Abstract
BRAF inhibitors were among the first systemic therapies to show clinical benefit in metastatic melanoma. Here, we review the spectrum of BRAF mutations in melanoma, their role in oncogenesis, clinicopathological associations and response to treatment. The differing biology and clinical features of V600E- and V600K-mutated melanoma are outlined. The molecular changes associated with BRAF fusion genes and their response to targeted therapies, as well as the role of immunotherapy in treatment sequencing with targeted therapies are discussed.
KEYWORDS : BRAF, BRAF fusion genes, dabrafenib, melanoma, mutation, trametinib, V600E, V600K, vemurafenib
Practice points.
BRAF mutations are found in just under half of patients with metastatic melanoma.
The incidence of BRAF mutations decreases with age. Almost all patients <30 years with cutaneous melanoma have BRAF-mutant melanoma.
The V600E mutation occurs in between 70–90% of patients with BRAF-mutant melanoma. The V600K is the second most common (10–30%), occurring more frequently in older patients and those with chronic sun-damaged skin.
The disease-free interval between primary and metastatic disease is shorter in patients with V600K compared with V600E melanoma; however, the evidence on overall survival in established metastatic disease is conflicting.
Combination therapy with BRAF and MEK inhibitors improves response, survival and cutaneous toxicity compared with BRAF inhibitor monotherapy, and are now standard of care.
Immunotherapy with anti-PD-1 antibodies is effective regardless of BRAF mutation status, and data suggest response rates are lower with ipilimumab when used after BRAF inhibitor failure, suggesting upfront immunotherapy is the best approach at present.
BRAF fusion genes result from translocations involving intact BRAF kinase domains. These cause MAPK pathway activation in vitro and respond to MEK inhibition in vivo, showing promise as a novel molecular target in the 30% of patients without other identifiable driver mutations.
Future gains in therapeutic benefit will result from combining targeted therapy with immunotherapy as well as optimizing the sequencing of targeted therapy and immunotherapy.
The MAPK & BRAF mutations in melanoma
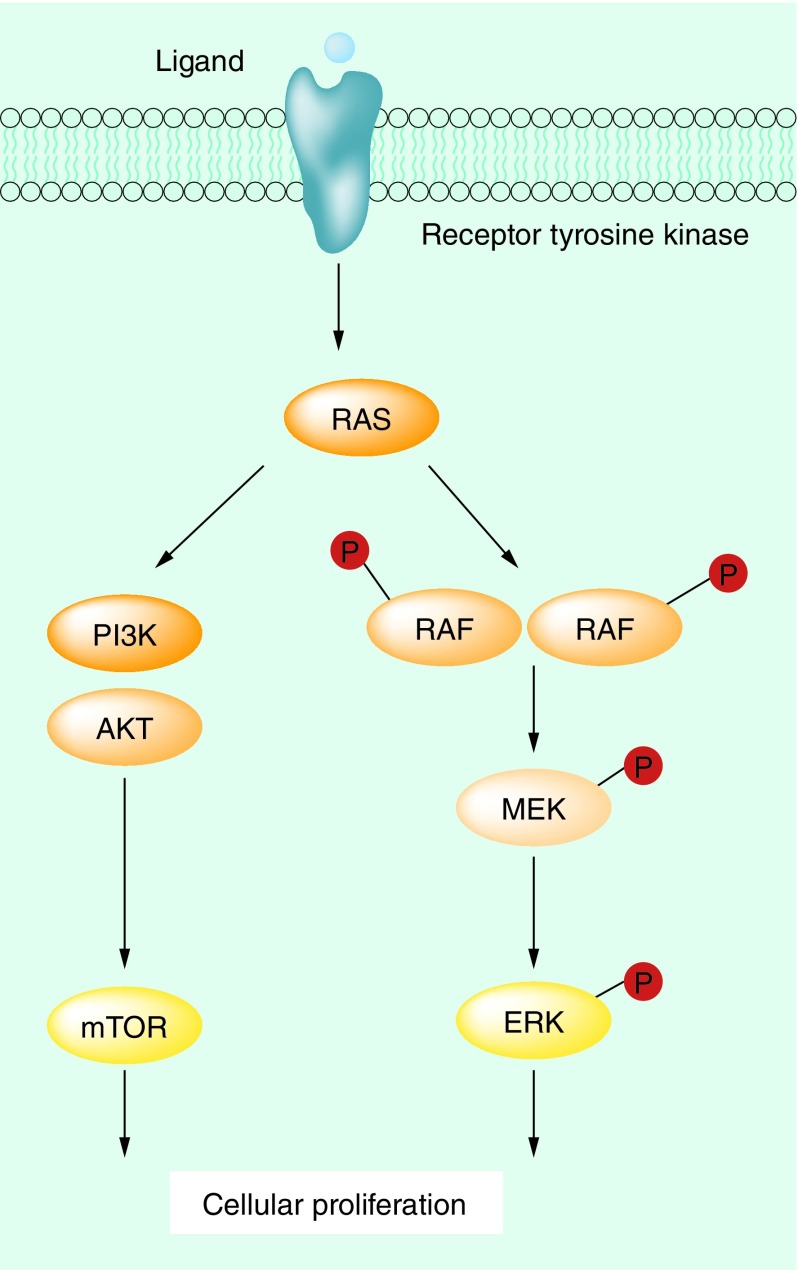
The MAPK pathway is one of the mechanisms by which basic cellular processes such as growth, proliferation and apoptosis are controlled (Figure 1) [1]. In normal cells, this pathway acts via extracellular signal-regulated kinase cascades, which ultimately control both cytoplasmic and nuclear targets involved in cellular proliferation in a highly regulated fashion, with feedback regulation by downstream elements. RAF is an upstream element of the MAPK pathway, sitting below RAS, and exists as three isoforms, ARAF, BRAF and CRAF. These cytoplasmic protein kinases are regulated by their interaction with RAS [2]. Membrane-anchored RAS GTPases lead to homo- and heterodimerization and activation of the RAF family proteins, which subsequently activates MEK by phosphorylation. The PI3K pathway, which is another key cellular growth regulation pathway, is activated by RAS–GTP, representing a cross-talk mechanism between these two key pathways of cellular growth [3].
Figure 1. . The MAPK pathway.
The MAPK pathway is overactive in the majority of melanomas, most often as a result of mutations in BRAF, NRAS and NF1 (a negative regulator of RAS) [4–7]. The most prevalent driver in melanoma is mutant BRAF, found in 40–50% of patients with metastatic disease. BRAF mutations occur in other cancers such as colon cancer, papillary thyroid cancer and serous ovarian cancer, but at a frequency much lower than in melanoma [4,8–9].
