Abstract
Melanoma is the deadliest form of skin cancer and the incidence continues to rise in the United States and worldwide. Activating mutations in RAS oncogenes are found in roughly a third of all human cancers. Mutations in NRAS occur in approximately a fifth of cutaneous melanomas and are associated with aggressive clinical behavior. Cells harboring oncogenic NRAS mutations exhibit activation of multiple signaling cascades, including PI3K/Akt, MEK-ERK and RAL, which collectively stimulate cancer growth. While strategies to target N-Ras itself have proven ineffective, targeting one or more N-Ras effector pathways has shown promise in preclinical models. Despite promising preclinical data, current therapies for NRAS mutant melanoma remain limited. Immune checkpoint inhibitors and targeted therapies for BRAF mutant melanoma are transforming the treatment of metastatic melanoma, but the ideal treatment for NRAS mutant melanoma remains unknown. Improved understanding of NRAS mutant melanoma and relevant N-Ras effector signaling modules will be essential to develop new treatment strategies.
KEYWORDS : BRAF, immunotherapy, melanoma, MEK, NRAS, PD-1
Practice points.
Aberrant signaling through the RAS-RAF pathway occurs in up to 75% of melanomas.
NRAS mutations are present in 15–30% of metastatic melanoma.
Despite the development of targeted therapies and immunomodulatory monoclonal antibodies, additional therapeutic approaches are needed for melanoma.
MEK inhibitors show modest activity in NRAS mutant melanoma.
Trials with MEK inhibitors in combination with other therapies are underway.
Efficacy of immunotherapy may be improved in NRAS mutant melanoma.
Management of melanoma in 2016
Melanoma is a malignant transformation of melanocytes, pigment-producing neural-crest derived cells that exist in the epidermis and mucosal surfaces in various other anatomic sites [1]. The predominant risk factor for melanoma is ultraviolet light exposure, typically through excess sun exposure or use of tanning beds, and melanoma is more common in individuals with fair or light complexion, although other factors (e.g., family history, genetic predisposition, multiple nevi) also influence the risk of developing cutaneous melanoma. While early disease is cured with surgical resection in the majority of patients, advanced or metastatic disease is incurable and historically has been associated with an extremely poor prognosis with few treatment options. Melanoma remains the deadliest form of skin cancer, and the incidence is rising with 76,100 new cases and 9710 deaths are estimated for 2014 in the USA [1].
The last decade has seen a revolution in the treatment of metastatic melanoma, and since 2011, there have been six drugs approved by the FDA for melanoma [2]. These drugs comprise two classes of therapies:
Drugs targeted at mediators of growth factors in the melanoma cell (vemurafenib, dabrafenib, trametinib); and
Immunomodulatory monoclonal antibodies that neutralize immune checkpoints and thereby permit tumor control by the patient's own immune system (ipilimumab, pembrolizumab, and nivolumab) [2,3].
Approved targeted therapies for melanoma at present are available for melanomas harboring BRAF driver mutations, which are present in roughly half of melanomas. Over 90% of mutations in BRAF occur at Val600 in the catalytic domain resulting in 500-fold activation of BRAF activity, which in turn drives MEK-ERK signaling, and is capable of transforming immortalized melanocytes [4,5]. BRAF inhibitors and MEK inhibitors are active in BRAF mutant melanoma [6,7], but combination BRAF-MEK inhibition yields higher response rates and improved progression-free survival and overall survival [8–10]. Despite an impressive response rate for combination BRAF-MEK inhibition (70–80%) resistance develops in most patients within 1 year.
Immunotherapeutic drugs include ipilimumab, a monoclonal antibody targeting CTLA-4, and the PD-1 inhibitors pembrolizumab and nivolumab [3]. These agents function to neutralize inhibitory T-cell signaling. Ipilimumab was the first of the new generation of melanoma drugs, approved in 2011 based on an overall survival benefit compared with gp100 peptide vaccination [11]. Ipilimumab is associated with a response rate of 10–15% as a single-agent, though approximately 20% of patients treated are alive at 5 years [12]. PD-1 inhibitors (pembrolizumab and nivolumab) have higher response rates (30–40%) and a more favorable toxicity profile compared with ipilimumab [13,14]. Recently, combination of CTLA-4 and PD-1 immune checkpoint inhibitors was shown to have an additive effect, improving response rates to up to 50–60% [13,15]. However, the toxicity was also markedly increased with combination therapy with >50% grade 3 or 4 adverse events, and it is not yet known if the combination of CTLA-4 and PD-1 improves overall survival compared with single agent therapy or sequential checkpoint blockade.
Despite these remarkable advances with the development of BRAF-MEK and immune checkpoint inhibitors, patients treated with targeted BRAF-MEK inhibitors eventually develop recurrent and refractory disease, and a limited number of patients respond to immunotherapy. Therefore, novel therapies and approaches to improve response rates and promote durable responses are needed. An attractive candidate for both the development of targeted therapies and the utilization and exploration of the impact of immune checkpoint inhibitors is NRAS mutant melanoma. While oncogenic Ras has historically been deemed ‘undruggable’, recent preclinical studies suggest that Ras may occupy a unique position in the oncogenic signaling network, making it an attractive candidate for both targeted agents and immune modulatory agents [16]. In this review, we will focus on the role of Ras-Raf signaling in melanoma, detail the unique clinical and pathologic features of NRAS mutant melanoma, and discuss clinical and preclinical approaches to treating NRAS mutant melanoma.
RAS & RAF signaling in melanoma
Three Ras proto-oncogenes have been identified, KRAS, NRAS and HRAS [17,18]. Oncogenic, activating mutations in RAS have been described in up to 30% of all human cancers [19–21]. Cellular homologues to these genes were first identified in the 1980s, initially with NRAS in neuroblastoma followed shortly thereafter by KRAS and HRAS [22,23]. These genes were later determined to encode small (low molecular weight) intracellular GTPases that mediate growth signals downstream of receptor tyrosine kinase activation and mediate diverse intracellular signal transduction cascades, including MAPK/ERK, PI3K/Akt, Ral-GDS and phospholipase-C-epsilon [17].
Ras proteins are active in the GTP-bound state, but are rendered inactive following GTP hydrolysis to GDP, a process promoted by RAS GTPase-activating proteins (GAPs). Guanine nucleotide exchange factors (GEFs) promote formation of GTP from GDP. Ras signaling is subject to tight spatial and temporal control with multiple regulators of GTPase activity, distinct subcellular localizations for the different Ras proteins, distinct post-translational modifications, and multiple effector pathways have been described for the different Ras family members [18,24].
The majority of mutations in RAS are in codons 12, 13 and 61 which influence GTPase activity. In normal cells, Ras signaling requires upstream stimulation of a receptor-tyrosine kinase, but cancer cells harboring an oncogenic RAS mutation, the GTP-bound Ras protein is locked in the active position providing continuous activation of downstream signaling pathways. Mutations in codons 12 and 13 result in Ras proteins with impaired GAP-mediated hydrolysis, where as mutations in codon 61 result in Ras proteins that cannot properly coordinate water molecules for the hydrolysis of GTP to GDP and are, therefore, locked in the GTP-bound state [25–27]. Codon 61 mutations have profound effect on intrinsic GTPase activity when Ras is bound to Raf [28].
