Abstract
Context:
Latent tuberculosis infection diagnosis and treatment is a strategic priority for eliminating tuberculosis in the U.S. The Centers for Disease Control and Prevention has recommended the short-course regimen of 3-month isoniazid-rifapentine administered by directly observed therapy. However, longer-duration regimens remain the most widely prescribed latent tuberculosis infection treatments. Limitation on adoption of 3-month isoniazid-rifapentine in the U.S. might be because of patients’ preference for self-administered therapy, providers’ lack of familiarity with 3-month isoniazid-rifapentine, or lack of resources to support directly observed therapy. This review examines the most recent evidence regarding 3-month isoniazid-rifapentine’s effectiveness, safety, and treatment completion when directly compared with other latent tuberculosis infection regimens primarily comprising 9-month isoniazid treatment.
Evidence acquisition:
Using Community Guide methodology, reviewers identified, evaluated, and summarized available evidence published during January 2006–June 2017. Analysis of the data was completed in 2017.
Evidence synthesis:
The analysis included 15 unique studies. Three-month isoniazid-rifapentine was determined to be equal to other latent tuberculosis infection regimens in effectiveness (OR 0.89, 95% CI=0.46, 1.70), and has higher treatment completion (87.5%, 95% CI=83.2%, 91.3%) compared with other latent tuberculosis infection regimens (65.9%, 95% CI=53.5%, 77.3%). Three-month isoniazid-rifapentine was associated with similar risk to other latent tuberculosis infection regimens for adverse events (relative risk=0.59, 95% CI=0.23, 1.52); discontinuing treatment because of adverse events (relative risk=0.48, 95% CI=0.17, 1.34); and death (relative risk=0.79, 95% CI=0.56, 1.11).
Conclusions:
The 3-month isoniazid-rifapentine regimen is as safe and effective as other recommended latent tuberculosis infection regimens and achieves significantly higher treatment completion rates.
CONTEXT
Tuberculosis (TB) is one of the leading causes of death from infectious diseases in the world, with an estimated 1.8 million deaths during 2015.1 Globally, approximately 1.7 billion people are estimated to be infected with Mycobacterium tuberculosis, the causative agent of TB among humans.2 Treatment of latent tuberculosis infection (LTBI) in people at high risk for progression to active TB is a principal strategy for controlling and eliminating TB.3–5 For individuals with LTBI, isoniazid (INH) treatment for 6–12 months has been reported to reduce the risk for progression to active TB disease by 60%–90%.6,7 However, because of the INH regimen’s long treatment duration, lack of patient tolerability, and risk for hepatotoxicity, its effectiveness in preventing TB disease has been hindered by inadequate acceptance and low treatment completion rates.3,8,9
One treatment regimen that has demonstrated promise in improving completion rates among people with LTBI is a 3-month combination of INH and rifapentine (3HP). In 2010, PREVENT TB (Tuberculosis Trials Consortium Study 26), a multicenter randomized clinical trial, tested the effectiveness of 3HP among persons with LTBI who were at high risk for progressing to active TB disease.10 Findings from this trial, and two other smaller trials,11,12 led the Centers for Disease Control and Prevention to recommend use of 3HP for treating LTBI.13 However, the recommendation was limited to administration of 3HP by directly observed therapy (DOT), and included significant limitations for using the regimen among people living with HIV/AIDS (PLWHA) and children aged 2–11 years.
A systematic review of the scientific literature examines current evidence on 3HP’s effectiveness and safety in preventing active TB among adults, adolescents, children aged 2 or more years, and PLWHA, as well as treatment completion rates for participants administered the regimen by DOT or self-administered therapy (SAT) when directly compared with other LTBI treatments—mostly 9 months of INH. The review also assesses the applicability of findings for 3HP administration across different populations and settings, considerations for implementation, and evidence gaps by using methodology developed for The Guide to Community Preventive Services.14,15
EVIDENCE ACQUISITION
A team of TB subject matter experts, a qualified systematic reviewer, and a librarian was formed to conceptualize and conduct this review. A review protocol was written before study initiation, but not published.
Analytic Framework
The analytic framework (Appendix Figure 1, available online) depicts the team’s conceptual approach to evaluating evidence regarding the effectiveness of 3HP in improving treatment completion rates and preventing TB disease. In brief, the team hypothesized that use of 3HP, either administered by DOT or SAT, to treat LTBI among people aged ≥12 years, children aged 2–11 years, and PLWHA would lead to increased treatment completion rates, and thus reduce TB-related morbidity and mortality. Additionally, administration of 3HP by SAT could lead to reduced costs and resources for public health programs, and administration by DOT could lead to increased patient–provider interaction because of weekly patient visits, which could potentially enhance reporting of adverse events. The team also postulated that key effect modifiers (e.g., tobacco use, low body weight, diabetes, and HIV treatment) could have a negative impact on the overall effectiveness of 3HP in preventing TB disease, as well as treatment completion.
