Introduction
Optogenetics describes the technique of expressing light-activatable proteins, called opsins, in a genetically and/or anatomically restricted population of cells, permitting temporally precise and selective manipulation of the activity of the targeted population. This makes it possible to dissect the physiological role of specific neuronal populations in different phases of pain processing (Also reviewed in [14; 22; 83]). Targeted neuronal expression of Channelrhodopsin-2 (ChR2), a light-activated cation channel cloned from the single-cell algae Chlamydomonas reinhardtii, provided the first evidence that optically activated channels can manipulate neuronal activity [13]. Since this discovery, a wide variety of opsins have been identified in nature or engineered to permit manipulation of neuronal activity (depolarization/activation or hyperpolarization/inhibition) [18; 45; 46; 56; 64; 115], intracellular signaling [1; 60; 96; 102; 103], and gene expression [32; 89; 97] in a temporally precise, and cell-type-specific manner (Reviewed in [33; 49; 62; 77]). Mutagenesis and genomic screening has expanded the opsin tool box to include opsins with faster kinetics [5; 48], permitting more precise control of activity patterns; bi-stable opsins [6], permitting sustained activation or inhibition with only brief light pulses; and opsins activated at longer wavelengths (red-shifted opsins) [20; 63], permitting improved light penetration through tissues. In addition to direct effects on membrane potential, other opsins, most of which have been molecularly engineered, can initiate more complex signaling processes, such as G-protein coupled receptor downstream signaling [1; 60; 102; 103], and regulation of gene expression [32; 89; 97]. These opsins extend the advantages of optogenetic manipulations beyond direct effects on membrane potential, furthering the utility of these approaches in manipulating both excitable and non-excitable cells. This ever-expanding set of optogenetic tools has provided many elegant approaches for manipulating activity and signaling in cells, and has led to an explosion of new findings in systems neuroscience. However, widespread implementation of optogenetics in studies of the peripheral nervous system (PNS) presents two major obstacles. The first is that there are challenges in producing consistent and robust genetically restricted expression of opsins in neurons of the PNS, as viral and transgenic tools are more limited and less established as compared to CNS applications. The second challenge is consistent and restricted delivery of light to the neurons expressing these opsins. Overcoming these obstacles is critical to realizing the full potential of optogenetic manipulations in the PNS.
Genetic expression of opsins in the PNS
The two primary methods used to target opsin expression to the PNS are viral delivery and transgenic mouse lines. Viral transduction offers the most experimental flexibility, with multiple readily available constructs containing different promoters, opsins and reporter tags, whereas the availability of transgenic mouse lines is more limited [70–72]. Adeno-associated viruses (AAVs) have been the primary method of opsin gene delivery to the PNS [4; 11; 52; 53; 59; 84; 108]. Multiple AAV serotypes have been identified and engineered to preferentially transduce different types of neurons. AAV5, 6, 8, 9 and PHP.S have been shown to have higher tropism for dorsal root ganglion (DRG) neurons than other serotypes [16; 17; 36; 52; 53; 59; 76; 84; 98; 99; 105; 108; 119]. Moreover, it may be possible to exploit different serotypes to induce differential expression patterns within sensory neuron populations [54; 109; 111; 119]. Cell type-specific promoters are possibly more effective in limiting expression to precise populations of DRG neurons [59; 73], although likely at the cost of decreased protein expression as less ubiquitous promotors can have weaker cellular expression.
Another strategy used to target more refined neuronal sub-populations utilizes a combination of viral vectors and transgenic animals that permits spatially restricted expression based on the location of virus injection, and cell-type specificity based on the expression of Cre recombinase in transgenic mice. The promoter for these DIO or FLEX viral constructs is often a strong ubiquitous promoter that drives robust Cre-dependent transgene expression. These viruses can be injected into discrete anatomical structures of transgenic mice that express Cre recombinase in a genetically-defined cell population, leading to Cre-dependent activation of opsin in that population [4; 41]. For example, a virus containing a Cre dependent ChR2 may infect all DRG neurons, but only DRG neurons expressing Cre under the control of the Nav1.8 promoter will express the opsin, hence restricting expression to Nav1.8+ cells. Herpes simplex virus (HSV) vectors also have significant potential for the study of pain and other aspects of somatosensation, owing to their natural tropism for sensory neurons and relatively large genome carrying capacity [66] (Reviewed in [27; 43]). This potential for larger genetic cargoes allows targeted expression of bigger and/or multiple different proteins in sensory neurons. Additional advantages of HSV over AAVs are rapid expression, in days compared to weeks for AAVs and they can be retrogradely transported, allowing for targeting to sensory neurons that innervate specific peripheral structures by delivering the virus to the desired peripheral target (e.g. skin, muscle, bladder, etc) [44; 73; 118].
A major advantage of restricting opsin expression to a specific anatomical region or target organ is that it allows for light delivery to be less specific with reduced off target effects. However, expression levels can be more variable from animal-to-animal with this approach, especially with viral injection into peripheral structures without dense afferent innervation. Unlike the brain, where highly conserved anatomy allows for targeted stereotaxic injections into discrete nuclei, peripheral structures like skin and visceral organs have more varied and less dense innervation that covers a much larger area, making animal-to-animal expression potentially less consistent. Intraganglionic [59] and intrathecal [4; 11] delivery of viral vectors can increase the number of transduced neurons, however anatomical specificity is lost, and the injection procedures are relativity more invasive. Intravenous and intraperitoneal delivery of AAVs (mostly AAV9) have both been used to express various proteins in the PNS [31; 36; 99]. Although these methods are less invasive than intraganglionic or intrathecal injection, they require larger viral volumes and normally result in poorer transduction efficiency. Recently, an AAV variant (AAV-PHP.S) [29] demonstrates dramatically increased DRG transduction efficiency (82% when delivered intravenously in adult mice compared to 46% with intravenous AAV9) has been developed [16]. Intravenous delivery of AAV-PHP.S resulted in expression throughout the periphery including cardiac ganglion, the enteric nervous system, and non-neuronal cells in the liver, lungs and heart, as well as in neurons within the CNS. By pairing this type of virus with promotor-specific expression or the Cre-dependent expression strategy described above, one can increase cell-type specificity, while benefitting from the increased expression levels.
