Highlights
-
•
Citric acid as a food additive is not natural citric acid; it is manufactured through fermentation using Aspergillus niger.
-
•
Aspergillus niger is a potent allergen.
-
•
Food additive manufactured citric acid may be causing allergic inflammatory cascades.
-
•
Manufactured citric acid may be contributing to the inflammation seen in asthma, juvenile idiopathic arthritis, autistic spectrum disorder, and fibromyalgia.
-
•
The safety of manufactured citric acid has never been studied since it was granted GRAS status.
Abstract
Citric acid naturally exists in fruits and vegetables. However, it is not the naturally occurring citric acid, but the manufactured citric acid (MCA) that is used extensively as a food and beverage additive. Approximately 99% of the world’s production of MCA is carried out using the fungus Aspergillus niger since 1919. Aspergilus niger is a known allergen. The FDA placed MCA under the category of GRAS without any research to substantiate this claim. In 2016, 2.3 million tons of MCA were produced, predominantly in China, and approximately 70% is used as a food or beverage additive. There have been no scientific studies performed to evaluate the safety of MCA when ingested in substantial amounts and with chronic exposure. We present four case reports of patients with a history of significant and repetitive inflammatory reactions including respiratory symptoms, joint pain, irritable bowel symptoms, muscular pain and enervation following ingestion of foods, beverages or vitamins containing MCA. We believe that ingestion of the MCA may lead to a harmful inflammatory cascade which manifests differently in different individuals based on their genetic predisposition and susceptibility, and that the use of MCA as an additive in consumable products warrants further studies to document its safety.
1. Introduction
Citric acid is a weak organic mono-constituent substance with the molecular formula C6H8O7 and REACH designated IUPAC name 2-hydroxypropane1,2,3-tricarboxylic acid (Fig. 1). Citric acid is listed as an ingredient in a significant percentage of prepared foods, beverages, and medications. The average consumer is under the impression that the added citric acid listed in the ingredients of prepared foods, beverages and vitamins is derived from natural sources such as lemons and limes. However, the ingredient list is quite misleading since the added citric acid is not procured through natural sources. More accurate terminology would list this substance as manufactured citric acid.
Fig. 1.
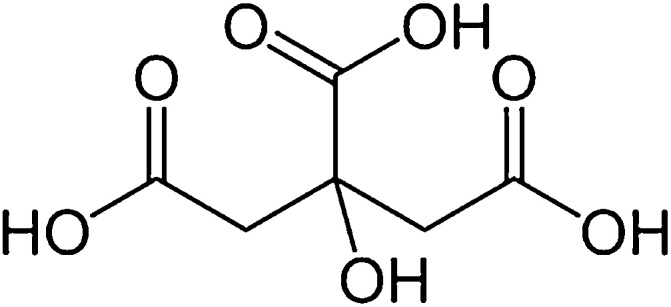
REACH registration dossier 15,451 EC Number: 201-069-1 CAS Number: 77-92-9, 5949-29-1 IUPAC Name: 2-hydroxypropane-1,2,3-tricarboxylic acid Molecular Formula: C6H8O7.
https://echa.europa.eu/registration-dossier/-/registered-dossier/15,451.
Manufactured citric acid (MCA) is a ubiquitous substance and one of the most common food additives in the world. Approximately 99% of the world production of MCA is through microbial processes using predominantly a mutant strain of the black mold Aspergillus niger [1]. This method has been the industry standard for production of MCA since 1919, long before the FDA’s involvement in evaluating food additives. When the FDA adopted the Food Additives Amendment in 1958, Congress excluded from the definition of Food Additive the common food ingredients in use before 1958, including MCA. Although the FDA has studied many food additives to ensure that they are within acceptable safety parameters, certain additives were granted GRAS (generally recognized as safe) status by the FDA due to lack of demonstrated harm over a history of prior use [2,3]. Thus, MCA was considered GRAS and did not undergo any FDA evaluation. MCA is one of the most common additives used today, with applications ranging from food to non-food industries. It is estimated that 70% is used in foods and beverages, 20% in the pharmaceutical and cosmetic industry, and 10% in cleaning detergents and softening agents [1]. In foods and beverages, it is used as a flavoring, a preservative, an acidulant, and to provide pH control. The growth of the processed foods industry, pharmaceuticals, and cosmetics is currently the driving force behind the rapid growth of the citric acid market globally.
