Abstract
Background:
Snus tobacco characteristics may attract non-tobacco users, including relatively low, but pharmacologically active, doses of nicotine. Lower nicotine doses may limit adverse drug effects while also producing a physiologically active response.
Objectives:
This pilot study is the first to profile the acute effects of snus on physiological and subjective assessments in a sample of never-tobacco users.
Methods:
Eleven never-tobacco users (five women; <100 uses/lifetime) were recruited from the community via university-approved advertisements. Using a within-subject design, participants consumed six pouches in ascending dose order (0, 1.6, 3.2, 4.8, 6.4, and 8.0 mg nicotine) within one session. The start of each snus bout was separated by 45 minutes, and pre- and post-pouch assessments included ratings of drug effects and physiological response.
Results:
Average heart rate and systolic blood pressure increased significantly from pre- to post-pouch use as a function of dose, though these increases were reliable for 8.0 mg nicotine only (p<.05). Collapsed across time, diastolic blood pressure was significantly higher for 8.0 mg nicotine than for all other doses (p<.05). Subjective ratings for “excessive salivation” and “satisfying” increased significantly from pre- to post-pouch use (p<.05), independent of dose.
Conclusion:
Significant increases in physiological response at some doses suggest that users were exposed to pharmacologically active doses of nicotine. The lack of reliable subjective effects may be the product of the dosing regimen or the relatively small sample size. Findings highlight the need for identification of doses of snus that may promote abuse among naïve users.
Keywords: snus, tobacco, abuse liability, never-tobacco users
Introduction
Snus is a spitless, moist tobacco product packaged in small pouches that originated in Sweden (1). Relative to traditional smokeless tobacco products (i.e., dip, chew), snus tobacco is cured so as to lower levels of toxins like tobacco-specific nitrosamines (2). While snus is not harmless (3, 4), risks of cancer and cardiovascular disease are notably lower for snus use than for use of many other tobacco products (5). Thus, some argue in favor of snus as a replacement product for cigarettes as part of a harm reduction strategy (6). Yet others are concerned that promotion of snus may increase tobacco-related harm overall, in part by attracting current non-tobacco users who eventually transition to regular use of snus and perhaps other tobacco products (i.e., “gateway” theory; 7).
Indeed, the United States (U.S.) tobacco industry has employed tactics to meet this objective, specifically the development and marketing of ‘starter’ products for nicotine naïve individuals (8, 9). Products like snus are available in configurations, packages, and/or flavors akin to gum and candy. These characteristics, which appeal to youth, may serve to enhance others’ perception of the user (“hip”; 10), ensure that product use is discreet (“get your fix while still not giving out the image that you’re a smoker or other tobacco user”; 10), and/or mask the harsh tobacco taste (“candy that gives you a little buzz”; 10). Perhaps as a consequence of this latter characteristic, the majority of users of traditional smokeless tobacco products report initiating use with a flavored product like mint or wintergreen (11). Starter products also often include reduced levels of pH, and thus reduced concentrations of free nicotine (12), with the goal of creating a more tolerable physiological experience for the naïve user (13). Not only do such products limit adverse side effects (e.g., nausea, mouth irritation), they also allow for the juices to be swallowed rather than spit (8, 11, 13). Youth find this characteristic appealing as well: “…you don’t have to spit or whatever and that’s like ideal for school…because you can’t go around spitting on the floors” (14). Consequently, industry tactics have arguably played a role in enticing non-tobacco users to try tobacco products.
Importantly, when starter products are used repeatedly by a non-tobacco user, tolerance to the effects of nicotine in those products may develop. At that point, the non-tobacco user may need to graduate to using tobacco products with higher concentrations of nicotine. This fact has not gone unnoticed by the tobacco industry, at least by one company that developed a “graduate theory” whereby new smokeless tobacco users slowly transition from “milder” to “stronger” products (8). For this strategy to work, of course, a starter product needs to contain doses of nicotine that produce a physiologically active response while simultaneously limiting unwanted side effects. In a pre-clinical model, the extracts from one snus product were shown to produce reinforcement-enhancing effects similar to those of low to moderate doses of nicotine alone (15). Moreover, the snus extract and nicotine alone solutions had comparable binding affinities at several nicotinic acetylcholine receptor subtypes known to play a role in nicotine addiction (15). No data are available that examine the effects of snus in nicotine naïve individuals. Thus, the purpose of this pilot study was to profile acute physiological and subjective effects of snus use in a sample of never-tobacco users.