PNS opsin expression can also be achieved efficiently using a transgenic approach, by breeding mice that express Cre recombinase under the control of a promotor specific to a cell-type of interest with mice that conditionally express opsins in a Cre-dependent manner [4; 24–26; 71; 88; 94; 95; 121]. This approach mitigates the inherent variability in expression levels one can encounter using viral approaches, allowing for more consistent opsin expression in a genetically-defined subset of cells. These genetic tools of course also allow for targeted expression of opsins in non-neuronal cells [3; 75; 82; 85]. This approach is limited by the availability of suitable opsin- and Cre-expressing lines. One important consideration when using these transgenic and knock-in Cre lines is that opsin expression is not restricted only to cells that express the gene driving the Cre recombinase in adulthood, as some genes may be transiently expressed during development. For example, a number of studies have found that although TRPV1 is transiently expressed in a broad population of DRG neurons during embryonic development, in adulthood TRPV1 expression is restricted to a much smaller population of DRG neurons [15; 51]. Therefore, use of TRPV1-Cre to conditionally express a Cre-dependent opsin would result in opsin expression in a larger population of DRG neurons than would be expected based in the adult pattern of TRPV1 expression [80]. The use of inducible Cre lines, where expression of Cre is temporally controlled and thus can be initiated in adulthood, could help circumvent this complication [34].
An additional caveat of these transgenic approaches is that some types of transgenic Cre lines may not contain all of the endogenous enhancer/repressor elements, or may have positional effects on expression based on where the transgene is inserted in the genome, resulting in “leaky” expression or reduced expression of the Cre recombinase relative to the desired population being targeted with the chosen promoter. As a result, these transgenic approaches may not necessarily grant exclusive access to a defined population, so they should always be validated for their specificity. Finally, it is important to consider that long-term expression of exogenous proteins, such as opsins, throughout development can lead to potential alterations in neuronal physiology [81; 93]. This can significantly complicate experimental interpretations, so limiting the length of time an opsin is expressed may be advantageous. All publications using these approaches should be considered with these shortcomings in mind, but with careful consideration of these caveats when designing experiments, transgenic and viral expressed opsins offer a powerful approach to target different cell types for optogenetic manipulation in the periphery.
Light sources for activation of opsins in peripheral tissues
For optogenetic studies in the brain, the skull provides a convenient, stable bony anchor point for the fixation of tethered fiber optics or recently developed wireless micro-scale light emitting diode (µLED)-based devices, for targeting light to specific regions of the brain. Peripheral structures, where similar stable anchor points are normally absent, present unique challenges for implementing optogenetic approaches. Several different methods have been employed to accomplish these difficult tasks. External illumination with LED arrays (e.g. in the floor of a behavior arena) or manual targeting of light delivered by light-coupled fiber optic cables have been used to target opsin-expressing nerve fibers in the paw during sensory behavior tests [4; 25; 52; 59; 82]. These approaches are sufficient for illumination of sensory afferents or other cell types near the skin surface. However, this approach requires a consistent source of light directed to the specific area of interest, which can be difficult in awake, freely moving animals. Furthermore, confounds of visible external illumination and movement of the experimenter may impact animal behavior. Additionally, this approach does not allow access to deeper structures such as viscera.
To overcome these limitations, tethered laser fiber optic devices, like those used in the CNS, have been adopted for use in the PNS. For illumination of spinal cord neurons or central processes of DRG neurons, fiber optic cables inserted into the epidural space or optical fibers mounted to the vertebrae via stabilizing blocks of dental acrylic have been used [12; 19; 85]. A similar approach, wherein peripheral nerve axons are targeted using cuffs that wrap around the nerve and are coupled to fiber optics, has also been shown to be effective [65; 79; 108]. For this direct nerve-interfacing approach to light delivery, it is critical that the material properties of these devices are compatible with the delicate tissues of the spinal cord and peripheral axons to avoid mechanical damage to these structures. Furthermore, the animals are still tethered to optical cables, which stresses the animal, inhibits normal locomotion and interactions of the animals, and restricts the type of behavior arenas that can be used. These factors limit the types of behavior experiments that can be performed and can complicate the interpretation of animal behavior. Having said that, these approaches have proven useful in advancing our understanding of the role of different neuronal subtypes
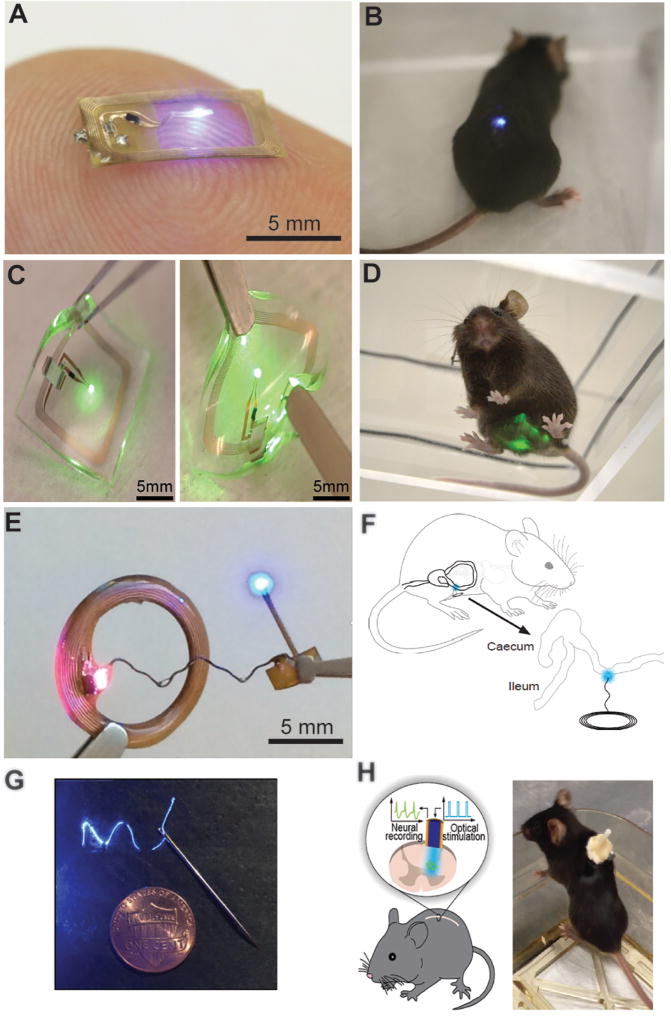
Recently, several fully wireless devices have been developed that allow for experiments in freely moving animals and resolve many of the limitations associated with fiber optic tethers [84; 88; 94; 95; 100] (Figure 1). The wireless systems use inductive coupling to directly power µLEDs, which eliminates the need for batteries and circuits to control frequency and power of illumination, dramatically decreasing the volume and mass of these devices. The µLEDs and powering antenna can be designed and fabricated to efficiently target specific structures like spinal cord, peripheral nerves, and viscera [84; 88; 94; 95]. The most recent versions of these devices utilize near-field communications (NFC) powering protocols and more durable encapsulation, which reduces the set-up cost and technical expertise required with previous versions and increases the longevity of these devices, respectively [94; 95; 100]. Angle dependencies between the receiving and emitting antenna are a limitation of these wirelessly powered devices, as angular mismatches can cause temporary loss of power. The addition of super capacitors or rechargeable batteries could bridge potential losses in power but would also add mass to implantable devices. Future generations of these wireless devices will make it possible to simultaneously record information from the animal while providing optical stimulation, as has been demonstrated in the CNS [67; 68] (Figure 1G and H). These devices could integrate different types of sensors (optical, temperature, electrical etc.) to allow for closed loop optogenetic stimulation akin to recent implementations in the CNS [58; 90] (Reviewed in [47]).