In melanoma, most BRAF mutations occur in exon 15. Over 70–90% of mutations involve a missense mutation at position 600 (T1796A), resulting in a substitution from valine to glutamic acid at amino acid 600 (termed V600E), creating a constitutively active protein that binds MEK [10]. V600K mutations are the second most common BRAF mutations, occurring in 10–30% of patients [8–9,11]. Other activating BRAF mutations include substitutions of valine at position 600 with other amino acids (V600M/D/R). Less common mutations include the double mutation 1799>1800TG>AA, termed V600E2 and mutations at positions 601 (K601E) and 597 (L597) [8–9,11]. Rare mutations such as D594A, D593V and K482M are associated with reduced BRAF activity, and these missense mutations promote enhanced MEK phosphorylation through BRAF/CRAF dimerization [12].
Most mutations in BRAF occur within the kinase domain, resulting in constitutive activity and increased MAPK signaling that can cause malignant transformation in vitro [4,10]. BRAF mutations are common in nevi, primary and metastatic melanoma, suggesting that this is an early event in the oncogenesis of melanoma that is preserved throughout tumor progression [13]. The intrapatient homogeneity of BRAF status has been confirmed in a study of patients with metastatic melanoma, where primary, regional nodal and distant metastatic tumors were assessed [13].
Clinicopathological associations of BRAF-mutant melanoma
BRAF mutations are most common in cutaneous melanoma, arising in skin that has had intermittent exposure to sunlight. Sites with less exposure to sunlight, such as acral and mucosal, have lower rates of BRAF mutation (10–15%) [14,15]. In contrast to cutaneous melanoma, uveal melanomas do not contain BRAF mutations [16].
Clinical features associated with a BRAF mutation include younger age, higher total body nevus count, truncal location, presence of mitoses, single or occult primary melanoma and histopathology (large epithelioid cytomorphology, heavy melanization, prominent upward epidermal scatter of melanocytes, nodular or superficial spreading subtypes) [8,15,17–20].
The interval between primary melanoma and the diagnosis of metastatic disease (the disease-free interval [DFI]) is thought to be similar between patients with a BRAF mutant or BRAF wild-type genotype. In historical cohorts of patients diagnosed prior to widespread availability of BRAF inhibitors, the DFI is similar between BRAF wild-type and mutated genotypes; however, retrospective studies are conflicting regarding measurement of the DFI, with some demonstrating no difference and others demonstrating a worse DFI in patients with a BRAF mutation [20–23].
Overall survival (OS) in established metastatic disease is a challenging area to study as there are few prospective historical cohorts of patients with BRAF mutation status who have not been treated with BRAF inhibitors. Retrospective cohorts of patients are conflicting due to confounding by treatment with BRAF inhibitors [8,24]; however, one cohort of patients treated prior to the availability of BRAF inhibitors did not show any difference in OS between BRAF mutant and BRAF wild-type melanoma [23]. The OS data for patients with stage III melanoma are similarly conflicting. No correlation between BRAF status and OS was found in retrospective cohorts [25]; however, one prospective study has demonstrated a worse OS with BRAF mutant stage III disease [22].
Clinical characteristics associated with different BRAF mutation genotypes
V600E BRAF mutations are associated with a younger age at diagnosis of first distant metastases compared with non-V600E mutations [26]. V600K melanoma is more likely to be associated with older age, male sex and head/neck primary tumor location compared with V600E melanoma [27], and V600K melanomas have higher levels of primary tumor site chronic sun damage than V600E melanomas [26]. Consistent with this, the prevalence of the V600K melanoma genotype varies by geographic region, ranging from <10% in Northern Europe [16,22,28] to 20–30% in Australia, Texas and Florida [21,29–30], reflecting differences in ambient UV exposure. Because of their rarity, the clinical correlates of other BRAF mutations, such as V600R and V600D remain to be determined. The difference in age-related incidence and cumulative UV damage between V600E and V600K melanoma suggest a difference in etiology.
The disease-free interval from diagnosis of primary melanoma to the first occurrence of distant metastases is significantly shorter for patients with a V600K/R mutation compared with V600E [23,26]; however, data on OS from the time of diagnosis of stage IV disease is conflicting, with some studies showing a shorter OS in V600K BRAF-mutant melanoma and others demonstrating similar OS in V600K and V600E BRAF-mutant melanoma [23,27].
BRAF mutation testing
Given the clinical importance of BRAF status for therapeutic decision making, BRAF mutation testing is now routine and required for all patients with advanced melanoma. BRAF testing is routinely performed on formalin-fixed paraffin embedded (FFPE) material. Although recent biopsies are preferable, the intrapatient homogeneity of BRAF mutation status means that in practice any tissue is satisfactory for testing, provided the clinical history is consistent (i.e., where one can be confident that the primary melanoma is the culprit lesion responsible for metastatic disease) and the sample has adequate tumor content [13,31]. Several methods of testing are available, including immunohistochemistry (IHC), Sanger sequencing, PCR-based approaches and next-generation sequencing. Most platforms focus on exon 15 mutations around codon 600, but different tests have the ability to detect different mutations with varying sensitivity and specificity. Current molecular testing platforms have the advantage of often testing for several mutations that are potentially actionable, including NRAS and KIT.
IHC using the VE1 antibody has been shown to be highly sensitive and specific for the V600E-mutated protein (formed via V600E or V600E2 mutation) [32,33]. This test does not detect non-V600E mutations such as the V600K and V600D mutations. The low cost and rapid turnaround of this test make it an attractive screening tool; furthermore, IHC has several advantages over molecular techniques; the volume of tumor content required is minimal and results are rapidly available at the time of pathological diagnosis. It is particularly useful for patients requiring urgent treatment without a known BRAF status, and can be performed in laboratories without molecular diagnostic facilities.
One specific molecular technique of note is the Cobas 4800© test, a companion diagnostic for the BRAF inhibitor vemurafenib [34]. This allele-specific PCR assay is highly sensitive for the 1799T>A V600E BRAF mutation, but less so for other V600 BRAF mutations such as V600K, V600R, V600D and V600E2 mutations [29]. While this test has had high take-up in many centers around the world due to its low cost, ease of use and quick turnaround time, the sensitivity for detecting ‘druggable’ non-V600E BRAF mutations (e.g., V600K/D/R/E2) is inadequate, and there is a lack of specificity in the result obtained (i.e., a positive or negative result is provided with no genotype details). As such, its use as a clinical diagnostic test to determine BRAF status in many patients is questionable given the prevalence of V600K BRAF mutations, and the potentially different outcomes that patients with different BRAF mutant genotypes may have with therapy.
To ensure the highest sensitivity and specificity of results, both IHC and molecular (DNA-based) BRAF mutation testing should be performed. IHC should be performed with appropriate controls. Certain samples (e.g., bone biopsies that require decalcification or cytology samples) may not provide reliable results for technical reasons. In these situations either molecular testing alone should be performed, or another tumor sample be tested. For molecular testing, all samples should be reviewed by an expert pathologist to confirm that it contains tumor, and for assessment of tumor purity. For tumor enrichment, the sample should be macrodissected and only samples with >10% tumor content should have DNA extracted and analyzed. Without such stringent quality control, false-negative results are likely to occur.