Despite substantial homology, there appear to be non-overlapping roles for the different Ras isoforms. N-Ras and H-Ras are dispensable for cell survival, as double (and single) knockout Hras and Nras knockout mice are viable and have no reproductive defects, whereas Kras -/- mice are embryonic lethal [29–32]. Nras-null mice appear to develop normally, but have impaired responses to viral pathogens owing to impaired T-cell activation [33]. Interestingly, NRAS overexpression has been shown to drive granulocytosis, T-cell expansion and early lethality [34].
NRAS is the second most common mutation in metastatic melanoma (15–30% of human melanomas) after BRAF, which is mutated in 40–50% of melanomas [4,21]. While mutations in KRAS are commonly found in lung adenocarcinoma, colon cancer and pancreatic cancer, mutations in NRAS occur in distinct tumor types including melanoma, acute myeloid leukemia and thyroid cancer [35] (Table 1). Oncogenic Ras stimulates downstream signaling through PI3K/Akt, MEK/ERK and Ral-GDS via RAF activation [36,37] (Figure 1). There are three Raf isoforms – A-Raf, B-Raf, and C-Raf – that are capable to activating MEK downstream of Ras activation, but have distinct cellular roles [38]. In normal melanocytes N-Ras signals through B-Raf dimers (rather than C-Raf/Raf–1), but Raf class-switching occurs in NRAS mutant melanoma whereby C-Raf signaling predominates as a consequence of ERK-mediated B-Raf inactivation [39]. While clinical data regarding the impact of BRAF inhibitors in NRAS melanoma are lacking, preclinical evidence indicates administration of BRAF inhibitors to NRAS mutant melanoma results in ERK activation and stimulation of tumor growth [40,41].
Table 1. . NRAS mutations in various malignancies.
| Cancer type | Frequency (%) | Ref. |
|---|---|---|
| Melanoma | 15–30 | [42–45] |
| Acute myeloid leukemia | 10 | [46,47] |
| Colon cancer | 1–2 | [48] |
| Thyroid cancer | 10 | [49] |
| Acute lymphoblastic leukemia | 10–15 | [50–52] |
| Multiple myeloma | 15–20 | [53] |
| Myelodysplastic syndrome | 5 | [54] |
| Juvenile myelomonocytic leukemia/chronic myelomonocytic leukemia | 20 | [55,56] |
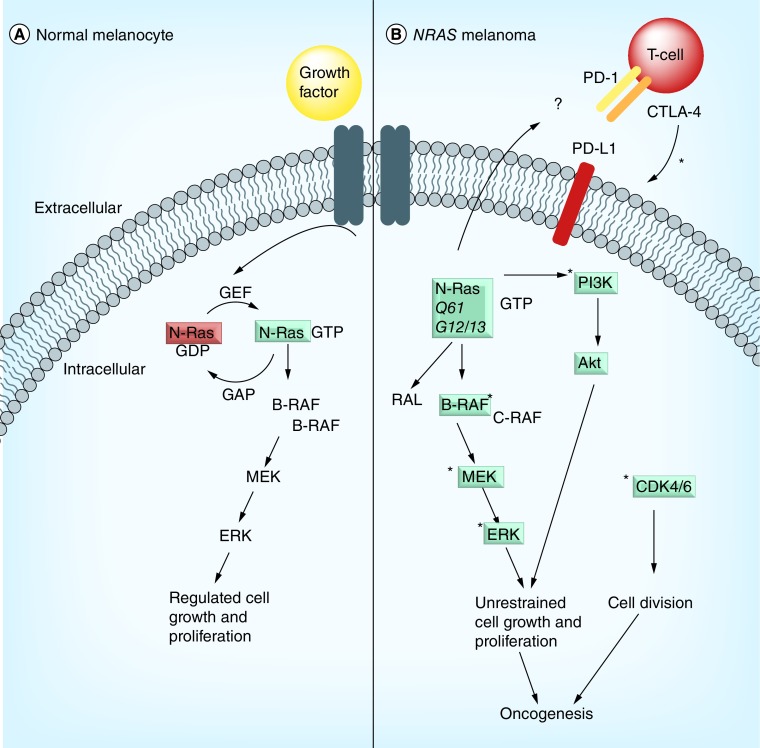
Figure 1. . N-Ras signaling and effector pathways.
(A) In normal melanocytes, N-Ras is inactive until a stimulus (e.g., growth factor) binds its receptor tyrosine kinase, which promotes exchange of GDP to GTP by GEFs, thereby activating N-Ras. In normal melanocytes, activation of N-Ras signaling is mediated by the Raf-MEK-ERK pathway. (B) In NRAS mutant melanoma, the N-ras protein is unable to hydrolyze GTP to GDP, and remains in the GTP-bound, active state. Oncogenic N-Ras stimulates various Ras effector pathway, including Raf, PI3K/Akt, and RAL. Ras-Raf-MEK-ERK signaling drives cellular proliferation, as does the PI3K/Akt pathway. Downstream signaling can be disrupted via inhibition of MEK signaling, and in cooperation with MEK inhibition. Disruption of CDK4/6 signaling cooperates to disable tumor growth in NRAS mutant melanoma. There is some suggestion the response to immune checkpoint blockade is enhanced in patients with NRAS mutant melanoma, but the reports are limited and a proposed mechanism is lacking. Treatment of NRAS mutant melanoma (*) involves targeting MEK, PI3K/Akt, CDK4/6, or immune checkpoints (PD1, CTLA-4).
Clinical & pathologic features of NRAS mutant melanoma
Interest in targeting NRAS mutant melanoma has grown substantially in recent years [36,57–58]. RAS mutations have been detected in up to 1/3 of melanomas [21,42–43], and mutations in codons 12, 13 and 61 of NRAS occur in 15–20% of melanomas, with some series reporting a frequency up to 30% [44,45]. Interestingly, in melanoma, 80–90% of all NRAS mutations are in codon 61 (and more frequently involve Gln to Arg), whereas mutations in codons 12 and 13 predominate in colon, pancreatic, lung and ovarian cancer comprising roughly 90% of all KRAS mutations [19,48,59]. NRAS mutations have been identified in multiple subtypes of melanoma including acral lentiginous melanoma and sinonasal mucosal melanomas with a frequency of ˜15% [60,61]. In an MD Anderson Cancer Center series, NRAS mutations were the most common genetic lesion in acral and mucosal melanomas, present in 20–25% of patient samples [44]. There are reports of regional differences in the incidence of NRAS and BRAF mutations, which may contribute to the variability in the frequency of NRAS mutations in various series [62,63].