Figure 1.
Peripheral Light Delivery Technology for Optogenetics. A and B) Wirelessly powered µLED device for stimulation of spinal afferents or spinal cord neurons. Reproduced from reference [95] with permission. C and D) µLED device for illuminating opsins in the bladder or lower abdomen. Additional encapsulation layers provide protection from mechanical forces and repetitive stresses associated with subcutaneous abdominal implants. Reproduced from reference [94] with permission. E) Multi-purpose wireless µLED device that can target opsins expressed in various brain regions and peripheral tissues. Reproduced from reference [100] with permission. F) Illustration of a multi-use wireless µLED device used to target colon afferents. Reproduced from reference [50] with permission. G and D) Demonstration of a nanowire system, for electrical recording and optical stimulation of spinal cord neurons [67].
Use of optogenetics in peripheral nociceptive neurons
The use of in vivo optogenetics to study the PNS has increased our understanding of the contributions of specific populations of sensory neurons in pain processing. Initial optogenetic studies that targeted large populations of sensory neurons demonstrated that ChR2-mediated stimulation of Nav1.8+ [25], TRPV1+ [88; 95], Advillin+ [88] or c-fiber sensory afferents [52; 84] resulted in nociceptive-like behaviors. These behaviors included hind paw withdrawal, jumping and real-time aversion in place preference assays. Place aversion was attenuated by analgesic administration, indicating that ChR2-mediated stimulation of these afferents produces pain [25]. ChR2-mediated activation of heterogenous nociceptor populations at the peripheral terminals [25; 52; 84], along the nerve [88] or at the level of central terminals [84; 88; 95] all resulted in nociceptive-like behaviors. While this may be unsurprising (activating nociceptors causes nociception), it is interesting to note that prolonged activation of Nav1.8+ or TRPV1+ sensory neurons caused long lasting behavioral hypersensitivity after the stimulus was removed [24; 53; 104]. It is well established that inflammation and nerve injury cause acute nociceptor sensitization [114]. One component of injury-induced nociceptor sensitization is the upregulation of ubiquitin specific peptidase 5 (USP5) in nociceptors, which stabilizes expression and increases activity of Cav3.2 T-type calcium channels [38]. The acute sensitization initiated by optical stimulation (10 Hz) of TRPV1-ChR2 neurons, is sufficient to increase USP5 expression, which results in increased Cav3.2 T-type activity and increased mechanical hypersensitivity that is dependent on the interaction of USP5 and Cav3.2 [104]. These experiments demonstrate that ChR2-mediated activation of TRPV1-ChR2 neurons alone can initiate mechanical hypersensitivity. It is interesting to note that while optical activation of Nav1.8 and TRPV1 nociceptor populations (10–30 min) causes nociceptive behaviors and sensitization that persists after the stimulus is removed, this short term optical activation does not appear to result in plasma extravasation, edema or neurogenic inflammation [25; 104]. More thorough testing is warranted to determine whether neuronal activity driven by optogenetic stimulation can generate neurogenic inflammation under different conditions.
Inhibitory opsins, which initiate neuronal hyperpolarization, can be used to silence or attenuate neuron activity. When expressed in sensory neurons, inhibitory opsins can decrease responses to noxious stimuli in naïve animals, and attenuate hypersensitivity associated with inflammation and nerve injury [11; 24; 52; 53; 94]. Stimulation of the virally transduced chloride pump halorhodopsin, in c-fibers, is sufficient to attenuate both thermal and mechanical sensitivity in naïve animals [53; 59], as well as hypersensitivity associated with chronic constriction injury (CCI) [52]. Similarly, the inhibitory proton pump archaerhodopsin (Arch), when expressed in sensory neurons under control of the Nav1.8 promoter, can attenuate pain in somatic models of inflammatory and neuropathic pain [24] as well as in a model of visceral inflammatory pain [94]. Viral approaches that produced expression restricted to A-delta fibers with Arch, exclusively reduce action potential firing in high threshold mechanoreceptors (HTMRs), not in c-fibers or low threshold mechanoreceptors (LTMRs) and decrease mechanical thresholds in rats after partial sciatic nerve ligation [11]. These studies illustrate the utility of inhibitory optogenetic techniques to noninvasively determine specific modalities of activation of genetically defined populations of nociceptors, before and after development of inflammatory and neuropathic pain. At the cellular level, it is clear that inhibitory opsins can effectively reduce firing of human sensory neurons in vitro [110] providing enticing evidence that optogenetic inhibition of neuronal activity could be effective in patients. These inhibitory approaches provide preclinical evidence for the feasibility of viral gene delivery of inhibitory opsins as a potential future form of neuromodulation that could provide nonpharmacologic analgesic therapy in chronic pain patients.
Optogenetic approaches have also allowed for identification of high and low threshold mechanoreceptor populations and characterization of their role in nociception. LTMRs encode innocuous tactile information, mostly thought to be distinct from nociceptor signaling. Accordingly, in a transgenic rat model, in which ChR2 is expressed in a population of Thy1+ LTMRS that associate with tactile end organs including Merkel discs and Meissner’s corpuscles, in vivo activation of Thy1-ChR2 neurons elicited non-nociceptive like withdrawals in naïve animals [55]. However, in the same animal model, optogenetic activation of Thy1+ neurons enhanced neuropathic-like pain behaviors after peripheral nerve injury [106]. This study identified a population of afferents (LTMRS associated with tactile organs in the skin) that appear to be responsible for mechanical allodynia associated with neuropathic injury.
