 10.3354/meps07008. [DOI] [Google Scholar]
- Lowe & Falter (2015).Lowe RJ, Falter JL. Oceanic forcing of coral reefs. Annual Review of Marine Science. 2015;7(1):43–66. doi: 10.1146/annurev-marine-010814-015834. [DOI] [PubMed] [Google Scholar]
- Maldonado, Ribes & Van Duyl (2012).Maldonado M, Ribes M, Van Duyl FC. Nutrient fluxes through sponges: biology, budgets, and ecological implications. Advances in Marine Biology. 2012;62:113–182. doi: 10.1016/B978-0-12-394283-8.00003-5. [DOI] [PubMed] [Google Scholar]
- McCook, Jompa & Diaz-Pulido (2001).McCook LJ, Jompa J, Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs. 2001;19(4):400–417. doi: 10.1007/s003380000129. [DOI] [Google Scholar]
- McMurray, Pawlik & Finelli (2017).McMurray SE, Pawlik JR, Finelli CM. Demography alters carbon flux for a dominant benthic suspension feeder, the giant barrel sponge, on Conch Reef, Florida Keys. Functional Ecology. 2017;31(11):2188–2198. doi: 10.1111/1365-2435.12908. [DOI] [Google Scholar]
- McMurray et al. (2018).McMurray SE, Stubler AD, Erwin PM, Finelli CM, Pawlik JR. A test of the sponge-loop hypothesis for emergent Caribbean reef sponges. Marine Ecology Progress Series. 2018;588:1–14. doi: 10.3354/meps12466. [DOI] [Google Scholar]
- Mohamed et al. (2008).Mohamed NM, Colman AS, Tal Y, Hill RT. Diversity and expression of nitrogen fixation genes in bacterial symbionts of marine sponges. Environmental Microbiology. 2008;10(11):2910–2921. doi: 10.1111/j.1462-2920.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- Mohamed et al. (2010).Mohamed NM, Saito K, Tal Y, Hill RT. Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges. ISME Journal. 2010;4(1):38–48. doi: 10.1038/ismej.2009.84. [DOI] [PubMed] [Google Scholar]
- Mueller et al. (2014a).Mueller B, De Goeij JM, Vermeij MJA, Mulders Y, Van Der Ent E, Ribes M, Van Duyl FC. Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC) PLOS ONE. 2014a;92(2):e90152. doi: 10.1371/journal.pone.0090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller et al. (2016).Mueller B, Den Haan J, Visser PM, Vermeij MJA, Van Duyl FC. Effect of light and nutrient availability on the release of dissolved organic carbon (DOC) by Caribbean turf algae. Scientific Reports. 2016;6(1):23248. doi: 10.1038/srep23248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, Meesters & Van Duyl (2017).Mueller B, Meesters EH, Van Duyl FC. DOC concentrations across a depth gradient on a Caribbean coral reef. PeerJ. 2017;5:e3456. doi: 10.7287/peerj.preprints.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller et al. (2014b).Mueller B, Van Der Zande R, Van Leent P, Meesters E, Vermeij M, Van Duyl FC. Effect of light availability on dissolved organic carbon (DOC) release by Caribbean reef algae and corals. Bulletin of Marine Science. 2014b;90(3):875–893. doi: 10.5343/bms.2013.1062. [DOI] [Google Scholar]
- Naumann et al. (2010a).Naumann M, Haas AF, Struck U, Mayr C, El-Zibdah M, Wild C. Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs. 2010a;29(3):649–659. doi: 10.1007/s00338-010-0612-7. [DOI] [Google Scholar]
- Naumann et al. (2010b).Naumann MS, Mayr C, Struck U, Wild C. Coral mucus stable isotope composition and labeling: experimental evidence for mucus uptake by epizoic acoelomorph worms. Marine Biology. 2010b;157(11):2521–2531. doi: 10.1007/s00227-010-1516-3. [DOI] [Google Scholar]
- Parnell & Jackson (2013).Parnell A, Jackson A. SIAR: stable isotope analysis in R package. Version 4.2http://CRAN.R-project.org/package=siar 2013