Clinically, NRAS mutant melanoma is associated with thicker primary melanomas and a poorer prognosis compared with BRAF mutant melanoma, with 75% occurring in tumors >1 mm in thickness compared with 40% of BRAF mutant melanomas [64]. NRAS mutations are more commonly seen in melanomas arising on the extremities. They are also less likely to exhibit ulceration, but are associated with a higher mitotic rate compared with BRAF mutant melanoma [64]. NRAS and BRAF mutant melanoma are both more likely to arise from skin not exposed to chronic sun damage, and both are associated with a higher rate of central nervous system metastases compared with patients with NRAS and BRAF wild-type melanoma [65]. NRAS mutation status has been reported as independent risk factor of shorter survival following a diagnosis of stage IV melanoma, but this has been challenged in other analyses [65,66].
NRAS mutations appear to be an early event in melanomagenesis. Mutational status of nevi-associated melanomas has demonstrated that the majority of samples share the same mutational profile, with 60–70% concordance between melanomas and associated nevi [67]. As a consequence, it has been postulated that mutations in NRAS (and BRAF) that are found in benign and malignant nevi result from UV damage. However, evaluation of specific base pair changes within the NRAS and BRAF genes do not demonstrate a mutational signature enriched for UV-induced cytosine to thymidine transitions [68]. Importantly, both NRAS mutant and NF1–inactivated melanomas demonstrate a high mutational burden with a strong UV signature [21]. NRAS and BRAF mutations are for the most part mutually exclusive, however coexisting mutations have been reported [69–71]. While NRAS mutations are rarely found in benign nevi, BRAF mutations are evident in the majority of benign nevi [72]. In contrast, mutations in NRAS have been identified in 80–90% of large and giant congenital melanocytic nevi (CMN) and 50–70% of small and medium congenital melanocytic nevi [73,74], while BRAF mutations are rare. Interestingly, development of neurocutaneous melanocytosis is seen in a minority of patients with CMN, but NRAS mutations were present in three-quarters of these patients [74].
NRAS mutations are diagnosed by sequencing of primary tumor samples, which is increasingly available as a part of many next-generation sequencing platforms [44]. Knowledge of a patient's NRAS mutational status does not currently impact management outside of the clinical trial setting, and therefore routine mutational profiling is rarely performed outside of academic centers. Most next-generation sequencing platforms or multiplexed PCR platforms evaluate for mutations in codons 12, 13 and 61. Immunohistochemical techniques for detection of common NRAS mutations has recently been described, but are not widely available [75].
Aberrations in proteins that influence the activity/function of Ras, can also promote melanomagenesis. Neurofibromatosis type 1 results from mutations in neurofibromin (NF1) and is characterized by the development of tumors of neuroectodermal origin, particularly cutaneous neurofibromas. NF1 is a Ras-GTPase activating protein (Ras-GAP) that functions normally to restrict the activity of Ras proteins, and loss of NF1 signaling results in enhanced Ras signaling. NF1 is, therefore, considered a ‘RAS-opathy’ as the growth of these tumors results from aberrant Ras signaling [76]. Somatic deletions and mutations in NF1 have been described in cutaneous melanoma and are associated with growth-dependent Ras-Raf-MEK signaling [77,78]. Recently, NF1 mutations were found to be enriched in patients with desmoplastic melanoma [79]. Loss of NF1 has also been shown to mediate resistance to BRAF inhibitors [80].
In patients with BRAF mutant melanoma treated with vemurafenib or dabrafenib, mutations in NRAS and KRAS have been demonstrated in roughly 10–20% of resistant cancers [81,82]. In many cases this was due to acquisition of a codon 61 NRAS mutation that was not detected in the original tumor [83]. RAS, NF1 and BRAF mutations are generally mutually exclusive and typically do not co-occur in human melanoma, but there is evidence to support the existence of BRAF mutant and NRAS mutant clones within the same tumor [21,69,71]. Acquired resistance to BRAF inhibitors occurs through several mechanisms, including NRAS mutation [83], upregulation of signaling through RTKs (including EGFR) [83], activation of COT (MAP3K8) [84], MEK mutations, loss of NF1 [80], amplification of BRAF, alternative BRAF splicing and stromal secretion of growth factors [85,86].
Ras signaling is also central to one of the unique side effects of BRAF inhibitors. Treatment with vemurafenib or dabrafenib is associated with the development of secondary malignancies, most commonly keratoacanthomas and cutaneous squamous cell carcinomas due to paradoxical activation of MAPK signaling in keratinocytes. These occur in up to a quarter of patients usually within the first 3–6 months after initiating therapy, and are more common with increasing age [87]. RAS mutations have been identified in the majority of these squamous cell carcinomas, in particular mutations in HRAS [88,89]. The incidence of these therapy-related squamous cell carcinomas is markedly reduced when BRAF inhibitors are given with MEK inhibitors.
Strategies for treating NRAS mutant melanoma
While BRAF mutant melanoma has been successfully targeted with BRAF inhibitors, and more recently with BRAF and MEK inhibitors in combination, no such strategy exists for NRAS mutant melanoma. Given the success of small molecules targeting BRAF and MEK in BRAF mutant melanoma, there is growing interest in developing small molecule inhibitors for the 50% of patients without BRAF mutations, especially those with NRAS mutations. Outside of a clinical trial, the treatment of NRAS mutant melanoma at this time remains no different than other BRAF wild-type melanomas [36,90]. Most current trial options are early phase trials, and there are no published large, randomized studies in NRAS mutant melanoma to date, although some Phase III trials are open and accruing. NRAS and BRAF mutant melanoma share a poor response systemic cytotoxic chemotherapy [66].
Despite decades of research since the initial discovery of oncogenic Ras, an effective therapy for RAS mutant cancer has remained elusive. The three primary approaches that have been evaluated for targeting Ras are: direct Ras inhibitors, Ras post-translational modification inhibitors, and Ras effector pathway inhibitors. A fourth potential option for treatment of RAS mutant cancers is immunotherapy, although it is not selective for Ras. Immune checkpoint inhibitors are now first-line for BRAF wild-type melanoma, and while predictors of response to immunotherapy remain poorly understood, there is some suggestion that NRAS mutant melanoma may have higher response rates to immunotherapeutic agents.
Targeting Ras itself, both using direct inhibitors and by disrupting key post-translational modifications have been largely unsuccessful. Efforts to develop direct enzyme inhibitors of Ras GTPases have been unsuccessful for two primary reasons [91]. First, given the low picomolar GTP concentration required for GTPase function relative to the high intracellular concentration of GTP, competitive small molecule inhibitors are not feasible. Second, targeting the molecular switch from GTP to GDP has proven challenging as GDP stabilizes the inactive molecule. In the 1990s, efforts to disrupt RAS signaling by inhibiting enzymes responsible for key post-translational modifications to RAS were evaluated. RAS requires post-translational modification by farnesylation of critical cysteine residue in the C-terminus for proper membrane targeting [92]. Farnesyl transferase inhibitors were developed and showed promising activity in preclinical studies [93], but yielded disappointing results in early phase clinical studies [94]. There have been no further studies of inhibitors of Ras post-translational modifications.