Historically, citric acid was first isolated by William Scheele in England in 1784 from lemon juice imported from Italy [2]. Subsequently, Italy controlled the industrial production of citric acid from lemon juice and commanded a high price for the next 100 years, with peak production in 1915–1916 at 17,500 tons, after which it started to decline due to cost.2 This led to attempts all over the world to find alternatives to its production with chemical and microbial techniques, including commercial production by sugar fermentation [2]. Citric acid was first manufactured using the fermentation process in 1919 in Belgium using Cytromices mold (now known as Penicillium), but this method was abandoned due to contamination and duration of fermentation [2]. In 1917, American food chemist James Currie had begun experimenting with a process of making citric acid from mold. Currie discovered that strains of Aspergillus niger provided high yields of citric acid through a fermentation process using low cost molasses as the raw material [4]. This system was very cost effective and rapidly adopted. Pfizer started to produce citric acid from Aspergillus niger in 1919, and this method is still used today across the world, particularly in China.
The molecular formula of the natural citric acid obtained from lemons and limes and that of MCA is the same, C6H8O7. However, the potential presence of impurities or fragments from the Aspergillus niger in MCA is a significant difference that may trigger deleterious effects when ingested. We have done several literature searches and have been unable to find any research evaluating the safety of long term or repetitive exposure to MCA, which has become ubiquitous in processed and pre-prepared foods, carbonated beverages, energy drinks, fruit drinks, nutritional supplements, pediatric and adult vitamins, confectioneries, processed dairy, common snacks, pharmaceuticals, cosmetics, detergents and cleansers. In certain common energy beverages, it is the second leading ingredient following water. We provide evidence with four case reports that ingestion of foods, beverages or supplements containing MCA may lead to increased inflammation, which in susceptible individuals affects the respiratory, gastrointestinal, neurological and musculoskeletal systems. Although MCA is an unnatural substance and is produced from Aspergillus niger, there has been a paucity of research to ascertain its safety with repetitive exposure over time. To our knowledge, this is the first scientific report revealing the potential inflammatory reactions related to ingestion of MCA.
2. Clinical findings
We have four case reports of individuals who demonstrate symptoms including: joint pain with swelling and stiffness, muscular pain, dyspnea, abdominal cramping and enervation that typically begin within 2–12 h of ingesting foods, beverages or vitamins containing MCA. Depending on the degree of symptoms, they resolve over a period of 8–72 h following ingestion. None of the four individuals in these case reports develop such symptoms when ingesting natural forms of citric acid such as in lemons and limes.
Table 1 lists 12 of the common foods and beverages that when consumed by these four individuals would consistently elicit the reported symptoms. It also lists the major ingredients found in these items, and the only ingredient found in all 12 items is MCA. The second most common ingredient is sugar, and none of the four subjects had any sensitivity to sugar. With the exception of the last item, all of the foods and beverages listed were consumed by all of the individuals described in the first three cases. Since manufacturers are not required to specify the exact amounts of listed ingredients, we cannot provide quantitative information regarding the amount of MCA ingested. However, in the United States product ingredients are listed in order of decreasing amounts. Although we cannot measure the exact amounts of MCA the subjects were exposed to, we know that the dose is higher in a diet soda drink where MCA is listed as the third leading ingredient after water and orange juice, and lower in ranch flavored potato chips where MCA is listed as the 18th of 25 ingredients. Subjects in the first three cases reacted to both the lower and higher doses of MCA. In the forth case, the exposure was only to an effervescent vitamin, which was a consistent dose. None of the subjects in any of the four cases initially knew that they were being exposed to MCA, nor that it might be problematic. It was later identified after their symptoms occurred and the foods and/or beverages consumed were examined. The exposures which resulted in the subjects’ responses were typically limited to one food product or beverage per episode.
Table 1.