Method
Participants
Participants were recruited from the community via print advertisements and word-of-mouth, and provided informed consent at an initial screening visit. All outlined procedures were approved by the university’s Institutional Review Board. Six men (four Caucasian; two African American), and five women (all Caucasian) completed the study. Participants were those aged ≥18 years (M = 21.5, SD = 2.0; Range = 19–26) who reported fewer than 100 lifetime uses of tobacco [cigarettes (n=5; M = 4.0, SD = 4.1), cigars (n=7; M = 4.4, SD = 6.0), and/or waterpipes (n=6; M = 6.2, SD = 8.0)] and no tobacco use in the past three months. Current non-tobacco use status was confirmed via a urinary cotinine reading of < 3 (M = 1.3, SD = 0.3) and an expired air carbon monoxide (CO) level ≤ 7 ppm (M = 2.2, SD = 0.9) samples. Exclusion criteria included chronic health or psychiatric conditions, use of illicit drugs in the past three months, regular medication use (except vitamins or birth control), and pregnancy (verified via urinalysis) or breast-feeding.
Study Procedures
Participants completed a single, ~5-hour session that was preceded by biochemically verified abstinence (urinary cotinine < 3 and expired air CO ≤ 7 ppm), as well as abstinence from food and drink for at least 1 hour. Participants were then connected to a monitor for continuous monitoring of heart rate and blood pressure throughout the session. Thirty minutes later, baseline subjective ratings of drug effects were evaluated, followed immediately by administration of a single snus pouch (0.0 mg nicotine). Participants were instructed to place the pouch between the lip and gum, in a randomized location (i.e., top versus bottom, right versus left), for 20 consecutive minutes. At the end of this bout, participants were instructed to discard the pouch and complete the same subjective measures as administered at baseline. These same procedures (pre-pouch subjective measures, snus use for 20 minutes, post-pouch subjective measures) were repeated five additional times, with 45 minutes separating the start of each bout. Thus, six total snus doses were administered within a single session, and doses were always presented in ascending dose order: 0.0, 1.6, 3.2, 4.8, 6.4, and 8.0 mg nicotine. Participants remained in the laboratory until adverse drug effects dissipated (e.g., dizziness, elevated heart rate). The session ended with payment of $80. Three months later, participants were contacted and asked to report on their use of any tobacco products in the interim.
Snus tobacco
Snus tobacco was purchased from Swedish Match AB (Stockholm, Sweden), a Swedish-based company, because a matched placebo was available: active (8 mg/g General White Large) and placebo (tobacco-free Onico White Large) tobacco-flavored pouches. To obtain the range of doses used in this study, loose tobacco was removed from the pouches, mixed to form a given dose of 1 gram weight (i.e., to model the original weight of the product prior to opening), and resealed with Periacryl® 90 Oral Tissue Adhesive (Glustitch, Inc.; Canada). Specifically, active and placebo tobacco were combined to create six doses: 0.0, 1.6, 3.2, 4.8, 6.4, and 8.0 mg nicotine. Nicotine doses are approximate, given that pouches were combined based upon weight (in grams). Thus, participants were exposed to a total of five nicotine-containing snus pouches during a single session, and one pouch that did not contain nicotine.
Outcome Measures
Physiological Measures.
Heart rate (HR) was measured every 20 seconds, and systolic and diastolic blood pressure (SBP and DBP, respectively) every 5 minutes, by non-invasive computerized equipment (Vital Care 506N3 Series; Criticare Systems, Inc.; Waukesha, WI).
Subjective Measures.
The Direct Effects of Nicotine Scale (DENS) and the Direct Effects of Tobacco Scale (DETS) were administered prior to and immediately following each snus pouch (16). Both scales consisted of visual analog scale (VAS) items, which are outlined in Tables I and II. Each word or phrase was centered above a horizontal line anchored on the left with “not at all” (score = 0) and on the right with “extremely” (score = 100). Scores represent the distance of the vertical mark from the left anchor, expressed as a percentage of line length.
Table I.