Current approaches to targeting RAS mutant cancer, including NRAS mutant melanoma, now focus on targeting various downstream effector pathways, such as MEK-ERK, PI3K/Akt, RAL, and others (Figure 1). The only clinical trials to date that have evaluated any drugs prospectively in NRAS mutant melanoma have investigated the impact of MEK inhibitors, which have shown clinical activity in other RAS mutant cancers [95]. There have been a handful of early phase trials of various MEK inhibitors (reviewed in [57]), although none were specifically for NRAS mutant melanoma. Nevertheless, a phase II study of binimetinib (MEK162) demonstrated some clinical activity in patients with NRAS mutant melanoma. Although there were no complete responses, 6 of 30 (20%) patients had a partial response to MEK162 [96].
Given the limited activity of single agent MEK inhibition in other cancers as well as BRAF mutant melanoma, newer trials are evaluating the efficacy of a MEK inhibitor in combination with other agents. Preclinical work has identified CDK4 as a resistance mechanism to single-agent MEK inhibition, and demonstrated that combined treatment was more effective than single agent MEK in xenograft models of NRAS mutant melanoma [97]. The combination of MEK and CDK4/6 inhibition moved into phase I studies in the preliminary data were presented at ASCO 2014. In this study, patients with metastatic NRAS mutant melanoma were treated with combination binimetinib (MEK162) and LEE011 (a CDK4/6 inhibitor) in which 7 of 22 patients had a clinical response and 11 patients had stable disease [98]. A separate, phase I/II, dose-escalation study evaluating a different MEK + CDK4/6 combination (trametinib with palbociclib) is currently enrolling patients (NCT02065063). Combination of MEK inhibition with PI3K/Akt/mTOR inhibition is another strategy under investigation based on compelling preclinical data, and further supported by the high levels of Akt3 overexpression in RAS mutant melanoma in the TCGA analysis [21,99–100]. There are now several trials underway evaluating various drug combinations for patients with NRAS mutant melanoma (Table 2), and many more preclinical studies evaluating novel drugs and drug combinations (Table 3).
Table 2. . Clinical trials for NRAS mutant melanoma.
| Clinical trial number | Phase | Population | Status | Drug(s)/notes |
|---|---|---|---|---|
| NCT01781572 | Ib/II | Locally advanced or metastatic NRAS mutant melanoma | Currently recruiting | Binimetinib + ribociclib (LEE011) [101] |
| NCT02065063 | I/II | Multiple cancers | Currently recruiting | Trametinib + palbociclib [102] |
| NCT01363232 | Ib/II | Multiple cancers | Ongoing, no longer recruiting | PI3K inhibitor BKM120 + the MEK1/2 inhibitor MEK162 [103] |
| NCT01337765 | Ib | Multiple cancers | CLOSED | PI3K/mTOR inhibitor BEZ235 in combination with the MEK1/2 inhibitor MEK162 [104] |
| NCT01449058 | Ib | Multiple cancers | Currently recruiting | BYL719 plus MEK162 [105] |
| NCT01941927 | II | BRAF wild-type mutation melanoma | Ongoing, no longer recruiting | Trametinib (2 mg) in combination with uprosertib GSK2141795 (25 mg) oral daily [106] |
| NCT01693068 | II | NRAS mutant locally advanced or metastasis malignant cutaneous melanoma | Ongoing, no longer recruiting | Pimasertib (MEK 1/2 inhibitor) vs dacarbazine [107] |
| NCT01763164 | III | Metastatic or unresectable cutaneous melanoma (NRAS Q61 mutant only) | Currently recruiting | NEMO: Phase III trial of binimetinib (MEK162) vs dacarbazine [108] |
| NCT01352273 | Ib | Adult patients with advanced solid tumors harboring RAS or BRAFV600E mutations | Completed | MEK162 and RAF265 in adult patients with advanced solid tumors harboring RAS or BRAFV600E mutations [109] |
Table 3. . Select preclinical studies and early phase clinical trials for NRAS mutant melanoma.
| Target | Agent(s) | Details | Ref. |
|---|---|---|---|
| MEK + PI3K/mTOR | Multiple | MEK and PI3K/mTOR1,2 inhibition is synergistic in xenograft model of NRAS melanoma | [110] |
| PI3K/mTOR + BRAF + MEK | Omipalisib (GSK2126458) | PI3K/mTOR (with dabrafenib + trametinib) | [111] |
| PKC delta | B106 | Novel PKC delta inhibitors demonstrated activity in NRAS melanoma cell lines and triggers apoptosis (B106, most selective and potent) | [112] |
| c-MET | PHA665752 | c-Met phosphorylation elevated in NRAS melanoma primary tumors | [113] |
| RAF | PRi | Enhanced efficacy of combination pan-RAF inhibitor (Amgen) with MEK inhibitor (trametinib) in NRAS melanoma cell lines | [114] |
| RAF | PLX7904 | Novel Raf inhibitors – PLX7904 showed activity in BRAF mutant melanoma with a secondary NRAS Q61 resistance mutation | [115] |
| ERK | SCH772984 | ERK 1/2 inhibitor, SCH772984, has single agent activity in both BRAF and NRAS mutant melanoma | [116] |
| Multitarget | Amuvatinib (MP–470) | Multitarget kinase inhibitor (activity against c-Kit, Axl, PDGFRA, Rad61) inhibited growth of NRAS (but not BRAF) mutant melanoma cell lines | [117] |
| HSP90 | XL888 | HSP90 inhibitor XL888 downregulated Wee1, AKT, and CDK4 in NRAS mutant melanoma in vitro and in vivo | [118] |
| GRM1 + mTOR | Riluzole | (GRM1 – metabotropic glutamate receptor 1 inhibitor) with mTOR inhibitor slowed melanoma growth regardless of BRAF status | [119] |
| MEK + TBK1 | Overexpression of TBK1 (an effector of the RAS-RAL pathway) promoted invasive behavior and inhibition/depletion of TBK1 decreased invasive features, which was enhanced with a MEK inhibitor | [120] | |
| mTOR + Wee1 | MK–1775 + Torin 1 | Wee1 inhibition potentiated inhibition of mTOR in NRAS mutant leukemia and melanoma | [121] |
| MEK/ERK + ROCK | GSK269962A + trametinib | MEK inhibitor (or ERK inhibitor) + ROCK inhibitor resulted in decreased proliferation and cell death in vitro | [122] |
| MEK + Plk1 | JTP–74057 + BI 6727 | Plk1 overexpressed in NRAS melanoma. MEK and Plk1 inhibition demonstrated activity in vitro and in xenograft model of NRAS melanoma (MEK inhibitor – JTP-74057 and Plk1 inhibitor – BI 6727 | [123] |
| MEK | PD325901 | GAB2 induces angiogenesis in NRAS mutant melanoma (and is sensitive to MEK inhibition) | [124] |
| Akt/NF-kB | BI–69A11 | BI–69A11 (inhibitor of Akt/sphingosine kinase-NF-kB) Inhibited tumor growth in a murine model of NRAS melanoma | [125] |
| MEK | Trametinib + metformin | Combination metformin and trametinib decreased NRAS melanoma growth in vitro and in xenograft model | [126] |
Immunotherapy in NRAS mutant melanoma
In BRAF wild-type melanoma, immunotherapy with either CTLA-4 inhibition or PD-1 inhibition is now first-line therapy, with recent reports suggesting PD-1 immune checkpoint inhibitors have higher response rates with a lower incidence of toxicity. Moreover, combination immune checkpoint blockade with ipilimumab and nivolumab demonstrated higher response rates than either drug alone, albeit with a much higher rate of grade 3 or 4 toxicities. Immunotherapy offers the possibility of prolonged and durable responses in the metastatic setting, but only in a small subset of patients. Efforts are underway to increase the number of patients who respond to immune checkpoint inhibitors. Presently, there are no biomarkers established for routine clinical use, patient characteristics, or pathologic features that are strongly predictive of response to immunotherapies, but this remains an area of active investigation.