Intervention Definition
INH and rifapentine are administered together as a combination regimen for treating LTBI. The regimen is taken once weekly under DOT or SAT for 12 weeks. Treatment is considered to be DOT when the medications are taken while observed by a healthcare worker or other trained individuals; when taken by patients on their own or administered by a family member, treatment is considered to be SAT.
Search for Evidence
A narrow search strategy developed with guidance from a professional librarian was used to select English-only 3HP intervention studies published during January 2006–June 2017. Electronic databases searched included MEDLINE, Embase, CINAHL, Cochrane Database Library, Scopus, and Clinicaltrials.gov. Additionally, reference lists of articles returned through the search strategy were reviewed and 3HP subject matter experts were consulted. The complete search strategy is available in the Appendix (Appendix Table 1, available online).
Inclusion/Exclusion Criteria
Studies were included in the review if (1) 3HP had been used as an LTBI treatment regimen; (2) study designs were RCTs, quasi-experimental studies, observational studies, or other designs with concurrent comparison groups; (3) the target population included people aged ≥12 years, children aged 2–11 years, or PLWHA; and (4) outcomes reported were prevention of TB disease, treatment completion, adverse events, discontinuation because of adverse events, or death. Studies that focused on individuals with suspected or confirmed TB disease were excluded.
Assessing and Summarizing the Body of Evidence
Studies included in this review were independently abstracted and assessed for suitability of study design by two independent reviewers using a data abstraction form adapted from The Guide for Community Preventive Services (www.thecommunityguide.org/methods/abstractionform.pdf).15 Data were collected on intervention characteristics, outcomes of interest, participant demographics, applicability, intervention benefits, potential harms, considerations for implementation, and evidence gaps. Discordance of data abstraction elements between reviewers was resolved by consensus.
Community Guide methods were used to assess each study for threats to internal and external validity, including inadequate descriptions of the intervention, target population, sampling frame, and inclusion or exclusion criteria; insufficient measurement of exposure or outcomes; lack of reporting of appropriate analytic methods; loss to follow-up; or intervention and comparison groups not being comparable at baseline. Studies were characterized as having good (zero to one limitation); fair (two to four limitations); or limited (five or more limitations) quality of execution. Studies categorized as limited were excluded from analysis.
The primary outcomes of interest were (1) the onset of active TB disease on the basis of acceptable standards reported in the included studies (e.g., clinical diagnosis of TB, detection of acid-fast bacilli on a sputum smear, culture-positive sputum, and abnormal chest radiograph) and (2) completion of treatment, defined for 3HP as administration of 11 or 12 treatment doses within a 16-week period. Author’s designations were accepted for completion of therapy for other LTBI treatment regimens. Secondary outcomes of interest included adverse events while on 3HP (Grades 3 and 4 toxicity); discontinuation of treatment because of adverse events; and death.
Statistical Analysis
A meta-analysis was conducted to assess the effectiveness of 3HP in preventing TB disease and the likelihood of participants on 3HP completing treatment, compared with other LTBI regimens. Additionally, adverse events, discontinuation because of adverse events, and deaths while on 3HP compared with other LTBI regimens were also examined. For studies reporting multiple comparison groups, a single pairwise group was created to calculate an overall effect estimate. A random effects model was used to calculate the pooled effect size because of heterogeneity among study participants and across designs. Studies were weighted by using the Mantel–Haenszel method for risk, odds, and incidence rate ratios,16 and the DerSimonian–Laird method was used to estimate weighted proportions.17 Statistical heterogeneity was assessed across studies by using I-squared (I2) statistics.18 I2 values ranged from 0% to 100%, and for this review, values ≥50% were considered to be of substantial heterogeneity.19
Subgroup analyses by study design, target population, and design suitability were also conducted, as appropriate, to assess whether changes occurred in intervention effect and to explore possible sources of heterogeneity. Additionally, stratified analyses were conducted based on the type of regimen compared with 3HP. Sensitivity analyses were conducted to assess whether different study-level factors influenced results of the pooled estimates. Publication bias was assessed by visual inspection of funnel plots and Egger’s test.18,20 All statistical analyses were conducted using the metafor or meta packages in R, version 3.3.2. Analyses of the data were completed in 2017.