Statistical Analysis Results for all Outcomes
| Dose | Time | Dose × Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Partial ŋ2 | F | p | Partial ŋ2 | F | p | Partial ŋ2 | |
| Physiological Measuresa | |||||||||
| Heart Rate | 5.28 | .012 | 0.40 | 29.31 | .001 | 0.79 | 4.34 | .018 | 0.35 |
| Systolic Blood Pressure | 8.34 | <.001 | 0.73 | 16.91 | .003 | 0.68 | 3.39 | .014 | 0.64 |
| Diastolic Blood Pressure | 3.38 | .021 | 0.45 | 1.91 | .204 | 0.19 | 0.69 | .634 | 0.11 |
| Subjective Measuresb | |||||||||
| Direct Effects of Nicotine Scale | |||||||||
| Nauseous | 2.25 | .157 | 0.23 | 3.83 | .082 | 0.30 | 2.32 | .113 | 0.26 |
| Dizzy | 1.35 | .284 | 0.16 | 3.10 | .112 | 0.11 | .264 | .734 | 0.65 |
| Lightheaded | 1.25 | .308 | 0.19 | 4.56 | .061 | 0.34 | .205 | .820 | 0.01 |
| Nervous | .982 | .349 | 0.37 | 1.03 | .337 | 0.34 | 1.49 | .254 | 0.17 |
| Sweaty | 1.13 | .316 | 0.12 | 1.06 | .331 | 0.11 | 1.09 | .331 | 0.08 |
| Headache | 2.00 | .170 | 0.23 | 3.20 | .116 | 0.25 | .638 | .505 | 0.02 |
| Salivation | .366 | .751 | 0.01 | 6.19 | .035 | 0.41 | 1.90 | .174 | 0.47 |
| Heart Pounding | 1.42 | .264 | 0.13 | .792 | .397 | 0.08 | .621 | .474 | 0.12 |
| Confused | 1.10 | .325 | 0.11 | 1.55 | .245 | 0.15 | 1.11 | .323 | 0.25 |
| Weak | 1.08 | .331 | 0.14 | .925 | .361 | 0.09 | .453 | .558 | 0.13 |
| Direct Effects of Tobacco Scale | |||||||||
| Was the product satisfying? | 1.98 | .154 | 0.27 | 5.38 | .046 | 0.37 | 1.93 | .156 | 0.05 |
| Was the product pleasant? | 1.97 | .145 | 0.28 | 3.61 | .090 | 0.29 | 1.10 | .367 | 0.002 |
| Did the product taste good? | 1.92 | .179 | 0.23 | 1.86 | .206 | 0.17 | 3.27 | .029 | 0.04 |
| Did the product make you dizzy? |
.698 | .493 | 0.09 | .742 | .411 | 0.08 | .282 | .835 | 0.04 |
| Did the product help calm you down? |
.775 | .504 | 0.09 | 2.77 | .130 | 0.24 | 2.83 | .062 | 0.26 |
| Did the product help you concentrate? |
.666 | .534 | 0.03 | 1.53 | .247 | 0.15 | 1.40 | .263 | 0.05 |
| Did the product make you feel more awake? |
.122 | .857 | 0.02 | 1.02 | .340 | 0.10 | .767 | .507 | 0.01 |
| Did the product reduce your hunger for food? |
.486 | .633 | 0.04 | .717 | .419 | 0.07 | .610 | .674 | 0.03 |
| Did the product make you sick? |
1.08 | .353 | 0.08 | .760 | .406 | 0.08 | 1.81 | .208 | 0.20 |
Bolded values denote statistical significance, p < .05.
dfbout = (1,8); dftime = (1,8); dfboutxtime = (1,8)
dfbout = (5,45); dftime = (1,9); dfboutxtime = (5,45)
Table II.
Mean (±SEM) difference scores from pre- to post-pouch at each dose
| Nicotine Dose: | 0.0 mg/g | 1.6 mg/g | 3.2 mg/g | 4.8 mg/g | 6.4 mg/g | 8.0 mg/g |
|---|---|---|---|---|---|---|
|
Physiological Measures Heart Rate |
−1.58 (1.33) | −0.68 (0.82) | −5.03 (6.70) | 0.32 (0.67) | 3.25 (1.13) | 4.93 (1.66) |
| Systolic Blood Pressure | −1.71 (3.17) | −1.35 (2.14) | 2.50 (1.54) | 2.97 (1.81) | 7.62 (1.64) | 6.73 (2.53) |
| Diastolic Blood Pressure | 2.21 (1.67) | −1.33 (1.56) | 0.96 (2.09) | 0.70 (2.16) | 1.03 (1.83) | 3.32 (2.07) |
| Subjective Measures | ||||||
| Direct Effects of Nicotine Scale | ||||||
| Nauseous | 0.00 (0.23) | 4.82 (3.30) | 1.00 (1.21) | −0.73 (1.13) | 10.91 (6.15) | 12.00 (6.69) |
| Dizzy | 1.09 (0.88) | 4.09 (2.35) | 6.00 (4.67) | 4.45 (3.90) | 6.09 (7.53) | 4.09 (1.93) |
| Lightheaded | 3.18 (1.31) | 7.91 (3.48) | 5.73 (5.04) | 6.82 (3.66) | 5.36 (7.85) | 5.64 (3.06) |