Increased expression of PD-L1 (the ligand expressed on some tumor cells that interacts with PD-1) is associated with increased response rates to PD-1 inhibition in many cancers. In melanoma, and some other cancers, higher expression of PD-L1 is associated with higher response rates [127,128]. However, PD-L1 positivity (or negativity) has not been predictive of response to PD-1 inhibitors equally across all studies and cancer types, prompting many to seek out other determinants and biomarkers to predict response to these agents.
BRAF status does not significantly influence the response to immune checkpoint inhibition, as response rates and progression-free survival data are comparable in patients with BRAF wild-type and BRAF mutant melanoma [13]. Differential expression of PD-L1 by specific driver mutation has been evaluated in cell culture, and failed to demonstrate a clear difference in PD-L1 expression based on BRAF and NRAS status in melanoma although PD-L1 expression appears to be variably affected by BRAF and MEK inhibitors [129].
Two, small retrospective studies suggest that responsiveness to immunotherapy may be influenced by NRAS mutational status. A retrospective analysis of patients that received high-dose IL–2 demonstrated a response rate of 47% in patients carrying NRAS mutations compared with 23% for those with BRAF mutations, and 12% that were NRAS-BRAF wild-type [130]. While the finding was statistically significant and compelling, it was a retrospective evaluation of a small patient population. Out of the 208 patients identified that were identified in the initial review, only 108 had available tissue for testing, there were only 7 out of 15 patients with NRAS mutations. Furthermore, there was no improvement in progression-free survival or overall survival in patients harboring NRAS mutations.
More recently a retrospective analysis of patients treated with immunotherapy (including high dose-IL2, ipilimumab, and PD-1/PD-L1 antibodies) was performed to evaluate response rate by mutational status [131]. Sixty of 229 patients had NRAS mutations (most in codon 61), and this cohort had a statistically significant higher response rate (28 vs 16%) and clinical benefit rate (50 vs 31%), with a trend towards significance in progression-free survival, overall survival and response to any-line of immunotherapy. Subgroup analysis revealed that the NRAS mutant cohort had a particularly impressive response to immune checkpoint inhibitors, most notably with respect to the clinical benefit rate and response rate to PD1/PD-1L therapy. Clinical benefit was seen in 8 out of 11 patients (73%) with NRAS mutant melanoma treated with PD-1/PD-1L therapy compared with 13 out of 37 (35%) in the non-NRAS mutant group (WT and BRAF mutant). The proportion of PD-L1-positive cells was also higher in NRAS mutant melanoma samples (from an independent cohort of archived samples), although this result was not statistically significant. It is conceivable that enhanced responses to immune checkpoint inhibition in this patient population is due to increased neoantigen formation in the background of a higher mutational burden of associated with NRAS mutant melanoma [21]. However, this is purely speculation and future studies in larger populations will be required to substantiate these findings, and to determine the mechanism whereby NRAS mutant melanoma might predict improved response rate to immunotherapies.
Conclusion & future perspective
The incidence of melanoma in the USA and worldwide has risen over the last two decades. Aberrant signaling through the Ras-Raf pathway is present in up to three-quarters of all melanomas, and anywhere from 15–30% of melanomas harbor mutations in NRAS. While the treatment of melanoma has been revolutionized over the last decade, there are no specific targeted agents for patients with NRAS mutant melanoma. Efforts to target NRAS mutant melanoma have failed to yield substantial clinical benefit to date, but there is a growing body of preclinical evidence identifying new potential drug targets. Moreover, several additional drug combinations are currently under investigation for NRAS driven melanoma. As the molecular understanding of NRAS mutant melanoma continues to evolve, new therapies are likely to emerge. NRAS mutant melanoma may have higher response rates to immunotherapies, and in particular PD-1/PD-L1 inhibition, but this will require validation in larger, prospective studies.
Improved understanding of the molecular basis of melanomagenesis, metastasis, clonal evolution, as well as identification of factors that predict both response and resistance will inform the evaluation of strategies to target NRAS mutant melanoma. Paired analysis of primary tumors and sites of metastases to better understand mutational stability and acquisition of new mutations will likely provide additional insight into mechanisms of resistance and response [132], as will identification of molecular determinants of resistance [82]. Once a successful drug or drug combination is identified, characterizing exceptional responders may identify additional factors that can guide selection of therapies [85]. In addition to profiling tumors for acquired mutations that influence response to therapies, identification of germline mutations or variants in genes that influence inflammatory response may also inform our understanding of the basis of response to immune-targeted agents, as was recently demonstrated with an patient with lung adenocarcinoma that had an exceptional response to anti-PD-L1 therapy and was found to have a germline JAK3 mutation [133]. With better understanding of both NRAS melanoma and determinants of immunotherapy response, combinations of targeted agents with immune checkpoint inhibitors may be viable option to improve both response rates and durability of responses, as has been suggested for BRAF mutant melanoma [134,135], although rational selection of agents and close monitoring with be essential to minimize both toxicity [136] and loss of efficacy.
Footnotes
Financial & competing interests disclosure
RW Jenkins has served as a consultant for Novartis and Astex Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346(6212):945–949. doi: 10.1126/science.1253735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan R, Lorusso P, Boerner S, Dummer R. Achievements and challenges of molecular targeted therapy in melanoma. Am. Soc. Clin. Oncol. Educ. Book. 2015;35:177–186. doi: 10.14694/EdBook_AM.2015.35.177. [DOI] [PubMed] [Google Scholar]
- 3.Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin. Oncol. 2015;42(3):429–435. doi: 10.1053/j.seminoncol.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated braf in metastatic melanoma. N. Engl. J. Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and mek inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 10.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 11.Hodi FS, O'day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of overall survival benefit with an immune checkpoint inhibitor.
- 12.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 15.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posch C, Ortiz-Urda S. Nras mutant melanoma – undrugable? Oncotarget. 2013;4(4):494–495. doi: 10.18632/oncotarget.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malumbres M, Barbacid M. Ras oncogenes: The first 30 years. Nat. Rev. Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 18.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 2008;9(7):517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes SA, Bindal N, Bamford S, et al. Cosmic: mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2011;39(Database issue):D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerami E, Gao J, Dogrusoz U, et al. The cbio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas N, Akbani R. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Cancer Genome Atlas Network evaluation of cutaneous melanoma, in which nearly 30% of evaluated melanomas harbored mutations in NRAS.