Targeted therapy for BRAF-mutant melanoma
BRAF inhibitors such as vemurafenib and dabrafenib, or MEK inhibitors such as trametinib, are used both alone and in combination as treatment for patients with BRAF mutant metastatic melanoma. The majority of patients achieve a rapid response to these drugs, making them an essential part of therapy, however, acquired resistance is common [28].
In Phase III trials, vemurafenib and dabrafenib have objective response rates of approximately 50% (although most patients have some degree of tumor regression) [35–39] and a median progression-free survival (PFS) of 6.9 months, superior to dacarbazine chemotherapy [38] (Table 1). OS with vemurafenib is 13.6 months compared with 9.7 months for dacarbazine, and dabrafenib has a median OS of 20.0 months [37]. The apparent superior survival with dabrafenib is thought to be due to continuation of drug beyond disease progression and the increased availability active second-line immunotherapy treatments (see below), rather than a true difference in efficacy with vemurafenib [40]. Both drugs have activity in patients with brain metastases [41,42]. Many toxicities with BRAF inhibitors are cutaneous, including squamous cell carcinoma (SCC), arising due to paradoxical activation of the MAPK pathway in BRAF wild-type cells [43].
Table 1. . Comparison of overall response rate, progression-free survival and overall survival rates for monotherapy and combination therapy.
| Variable | Vemurafenib monotherapy (BRIM-3 [28,39]) | Dabrafenib monotherapy (BREAK-3 [36–38]) | Dabrafenib/trametinib (Combi-D [44]) | Dabrafenib/trametinib (Combi-V [45]) | Vemurafenib/cobimetinib (CoBRIM [46]) |
|---|---|---|---|---|---|
| Patients (n) | 337† | 187‡ | 211§ | 352¶ | 247# |
| ORR (%) | 48 | 50 | 67 | 64 | 68 |
| PFS (months) | 6.9 | 6.9 | 9.3 | 11.4 | 9.9 |
| OS | All genotypes: 13.6 months; BRAF V600E: 13.3 months [39]; BRAF V600K: 14.5 months | V600E only 20.0 months | 25.1 months | 72% (at 12 months) | 81% (at 9 months) |
†Number of vemurafenib patients treated.
‡Number of dabrafenib patients treated.
§Number of dabrafenib/trametinib patients treated.
¶Number of dabrafenib/trametinib patients treated.
#Number of vemurafenib/cobimetinib patients treated.
ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival.
Trametinib monotherapy improves both PFS and OS compared with chemotherapy in patients with V600E or V600K mutations [47]. The overall response rate with trametinib is lower than that seen with BRAF inhibitors, at approximately 20%. Trametinib monotherapy is not associated with cutaneous toxicities such as SCC or hyperkeratosis despite commonly causing papulopustular rashes; however, diarrhea and peripheral edema are frequently observed. The lower response rate and inferior survival compared with BRAF inhibitors means that trametinib monotherapy is not used commonly in clinical practice; however, combination therapy is now the standard of care.
Compared with BRAF inhibitors alone, the combination of BRAF and MEK inhibitors improves the objective response rate (ORR) and survival and also attenuates cutaneous toxicity, particularly hyperkeratosis and cutaneous SCC (Table 1) [45,48–49]. The first Phase III trial, the COMBI-D trial, showed that the median OS was superior with dabrafenib/trametinib (25.1 months) compared with dabrafenib monotherapy (18.7 months; HR: 0.71; p = 0.0107). Similarly, median PFS was superior with dabrafenib/trametinib (11.0 months) compared with dabrafenib monotherapy (8.8 months, HR: 0.67, p = 0.0004) [44]. Almost every patient has a degree of tumor regression on dabrafenib/trametinib, and the ORR is between 64–67% [44–45]. The dabrafenib/trametinib combination also improves response and survival when compared with vemurafenib monotherapy, as seen in the COMBI-V trial [49], and the combination of vemurafenib and the MEK inhibitor, cobimetinib, also improved response and survival compared with vemurafenib in the coBRIM trial [46]. Other combinations of BRAF/MEK inhibitors such as encorafenib/binimetinib continue to be tested in clinical trials, but there is no doubt that combination BRAF/MEK inhibitors are now standard of care for V600 BRAF-mutant melanoma [50].
BRAF and MEK inhibitors are active for both V600E and other V600 genotypes [36,51–52]. The Phase II trial of dabrafenib showed a higher ORR (59 vs 13%), longer median PFS (6.3 vs 4.5 months) but similar OS (13.1 and 12.9 months) for patients with V600E and V600K melanoma, respectively [53]. Similarly, the Phase III trial of vemurafenib showed a higher ORR (59 and 45%), longer median PFS (6.9 and 5.9 months), but similar median OS (13.3 and 14.5 months) in patients with V600E and V600K mutant melanoma [39]. Neither study formally compared efficacy between the genotypes, but a retrospective multivariate statistical comparison confirmed this, demonstrating that V600K/R melanoma has a significantly lower ORR and shorter PFS than V600E melanoma, with similar OS [54]. The mechanisms behind these differences in efficacy may relate to the increased mutational load in V600K melanoma and may have future implications on selection of subsequent lines of treatment and response monitoring [55].
Cases reports exist of BRAF and MEK inhibitor activity in rarer V600 and non-V600 mutations. A case series of six V600R patients reported an 86% response rate to either dabrafenib or vemurafenib [52]. Other V600 BRAF mutations (e.g., V600D/M) are predicted to be sensitive to BRAF inhibitors; however, to date there are no published data [56]. In the phase I BREAK-1 study of dabrafenib, two patients with a K601E mutation had no objective tumor response and one patient with a V600-K601E mutation did not achieve a measurable tumor response [57]. Despite resistance to BRAF inhibitors, these patients may benefit from MEK inhibition, and activity has been reported of trametinib in the patients with K601E as well as the L597Q/S/V mutations [58–60]. The L597R mutation has been shown to respond to BRAF and MEK inhibitors alike [61].
Therapeutic resistance with targeted therapy
Despite the initial response observed in most patients treated with targeted therapy, acquired resistance is almost inevitable and occurs in approximately half of all patients within the first year of therapy [56]. Patients with complete response to targeted therapy are in the minority, but have the best survival, and the strongest predictor of long-term survival is the pretreatment lactate dehydrogenase level [54,62].
The spectrum of BRAF inhibitor resistance mechanisms in BRAF-mutant melanoma is diverse. Acquired resistance occurs due to MAPK pathway reactivation in the vast majority of tumors [63,64]. Known resistance mechanisms may be identified in most progressing tumors [51]. Although some mechanisms dominate, heterogeneity of resistance mechanisms is noted both within patients and tumors alike [65].