A novel population of HTMRs was uniquely identified using a combination of optogenetics and ablation techniques. Transgenic mice that express ChR2 in Calca+ neurons, a precursor peptide for CGRP, were injected with resiniferatoxin to eliminate TRPV1+ fibers. The remaining ChR2+ / Calca+ / TRPV1- cells were stimulated by blue light and characterized [40]. This population of cells has unique circumferential endings, responds to mechanical stimulation, and can be activated by as little as pulling a single hair. This study highlights the ability to combine optogenetic and ablation techniques to classify populations of cells that have yet to be uniquely genetically identified.
The cellular specificity and temporal precision of optogenetics has enabled a more precise investigation of the labeled line theory, where individual fibers are proposed to respond to distinct nociceptive modalities (heat, mechanical, cold etc.). A comparative study looked at the differences between the two major divisions of unmyelinated C-fiber nociceptors; peptidergic, TRPV1+ neurons primarily which have been reported to be responsible for sensing noxious heat and nonpeptidergic, MrgD+ neurons are responsible for sensing noxious mechanical stimuli [4]. While ablation studies have previously determined the sensory modalities of these neuronal populations, optogenetics allows for non-invasive activation of these subtypes without damaging or injuring the sensory fibers as would occur with ablation studies [120]. Cutaneous illumination of MrgD-ChR2 fibers resulted in primarily hind paw lifting responses, indicative of mechanical withdrawal, compared to activation of TRPV1-ChR2, which resulted in primarily licking responses, a behavior that mimics responses elicited by noxious heat [4]. These results suggest that activation of specific populations of sensory neurons results in differential nociceptive responses, which supports the labeled line theory. Another study compared the effects of activation of the SNS population of sensory neurons (sensory neurons that express Nav 1.8 including most C-fibers, and 40% of NF200+ myelinated fibers) to activation of the TRPV1 lineage population (most C-fibers) on bladder function and pain [26]. ChR2-mediated activation of only SNS+ fibers elicited nociceptive responses to non-noxious stimuli. In addition, optical stimulation of SNS+ fibers resulted in more intense bladder contractions, compared to stimulation of TRPV1+ afferents, illustrating the potentially differential effects that activation of distinct but overlapping populations of nociceptors can have on sensory processes.
Optogenetic strategies also allow for the determination of how cross talk between different sensory modalities influences sensory perception, including the ability to test aspects of the gate control theory, where A-beta LTMRS are proposed to activate inhibitory spinal interneurons which decreases nociceptive transmission [78]. Activation of ChR2 in a subset of A-beta LMTRs (MafA+) that innervate D-hair, Merkel cells, and Meissner’s corpuscles reduced mechanical sensitivity evoked by stimulation of HTMR fibers (Npy2r+). These Npy2r+ cells have distinctive high frequency firing responses to mechanical stimulation and are responsible for pinprick nociceptive responses [2]. Stimulation of MafA+ fibers also resulted in less c-fos expression in the spinal cord dorsal horn evoked by Npr2r+ stimulation, so presumably MafA+ fibers activate an inhibitory circuit at the spinal cord to reduce the input from Npy2r+ fibers. These results, consistent with the concept of gate control, may help to explain why rubbing a painful area and activating innocuous touch sensors helps relieve pain. These studies highlight the utility of optogenetics in deciphering the roles of different fiber types in nociception.
Use of optogenetics in peripheral non-neuronal cells to study pain
Non-neuronal cells like keratinocytes, astrocytes, and endothelial cells express different types of sensory transduction channels. These cell types often associate with neurons, and there is evidence supporting the idea that these cells can signal to other cell types, including neurons (reviewed in [10; 23; 37; 69]). It has previously been difficult to measure signaling between these non-neuronal cells and neurons because of overlap in signaling receptors and signaling molecules in these cell types. Optogenetic techniques, however, offer the opportunity to manipulate specific types of non-neuronal cells without influencing neurons, to identify the role of non-neuronal cell signaling in nociceptive and sensory signaling processes [3; 74; 75; 82; 85].
The first genetic strategies to specifically manipulate keratinocytes did not use optogenetics but used a chemogenetic strategy to selectively express TRPV1 in keratinocytes of a global TRPV1−/− mouse [87]. In this study, activation of keratinocytes with a TRPV1 agonist could initiate nociceptive responses. Subsequently, optogenetic techniques, which allow more precise temporal activation, were utilized to specifically manipulate keratinocytes in vitro and in vivo. Activation of ChR2 in these cells was sufficient to generate action potential firing in nociceptive neurons [3]. In addition, stimulation of the inhibitory opsin halorhodopsin in keratinocytes reduced the number of action potentials evoked in sensory neurons by mechanical activation of keratinocytes [3]. Further, depolarization of keratinocytes by mechanical stimulation or by ChR2 activation initiates release of ATP. Interestingly, this ATP release appears to produce P2X4-dependent increases in sensory neuron firing [82], a fascinating finding given the expression of many P2X channels in sensory neurons that respond to ATP including P2X2 and P2X3, which are involved in other pain states [7; 21; 86]. In vivo optical activation of ChR2 in keratinocytes resulted in nociceptive behaviors in the absence of injury [3; 82]. Conversely, activation of Arch, an optically activated inhibitory proton pump, in keratinocytes decreased ATP release and nocifensive responses to mechanical stimulation [82]. Similarly, optogenetic activation of colonic epithelium with ChR2 can activate nociceptive sensory afferents in a similar manner to stretch-mediated activation, in a mechanism that at least in part relies on ATP signaling [74]. While these studies describe novel mechanisms of mechanosensation, they do not supplant or refute the extensive existing literature that clearly demonstrates mechanosensitivity in nociceptive neurons (Reviewed in [30; 91]) but suggest other mechanisms that collectively may influence the overall perception of mechanical stimuli.
Optogenetic approaches have also been deployed to clarify the role of glial cells in the activation of pain pathways. While it had been previously understood that microglia are important in enhancement of nociceptive sensitization and/or central sensitization, optogenetic techniques allowed for the exclusive manipulation of astrocytes, demonstrating that astrocyte activation alone can trigger a cascade that activates microglia [85]. This paper is also noteworthy because it provided direct evidence that ChR2 activation in astrocytes, which are non-excitable cells, can indeed promote the release of ATP and induce expression of cytokines, suggesting that at least ChR2-mediated activation of astrocyte signaling is possible.