| Nervous | −2.36 (1.16) | 0.18 (0.18) | 2.18 (1.77) | −0.45 (0.59) | 7.18 (6.22) | 0.45 (0.59) |
| Sweaty | 0.00 (0.00) | 0.27 (0.19) | 1.18 (0.77) | −0.82 (0.72) | 6.64 (6.34) | −0.73 (1.38) |
| Headache | 1.09 (1.45) | 2.45 (1.69) | 7.27 (4.42) | −0.09 (1.92) | 6.45 (6.90) | 2.82 (2.85) |
| Salivation | 20.55 (7.49) | 16.73 (6.28) | 9.09 (3.80) | 7.09 (2.93) | 14.18 (10.65) | 14.45 (8.20) |
| Heart Pounding | 0.27 (0.69) | −0.82 (1.23) | 0.18 (0.62) | 0.36 (0.20) | 5.00 (6.01) | −0.18 (1.48) |
| Confused | 2.09 (1.83) | 2.18 (1.62) | 2.82 (3.05) | −1.18 (1.51) | 6.82 (6.05) | −0.45 (0.99) |
| Weak | −1.00 (1.190) | 0.55 (1.17) | 0.64 (0.78) | 3.09 (1.38) | 4.36 (6.20) | 2.18 (1.90) |
| Direct Effects of Tobacco Scale | ||||||
| Was the product satisfying? | 13.45 (6.22) | −3.45 (4.68) | 0.00 (2.17) | 2.55 (2.30) | 6.00 (4.63) | −0.55 (0.41) |
| Was the product pleasant? | 11.00 (6.60) | −3.18 (4.71) | −1.09 (4.88) | 6.73 (3.64) | 5.45 (3.41) | 3.82 (3.54) |
| Did the product taste good? | 17.45 (7.53) | −3.36 (4.78) | 0.73 (3.04) | 9.27 (4.82) | 9.27 (5.65) | 3.55 (3.5)) |
| Did the product make you dizzy? |
−0.27 (2.43) | 4.36 (2.52) | 4.36 (4.58) | 7.64 (3.75) | 5.00 (8.42) | 9.45 (8.87) |
| Did the product help calm you down? |
11.36 (6.28) | 1.09 (4.19) | 4.18 (2.37) | 15.00 (7.16) | 4.00 (7.57) | −0.27 (0.52) |
| Did the product help you concentrate? |
2.64 (6.84) | −2.09 (2.89) | 2.82 (3.09) | 12.18 (5.74) | 2.91 (5.05) | 4.18 (3.58) |
| Did the product make you feel more awake? |
2.18 (5.50) | −1.64 (5.78) | 4.82 (4.54) | 9.09 (4.53) | 1.91 (5.19) | 1.27 (2.04) |
| Did the product reduce your hunger for food? |
3.27 (5.76) | −1.73 (5.14) | 2.00 (1.53) | 11.00 (8.22) | 2.82 (7.14) | 7.36 (6.33) |
| Did the product make you sick? |
−3.82 (2.78) | −0.82 (1.38) | −0.64 (1.74) | 0.55 (0.67) | 8.64 (6.81) | 17.91 (10.83) |
Tobacco Initiation.
Participants were contacted three months after the completion of their session to inquire about their use of any tobacco products.
Data Analysis
Due to equipment malfunction, HR and BP data for two of the 11 completed participants were incomplete; therefore, nine participants’ data were used for analysis of physiological measures. For each snus bout, HR data were organized into two bins by averaging 5 minutes prior to each pouch (pre-pouch) and 5 minutes post-pouch use. These same timepoints (pre- and post-pouch) were used for BP and subjective measures. All measures were analyzed using a Dose (six levels) by Time (two levels) repeated-measures analysis of variance. Huynh-Feldt corrections were used to adjust for violations of the sphericity assumption, (17) and Tukey’s Honestly Significant Difference (HSD) test was used to examine differences between means. For all comparisons, significance is reported at p < .05.
Results
Statistical analysis results for all outcome measures are presented in Table I. Difference scores from pre- to post-doses are presented in Table II.
Physiological Measures
Heart Rate.
A significant Dose X Time interaction was observed for HR. As shown in Figure 1A, HR levels generally decreased from pre- to post-dose for the initial snus doses, but then increased toward the end of session. Increases in HR from pre- to post-pouch were significant only for the sixth and final dose (8.0 mg nicotine) (Tukey’s HSD; p < .05).
Figure.