BRAF-related resistance mechanisms include gains of copy number or aberrant splice isoforms [66,67]. NRAS mutations leading to aberrant MEK phosphorylation by CRAF may reactivate the MAPK pathway and cause resistance to BRAF/MEK inhibitors [68]. MEK mutations may achieve a similar effect [69]. MAPK pathway independent resistance may occur via activation of the PI3K/AKT pathway or via paracrine secretion of growth factors from tumor stromal cells [70,71]. This last mechanism has been observed in association with vemurafenib and leads to activation of multiple upstream receptor tyrosine kinases.
In patients treated with BRAF inhibitors, BRAF splice variants are the most common mutations detected, followed by NRAS mutations, MEK1/2 mutations and BRAF amplification [70]. MAPK pathway signaling demonstrates reactivation in the majority of cases. In order to overcome the resistance mechanisms that cause upstream reactivation of the MAPK pathway, pan-RAF inhibitors such as CCT196969 and CCT241161 have been developed, which inhibit MEK/ERK in BRAF and NRAS mutant melanoma [72]. These drugs will soon enter Phase I clinical trials.
Compared with BRAF inhibitors resistance to MEK inhibitors is less well-studied. In preclinical studies, multiple mechanisms overlap including BRAF amplification, NRAS and MEK mutations. Consistent with these in vitro findings, no responses were seen when the MEK inhibitor, trametinib, was used in patients whom had failed a BRAF inhibitor [73]. Of note, at least in preclinical models, tumors that have developed a BRAF splice variant remain sensitive to MEK inhibitors [70].
The spectrum of acquired resistance mechanisms with combined BRAF/MEK inhibitors occurs via MAPK activation mechanisms. However, in contrast to treatment with BRAF inhibitor monotherapy, the most common mechanisms are BRAF amplifications, MEK1/2 mutations and NRAS mutations. MAPK pathway signaling is reactivated in the vast majority of cases [69]. BRAF splice variants appear less common in resistance to combined BRAF/MEK inhibitors; however, cases of resistance have been reported [64].
Immunotherapy for BRAF-mutant melanoma
Immunotherapy forms part of the standard of care for most patients with metastatic melanoma regardless of BRAF mutation status. Ipilimumab is a monoclonal antibody directed against CTLA-4, a T-cell inhibitory receptor whose normal function is to interrupt the cytotoxic T-cell-mediated reaction. Pembrolizumab and nivolumab are monoclonal antibodies that bind to the T-cell PD-1 receptor. PD-1 is stimulated by upregulation of its ligand, PD-L1, in the tumor microenvironment, leading to inhibition of T-cell proliferation and cytokine production.
Ipilimumab was the first therapy in metastatic melanoma to demonstrate an OS benefit. In the second-line setting, it has been shown to improve OS compared with a gp100 vaccine. While a small proportion of patients achieve an objective response to therapy, survival can be durable. The 2- and 3-year OS rates with ipilimumab was 20 and 16%, which is higher with gp100 vaccine [74]. In the first-line setting, the combination of ipilimumab, given at a higher dose than the currently accepted standard, and dacarbazine improves OS compared with dacarbazine alone; however, the high rate of adverse liver function test abnormalities has meant that this combination is not used in routine clinical practice [75,76]. The ORR for ipilimumab is between 11 and 15%; however, despite this low rate, durable survival is observed in approximately 21% of all patients [75–77]. Patients who achieve durable benefit include patients from all Response Evaluation Criteria in Solid Tumors (RECIST) response categories, including those with complete response, partial response, stable disease or progressive disease, and the atypical or ‘immune’ patterns of responses seen with ipilimumab, whereby some tumors grow before regressing, have led to the development of the immune-related response criteria [78].
The anti-PD-1 antibodies, pembrolizumab and nivolumab, have both been shown to be superior to ipilimumab for both efficacy and toxicity in Phase III trials [79–81]. Pembrolizumab and nivolumab appear to have similar efficacy and toxicity, and both have activity after ipilimumab failure [82,83]. Combination of nivolumab and ipilimumab appears to have greater efficacy than either of the drugs alone, but the Phase III trial was not powered to show an improvement in survival compared with nivolumab alone, and the toxicity with combination immunotherapy is significant [80]. This is likely to be an important factor in selecting treatment for individual patients in the future.
The rationale for combination immunotherapy continues to be tested both for efficacy and tolerability and studies of combination pembrolizumab and ipilimumab are currently underway (NCT 02089685). Although long-term data are still awaited from the anti-PD-1 antibody trials, it is widely expected that a tail of survival will be observed, albeit with a higher rate of long-term survivors compared with targeted therapy or ipilimumab.
Trials of anti-PD-1 antibodies have included both BRAF wild-type and mutated patients [79–82,84], and a pooled analysis of 440 patients treated on four clinical trials of nivolumab showed that the response rate was similar in BRAF wild-type and mutant melanoma patients at 34.6 and 29.7%, respectively [85]. Similar data are not readily available for ipilimumab treatment as trials were conducted prior to widespread BRAF testing [74,75]; however, retrospective analyses have shown that the response rate is similar with V600E BRAF mutant and BRAF wild-type tumors [86].
Optimal sequencing of targeted therapy & immunotherapy
There is a paucity of data on the optimal sequence of targeted therapy and immunotherapy for BRAF mutant metastatic melanoma. Selected patients who have received ipilimumab will have a durable response regardless of whether they have residual disease or a complete response by RECIST [77]. Although it is clear that a subset of patients treated with targeted therapy have durable survival, confounding factors such as crossover to other therapies in clinical trials have meant that this effect is difficult to measure [62]. Long-term survival data for anti-PD-1 antibodies are awaited; however, Phase I data for nivolumab have shown that the majority of patients have responses lasting at least 1 year [87].
The response rate to dabrafenib when used in first-line setting is 50%, whereas patients who crossed over to dabrafenib after disease progression (that is, second-line setting) had a response rate of 46% [35,37–38]. Furthermore, the response rates across the Phase I–III trials of both vemurafenib and dabrafenib are similar, regardless of line of therapy, further suggesting that BRAF inhibitors have similar activity regardless of when they are used [35–36,53,57,88–89]. In contrast to targeted therapy, available data suggest that that anti-PD-1 antibodies have the best activity in the first-line setting. Nivolumab monotherapy has an objective response rate of 40% in previously untreated patients, but following treatment with ipilimumab, the response rate is lower at 32% [79,83]. Similar data exist for pembrolizumab [81–82].