Identified Foods and Beverages.
| Foods/Beverages | MCA | Sugar | Salt | Sodium Bicarbonate | Phenylalanine | Caffeine | Whey | Artificial Sweetener | Artificial Color |
|---|---|---|---|---|---|---|---|---|---|
| Diet Soda Drink | X | X | X | X | X | ||||
| Energy Drink (Diet) | X | X | X | X | X | X | |||
| Energy Drink (Regular) | X | X | X | X | X | ||||
| Energy Snack Bar | X | X | X | ||||||
| Grape Leaves (preserved) | X | ||||||||
| Hummus (pre-prepared) | X | X | |||||||
| Instant Oatmeal | X | X | X | X | X | ||||
| Jelly Beans | X | X | X | ||||||
| Potato Chips (Ranch Flavor) | X | X | X | X | |||||
| Tonic Water | X | X | |||||||
| Tortilla Chip | X | X | |||||||
| Vitamin C (Effervescent) | X | X | X |
3. Case report 1
Case 1 is a 52 year-old Caucasian woman with a history of post-surgical hypothyroidism well-controlled with Synthroid and Cytomel, who is otherwise healthy. She reports developing severe diffuse joint and muscle pain in the upper and lower extremities with associated joint swelling, abdominal bloating with cramping and feeling enervated within 6–12 h of ingesting foods that contain MCA. Her symptoms began in her late 30’s. Unaware of the etiology of her symptoms, she sought consultations with rheumatologists, immunologists and allergists, none of whom found an explanation. Over a five year period, she underwent extensive work-ups for auto-immune disease, rheumatoid arthritis, vitamin deficiencies, as well as adrenal and thyroid imbalance, all of which were negative. Due to the debilitating nature of her symptoms, she attempted to eliminate certain foods from her diet such as gluten, dairy, and yeast. However, her symptoms were minimally altered. After years of trial and error, she noted that her symptoms followed ingesting certain pre-prepared foods, the commonality being presence of citric acid in the listed ingredients.
By age 47, she began avoiding all foods with MCA and noted a remarkable absence of her symptoms. Subsequently, when she would feel the symptoms reported above after consuming pre-prepared foods or beverages, she would check the listed ingredients and always find that at least one of the foods consumed within the previous 12 h contained MCA. The extent of her joint pain, abdominal discomfort and enervation was directly correlated with the amount of MCA ingested at a given time. If she consumed a meal in which a food item contained MCA and consumed a drink in which MCA was one of the leading ingredients, her symptoms were worse and lasted longer than if she consumed a single food item in which MCA was listed as a more minor ingredient. Even pre-prepared organic foods that were free of all additives except MCA would elicit her symptoms.
4. Case report 2
Case 2 is a 68 year-old very healthy Caucasian male with allergic asthma previously treated with Prednisone, and adult onset Addison’s Disease due to prolonged Prednisone exposure. He reports developing a triad of symptoms including dyspnea, significant enervation, and stiffness with edema of his prosthetic knee within 12 h of ingesting pre-prepared foods or beverages with citric acid listed in the ingredients. His symptoms resolve over a 36-48-hour period. At 68 years of age, he has a very active life style including a demanding career, significant travel and a healthy exercise routine. At 36 years of age, he developed ABPA (Allergic Bronchopulmonary Aspergillosis), which resolved leaving only a small area of fibrosis in his right upper pulmonary lobe. This is typically of no consequence, even with participation in heavy sports. He underwent a successful total left knee replacement at 67 years of age.
His symptoms related to ingesting foods or beverages containing MCA involve his relatively compromised systems including his lungs and his healing prosthetic knee, in addition to an overall sense of enervation. He describes that his enervation is not sleepiness, but enervation similar to that reported by persons with chronic fatigue syndrome. He eats an organic vegan diet and eliminates foods with preservatives in effort to improve his overall health. He notes that inadvertently ingesting pre-prepared foods with any amount of MCA results in recurrence of his symptoms, the severity of which is correlated with the amount of consumed foods or beverages containing MCA. During the week when he initially discovered the MCA correlation with his symptom, he had been consuming two very common energy beverages. He recalls experiencing greater swelling around his prosthetic knee and feeling quite depleted, not more energized. The more he consumed of the energy drinks, the worse he felt. Upon checking the labels, he found that the two energy beverages listed MCA as the second leading ingredient after water. Similar to the patient in Case 1, he notes that ingesting pre-prepared organic foods that are free of all additives except MCA also elicits his triad of symptoms.