It is important to understand the limitations of using opsins in non-excitable cells. ChR2 is a non-specific cation channel and it is not clear how expression and activation of this channel in non-excitable cells influences endogenous signaling. In excitable cells it has been well established that ChR2 activation directly depolarizes the membrane, leading to activation of voltage gated sodium channels and the generation of action potentials. However, this does not occur in non-excitable cells and the effects of a depolarizing stimulus are unclear [3; 82]. Activation of ChR2 may result in other changes in intracellular signaling events in non-excitable cells, possibly related to increases in intracellular calcium; however, this is likely to be opsin- and cell-dependent. It is therefore critical to directly determine the effects of optogenetic “activation” or “inhibition” in every case to understand and compare this non-physiologic signaling to a physiologic signaling mechanism. For example, ChR2 activation in keratinocytes produces a similar amount of ATP release as mechanical stimulation of these cells [82]. While ChR2 may mimic activation of other sensory cation channels (TRP, P2X channels), there are many different types of newly-developed opsins that endow light sensitivity to G-protein coupled receptors (GPCRs), thus better mimicking physiologic processes mediated by GPCR signaling [1; 60; 102; 103]. Additionally, the development of optically activated sensory channels and intracellular signaling proteins could help clarify the relative contributions of non-neuronal cells vs. neurons in sensory transduction.
Clinical neuromodulation and pain
Direct electrical and pharmacological neuromodulation of the PNS are currently used to treat different pain conditions via implantable stimulators and injections, respectively [39; 42; 92; 101; 117]. Both techniques block or attenuate sensory afferent neurotransmission and therefore reduce nociceptive input and pain sensation in patients. Compared to electrically induced neuronal inhibition, pharmacological approaches can be somewhat more selective for nociceptive neurons (topical capsaicin for arthritis, or intravesicular capsaicin for interstitial cystitis). However, these approaches are generally not very specific and target whole nerve bundles or spinal cord circuits (e.g., electrical stimulation or lidocaine nerve block). Additionally, these therapies often employ continuous electrical stimulation or a single delivery of a nerve blocking agent, which offers patients little control over the timing of their treatments. Optogenetic neuromodulatory approaches have the potential to improve both target specificity and temporal control of pain management in chronic pain patients. Implementation of optogenetics as next-generation neuromodulation therapies in patients faces some notable hurdles in the development of safe, effective gene therapy vectors to provide opsin expression, and robust, biocompatible light-delivery systems to provide control over the location, duration, and intensity of opsin activation.
Clinical trials utilizing opsins are already underway to treat Retinitis pigmentosa, a genetic disease that leads to progressive loss of photoreceptors in the retina [9; 107]. These trials use AAV-mediated gene transfer to express ChR2 in retinal ganglion cells of patients. This approach has been shown to partially restore vision in a photoreceptor-deficient mouse model [8]. Another group is testing a similar intervention for Retinitis pigmentosa using a red-shifted opsin [9]. The United States Food and Drug Administration recently approved an AAV-mediated gene therapy, Luxturna, to treat the inherited retinal disease caused by mutations to the PTR65 gene, which further supports the safety and efficacy of AAV mediated gene transfer. In addition to AAV vectors, HSV vector strategies have been used successfully to deliver gene products to sensory neurons in humans through intradermal injections [35; 116]. HSV-mediated gene transfer is therefore a promising potential approach for delivering opsins to the PNS in patient populations.
Optogenetics has potential clinical applications beyond silencing nociceptive neurons. For example, optogenetic approaches have been used in pre-clinical studies to improve nerve regeneration [113] and to improve gut motility [50]. Opsins can also be utilized to bi-directionally regulate gene expression, through light activatable transcription factors [57; 89; 112]; an intriguing application of this idea would be to tune the expression of genes that contribute to increased excitability after the development of chronic pain [28; 61]. Advancements in light delivery and opsin technology will also improve the potential efficacy of optogenetic-mediated therapies. Opsins activated by longer wavelengths of light that more efficiently penetrate tissue could allow for external light sources that would eliminate complications associated with implantation of light delivery devices. The use of step-function opsins, which are activated by a single pulse of light, could also eliminate the need for constant illumination, improving device lifetimes and mitigating concerns around tissue heating.
Summary
The advent of optogenetics has provided a technological revolution that has dramatically improved our understanding of the cells and circuits that mediate nociception and pain. This technology certainly offers a revolutionary approach to manipulate cellular function with exceptional spatial and temporal precision, allowing us to answer questions that were previously impossible to answer. Refinement of gene therapy approaches and light delivery systems are required to unlock the full potential of optogenetics as next-generation neuromodulatory therapies for chronic pain, but promising early studies in human cells and ongoing initial clinical trials of optogenetics for other conditions, portend a bright future for optogenetic therapies in patients.
Acknowledgments
We would thank Judy Golden and Bryan Copits as well as the entire Gereau Lab for their helpful insights and suggestions in the preparation of this manuscript. This work was funded by an NIH SPARC award (NIBIB U18EB021793) and an NIH Director’s Transformative Research Award (R01NS081707) to RWG, and NRSA F32 DK115122 awarded to ADM. RWG is a co-founder of Neurolux, a company that manufactures wireless optoelectronic research devices, some of which were described in this article.