EVIDENCE SYNTHESIS
Search Yield
A total of 292 articles published during January 2006–June 2017 were identified (Figure 1). Of that total, 254 were screened for relevancy on the basis of their titles and abstracts; of those, 30 full-text articles were screened for inclusion. In total, 19 articles10–12,21–36 representing 15 unique studies were included in the meta-analysis10–12,21,23–33 (evidence tables available online in the Appendix). The Sterling and colleagues10 study in 2011 had subsequent follow-up studies on pediatric36 and HIV populations,35 as well as additional substudies on hepatotoxicity22 and systemic drug reactions.34 Therefore, to minimize the chance of double-counting participants, only the larger Sterling and colleagues10 study in 2011 was included in the overall pooled analyses, but the smaller, follow-up studies were included in any subgroup analyses related to pediatric and HIV populations.
Figure 1.
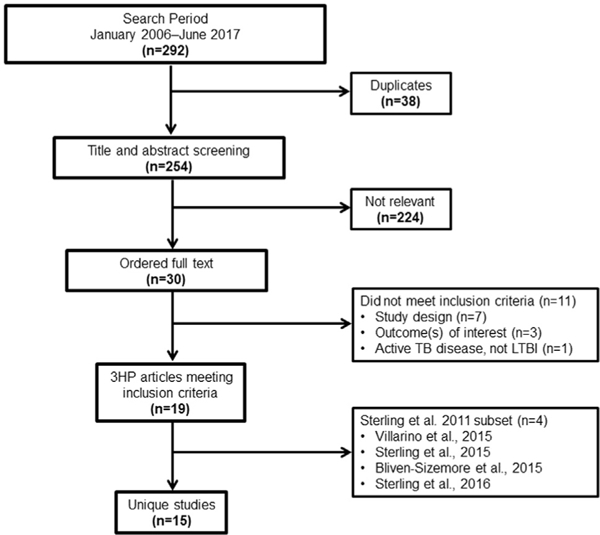
PRISMA flow diagram.
3HP, 3-month isoniazid-rifapentine; LTBI, latent tuberculosis infection; TB, tuberculosis.
Risk of Bias Assessment
Appendix Figure 2 (available online) displays the percentage of studies assigned a limitation using Community Guide standards. Overall, the body of evidence had a low risk of bias for most of the elements assessed. The majority of studies adequately described the intervention being examined, target population and sampling frame, and outcome measurement. However, at least one third of studies had moderate to high risk of bias for failure to report or conduct appropriate data analyses to address biases,12,24,26–28,32 and concerns related to possible selection bias.23,27,28,32,33 As expected, observational studies were more likely to be prone to bias when compared with RCTs.
Study and Population Characteristics
Of the 19 included studies, 16 were partially or fully conducted in the U.S.10,21–25,27–36; other locations included Canada,10,22,34–36 Spain,10,21,22,34–36 Taiwan,26 Hong Kong,21,35,36 Brazil,10,12,22,33–35 Peru,35 and South Africa (Appendix Table 2, available online).11,21 The majority of studies targeted individuals aged ≥12 years, with three studies each focused exclusively on children and adolescents23,25,36 and two focused on PLWHA.11,35 Nine studies reported their funding source, with the majority of those being funded by NIH or Centers for Disease Control and Prevention.10–12,21,22,27,31,33–36
In six studies reporting, 61% of adults in the included studies reported being at least high school graduates.10–12,21,33,35 Treatment was most frequently administered by DOT (17 studies),10–12,22–31,33–36 with one study reporting administration by SAT32 and another study reporting use of both DOT and SAT.21 Eight studies reported on DOT administered in healthcare settings.23,24,26,28–30,32,33 The most frequent comparator with 3HP was reported as 9 months of INH (9H),10,22,26,27,29,32–36 followed by multiple other LTBI regimens (e.g., 2-month rifampin-pyrazinamide [RIF-PZA]; 4-month rifampin; 4-month INH-rifampin; 6-month INH; and daily INH for ≤6 years). Six studies did not include a concurrent comparison group.21,23–25,28,31
The median age for participants in the included studies was 36.9 years (Appendix Table 3, available online). All studies reported the participants’ sex, with the majority of studies having more males than females. Forty percent of study participants identified as white, 24.7% as black, and 18.6% as Asian or Pacific Islander. Hispanics accounted for approximately 52.8% of participants in the studies that reported ethnicity. Few studies reported on comorbidities such as diabetes and hepatitis C. Most participants (36.0%) were known contacts of active TB patients or were immunosuppressed (34.0%).