- Parnell et al. (2013).Parnell AC, Phillips DL, Bearhop S, Semmens BX, Ward EJ, Moore JW, Jackson AL, Grey J, Kelly DJ, Inger R. Bayesian stable isotope mixing models. Environmetrics. 2013;24:387–399. doi: 10.1002/env.2221. [DOI] [Google Scholar]
- Pawlik, Burkepile & Thurber (2016).Pawlik JR, Burkepile DE, Thurber RV. A vicious circle? Altered carbon and nutrient cycling may explain the low resilience of Caribbean coral reefs. Bioscience. 2016;66(6):470–476. doi: 10.1093/biosci/biw047. [DOI] [Google Scholar]
- Peterson & Fry (1987).Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics. 1987;18(1):293–320. doi: 10.1146/annurev.ecolsys.18.1.293. [DOI] [Google Scholar]
- Phillips et al. (2014).Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology. 2014;92(10):823–835. doi: 10.1139/cjz-2014-0127. [DOI] [Google Scholar]
- R Core Team (2017).R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Raven et al. (1995).Raven J, Walker D, Johnston A, Handley L, Kubler J. Implications of 13C natural abundance measurements for photosynthetic performance by marine macrophytes in their natural environment. Marine Ecology Progress Series. 1995;123:193–205. doi: 10.3354/meps123193. [DOI] [Google Scholar]
- Ribes et al. (2015).Ribes M, Dziallas C, Coma R, Riemann L. Microbial diversity and putative diazotrophy in High- and Low-Microbial-Abundance Mediterranean sponges. Applied and Environmental Microbiology. 2015;81(17):5683–5693. doi: 10.1128/aem.01320-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk et al. (2009).Risk MJ, Lapointe BE, Sherwood OA, Bedford BJ. The use of δ15N in assessing sewage stress on coral reefs. Marine Pollution Bulletin. 2009;58(6):793–802. doi: 10.1016/j.marpolbul.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Risk, Sammarco & Schwarcz (1994).Risk MJ, Sammarco PW, Schwarcz HP. Cross-continental shelf trends in d13C in coral on the Great Barrier Reef. Marine Ecology Progress Series. 1994;106:121–130. doi: 10.3354/meps106121. [DOI] [Google Scholar]
- Rix et al. (2016a).Rix L, De Goeij JM, Mueller CE, Struck U, Middelburg JJ, Van Duyl FC, Al-Horani FA, Wild C, Naumann MS, Van Oevelen D. Coral mucus fuels the sponge loop in warm- and cold-water coral reef ecosystems. Scientific Reports. 2016a;6(1):18715. doi: 10.1038/srep18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rix et al. (2016b).Rix L, De Goeij JM, Van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS. Differential recycling of coral and algal dissolved organic matter via the sponge loop. Functional Ecology. 2016b;31(3):778–789. doi: 10.1111/1365-2435.12758. [DOI] [Google Scholar]
- Rue, Martino & Chopin (2009).Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2009;71(2):319–392. doi: 10.1111/j.1467-9868.2008.00700.x. [DOI] [Google Scholar]
- Schläppy et al. (2010).Schläppy M-L, Schottner SI, Lavik G, Kuypers MMM, De Beer D, Hoffmann F. Evidence of nitrification and denitrification in high and low microbial abundance sponges. Marine Biology. 2010;157(3):593–602. doi: 10.1007/s00227-009-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simister et al. (2013).Simister RL, Taylor MW, Rogers KM, Schupp PJ, Deines P. Temporal molecular and isotopic analysis of active bacterial communities in two New Zealand sponges. FEMS Microbiology Ecology. 2013;85(1):195–205. doi: 10.1111/1574-6941.12109. [DOI] [PubMed] [Google Scholar]
- Southwell (2007).Southwell MW. Sponge impacts on coral reef nitrogen cycling, Key Largo, Florida. 2007. PhD thesis. University of North Carolina, Chapel Hill.