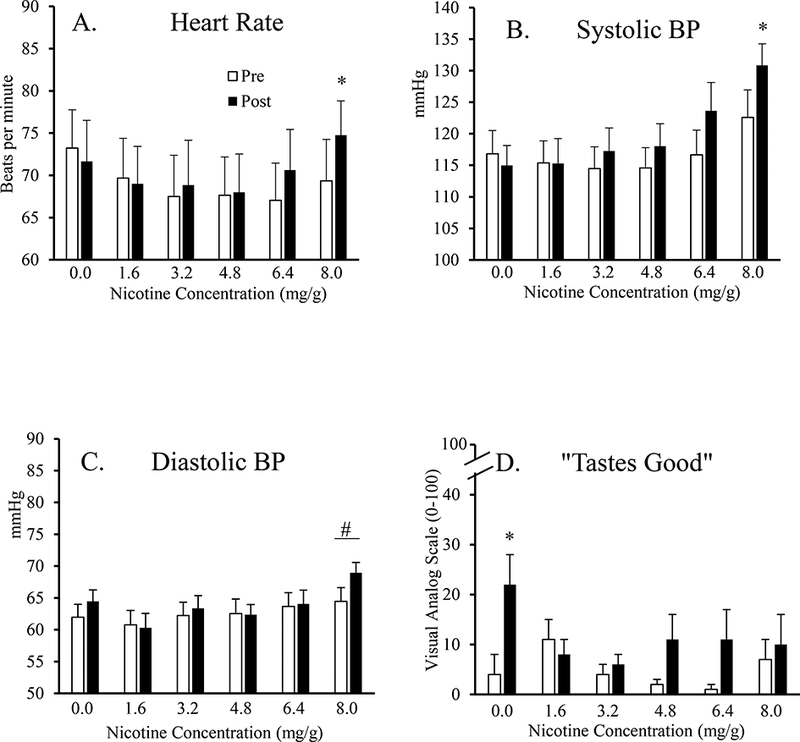
Heart rate (HR; Panel A), systolic blood pressure (SBP; Panel B), diastolic blood pressure (DBP; Panel C), and the DETS item “tastes good” (Panel D) as a function of Dose 6 levels) and Time (2 levels). Asterisks represent significant increases from pre-pouch values (white bars) to post-pouch values (black bars) for that dose (p < .05). Pound symbol represents a significant main effect of dose, with DBP for 8.0 mg nicotine being significantly higher than all other doses (p < .05).
Systolic Blood Pressure.
A significant Dose X Time interaction was also observed for SBP. Figure 1B shows that SBP increased from pre- to post-pouch at nearly every active dose. Collapsed across dose, average SBP was 116.9 mmHg (SEM = 1.8) at pre-pouch and 120.1 mmHg (SEM = 1.9) post-pouch. Still, these increases were reliable only for the sixth and final dose (8.0 mg nicotine) (Tukey’s HSD; p < .05).
Diastolic Blood Pressure.
For DBP, a significant main effect of Dose was observed (see Figure 1C). Collapsed across time, average DBP was 62.6 mmHg (SEM = 1.6) for 0.0 mg nicotine, 59.2 mmHg (SEM = 1.7) for 1.6 mg nicotine, 62.8 mmHg (SEM = 1.6) for 3.2 mg nicotine, 61.7 mmHg (SEM = 1.4) for 4.8 mg nicotine, 63.1 mmHg (SEM = 1.5) for 6.4 mg nicotine, and 66.9 mmHg (SEM = 1.5) for 8.0 mg nicotine. Average DBP for the 8.0 mg nicotine dose was significantly higher than that for all other doses (Tukey’s HSD; p < .05).
Subjective Measures
Direct Effects of Nicotine Scale.
None of the DENS items were significant for a Dose X Time interaction or a main effect of Dose. In addition, only one DENS item was significant for a main effect of Time, specifically “excessive salivation”. Collapsed across dose, average ratings were 6.7 (SEM = 1.6) pre-pouch and 20.6 (SEM = 3.1) post-pouch (Tukey’s HSD; p < .05).
Direct Effects of Tobacco Scale.
As displayed in Table I, only one DETS item was observed to be significant for a Dose X Time interaction. Specifically, ratings for the item “taste good”, shown in Figure 1D, generally increased from pre- to post-pouch for each dose (see Table II for difference scores). Still, these increases were reliable only for the 0.0 mg nicotine dose (Tukey’s HSD; p < .05).
A significant main effect of Time was observed for the DETS item “Was the product satisfying?” Average ratings, collapsed across dose, increased from pre- (M = 4.6, SEM = 1.3) to post-pouch (M = 7.7, SEM = 1.6) (Tukey’s HSD, p < .05). No DETS items were significant for a main effect of Dose.
Tobacco Initiation.
All eleven participants stated that they had not used any tobacco products since the completion of their study session.
Discussion
U.S.-based tobacco companies may have designed snus to serve as a starter product for individuals who are naïve to nicotine (8, 9). While the effects of nicotine in naïve users have been characterized previously (18, 19), this work included products different from snus (e.g., nicotine gum, nasal spray, etc.). Given that snus may deliver nicotine in a different form or with a different speed than these other products, it is important to evaluate the effects of this specific nicotine-containing product separately (20). As a first step toward this goal, the current pilot study was designed to characterize the acute physiological and subjective effects of snus in a representative sample of never-tobacco users.