Primary Outcomes
Figure 2 displays the forest plot for prevention of TB disease from five studies that provided enough information to calculate ORs.10–12,26,32 The pooled random effects estimate for TB prevention for 3HP was 0.89 (95% CI=0.46, 1.70), compared with other LTBI treatments (mostly the 9H regimen), indicating that 3HP is comparable with other treatments regarding effectiveness. However, the difference was not statistically significant; thus, superiority of 3HP compared with other LTBI treatments was not demonstrated. TB incidence rate ratio for three studies reporting person-time10–12 for 3HP compared with other regimens was 0.86 (95% CI=0.39, 1.11; Appendix Figure 3, available online).
Figure 2.

Prevention of tuberculosis disease among participants receiving treatment with 3-month isoniazid-rifapentine, compared with other latent tuberculosis infection regimens.
3HP, 3-month isoniazid-rifapentine; TB, tuberculosis.
Subgroup analyses were also conducted to examine the effect of 3HP on different populations (i.e., people aged ≥12 years, children aged 2–17, and PLWHA). One study, linked to the study by Sterling and colleagues, targeted children and adolescents aged 2–17 years and reported an OR of 0.13 (95% CI=0.01, 2.54)10,36; two studies that targeted PLWHA reported an OR of 0.74 (95% CI=0.23, 2.43).11,35
Results from stratified analyses based on comparator regimens are presented in Table 1. When compared with the 9H regimen, individuals on 3HP had lower odds of developing active TB, but the difference was not statistically meaningful (OR=0.47, 95% CI=0.20, 1.12).10,26,32 Similarly, no statistically meaningful difference was reported when 3HP was compared with 2–3 months of RIF-PZA (OR=2.84, 95% CI=0.29, 27.5).12 Of note, RIF-PZA is no longer recommended for LTBI treatment because of high rates of hospitalization and death from liver injury.37 Equal effectiveness for the prevention of TB disease was also reported when 3HP was compared with 6-month INH,11 4-month rifampin-INH,11 and continuous daily INH (≤6 years).11
Table 1.
Stratified Results for Each LTBI Regimen Compared to 3HP
| Outcome/Number of studies | Comparator regimen | Measure | Effect size, 95% CI | I2, p-value |
|---|---|---|---|---|
| Prevention of TB disease | ||||
| 1 | 6-month INH | OR | 1.09 (0.60, 1.99) | – |
| 3 | 9-month INH | OR | 0.47 (0.20, 1.12) | 0% (p=0.76) |
| 1 | Continuous daily INH (≤6 years) | OR | 1.54 (0.68, 3.51) | – |
| 1 | 4-month RIF-INH | OR | 1.00 (0.56, 1.81) | – |
| 0 | 4-month RIF | OR | – | – |
| 1 | 2–3-month RIF-PZA | OR | 2.84 (0.29, 27.5) | – |
| Adverse events while on 3HP | ||||
| 1 | 6-month INH | RR | 0.68 (0.40, 1.15) | – |
| 4 | 9-month INH | RR | 0.46 (0.02, 1.71) | 75% (p< 0.0001) |
| 1 | Continuous daily INH (≤6 years) | RR | 0.29 (0.20, 0.44) | – |
| 1 | 4-month RIF-INH | RR | 0.88 (0.50, 1.54) | – |
| 0 | 4-month RIF | RR | – | – |
| 1 | 2–3-month RIF-PZA | RR | 0.09 (0.02, 0.40) | – |
| Treatment discontinuation while on 3HP | ||||
| 1 | 6-month INH | RR | 1.00 (0.25, 3.95) | – |
| 3 | 9-month INH | RR | 1.08 (0.66, 1.76) | 28% (p=0.25) |
| 1 | Continuous daily INH (≤6 years) | RR | 0.04 (0.01, 0.11) | – |
| 1 | 4-month RIF-INH | RR | 0.50 (0.15, 1.65) | – |
| 0 | 4-month RIF | RR | – | – |
| 1 | 2–3-month RIF-PZA | RR | 0.16 (0.02, 1.29) | |
| Deaths while on 3HP | ||||
| 1 | 6-month INH | RR | 0.68 (0.37, 1.23) | – |
| 2 | 9-month INH | RR | 0.75 (0.47, 1.20) | 0% (p=0.81) |
| 1 | Continuous daily INH (≤6 years) | RR | 1.06 (0.47, 2.41) | – |
| 1 | 4-month RIF-INH | RR | 1.07 (0.55, 2.07) | – |
| 0 | 4-month RIF | RR | – | – |
| 1 | 2–3-month RIF-PZA | RR | 0.31 (0.03, 2.98) | – |
| Treatment completion | ||||
| 1 | 6-month INH | OR | 4.34 (2.36, 7.99) | – |
| 6 | 9-month INH | OR | 5.06 (2.31, 11.1) | 92% (p< 0.001) |
| 1 | Continuous daily INH (≤6 years) | OR | 3.53 (1.77, 7.06) | – |
| 1 | 4-month RIF-INH | OR | 1.22 (0.59, 2.52) | – |
| 1 | 4-month RIF | OR | 0.98 (0.42, 2.28) | – |
| 1 | 2–3-month RIF-PZA | OR | 0.91 (0.41, 2.02) | – |
3HP, 3-month isoniazid-rifapentine; I2, statistical test for heterogeneity, where N>1; INH, isoniazid; LTBI, latent tuberculosis infection; RIF, rifampin; RIF-INH, rifampin-isoniazid; RIF-PZA, rifampin-pyrazinamide; RR, relative risk.