5. Case report 3
Case 3 is a 75 year-old Caucasian woman with a history of atrial fibrillation, hypothyroidism, and Restless Leg Syndrome (RLS). She is on Digoxin and Xeralto for the atrial fibrillation, and is euthyroid on Synthroid. Her RLS is well-controlled on Mirapex. At 73 years of age, she reported a long-standing history of severe diffuse upper and lower extremity joint and muscle pain with associated swelling. Similar to the patient in Case 1, she underwent an extensive work-up for auto-immune disease, rheumatoid arthritis, vitamin deficiencies, and serum metal levels, all of which were negative. Due to the severity of her symptoms and lack of effective medical intervention, she began a long process of food elimination. This consisted of continuing a healthy vegetarian diet but eliminating all processed and pre-prepared foods. While preparing her foods from fresh organic ingredients, her symptoms improved to an almost negligible level.
Over time, as she began incorporating very few pre-prepared foods back into her diet, she began developing her symptoms of diffuse severe joint and muscle pain and swelling. The pre-prepared foods in her diet consisted of freshly prepared organic items with a minimal number of additives. Through a process of slowly eliminating various pre-prepared foods, she was able to identify the offending foods as those with citric acid listed in the ingredients. Her symptoms would begin within 6 h of exposure and resolve within 72 h of exposure. Also similar to Case 1, when she would feel the symptoms reported above after consuming pre-prepared foods or beverages, she would check the listed ingredients and always find that at least one of the foods consumed within the previous 6–12 h contained MCA. Similar to the patients in Case 1 and in Case 2, the severity of her symptoms after ingesting foods containing MCA directly correlated with the amount consumed.
6. Case report 4
Case 4 is a 43 year-old Indian woman without any past medical history, except for undergoing in-vitro fertilization at 39 years of age resulting in a successful full-term pregnancy. She does not take any medications and has no allergies. She is a very health-conscious vegetarian, consuming only a raw diet and prepares all of her food from fresh ingredients at home. From 41 through 42 years of age, she began ingesting an effervescent form of Vitamin C (ascorbic acid) tablets on a regular (not daily) basis. She developed severe enervation and mental fatigue during that two year period. Her medical work-up for these symptoms was negative. Her symptoms limited her ability to perform her daily professional tasks as a physician. Unlike the other three cases, her symptoms would develop within two hours after drinking the effervescent Vitamin C, and resolve within 8–12 h. Since she did not consume any other medications or supplements, she was able to determine that her symptoms were limited to the days when she consumed the effervescent Vitamin C, and decided to discontinue the supplement. Her symptoms of enervation and mental fatigue resolved shortly after the discontinuation. Weeks later, she elected to try a different brand of Vitamin C pills. The only difference between the two formulations was the absence of MCA in the pill form as compared to the effervescent form. She did not experience any symptoms of mental fatigue or enervation with the new form of Vitamin C pills that did not contain MCA. She could not provide any information regarding MCA in foods since she consumed a raw diet and did not consume pre-prepared foods that would potentially contain additives.
7. Discussion
Aspergillus was first described and named in 1729 by the Italian priest and biologist, Pier Antonio Micheli. He studied the fungus under the microscope and noted that it had the shape of an aspergillum (holy water sprinkler) and named the genus accordingly.5 Aspergillus niger is an asexual saprophytic fungus thatis very thermotolerant and can thrive in freezing conditions and very hot weather [5]. It produces its spores on an asexual structure called the conidium. The Aspergillus genus includes several hundred fungal species [6], of which 16 are known to be harmful to humans, causing allergies, infections and diseases. The three most common species known to affect humans are the A. fumigatus, A. flavus, and A. niger [7]. Because aspergillosis is not a reportable infection in the United States, the exact number of cases is difficult to determine. Milder, allergic forms of aspergillosis are thought to be more common than the invasive form of the infection [7]. Furthermore, the incubation period for aspergillosis is unclear and seemingly varies depending on the load of Aspergillus and the individual’s immune response [7].