References
- 1.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458(7241):1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 2.Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG. Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron. 2017;93(1):179–193. doi: 10.1016/j.neuron.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, Ross SE, Koerber HR, Davis BM, Albers KM. Keratinocytes can modulate and directly initiate nociceptive responses. eLife. 2015;4 doi: 10.7554/eLife.09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, Seguela P. Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain. 2017;158(12):2329–2339. doi: 10.1097/j.pain.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 5.Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(18):7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nature neuroscience. 2009;12(2):229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 7.Bernier LP, Ase AR, Seguela P. P2X receptor channels in chronic pain pathways. British journal of pharmacology. 2018;175(12):2219–2230. doi: 10.1111/bph.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50(1):23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biologics G. In: Dose-escalation Study to Evaluate the Safety and Tolerability of GS030 in Subjects With Retinitis Pigmentosa (PIONEER) CgI NCT03326336, editor. Vol. 2018. NIH; 2018. [Google Scholar]
- 10.Birder L, Andersson KE. Urothelial signaling. Physiological reviews. 2013;93(2):653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boada MD, Martin TJ, Peters CM, Hayashida K, Harris MH, Houle TT, Boyden ES, Eisenach JC, Ririe DG. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain. 2014;155(12):2646–2655. doi: 10.1016/j.pain.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonin RP, Wang F, Desrochers-Couture M, Ga Secka A, Boulanger ME, Cote DC, De Koninck Y. Epidural optogenetics for controlled analgesia. Molecular pain. 2016;12 doi: 10.1177/1744806916629051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 14.Carr FB, Zachariou V. Nociception and pain: lessons from optogenetics. Frontiers in behavioral neuroscience. 2014;8:69. doi: 10.3389/fnbeh.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(28):10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nature neuroscience. 2017;20(8):1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161(3):622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen AJ, Iyer SM, Francois A, Vyas S, Ramakrishnan C, Vesuna S, Deisseroth K, Scherrer G, Delp SL. In Vivo Interrogation of Spinal Mechanosensory Circuits. Cell reports. 2016;17(6):1699–1710. doi: 10.1016/j.celrep.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, Ogawa M, Ramanlal SB, Bandler RC, Allen BD, Forest CR, Chow BY, Han X, Lin Y, Tye KM, Roska B, Cardin JA, Boyden ES. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nature neuroscience. 2014;17(8):1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407(6807):1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 22.Copits BA, Pullen MY, Gereau RWt. Spotlight on pain: optogenetic approaches for interrogating somatosensory circuits. Pain. 2016;157(11):2424–2433. doi: 10.1097/j.pain.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS. Role of the endothelium in inflammatory bowel diseases. World journal of gastroenterology. 2011;17(5):578–593. doi: 10.3748/wjg.v17.i5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daou I, Beaudry H, Ase AR, Wieskopf JS, Ribeiro-da-Silva A, Mogil JS, Seguela P. Optogenetic Silencing of Nav1.8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain. eNeuro. 2016;3(1) doi: 10.1523/ENEURO.0140-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Seguela P. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(47):18631–18640. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBerry JJ, Samineni VK, Copits BA, Sullivan CJ, Vogt SK, Albers KM, Davis BM, Gereau Iv RW. Differential Regulation of Bladder Pain and Voiding Function by Sensory Afferent Populations Revealed by Selective Optogenetic Activation. Frontiers in integrative neuroscience. 2018;12:5. doi: 10.3389/fnint.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delbeke J, Hoffman L, Mols K, Braeken D, Prodanov D. And Then There Was Light: Perspectives of Optogenetics for Deep Brain Stimulation and Neuromodulation. Frontiers in neuroscience. 2017;11:663. doi: 10.3389/fnins.2017.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M. Epigenetic mechanisms of chronic pain. Trends in neurosciences. 2015;38(4):237–246. doi: 10.1016/j.tins.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature biotechnology. 2016;34(2):204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. The Journal of clinical investigation. 2010;120(11):3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P, Colle MA, Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(7):1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards WF, Young DD, Deiters A. Light-activated Cre recombinase as a tool for the spatial and temporal control of gene function in mammalian cells. ACS chemical biology. 2009;4(6):441–445. doi: 10.1021/cb900041s. [DOI] [PubMed] [Google Scholar]
- 33.Eleftheriou C, Cesca F, Maragliano L, Benfenati F, Maya-Vetencourt JF. Optogenetic Modulation of Intracellular Signalling and Transcription: Focus on Neuronal Plasticity. Journal of experimental neuroscience. 2017;11 doi: 10.1177/1179069517703354. 1179069517703354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, Wolfe D. Gene therapy for pain: results of a phase I clinical trial. Annals of neurology. 2011;70(2):207–212. doi: 10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature biotechnology. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7(4):482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, Bladen C, Chen L, Hamid J, Pizzoccaro A, Deage M, Francois A, Bourinet E, Zamponi GW. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron. 2014;83(5):1144–1158. doi: 10.1016/j.neuron.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 39.Geurts JW, Joosten EA, van Kleef M. Current status and future perspectives of spinal cord stimulation in treatment of chronic pain. Pain. 2017;158(5):771–774. doi: 10.1097/j.pain.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 40.Ghitani N, Barik A, Szczot M, Thompson JH, Li C, Le Pichon CE, Krashes MJ, Chesler AT. Specialized Mechanosensory Nociceptors Mediating Rapid Responses to Hair Pull. Neuron. 2017;95(4):944–954. e944. doi: 10.1016/j.neuron.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(37):9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goroszeniuk T, Pang D. Peripheral neuromodulation: a review. Current pain and headache reports. 2014;18(5):412. doi: 10.1007/s11916-014-0412-9. [DOI] [PubMed] [Google Scholar]