A single retrospective review demonstrated that patients who received BRAF inhibitors following immunotherapy agents (mainly ipilimumab) had a similar response rate when used in first-line setting when other variables were taken into account [90]. However, when ipilimumab was given after BRAF inhibitors, only half of all patients were able to complete four cycles of ipilimumab. Despite the inherent biases in this study due to the retrospective nature, the response rates observed were lower than that expected in first-line ipilimumab treatment, suggesting that ipilimumab is best given prior to targeted therapy, particularly in well patients. Survival with anti-PD-1 antibodies in those failing BRAF inhibitors is similar to those that are BRAF inhibitor naive, but the best sequence is not yet clear [85]. The data should be interpreted in light of the highly selected patient population in clinical trials of anti-PD-1 antibodies as these patients may have different baseline characteristics compared with those included in the retrospective analysis of ipilimumab sequencing, therefore, prospective sequencing trials will be required to answer this question definitively.
In patients with asymptomatic and/or low volume disease, given the greater potential for durable response with immunotherapy than targeted therapy, upfront immunotherapy is generally preferred. The characteristics of targeted therapies and their response rates after previous treatment mean that they are useful as salvage therapy regardless of treatment sequence or upfront therapy in those with symptomatic or rapidly progressing disease. A trial is currently underway comparing combination of dabrafenib/trametinib followed by ipilimumab/nivolumab compared with the reverse sequence (NCT02224781). This trial will help to elucidate some of the controversies surrounding retrospective analyses of treatment sequence regimens.
In order to improve treatment outcomes further, combination immunotherapy and targeted therapy strategies are being pursued. The translational evidence underlying this approach suggests that BRAF inhibition leads to tumor infiltrating lymphocytes after initiation of treatment and thus modulate immune responses within the tumor microenvironment [91–93]. This may also occur by facilitating tumor clearance, release of antigenic debris and modulation of immune responses [94]. A clinical trial of the BRAF inhibitor, vemurafenib, combined with ipilimumab, was initiated but closed due to the severe hepatotoxicity [95]. A clinical trial of dabrafenib plus ipilimumab with or without trametinib was commenced (NCT01767454); however, the triplet combination was ceased early due to two of the seven patients developed colon perforation soon after commencing ipilimumab [96]. The doublet combination (dabrafenib plus ipilimumab) continued into the dose expansion phase and results are awaited.
Combination BRAF/MEK inhibitor and anti-PD-1 antibody trials have already begun, such as the combination of dabrafenib, trametinib and the anti-PD-1 antibody pembrolizumab (NCT02130466). The ligand of the PD-1 receptor, PD-L1, is also being investigated as a therapeutic target. Early phase trials of anti-PD-L1 therapy combined with dabrafenib/trametinib have shown an acceptable safety profile and evidence of clinical activity [97].
BRAF fusion genes as a novel molecular target
The classification of melanoma based upon the molecular profile of a patient’s tumor has changed the way melanoma is treated. While BRAF and MEK inhibitors in combination have become standard care for BRAF-mutant melanoma [45,48]; MEK inhibitors have activity in patients with NRAS-mutant melanoma [98]. Multikinase inhibitors such as imatinib or sunitinib may have activity in KIT mutant melanoma [99–103]; however, the number of patients treated with these agents remains low, precluding detailed survival analysis. Preclinical data suggest that MEK inhibitors may be effective for patients with NF1 mutations [104]. Approximately 30% of melanomas do not have clear driver mutations, and for these patients, the only available current treatments are immunotherapy and chemotherapy [105,106].
BRAF kinase fusions, occurring as a result of rearrangements that fuse the BRAF kinase domain to 5' partner genes, have been shown to activate the MAPK pathway in several cancers including pilocytic astrocytoma, and gastric, thyroid and prostate cancers [88,107–111]. In melanoma, BRAF fusions may occur in 4–8% of BRAF/NRAS/KIT wild-type patients [112,113]. Similar to tumors with wild-type BRAF, type 1 RAF inhibitors (e.g., vemurafenib, dabrafenib) appear ineffective and may paradoxically activate the MAPK pathway; however, sensitivity to MEK inhibitors has been demonstrated in vitro [112–114].
Retrospective (posthumous) evidence of clinical activity of sorafenib, an nonselective RAF inhibitor that particularly inhibits CRAF, has been shown, in a patient with metastatic melanoma with an AGK–BRAF fusion [112,115]. Recently, case reports of activity of trametinib in melanoma patients with BRAF fusions have been published [116]. A PPFIBP2-BRAF inframe fusion was reported in a patient with metastatic melanoma, who, following treatment with trametinib, had a measurable response in intracranial and extracranial sites. A previously reported fusion protein with constitutive kinase activity in pilocytic astrocytoma, KIAA1549-BRAF, was found in another patient where treatment with an MEK inhibitor was found to slow down disease progression. These cases provide an important proof-of-concept for BRAF fusion proteins as a potential target, which may inform the design of future clinical trials. Other BRAF fusion proteins have been characterized [38,42]; however, the optimal therapy for targeting individual gene fusion products remains to be studied.
Conclusion
BRAF mutations are found in almost half of all patients with metastatic disease. The most commonly found mutation, the V600E missense mutation, has been proven in clinical trials to respond to BRAF inhibitors such as vemurafenib and dabrafenib. Other V600 BRAF mutations such as V600K have been shown to respond to BRAF inhibitors, while a subset of non-V600 mutations respond to MEK inhibitors. The choice of mutation testing is paramount as many current methods may not detect non-V600E mutations, which may respond to BRAF inhibitors. In V600 BRAF-mutant melanoma, the combination of BRAF/MEK inhibitors is now the standard of care, delaying resistance and abrogating many toxicities, which develop with monotherapy, but acquired drug resistance is common, complex and incompletely understood. The optimal sequencing or combination of BRAF/MEK inhibitors with immunotherapy is poorly understood and several trials are underway to evaluate the best treatment approach for patients with BRAF-mutant melanoma, and efforts continue to target other aberrations for those with BRAF wild-type melanoma.
Future perspective
Therapies targeting the MAPK pathway in melanoma will remain an important therapeutic option for the treatment of BRAF-mutated melanoma for the foreseeable future. Despite the advent of immunotherapy with ipilimumab and anti-PD-1 antibodies, which have shown significant activity both alone and in combination, targeted therapy has a role in patients who are unable to tolerate the side effects of immunotherapy, where a rapid response to treatment is required, or where immunotherapy is unsuccessful.
The discovery of BRAF fusion proteins and their activation of the MAPK pathway demonstrates that the full spectrum of cellular machinery driving oncogenesis in melanoma is yet to be fully elucidated and may provide further therapeutic strategies in metastatic melanoma. Early phase clinical trials with agents that have shown preclinical activity in BRAF inhibitor refractory disease open the possibility to further research in this area, particularly for patients who have developed treatment resistance.
The combination of targeted therapy and immunotherapy may be an important mechanism to increase the potential for immunotherapy to induce durable responses in a larger proportion of patients due to their modulation of immune responses. Future research on methods for potentiating the antitumor immune responses and facilitating tumor clearance by T cells will be the key to treatment of patients with BRAF mutations and acquired resistance to BRAF/MEK inhibitors.