Nine studies reported data from which ORs for treatment completion could be calculated.10–12,26,27,29,30,32,33 Two studies were excluded from this analysis: one study had been terminated early (resulting in insufficient data about treatment completion),12 and another study was excluded because it targeted an incarcerated population.27 Therefore, seven studies were analyzed for treatment completion with an OR of 2.97 (95% CI 2.10, 4.21), indicating that the odds of completing treatment was 3 times higher for 3HP compared with other LTBI regimens (Figure 3). Substantial heterogeneity was identified for this outcome, with I2 >50%, but the p-value was not statistically significant.
Figure 3.

Treatment completion among participant receiving treatment with 3-month isoniazid-rifapentine compared to other latent tuberculosis infection regimens.
3HP, 3-month isoniazid-rifapentine.
From 13 studies, the proportion of participants on 3HP who completed treatment was 87.5% (95% CI=83.2%, 91.3%),10,11,21,23–26,28–33 with seven studies reporting the proportion of participants completing treatment for other LTBI regimens at 65.9% (95% CI=53.5%, 77.3%).10,11,26,29,30,32,33 Subgroup analyses on the basis of target population was also conducted, and 82.2% (95% CI=78.0%, 87.0%) of healthy people aged ≥12 years completed 3HP treatment10,21,26,29–31,33; treatment completion was 95.5% for three studies targeting children and adolescents23,25,36 and 93.0% for two studies targeting PLWHA.11,35 A subgroup analysis was conducted on the basis of administration mode for studies occurring in the U.S. Ten studies administered 3HP by DOT, with 86.6% (95% CI=81.3%, 91.1%) of DOT participants completing treatment.10,21,23–25,28–31,33 For SAT, the pooled effect estimate for two studies with three intervention arms was 81.9% (95% CI=73.8%, 88.9%).21,32
Secondary Outcomes
All secondary outcomes favored the 3HP regimen, but showed no statistical difference when compared with other LTBI regimens (Appendix Table 4, available online). For five studies reporting treatment discontinuation because of adverse events, a 52% (p=0.16) reduced risk for discontinuing treatment occurred among participants on 3HP, compared with other LTBI regimens10–12,26,32; a similar result was reported regarding risk for experiencing adverse events while on 3HP (five studies, 41% risk reduction, p=0.27).10–12,26,32 Four studies reported a 21% (p=0.18) reduction in risk for death while on 3HP, compared with other LTBI regimens.10–12,26 The percentage of participants who experienced an adverse event, discontinued treatment, or died while on 3HP was 8%, 4%, and 0.7%, respectively.
Special Populations
Three included studies focused on solid-organ transplant candidates. Although the combined sample size for the three studies was small (n=72),29,33,37 participants had initiated treatment before transplantation and none progressed to active TB while on treatment (93% completed treatment). One study targeting an incarcerated population reported that 85% of inmates started on 3HP completed treatment, compared with 18% of those started on 9H.32
Publication Bias
Funnel plots for all outcomes assessed are included in the Appendix (Appendix Figures 4–8, available online). Based on Egger’s test, no evidence of publication bias was identified for studies reporting data on the prevention of TB disease (z=0.67, p=0.50), although the number of included studies for this outcome might have resulted in low statistical power; visual inspection of the funnel plot indicates possible publication bias. For studies reporting treatment completion, Egger’s test (z=3.46, p=0.001) and visual inspection of the funnel plot both suggest possible publication bias—this is likely because of heterogeneity in study size and design. Egger’s test for all secondary outcomes were not statistically significant, but visual inspection of the funnel plots indicate asymmetry for discontinuation of treatment while on 3HP.