Snus use increased HR and BP levels, consistent with previous work that included administration of nicotine via snus in cigarette smokers (21, 22, 23) and via gum in never smokers (18, 19). Elevation of physiological parameters as a function of nicotine dose may be indicative of nicotine exposure (25, 26, 27, 28). Still, the increases observed here were reliable only for the 8.0 mg nicotine dose. As for subjective outcomes, this sample of never-tobacco users reported largely negligible effects following consumption of five nicotine-containing snus pouches over ~4 hours (the first hour included baseline assessments and administration of a placebo dose). A similar pattern of results has been reported when snus is administered acutely to overnight abstinent cigarette smokers (e.g., 21).
It is worth noting that individual differences were observed between the never-tobacco users sampled here. Consistent with other work (29), some participants reported relatively high ratings of aversive effects (e.g., “dizzy”, “nauseous”) and relatively low ratings of positive effects (e.g., “satisfying”, “tastes good”) at the same dose (e.g., 8 mg), while other participants reported the opposite. These differences were not explained by an analysis of those demographic characteristics measured in this study (e.g., age, gender, lifetime uses of tobacco), though previous work has identified covariates such as impulsivity, anxiety, smoking status, and genotype (30, 31). Others have highlighted the importance of understanding better such individual differences given that nicotine is a relatively weak reinforcer (e.g., 29, 31).
The overall lack of significant effects may have been due to the dosing regimen. Previous work demonstrates that, in overnight abstinent cigarette smokers, average plasma nicotine levels increase by ~7–22 ng/mL following administration of between one and four snus pouches of the same brand and approximate dose (i.e., ~8 mg nicotine; 22, 23, 24). Additionally, when a single 8.7 mg snus pouch is consumed by overnight abstinent cigarette smokers, the average time to reach maximum plasma nicotine concentration is 37 minutes and subjective effects remain elevated for up to 30 minutes (22). In this study, never tobacco users were administered a total dose of 24 mg nicotine over the last 4 hours of the 5 hour session, with ~20–25 minutes separating the end of a pouch and the start of the next pouch. Thus, participants may have been experiencing peak effects for one pouch when the next pouch was administered, or carryover effects. Future work should consider implementing longer time intervals between pouch administration, and perhaps altering the duration of pouch use. In fact, that the duration of use may influence the effects of snus has been highlighted by industry representatives (“…keep your first one in for about a minute – then remove…After four or five…you’ll want to keep a pouch in as long as the flavor lasts…”) (8). Nonetheless, the influence of these dosing procedures on nicotine exposure should be evaluated using plasma nicotine measurements. Such measurements were not included here, and thus the extent to which participants were exposed to physiologically active doses of nicotine is unknown. Nicotine gum administered to never smokers has resulted in plasma nicotine levels of 1.3 ng/ml, 4.0 ng/ml, and 11.5 ng/ml for doses of 2, 4, and 8 mg nicotine, respectively (18). These levels of plasma nicotine were elevated significantly above baseline levels for the 4 and 8 mg doses (18). In this study, it is arguable that the doses of 1.6 mg and 3.2 mg nicotine were not physiologically active.
Another potential study limitation was the relatively small sample size, which may have precluded the detection of significant effects. In the absence of effect sizes for examination of snus in never tobacco users, sample size was determined from previous work that included administration of other forms of nicotine (e.g., gum) in never smokers (18, 19) and administration of snus in tobacco users (21, 22, 23). Table I shows that effect sizes for subjective measures were notably small. It is worth noting that subjective measures might be less sensitive to the effects of nicotine at low doses, and thus other measures indicative of abuse liability (e.g., discrimination) should be considered (29, 31). Moreover, a larger sample size may have revealed dose-dependent effects as a function of individual response to nicotine (e.g., those who preferred versus did not prefer snus based on their subjective ratings of positive versus negative effects), as has been showed previously (29).
Future work should also investigate the role that flavorings (e.g., mint, fruit, etc.) play in the acceptability and uptake of snus products among naïve users (11). A recent survey of youth aged 12–17 years demonstrates that, among past 30-day users of non-cigarette tobacco, the majority of respondents reported that their reason for snus use was product flavorings (32). Such flavorings may serve the purpose of masking the harsh tobacco taste among individuals who have no history of tobacco use. As highlighted by tobacco industry representatives, “…new users of smokeless tobacco…are most likely to begin with products that are milder tasting, more flavored…” (33). Finally, consideration should be given to the brand of snus evaluated. Brands currently available to consumers in the U.S., some of which originated in Sweden, differ by nicotine content (34) and flavor profile (35). In this study, the brand was chosen based on availability of a matching placebo; however, this brand may not be the most preferred among U.S. samples (36, 37). As mentioned above, different tobacco products may result in a different profile of effects and thus a different likelihood of abuse (20).