Aspergillus niger is one of the most abundant species of Aspergillus in nature as it can grow on a large variety of substances, even in environments with very little nutrients available [7], Although Aspergillus niger is not as deadly as other Aspergillus species, it can cause sickness and allergic reactions. Aspergillus niger is considered very harmful to those with a weak immune system or those who have a sensitive allergy to fungi. Most people can handle the inhalation of a moderate amount of Aspergillus niger spores. Those who suffer from leukemia, AIDS, immunosuppression (transplant patients), severe fungal allergies and other immune deficiencies could become very sick from the intake of the spores [7]. Allergic reactions can be severe in individuals who are very allergic to fungi.
When inhaled, A. niger can cause hypersensitivity reactions such as asthma and allergic alveolitis. It has been a significant cause of asthma in certain parts of the world such as Iran [8]. It has also been the cause of otomycosis [9], cutaneous infections [10], pneumonia [11], and invasive pulmonary aspergillosis [12]. The ability of A. niger to elicit an inflammatory response is not limited to the live spores. A. niger that is killed at 68 °C for 4 h to ensure destruction of the spores has been shown to elicit an immune response with measurable quantities of the pro-inflammatory cytokine IL-6 [13]. Therefore, remnant fragments of A. niger that is killed could still elicit inflammatory responses.
Aspergillus niger contains several toxins, some harmless and others harmful to certain people. The main toxins it contains are ochratoxin A (OTA) and malformin C. OTA is a known carcinogenic mycotoxin with nephrotoxic and immunotoxic potential in animals [14]. Individuals are at risk of exposure to OTA if they ingest food contaminated with it such as wine, beer, coffee, dried vine fruit, pork, poultry, dairy, spices, and cacao [14]. Toxicity from OTA is considered serious enough that it is among the 20 mycotoxins monitored in food [15,16]. Animals exposed to OTA develop DNA adducts, which are covalent modifications of the DNA. This is believed to interfere with the DNA repair system and cell cycle control and may serve as the initiating point of carcinogenesis [14].
Despite its contribution to pathogenesis, A. niger is widely used in the food industry for the production of citric acid and gluconic acid, and many enzymes such as a-amylase, amyloglucosidase, cellulases, lactase, invertase, pectinases, and acid proteases [5]. Over the past several decades, there have been significant genetic modifications of A. niger to increase MCA production and decrease production of unwanted byproducts resulting in genetically modified mutant variants of this mold. The two main types of modification include gamma radiation-induced mutagenesis of A. niger to increase its fermentation activity and genetic modification in the laboratory to enhance the pathway to increase production of MCA and decrease in other non-MCA producing pathways [17]. Nearly all MCA begins with highly processed glucose from corn syrup derived from corn, and less so from beet sugar, cane molasses, and fruit waste [18].
Although Brazil and India produce MCA, China is the largest single participant in MCA production. China accounted for 59% of the world’s production and 74% of the world’s exports of MCA in 2015 [19]. By 2015, Asia was the largest consumer of MCA, accounting for 28% of world consumption, followed closely by North America accounting at 23% and Western Europe accounting at 22% of world consumption, while China accounted for 12% of the world consumption. Due to new biotechnological production units mostly located in China, the global supply of MCA in the last two decades rose to 2.3 million tons in 2016 becoming the single largest chemical obtained via biomass fermentation and the most widely used organic acid [20]. It is expected that China will not only remain the largest producer of MCA during 2015–2020, but that Chinese manufacturers will expand to establish manufacturing plants in other countries to secure more of the MCA global market [19]. The global citric acid market production has been growing at a rate of 3.5% during 2009–2016, and is expected to be at 2.7 million tons by 2022 [21]. According to Credence Research, the global MCA market is projected to reach USD 3.66 Billion by 2022 [22].