- 22.Hall A, Marshall CJ, Spurr NK, Weiss RA. Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature. 1983;303(5916):396–400. doi: 10.1038/303396a0. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Goldfarb M, Suard Y, et al. Three human transforming genes are related to the viral RAS oncogenes. Proc. Natl Acad. Sci. USA. 1983;80(8):2112–2116. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock JF. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003;4(5):373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 25.Buhrman G, Holzapfel G, Fetics S, Mattos C. Allosteric modulation of ras positions q61 for a direct role in catalysis. Proc. Natl Acad. Sci. USA. 2010;107(11):4931–4936. doi: 10.1073/pnas.0912226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adari H, Lowy DR, Willumsen BM, Der CJ, Mccormick F. Guanosine triphosphatase activating protein (gap) interacts with the p21 ras effector binding domain. Science. 1988;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- 27.Frech M, Darden TA, Pedersen LG, et al. Role of glutamine–61 in the hydrolysis of gtp by p21h-RAS: an experimental and theoretical study. Biochemistry. 1994;33(11):3237–3244. doi: 10.1021/bi00177a014. [DOI] [PubMed] [Google Scholar]
- 28.Buhrman G, Kumar VS, Cirit M, Haugh JM, Mattos C. Allosteric modulation of RAS-GTP is linked to signal transduction through raf kinase. J. Biol. Chem. 2011;286(5):3323–3331. doi: 10.1074/jbc.M110.193854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson L, Greenbaum D, Cichowski K, et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11(19):2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umanoff H, Edelmann W, Pellicer A, Kucherlapati R. The murine N-RAS gene is not essential for growth and development. Proc. Natl Acad. Sci. USA. 1995;92(5):1709–1713. doi: 10.1073/pnas.92.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koera K, Nakamura K, Nakao K, et al. K-ras is essential for the development of the mouse embryo. Oncogene. 1997;15(10):1151–1159. doi: 10.1038/sj.onc.1201284. [DOI] [PubMed] [Google Scholar]
- 32.Esteban LM, Vicario-Abejon C, Fernandez-Salguero P, et al. Targeted genomic disruption of H-RAS and N-RAS, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol. Cell Biol. 2001;21(5):1444–1452. doi: 10.1128/MCB.21.5.1444-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez De Castro I, Diaz R, Malumbres M, et al. Mice deficient for N-RAS: impaired antiviral immune response and T-cell function. Cancer Res. 2003;63(7):1615–1622. [PubMed] [Google Scholar]
- 34.Lassen LB, Ballarin-Gonzalez B, Schmitz A, Fuchtbauer A, Pedersen FS. Fuchtbauer EM. NRAS overexpression results in granulocytosis, T-cell expansion and early lethality in mice. PLoS ONE. 2012;7(8):e42216. doi: 10.1371/journal.pone.0042216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson DB, Smalley KS, Sosman JA. Molecular pathways: targeting NRAS in melanoma and acute myelogenous leukemia. Clin. Cancer Res. 2014;20(16):4186–4192. doi: 10.1158/1078-0432.CCR-13-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedorenko IV, Gibney GT, Smalley KS. Nras mutant melanoma: biological behavior and future strategies for therapeutic management. Oncogene. 2013;32(25):3009–3018. doi: 10.1038/onc.2012.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zipfel PA, Brady DC, Kashatus DF, Ancrile BD, Tyler DS, Counter CM. RAL activation promotes melanomagenesis. Oncogene. 2010;29(34):4859–4864. doi: 10.1038/onc.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desideri E, Cavallo AL, Baccarini M. Alike but different: RAF paralogs and their signaling outputs. Cell. 2015;161(5):967–970. doi: 10.1016/j.cell.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 39.Dumaz N, Hayward R, Martin J, et al. In melanoma, Ras mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 2006;66(19):9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 40.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type raf to activate the mapk pathway and enhance growth. Nature. 2010;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 41.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. Raf inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ball NJ, Yohn JJ, Morelli JG, Norris DA, Golitz LE, Hoeffler JP. RAS mutations in human melanoma: a marker of malignant progression. J. Invest. Dermatol. 1994;102(3):285–290. doi: 10.1111/1523-1747.ep12371783. [DOI] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas Network. Electronic Address IMO, Cancer Genome Atlas N. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siroy AE, Boland GM, Milton DR, et al. Beyond BRAF(v600): clinical mutation panel testing by next-generation sequencing in advanced melanoma. J. Invest. Dermatol. 2015;135(2):508–515. doi: 10.1038/jid.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edlundh-Rose E, Egyhazi S, Omholt K, et al. Nras and Braf mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16(6):471–478. doi: 10.1097/01.cmr.0000232300.22032.86. [DOI] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat. Rev. Cancer. 2011;11(11):761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bos JL. RAS oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 50.Flex E, Petrangeli V, Stella L, et al. Somatically acquired Jak1 mutations in adult acute lymphoblastic leukemia. J. Exp. Med. 2008;205(4):751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perentesis JP, Bhatia S, Boyle E, et al. RAS oncogene mutations and outcome of therapy for childhood acute lymphoblastic leukemia. Leukemia. 2004;18(4):685–692. doi: 10.1038/sj.leu.2403272. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early t-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chng WJ, Gonzalez-Paz N, Price-Troska T, et al. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia. 2008;22(12):2280–2284. doi: 10.1038/leu.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011;364(26):2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricci C, Fermo E, Corti S, et al. RAS mutations contribute to evolution of chronic myelomonocytic leukemia to the proliferative variant. Clin. Cancer Res. 2010;16(8):2246–2256. doi: 10.1158/1078-0432.CCR-09-2112. [DOI] [PubMed] [Google Scholar]
- 56.Ward AF, Braun BS, Shannon KM. Targeting oncogenic RAS signaling in hematologic malignancies. Blood. 2012;120(17):3397–3406. doi: 10.1182/blood-2012-05-378596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson DB, Puzanov I. Treatment of NRAS-mutant melanoma. Curr. Treat. Options Oncol. 2015;16(4):15. doi: 10.1007/s11864-015-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelleher FC, McArthur GA. Targeting NRAS in melanoma. Cancer J. 2012;18(2):132–136. doi: 10.1097/PPO.0b013e31824ba4df. [DOI] [PubMed] [Google Scholar]
- 59.Burd CE, Liu W, Huynh MV, et al. Mutation-specific ras oncogenicity explains nras codon 61 selection in melanoma. Cancer Discov. 2014;4(12):1418–1429. doi: 10.1158/2159-8290.CD-14-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zebary A, Omholt K, Vassilaki I, et al. Kit, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72(3):284–289. doi: 10.1016/j.jdermsci.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Zebary A, Jangard M, Omholt K, Ragnarsson-Olding B, Hansson J. Kit, NRAS and BRAF mutations in sinonasal mucosal melanoma: a study of 56 cases. Br. J. Cancer. 2013;109(3):559–564. doi: 10.1038/bjc.2013.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Den Hurk K, Balint B, Toomey S, et al. High-throughput oncogene mutation profiling shows demographic differences in BRAF mutation rates among melanoma patients. Melanoma Res. 2015;25(3):189–199. doi: 10.1097/CMR.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 63.Sheen YS, Liao YH, Liau JY, et al. Prevalence of BRAF and NRAS mutations in cutaneous melanoma patients in taiwan. J. Formos. Med. Assoc. 2016;115(2):121–127. doi: 10.1016/j.jfma.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24(4):666–672. doi: 10.1111/j.1755-148X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 65.Jakob JA, Bassett RL, Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118(16):4014–4023. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides evidence of prognostic impact of NRAS mutations.