Though the uptake of snus products among U.S. adult cigarette smokers is relatively low (38), concerns remain over their use by novices (39, 40). For instance, snus use among never-smokers may predict cigarette-smoking initiation (7, 41). Such findings highlight the need for further assessment of abuse liability of snus products. In this study, all doses produced minimal subjective effects, and only the highest dose increased physiological effects significantly. Still, these findings can help guide the design of future studies intended to identify doses of snus that promote abuse.
Acknowledgements
The authors thank Claire Wilkison, Michael Phillips, Gemma David, and Ian Loy for their diligent efforts with data collection.
This study was supported by funds provided by the [University’s] Department of Psychology to author XXX. In addition, the stipend for authors YYY and ZZZ are provided by NIGMS T32 GM081741.
This study was supported by funds provided by the WVU Department of Psychology to author MDB. In addition, the stipend for authors JEO and NJF is provided by NIGMS T32 GM081741.
References
- (1).Rutqvist LE, Curvall M, Hassler T, Ringberger T, & Wahlberg I (2011). Swedish snus and the GothiaTek standard. Harm Reduction Journal, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hatsukami DK, Joseph AM, LeSage M, & Hecht SS (2007). Developing the science base for reducing tobacco harm. Nicotine and Tobacco Research, 9, 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Andersson G, & Axell T (1989). Clinical appearance of lesions associated with the use of loose and portion-bag packed Swedish moist snuff: a comparative study. Journal of Oral Pathology & Medicine, 1, 2–7. [DOI] [PubMed] [Google Scholar]
- (4).Luo J, Ye W, Zendehdel K, & Nyren O (2007). Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. The Lancet, 369, 16–22. [DOI] [PubMed] [Google Scholar]
- (5).Lee PN (2013). The effect on health of switching from cigarettes to snus – a review. Regulatory Toxicolology and Pharmacology, 66, 1–5. [DOI] [PubMed] [Google Scholar]
- (6).Foulds J, Ramstrom L, Burke M, & Fagerstrom K (2003). Effects of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control, 12, 349–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Soneji S, Sargent JD, Tanski SE, & Primack BA (2015). Associations between initial water pipe tobacco smoking and snus use and subsequent cigarette smoking: results from a longitudinal study of US adolescents and young adults. JAMA Pediatrics, 169, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Connolly GN (1995). The marketing of nicotine addiction by one oral snuff manufacturer. Tobacco Control, 5, 73–79. [Google Scholar]
- (9).Henningfield JE, Rose CA, & Zeller M (2006). Tobacco industry litigation position on addiction: continued dependence on past views. Tobacco Control, 15 (Suppl 4), iv27–iv36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Choi K, Fabian L, Mottey N, Corbett A, & Forster J (2012). Young adults’ favorable perceptions of snus, dissolvable tobacco products, and electronic cigarettes: Findings from a focus group study. American Journal of Public Health, 102, 2088–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Oliver AJ, Jensen JA, Vogel RI, Anderson AJ, & Hatsukami DK (2013). Flavored and nonflavored smokeless tobacco products: rate, pattern of use, and effects. Nicotine and Tobacco Research, 15, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Henningfield JE, Radzius A, & Cone EJ (1995). Estimation of available nicotine content of six smokeless tobacco products. Tobacco Control, 4, 57–61. [Google Scholar]
- (13).Carpenter CM, Connolly GN, Ayo-Yusuf OA, & Wayne GF (2009). Developing smokeless tobacco products for smokers: an examination of tobacco industry documents. Tobacco Control, 18, 54–59. [DOI] [PubMed] [Google Scholar]
- (14).Liu ST, Nemeth JM, Klein EG, Ferketich AK, Kwan M, & Wewers ME (2014). Adolescent and adult perceptions of traditional and novel smokeless tobacco products and packaging in rural Ohio. Tobacco Control, 23, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Harris AC, Tally L, Schmidt CE, & LeSage MG (2015). Animal models to assess the abuse liability of tobacco products: Effects of smokeless tobacco extracts on intracranial self-stimulation. Drug and Alcohol Dependence, 147, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Cobb CO, Blank MD, Morlett A, & Eissenberg T (2015). Comparison of puff topography, toxicant exposure, and subjective effects in low- and high-frequency waterpipe users: A double-blind, placebo-control study. Nicotine and Tobacco Research, 17, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Huynh H, & Feldt LS (1976). Estimation of the box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Education Statistics, 1, 69–82. [Google Scholar]
- (18).Heishman SJ, & Henningfield JE (2000). Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology, 152, 321–333. [DOI] [PubMed] [Google Scholar]