Given the thermotolerance of A. niger, there is great potential that byproducts of A. niger remain in the final MCA product. Furthermore, given the pro-inflammatory nature of A. niger even when heat-killed [13], repetitive ingestion of MCA may trigger sensitivity or allergic reactions in susceptible individuals. Over the last two decades, there has been a significant rise in the incidence of food allergies. Among children aged 0–17 years, the prevalence of food allergies increased from 3.4% in 1997–1999 to 5.1% in 2009–2011 [23,24]. The significant rise in the global use of MCA in foods and the rise in the incidence of food allergies pose concern over a possible potential relationship. Given the increase in the use of MCA and the increase in the incidence of allergies, it is conceivable that MCA production contaminants are eliciting a low grade inflammatory response which results in chronic low grade allergies. Recent studies have revealed that the incidences of allergic and autoimmune diseases have been increasing in parallel, making them a serious health-care burden [25]. Food allergies and sensitivities have been documented to occur with greater frequency in conditions such as asthma, Autism Spectrum Disorder (ASD), Juvenile Idiopathic Arthritis (JIA) and Fibromyalgia (FM) [26,27]. Numerous research studies have identified elevation in certain pro-inflammatory cytokines such as IL-1B, IL-6, IL-8, and TNF-a as a significant and common thread of the inflammatory process in asthma [[28], [29], [30], [31]], ASD [[32], [33], [34], [35]], JIA [[36], [37], [38]], and FM [[39], [40], [41]].
8. Conclusion
We recognize the limitations of the level of evidence from our four case reports. We cannot conclusively affirm that MCA is the causative factor in the subjects’ inflammatory symptoms. However, our findings demonstrate a significant likelihood that MCA may be the culprit and are suggestive of valid concerns which warrant proper double blind studies to determine presence or absence of harm.
We hypothesize that since MCA is a product of Aspergillus niger, there are contaminants from the production process that remain in the final product. We hypothesize that there are proteins or other by-products of the A. niger or substances from the manufacturing process which remain in MCA after its production process and these lead to an inflammatory process, and possibly unlike natural citric acid, MCA is highly inflammatory itself. We further hypothesize that when we consume foods with MCA, we are consuming the proteins or by-products of the A. niger or the highly concentrated unnatural form of citric acid, and with repeat exposure over time we are either developing elevation in pro-inflammatory cytokines such as IL-6 or building antibodies against the A. niger proteins that lead to inflammatory symptoms, or the MCA itself may contain yet unidentified substances or by- products from the production process that are inflammatory to our body.
Given the ubiquitous presence of MCA and repetitive exposure to it through ingesting common foods and beverages, we may be re-introducing small amounts of A. niger proteins or byproducts into our bodies, and repeatedly eliciting an insidious low grade immune response. With the repetitive exposure and insult, the immune system maintains a low grade inflammatory response. Over time, the chronic inflammatory state can impact various systems in the body depending on the individual’s weaker or compromised organ system. Ingestion of the MCA leads to an inflammatory cascade which manifests differently in different individuals based on their genetic predisposition, susceptibility and underling medical history, as well as the degree of stress exerted by environmental factors. We further hypothesize that these inflammatory reactions may play a causative role in allergic asthma, FM, JIA, and possibly CFS, and lead to increased inflammation in the musculoskeletal system leading to idiopathic joint and muscle inflammation/pain and inflammation in the gastro-intestinal system leading to conditions such as irritable bowel syndrome.
Unlike naturally occurring citric acid, manufactured citric acid is ubiquitous in the average diet of both adults and children. With the expected continued increase in its production to meet the demand of an expanding global market, it is imperative to ascertain its safety. Due to its GRAS status, manufactured citric acid has escaped proper scrutiny for nearly a century. Since it is not a natural substance but created using Aspergillus niger, a black mold proven to cause allergic reactions and disease in humans, it is difficult to understand how it has been protected under GRAS classification and has not been empirically studied. It only seems prudent that a thorough investigation of the manufactured form of citric acid be undertaken. With an unexplained increase in inflammatory diseases, it is difficult to justify its ubiquitous use without proper investigation. Additional research is mandatory to evaluate the potential of MCA to cause inflammatory symptoms in the body, or to contain Aspergillus proteins or by-products from the manufacturing process which may be inflammatory with repetitive exposure. We conclude that there is enough anecdotal data to support the need for thorough evaluation of the safety and risks associated with the ubiquitous use of the currently manufactured citric acid in our foods, beverages and other ingested substances, and to ensure that the final product is highly purified, non-inflammatory and void of pro-inflammatory contaminants.