Applicability of Findings
Major findings about effectiveness, safety, and treatment completion of 3HP from this review are broadly applicable to public health programs that provide care for individuals with LTBI in the U.S. and globally. Although more males than females were participants in the included studies, the review findings are applicable to both sexes; similarly, 3HP treatment should be broadly applicable to all racial and ethnic groups. However, findings regarding treatment administration (SAT versus DOT) or use of 3HP among certain populations might not be applicable in non-U.S. healthcare settings because the majority of data came from U.S. study sites. Moreover, data were limited regarding whether educational attainment and other environmental and social determinants influenced treatment completion.
Implementation Considerations
Programs considering implementing the 3HP treatment regimen for LTBI should consider patient, provider, and health system–level factors. Patients should be educated regarding 3HP’s advantages, compared with other LTBI treatment regimens (e.g., higher treatment completion rates and shorter number of days on treatment), as well as potential treatment effects for any regimen. Relatively low risk for hepatotoxicity exists while on 3HP. Systemic drug reactions occur more frequently when compared with 9H; patients who experience such reactions usually recover within 24 hours after removal from treatment.10,34
Providers should receive education about 3HP as an equal alternative to 9H, along with social and environmental factors to consider when prescribing 3HP by SAT for certain populations (e.g., homeless people, substance and drug users, and those with mental health disorders). Health systems and programs should also consider whether to administer 3HP by DOT, SAT, or both; how to monitor adverse events among individuals receiving the regimen under SAT; the resources needed to administer treatment and monitor patients; drug procurement costs; and policies regarding patients who might have difficulty with medication adherence.
DISCUSSION
On the basis of standards developed by The Community Guide,14 sufficient evidence exists that 3HP is effective for TB prevention. This review did not find the 3HP regimen to be statistically more effective than other LTBI regimens. Strong evidence does exist that using 3HP improves treatment completion rates, compared with other LTBI treatment regimens. 3HP treatment completion is slightly lower under SAT than DOT, but completion with 3HP-SAT still remains high, compared with other LTBI treatments. Using 3HP was associated with reduced risk for adverse events, lower rates of treatment discontinuation because of adverse events, and fewer deaths; however, these findings were not statistically significant.
Limitations
This review had certain limitations. First, the vast majority of evidence is for 3HP by DOT; therefore, information regarding implementation concerns for 3HP by SAT, both in research and real-world settings, is limited. Second, a majority (64.7%) of the included studies were observational cohort designs, which potentially had design-related problems, including possible selection bias and confounding. Third, the majority of included studies did not report the proportion of participants offered 3HP as LTBI treatment; therefore, assessing whether 3HP’s high completion rates should be attributed to shorter treatment duration with once weekly administration alone or to other unreported factors related to selection for 3HP is difficult to determine. Fourth, follow-up durations among participants in the included studies greatly varied and might have influenced when progression from infection to disease was noted. Fifth, this review was limited to articles in English; this might have resulted in the search for evidence to miss high-quality articles published in other languages. Finally, absence of cost and cost-effectiveness data in the included studies precluded the authors’ ability to conduct an economic evaluation of use of the 3HP regimen.
Publication of 3HP studies has increased since 2011, but these publications report mainly on studies administering 3HP by DOT. More research and practice-based evidence is needed regarding SAT of 3HP, use of electronic or video DOT, and other innovative modes of administration. Additional information is also needed regarding 3HP’s safety among pregnant women and children aged 2 years or younger. Drug–drug interaction for people on antiretroviral therapy is another area for investigation. Few studies provided information regarding 3HP’s acceptance rate, compared with other LTBI treatment regimens, as well as patients’ overall experience with care while receiving treatment. Data focused on populations with comorbidities, individuals on tumor necrosis factor-α inhibitors, and types of healthcare worker administering DOT were scarce.
CONCLUSIONS
The effectiveness of 3HP for preventing active TB disease and achieving high treatment completion rates among people with LTBI was examined in this review. Findings from this review are consistent with those published in a recent systematic review examining 3HP’s efficacy and completion rates, compared with other treatment regimens.38 Using studies published during 1968–June 2016, the Pease et al.38 review identified favorable, but not statistically significant, results for 3HP’s efficacy in preventing TB, compared with 9H. Additionally, people on short-course regimens (e.g., 3HP) had higher treatment completion rates.