- Southwell, Popp & Martens (2008).Southwell MW, Popp BN, Martens CS. Nitrification controls on fluxes and isotopic composition of nitrate from Florida Keys sponges. Marine Chemistry. 2008;108(1–2):96–108. doi: 10.1016/j.marchem.2007.10.005. [DOI] [Google Scholar]
- Southwell et al. (2008).Southwell MW, Weisz JB, Martens CS, Lindquist N. In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnology and Oceanography. 2008;53(3):986–996. doi: 10.4319/lo.2008.53.3.0986. [DOI] [Google Scholar]
- Taylor et al. (2007).Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiology and Molecular Biology Reviews. 2007;71(2):295–347. doi: 10.1128/mmbr.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker et al. (2010).Thacker RW, Diaz MC, De Voogd NJ, Van Soest RWM, Freeman CJ, Mobley AS, LaPietra J, Cope K, McKenna S. Preliminary assessment of sponge biodiversity on Saba Bank, Netherlands Antilles. PLOS ONE. 2010;5(5):e9622. doi: 10.1371/journal.pone.0009622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker & Freeman (2012).Thacker RW, Freeman CJ. Sponge-microbe symbioses: recent advances and new directions. Advances in Marine Biology. 2012;62:57–111. doi: 10.1016/B978-0-12-394283-8.00002-3. [DOI] [PubMed] [Google Scholar]
- Thibodeau et al. (2013).Thibodeau B, Miyajima T, Tayasu I, Wyatt ASJ, Watanabe A, Morimoto N, Yoshimizu C, Nagata T. Heterogeneous dissolved organic nitrogen supply over a coral reef: first evidence from nitrogen stable isotope ratios. Coral Reefs. 2013;32(4):1103–1110. doi: 10.1007/s00338-013-1070-9. [DOI] [Google Scholar]
- Van Beek & Meesters (2014).Van Beek IJM, Meesters EHWG. Wageningen: Wageningen University and Research; 2014. Saba Bank Research expedition 2013-Progress Report. Report nr CO86/14. [Google Scholar]
- Vander Zanden & Rasmussen (2001).Vander Zanden MJ, Rasmussen JB. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography. 2001;46(8):2061–2066. doi: 10.4319/lo.2001.46.8.2061. [DOI] [Google Scholar]
- Van Duyl (2016).Van Duyl FC. Net coral community calcification and hydrodynamics (Saba Bank/Saba) 2016. http://www.dcbd.nl/document/nioz-saba-banksaba-cruise-report-net-coral-community-calcification-and-hydrodynamics-saba http://www.dcbd.nl/document/nioz-saba-banksaba-cruise-report-net-coral-community-calcification-and-hydrodynamics-saba NIOZ-Cruise report 17/24–31 October 2015.
- Van Duyl & Gast (2001).Van Duyl FC, Gast GJ. Linkage of small-scale spatial variations in DOC, inorganic nutrients and bacterioplankton growth with different coral reef water types. Aquatic Microbial Ecology. 2001;24:17–26. doi: 10.3354/ame024017. [DOI] [Google Scholar]
- Van Duyl et al. (2008).Van Duyl FC, Hegeman J, Hoogstraten A, Maier C. Dissolved carbon fixation by sponge-microbe consortia of deep water coral mounds in the northeastern Atlantic Ocean. Marine Ecology Progress Series. 2008;358:137–150. doi: 10.3354/meps07370. [DOI] [Google Scholar]
- Van Duyl et al. (2011).Van Duyl FC, Moodley L, Nieuwland G, Van Ijzerloo L, Van Soest RWM, Houtekamer M, Meesters EH, Middelburg JJ. Coral cavity sponges depend on reef-derived food resources: stable isotope and fatty acid constraints. Marine Biology. 2011;158(7):1653–1666. doi: 10.1007/s00227-011-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafar, Wafar & David (1990).Wafar M, Wafar S, David JJ. Nitrification in reef corals. Limnology and Oceanography. 1990;35(3):725–730. doi: 10.4319/lo.1990.35.3.0725. [DOI] [Google Scholar]
- Webb et al. (1975).Webb KL, DuPaul WD, Wiebe W, Sottile W, Johannes RE. Enewetak (Eniwetok) Atoll: aspects of the nitrogen cycle on a coral reef. Limnology and Oceanography. 1975;20(2):198–210. doi: 10.4319/lo.1975.20.2.0198. [DOI] [Google Scholar]
- Weisz et al. (2007).Weisz JB, Hentschel U, Lindquist N, Martens CS. Linking abundance and diversity of sponge-associated microbial communities to metabolic differences in host sponges. Marine Biology. 2007;152(2):475–483. doi: 10.1007/s00227-007-0708-y. [DOI] [Google Scholar]
- Wilkinson (1983).Wilkinson CR. Net primary productivity in coral reef sponges. Science. 1983;219(4583):410–412. doi: 10.1126/science.219.4583.410. [DOI] [PubMed] [Google Scholar]
- Wilkinson & Fay (1979).Wilkinson C, Fay P. Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature. 1979;279(5713):527–529. doi: 10.1038/279527a0. [DOI] [Google Scholar]
- Williams & Gordon (1970).Williams PM, Gordon LI. Carbon-13:Carbon-12 ratios in dissolved and particulate organic matter in the sea. Deep Sea Research and Oceanographic Abstracts. 1970;17(1):19–27. doi: 10.1016/0011-7471(70)90085-9. [DOI] [Google Scholar]
- Wiltink (2016).Wiltink M. Changing benthic communities on Saba Bank. Is Saba Bank becoming a ‘Sponge reef’? 2016. Master Thesis, University of Amsterdam.