Conflict of interest
No competing interests nor conflict of interest
Contributor Information
Iliana E. Sweis, Email: i_sweis@yahoo.com.
Bryan C. Cressey, Email: bccressey@icloud.com.
References
- 1.Max B., Salgado J.M., Rodriguez N. Biotechnological production of citric acid. Braz. J. Microbiol. 2010;4(October-Decembr (4)):862–875. doi: 10.1590/S1517-83822010000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel T., Pandya H. Citric acid production fermentation process. IJARIIE. 2017;3(2):3983–3991. [Google Scholar]
- 3.Vandenberghe L.P.S., Soccol C.R., Pandey A. Microbial production of citric acid. Braz. Arch. Biol. Technol. 1999;42(3) (Online version ISSN 1678-4324) [Google Scholar]
- 4.Currie J.N. The citric acid fermentation of A. niger. J. Biol. Chem. 1917;31(5) [Google Scholar]
- 5.Bennett J.W. Aspergillus: Molecular Biology and Genomics. Caister Academic Press; 2010. An overview of the genus aspergillus (PDF) ISBN 978-1-904455-53-0. [Google Scholar]
- 6.Geiser D. Sexual structures in Aspergillus: morphology, importance and genomics. Med. Mycol. 2009;47(Suppl. 1):S21–S26. doi: 10.1080/13693780802139859. (s1) [DOI] [PubMed] [Google Scholar]
- 7.https://www.cdc.gov/fungal/diseases/aspergillosis/causes.html.
- 8.Zanjani L.S. 2012. Sensibilisation of Asthmatic Patients to Extract Antigens From Strains of Aspergillus fumigatus, Aspergillus flavus and Asperillus Niger.http://europepmc.org/abstract/med/23177815 Accessed 11 November 2017. [DOI] [PubMed] [Google Scholar]
- 9.Araiza J., Canseco P., Bonifaz A. Otomycosis: clinical and mycological study of 97 cases. Rev. Laryngol. Otol. Rhinol. (Bord.) 2006;127:251–254. [PubMed] [Google Scholar]
- 10.Loudon K.W., Coke A.P., Burnie J.P. Kitchens as a source of Aspergillus niger infection. J. Hosp. Infect. 1996;32:191–198. doi: 10.1016/s0195-6701(96)90145-0. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa Y., Shimazu K., Ebihara M. Aspergillus niger pneumonia with fatal pulmonary oxalosis. J. Infect. Chemother. 1999;5:97–100. doi: 10.1007/s101569900005. [DOI] [PubMed] [Google Scholar]
- 12.Person A.K., Chudgar S.M., Norton B.L. Aspergillus niger: an unusual cause of invasive pulmonary aspergillosis. J. Med. Microbiol. 2010;59:834–838. doi: 10.1099/jmm.0.018309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chacko S. Comparison of pro-inflammatory cytokines (IL-6,IL-1α and IL-1β) released by MPI and MARCO (-/-) knockout cells when stimulated by heat killed fungi- Candida albicans and Aspergillus niger. Plymouth Stud. Sci. 2016;9(1):4–23. [Google Scholar]
- 14.Hope J.H., Hope B.E. A review of the diagnosis and treatment of ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J. Environ. Public Health. 2012;2012 doi: 10.1155/2012/835059. 10 pages, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark H.A., Snedeker S.M. Ochratoxin A: its cancer risk and potential for exposure. J. Toxicol. Environ. Health B Crit. Rev. 2006;9(3):265–296. doi: 10.1080/15287390500195570. [DOI] [PubMed] [Google Scholar]
- 16.Varga J., Kevel E., Rinyu E. Ochratoxin production by Aspergillus species. Appl. Environ. Microbiol. 1996:4461–4464. doi: 10.1128/aem.62.12.4461-4464.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awan M.S., Tabbasam N., Ayub N. Gamma radiation induced mutagenesis in Aspergillus niger to enhance its microbial fermentation activity for industrial enzyme production. Mol. Biol. Rep. 2011;38:1367–1374. doi: 10.1007/s11033-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon G.S., Brar S.K., Verma M. Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger. Biochem. Eng. J. 2011;54:83–92. [Google Scholar]
- 19.https://www.ihs.com/products/citric-acid-chemical-economics-handbook.html.