This review not only examines the effectiveness and treatment completion rates of the 3HP regimen, but also assesses risks related to adverse events, discontinuation caused by adverse events, and death; applicability; considerations for implementation; and evidence gaps in research and practice. Furthermore, the review also compares treatment completion rates for administration by SAT and DOT.
As public health programs throughout the U.S. receive diminishing resources, the elimination of TB becomes an increasingly challenging goal. Progress toward elimination appears to have stalled in recent years.39 If programs aim to increase screening and treatment of high-risk individuals with LTBI, updated LTBI treatment guidelines promoting the use of effective, short-course LTBI regimens are needed. Regimens of 6–9 months of INH, historically the most commonly used LTBI treatment regimens, are highly effective in preventing TB disease, but their overall effectiveness is limited by reduced acceptance and low treatment completion rates.9,40
The scope of this review was limited to including only evidence that directly compared 3HP with other LTBI regimens. Therefore, most of the safety and effectiveness studies included in this review compared 3HP with 9H, and not other short-course regimens. This review found no evidence directly comparing the safety and effectiveness of 3HP with 4-month rifampin. However, evidence from this review identified one observational study30 that reported treatment completion for both 3HP and 4-month rifampin, and found high completion rates for both regimens—indicating that a shorter treatment duration is likely a key factor for patients completing treatment.
The findings of this systematic review indicate that the short-course 3HP treatment regimen has equal safety and effectiveness as other LTBI regimens and achieves higher treatment completion rates when administered by DOT and SAT. The included evidence indicates that 3HP is safe and effective for healthy adults, children aged more than 2 years, adolescents, and PLWHA; no studies included in this review reported on children aged less than 2 years or people on tumor necrosis factor-α inhibitors. Moreover, 3HP was well tolerated among solid-organ transplant candidates.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Joanna Taliano, MA, MLS (Stephen B. Thacker Library, Centers for Disease Control and Prevention [CDC]) for conducting the literature search; C. Kay Smith, MEd (National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC) for reviewing and providing editorial services; and Andrew Hill, PhD, Roque Miramontes, PA-C, MPH, Carla Winston, PhD, and Philip LoBue, MD (Division of Tuberculosis Elimination, CDC) for their support and guidance through each step of the systematic review process. This review was supported by the CDC.
No financial disclosures were reported by the authors of this paper.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2018.04.030.
REFERENCES
- 1.WHO. Global Tuberculosis Report 2016. Geneva, Switzerland: WHO, 2016. Report No.: 924156539X. www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society, Centers for Disease Control and Prevention (CDC). Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161(suppl 4 pt 2):S221–S247. 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 4.Geiter L Ending Neglect: The Elimination of Tuberculosis in the United States Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 5.Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173(8):927–931. 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 6.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 7.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982;60(4):555–564. [PMC free article] [PubMed] [Google Scholar]
- 8.Horsburgh CR, Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137(2):401–409. 10.1378/chest.09-0394. [DOI] [PubMed] [Google Scholar]
- 9.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003;168(4):443–447. 10.1164/rccm.200303-390OC. [DOI] [PubMed] [Google Scholar]
- 10.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–2166. 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 11.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20. 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schechter M, Zajdenverg R, Falco G, et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med. 2006;173(8):922–926. https://doi. org/10.1164/rccm.200512-1953OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60(48):1650–1603. [PubMed] [Google Scholar]
- 14.Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. Am J Prev Med 2000;18(1)(suppl):35–43. 10.1016/S0749-3797(99)00119-1. [DOI] [PubMed] [Google Scholar]