Other combinations of targeted therapies such as cyclin-dependent kinase inhibitors together with BRAF and MEK inhibitors are being tested in early phase trials (NCT01820364) [117]. Combinations of targeted therapies against less common BRAF mutations together with immunotherapy may allow for effective treatment of patients with inherent or acquired resistance to immunotherapy agents. This will require application of our evolving knowledge of the tumor microenvironment.
Both targeted therapy combinations and immunotherapy agents are currently being explored for use in the adjuvant setting, where the largest gains in efficacy are likely to be seen in the future. The ultimate goal of treatment, particularly in the age of immunotherapy, will be to manipulate and induce long-lasting antitumor immune responses that ultimately prevent systemic disease relapse.
Footnotes
Financial & competing interests disclosure
MS Carlino is on the following advisory boards: Novartis, MSD, BMS and Amgen. AM Menzies has received honoraria and travel support from GSK and BMS; and honoraria from Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 2.Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 2001;93(1–2):53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Viciana P, Warne PH, Dhand R, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370(6490):527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]; •• Seminal paper describing the frequency of V600E BRAF mutations in melanoma and the abnormal function of resultant elevated kinase activity.
- 5.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62(23):6997–7000. [PubMed] [Google Scholar]
- 6.Akbani R, Akdemir KC, Aksoy A, et al. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive The Cancer Genome Atlas (TCGA) collaboration paper outlining the genomic classification of the most commonly mutated genes in cutaneous melanoma and their prognostic implications.
- 7.Krauthammer M, Kong Y, Bacchiocchi A, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat. Genet. 2015;47(9):996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011;29(10):1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]; •• Clinicopathologic study assessing the frequency and type of BRAF mutations in a consecutive cohort of patients with metastatic melanoma. Describes the clinical characteristics associated with BRAF mutations and associated prognostic patterns.
- 9.Jakob JA, Bassett RL, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118(16):4014–4023. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan PTC, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 11.Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrolment in genotype-driven therapeutic trials. PLoS ONE. 2012;7(4):e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies AM, Lum T, Wilmott JS, et al. Intrapatient homogeneity of BRAF V600E expression in melanoma. Am. J. Surg. Path. 2014;38(3):377–382. doi: 10.1097/PAS.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 14.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]; •• Paper describing the frequency of BRAF and NRAS mutations according to the level of UV light. Established the association between BRAF and NRAS mutations and lack of chronic sun damage.
- 15.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J. Natl Cancer Inst. 2003;95(24):1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 16.Rimoldi D, Salvi S, Liénard D, et al. Lack of BRAF mutations in uveal melanoma. Cancer Res. 2003;63(18):5712–5715. [PubMed] [Google Scholar]
- 17.Hacker E, Hayward NK, Dumenil T, James MR, Whiteman DC. The association between MC1R genotype and BRAF mutation status in cutaneous melanoma: findings from an Australian population. J. Invest. Dermatol. 2010;130(1):241–248. doi: 10.1038/jid.2009.182. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAFT1799A(V600E) mutation in primary melanoma. J. Invest. Dermatol. 2006;127(4):900–905. doi: 10.1038/sj.jid.5700632. [DOI] [PubMed] [Google Scholar]
- 19.Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol. Biomark. Prev. 2007;16(5):991–997. doi: 10.1158/1055-9965.EPI-06-1038. [DOI] [PubMed] [Google Scholar]
- 20.Edlundh-Rose E, Egyházi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16(6):471–478. doi: 10.1097/01.cmr.0000232300.22032.86. [DOI] [PubMed] [Google Scholar]
- 21.Meckbach D, Bauer J, Pflugfelder A, et al. Survival according to BRAF-V600 tumor mutations – an analysis of 437 patients with primary melanoma. PLoS ONE. 2014;9(1):e86194. doi: 10.1371/journal.pone.0086194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiag P, Aegerter P, Lebbe C, et al. Prognostic value of BRAFV600 mutations in American Joint Committee on Cancer (AJCC) stage 3 cutaneous melanoma patients in the MelanCohort prospective cohort. ASCO Meet. Abstr. 2015;33(15 Suppl.):9037. [Google Scholar]
- 23.Carlino MS, Haydu LE, Kakavand H, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br. J. Cancer. 2014;111(2):292–299. doi: 10.1038/bjc.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brissy S, Gaudy-Marqueste C, Mallet S, et al. BRAF mutation as a pejorative marker in metastatic melanoma. ASCO Meet. Abstr. 2012;30(15 Suppl.):8555. [Google Scholar]
- 25.Rutkowski P, Gos A, Jurkowska M, et al. Molecular alterations in clinical stage III cutaneous melanoma: correlation with clinicopathological features and patient outcome. Oncol. Lett. 2014;8(1):47–54. doi: 10.3892/ol.2014.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin. Cancer Res. 2012;18(12):3242–3249. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]; •• Clinicopathologic study establishing the age-related prevalence of BRAF mutations, frequency of BRAF mutation subtypes and associated prognosis.
- 27.Bucheit AD, Syklawer E, Jakob JA, et al. Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma. Cancer. 2013;119(21):3821–3829. doi: 10.1002/cncr.28306. [DOI] [PubMed] [Google Scholar]
- 28.Menzies AM, Long GV. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol. 2014;15(9):e371–e381. doi: 10.1016/S1470-2045(14)70072-5. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Algarra S, Labiano T, Echeveste JI, et al. Use of Cobas 4800 BRAF mutation test for the analysis of BRAF V600 mutations in cytological samples (CS) from metastatic melanoma (MM) J. Clin. Oncol. 2012;30(15 Suppl.):8572. [Google Scholar]
- 30.Cheng S, Chu P, Hinshaw M, et al. Frequency of mutation associated with targeted therapy in malignant melanoma patients. J. Clin. Oncol. 2011;29(15 Suppl.) Abstract 8597. [Google Scholar]
- 31.Menzies AM, Wilmott JS, Long GV, Scolyer RA. Intra-patient heterogeneity of BRAF mutation status: fact or fiction? Br. J. Cancer. 2014;111(8):1678–1679. doi: 10.1038/bjc.2013.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busam KJ, Hedvat C, Pulitzer M, von Deimling A, Jungbluth AA. Immunohistochemical analysis of BRAF(V600E) expression of primary and metastatic melanoma and comparison with mutation status and melanocyte differentiation antigens of metastatic lesions. Am. J. Surg. Pathol. 2013;37(3):413–420. doi: 10.1097/PAS.0b013e318271249e. [DOI] [PubMed] [Google Scholar]
- 33.Long GV, Wilmott JS, Capper D, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am. J. Surg. Pathol. 2013;37(1):61–65. doi: 10.1097/PAS.0b013e31826485c0. [DOI] [PubMed] [Google Scholar]; •• Established the sensitivity and specificity of the V600E BRAF mutant-specific antibody, VE1 in the diagnosis of BRAF-mutated melanoma.