- 20.Ciriminna Rosaria. Citric acid: emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017;11:22. doi: 10.1186/s13065-017-0251-y. http://www.imarcgroup.com/global-citric-acid-market PMC. Web. 19 Oct. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IMARC Group . 2017. Citric Acid Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast. 2017-2022. Accessed online 11/05/2017. [Google Scholar]
- 22.http://www.credenceresearch.com/report/citric-acid-market. Accessed online 5 November 2017.
- 23.Jackson K.D. 2013. Trends in Allergic Conditions Among Children: United States. 1997–2011. NCHS Data Brief; No. 121, May. [PubMed] [Google Scholar]
- 24.Liu A.H., Jaramillo R., Sicherer S.H. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2010;126(4):798–806. doi: 10.1016/j.jaci.2010.07.026. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson C.R., Anderson W.J., Helms P.J. Coincidence of immune-mediated diseases driven by Th1 and Th2 subsets suggests a common aetiology. A population-based study using computerized general practice data. Clin. Exp. Allergy. 2002;32(1):37–42. doi: 10.1046/j.0022-0477.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 26.Lyall k Asthma and allergies in children with autism Spectrum disorders: results from the CHARGE study. Autism Res. 2015;8:567–574. doi: 10.1002/aur.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puccio F., Rojas R., Mosquera I. Food allergy is an important diseases associated to fibromyalgia. Clin. Transl. Allergy. 2013;3(Suppl. 3) P120. [Google Scholar]
- 28.Barnes P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Invest. 2008;118(11):3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalinina E., Denisenko Y., Vitkina T. The mechanisms of the regulation of immune response in patients with comorbidity of chronic obstructive pulmonary disease and asthma. Can. Respir. J. 2016;2016 doi: 10.1155/2016/4503267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alwakil I.M., Al Kabeer A.M., Kabil T.H. Serum Interleukin-6 and Interleukin-8 in bronchial asthma. AAMJ. 2011;9(Suppl. 1) N. 3, September. [Google Scholar]
- 31.Shannon J., Ernst P., Alwakil I. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest J. 2008;133(February (2)):420–426. doi: 10.1378/chest.07-1881. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K., Matsuzaki H., Iwata K. Plasma cytokine profiles in subjects with high-functioning autism Spectrum disorders. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krakowiak P., Goines P., Tancredi D. Neonatal cytokine profile associated with autism Spectrum disorder. Biol. Psychiatry. 2017;81(5):442–451. doi: 10.1016/j.biopsych.2015.08.007. 1 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricci S., Businaro R., Ippoliti F. Altered cytokine and BDNF levels in autism spectrum disorder. Neurotox. Res. 2013;24:491–501. doi: 10.1007/s12640-013-9393-4. [DOI] [PubMed] [Google Scholar]
- 35.Chez M., Dowling T., Patel P. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 2007;36(6):361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Put K., Avau A., Brisse K. Cytokines in systemic juvenile idiopathic and haemophagocytic lymphohistiocytosis. Rheumatology. 2015;54(8):1507–1517. doi: 10.1093/rheumatology/keu524. 1 August. [DOI] [PubMed] [Google Scholar]
- 37.Pascual V., Allantaz F., Arce E. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005;201(9):1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prahalad S., Martins T., Tebo A. Elevated serum levels of soluble CD154 in children with idiopathic juvenile arthritis. Pediatr. Rheumatol. 2008;6(8) doi: 10.1186/1546-0096-6-8. BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross R., Jones K., Bennett R. Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol. J. 2010;3:9–18. doi: 10.2174/1874226201003010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazzichi L., Rossi A., Massimetti G. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin. Exp. Rheumatol. 2007;25(March-April (2)):225–230. [PubMed] [Google Scholar]
- 41.Salemi S., Rethage J., Wollina U. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J. Reumatol. 2003;30(January (1)):146–150. [PubMed] [Google Scholar]