- 66.Carlino MS, Haydu LE, Kakavand H, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br. J. Cancer. 2014;111(2):292–299. doi: 10.1038/bjc.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shitaraa D, Tell-Marti G, Badenas C, et al. Mutational status of nevus associated-melanomas. Br. J. Dermatol. 2016;173(3):671–680. doi: 10.1111/bjd.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sensi M, Nicolini G, Petti C, et al. Mutually exclusive NRASQ61R and BRAFV600E mutations at the single-cell level in the same human melanoma. Oncogene. 2006;25(24):3357–3364. doi: 10.1038/sj.onc.1209379. [DOI] [PubMed] [Google Scholar]
- 70.Zebary A, Omholt K, Van Doorn R, et al. Somatic BRAF and NRAS mutations in familial melanomas with known germline CDKN2A status: a genomel study. J. Invest. Dermatol. 2014;134(1):287–290. doi: 10.1038/jid.2013.270. [DOI] [PubMed] [Google Scholar]
- 71.Jovanovic B, Egyhazi S, Eskandarpour M, et al. Coexisting nras and braf mutations in primary familial melanomas with specific CDKN2A germline alterations. J. Invest. Dermatol. 2010;130(2):618–620. doi: 10.1038/jid.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16(4):267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 73.Charbel C, Fontaine RH, Malouf GG, et al. NRAS mutation is the sole recurrent somatic mutation in large congenital melanocytic nevi. J. Invest. Dermatol. 2014;134(4):1067–1074. doi: 10.1038/jid.2013.429. [DOI] [PubMed] [Google Scholar]
- 74.Salgado CM, Basu D, Nikiforova M, et al. BRAF mutations are also associated with neurocutaneous melanocytosis and large/giant congenital melanocytic nevi. Pediatr. Dev. Pathol. 2015;18(1):1–9. doi: 10.2350/14-10-1566-OA.1. [DOI] [PubMed] [Google Scholar]
- 75.Massi D, Simi L, Sensi E, et al. Immunohistochemistry is highly sensitive and specific for the detection of NRASQ61R mutation in melanoma. Mod. Pathol. 2015;28(4):487–497. doi: 10.1038/modpathol.2014.137. [DOI] [PubMed] [Google Scholar]
- 76.Ratner N, Miller SJ. A rasopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat. Rev. Cancer. 2015;15(5):290–301. doi: 10.1038/nrc3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with ras activation and mek dependence. Cancer Res. 2014;74(8):2340–2350. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maertens O, Johnson B, Hollstein P, et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov. 2013;3(3):338–349. doi: 10.1158/2159-8290.CD-12-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiesner T, Kiuru M, Scott SN, et al. NF1 mutations are common in desmoplastic melanoma. Am. J. Surg. Pathol. 2015 doi: 10.1097/PAS.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whittaker SR, Theurillat JP, Van Allen E, et al. A genome-scale rna interference screen implicates NF1 loss in resistance to raf inhibition. Cancer Discov. 2013;3(3):350–362. doi: 10.1158/2159-8290.CD-12-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to b-RAF(V600E) inhibition by RTK or n-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to raf inhibition through map kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wheler J, Yelensky R, Falchook G, et al. Next generation sequencing of exceptional responders with BRAF-mutant melanoma: implications for sensitivity and resistance. BMC Cancer. 2015;15:61. doi: 10.1186/s12885-015-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spagnolo F, Ghiorzo P, Orgiano L, et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco. Targets Ther. 2015;8:157–168. doi: 10.2147/OTT.S39096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anforth R, Menzies A, Byth K, et al. Factors influencing the development of cutaneous squamous cell carcinoma in patients on BRAF inhibitor therapy. J. Am. Acad. Dermatol. 2015;72(5):809–815. doi: 10.1016/j.jaad.2015.01.018. e801. [DOI] [PubMed] [Google Scholar]
- 88.Anforth R, Tembe V, Blumetti T, Fernandez-Penas P. Mutational analysis of cutaneous squamous cell carcinomas and verrucal keratosis in patients taking BRAF inhibitors. Pigment Cell Melanoma Res. 2012;25(5):569–572. doi: 10.1111/j.1755-148X.2012.01031.x. [DOI] [PubMed] [Google Scholar]
- 89.Oberholzer PA, Kee D, Dziunycz P, et al. Ras mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J. Clin. Oncol. 2012;30(3):316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson DB, Puzanov I. Treatment of NRAS-mutant melanoma. Curr. Treat. Options Oncol. 2015;16(4):330. doi: 10.1007/s11864-015-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gysin S, Salt M, Young A, Mccormick F. Therapeutic strategies for targeting RAS proteins. Genes Cancer. 2011;2(3):359–372. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of gtpases as anticancer targets. Nat. Rev. Drug Discov. 2007;6(7):541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 93.Kohl NE, Wilson FR, Mosser SD, et al. Protein farnesyltransferase inhibitors block the growth of RAS-dependent tumors in nude mice. Proc. Natl Acad. Sci. USA. 1994;91(19):9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gajewski TF, Salama AK, Niedzwiecki D, et al. Phase II study of the farnesyltransferase inhibitor r115777 in advanced melanoma (calgb 500104) J. Transl. Med. 2012;10:246. doi: 10.1186/1479-5876-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Z, Golay HG, Barbie DA. Targeting pathways downstream of KRAS in lung adenocarcinoma. Pharmacogenomics. 2014;15(11):1507–1518. doi: 10.2217/pgs.14.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ascierto PA, Schadendorf D, Berking C, et al. Mek162 for patients with advanced melanoma harbouring NRAS or val600 braf mutations: a non-randomised, open-label Phase 2 study. Lancet Oncol. 2013;14(3):249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]; •• This is the first study to demonstrate activity of a targeted therapy in NRAS-mutant melanoma.
- 97.Kwong LN, Costello JC, Liu H, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat. Med. 2012;18(10):1503–1510. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This preclinical study utilizing an inducible NRAS murine melanoma model demonstrated synergy with use of MEK and CDK4 inhibitors.