- 15.Zaza S, Wright-De Aguero LK, Briss PA, et al. Data collection instrument and procedure for systematic reviews in the Guide to The Community Preventive Services. Am J Prev Med 2000;18(1) (suppl):44–74. 10.1016/S0749-3797(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Thompson SG. Quantifying heterogeneity in a meta‐ analysis. Stat Med. 2002;21(11):1539–1558. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0. Updated March 2011. The Cochrane Collaboration. http://training.cochrane.org/handbook. [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belknap R, Holland D, Feng PJ, et al. Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection. Ann Intern Med. 2017;167(10):689–697. 10.7326/M17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliven-Sizemore EE, Sterling TR, Shang N, et al. Three months of weekly rifapentine plus isoniazid is less hepatotoxic than nine months of daily isoniazid for LTBI. Int J Tuberc Lung Dis. 2015;19(9):1039–1044. 10.5588/ijtld.14.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz AT, Starke JR. Safety and adherence for 12 weekly doses of isoniazid and rifapentine for pediatric tuberculosis infection. Pediatr Infect Dis J. 2016;35(7):811–813. https://doi.org/10.1097/INF.000000 0000001164. [DOI] [PubMed] [Google Scholar]
- 24.de Castilla DL, Rakita RM, Spitters CE, Narita M, Jain R, Limaye AP. Short-course isoniazid plus rifapentine directly observed therapy for latent tuberculosis in solid-organ transplant candidates. Transplantation. 2014;97(2):206–211. 10.1097/TP.0b013e3182a94a2f. [DOI] [PubMed] [Google Scholar]
- 25.Hatzenbuehler LA, Starke JR, Graviss EA, Smith EO, Cruz AT. School-based study to identify and treat adolescent students at risk for tuberculosis infection. Pediatr Infect Dis J. 2016;35(7):733–738. 10.1097/INF.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 26.Huang YW, Yang SF, Yeh YP, Tsao TC, Tsao SM. Impacts of 12-dose regimen for latent tuberculosis infection: treatment completion rate and cost-effectiveness in Taiwan . Medicine (Baltimore). 2016;95(34): e4126 10.1097/MD.0000000000004126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juarez-Reyes M, Gallivan M, Chyorny A, O’Keeffe L, Shah NS. Completion rate and side-effect profile of three-month isoniazid and rifapentine treatment for latent tuberculosis infection in an urban county jail. Open Forum Infect Dis. 2016;3(1):ofv220 10.1093/ofid/ofv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoll BM, Nog R, Wu Y, Dhand A. Three months of weekly rifapentine plus isoniazid for latent tuberculosis treatment in solid organ transplant candidates. Infection. 2017;45(3):335–339. https://doi. org/10.1007/s15010-017-1004-5. [DOI] [PubMed] [Google Scholar]
- 29.Lines G, Hunter P, Bleything S. Improving treatment completion rates for latent tuberculosis infection: a review of two treatment regimens at a community health center. J Health Care Poor Underserved. 2015;26(4):1428–1439. 10.1353/hpu.2015.0126. [DOI] [PubMed] [Google Scholar]
- 30.McClintock AH, Eastment M, McKinney CM, et al. Treatment completion for latent tuberculosis infection: a retrospective cohort study comparing 9 months of isoniazid, 4 months of rifampin and 3 months of isoniazid and rifapentine. BMC Infect Dis 2017;17(146):1–8. 10.1186/s12879-017-2245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandul AL, Nwabunie N, Holcombe JM, et al. High rate of treatment completion in program settings with 12-dose weekly isoniazid and rifapentine (3HP) for latent mycobacterium tuberculosis infection. Clin Infect Dis. 2017;65(7):1085–1093. 10.1093/cid/cix505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simkins J, Abbo LM, Camargo JF, Rosa R, Morris MI. Twelve-week rifapentine plus isoniazid versus 9-month isoniazid for the treatment of latent tuberculosis in renal transplant candidates. Transplantation. 2017;101(6):1468–1472. 10.1097/TP.0000000000001329. [DOI] [PubMed] [Google Scholar]
- 33.Stennis NL, Burzynski JN, Herbert C, Nilsen D, Macaraig M. Treatment for tuberculosis infection with 3 months of isoniazid and rifapentine in New York City health department clinics. Clin Infect Dis. 2016;62(1):53–59. 10.1093/cid/civ766. [DOI] [PubMed] [Google Scholar]
- 34.Sterling TR, Moro RN, Borisov AS, et al. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT Tuberculosis Study. Clin Infect Dis. 2015;61(4):527–535. 10.1093/cid/civ323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterling TR, Scott NA, Miro JM, et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS. 2016;30(10):1607–1615. 10.1097/QAD.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and Isoniazid. JAMA Pediatr. 2015;169(3):247–255. 10.1001/jamapediatrics.2014.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC), American Thoracic Society. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection—United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52(31):735–739. [PubMed] [Google Scholar]
- 38.Pease C, Hutton B, Yazdi F, et al. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: a systematic review with network meta-analyses. BMC Infect Dis. 2017;17(1):265 https://doi.org/10. 1186/s12879-017-2377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence—United States, 2013–2015. MMWR Morb Mortal Wkly Rep 2016;65(11):273–278. https://doi.org/10. 15585/mmwr.mm6511a2. [DOI] [PubMed] [Google Scholar]
- 40.Haley CA. Treatment of latent tuberculosis infection. TNMI7. Micro-biol Spectr. 2017;5(2):0039–2016. https://doi.org/10.1128/microbiol spec.TNMI7-0039-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