- Wyatt et al. (2013).Wyatt ASJ, Lowe RJ, Humphries S, Waite AM. Particulate nutrient fluxes over a fringing coral reef: source-sink dynamics inferred from carbon to nitrogen ratios and stable isotopes. Limnology and Oceanography. 2013;58(1):409–427. doi: 10.4319/lo.2013.58.1.0409. [DOI] [Google Scholar]
- Yahel et al. (2003).Yahel G, Sharp JH, Marie D, Hase C, Genin A. In situ feeding and element removal in the symbiont-bearing sponge Theonella swinhoei: bulk DOC is the major source for carbon. Limnology and Oceanography. 2003;48(1):141–149. doi: 10.4319/lo.2003.48.1.0141. [DOI] [Google Scholar]
- Zuur, Hilbe & Ieno (2013).Zuur AF, Hilbe JM, Ieno EN. A beginner’s guide to GLM and GLMM with R: a frequentist and Bayesian perspective for ecologists. Newburgh: Highland Statistics Ltd; 2013. [Google Scholar]
- Zuur, Ieno & Saveliev (2017).Zuur AF, Ieno EN, Saveliev AA. Beginner’s guide to spatial, temporal and spatial-temporal Ecological data analysis with R-INLA statistics. Vol. I. Using GLM and GLMM. Newburgh: Highland Statistics Ltd.; 2017. p. 357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variations in inorganic nitrate (NO3−) in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
Variations in soluble reactive phosphate (PO43−) in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
Variations in dissolved organic carbon (DOC) in surface water along the S-SE and E-NE side of the Saba Bank in 2011 and 2013 with standard deviations.
(Sheet Stations) Names and positions of stations sampled on the Saba Bank in October 2011 and October 2013.(Sheet Sponges SI) Stable isotope signatures of sponges collected on the Saba Bank in October 2011 and October 2013. Position of station numbers are presented on sheet Stations.(Sheet Macroalgae SI) Stable isotope signatures of benthic primary producers collected on the Saba Bank in October 2011 and October 2013. Position of station numbers are presented on sheet Stations. (Sheet POM SI) Stable isotope signatures of particulate organic matter (POM) collected in surface water on the Saba Bank in October 2011 andOctober 2013. Position of station numbers are presented on sheet Stations.(sheet Nutriënt concentrations) Organic and inorganic nutrient concentrations in surface water at stations sampled in October 2011 and 2013 on the Saba Bank. In 2011 replicate water samples were analysed. PO43− is soluble reactive phosphate; Sum of ammonium (NH4+) nitrite (NO2−) and nitrate (NO3−) represents dissolved inorganic nitrogen (DIN); DOC is dissolved organic carbon; DON is dissolved organic nitrogen obtained by distracting DIN from total dissolved nitrogen (TDN); TOC is total organic carbon; TON is total organic nitrogen obtained by distracting DIN from total nitrogen (TN); POC is particulate organic carbon; PON is particulate organic nitrogen. Position of station numbers are presented on sheet Stations.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.