- 43.Goss JR, Gold MS, Glorioso JC. HSV vector-mediated modification of primary nociceptor afferents: an approach to inhibit chronic pain. Gene therapy. 2009;16(4):493–501. doi: 10.1038/gt.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene therapy. 2001;8(7):551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 45.Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349(6248):647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain cell biology. 2008;36(1–4):129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron. 2015;86(1):106–139. doi: 10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nature neuroscience. 2010;13(3):387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 49.Guru A, Post RJ, Ho YY, Warden MR. Making Sense of Optogenetics. The international journal of neuropsychopharmacology. 2015;18(11):pyv079. doi: 10.1093/ijnp/pyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hibberd TJ, Feng J, Luo J, Yang P, Samineni VK, Gereau RWt, Kelley N, Hu H, Spencer NJ. Optogenetic Induction of Colonic Motility in Mice. Gastroenterology. 2018 doi: 10.1053/j.gastro.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(10):2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nature biotechnology. 2014;32(3):274–278. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iyer SM, Vesuna S, Ramakrishnan C, Huynh K, Young S, Berndt A, Lee SY, Gorini CJ, Deisseroth K, Delp SL. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Scientific reports. 2016;6:30570. doi: 10.1038/srep30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacques SJ, Ahmed Z, Forbes A, Douglas MR, Vigenswara V, Berry M, Logan A. AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Molecular and cellular neurosciences. 2012;49(4):464–474. doi: 10.1016/j.mcn.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Ji ZG, Ito S, Honjoh T, Ohta H, Ishizuka T, Fukazawa Y, Yawo H. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. PloS one. 2012;7(3):e32699. doi: 10.1371/journal.pone.0032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES. Independent optical excitation of distinct neural populations. Nature methods. 2014;11(3):338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konermann S, Brigham MD, Trevino A, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro. 2014;1(1) doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B, Yang XY, Qian FP, Tang M, Ma C, Chiang LY. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain research. 2015;1609:12–20. doi: 10.1016/j.brainres.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X, Wang Y, van Westen GJ, Payen MP, Augusto E, Goncalves N, Tome AR, Li Z, Wu Z, Hou X, Zhou Y, A PI, Boyden ES, Cunha RA, Qu J, Chen JF. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Molecular psychiatry. 2015;20(11):1481. doi: 10.1038/mp.2015.43. [DOI] [PubMed] [Google Scholar]
- 61.Ligon CO, Moloney RD, Greenwood-Van Meerveld B. Targeting Epigenetic Mechanisms for Chronic Pain: A Valid Approach for the Development of Novel Therapeutics. The Journal of pharmacology and experimental therapeutics. 2016;357(1):84–93. doi: 10.1124/jpet.115.231670. [DOI] [PubMed] [Google Scholar]
- 62.Lin JY. A user's guide to channelrhodopsin variants: features, limitations and future developments. Experimental physiology. 2011;96(1):19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nature neuroscience. 2013;16(10):1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophysical journal. 2009;96(5):1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Llewellyn ME, Thompson KR, Deisseroth K, Delp SL. Orderly recruitment of motor units under optical control in vivo. Nature medicine. 2010;16(10):1161–1165. doi: 10.1038/nm.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lo L, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72(6):938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu C, Park S, Richner TJ, Derry A, Brown I, Hou C, Rao S, Kang J, Mortiz CT, Fink Y, Anikeeva P. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Science advances. 2017;3(3):e1600955. doi: 10.1126/sciadv.1600955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu L, Gutruf P, Xia L, Bhatti DL, Wang X, Vazquez-Guardado A, Ning X, Shen X, Sang T, Ma R, Pakeltis G, Sobczak G, Zhang H, Seo DO, Xue M, Yin L, Chanda D, Sheng X, Bruchas MR, Rogers JA. Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(7):E1374–E1383. doi: 10.1073/pnas.1718721115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445(7130):858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 70.Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, Gu H, Mills M, Cheng A, Tasic B, Nguyen TN, Sunkin SM, Benucci A, Nagy A, Miyawaki A, Helmchen F, Empson RM, Knopfel T, Boyden ES, Reid RC, Carandini M, Zeng H. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85(5):942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Majima T, Funahashi Y, Takai S, Goins WF, Gotoh M, Tyagi P, Glorioso JC, Yoshimura N. Herpes Simplex Virus Vector-Mediated Gene Delivery of Poreless TRPV1 Channels Reduces Bladder Overactivity and Nociception in Rats. Human gene therapy. 2015;26(11):734–742. doi: 10.1089/hum.2015.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makadia PA, Najjar SA, Saloman JL, Adelman P, Feng B, Margiotta JF, Albers KM, Davis BM. Optogenetic activation of colon epithelium of the mouse produces high frequency bursting in extrinsic colon afferents and engages visceromotor responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018 doi: 10.1523/JNEUROSCI.0837-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509(7502):617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(4):715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McIsaac RS, Bedbrook CN, Arnold FH. Recent advances in engineering microbial rhodopsins for optogenetics. Current opinion in structural biology. 2015;33:8–15. doi: 10.1016/j.sbi.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 79.Michoud F, Sottas L, Browne LE, Asboth L, Latremoliere A, Sakuma M, Courtine G, Woolf CJ, Lacour SP. Optical cuff for optogenetic control of the peripheral nervous system. Journal of neural engineering. 2018;15(1):015002. doi: 10.1088/1741-2552/aa9126. [DOI] [PubMed] [Google Scholar]
- 80.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. The EMBO journal. 2011;30(3):582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyashita T, Shao YR, Chung J, Pourzia O, Feldman DE. Long-term channelrhodopsin-2 (ChR2) expression can induce abnormal axonal morphology and targeting in cerebral cortex. Frontiers in neural circuits. 2013;7:8. doi: 10.3389/fncir.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife. 2018;7 doi: 10.7554/eLife.31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K, Delp SL. Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Science translational medicine. 2016;8(337):337rv335. doi: 10.1126/scitranslmed.aad7577. [DOI] [PubMed] [Google Scholar]
- 84.Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon AS. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nature methods. 2015;12(10):969–974. doi: 10.1038/nmeth.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nam Y, Kim JH, Kim JH, Jha MK, Jung JY, Lee MG, Choi IS, Jang IS, Lim DG, Hwang SH, Cho HJ, Suk K. Reversible Induction of Pain Hypersensitivity following Optogenetic Stimulation of Spinal Astrocytes. Cell reports. 2016;17(11):3049–3061. doi: 10.1016/j.celrep.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 86.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80(1–2):273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 87.Pang Z, Sakamoto T, Tiwari V, Kim YS, Yang F, Dong X, Guler AD, Guan Y, Caterina MJ. Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain. 2015;156(4):656–665. doi: 10.1097/j.pain.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 88.Park SI, Brenner DS, Shin G, Morgan CD, Copits BA, Chung HU, Pullen MY, Noh KN, Davidson S, Oh SJ, Yoon J, Jang KI, Samineni VK, Norman M, Grajales-Reyes JG, Vogt SK, Sundaram SS, Wilson KM, Ha JS, Xu R, Pan T, Kim TI, Huang Y, Montana MC, Golden JP, Bruchas MR, Gereau RWt, Rogers JA. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature biotechnology. 2015;33(12):1280–1286. doi: 10.1038/nbt.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pathak GP, Spiltoir JI, Hoglund C, Polstein LR, Heine-Koskinen S, Gersbach CA, Rossi J, Tucker CL. Bidirectional approaches for optogenetic regulation of gene expression in mammalian cells using Arabidopsis cryptochrome 2. Nucleic acids research. 2017;45(20):e167. doi: 10.1093/nar/gkx260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nature neuroscience. 2013;16(1):64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015;87(6):1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, Cohen SR, Wu CL. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesthesia and analgesia. 2006;102(1):248–257. doi: 10.1213/01.ANE.0000181289.09675.7D. [DOI] [PubMed] [Google Scholar]