- 34.Halait H, Demartin K, Shah S, et al. Analytical performance of a real-time PCR-based assay for V600 mutations in the BRAF gene, used as the companion diagnostic test for the novel BRAF inhibitor vemurafenib in metastatic melanoma. Diagn. Mol. Pathol. 2012;21(1):1–8. doi: 10.1097/PDM.0b013e31823b216f. [DOI] [PubMed] [Google Scholar]
- 35.Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, Phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]; •• Phase III trial of dabrafenib compared with dacarbazine demonstrating improved progression-free survival compared with dacarbazine.
- 36.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Phase III trial of vemurafenib, the first US FDA approved BRAF inhibitor, demonstrating improved overall survival compared with dacarbazine.
- 37.Hauschild A, Grob JJ, Demidov LV, et al. An update on BREAK-3, a Phase III, randomized trial: dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E-positive mutation metastatic melanoma (MM) J. Clin. Oncol. 2013;31(15 Suppl.):9013. [Google Scholar]
- 38.Hauschild Axel. The European Society for Medical Oncology Congress. Madrid, Spain: 2014. An update on overall survival (OS) and follow-on therapies in BREAK-3, a Phase III, randomized trial: dabrafenib (D) vs. dacarbazine (DTIC) in patients (pts) with BRAF V600E mutation-positive metastatic melanoma (MM) Presented at. [Google Scholar]
- 39.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a Phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Extended follow-up data from phase III study of vemurafenib showing activity of vemurafenib in V600E and V600K mutant melanoma.
- 40.Chan M, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120(20):3142–3153. doi: 10.1002/cncr.28851. [DOI] [PubMed] [Google Scholar]
- 41.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, Phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]; •• Key clinical trial demonstrating activity of dabrafenib in brain metastases.
- 42.Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAFV600 mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur. J. Cancer. 2014;50(3):611–621. doi: 10.1016/j.ejca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Menzies AM, Kefford R, Long GV. Paradoxical oncogenesis: are all BRAF inhibitors equal? Pigment Cell Melanoma Res. 2013;26(5):611–615. doi: 10.1111/pcmr.12132. [DOI] [PubMed] [Google Scholar]
- 44.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, Phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 45.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]; •• Phase III study showing progression-free survival advantage with combined BRAF/MEK inhibitors vemurafenib/cobimetanib compared with vemurafenib alone.
- 46.Larkin JMG, Yan Y, McArthur G, et al. Update of progression-free survival (PFS) and correlative biomarker analysis from coBRIM: phase III study of cobimetinib (COBI) plus vemurafenib (VEM) in advanced BRAF-mutated melanoma. J. Clin. Oncol. 2015;33(15 Suppl.):9006. [Google Scholar]
- 47.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 48.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]; •• Phase III clinical trial showing improved progression-free survival of combination of dabrafenib/trametinib compared with dabrafenib alone.
- 49.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]; •• Phase III trial demonstrating superior overall survival with combination of dabrafenib/trametinib compared with vemurafenib monotherapy.
- 50.Sullivan RJ, Weber JS, Patel SP, et al. A Phase Ib/II study of BRAF inhibitor (BRAFi) encorafenib (ENCO) plus MEK inhibitor (MEKi) binimetinib (BINI) in cutaneous melanoma patients naive to BRAFi treatment. J. Clin. Oncol. 2015;3(15 Suppl.):9007. [Google Scholar]
- 51.Infante JR, Falchook GS, Lawrence DP, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436) J. Clin. Oncol. 2011;29(18 Suppl.):CRA8503. [Google Scholar]
- 52.Klein O, Clements A, Menzies AM, O’Toole S, Kefford RF, Long GV. BRAF inhibitor activity in V600R metastatic melanoma. Eur. J. Cancer (Oxf. Engl.: 1990) 2013;49(5):1073–1079. doi: 10.1016/j.ejca.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J. Clin. Oncol. 2013;31(26):3205–3211. doi: 10.1200/JCO.2013.49.8691. [DOI] [PubMed] [Google Scholar]
- 54.Menzies AM, Wilmott JS, Drummond M, et al. Clinicopathologic features associated with efficacy and long-term survival in metastatic melanoma patients treated with BRAF or combined BRAF and MEK inhibitors. Cancer. 2015 doi: 10.1002/cncr.29586. doi:10.1002/cncr.29586. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Mar VJ, Wong SQ, Li J, et al. BRAF/NRAS wild-type melanomas have a high mutation load correlating with histological and molecular signatures of UV damage. Clin. Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0398. doi: 10.1158/1078-0432.CCR-13-0398. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Carlino MS, Long GV, Kefford RF, Rizos H. Targeting oncogenic BRAF and aberrant MAPK activation in the treatment of cutaneous melanoma. Crit. Rev. Oncol. Hematol. 2015 doi: 10.1016/j.critrevonc.2015.08.021. doi:10.1016/j.critrevonc.2015.08.021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a Phase 1 dose-escalation trial. Lancet Lond. Engl. 2012;379(9829):1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bowyer SE, Rao AD, Lyle M, et al. Activity of trametinib in K601E and L597Q BRAF mutation-positive metastatic melanoma. Melanoma Res. 2014;24(5):504–508. doi: 10.1097/CMR.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 59.Dahlman KB, Xia J, Hutchinson K, et al. BRAF L597 mutations in melanoma are associated with sensitivity to MEK inhibitors. Cancer Discov. 2012;2(9):791–797. doi: 10.1158/2159-8290.CD-12-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a Phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahadoran P, Allegra M, Le Duff F, et al. Major clinical response to a BRAF inhibitor in a patient with a BRAF L597R-mutated melanoma. J. Clin. Oncol. 2013;31(19):e324–e326. doi: 10.1200/JCO.2012.46.1061. [DOI] [PubMed] [Google Scholar]
- 62.Daud A, Weber JS, Sosman JA, et al. Updated overall survival (OS) results for BRF113220, a phase I–II study of dabrafenib alone versus combined dabrafenib and trametinib in patients with BRAF V600 metastatic melanoma (MM) J. Clin. Oncol. 2015;33(15 Suppl.):9036. [Google Scholar]
- 63.Holderfield M, Nagel TE, Stuart DD. Mechanism and consequences of RAF kinase activation by small-molecule inhibitors. Br. J. Cancer. 2014;111(4):640–645. doi: 10.1038/bjc.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagle N, Van Allen EM, Treacy DJ, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4(1):61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spagnolo F, Ghiorzo P, Queirolo P. Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget. 2014;5(21):10206–10221. doi: 10.18632/oncotarget.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi H, Moriceau G, Kong X, et al. Melanoma whole exome sequencing identifies V600EB-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat. Commun. 2012;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Established the amplification of (V600E)BRAF driven BRAF inhibitor resistance in vitro.