- 98.Sosman Ja KM, Lolkema M, et al. A Phase 1b/2 study of lee011 in combination with binimetinib (mek162) in patients with nras-mutant melanoma: early encouraging clinical activity. J. Clin. Oncol. 2014;2014;32(5s) suppl; abstr 9009. [Google Scholar]
- 99.Jaiswal BS, Janakiraman V, Kljavin NM, et al. Combined targeting of BRAF and CRAF or BRAF and PI3K effector pathways is required for efficacy in nras mutant tumors. PLoS ONE. 2009;4(5):e5717. doi: 10.1371/journal.pone.0005717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Posch C, Moslehi H, Feeney L, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo . Proc. Natl Acad. Sci. USA. 2013;110(10):4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.https://clinicaltrials.Gov/ct2/show/nct01781572 Clinicaltrials database: NCT01781572.
- 102.https://clinicaltrials.Gov/ct2/show/nct02065063 Clinicaltrials database: NCT02065063.
- 103.https://clinicaltrials.Gov/ct2/show/nct01363232 Clinicaltrials database: NCT01363232.
- 104.https://clinicaltrials.Gov/ct2/show/nct01337765 Clinicaltrials database: NCT01337765.
- 105.https://clinicaltrials.Gov/ct2/show/nct01449058 Clinicaltrials database: NCT01449058.
- 106.https://clinicaltrials.Gov/ct2/show/nct01941927 Clinicaltrials database: NCT01941927.
- 107.https://clinicaltrials.Gov/ct2/show/nct01693068 Clinicaltrials database: NCT01693068.
- 108.https://clinicaltrials.Gov/ct2/show/nct01763164 Clinicaltrials database: NCT01763164.
- 109.https://clinicaltrials.Gov/ct2/show/nct01352273 Clinicaltrials database: NCT01352273.
- 110.Posch C, Moslehi H, Feeney L, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo . Proc. Natl Acad. Sci. USA. 2013;110(10):4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/MTOR inhibitors overcome acquired resistance to the BRAF inhibitor gsk2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol. Cancer Ther. 2012;11(4):909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 112.Takashima A, English B, Chen Z, et al. Protein kinase cdelta is a therapeutic target in malignant melanoma with NRAS mutation. ACS Chem. Biol. 2014;9(4):1003–1014. doi: 10.1021/cb400837t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chattopadhyay C, Ellerhorst JA, Ekmekcioglu S, Greene VR, Davies MA, Grimm EA. Association of activated c-Met with NRAS-mutated human melanomas. Int. J. Cancer. 2012;131(2):E56–65. doi: 10.1002/ijc.26487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Atefi M, Titz B, Avramis E, et al. Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol. Cancer. 2015;14:27. doi: 10.1186/s12943-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le K, Blomain ES, Rodeck U, Aplin AE. Selective raf inhibitor impairs ERK1/2 phosphorylation and growth in mutant NRAS, vemurafenib-resistant melanoma cells. Pigment. Cell Melanoma Res. 2013;26(4):509–517. doi: 10.1111/pcmr.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong DJ, Robert L, Atefi MS, et al. Antitumor activity of the ERK inhibitor sch722984 against BRAF mutant, NRAS mutant and wild-type melanoma. Mol Cancer. 2014;13:194. doi: 10.1186/1476-4598-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fedorenko IV, Fang B, Koomen JM, Gibney GT, Smalley KS. Amuvatinib has cytotoxic effects against NRAS-mutant melanoma but not braf-mutant melanoma. Melanoma Res. 2014;24(5):448–453. doi: 10.1097/CMR.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haarberg HE, Paraiso KH, Wood E, et al. Inhibition of Wee1, Akt, and Cdk4 underlies the efficacy of the Hsp90 inhibitor xl888 in an in vivo model of NRAS-mutant melanoma. Mol Cancer Ther. 2013;12(6):901–912. doi: 10.1158/1535-7163.MCT-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosenberg SA, Niglio SA, Salehomoum N, et al. Targeting glutamatergic signaling and the PI3 kinase pathway to halt melanoma progression. Transl Oncol. 2015;8(1):1–9. doi: 10.1016/j.tranon.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vu HL, Aplin AE. Targeting Tbk1 inhibits migration and resistance to MEK inhibitors in mutant NRAS melanoma. Mol. Cancer Res. 2014;12(10):1509–1519. doi: 10.1158/1541-7786.MCR-14-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weisberg E, Nonami A, Chen Z, et al. Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies. Leukemia. 2015;29(1):27–37. doi: 10.1038/leu.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vogel CJ, Smit MA, Maddalo G, et al. Cooperative induction of apoptosis in NRAS mutant melanoma by inhibition of MEK and rock. Pigment Cell Melanoma Res. 2015;28(3):307–317. doi: 10.1111/pcmr.12364. [DOI] [PubMed] [Google Scholar]
- 123.Posch C, Cholewa BD, Vujic I, et al. Combined inhibition of MEK and plk1 has synergistic antitumor activity in NRAS mutant melanoma. J. Invest. Dermatol. 2015;135(10):2475–2483. doi: 10.1038/jid.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Y, Wu J, Demir A, et al. Gab2 induces tumor angiogenesis in NRAS-driven melanoma. Oncogene. 2013;32(31):3627–3637. doi: 10.1038/onc.2012.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng Y, Lau E, Scortegagna M, et al. Inhibition of melanoma development in the nras((q61k)): Ink4a(-/-) mouse model by the small molecule bi–69a11. Pigment Cell Melanoma Res. 2013;26(1):136–142. doi: 10.1111/pcmr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vujic I, Sanlorenzo M, Posch C, et al. Metformin and trametinib have synergistic effects on cell viability and tumor growth in NRAS mutant cancer. Oncotarget. 2015;6(2):969–978. doi: 10.18632/oncotarget.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and mpdl3280a according to the tumor expression of programmed death-ligand–1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE. 2015;10(6):e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study to demonstrate activity of PD1 blockade in melanoma.
- 129.Atefi M, Avramis E, Lassen A, et al. Effects of mapk and PI3K pathways on PD-L1 expression in melanoma. Clin. Cancer Res. 2014;20(13):3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J. Immunother. 2012;35(1):66–72. doi: 10.1097/CJI.0b013e3182372636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnson DB, Lovly CM, Flavin M, et al. Impact of nras mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol. Res. 2015;3(3):288–295. doi: 10.1158/2326-6066.CIR-14-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This small, retrospective study suggests a higher response rate to immune checkpoint blockade in patients with NRAS mutant melanoma.
- 132.Varada S, Mahalingam M. Mutation stability in primary and metastatic melanoma: what we know and what we don't. Histol. Histopathol. 2015;30(7):763–770. doi: 10.14670/HH-11-584. [DOI] [PubMed] [Google Scholar]
- 133.Van Allen EM, Golay HG, Liu Y, et al. Long-term benefit of PD-L1 blockade in lung cancer associated with Jak3 activation. Cancer Immunol Res. 2015;3(8):855–863. doi: 10.1158/2326-6066.CIR-15-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tomei S, Bedognetti D, De Giorgi V, et al. The immune-related role of BRAF in melanoma. Mol. Oncol. 2015;9(1):93–104. doi: 10.1016/j.molonc.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cooper ZA, Juneja VR, Sage PT, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol. Res. 2014;2(7):643–654. doi: 10.1158/2326-6066.CIR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 2013;368(14):1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]