- 93.Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, Gold MS. Gi-DREADD Expression in Peripheral Nerves Produces Ligand-Dependent Analgesia, as well as Ligand-Independent Functional Changes in Sensory Neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(42):10769–10781. doi: 10.1523/JNEUROSCI.3480-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samineni VK, Mickle AD, Yoon J, Grajales-Reyes JG, Pullen MY, Crawford KE, Noh KN, Gereau GB, Vogt SK, Lai HH, Rogers JA, Gereau RWt. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Scientific reports. 2017;7(1):15865. doi: 10.1038/s41598-017-16129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samineni VK, Yoon J, Crawford KE, Jeong YR, McKenzie KC, Shin G, Xie Z, Sundaram SS, Li Y, Yang MY, Kim J, Wu D, Xue Y, Feng X, Huang Y, Mickle AD, Banks A, Ha JS, Golden JP, Rogers JA, Gereau RWt. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain. 2017;158(11):2108–2116. doi: 10.1097/j.pain.0000000000000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schierling B, Noel AJ, Wende W, Hien le T, Volkov E, Kubareva E, Oretskaya T, Kokkinidis M, Rompp A, Spengler B, Pingoud A. Controlling the enzymatic activity of a restriction enzyme by light. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(4):1361–1366. doi: 10.1073/pnas.0909444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schindler SE, McCall JG, Yan P, Hyrc KL, Li M, Tucker CL, Lee JM, Bruchas MR, Diamond MI. Photo-activatable Cre recombinase regulates gene expression in vivo. Scientific reports. 2015;5:13627. doi: 10.1038/srep13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuster DJ, Belur LR, Riedl MS, Schnell SA, Podetz-Pedersen KM, Kitto KF, McIvor RS, Vulchanova L, Fairbanks CA. Supraspinal gene transfer by intrathecal adeno-associated virus serotype 5. Frontiers in neuroanatomy. 2014;8:66. doi: 10.3389/fnana.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS, Elde RP, Fairbanks CA, Vulchanova L. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Frontiers in neuroanatomy. 2014;8:42. doi: 10.3389/fnana.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin G, Gomez AM, Al-Hasani R, Jeong YR, Kim J, Xie Z, Banks A, Lee SM, Han SY, Yoo CJ, Lee JL, Lee SH, Kurniawan J, Tureb J, Guo Z, Yoon J, Park SI, Bang SY, Nam Y, Walicki MC, Samineni VK, Mickle AD, Lee K, Heo SY, McCall JG, Pan T, Wang L, Feng X, Kim TI, Kim JK, Li Y, Huang Y, Gereau RWt, Ha JS, Bruchas MR, Rogers JA. Flexible Near-Field Wireless Optoelectronics as Subdermal Implants for Broad Applications in Optogenetics. Neuron. 2017;93(3):509–521. e503. doi: 10.1016/j.neuron.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegel S, Noblett K, Mangel J, Griebling TL, Sutherland SE, Bird ET, Comiter C, Culkin D, Bennett J, Zylstra S, Berg KC, Kan F, Irwin CP. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulation with InterStim therapy compared to standard medical therapy at 6-months in subjects with mild symptoms of overactive bladder. Neurourology and urodynamics. 2015;34(3):224–230. doi: 10.1002/nau.22544. [DOI] [PubMed] [Google Scholar]
- 102.Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RWt, Bruchas MR. Spatiotemporal control of opioid signaling and behavior. Neuron. 2015;86(4):923–935. doi: 10.1016/j.neuron.2015.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spoida K, Masseck OA, Deneris ES, Herlitze S. Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(17):6479–6484. doi: 10.1073/pnas.1321576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stemkowski P, Garcia-Caballero A, Gadotti VM, M'Dahoma S, Huang S, Black SAG, Chen L, Souza IA, Zhang Z, Zamponi GW. TRPV1 Nociceptor Activity Initiates USP5/T-type Channel-Mediated Plasticity. Cell reports. 2016;17(11):2901–2912. doi: 10.1016/j.celrep.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 105.Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS, Morrison JH, Beutler AS. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tashima R, Koga K, Sekine M, Kanehisa K, Kohro Y, Tominaga K, Matsushita K, Tozaki-Saitoh H, Fukazawa Y, Inoue K, Yawo H, Furue H, Tsuda M. Optogenetic Activation of Non-Nociceptive Abeta Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats. eNeuro. 2018;5(1) doi: 10.1523/ENEURO.0450-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Theraputics R. CgI NCT02556736, editor. RST-001 Phase I/II Trial for Retinitis Pigmentosa. 2015;2016 ClinicalTrials.com: NIH.
- 108.Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Optogenetic control of targeted peripheral axons in freely moving animals. PloS one. 2013;8(8):e72691. doi: 10.1371/journal.pone.0072691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Molecular pain. 2009;5:52. doi: 10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valtcheva MV, Copits BA, Davidson S, Sheahan TD, Pullen MY, McCall JG, Dikranian K, Gereau RWt. Surgical extraction of human dorsal root ganglia from organ donors and preparation of primary sensory neuron cultures. Nature protocols. 2016;11(10):1877–1888. doi: 10.1038/nprot.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF, Wilcox GL, McIvor RS, Fairbanks CA. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Molecular pain. 2010;6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nature methods. 2012;9(3):266–269. doi: 10.1038/nmeth.1892. [DOI] [PubMed] [Google Scholar]
- 113.Ward PJ, Jones LN, Mulligan A, Goolsby W, Wilhelm JC, English AW. Optically-Induced Neuronal Activity Is Sufficient to Promote Functional Motor Axon Regeneration In Vivo. PloS one. 2016;11(5):e0154243. doi: 10.1371/journal.pone.0154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nature neuroscience. 2014;17(2):153–163. doi: 10.1038/nn.3602. [DOI] [PubMed] [Google Scholar]
- 115.Wietek J, Broser M, Krause BS, Hegemann P. Identification of a Natural Green Light Absorbing Chloride Conducting Channelrhodopsin from Proteomonas sulcata. The Journal of biological chemistry. 2016;291(8):4121–4127. doi: 10.1074/jbc.M115.699637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolfe D, Mata M, Fink DJ. A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene therapy. 2009;16(4):455–460. doi: 10.1038/gt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolter T. Spinal cord stimulation for neuropathic pain: current perspectives. Journal of pain research. 2014;7:651–663. doi: 10.2147/JPR.S37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yokoyama H, Oguchi T, Goins WF, Goss JR, Nishizawa O, de Groat WC, Wolfe D, Krisky DM, Glorioso JC, Yoshimura N. Effects of herpes simplex virus vector-mediated enkephalin gene therapy on bladder overactivity and nociception. Human gene therapy. 2013;24(2):170–180. doi: 10.1089/hum.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu H, Fischer G, Ferhatovic L, Fan F, Light AR, Weihrauch D, Sapunar D, Nakai H, Park F, Hogan QH. Intraganglionic AAV6 results in efficient and long-term gene transfer to peripheral sensory nervous system in adult rats. PloS one. 2013;8(4):e61266. doi: 10.1371/journal.pone.0061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. The Journal of physiology. 2013;591(4):1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature methods. 2011;8(9):745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]