- 67.Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480(7377):387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Established the V600E BRAF splice variant as a mechanism of BRAF inhibitor resistance.
- 68.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Established NRAS upregulation as a mechanism of BRAF inhibitor resistance.
- 69.Long GV, Fung C, Menzies AM, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat. Commun. 2014;5:5694. doi: 10.1038/ncomms6694. [DOI] [PubMed] [Google Scholar]; •• Established activating MEK mutations as a mechanism for BRAF inhibitor resistance in the majority of cases resistant to BRAF inhibitors. Demonstrated the heterogeneity of resistance mechanisms, which makes selection of sequenced-targeted therapy difficult in clinical practice.
- 70.Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin. Cancer Res. 2014;20(7):1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]; •• Comprehensive study establishing the reactivation of MAPK signaling in 79% of melanomas resistant to BRAF/MEK inhibitor treatment. Demonstrated that the lack of MEK or BRAF/MEK activity in resistant tumors is associated with poor prognosis.
- 71.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Girotti MR, Lopes F, Preece N, et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell. 2015;27(1):85–96. doi: 10.1016/j.ccell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 2013;31(4):482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDermott D, Haanen J, Chen T-T, Lorigan P, O’Day S MDX010–20 Investigators. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a Phase III trial (MDX010–20) Ann. Oncol. 2013;24(10):2694–2698. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 75.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Pivotal Phase III trial demonstrating improved overall survival with ipilimumab compared with gp100 vaccine alone.
- 76.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]; •• Pivotal Phase III trial demonstrating improved overall survival with ipilimumab compared with dacarbazine.
- 77.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 79.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]; •• Established the superiority of nivolumab over dacarbazine for overall survival.
- 80.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Phase II study demonstrating improved progression-free survival of combined nivolumab and ipilimumab compared with ipilimumab alone. Demonstrated the importance of PD-L1 as a biomarker, as patients without PD-L1 had a trend toward improved progression-free survival.
- 81.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]; •• Phase III study demonstrating improved progression-free survival and 1-year overall survival with pembrolizumab compared with ipilimumab.
- 82.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, Phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, Phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 84.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD1) in melanoma. N. Engl. J. Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of nivolumab in patients with BRAF v600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 86.Shahabi V, Whitney G, Hamid O, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Cancer Immunol. Immunother. CII. 2012;61(5):733–737. doi: 10.1007/s00262-012-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ribas A, Kim KB, Schuchter LM. BRIM-2: an open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. J. Clin. Oncol. 2011;29(15 Suppl.):8509. [Google Scholar]
- 89.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695–1701. doi: 10.1002/cncr.28620. [DOI] [PubMed] [Google Scholar]
- 91.Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 2013;19(5):1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Preclinical study demonstrating the enhancement of melanoma antigen expression with BRAF inhibitor treatment, providing the basis upon which combination of BRAF inhibitor and immunotherapy trials were proposed.
- 92.Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012;18(5):1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]; •• Preclinical study demonstrating T-cell infiltration of tumors following treatment with dabrafenib or vemurafenib.
- 93.Kakavand H, Wilmott JS, Menzies AM, et al. PD-L1 expression and tumor-infiltrating lymphocytes define different subsets of MAPK inhibitor-treated melanoma patients. Clin. Cancer Res. 2015;21(14):3140–3148. doi: 10.1158/1078-0432.CCR-14-2023. [DOI] [PubMed] [Google Scholar]
- 94.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 2013;368(14):1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 96.Puzanov I. Combining targeted and immunotherapy: BRAF inhibitor dabrafenib (D) ± the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation-positive unresectable or metastatic melanoma (MM) J. Transl. Med. 2015;13(Suppl. 1):K8. [Google Scholar]
- 97.Ribas A. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J. Clin. Oncol. 2015;33(15 Suppl.):3003. [Google Scholar]
- 98.Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14(3):249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 99.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. 2013;31(26):3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29(21):2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 102.Su Y, Vilgelm AE, Kelley MC, et al. RAF265 inhibits the growth of advanced human melanoma tumors. Clin. Cancer Res. 2012;18(8):2184–2198. doi: 10.1158/1078-0432.CCR-11-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Minor DR, Kashani-Sabet M, Garrido M, O’Day SJ, Hamid O, Bastian BC. Sunitinib therapy for melanoma patients with KIT mutations. Clin. Cancer Res. 2012;18(5):1457–1463. doi: 10.1158/1078-0432.CCR-11-1987. [DOI] [PubMed] [Google Scholar]
- 104.Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014;74(8):2340–2350. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Study demonstrating the importance of NF1 loss in melanomagenesis. This genotype demonstrates resistance to BRAF inhibition and potential for MEK inhibition as a treatment strategy.
- 105.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia J, Jia P, Hutchinson KE, et al. A meta-analysis of somatic mutations from next generation sequencing of 241 melanomas: a road map for the study of genes with potential clinical relevance. Mol. Cancer Ther. 2014;13(7):1918–1928. doi: 10.1158/1535-7163.MCT-13-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 2005;115(1):94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Forshew T, Tatevossian RG, Lawson ARJ, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J. Pathol. 2009;218(2):172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 109.Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. (Berl.) 2011;121(6):763–774. doi: 10.1007/s00401-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 110.Palanisamy N, Ateeq B, Kalyana-Sundaram S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat. Med. 2010;16(7):793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549: BRAF fusion variants. J. Neuropathol. Exp. Neurol. 2012;71(1):66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Botton T, Yeh I, Nelson T, et al. Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell Melanoma Res. 2013;26(6):845–851. doi: 10.1111/pcmr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hutchinson KE, Lipson D, Stephens PJ, et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin. Cancer Res. 2013;19(24):6696–6702. doi: 10.1158/1078-0432.CCR-13-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sievert AJ, Lang S-S, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl Acad. Sci. USA. 2013;110(15):5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Passeron T, Lacour J-P, Allegra M, et al. Signalling and chemosensitivity assays in melanoma: is mutated status a prerequisite for targeted therapy? Exp. Dermatol. 2011;20(12):1030–1032. doi: 10.1111/j.1600-0625.2011.01385.x. [DOI] [PubMed] [Google Scholar]
- 116.Menzies AM, Yeh I, Botton T, Bastian BC, Scolyer RA, Long GV. Clinical activity of the MEK inhibitor trametinib in metastatic melanoma containing BRAF kinase fusion. Pigment Cell Melanoma Res. 2015;28(5):607–610. doi: 10.1111/pcmr.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taylor M, Sosman J, Gonzalez R, et al. 10860-phase Ib/II study of LEE011 (CDK4/6 Inhibitor) and LGX818 (BRAF inhibitor) in BRAF-mutant melanoma. Ann. Oncol. 2014;25(Suppl. 4):iv374–iv375. [Google Scholar]