- (19).Heishman SJ, Snyder FR, & Henningfield JE (1993). Performance, subjective, and physiological effects of nicotine in non-smokers. Drug and Alcohol Dependence, 34, 11–18. [DOI] [PubMed] [Google Scholar]
- (20).Fagerstrom K, & Eissenberg T (2012). Dependence on tobacco and nicotine products: A case for product-specific assessment. Nicotine and Tobacco Research, 14, 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cobb CO, Weaver MF, & Eissenberg T (2010). Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tobacco Control, 19, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lunell E, & Curvall M (2011). Nicotine delivery and subjective effects of Swedish portion snus compared with 4 mg nicotine polacrilex chewing gum. Nicotine and Tobacco Research, 13, 573–578. [DOI] [PubMed] [Google Scholar]
- (23).Lunell E, & Lunell M (2005). Steady-state nicotine plasma levels following use of four different types of Swedish snus compared with 2-mg Nicorette chewing gum: A crossover study. Nicotine and Tobacco Research, 7, 397–403. [DOI] [PubMed] [Google Scholar]
- (24).Gray JN, Breland AB, Weaver M, & Eissenberg T (2008). Potential reduced exposure products (PREPs) for smokeless tobacco users: clinical evaluation methodology. Nicotine and Tobacco Research, 10, 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Jolma CD, Samson RA, Klewer SE, Donnerstein RL, & Goldberg SJ (2002). Acute cardiac effects of nicotine in healthy young adults. Echocardiography, 19, 443–448. [DOI] [PubMed] [Google Scholar]
- (26).Martin JS, Beck DT, Gurovich AN, & Braith RW (2010). The acute effects of smokeless tobacco on central aortic blood pressure and wave reflection characteristics. Experimental Biology and Medicine, 235, 1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ramakrishnan S, Thangjam R, Roy A, & Bhargava B (2011). Acute effects of tobacco chewing on the systemic, pulmonary, and coronary circulation. American Journal on Cardiovascular Drugs, 11, 109–114. [DOI] [PubMed] [Google Scholar]
- (28).Benowitz NL, Porchet H, Sheiner L, & Jacob P (1988). Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clinical Pharmacology & Therapeutics, 44, 23–28. [DOI] [PubMed] [Google Scholar]
- (29).Duke AN, Johnson MW, Reissig CJ, & Griffiths RR (2015). Nicotine reinforcement in never-smokers. Psychopharmacology, 232, 4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Falco AM, & Bevins RA (2015). Individual differences in the behavioral effects of nicotine: A review of the preclinical animal literature. Pharmacology, Biochemistry and Behavior, 138, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Perkins KA (2009). Discriminative stimulus effects of nicotine in humans. Handbook of Experimental Pharmacology, 192, 369–400. [DOI] [PubMed] [Google Scholar]
- (32).Ambrose BK Day HR, Rostron B, & Villanti AC (2015). Flavored tobacco product use among US youth aged 12–17 years, 2013–2014. Journal of the American Medical Association, 314, 1871–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).U.S. Smokeless Tobacco Company, David Weiss Associates. (1984). The ‘Graduation Theory’. Bates No. USSTC1945141-USSTC1945142. Retrieved from http://legacy.library.ucsf.edu/tid/lfc46b00.
- (34).Stepanov I, Jensen J, Hatsukami D, & Hecht SS (2008). New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine and Tobacco Research, 10, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Villanti AC, Richardson A, Vallone DM, & Rather JM (2013). Flavored tobacco product use among U.S. young adults. American Journal of Preventive Medicine, 44, 388–391. [DOI] [PubMed] [Google Scholar]
- (36).Hatsukami DK, Jensen J, Anderson A, & Severson H (2011). Oral tobacco products: Preference and effects among smokers. Drug and Alcohol Dependence, 118, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Hatsukami DK, Zhang Y, O’Connor RJ, & Severson HH (2013). Subject responses to oral tobacco products: Scale validation. Nicotine and Tobacco Research, 15, 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Biener L, Roman AM, McInerney SA, & Romito L (2014). Snus use and rejection in the USA. Tobacco Control. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Choi K, & Forster J (2013). Awareness, perceptions and use of snus among young adults from the upper Midwest region of the USA. Tobacco Control, 22, 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Rodu B, Plurphanswat N, Hughes JR, & Fagerstrom K (2015). Associations of proposed relative-risk warning labels for snus with perceptions and behavioral intentions among tobacco users and nonusers. Nicotine and Tobacco Research. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Taylor N, Choi K, & Forster J (2015). Snus use and smoking behaviors: preliminary findings from a prospective cohort study among US Midwest young adults. American Journal of Public Health, 105, 583–685. [DOI] [PMC free article] [PubMed] [Google Scholar]


